ABSTRACT
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance (IR). T2DM is correlated with obesity and most T2DM medications have been developed for enhancing insulin sensitivity. Silk protein fibroin (SPF) from spiders has been suggested as an attractive biomaterial for medical purposes. We generated transgenic rice (TR) expressing SPF and fed it to diabetic BKS.Cg-m+/+Leprdb mice to monitor the changes in blood glucose levels and adipose tissue proteins associated with energy metabolism and insulin signaling. In the present study, the adipocyte size in abdominal fat in TR-SPF-fed mice was remarkably smaller than that of the control. Whereas the adenosine monophosphate-activated protein kinase (AMPK)-activated protein kinase and insulin receptor substrate 1 (IRS1) protein levels were increased in abdominal adipose tissues after TR-SPF feeding, levels of six-transmembrane protein of prostate 2 (STAMP2) proteins decreased. Phosphorylation of AMPK at threonine 172 and IRS1 at serine 307 and tyrosine 632 were both increased in adipose tissues from TR-SPF-fed mice. Increased expression and phosphorylation of IRS1 at both serine 307 and tyrosine 632 in adipose tissues indicated that adipocytes obtained from abdominal fat in TR-SPF-fed mice were more susceptible to insulin signaling than that of the control. STAMP2 protein levels decreased in adipose tissues from TR-SPF-fed mice, indicating that STAMP2 proteins were reducing adipocytes that were undergoing lipolysis. Taken together, this study showed that TR-SPF was effective in reducing blood glucose levels in diabetic mice and that concurrent lipolysis in abdominal adipocytes was associated with alterations of AMPK, IRS1, and STAMP2. Increased IRS1 expression and its phosphorylation by TR-SFP were considered to be particularly important in the induction of lipolysis in adipocytes, as well as in reducing blood glucose levels in this animal model.
Keywords: Spider silk protein fibroin, Transgenic rice, IRS1, Adipocytes, Diabetic mouse
INTRODUCTION
During the last few decades, the prevalence of diabetes mellitus (DM) has increased worldwide and is one of the leading causes of high mortality and morbidity. DM is a metabolic syndrome characterized by an elevated blood glucose levels and is largely classified into two types: type 1 DM (T1DM) and type 2 DM (T2DM) (Thomas & Philipson, 2015). T1DM is associated with failure in insulin production resulting from the destruction of pancreatic β-cells while T2DM often is characterized by insulin resistance (IR). The therapeutic regimes and outcomes for T2DM is more complicated and unguaranteed, respectively, than those of T1DM. T2DM is often correlated with obesity (Boles et al., 2017) and most T2DM medications have been developed for enhancing insulin sensitivity. For instance, Metformin is the most representative antidiabetic drug, especially for obese individuals (Sanchez-Rangel & Inzucchi, 2017). Metformin functions by activating one of the enzymes involved in expressing gluconeogenic genes, known as 5-adenosine monophosphate-activated protein kinase (AMPK) (Rena et al., 2017).
Silk protein fibroin (SPF) from spiders has been suggested as an attractive biomaterial for industrial and medical purposes because of its unique properties (Altman et al., 2003; Kluge et al., 2008). SPF is mainly composed of glycine and alanine. The biological effects of SPF include the enhancement of glucose (Hyun et al., 2004; Do et al., 2012) and lipid metabolism (Jung et al., 2010), anti-viral activity (Gotoh et al., 2000), DNA damage protection (Park et al., 2002), and blood pressure-depressing activity (Igarashi et al., 2006). Recombinant spider SPF exhibits non-cytotoxic and non-inflammatory effects in NIH 3T3 cells (Lee et al., 2016) and also reduces blood glucose levels in diabetic mice (Lee et al., 2014).
In the present study, we generated transgenic rice (TR) expressing SPF and fed it to diabetic BKS.Cg-m+/+Leprdb mice to monitor the changes in blood glucose levels and adipose tissue proteins associated with energy metabolism and insulin signaling. The purpose of this study was to investigate whether cellular and biochemical alterations in adipocytes were correlated with changes in blood glucose levels in TR-SPF-fed mice.
