A prospective multicenter clinical study involving subjects from 21 sites across the United States was conducted to validate the performance of a new in vitro diagnostic nucleic acid amplification test (NAAT) for the detection of Mycoplasma genitalium.
KEYWORDS: Mycoplasma genitalium, sexually transmitted infection, Aptima, Aptima Mycoplasma genitalium Evaluation Study (AMES)
ABSTRACT
A prospective multicenter clinical study involving subjects from 21 sites across the United States was conducted to validate the performance of a new in vitro diagnostic nucleic acid amplification test (NAAT) for the detection of Mycoplasma genitalium. Seven urogenital specimen types (n = 11,556) obtained from 1,778 females, aged 15 to 74 years, and 1,583 males, aged 16 to 82 years, were tested with the Aptima Mycoplasma genitalium assay, an investigational transcription-mediated amplification (TMA) NAAT for the detection of M. genitalium 16S rRNA. Infected status for enrolled subjects was established using results obtained from testing either self-collected vaginal swab or clinician-collected male urethral swab specimens with a composite reference method consisting of three transcription-mediated amplification NAATs targeting unique regions of M. genitalium 16S or 23S rRNA. M. genitalium prevalence was 10.2% in females and 10.6% in males; prevalence was high in both symptomatic and asymptomatic subjects for both sexes. Compared to the subject infected status standard, the investigational test had sensitivity and specificity estimates, respectively, of 98.9% and 98.5% for subject-collected vaginal swabs, 92.0% and 98.0% for clinician-collected vaginal swabs, 81.5% and 98.3% for endocervical swabs, 77.8% and 99.0% for female urine, and 98.2% and 99.6% for male urethral swabs, 88.4% and 97.8% for self-collected penile meatal swabs, and 90.9% and 99.4% for male urine specimens. For all seven specimen types, within-specimen positive and negative agreements between the investigational test and the composite reference standard ranged from 94.2% to 98.3% and from 98.5 to 99.9%, respectively. These results provide clinical efficacy evidence for the first FDA-cleared NAAT for M. genitalium detection in the United States.
INTRODUCTION
Mycoplasma genitalium is a fastidious bacterium in the class Mollicutes. Its minute 580-kb genome is the smallest known among prokaryotes capable of self-replication (1, 2). M. genitalium was first cultured in 1981 using urethral specimens from men with nongonococcal urethritis (NGU) (3). Slow growth in vitro and burdensome culture requirements have precluded routine diagnosis using this method (4). Nucleic amplification assay tests (NAATs) based on PCR and transcription-mediated amplification (TMA) chemistries have been necessary for the study of associations between infection in humans and disease (5–9). Since the availability of such molecular assays, the organism has been associated with many disease syndromes, such as urethritis in men and cervicitis and adverse reproductive sequelae such as endometritis and pelvic inflammatory disease in women. In addition to the cervix, vagina, and male urethra, M. genitalium is also found in the oropharynx and rectum (10–20). An association of M. genitalium infection with risk for HIV infection has been reported also (21). Increasing the concerns about treatment of the syndromes associated with M. genitalium are the reports of rising rates of resistance to azithromycin and moxifloxacin (22, 23), the primary agents used to treat these conditions. The overall importance of M. genitalium as a sexually transmitted pathogen has been comprehensively reviewed (24).
The development of the Aptima Mycoplasma genitalium (AMG) assay, an in vitro diagnostic (IVD) transcription-mediated amplification (TMA) assay that targets the 16S RNA of M. genitalium, has led to its experimental use to study the epidemiology and clinical outcomes associated with infection with the organism, as well as to a comparison with other molecular amplification assays (15, 16, 25–30). The assay has been used with sequencing to demonstrate high levels of macrolide antibiotic resistance in M. genitalium infections originating in the United States (31). Following receipt of the Conformité Européene (CE) mark from the European Union (32, 33), the AMG assay was clinically validated for detection of M. genitalium in urogenital specimens collected from subjects enrolled in a prospective, multicenter study encompassing multiple regions of the United States. The manuscript reports a summary of the U.S. study results.
MATERIALS AND METHODS
Study design and ethics approval.
This cross-sectional study was conducted in accordance with the ethical principles derived from the Declaration of Helsinki and Belmont Report and in compliance with the U.S. Food and Drug Administration (FDA) and Good Clinical Practice Guidelines (cGCP) set forth by the International Conference on Harmonization (ICH-E6). The study protocol (A10109-MGENPS-CSP-01) was approved by the local institutional review board at every site. Written informed consent was obtained from each subject at the time of enrollment, prior to specimen collection. Participants were compensated for study participation.
