Abstract
Objective
The purpose of the present study was to determine the effect of abdominal binder usage on mobilization, postoperative pain, and distress after cesarean delivery.
Materials and Methods
This prospective randomized controlled study was conducted between September 1, 2017 and January 31, 2018 at Bakirkoy Dr. Sadi Konuk Education and Research Hospital, Istanbul, Turkey. A total of 89 women undergoing elective cesarean were randomized to the study (binder, n=45) or control (no binder, n=44) groups. Patients in the study group were fitted with a binder before leaving the operating room. Mobilization (6-minute walk test), postoperative pain (measured by Short-Form McGill Pain Questionnaire and Visual Analog Scale), and perceived distress status of both groups were evaluated within 8th (first mobilization time), 24th, and 48th h of surgery.
Results
We found that the binder group (BG) walked longer than the control group during the 6-minute walking distance test. At the first mobilization time (postoperative 8th h), the BG (99.4±27.3 m) covered significantly more distance than the control group (81.0±22.2 m) (p=0.001) in the walking distance test. At postoperative 24th h, the McGill pain score in the BG was significantly lower than that in the control group (p=0.004). For all three test times, the Symptom Distress Scale of the BG was lower than that of the control group (postoperative 8th h p=0.024, 24th h p<0.001, and 48th h p<0.001).
Conclusion
The evidence is consistent with abdominal binder usage after cesarean section decreasing the feeling of distress and increasing mobility.
Keywords: Abdominal binder, cesarean section, mobilization, pain
Introduction
Cesarean section (CS) is the most frequent abdominal surgery worldwide [1], and pain is the most common complaint associated with it [2]. In the early stages of the postpartum period, when pain cannot be managed effectively, patients worry about injuries to the surgical site [3]. Pain and worry in turn cause the patients to avoid mobilization, thereby increasing the risk of thromboembolism and disrupting the maternal bonding with the infant.
In the literature, there are few studies on the use of non-pharmacological interventions to reduce pain and improve mobilization [4]. One of the most promising non-pharmacological interventions is postoperative abdominal binder usage. An abdominal binder is a wide belt that surrounds the abdomen and supports the surgical site. The binder is hypothesized to improve postoperative pulmonary function by limiting the movement of abdominal wall muscles [5]. In particular, it might speed up the return of the uterus and the other organs to their pre-pregnancy positions by compressing the abdomen, and it might help tissue repair by increasing blood flow [6]. The benefits of abdominal binder use for reducing pain and improving mobility after major abdominal surgeries are well-established in the literature [7, 8]. However, for cesarean delivery, there are few studies on the impact of binder use on postoperative pain and symptom distress, and to our knowledge, there are no studies on its impact on mobilization.
The aim of the present study was to investigate the effects of abdominal binder use after cesarean delivery on postoperative pain, distress, and mobilization.
Materials and Methods
Study Design and Ethical Approval
This study was designed as a prospective randomized controlled trial and conducted at Bakirkoy Dr. Sadi Konuk Education and Research Hospital, Istanbul, Turkey. The study was approved by the hospital ethics committee (2017-09-22/246). Written informed consent was obtained from the subjects. As soon as the patients were admitted to the obstetric service, they were randomly assigned to one of the two groups by the successive opening of sequentially numbered, opaque and stamped envelopes. Patients in the intervention group used an abdominal binder, and patients in the control group did not. The allocation of the patients to the two groups was performed by V.S.E. who was blinded to data collection.
Patients and Methods
The study comprised 104 women who underwent elective CS in our clinic between September 22, 2017 and January 23, 2018. Exclusion criteria included women with more than two previous pregnancies, not delivering the current pregnancy at term, with emergency cesarean, who underwent general anesthesia instead of spinal anesthesia, with chronic diseases, and who underwent non-routine or additional surgical procedures, such as hysterectomy, tubal ligation, and classical uterine incision.
