Abstract
Objective
Pulmonary disease is a leading cause of morbidity and mortality in rheumatoid arthritis (RA). In this study, we investigated the prevalence and progression of interstitial lung abnormalities (ILA) in a prospective cohort study of 18 subjects with early RA.
Methods
Eighteen adults diagnosed with anti-citrullinated protein-antibody-positive RA within the prior year underwent baseline high-resolution computed tomography (HRCT), symptom assessment, and pulmonary function and laboratory testing. The follow-up HRCT and clinical assessment were completed after 1 year.
Results
Seven of the 18 patients (39%) had baseline HRCT abnormalities including septal thickening, honeycombing, ground glass opacities, and/or traction bronchiectasis. At follow-up, 6 out of the 7 subjects (86%) with ILAs at baseline exhibited progression, while 10 out of 11 (91%) without ILAs at baseline remained stable. A higher Clinical Chronic Obstructive Pulmonary Disease Questionnaire score was associated with both the presence and progression of HRCT abnormalities (10 vs 2, p=0.045; 10 vs 2, p=0.009, respectively). C-reactive protein (CRP) trended higher in patients with radiologic abnormalities (3.5 mg/L vs 1.1 mg/L, p=0.08) and was significantly higher in those with progression (3.5 mg/L vs 1 mg/L, p=0.024). Smoking, pulmonary function, and autoantibodies were not associated with HRCT abnormalities.
Conclusion
ILAs are prevalent in patients with early RA. If identified at baseline, radiographic progression of ILAs after 1 year is likely, while those without ILAs at baseline are unlikely to develop new ILAs. In addition, early respiratory symptoms and higher CRP levels may correlate with the presence and progression of underlying ILAs.
Keywords: Rheumatoid arthritis, interstitial lung disease, autoantibodies, computed tomography, disease progression
Introduction
The characteristic manifestation of rheumatoid arthritis (RA) is joint disease, although extra-articular involvement is commonly seen (1). Lung involvement has been reported in up to 67% of patients with RA, and it can involve the pleura, airways, parenchyma, and vasculature (2). In particular, parenchymal disease in the form of interstitial lung disease (ILD) portends a poor prognosis and alone can account for 10%–20% of RA-related mortality (3). Currently, there are no formal guidelines for clinical screening of ILD in RA patients, but identifying RA patients who will develop ILD can improve clinical prognosis.
On high-resolution computerized tomography (HRCT), RA-associated ILD can present as multiple radiographic patterns, including usual interstitial pneumonia (UIP) characterized by fibrosis and honeycombing, as well as non-specific interstitial pneumonia (NSIP) distinguished by extensive ground glass opacities (4–5). Imaging features of reticular thickening, honeycombing, traction bronchiectasis, and/or ground glass opacities, without a diagnosis of UIP or NSIP, have been termed interstitial lung abnormalities (ILA), and they are thought to represent subclinical disease (6).
Multiple cross-sectional studies using HRCT have identified a high prevalence of ILAs in patients with early RA (7–14). However, the time course and significance of subtle findings of ILAs, often in the absence of significant respiratory symptoms, are not known. In addition, there is limited knowledge about the factors that may be associated with the prevalence and progression of ILAs in RA.
Utilizing a unique cohort of patients with both recent diagnosis of RA and anti-citrullinated protein/peptide antibody (ACPA) positivity, this study evaluated the prevalence and progression of ILAs by employing a novel scoring system for HRCT findings, as well as factors associated with these findings.
Methods
Study subjects and overall design
Eighteen consecutive individuals meeting study criteria were recruited from the rheumatology clinic at our institution. Study eligibility criteria included age >18 years, RA diagnosed within the past year (irrespective of symptom duration or prior treatments) by a rheumatology specialist and serum ACPA positivity as measured by the anti-cyclic citrullinated peptide (CCP) assay. Patients with existing respiratory diagnoses or additional connective tissue diseases were excluded.
From September 2011 to February 2013, a total of 18 patients with RA were enrolled. All 18 patients returned for a second visit at a median follow-up of 14 months, range 12 to 32 months.
Respiratory symptom assessment
At the initial study visit, all subjects completed a Clinical Chronic Obstructive Pulmonary Disease Questionnaire (CCQ) as a standardized measure for assessing respiratory symptoms (15). In addition, all subjects underwent HRCT, pulmonary function testing (PFT), as well as laboratory testing as described below. After at least a year from the initial visit, all subjects returned for a second HRCT.