Study population.
Sexually active female and male subjects of ≥14 years of age with (symptomatic) or without (asymptomatic) symptoms of sexually transmitted infections (STIs) (e.g., abnormal discharge, genital itching, pain/discomfort during sexual intercourse or urination, or pain/discomfort in groin or lower belly) were eligible for enrollment. Subjects were enrolled at 21 U.S. sites (clinical research centers and emergency medicine, family planning, public health, STI, family medicine/obstetric-gynecologic [OB-GYN] facilities) between July 2017 and April 2018. Exclusion criteria included antibiotic treatment (i.e., with macrolides, fluoroquinolones, tetracyclines, or clindamycin) within 21 days of enrollment or previous enrollment in this study.
Sample collection.
Four specimens were collected in the clinic from each female subject in the following order: one self-collected first-catch urine specimen, one self-collected vaginal swab specimen, one clinician-collected vaginal swab specimen, and one clinician-collected endocervical swab specimen. Three specimens were collected in the clinic from each male subject in the following order: one self-collected penile meatal swab specimen, one clinician-collected urethral swab specimen, and one self-collected first-catch urine specimen. Vaginal and penile meatal specimens were collected using Aptima multitest swabs and placed in Aptima tubes containing specimen transfer medium (STM). Urethral and endocervical specimens were collected using an Aptima unisex swab and placed in Aptima tubes containing STM. First-catch urine specimens (i.e., approximately 20 to 30 ml of the initial urine stream collected in a urine collection cup free of any preservatives) were processed for testing using an Aptima urine specimen collection kit and placed in Aptima tubes containing urine transport medium (UTM).
TMA and specimen testing.
The design, format, and comparative analytical performance of the AMG transcription-mediated amplification (TMA) assays and three alternate (Alt) TMA assays used for the composite reference standard have been described previously (34). All specimens were tested first with the AMG assay on an automated Panther system in one of three U.S. laboratories before being transported to Hologic for reference testing. Reference testing was performed using three research-use-validated alternate TMA assays developed by Hologic to capture, amplify, and detect unique regions of the 16S rRNA (Alt TMA assay-1) or 23S rRNA (Alt TMA assay-2 and Alt TMA assay-3) of M. genitalium; the Alt TMA assay-1 detected a different region of the 16S rRNA than the AMG assay. All three Alt TMA assays have similar analytical and clinical sensitivities (34). Alt TMA assays were performed on a Panther system (Alt TMA assays-1 and -2) or a manual direct tube sampling (DTS) system (Alt TMA-3) using validated laboratory-developed assay software. Each specimen was tested using Alt TMA assays -1 and -2; if the results of the two tests were discordant, the result from Alt TMA assay-3 testing was used as a tiebreaker. If two Alt TMA assay results were positive, the reference result was classified positive; if two Alt TMA results were negative, the reference result was negative (Table 1). Operators performing Alt TMA assays were blinded to AMG test results and all patient identifying information.
TABLE 1.
Algorithm for establishing patient infected status using alternate TMA assay consensus results
| Reference specimen resulta |
Patient infected status | ||
|---|---|---|---|
| Alt TMA assay-1b | Alt TMA assay-2 | Alt TMA assay-3 | |
| Positive | Positive | NA | Positive |
| Negative | Invalid/missing | Unknown | |
| Negative | Positive | Positive | |
| Negative | Negative | Negative | |
| Invalid/missing | Invalid/missing | Unknown | |
| Invalid/missing | Positive | Positive | |
| Invalid/missing | Negative | Unknown | |
| Negative | Positive | Invalid/missing | Unknown |
| Positive | Positive | Positive | |
| Positive | Negative | Negative | |
| Negative | NA | Negative | |
| Invalid/missing | Invalid/missing | Unknown | |
| Invalid/missing | Positive | Unknown | |
| Invalid/missing | Negative | Negative | |
| Invalid/missing | Positive | Invalid/missing | Unknown |
| Positive | Positive | Positive | |
| Positive | Negative | Unknown | |
| Negative | Invalid/missing | Unknown | |
| Negative | Positive | Unknown | |
| Negative | Negative | Negative | |
| Invalid/missing | NA | Unknown | |
The reference specimen is the urethral swab sample for male subjects and the self-collected vaginal swab sample for female subjects. NA, not applicable.
Alt TMA, alternate transcription-mediated amplification.
Statistical methods.