All patients received evidence-based and standardized preoperative and postoperative care. The surgeons were blinded to group assignments and were instructed to perform CS based on the same rules. According to these instructions, Pfannenstiel incision was performed for the skin, transverse incision was performed for the subcutaneous tissue, and sharp incision was performed for opening the peritoneum. After pushing the peritoneum of the bladder downward, Kerr incision was performed to the uterus. After delivery, continuous one-layer suturation of the uterus was performed with polyglactin no. 1 (Vicryl) while the uterus was inside of the pelvis of the patient. Parietal peritoneum was sutured with polyglactin no. 2-0 (Vicryl Rapide), and transverse incision on the rectus sheath was closed using polyglactin no. 1 (Vicryl). Finally, the skin was closed with polyglactin no. 4-0 (Vicryl Rapide). After completing the CS, the abdominal binder was fitted to the lower abdomen by covering the incision. The binder used in the study was manufactured using an elastic, breathable nylon thread fabric, with a height of 24 cm. All patients were mobilized for the first time at 8th h after CS. Analgesia protocol and dietary intakes were standardized for both groups. Antibiotic prophylaxis, an antiemetic, a prokinetic agent, paracetamol, and additional nonsteroidal analgesia, if necessary, were administered in the postoperative period.
Outcome Measures
The outcome measures were the pain level as assessed by the Short-Form McGill Pain Questionnaire (SF-MPQ) and Visual Analog Scale (VAS), postoperative physical function as measured by the 6-minute walk test (6MWT), and perceived distress as measured by the Symptom Distress Scale (SDS). All measurements (6MWT, SF-MPQ, VAS, and SDS) were repeated at the time of the first mobilization (postoperative 8th h) and postoperative days 1 and 2. Hemoglobin levels were tested before surgery and the first day after surgery.
6-minute Walk Test
The 6MWT was used to evaluate the physical functions of the patients. The researcher recorded the distance the patient walked in 6 min in meters. The test was conducted on an 80-meter elliptical hospital corridor [9].
Short-Form McGill Pain Questionnaire
Pain was assessed using the SF-MPQ, which consists of 15 adjectives that describe both the sensory and the affective attributes of pain. The adjectives are rated on a 4-point intensity scale (0=none and 3=severe), and a higher total score (range: 0–45) corresponds to a higher level of pain [10].
Visual Analog Scale
The acuity of pain was evaluated by the VAS. Patients were instructed to place a mark on a 10 cm line based on the severity of their pain, with 0 cm representing no pain and 10 cm being the worst pain they had ever experienced.
Symptom Distress Scale
Patient distress was evaluated using the SDS. SDS is associated with the following 14 symptoms: nausea; vomiting; pain; anorexia; trouble sleeping; fatigue; difficulty breathing; coughing; lacrimation; restlessness; and changes in the ability to concentrate, body temperature, bowel elimination, and physical appearance. Each item is rated on a 5-point Likert-type scale (0=no occurrence or distress and 4=greatest occurrence or distress).
Statistical Analysis
The MedCalc statistical software, version 18.9 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018) was used for power analysis. When the size of the study reached 15 patients in each group, interim analysis was conducted. Based on the information obtained from the 6MWT results at postoperative 8 h of patients using a binder (X̄ =95.1, s. s. =31.3) and not using a binder (X̄ =78.3, s. s. =24.2), the required total sample size was found to be 90, with 45 in each group, to reach 80% power with a two-tailed alpha level of 0.05. Ten additional subjects were recruited to account for possible attrition.
The Statistical Package for the Social Sciences (SPSS), version 23.0 (IBM Corp.; Armonk, NY, USA) was used for statistical analysis. For each variable, the mean, median, standard deviation, maximum, minimum, and sample size values were calculated. Shapiro–Wilk test was used to check whether the variables were distributed normally, and Levene’s test was used to assess the homogeneity of variances across the groups. Independent samples t-test was used when normality and variance homogeneity were satisfied, and Mann–Whitney U test was used otherwise.