High-resolution computerized tomography
HRCT of the chest without intravenous contrast was performed in the supine and prone positions using 1 mm collimation at 40 mm intervals for the high-resolution component and 5 mm collimation at 5 mm intervals for routine computerized tomography (GE 750HD CT, General Electric Medical Systems, Chicago, USA). A single radiologist (PJ), blinded to the patient identity and characteristics, but not to the sequence of the scans, read, and scored the images as described below.
HRCT scoring system
A radiologic quantitative scoring system was created based on the modification of a previously published scoring system for ILD (16). Each HRCT was divided into three zones axially, Zone 1 being defined as above the under-surface of the aortic arch, Zone 3 defined as below the inferior right pulmonary vein, and Zone 2 between the two landmarks. Given that we suspected the abnormalities would be subtle in majority of cases, the images were further divided as follows for further quantification. Two representative slices were selected from each zone for scoring per the radiologist’s discretion. In addition, each slice is divided into left and right lung for a total of 12 sections and assessed for the presence of ILAs defined by the presence of one or more of the following: (1) septal thickening, (2) honeycombing, (3) ground glass opacity (GGO), and (4) traction bronchiectasis. Each lung zone was scored based on a semi-quantitative estimate with a 4-point scale (0=no involvement, 1=1%–25% involvement, 2=26%–50% involvement, 3=51%–75% involvement, or 4=76%–100% involvement). The maximum possible score for each subject is 192, shown in Table 1. Subjects were considered to have positive radiographic findings if any abnormalities were seen (score of 1 or above).
Table 1.
Protocol for scoring CT chest.
| CT Scoring Protocol | |||
|---|---|---|---|
| Zone 1 | Slice 1 Left | Slice 1 Right | 12 Total Sections |
| Slice 2 Left | Slice 2 Right | ||
| Zone 2 | Slice 3 Left | Slice 3 Right | |
| Slice 4 Left | Slice 4 Right | ||
| Zone 3 | Slice 5 Left | Slice 5 Right | |
| Slice 6 Left | Slice 6 Right | ||
| Abnormal Findings: | Score 0=No involvement | 16 Point Maximum Per Section | |
| Septal Thickening | Score 1=1–25% | ||
| Honeycombing | Score 2=26–50% | ||
| Ground-Glass Opacities | Score 3=51–75% | ||
| Bronchiectasis | Score 4=76–100% | ||
| 192 Total Maximum Points |
Zone 1 is above the aortic arch. Zone 2 is between the aortic arch and the inferior pulmonary veins. Zone 3 is below the inferior pulmonary veins.
Pulmonary function testing
Measured pulmonary function values included the forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1-to-FVC ratio, total lung capacity, and diffusion capacity of carbon monoxide (DLCO). Values were expressed as the percentage of predicated values and interpreted according to the American Thoracic Society recommendations (17).
Serologic testing
At the baseline study visit, blood samples were collected from each subject and tested for the following biomarkers: (1) antibodies to CCP (CCP3.1 IgG/IgA ELISA; Inova Diagnostics, San Diego, USA); (2) high-sensitivity c-reactive protein (hsCRP) using nephelometry (Dade-Behring BNII; Dade-Behring, Deerfield, USA); and (3) rheumatoid factor (RF) by nephelometry (Dade-Behring BNII; Dade-Behring, Deerfield, USA).
Sputum antibody testing
Induced sputum samples were collected from each subject at the initial visit. The samples were processed at the University of Colorado Division of Rheumatology Clinical and Research Laboratory according to a previously published protocol (18). All samples were tested for RF isotypes IgM and IgG by ELISA (Quanta Lite kits; Inova Diagnostics, San Diego, USA) and anti-CCP3.1 (IgG/IgA ELISA; Inova Diagnostics, San Diego, USA).