Prevalence (based on reference test result and patient infected status [PIS]), sensitivity, specificity, positive predictive value, and negative predictive value were calculated according to standard equations (35). Confidence intervals (CIs) for sensitivity and specificity were calculated using the score method. The confidence intervals for positive and negative predictive values were calculated using the exact method. Samples with inconclusive reference results and samples with invalid or missing investigational assay results were excluded from the analyses. The positive likelihood ratio (PLR) was calculated as sensitivity/(1 − specificity), and negative likelihood (NLR) was calculated as (1 − sensitivity)/specificity. Analyses were performed with SAS software (version 9.4; SAS Institute, Inc., Cary, NC).
PIS.
To determine the clinical performance of the investigational assay in the assessed specimen types, Aptima TMA results were assessed relative to the patient infected status (PIS). The PIS was based on reference (composite Alt TMA assay) results from testing of the urethral swab sample for male subjects and of the patient-collected vaginal swab sample for female subjects; based on prior studies, vaginal swab and urethral samples are the most sensitive specimen types for the detection of M. genitalium (25, 36, 37). Table 1 shows the algorithm for establishing the PIS. Unless otherwise specified, for each specimen type, assay performance for the detection of M. genitalium was calculated relative to the PIS.
RESULTS
Study design and subject accountability.
Figure 1 shows the patient accountability log, as well as the final numbers of evaluable samples for each specimen type. There were 3,393 subjects enrolled in the study, including 1,789 females and 1,604 males. Of these, 32 subjects were withdrawn for various reasons, including ineligibility or self-termination of participation. An additional 61 subjects with insufficient test results available for establishing a PIS were excluded, leaving 3,300 subjects (1,737 females and 1,563 males) evaluable for analysis. A total of 11,556 specimens were collected and analyzed using four female specimen types (n = 6,880) and three male specimen types (n = 4,676).
FIG 1.
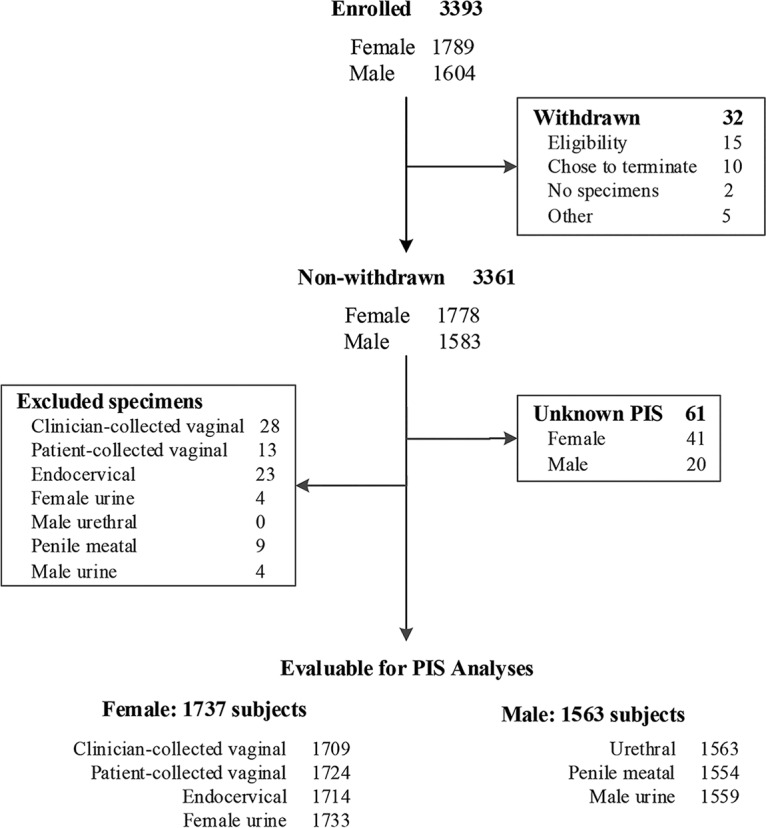
Overall study design and subject accountability. Subjects were not evaluable for the analysis versus PIS if they had an unknown PIS. Specimens with missing or invalid Aptima results were excluded from all analyses.
Demographic characteristics of enrolled subjects for sex, age, and race/ethnicity, as well as symptom status and M. genitalium prevalence for each group, are shown in Table 2. The majority of subjects were between 18 and 40 years of age (83.7% female; 70.1% male), Black (∼61% for both sexes), non-Hispanic (∼77% for both sexes), and from southeast or southwest clinical centers (∼74% for both sexes). For females, 60.6% were symptomatic; 55.4% of males were symptomatic. For females, M. genitalium prevalence was 11.6% and 7.9% among symptomatic and asymptomatic subjects, respectively; for males, M. genitalium prevalence was 12.0% and 8.8% among symptomatic and asymptomatic subjects, respectively. The highest prevalence of infection was in young adults of both sexes aged 18 to 20 years (female, 26.2%; male, 15.1%).