Results
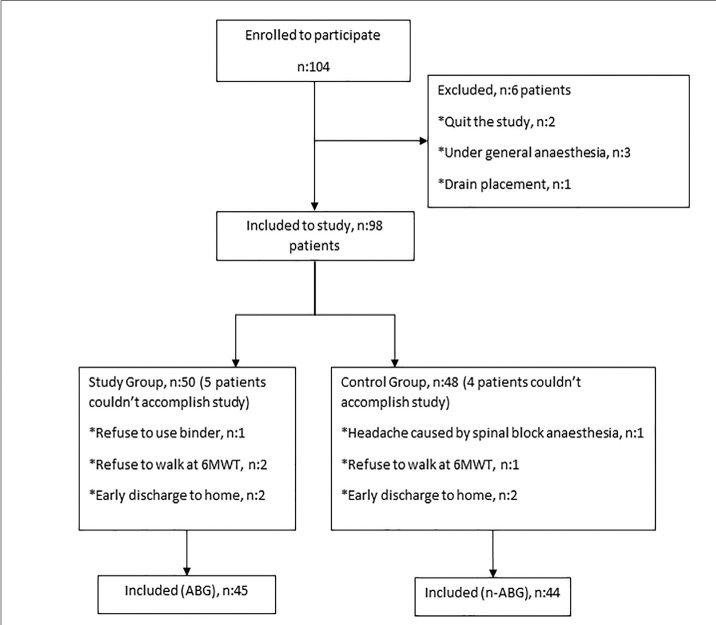
The flowchart of the study is presented in Figure 1. Of the 104 patients who initially enrolled in the analysis, 89 were included in the study. Reasons for leaving are listed in the flowchart (Figure 1). By the end of the study, the abdominal binder group (BG) had 45 patients, and the no-abdominal binder group (N-BG) had 44. The demographic differences between the two groups were not statistically significant. There was also no detectable difference between the two groups for body mass index values in the pre-pregnancy period and the weight gain during pregnancy (Table 1).
Figure 1.
Flow diagram of the groups. 6MWT: 6-minute walk test; ABG: abdominal binder group; n-ABG: no binder group
Table 1.
Demographic characteristics of the groups
| Binder group (n=45) | Control group (n=44) | p | |
|---|---|---|---|
| Age | 27.1±6.0 | 26.3±6.9 | 0.1 |
| Gravity | 2.1±1.3 | 2.3±1.7 | 0.95 |
| Parity | 0.9±0.8 | 0.8±0.7 | 0.68 |
| Pre-pregnancy BMI | 28.9±1.5 | 27.1±1.8 | 0.08 |
| Pre-partum BMI | 33.1±1.5 | 32.7±1.8 | 0.199 |
| Gain weight during pregnancy (kg) | 12.4±2.6 | 12.2±2.9 | 0.492 |
| Gestational age (week) | 38.6±1.7 | 38.4±1.5 | 0.8 |
BMI: body mass index.
Data are presented as mean±SD. Mann–Whitney U test or Student’s t-test was used.
The 6MWT distances of the two groups are listed in Table 2. For both groups, walking distance increases as time passes after the operation (postoperative day 2 (POD2) walking distance>postoperative day 1 (POD1) walking distance >PO8 hours walking distance).
Table 2.
Postoperative 8th h/POD1/POD2 6MWT results
| Group | Binder group (n=45) | Control group (n=44) | p |
|---|---|---|---|
| PO 8th h 6MWT (m) | 99.4±27.3 | 81.0±22.2 | 0.001* |
| 995 (40–160) | 80 (30–130) | ||
| POD1 6MWT (m) | 155.3±23.6 | 146.7±28.6 | 0.122 |
| 150 (100–200) | 140 (80–200) | ||
| POD2 6MWT (m) | 215.3±30.9 | 213.4±28.4 | 0.761 |
| 210 (150–280) | 210 (160–280) |
PO: postoperative; POD: postoperative day; 6MWT: 6-minute walk test.
Data are presented as mean±SD and median (minimum–maximum). Mann–Whitney U test or Student’s t-test was used.
Statistically significant comparisons.
For all time periods, the 6MWT distance of the study group was longer than that of the control group. The difference was significant 8 h after CS (99.4±27.3 vs. 81.0±22.2, p=0.001), but it was not significant on POD1 and POD2.
Results of the SF-MPQ total pain, sensory, and affective subscales and VAS scores are presented in Table 3. The VAS scores of the study group were found to be lower than those of the control group at postoperative 8 h (p=0.001) and on POD1 (p=0.03). The SF-MPQ total pain scores of the study group were significantly lower than those of the control group on POD1 (p=0.004). Sensory subscale scores were significantly lower in the study group on POD1 (p<0.001) and POD2 (p=0.025). Although the effective subscale scores were lower for the study group, the difference was not statistically significant.
Table 3.