Statistical analysis
All values are expressed as median with the interquartile range (IQR) for non-normally distributed data. In comparing baseline characteristics between subjects with ILAs present or absent on initial HRCT, a univariate analysis was performed. Variables with continuous values were statistically analyzed using two-sided Mann-Whitney U test. Variables with categorical values were analyzed using Fisher’s exact test. The relationship of variables (CCQ score, RF level, anti-CCP3.1 level, and hsCRP level) and either the presence or progression of ILAs was analyzed using a multivariate regression model. For sputum antibodies, which are considered to be experimental variables in this study, a two-sided Mann-Whitney U test was performed. For all analysis, a two-tailed p<0.05 was considered statistically significant.
Ethical considerations
This study was approved by the Institutional Review Board of the Cedars-Sinai Medical Center (approval #4422) in accordance to human subject rights. All subjects provided written informed consent.
Results
Imaging findings on HRCT
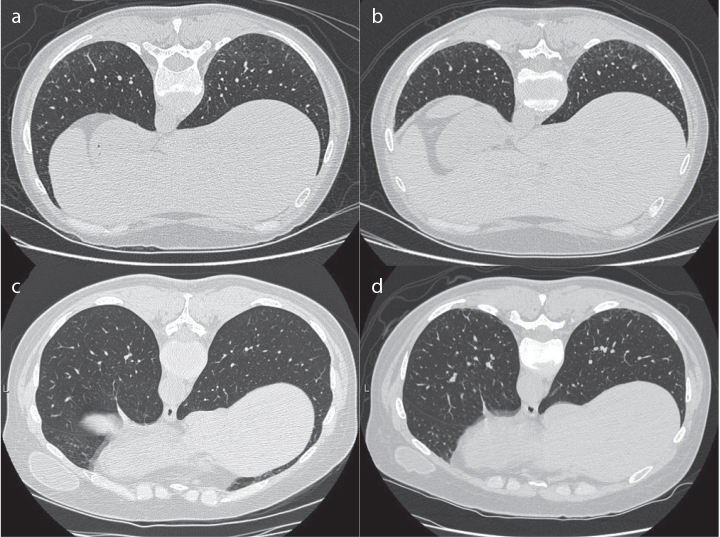
Of the 18 total subjects, 7 (39%) had ≥1 ILA on HRCT at the initial study visit; 7 (39%) had septal thickening, 3 (17%) had honeycombing, 2 (11%) had ground glass opacities, and 1 (6%) had bronchiectasis. At the follow-up visit (median 14 months), 6 of the 7 subjects (86%) with baseline ILAs demonstrated progression of ILAs as defined by an increased CT score, with a median increase of 4 points, range from 1 to 7 points (Table 2). The remaining 1 subject had a decrease from 2 to 0 points on follow-up. In addition, 1 of 11 (9%) subjects without lung abnormalities on their initial HRCT scan demonstrated progression of ILAs at follow-up with a CT score increase from 0 to 6 points at visit 2. The other 10 subjects without ILAs at baseline remained without any lung abnormalities on follow-up CT imaging. Representative HRCT images are shown in Figure 1.
Table 2.
HRCT scores in subjects with radiographic progression from visit 1 (gray) to visit 2 (white).
| Subject | SEP | HON | GGO | BRO | TOTAL |
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 0 | 5 |
| 8 | 4 | 0 | 0 | 12 | |
| 2 | 1 | 0 | 0 | 0 | 1 |
| 2 | 0 | 0 | 0 | 2 | |
| 3 | 3 | 0 | 0 | 0 | 3 |
| 4 | 0 | 0 | 0 | 4 | |
| 4 | 9 | 9 | 0 | 0 | 18 |
| 9 | 9 | 2 | 9 | 29 | |
| 5 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 6 | |
| 6 | 6 | 0 | 0 | 0 | 6 |
| 10 | 2 | 0 | 0 | 12 | |
| 7 | 10 | 0 | 10 | 0 | 20 |
| 12 | 12 | 0 | 0 | 24 |
SEP: Septal thickening; HON: Honeycombing; GGO: ground glass opacities; BRO: bronchiectasis.
Figure 1. a–d.
Representative HRCT images. Subject with baseline HRCT score of 20 (left) vs 13 months follow-up score of 24 (right) (a, b); subject with baseline HRCT score of 0 (left) vs 13 months follow-up score of 6 (right) (c, d).
Baseline characteristics
The characteristics of the subjects at baseline are presented in Table 3. The median age was 52 years, 61% were female, and 39% were smokers. The median duration of RA symptoms prior to the baseline visit was 1 year. 61% have used methotrexate, and 56% have used a biologic agent. There were no significant differences in baseline demographic data, prior medication usage, or PFT values between those subjects with ILAs at baseline and those subjects without them.
