Abstract
Objective
To compare two medical methods for second-trimester abortion, mifepristone followed by misoprostol versus mifepristone followed by ethacridine lactate and oxytocin for success rate, induction to abortion time and acceptability.
Materials and Methods
This is a randomized trial conducted from July 2014 to May 2016 and enrolled 120 women undergoing second trimester abortion (13–20 weeks). All patients received 200mg mifepristone orally and were randomized to receive further treatment after 36 hrs. Patients in Group M (n=60) received 400 microgram of misoprostol vaginally every 3 hours (maximum - 5 doses) and Group E (n=60) had extra-amniotic ethacridine lactate instillation followed by oxytocin infusion (max-100miu).
Results
Baseline demographic characteristics were comparable in both the groups. Success rate was 100% in group M and 98.3% in group E (p=0.31). Mean induction to abortion time was significantly shorter in group M than group E (8.2+2.3hours & 10.9+2.6 hours respectively; p=0.001). Majority of women reported side effects, 96.7% women in group M and 75% women in group E (p=0.001). Fall in hemoglobin after procedure was significantly higher in group M (0.70+0.33gram %) than group E (0.52+0.23 gram %) (p=0.001). Perception of intensity of pain was significantly more in group M but patient satisfaction in both groups was similar.
Conclusion
Both methods are comparable for success rate, induction interval was more for ethacridine lactate compared to misoprostol.
Keywords: Misoprostol, ethacridine lactate, second trimester abortion
Introduction
Abortion is a major social and health issue, particularly in the developing countries. In India, over 6 lac abortions are performed each year, and of these, 10%–15% are performed in the second-trimester [1]. Mifepristone followed by misoprostol is a safe and effective regimen in the second-trimester abortion. A systematic review of 40 randomized controlled trials addressing various regimens for the abortion between 12 and 28 weeks of gestation concluded that the combination of mifepristone and misoprostol appeared to have the highest efficacy and shortest induction time [2]. The World Health Organization (WHO) [3] and Royal College of Obstetrics & Gynecology (RCOG) [4] strongly recommend the use of the anti-progestin and mifepristone, followed by misoprostol, as the medical method for second-trimester abortion. One of the drawbacks of this regimen are misoprostol side effects, which can occur in up to 30% of cases [5], and the possibility of uterine rupture in women with a previous uterine scar [6, 7].
Despite the available literature, many clinicians in India and South Asia continue to use ethacridine lactate (EL) in the second-trimester abortion. EL is an organic compound based on acridine. In India, the extra-amniotic instillation of EL followed by a high dose oxytocin infusion is popular, whereas in China, the intra-amniotic instillation is commonly used. The extra-amniotic instillation causes stripping of the amniotic membranes from the uterine wall, which leads to the release of prostaglandin and oxytocin and mechanical stimulation of the uterus. It is a well-accepted method of pregnancy termination due to its efficacy and a good safety profile. The induction time, when used with a high dose oxytocin infusion, is 15–20 hours with a success rate of approximately 90% [8–10]. High doses of oxytocin are required as the uterine myometrium is not sensitized to oxytocin in early pregnancy. In a systematic review of Chinese trials, authors reported that the failure of abortion with intra-amniotic EL was 7.4%–20.7% compared to the 2%–5.9% failure rate in studies using mifepristone and misoprostol [11]. Zhuang et al. showed that mifepristone may shorten the induction-to-abortion time when used with intra-amniotic EL [12].
An earlier study showed that priming the uterus with mifepristone before oxytocin improved success rates to 92.3%, versus 52.9% when oxytocin was used alone, and that it reduced the induction time (11.3±6 to 17.6±6.5 hours) [13]. As with misoprostol and oxytocin, we postulate that the mifepristone priming prior to extra-amniotic instillation of EL, followed by oxytocin infusion, would reduce the induction time.