SF-MPQ and VAS scores of the study and control groups
| Total score | Sensory | Affective | VAS score | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Binder | Control | Binder | Control | Binder | Control | Binder | Control | |
| PO 8 h | 7.8±3.2 | 9.98±6.3 | 6.3±2.3 | 7.9±4.7 | 1.48±1.2 | 2.02±1.7 | 44.4±8.03 | 51.4±11‡ |
| 7 (1–22) | 7 (3–28) | 6 (1–17) | 6 (2–21) | 1 (0–5) | 1 (0–7) | 43 (30–65) | 50 (32–75) | |
| POD1 | 4.75±2.1* | 6.93±4.3* | 3.8±1.7¥ | 5.8±3.4¥ | 0.95±0.9 | 1.1±1.2 | 24.7±5.7€ | 31.1±6.4€ |
| 4 (0–10) | 5 (0–21) | 3 (0–8) | 5 (0–18) | 1 (0–4) | 1 (0–4) | 25 (15–40) | 32 (15–45) | |
| POD2 | 2.1±1.2 | 2.76±2 | 1.5±0.9£ | 2.2±1.6£ | 0.55±0.7 | 0.58±0.7 | 23.3±4.8 | 23.4±4.4 |
| 2 (0–5) | 2 (0–9) | 1 (0–4) | 2 (0–7) | 0 (0–2) | 0 (0–2) | 23 (14–35) | 23 (13–33) | |
SF-MPQ: Short-Form McGill Pain Questionnaire; VAS: Visual Analog Scale; PO: postoperative; POD: postoperative day.
Data are presented as mean±SD and median (minimum–maximum). Mann–Whitney U test or Student’s t-test was used.
p=0.004 (total pain score POD1 study vs. control).
p<0.001 (sensory subscale POD1 study vs. control).
p=0.025 (sensory subscale POD2 study vs. control).
p=0.001 (VAS score postoperative 8th h study vs. control).
p=0.03 (VAS score POD1 study vs. control).
Significant values are identified with markers.
SDSs (Table 4) were significantly lower in the study group than in the control group for all time frames (postoperative 8 h p=0.024, POD1 p<0.001, and POD2 p<0.001).
Table 4.
Symptom Distress Scale of the study and control groups
| Symptom Distress Scale | |||
|---|---|---|---|
|
|
|||
| Groups | Binder group | Control group | p |
| PO 8 h | 11.8±3.8 | 12.9±4.4 | 0.024* |
| 12 (5–24) | 12 (5–27) | ||
| POD1 | 10.7±3.1 | 13.6±3.7 | <0.001* |
| 10 (6–21) | 13 (7–23) | ||
| POD2 | 8.8±3.1 | 12.6±3.4 | <0.001* |
| 9 (4–16) | 12 (6–22) | ||
PO: postoperative; POD: postoperative day.
Data are presented as mean±SD and median (minimum–maximum). Mann–Whitney U test or Student’s t-test was used.
Statistically significant comparisons.
Preoperative hemogram levels were similar (p=0.249) for the binder (11.7±1.4 mg/dL) and control groups (11.3±1.4 mg/dL). Postoperative 24 h hemogram levels of the patients were also similar (p=0.611), 10.6±1.2 mg/dl for the BG and 10.4±1.5 mg/dL for the control group.
Discussion
Early mobilization after CS is critical for the return of physiological features to their pre-pregnancy levels. However, a large number of patients refuse to walk for mobilization after CS due to the fear of pain and damage to the surgical incision area. To the best of our knowledge, the present study is the first randomized controlled clinical trial on the effects of abdominal binder usage on mobilization. We hypothesized that abdominal binder usage would protect the movement of the abdominal wall and decrease pain due to physical functions. Our results show that abdominal binder use increases mobilization, decreases pain and distress, and does not affect postpartum bleeding.
Physical function (defined as the distance covered in a 6-minute walk) was investigated in two trials after laparotomy (binders versus no binders). Cheifetz et al. compared walking distance with 6MWT on postoperative days 1 and 5 after major abdominal surgery and found that abdominal binder using the group’s distance is significantly longer [7]. On the other hand, Olsen et al. detected no difference between the mobilization of the two groups [8].