Table 3.
Baseline subject characteristics.
| All Subjects (n=18) | ILA Absent (n=11) | ILA Present (n=7) | p | |
|---|---|---|---|---|
| Female, n (%) | 11 (61%) | 7 (64%) | 4 (47%) | 1.00 |
| Age, years | 52 (47–57) | 49 (38–56) | 52 (47–63) | 0.36 |
| RA symptom duration, years | 1 (0.2–3) | 1 (1–2) | 1 (1–2) | 0.68 |
| Ever smoker, n (%) | 7 (39%) | 4 (57%) | 3 (43%) | 1.00 |
| Methotrexate use (current or prior), n (%) | 11 (61%) | 7 (64%) | 4 (47%) | 1.00 |
| Biologic agent use (current or prior), n (%) | 10 (56%) | 7 (64%) | 3 (43%) | 0.63 |
| FEV1, % predicted | 105 (98–110) | 105 (101–109) | 95 (93–114) | 0.41 |
| FEV1/FVC ratio | 77 (75–81) | 79 (77–81) | 75 (68–82) | 0.36 |
| TLC, %predicted | 107 (96–112) | 109 (103–112) | 96 (92–112) | 0.17 |
| DLCO, % predicted | 82 (74–90) | 81 (74–92) | 83 (67–87) | 0.34 |
| HRCT score | 0 (0–3) | 0 (0–0) | 5 (2–20) | n/a |
All values are presented as median (interquartile range) unless otherwise noted.
“ILA present” is defined as having total score of 1 or higher on baseline HRCT. “ILA absent” is defined as baseline HRCT total score of 0.
ILA: interstitial lung abnormalities; RA: rheumatoid arthritis; HRCT: high-resolution computed tomography; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; TLC: total lung capacity; DLCO: diffusion capacity of lungs for carbon monoxide.
Factors associated with ILA presence and progression
CCQ scores, autoantibody levels, and hsCRP levels were compared between subjects with and without ILA at baseline, and between subjects with or without progression on follow-up HRCT (Table 4). The CCQ scores were higher in both the subjects with baseline ILA findings compared to those without ILAs (median 10 vs 2, p=0.07), and in subjects with radiographic progression compared to those without it (median 10 vs 2, p=0.04), although this association was only statistically significant in the latter. Neither anti-CCP3.1 nor RF positivity or levels demonstrated significant associations with imaging findings. The hsCRP level trended positively with both the ILA presence (Median 4 vs 1, p=0.06) and progression (Median 4 vs 1, p=0.08). However, neither comparison met statistical significance.
Table 4.
Comparison of subject characteristics in association with ILA presence or progression.
| All Subjects (n=18) | ILA Absent (n=11) | ILA Present (n=7) | p | Non-Progressor (n=11) | Progressor (n=7) | p | |
|---|---|---|---|---|---|---|---|
| CCQ score | 5 (1–10) | 2 (1–5) | 10 (5–12) | 0.07 | 2 (0–5) | 10 (5–12) | 0.04 |
| CCP3.1 level | 162 (22–401) | 182 (34–401) | 143 (9–401) | 0.57 | 83 (1–279) | 201 (22–401) | 0.63 |
| RF level | 19 (10–81) | 16 (10–81) | 30 (10–518) | 0.12 | 15 (10–55) | 51 (10–518) | 0.16 |
| hsCRP level | 1 (1–4) | 1 (1–3) | 4 (1–20) | 0.06 | 1 (1–3) | 4 (1–20) | 0.08 |
All values are presented as median (interquartile range).
“ILA present” is defined as having total score of 1 or higher on baseline HRCT. “ILA absent” is defined as baseline HRCT total score of 0. “Progressor” is defined as having an increase in total score of 1 point or more on follow-up HRCT by 1 point or more. “Non-progressor” is defined as stable or decreased score on follow-up HRCT.
ILA: interstitial lung abnormalities; CCQ: Clinical COPD Questionnaire; CCP: cyclic citrullinated peptide; RF: rheumatoid factor; hsCRP: high-sensitivity c-reactive protein.