The aim of the study was to evaluate a regimen that would be comparable with the standard regimen of mifepristone followed by misoprostol in terms of successful abortion and the induction time. This study compares two second-trimester abortion methods of mifepristone followed by EL and oxytocin with the commonly used protocol of mifepristone followed by misoprostol.
Materials and Methods
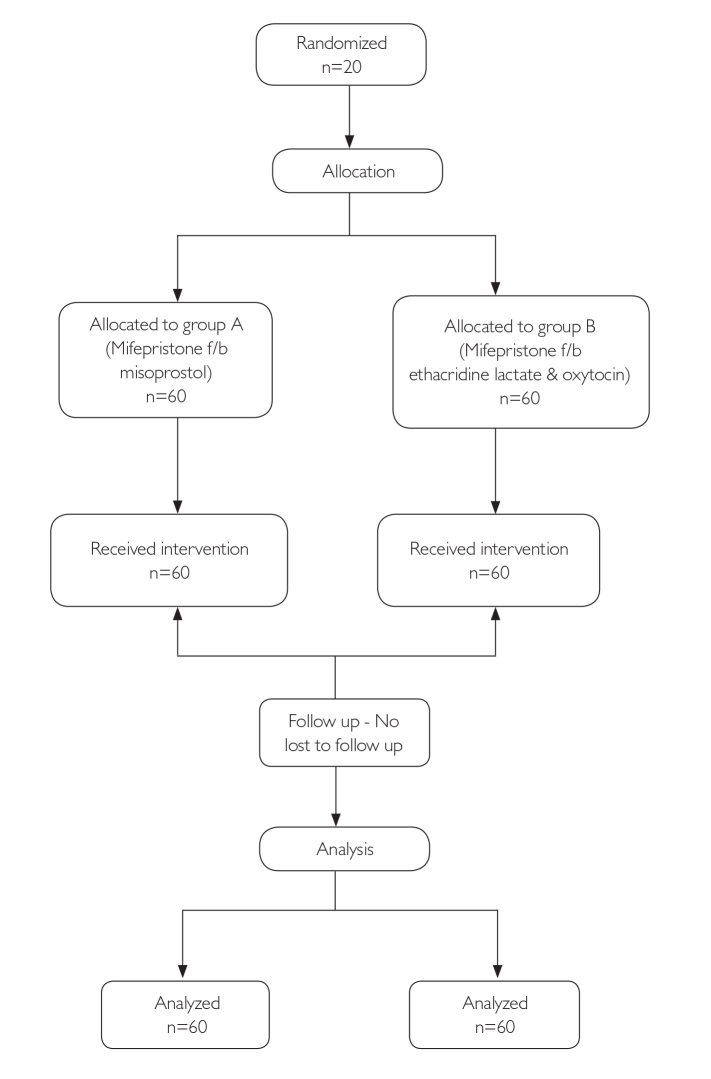
This was a prospective randomized trial with the 1:1 ratio allocation, conducted in a unit of the Department of Obstetrics and Gynecology at a tertiary care center over a period of 2 years (from July 2014 to May 2016). The study included healthy women who underwent a second-trimester abortion (13–20 weeks of gestation) for medical or social reasons. The gestational age was defined by the last menstrual period and ultrasound estimation of the fetus size. Exclusion criteria included women with a history of renal or hepatic disease, bronchial asthma, hematological disorder, glaucoma and heart disease, chronic adrenal failure, long-term steroid treatment, moderate or severe anemia (hemoglobin [Hb]<10gram/dL); previous cesarean or other uterine scars (hysterotomy, myomectomy, unknown scars); symptomatic reproductive tract infections; hypersensitivity to mifepristone/misoprostol/EL/oxytocin; and the presence of rupture of membranes. Eligible women were recruited after informed consent. Demographic and clinical data were collected in structured proformas. Women were randomized to one of the two study arms using a computer-generated randomization table. Using the Epi Info software version 7.0 developed by the Centre for Disease Control Program, Atlanta, USA, six random numbers were generated between 1 and 120. A generated-random-number list was put in an envelope and labeled as M. The remaining 60 numbers list was put in another envelope labeled as E. According to the inclusion and exclusion criteria, recruited patients were assigned identification numbers sequentially from 1 to 120, and an appropriate group was decided based on the envelope list. All women received 200 mg of mifepristone (Mt Pill 200 mg tablet; Cipla Ltd.) orally. Thirty-six hours after mifepristone administration, women were admitted to hospital, and according to randomization, women in Group M were given 400 micrograms of misoprostol (Misoprost 200 MCG Tablet; Cipla Ltd.) vaginally every 3 hours to a maximum of five doses. For women in group E, 0.1% EL (Vecredil; Jagsonpal Pharmaceuticals Ltd.) was instilled transcervically through the Foley’s catheter in the extra-amniotic space slowly over 10 minutes under aseptic conditions. The volume instilled was 10 ml per week of gestation with a maximum of 150 mL. The Foley’s catheter was removed after 6 hours, unless expelled spontaneously. This was followed by oxytocin infusion (Pitocin AMP; Pfizer) in 500 mL of ringer lactate, starting with 10 mIU/min and increased to the rate of 10 mIU/min every half hour up to a maximum of 100 mIU/min, depending on response. Oxytocin drip was to be discontinued after 12 hours if the patient did not respond. Once fetus was expelled, 1 hour was given for a spontaneous expulsion of the placenta. Ultrasound was done after the expulsion of the placenta to look for retained products. A curettage was done for retained products. Figure 1 depicts the flow of participants in the study.