In our study, when the patients were mobilized for the first time at postoperative 8th h, the walking distance of the study group at 6MWT was significantly longer than that of the control group. On the other hand, the distances on POD1 and POD2 after CS were not different for the two groups. Using an abdominal binder after CS increased the walking distance by 20% as compared with the N-BG at 8th h following the cesarean birth. The increase in postoperative mobilization in turn reduces the threat of thromboembolism. While in patients who had major abdominal surgery the positive effects of abdominal binder usage on walking distance were detected on postoperative day 5 [8], in our study, the positive effects on patients who had CS were seen in the early stages of the postoperative period (at the time of the first mobilization). Moreover, the 6MWT values of the patients in our study group on POD1 and POD2 showed no difference when compared with the control group. Arguably, the reason for this difference is that the healing period after CS is shorter than that after major abdominal surgeries. Therefore, patients can return to their preoperative performance levels quickly, and there is no difference at 6MWT on POD1 and POD2.
Abdominal binders generalize pain over the abdomen rather than just the incision line by applying pressure on the incision line itself. The feeling of discomfort in patients while sitting, standing, and walking becomes lesser as a result. Today, especially for patients who had ventral hernia correction, abdominal binders are being used in an almost routine manner. A systematic review of the effects of postoperative binder usage on pain has also revealed that using abdominal binders reduces postoperative pain [11]. Similarly, Ghana et al. and Gustafson et al. showed that abdominal binder usage after CS reduces the feeling of pain and distress [6, 12]. In contrast, another previous study has suggested that using an abdominal binder does not have an impact on VAS scores or SDS scores [4].
In our study, we compared the distress scores of both groups (binders versus no binders) after giving birth by CS and detected that abdominal binder usage reduces distress at all time periods. The same conclusion cannot be made for the pain scores. The SF-MPQ scores of the study group were lower only on POD1, and no significant difference was observed at postoperative 8th h. This finding suggests that the increase in mobilization of patients using an abdominal binder at 8th h might be caused by the lowered distress score rather than the pain score.
No adverse effects of using abdominal binders were observed in our study. However, some researchers hypothesize that the increased abdominal pressure due to abdominal binder use may lower lung capacity [13]. Another side effect that has been suggested is that using abdominal binders decreases the cardiac output by increasing the venous volume in the lower extremities [14]. Furthermore, the researchers recommend limiting the use of abdominal binders in non-ambulatory patients since it might increase the risk of thrombosis [5]. Therefore, patients who had respiratory and cardiac diseases were excluded from the study to avoid possible complications. All the participants in the study group were quite willing to use abdominal binders. This situation might have contributed to the decrease in distress levels during the hospitalization period.
Postoperative pain, distress, and mobilization are not the only areas that the abdominal binders are also beneficial. They have some additional potential advantages, such as quick healing of the scar and prevention of hematoma and seroma in the incision area [15]. In our study, we did not detect complications in the incision area for both groups.
Our study has few limitations. First, blinding of the group assignment was impossible due to the nature of the intervention, and the placebo of using an abdominal binder might have had effect on the results of the study. Second, pain is a subjective phenomenon, and the psychological and emotional states of the patient may change her perception of this symptom [16]. To minimize the personal differences in pain perception, pain tests could be performed preoperatively and compared with the postoperative test results. Similarly, mobilization habits of the patients could be scaled preoperatively and could be compared with the postoperative score for a more objective comparison. However, both preoperative measurements were not available in the present study. Finally, the BG was encouraged to use their binder in every chance they have. However, they were allowed to have breaks, and it was not possible to note over use or not enough usage. Besides, to our knowledge, this prospective randomized controlled trial is the first study comparing the mobilization rate of women using a postoperative abdominal binder and women who were not using it.
In conclusion, abdominal binder usage after CS can be suggested to patients as it increases the walking distance, has a positive effect on pain, and lowers the symptom associated with distress.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Istanbul Bakirkoy Dr. Sadi Konuk Training and Research Hospital (2017-09-22/246).