Sputum antibodies
Sputum RF IgM and RF IgG levels were higher in patients with ILAs present on HRCT at baseline; however, these differences, as well as CCP3.1 levels, were not statistically significant (Table 5).
Table 5.
Comparison of sputum antibody levels.
| All Subjects (n=18) | ILA Absent (n=11) | ILA Present (n=7) | p | |
|---|---|---|---|---|
| Sputum CCP3.1 level | 112 (53–210) | 108 (36–232) | 116 (74–202) | 0.59 |
| Sputum RF IgM level | 7 (2–101) | 6 (2–11) | 61 (6–102) | 0.17 |
| Sputum RF IgG level | 47 (31–81) | 43 (20–79) | 60 (34–87) | 0.24 |
All values are presented as median (interquartile range).
ILA: interstitial lung abnormalities; CCP: cyclic citrullinated peptide; RF: rheumatoid factor; IgM: immunoglobulin M; IgG: immunoglobulin G.
Discussion
ILD is one of the most clinically impactful extra-articular manifestations in RA, causing significant morbidity and mortality. However, standardized approaches have not been established to evaluate for lung disease, either ILAs or ILD, at baseline, as well progression over time in patients with RA. This is in part due to the lack of clear understanding of the exact factors that predict who will develop ILA or ILD or who will progress to require clinical intervention.
There has been strong evidence in several cross-sectional studies that lung abnormalities on HRCT can be seen early and frequently, even in asymptomatic patients (7–14). Gabbay et al. (7) found that 58% of 36 patients with early RA had at least one radiologic abnormality. More recently, Chen et al. (13) published a study involving 103 patients, one of the largest cohorts studied thus far. The authors found that 61% of the patients exhibited abnormalities on CT imaging, with GGO being the more frequent finding. In the general population, an analysis of CT imaging in over 11,000 subjects found that ILAs were present at a rate of only 7%–9% (19), but the presence of ILAs was associated with increased all-cause mortality, highlighting the potential clinical implication of these findings.
Our study provides a novel perspective into lung disease in patients with early RA. Prior to this investigation, to the best of our knowledge, there has only been one other study that assessed radiologic progression of early ILA over time. Guchuico et al. (9) found that 33% of their 64 patients with RA had subclinical ILD on CT imaging. Out of these patients, 57% demonstrated radiologic progression after a mean follow-up of 1.5 to 2 years. This group identified smoking as a risk factor for having RA-ILD, and methotrexate use was correlated with progression. It is of note that our patient population was different than that studied by Guchuico et al. (20) in that the median RA symptom duration was only 1 year compared to 11.3–13.7 years in their study. Hence, we are capturing patients who are earlier in their disease course. Prior evidence has shown that even when treating naïve RA patients, there is a higher prevalence of ILAs in patients with early RA who were ACPA positive. Other studies have identified lung abnormalities that even predate the development of arthritis in seropositive patients (21–22). Thus, despite the shorter disease duration, our patient population would be considered at high risk for extra-articular disease given that they were also pre-selected as ACPA positive.
In choosing a scoring system to assess the prevalence and progression of ILAs in our patients, we reviewed several previously published studies, including those on RA-related ILD and idiopathic pulmonary fibrosis (9, 16, 23–24). Given that we suspected the radiographic findings would be very subtle early in the disease course, existing scoring metrics lacked appropriate sensitivity to detect minimal abnormalities or very small changes. Therefore, in this study, we divided each CT scan into 12 zones to be graded on a quartile scale. Given that the NSIP pattern can also be observed in RA, GGOs were included as a grading parameter in addition to septal thickening, bronchiectasis, and honeycombing.
According to this scoring algorithm, our study identified radiographic abnormalities in 39% of these early ACPA positive subjects, which is in compliance with the prevalence seen in prior studies. In our patients, the maximum HRCT score was 29 out of a possible 192 points. Therefore, we believe that we are capturing radiographic changes at a very early stage in these patients. This is supported by the fact that none of our subjects has had any prior respiratory diagnosis nor showed any significant obstruction or restriction on PFT.