MATERIALS AND METHODS
1. Reagents and antibodies
Bouin’s solution, eosin and hematoxylin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Complete Protease Inhibitor Cocktail Tablet was from Roche Applied Science (Mannheim, Germany). The western enhanced chemiluminescence (ECL) detection reagent was purchased from Bio-Rad (Hercules, CA, USA). VECTASTAIN® Elite ABC Kit was from Vector Laboratories (Burlingame, CA, USA). Anti-AMPKα and Phospo-AMPKα(Thr172) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-insulin receptor substrate (IRS1), Phospo-IRS-1 (Ser307) and Phospo-IRS-1 (Tyr632) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-STEAP4 (STAMP2) antibody was from Proteintech (Rosemont, IL, USA). Anti-β-Tubulin antibody was from Sigma Chemical Co. (St. Louis, MO, USA).
2. Production of transgenic rice
cDNAs encoding a partial repetitive region and the C-terminal domain of AvMaSp (Lee et al., 2012) were PCR-amplified from pGEMT-AvMaSp using the forward primer 5′-GGATCCATGGCCGCCGCAGCCGCA-3′ (with BamHI) and reverse primer 5′-GTCGACTAACGCTGCGGCAGAGGC-3′ (with SalI). The AvMaSp fragment was inserted into the binary pPZP-3′PINII-Bar vector (pAvMaSp-Bar) containing the seed-specific GluC promoter and the Tnos terminator sequence. To generate transgenic rice containing the partial AvMaSp gene, pAvMaSp-Bar was introduced into Agrobacterium tumefaciens (EHA105) by electroporation. A modified version of a general rice transformation protocol was used (ToKi, 1997; Mohanty et al., 1999). Rice grains were prepared using a rice milling machine and processed to a fine powder using a blender for oral administration. The compositions of both control (Dong-jin rice) and transgenic rice powders were the same, including equal proportions of casein, sucrose, corn oil, cellulose, vitamin mixture, mineral mixture, choline bitartrate, DL-methionine, and t-butylhydroquinone.
3. Animals and sampling
Diabetic male BKS.Cg-m+/+Leprdb mice (8 weeks of age) were purchased from Samtako Bio-Korea Co. (Osan, Korea). The mice were housed in a climate-controlled (21±2℃) animal room at a constant 12-h light/dark cycle. All procedures were performed in accordance with protocols approved by the Dong-A University Animal Care and Use Committee (DIACUC-19-13). Each control or transgenic rice powder was provided in a separate feeding container ad libitum once per day. After 4 weeks of feeding, the mice (n=15 per group) were sacrificed by carbon dioxide asphyxiation and whole blood was collected by cardiac puncture. The abdominal fat samples were finely dissected, weighed, and immediately used for the protein analysis and histological study.
4. Determination of blood glucose level
Plasma glucose concentrations were measured in tail blood using a GlucoDr Blood Glucose Test Strip (Hasuco, Seoul, Korea).
5. Western blot analysis
Small pieces of abdominal adipose tissues were homogenized in lysis buffer [300 mM NaCl, 0.5% Triton X-100, 50 mM Tris-HCl (pH 7.4), 25 mM NaF, 1 mM Na3VO4, 10 mM Na4P2O7, and protease inhibitor] for 40 min on ice. The lysates were centrifuged at 13,000 ×g at 4°C for 20 min, the supernatants were collected and protein concentration was measured using the BCA protein assay kit. 30 μg of protein extract with sodium dodecyl sulfate (SDS)-loading buffer was electrophoretically separated on a 8%–15% gradient SDS-PAGE gel, and transferred onto a nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk dissolved in Tris-buffered saline (TBS) buffer containing 0.05% Tween-20 at RT for 1 h. The blots were incubated with primary antibodies, followed by incubation with appropriate HRP-conjugated secondary antibodies. The signals were detected with ECL detection reagent in the LAS-4000 (Fuji, Tokyo, Japan). β-Tubulin was used as internal control for total cellular proteins.