Figure 1.
Flow of participants in the study.
Hb was measured on the day of induction (pre-procedure) and again after the successful abortion (24 hours post-procedure). The Hb difference (pre-procedure vs. post-procedure) was taken as an estimate of blood loss. Anti-D 300 microgram was administered in patients with the Rh-negative blood group.
The primary outcome measure was the success rate, defined as the percentage of abortions within 24 hours after the use of the first dose of misoprostol or instillation of EL. The secondary outcome was the induction time, defined as the time from the first use of misoprostol or EL to the expulsion of fetus and placenta. Other outcome measures included a composite of side effects (nausea, vomiting, flatulence, dyspepsia, diarrhea, constipation, headache, heavy bleeding, hypersensitivity, pyrexia); patient satisfaction (measured on a visual scale of 0–10; 0, not satisfied and 10, strongly satisfied); pain perception (measured by a visual analog scale [VAS] 0–10; 0, no pain to 10, maximum pain) and fall in Hb (difference in pre- and post-procedure Hb). The patient satisfaction and pain perception were measured after the completion of abortion.
Sample Size Calculation
The study design was a non-inferiority trial and for calculating the sample size, results of a similar study (Matan Elami-Suzin et al., 2013) [14] were considered. These authors showed that the abortion rate using mifepristone followed by misoprostol or oxytocin for the second-trimester abortion was 100% and 95.8%, respectively. Assuming that similar success rates would be obtained for the present study, a difference of 2% between the groups is clinically negligible, with the 5% level of significance (one-tailed) and power of 80%, a sample size of 60 in each group was considered to be sufficient to prove equivalence. The sample size calculation was done using the R-software version 3.24.
Statistical Analysis
Comparison of the success rate between the two groups was done by using the chi-squared/Fisher’s exact test as appropriate. The induction abortion interval between the two groups was compared using Student’s t-independent test. All qualitative variables related to the side effects were compared between the two groups using the chi-squared test. Other quantitative variables related to side effects were compared using Student’s t-independent test. For all the statistical tests, a p<0.05 was considered to be statistically significant. A Cox regression model was used to evaluate the factors affecting the success rate. All statistical analysis was carried out using the SPSS program.
The study protocol was approved by the institutional ethics committee, and the study is registered with the Central Trial Registration, India.