When assessing radiographic progression, our study considered any increase in the HRCT score to be a positive finding. Two of the subjects had only a 1-point increase over the study period, while the rest ranged from 4 to 7 points. A larger study of clinical outcomes is certainly needed to determine the minimally clinically significant difference in our scoring system. However, our study suggests that once these ILAs are identified, they will likely increase after 1 year, as seen in 86% of our subjects, and it is possible that radiologic abnormalities may continue to progress over time to eventually give rise to clinically apparent disease. On the other hand, of those subjects with normal CT scans, the majority (91%) did not show evidence of progression at the second visit. While it is unknown whether these patients are going to develop pulmonary involvement in the future, it appears that they may be at a lower risk in the short term.
Using the CCQ symptom scores and laboratory findings, we assessed the association between subject characteristics and the presence or progression of ILAs. While only the CCQ score demonstrated significant association with ILA progression, both the CCQ score and hsCRP levels showed a positive trend toward an association with baseline ILAs and radiographic progression. Although we cannot draw conclusions due to the small sample size, our analysis also showed a trend toward higher levels of sputum RF IgM and IgG in individuals with ILAs, and therefore, additional studies are needed to understand the relationship between sputum antibodies and ILD. Overall, our findings suggest that early and subtle respiratory symptoms, as well as higher levels of systemic inflammation, may be a clue toward underlying early parenchymal lung disease in patients with RA. Notably, respiratory symptoms are not often routinely assessed in rheumatology clinics and may thus explain the findings of subclinical lung disease in RA. If the relationship between these symptoms and the development or progression of lung disease is validated in further studies, screening for early respiratory symptoms in rheumatology clinics may be clinically useful.
Our study has several limitations. The subjects were recruited by rheumatologists who were knowledgeable of the study aims, and this may lead to a subtle selection bias toward patients in who respiratory disease is suspected. Neither the CCQ nor our novel HRCT scoring system has been previously validated in this subject cohort. The HRCT images were read and scored by only 1 thoracic radiologist, who was not blinded to our study aim or to the sequence of images. Thus, he might have been more biased toward finding a change between initial and subsequent scans and hence increase the possibility of finding differences. However, because this is a pilot study gathering hypothesis generating data, we felt that the presumed increased sensitivity for finding variables was justified. Lastly, our small sample size precludes robust analyses of the association between subject variables and radiographic findings, and statistical analyses may overestimate the effect in this setting. Thus, while our findings can only be taken as suggestive or preliminary, they do provide new and intriguing findings regarding longitudinal lung changes in patients with early RA.
Despite the limitations, the implication from our findings is that clinicians should have a high index of suspicion for underlying pulmonary abnormalities in patients with early RA and ACPA positivity, especially if respiratory symptoms or high inflammatory markers are present. If radiographic abnormalities are observed, findings from our small cohort suggest that these abnormalities may progress over time, and further follow-up should be considered. As discussed above, additional studies are needed to more clearly define appropriate screening approaches that can be implemented in rheumatology clinics to identify lung disease, to identify which patients are at the highest risk for progressive lung disease (and especially disease that may have important clinical outcomes), and ultimately, to help develop effective treatments for RA-related lung disease.
Main Points.
Radiographic interstitial lung abnormalities (ILAs) were observed in 39% of rheumatoid arthritis subjects without respiratory symptoms.
At 1 year follow up, majority of patients with baseline ILAs exhibited radiographic progression.
Both the presence of baseline ILAs and their progression trended with higher c-reactive protein levels.
These observed ILAs raises concern for the development of interstitial lung disease in the future, but further studies are needed to determine their clinical significance.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Institutional Review Board of the Cedars-Sinai Medical Center (approval #4422).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.H.D., K.D.D., M.K.D.; Design - M.H.D., K.D.D., M.K.D.; Supervision - M.H.W.; Resources - M.H.D., K.D.D., M.K.D.; Materials - M.H.D., K.D.D.; Data Collection and/or Processing - H.D., P.J.J., M.H.K.; Analysis and/or Interpretation - H.D., P.J.J.; Literature Search - H.D.; Writing Manuscript - H.D.; Critical Review - H.D., P.J.J., M.K.D., K.D.D., M.H.W.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received financial support from the National Institute of Health - National Center for Advancing Translational Sciences: UL1TR000124; National Institute of Health - National Institute of Allergy and Infectious Diseases: R56 AI103023; National Institute of Health - National Institute of Arthritis and Musculoskeletal and Skin Diseases: K23 AR066714; Rheumatology Research Foundation; Walter S. and Lucienne Driskill Foundation.