6. Immunohistochemistry
Abdominal adipose tissues were fixed in Bouin’s solution, embedded in paraffin, and sectioned at 5 μm thickness. Tissue sections were placed on glass slides, deparaffinized, hydrated, and treated with 1% hydrogen peroxide (H2O2) for 10 min to suppress endogenous peroxidase activity. Antigen retrieval was performed by heating the sections in 1 mM citric acid solution (pH 6.0). Sections were incubated with the primary antibody for AMPKa, IRS1, pIRS1 (Tyr632) and STAMP2 at 4°C overnight and reacted with biotinylated horse anti-mouse IgG for 1 h at RT, as per the manufacturer’s instructions for Vectastain Elite ABC kit. The stained sections were developed with liquid diaminobenzidine and counterstained with hematoxylin. The results were observed using a ScanScope digital slide scanning system (Aperio Technologies, Vista, CA, USA).
7. Statistics
Data were expressed as the mean±SD of at least 3 independent experiments. The difference in means between 2 groups was analyzed using the Student’s t-test. Mean values were considered significantly different at p<0.05.
RESULTS AND DISCUSSION
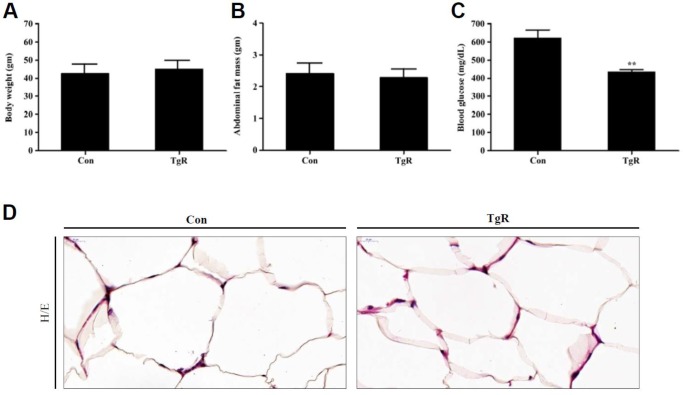
In the present study, feeding diabetic mice with TR-SPF caused significant reduction of their blood glucose levels (Fig. 1C) without changes in body weight (Fig. 1A) and abdominal fat mass (Fig. 1B). However, histological observation showed that the adipocyte size in abdominal fat in TR-SFP-fed mice was remarkably smaller than that of the control (Fig. 1D). These results indicated that although the fat mass and weight were not noticeably reduced after TR-SPF feeding, subtle cellular and molecular metabolic changes in adipocytes may correlate with lipolysis in adipocytes in these diabetic mice. In T2DM, the potent involvement of adipocytes in balancing glucose metabolism has been implicated together with that of the liver, skeletal muscle, and pancreas (Czech, 2017). Obesity is correlated with the occurrence of T2DM (Boles et al., 2017). Therefore, lipolysis in adipocytes is an important event that modulates IR in T2DM (Morigny et al., 2016). In this context, this study focused on investigating the cellular and biochemical changes in adipocytes during their size reduction after exposure to TR-SPF.
Fig. 1. Changes in body weight (A), abdominal fat mass (B), blood glucose levels (C), and abdominal fat histology (D) after feeding diabetic mice TR-SPF for 4 weeks. The data are expressed as mean±SD of three independent experiments performed in triplicates. **p <0.01 compared with those of control (CON). TgR denotes TR-SPF-fed group. (D) Hematoxylin-eosin (H/E) staining. Original magnifications: 800×. TR, transgenic rice; SPF, silk protein fibroin.