Results
This study was conducted to compare two medical methods used in the second-trimester abortion: mifepristone followed by misoprostol (Group M) vs. mifepristone followed by EL and oxytocin (Group E). A total of 120 women fulfilling the eligibility criteria were enrolled in the study and randomized to two groups. No woman aborted after mifepristone, and none was lost at follow-up after mifepristone.
Baseline Characteristics
The two groups did not differ in age, parity, gestation age, and indication of abortion (Table 1). In Group M, the mean requirement of misoprostol dose was 3±0.9 tablets (minimum, 1; maximum, 5), and a single dose of misoprostol included 400 micrograms. In Group E, the average duration of oxytocin infusion was 4.97±2.57 hours (minimum, 1; maximum, 12.25), and the average maximum oxytocin concentration required was 81.55±24.26 mIU/min (minimum, 20; maximum, 100).
Table 1.
Demographics of women undergoing pregnancy termination
| Variable | Group M (n=60) | Group E (n=60) | p | |
|---|---|---|---|---|
| Age (years) | 29.10±3.5 | 27.45±3.8 | 0.15 | |
| Gestational age (weeks) | 16.94±1.85 | 16.57±1.79 | 0.27 | |
| Parity | Primigravida | 11 (18%) | 11 (18%) | 0.42 |
| Multigravida | 49 (82%) | 49 (82%) | ||
| Baseline Hb (gram %) | 10.66±0.86 | 10.51±0.95 | 0.39 | |
| Indication | Unwanted preg-nancy | 32 (54%) | 39 (65%) | 0.2 |
| Congenital mal-formations/Anomalies | 26 (43%) | 21 (35%) | ||
| Missed abortion | 2 (3%) | 0 |
Hb: hemoglobin
Outcome Measures
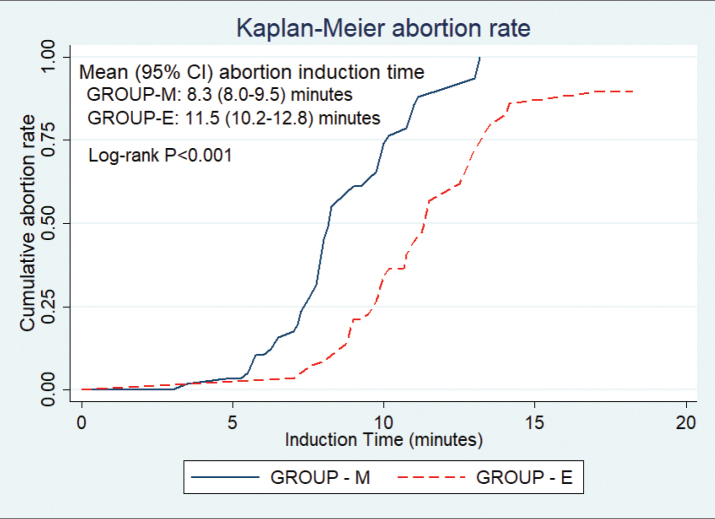
Both study arms recorded high success rates: 60/60 patients (100%) in Group M and 59/60 patients (98%) in Group E aborted within 24 hours, and the difference was not statistically significant. No patient aborted after mifepristone. None of the patients in Group M needed an alternative treatment because of side effects. The time to expulsion of fetus was significantly shorter for Group M compared to Group E (8.2±2.3 vs. 10.9±2.6 hours; p≤0.001). The Cox regression model was used taking into account “time until expulsion,” controlling for the type of treatment, age, and parity of women and the gestation age at pregnancy termination. The factors significantly associated with shorter time until expulsion was parity (adjusted HR 3.2; p=0.001; confidence interval [CI] 1.59–6.4) and use of misoprostol (adjusted HR 3.23; p<0.001, CI, 2.05–5.08) (Figure 2). The outcome measures are presented in Table 2.
Figure 2.
The Kaplan–Meier time curve for the induction-to-abortion time.
Table 2.