References
- 1.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extra-articular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–7. [PubMed] [Google Scholar]
- 2.Bilgici A, Ulusoy H, Kuru O, Celenk C, Unsal M, Danaci M. Pulmonary involvement in rheumatoid arthritis. Rheumatol Int. 2005;25:429–35. doi: 10.1007/s00296-004-0472-y. [DOI] [PubMed] [Google Scholar]
- 3.Sihvonen S, Korpela M, Laippala P, Mustonen J, Pasternack A. Death rates and causes of death in patients with rheumatoid arthritis: a population-based study. Scand J Rheumatol. 2004;33:221–7. doi: 10.1080/03009740410005845. [DOI] [PubMed] [Google Scholar]
- 4.Shaw M, Collins BF, Ho LA, Raghu G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev. 2015;24:1–16. doi: 10.1183/09059180.00008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka N, Kim JS, Newell JD, Brown KK, Cool CD, Meehan R, et al. Rheumatoid arthritis-related lung disease: CT findings. Radiology. 2004;232:81–91. doi: 10.1148/radiol.2321030174. [DOI] [PubMed] [Google Scholar]
- 6.Doyle TJ, Dellaripa PF, Batra K, Frits ML, Iannaccone CK, Hatabu H, et al. Functional impact of a spectrum of interstitial lung abnormalities in rheumatoid arthritis. Chest. 2014;146:41–50. doi: 10.1378/chest.13-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997;156:528–35. doi: 10.1164/ajrccm.156.2.9609016. [DOI] [PubMed] [Google Scholar]
- 8.Metafratzi ZM, Georgiadis AN, Ioannidou CV, Alamanos Y, Vassiliou MP, Zikou AK, et al. Pulmonary involvement in patients with early rheumatoid arthritis. Scan J Rheumatol. 2007;36:338–44. doi: 10.1080/03009740701393957. [DOI] [PubMed] [Google Scholar]
- 9.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168:159–66. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 10.Mori S, Cho I, Koga Y, Sugimoto M. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol. 2008;35:1513–21. [PubMed] [Google Scholar]
- 11.Habib HM, Eisa AA, Arafat WR, Marie MA. Pulmonary involvement in early rheumatoid arthritis patients. Clin Rheumatol. 2011;30:217–21. doi: 10.1007/s10067-010-1492-5. [DOI] [PubMed] [Google Scholar]
- 12.Wilsher M, Voight L, Milne D, Teh M, Good N, Kolbe J, et al. Prevalence of airway and parenchymal abnormalities in newly diagnosed rheumatoid arthritis. Respir Med. 2012;106:1441–6. doi: 10.1016/j.rmed.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Shi Y, Wang X, Huang H, Ascherman D. Asymptomatic preclinical rheumatoid arthritis-associated interstitial lung disease. Clin Dev Immunol. 2013;2013 doi: 10.1155/2013/406927. 406927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robles-Perez A, Luburich P, Rodriguez-Sanchon B, Dorca J, Nolla JM, Molina-Molina M, et al. Preclinical lung disease in early rheumatoid arthritis. Chron Respir Dis. 2016;13:75–81. doi: 10.1177/1479972315620746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postmas DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes. 2013;28:1–13. doi: 10.1186/1477-7525-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mooney JJ, Elicker BM, Urbania TH, Agarwal MR, Ryerson CJ, Nguyen ML, et al. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest. 2013;114:586–92. doi: 10.1378/chest.12-2623. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. ATS/ERS task force: Standardization of lung function testing, interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 18.Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65:2545–54. doi: 10.1002/art.38066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–81. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody positive rheumatoid arthritis. Arthritis Rheumatol. 2014;66:31–9. doi: 10.1002/art.38201. [DOI] [PubMed] [Google Scholar]
- 21.Fischer A, Solomon JJ, Du Bois RM. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir Med. 2012;106:1040–7. doi: 10.1016/j.rmed.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airway abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheumatol. 2012;64:1756–61. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–54. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 24.Oda K, Ishimoto H, Yatera K, Naito K, Ogoshi T, Yamasaki K, et al. High-resolution CT scoring system-based grading scale predicts the clinical outcomes in patients with idiopathic pulmonary fibrosis. Respir Res. 2014;15:10. doi: 10.1186/1465-9921-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]