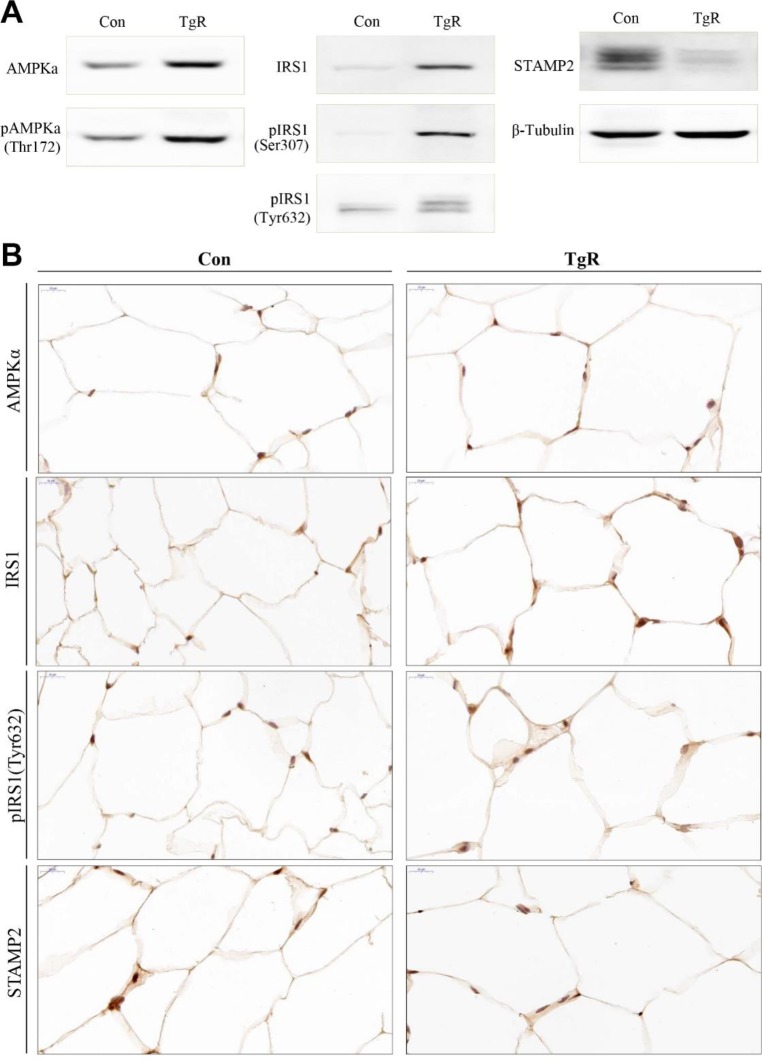
In both hepatocytes and adipocytes, AMPK and IRS1 are known to associate with IR and the pathogenesis of T2DM (Musi & Goodyear, 2006). Recently, the six-transmembrane protein of prostate 2 (STAMP2) has been also emphasized in the regulation of IR and adipocyte differentiation (Sikkeland & Saatcioglu, 2013; Kim et al., 2015). In this study, whereas the AMPK and IRS1 protein levels were increased in abdominal adipose tissues after TR-SPF feeding, levels of STAMP2 proteins decreased (Fig. 2A). In addition, phosphorylation of AMPK at threonine 172 (pAMPK thr172) and IRS1 at serine 307 and tyrosine 632 (pIRS1 ser307/tyr632) were both increased in adipose tissues from TR-SPF-fed mice (Fig. 2A). The changes in these proteins were confirmed in adipose tissues by immunohistochemical localization (Fig. 2B). AMPK is a threonine/serine kinase that plays an important role in energy metabolism and is a sensor of the cellular energy state that responds to metabolic stresses and other regulatory signals. In hepatocytes, inhibition of AMPK is known to cause IR, whereas activation of AMPK increases insulin sensitivity. Recently, it has been demonstrated that AMPK is activated in adipocytes in which lipolysis is stimulated (Gauthier et al., 2008). Consistent with this, our results also showed that AMPK expression and AMPK phosphorylation were increased in adipose tissues from TR-SPF-fed mice. This indicated that feeding TR-SPF to a diabetic mouse provoked lipolysis in adipocytes through the activation of AMPK and thereby reduced adipocyte size. IRS1 serves as a hub for transmitting the insulin signal. Most of the phosphorylation of IRS1 at serine/threonine residues has been associated with inhibition of insulin signaling. However, phosphorylation of IRS1 at serine/threonine residues has also been shown to enhance the phosphorylation of tyrosine in IRS1 in response to insulin and hence to stimulate insulin signaling (Danielsson et al., 2005). Increased expression and phosphorylation of IRS1 at both serine 307 and tyrosine 632 in adipose tissues in this study indicated that adipocytes obtained from abdominal fat in TR-SPF-fed mice were more susceptible to insulin signaling than that of the control. Previously, it has been suggested that STAMP family proteins are associated with adipocyte differentiation (Sikkeland & Saatcioglu, 2013). Hepatic STAMP2 alleviates high fat diet-induced hepatic steatosis and IR (Kim et al., 2015). In addition, STAMP2 expression is elevated in fed mice compared to fasting mice (Wellen et al., 2007). In the present study, STAMP2 protein levels decreased in adipose tissues from TR-SPF-fed mice, indicating that STAMP2 proteins were reducing adipocytes that were undergoing lipolysis. However, this cellular and molecular event in adipocytes needs to be further elucidated in the near future.