Outcomes of pregnancy termination
| Variable | Group M (n=60) | Group E (n=60) | p |
|---|---|---|---|
| Success rate (24 hours) | 100% | 98% | 0.31 |
| Induction-to-Abortion Interval (hours) | 8.2±2.3 | 10.9±2.6 | 0.001 |
| Hb difference (gram %) | 0.70±0.33 | 0.52±0.23 | 0.001 |
| Side effects | 97% | 75% | 0.001 |
| Pain score (VAS) | 5.97±1.23 | 5.14±1.27 | 0.001 |
| Satisfaction score | 5.90±0.85 | 6.0±1.08 | 0.57 |
VAS: visual analogue scale; Hb: hemoglobin
The two groups were compared with regard to the amount of blood lost as determined by the decrease in Hb (pre-procedure Hb vs. post-procedure Hb). The mean pre- and post-procedure Hb was comparable in both groups (10.66±0.86 to 9.97±0.94; p=0.001 and in Group B, 10.51±0.95 to 10±0.91; p=0.001). The Hb decrease was significantly greater in Group M than in Group E (0.70±0.33 gm/dL and 0.52±0.23 gm/dL, respectively; p=0.001). Post-abortion, dilatation and curettage were required in 78% (47/60) cases in Group M and 70% (42/60) in Group E. No patient required blood transfusion. There were no major or minor complications encountered in either group during the abortion. The composite side effects defined as headache, fever, and gastrointestinal side effects (nausea, vomiting, diarrhea) were noted. Side effects were significantly higher in Group M than in Group E (97% and 75%, respectively; p=0.001). In Group M, the mean VAS score for the pain experienced was significantly greater than in Group E (5.97±1.23 vs. 5.14±1.27; p=0.001). Although the difference was statistically significant, the actual difference in score was small, and this may not be considered clinically important. Todd et al. [15] suggested that a minimum change of 13 mm out of 100 mm of VAS should be considered clinically significant. If it is <13 mm, it may not have any clinical importance.
Patient satisfaction with the method of abortion was also rated on a scale of 0–10. In Group M, the mean score was 5.90±0.85, and in Group E, it was 6.0±1.08. This was not statistically significant (p=0.57).
Discussion
Historically, the morbidity associated with second-trimester medical abortions was due to prolonged induction times. The regimen of anti-progesterone-mifepristone followed by prostaglandin-misoprostol has shortened the induction-to-abortion time and improved the rates of complete abortion. This protocol was approved by WHO [3], as well as various professional bodies such as ACOG [16] and RCOG [4]. The extra-amniotic instillation of EL followed by oxytocin infusion is still a protocol used in India. Its drawback is a long induction time, which requires a longer hospital stay and physical and emotional trauma to women. As priming with mifepristone before misoprostol reduces the induction time compared to use of misoprostol alone [17], we were able to show some benefits with EL. However, without a direct comparative trial, there may be some reservations.
There are concerns about the use of oxytocics in women with scarred uterus. The risk of uterine rupture with misoprostol has been reported in women undergoing a second-trimester abortion, with or without the scar in the uterus [6, 7, 18, 19]. There is a report of the rupture in unscarred uterus following the use of EL and oxytocin also [20]. There have also been reports of rupture in the scarred as well as unscarred uterus, following the use of high dose oxytocin alone and with other oxytocic agents for second-trimester abortion [8, 21]. Whatever method is used, careful monitoring is required in all patients.
We compared the two methods of second-trimester medical abortion in patients with similar demographic characteristics. We reported high success rates for both methods. For regimen constituted by mifepristone followed misoprostol, the induction time was 8.2±2.3 hours, and this was a significantly shorter than the induction time of 10.9±2.6 hours for Group E. Moreover, the induction time in Group E was much shorter than the one published in the literature for extra-amniotic instillation of EL with or without another oxytocic agent. Deliwala et al. [22] reported an average induction time of 27.6 hours with the use of extra-amniotic EL with oxytocin. Tayade et al. [23] used extra-amniotic EL along with misoprostol for first- and second-trimester abortion and found the induction time of 16.43 hours. Nanda et al. [24] studied the use of EL with prostaglandin alpha in the mid-trimester abortion and reported the induction time of 32.28 hours. The shorter induction time in the EL group of our study can be explained by the pretreatment effect of mifepristone. To the best of our knowledge, this is the first study in which mifepristone is used prior to extra-amniotic EL instillation. The success rate and acceptability of the two protocols was not significantly different.