Informed Consent: Informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – I.K.; Design - I.K., I.A.; Supervision - M.O., S.Y.K.; Resources - M.O..; Materials - V.S.E.; Data Collection and/or Processing - V.S.E.; Analysis and/or Interpretation - O.I.; Literature Search - M.E.; Writing Manuscript - I.K., O.I.; Critical Review - M.E., S.Y.K.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Molina G, Weiser TG, Lipsitz SR, et al. Relationship Between Cesarean Delivery Rate and Maternal and Neonatal Mortality. Jama. 2015;314:2263–70. doi: 10.1001/jama.2015.15553. [DOI] [PubMed] [Google Scholar]
- 2.Karlstrom A, Engstrom-Olofsson R, Norbergh KG, Sjoling M, Hildingsson I. Postoperative pain after cesarean birth affects breastfeeding and infant care. J Obstet Gynecol Neonatal Nurs. 2007;36:430–40. doi: 10.1111/j.1552-6909.2007.00160.x. [DOI] [PubMed] [Google Scholar]
- 3.Havey R, Herriman E, O’Brien D. Guarding the gut: early mobility after abdominal surgery. Crit Care Nurs Q. 2013;36:63–72. doi: 10.1097/CNQ.0b013e3182753237. [DOI] [PubMed] [Google Scholar]
- 4.Gillier CM, Sparks JR, Kriner R, Anasti JN. A randomized controlled trial of abdominal binders for the management of postoperative pain and distress after cesarean delivery. Int J Gynaecol Obstet. 2016;133:188–91. doi: 10.1016/j.ijgo.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Larson CM, Ratzer ER, Davis-Merritt D, Clark JR. The effect of abdominal binders on postoperative pulmonary function. Am Surg. 2009;75:169–71. [PubMed] [Google Scholar]
- 6.Ghana S, Hakimi S, Mirghafourvand M, Abbasalizadeh F, Behnampour N. Randomized controlled trial of abdominal binders for postoperative pain, distress, and blood loss after cesarean delivery. Int J Gynaecol Obstet. 2017;137:271–6. doi: 10.1002/ijgo.12134. [DOI] [PubMed] [Google Scholar]
- 7.Cheifetz O, Lucy SD, Overend TJ, Crowe J. The effect of abdominal support on functional outcomes in patients following major abdominal surgery: a randomized controlled trial. Physiother Can. 2010;62:242–53. doi: 10.3138/physio.62.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagevik Olsén M, Josefson K, Wiklund M. Evaluation of abdominal binder after major upper gastrointestinal surgery. Adv Physiother. 2009;11:104–10. doi: 10.1080/14038190802141073. [DOI] [Google Scholar]
- 9.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 10.Bicici B, Gunes UY. The validity and reliability of the Turkish version of short-form McGill Pain Questionnaire in patients with leukemia. J Clin Nurs. 2012;21:3328–34. doi: 10.1111/j.1365-2702.2012.04107.x. [DOI] [PubMed] [Google Scholar]
- 11.Gustafson JL, Dong F, Duong J, Kuhlmann ZC. Elastic Abdominal Binders Reduce Cesarean Pain Postoperatively: A Randomized Controlled Pilot Trial. Kans J Med. 2018;11:1–19. [PMC free article] [PubMed] [Google Scholar]
- 12.Haferbier Gustafson JL, Kuhlmann ZC, Frazier LM, Duong J. Do Elastic Abdominal Binders Reduce Postoperative Pain and Blood Loss?: A Randomized Controlled Trial [146] Obstet Gynecol. 2015;125:51S. [Google Scholar]
- 13.Toft MH, Bulow J, Simonsen L. Haemodynamic and respiratory effects of an abdominal compression binder. Clin Physiol Funct Imaging. 2008;28:398–402. doi: 10.1111/j.1475-097X.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- 14.Laurikka JO, Toivio I, Tarkka MR. Effects of a novel pneumatic vest on postoperative pain and lung function after coronary artery bypass grafting. Scand Cardiovasc J. 1998;32:141–4. doi: 10.1080/14017439850140094. [DOI] [PubMed] [Google Scholar]
- 15.Kaafarani HM, Hur K, Hirter A, et al. Seroma in ventral incisional herniorrhaphy: incidence, predictors and outcome. Am J Surg. 2009;198:639–44. doi: 10.1016/j.amjsurg.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Merskey H, Bogduk N, editors. International Association for the Study of Pain (IASP) Seattle: Seattle: IASP Press; 1994. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. [Google Scholar]