Fig. 2. Alterations in the levels of AMPK, pAMPK (Thr172), IRS1, pIRS1 (Ser307), pIRS1 (Tyr632), and STAMP2 proteins and immunocytochemical localizations of AMPK, IRS1, pIRS1 (Tyr632), and STAMP2 in abdominal fat tissues after feeding diabetic mice TR-SPF for 4 weeks. (A) SDS-PAGE followed by western blotting was performed using 25 μg of protein lysates from fat tissues. β-Tubulin was used as an internal control for all cellular proteins. (B) Abdominal fat tissues were fixed, paraffin-embedded, and immunostained with corresponding antibody, and observed under a light microscope. Original magnification: 800×. AMPK, adenosine monophosphate-activated protein kinase; IRS1, insulin receptor substrate 1; STAMP2, six-transmembrane protein of prostate 2; TR, transgenic rice; SPF, silk protein fibroin.
Taken together, this study showed that TR-SPF was effective in reducing blood glucose levels in diabetic mice and that concurrent lipolysis in abdominal adipocytes was associated with alterations of AMPK, IRS1, and STAMP2. Among these, increased IRS1 expression and its phosphorylation by TR-SFP were considered to be particularly important in the induction of lipolysis in adipocytes, as well as in reducing blood glucose levels in this animal model. Finally, the underlying mechanism(s) by which TR-SPF was able to lower blood glucose levels in diabetic mice will become more obvious with further comparative studies in hepatic tissues.
Acknowledgments
This study was supported by a grant from the Next-Generation BioGreen 21 Program (Project No. PJ01368402), Rural Development Administration, Korea.
CONFLICT OF INTERESTS
The authors declare no potential conflict of interest.
REFERENCES
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based bi-omaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Boles A, Kandimalla R, Reddy PH. Dynamics of diabetes and obesity: Epidemiological perspective. Bi-ochim Biophys Acta Mol Basis Dis. 2017;1863:1026–1036. doi: 10.1016/j.bbadis.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23:804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson A, Ost A, Nystrom FH, Stralfors P. At-tenuation of insulin-stimulated insulin receptor sub-strate-1 serine 307 phosphorylation in insulin re-sistance of type 2 diabetes. J Biol Chem. 2005;280:34389–34392. doi: 10.1074/jbc.C500230200. [DOI] [PubMed] [Google Scholar]
- Do SG, Park JH, Nam H, Kim JB, Lee JY, Oh YS, Suh JG. Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic β cell mass in C57BL/KsJ-db/db mice. J Vet Sci. 2012;13:339–344. doi: 10.4142/jvs.2012.13.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-acti-vated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh K, Izumi H, Kanamoto T, Tamada Y, Nakashima H. Sulfated fibroin a novel sulfated peptide de-rived from silk inhibits human immunodeficiency vi-rus replication in vitro. Biosci Biotechnol Bio-chem. 2000;64:1664–1670. doi: 10.1271/bbb.64.1664. [DOI] [PubMed] [Google Scholar]
- Hyun CK, Kim IY, Frost SC. Soluble fibroin en-hances insulin sensitivity and glucose metabolism in 3T3-L1 adipocytes. J Nutr. 2004;134:3257–3263. doi: 10.1093/jn/134.12.3257. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Yoshioka K, Mizutani K, Miyakoshi M, Murakami T, Akizawa T. Blood pressure-depressing activity of a peptide derived from silkworm fibroin in spontaneously hypertensive rats. Biosci Biotechnol Bi-ochem. 2006;70:517–520. doi: 10.1271/bbb.70.517. [DOI] [PubMed] [Google Scholar]