In the present study, the blood loss was assessed by a decrease in Hb after the procedure, and it was significantly higher in Group M. No patient required blood transfusion. We did a pelvis ultrasonography for every patient after the expulsion of placenta, and this might be the reason for a high curettage rate in our study. Gastrointestinal side effects and fever were significantly higher in Group M, and this was similar to results shown in a systemic review of literature of Chinese trials [11]. The pain perception and overall satisfaction were similar in both the groups. The EL instillation is a cheap, effective, and safe method, and the preferred method for the mid-trimester abortion in few countries such as China [24]. It has also been considered as a safer alternative in several other studies [24–26].
Few studies have been published where the advantage of mifepristone has been taken in protocols other than those using misoprostol to improve the induction-to-abortion time. Elami-Suzin et al. [14] conducted a randomized trial comparing mifepristone followed by misoprostol or oxytocin for second-trimester abortion. The success rate defined as the expulsion within 36 hours was 100% for the mifepristone-misoprostol and 95.8% for the mifepristone-oxytocin group, statistically not different. However, the induction time was significantly higher for the mifepristone-oxytocin group, 11.3±7.4 hours, vs. 7±4.9 hours for the mifepristone-misoprostol group. An addition of mifepristone to oxytocin did reduce the induction time, which is evident when we compare a study by Ramin et al. [27] that reported an induction time of 21.7±11 hours for oxytocin used alone, and the success rate was 62%.
Mifepristone has been used in combination with intra-amniotic EL in few studies to shorten the induction time. Mei et al. [28], in a randomized controlled trial, comparing the intra-amniotic 100 ml EL to intra-amniotic EL followed by 150 mg mifepristone orally reported a significantly shorter induction time with the use of mifepristone; 36.45±8.05 vs. 49.03±9.30 hours (p=0.00). No difference was found in the success rates and cervical laceration in the two groups. The benefit of use of mifepristone with intra-amniotic EL was also reported by Zhuang et al. [12]. They found that 25.94% women delivered within 24 hours in the mifepristone group, while only 10.18% delivered in the non-mifepristone group (p≤0.001); the complication rate was similar in both groups. Chen et al. [29] studied the use of mifepristone combined with intra-amniotic EL in the second-trimester pregnancy termination in 443 women with placenta previa and/or a prior cesarean delivery. All patients had delivered vaginally, and the mean induction time was 34±9.4 to 38.56±12.4 hours. There was no incidence of uterine rupture in any patient. We could not find a study that used mifepristone with extra-amniotic EL for second-trimester abortion.
The randomized prospective nature was one of the strengths of this study. Although the sample size was pre-calculated, a larger sample or a multi-centric study to get a larger sample would have produced more robust results. This was perhaps one of the possible shortcomings of our study.
After analyzing our data, we conclude that both the regimens are safe and equally acceptable by the patients in the second-trimester abortion. We suggest the use of mifepristone, 36–48 hours prior to the extra-amniotic EL instillation for the second-trimester abortion. This gives a success rate comparable to mifepristone and misoprostol, although the induction-to-abortion interval is greater with EL. The induction-to-abortion time in the present study for EL is much shorter than that reported in other studies. In addition, this may be an effective alternative method for women in whom there is concern for the use of misoprostol.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of All India Institute of Medical Sciences, New Delhi, India (IESC/T-269/21.06.2014). Clinical trial registration - Registered with Central Trial Registration India (CTRI/2018/01/011157).
Informed Consent: Informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.D., S.G.; Design - V.D., V.P.; Supervision - V.D., D.D.; Resources - V.D., K.A.S.; Materials - K.K., S.G.; Data Collection and/or Processing - S.G.; Analysis and/or Interpretation - V.D., S.G., V.P.; Literature Search - K.K., S.G.; Writing Manuscript - K.K., V.D.; Critical Review - V.D., D.D.
Conflict of Interest: The authors declared no conflicts of interest.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Lalitkumar S, Bygdeman M, Danielsson KG. Mid-trimester induced abortion: a review. Hum Reprod Update. 2007;13:37–52. doi: 10.1093/humupd/dml049. [DOI] [PubMed] [Google Scholar]
- 2.Wildschut H, Both MI, Medema S, Thomee E, Wildhagen MF, Kapp N. Medical methods for mid-trimester termination of pregnancy. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD005216.pub2. doi: 10.1002/14651858.CD005216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safe abortion: technical and policy guidance for health systems. Second edition. World Health Organization; 2012. [PubMed] [Google Scholar]
- 4.Evidence based clinical guideline number 7. The care of Women requesting induced abortion. RCOG 2011. [Google Scholar]
- 5.Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: pharmacokinetics profiles, effects on uterus and side-effect. Int J Gynecol Obstet. 2007;99:160–7. doi: 10.1016/j.ijgo.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Berghahn L, Christensen D, Droste S. Uterine rupture during second trimester abortion associated with misoprostol. Obstet Gynecol. 2001;98:976–7. doi: 10.1097/00006250-200111001-00037. [DOI] [PubMed] [Google Scholar]
- 7.Goyal V. Uterine rupture in second trimester misoprostol-induced abortion after cesarean delivery: a systematic review. Obstet Gynecol. 2009;113:1117–23. doi: 10.1097/AOG.0b013e31819dbfe2. [DOI] [PubMed] [Google Scholar]
- 8.Yapar EG, Senoz S, Urkutur M, Batioglu S, Gökmen O. Second trimester pregnancy termination in-cluding fetal death: comparison of five different methods. Eur J Obstet Gynecol Reprod Biol. 1996;69:97–102. doi: 10.1016/0301-2115(95)02548-0. [DOI] [PubMed] [Google Scholar]
- 9.Zauva BL, Gupta I, Dhall GI. Mid trimester abortion by extraamniotic ethacridine lactate vs normal saline. Aust N Z J Obstet Gynecol. 1989;29:299–302. doi: 10.1111/j.1479-828X.1989.tb01747.x. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Sachdeva L, Gupta R. Ethacridine lactate a safe and effective drug for termination of preg-nancy. Indian J Mater Child Health. 1993;4:59–61. [PubMed] [Google Scholar]
- 11.Hou SP, Fang AH, Chen QF, Huang YM, Chen OJ, Cheng LN. Termination of second-trimester preg-nancy by mifepristone combined with misoprostol versus intra-amniotic injection of ethacridine lac-tate (Rivanol®): a systematic review of Chinese trials. Contraception. 2011;84:214–23. doi: 10.1016/j.contraception.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang Y, Chen XY, Huang L. Mifepristone may shorten the induction abortion time for termination of pregnancies by ethacridine lactate. Contraception. 2012;85:211–4. doi: 10.1016/j.contraception.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Meir A, Erez Y, Feigenberg T, Hamani Y, Laufer N, Rojansky N. Mifepristone followed by high dose oxytocin drip for second trimester abortion. J Reprod Med. 2009;54:511–6. [PubMed] [Google Scholar]
- 14.Elami-Suzin M, Freeman MD, Porat N, Rojansky N, Laufer N, Meir AB. Mifepristone Followed by Misoprostol or Oxytocin for Second-Trimester Abortion: A Randomized Controlled Trial. Obstet Gynecol. 2013;122:815–20. doi: 10.1097/AOG.0b013e3182a2dcb7. [DOI] [PubMed] [Google Scholar]