- Jung EY, Lee HS, Lee HJ, Kim JM, Lee KW, Suh HJ. Feeding silk protein hydrolysates to C57BL/KsJ-db/db mice improves blood glucose and lipid profiles. Nutr Res. 2010;30:783–790. doi: 10.1016/j.nutres.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Kim HY, Park SY, Lee MH, Rho JH, Oh YJ, Jung HU, Yoo SH, Jeong NY, Lee HJ, Suh S, Seo SY, Cheong J, Jeong JS, Yoo YH. Hepatic STAMP2 alleviates high fat diet-induced hepatic steatosis and insulin resistance. J Hepatol. 2015;63:477–485. doi: 10.1016/j.jhep.2015.01.025. [DOI] [PubMed] [Google Scholar]
- Kluge JA, Rabotyagova O, Leisk GG, Kaplan DL. Spider silks and their applications. Trends Biotechnol. 2008;26:244–251. doi: 10.1016/j.tibtech.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim BY, Kim DH, Jin BR. Molecular clon-ing and characterization of the partial major ampul-late silk protein gene from the spider Araneus ven-tricosus. J Asia Pac Entomol. 2012;15:641–646. doi: 10.1016/j.aspen.2012.08.004. [DOI] [Google Scholar]
- Lee KS, Kim BY, Kim DH, Jin BR. Spider silk fibroin enhances insulin secretion and reduces blood glucose levels in diabetic mice. J Asia-Pacific Entomol. 2014;17:907–909. doi: 10.1016/j.aspen.2014.10.005. [DOI] [Google Scholar]
- Lee KS, Kim BY, Kim DH, Jin BR. Recombinant spider silk fibroin protein produces a non-cytotoxic and non-inflammatory response. J Asia-Pacific Ento-mol. 2016;19:1015–1018. doi: 10.1016/j.aspen.2016.09.004. [DOI] [Google Scholar]
- Mohanty A, Sarma NP, Tyagi AK. Agrobacterium-mediated high frequency transformation of an elite indica rice variety Pusa Basmati 1 and transmission of the transgenes to R2 progeny. Plant Sci. 1999;147:127–137. doi: 10.1016/S0168-9452(99)00103-X. [DOI] [Google Scholar]
- Morigny P, Houssier M, Mouisel E, Langin D. Adi-pocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. doi: 10.1016/j.biochi.2015.10.024. [DOI] [PubMed] [Google Scholar]
- Musi N, Goodyear LJ. Insulin resistance and im-provements in signal transduction. Endocrine. 2006;29:73–80. doi: 10.1385/ENDO:29:1:73. [DOI] [PubMed] [Google Scholar]
- Park KJ, Jin HH, Hyun CK. Antigenotoxicity of pep-tides produced from silk fibroin. Process Biochem. 2002;38:411–418. doi: 10.1016/S0032-9592(02)00136-X. [DOI] [Google Scholar]
- Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia 2017. 2017;60:1586–1593. doi: 10.1007/s00125-017-4336-x. [DOI] [PubMed] [Google Scholar]
- Sikkeland J, Saatcioglu F. Differential expression and function of stamp family proteins in adipocyte differentiation. PLOS ONE. 2013;8:e68249. doi: 10.1371/journal.pone.0068249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CC, Philipson LH. Update on diabetes classification. Med Clin North Am. 2015;99:1–16. doi: 10.1016/j.mcna.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Toki S. Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol Bio Rep. 1997;15:16–21. doi: 10.1007/BF02772109. [DOI] [Google Scholar]
- Wellen KE, Fucho R, Gregor MF, Furuhashi M, Morgan C, Lindstad T, Vaillancourt E, Gorgun CZ, Saatcioglu F, Hotamisligil GS. Coordinated regulation of nu-trient and inflammatory responses by STAMP2 is es-sential for metabolic homeostasis. Cell. 2007;129:537–548. doi: 10.1016/j.cell.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]