- 15.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–9. doi: 10.1016/S0196-0644(96)70238-X. [DOI] [PubMed] [Google Scholar]
- 16.ACOG Practice Bulletin No. 135: Second-trimester abortion. Obstet Gynecol. 2013;121:1394–406. doi: 10.1097/01.AOG.0000431056.79334.cc. [DOI] [PubMed] [Google Scholar]
- 17.Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and Misoprostol Compared with Misoprostol Alone for Second-Trimester Abortion: A Randomized Controlled Trial. Obstet Gynecol. 2011;118:601–8. doi: 10.1097/AOG.0b013e318227214e. [DOI] [PubMed] [Google Scholar]
- 18.Cuellar TM. Silent uterine rupture with the use of misprostol for second-trimester termination of pregnancy: a case report. Obstet Gynecol Int. 2011 doi: 10.1155/2011/584652. doi: 10.1155/2011/584652. Epub 2011 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egbe TO, Halle-Ekane GE, Tchente CN, Nyemb JE, Belley-Prosi E. Management of uterine rupture: a case report and review of literature. BMC Res Notes. 2016;9:492. doi: 10.1186/s13104-016-2295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhotra N, Chanana C. Silent rupture of unscarred uterus: an unusual presentation at second tri-mester abortion. Arch Gynecol Obstet. 2007;275:283–5. doi: 10.1007/s00404-006-0225-0. [DOI] [PubMed] [Google Scholar]
- 21.Biale Y, Lewenthal H. Uterine rupture during induced mid-trimester abortions. Eur J Obstet Gynecol Reprod Biol. 1985;19:175–82. doi: 10.1016/0028-2243(85)90152-2. [DOI] [PubMed] [Google Scholar]
- 22.Deliwala K, Shah H, Vyas R. Therapeutic efficacy of misoprostol versus ethacridine lactate in second trimester abortion-Randomised controlled study. Nat J Med Res. 2013;3:16–8. [Google Scholar]
- 23.Tayade SA, Samal S, Tayade AT. Prostaglandin Analogues and Ethacridine Lactate for 1st and 2nd Trimester Induced Abortion. Int J Biol Med Res. 2011;2:1075–7. [Google Scholar]
- 24.Nanda S, Paul A. Comparison of efficacy and safety of mifepristone-misoprostol combination with ethacridine lactate in mid-trimester termination of pregnancy. Int J Med Med Sci. 2013;5:307–11. [Google Scholar]
- 25.Chaudhuri S, Mitra SN, Chaudhuri N, Chattopadhya D, Banerjee D, Bose S. A comparison of in-travaginal misoprostol with extraamniotic ethacridine lactate for second trimester MTP. J Obstet Gynecol India. 2006;56:518–21. [Google Scholar]
- 26.Berg C, Ludwig M, Sturm N, Diedrich K, Gembruch U, Geipel A. Intraamniotic ethacridine lactate instillation versus vaginal PGE1 in second trimester termination of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006;126:193–6. doi: 10.1016/j.ejogrb.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Ramin KD, Ogburn PL, Danilenko DR, Ramsey PS. High-dose oral misoprostol for mid-trimester pregnancy interruption. Gynecol Obstet Invest. 2002;54:176–9. doi: 10.1159/000067889. [DOI] [PubMed] [Google Scholar]
- 28.Mei Q, Li XL, Liu H, Zhou H. Effectiveness of mifepristone in combination with ethacridine lactate for second trimester pregnancy termination. Eur J Obstet Gynecol Rep Biology. 2014;178:12–5. doi: 10.1016/j.ejogrb.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Lin F, Wang X, Jiang Y, Wu S. Mifepristone combined with ethacridine lactate for the sec-ond trimester pregnancy termination in women with placenta previa and/or prior cesarean deliveries. Arch Gynecol Obstet. 2017;295:119–24. doi: 10.1007/s00404-016-4205-8. [DOI] [PubMed] [Google Scholar]