TABLE 2.
Prevalence of M. genitalium urogenital infection by subject demographic status and geographic region
| Category | No. of specimens |
M. genitalium prevalence |
|||
|---|---|---|---|---|---|
| Female |
Male |
||||
| No. positive/total no. | % positive (95% CI) | No. positive/total no. | % positive (95% CI) | ||
| Subject age (yr) | |||||
| 15–17 | 4 | 0/3 | 0 (0.0–56.1) | 0/1 | 0 (0.0–79.3) |
| 18–20 | 242 | 39/149 | 26.2 (19.8–33.8) | 14/93 | 15.1 (9.2–23.7) |
| 21–30 | 1,379 | 94/805 | 11.7 (9.6–14.1) | 81/574 | 14.1 (11.5–17.2) |
| 31–40 | 929 | 36/500 | 7.2 (5.2–9.8) | 53/429 | 12.4 (9.6–15.8) |
| 41–50 | 345 | 6/157 | 3.8 (1.8–8.1) | 11/188 | 5.9 (3.3–10.2) |
| 51–60 | 298 | 1/94 | 1.1 (0.2–5.8) | 5/204 | 2.5 (1.1–5.6) |
| 61–70 | 90 | 0/25 | 0 (0.0–13.3) | 1/65 | 1.5 (0.3–8.2) |
| 71–82 | 13 | 0/4 | 0 (0.0–49.0) | 0/9 | 0 (0.0–29.9) |
| All females (15–74) | 1,737 | 176/1,737 | 10.1 (8.8–11.6) | ||
| All males (16–82) | 1,563 | 165/1,563 | 10.6 (9.1–12.2) | ||
| Symptom statusa | |||||
| Symptomatic | 1,919 | 122/1,053 | 11.6 (9.8–13.7) | 104/866 | 12.0 (10.0–14.3) |
| Asymptomatic | 1,381 | 54/684 | 7.9 (6.1–10.2) | 61/697 | 8.8 (6.9–11.1) |
| Subject race/ethnicityb | |||||
| Asian | 47 | 5/29 | 17.2 (7.6–34.5) | 0/18 | 0 (0.0–17.6) |
| Black | 2,025 | 127/1,059 | 12.0 (10.2–14.1) | 125/966 | 12.9 (11.0–15.2) |
| White | 1,131 | 40/591 | 6.8 (5.0–9.1) | 37/540 | 6.9 (5.0–9.3) |
| Unknown/other race | 146 | 6/79 | 7.6 (3.5–15.6) | 6/67 | 9.0 (4.2–18.2) |
| Hispanic | 720 | 23/381 | 6.0 (4.1–8.9) | 23/339 | 6.8 (4.6–10.0) |
| Non-Hispanic | 2,556 | 151/1,347 | 11.2 (9.6–13.0) | 140/1,209 | 11.6 (9.9–13.5) |
| Unknown ethnicity | 24 | 2/9 | 22.2 (6.3–54.7) | 2/15 | 13.3 (3.7–37.9) |
| U.S. geographic areac | |||||
| Mid-Atlantic | 260 | 16/142 | 11.3 (7.1–17.5) | 13/118 | 11.0 (6.6–17.9) |
| Midwest | 288 | 23/190 | 12.1 (8.2–17.5) | 14/98 | 14.3 (8.7–22.6) |
| Northeast | 225 | 13/106 | 12.3 (7.3–19.9) | 11/119 | 9.2 (5.2–15.8) |
| Northwest | 65 | 0/12 | 0 (0.0–24.2) | 3/53 | 5.7 (1.9–15.4) |
| Southeast | 1,424 | 72/703 | 10.2 (8.2–12.7) | 84/721 | 11.7 (9.5–14.2) |
| Southwest | 1,038 | 52/584 | 8.9 (6.9–11.5) | 40/454 | 8.8 (6.5–11.8) |
Symptom status was determined based on subject-reported symptoms.
Subjects could report multiple responses.
Mid-Atlantic: Maryland, North Carolina, and Washington DC; Midwest: Indiana, Michigan, Nebraska, and Ohio (2 sites); Northeast: Connecticut and New Jersey; Northwest: Washington; Southeast: Alabama, Georgia, Florida (3 sites), and Louisiana; Southwest: California (2 sites) and Texas (2 sites).
Prevalence and clinical performance.
The M. genitalium prevalence and the clinical performance of the investigational assay for the detection of M. genitalium are shown in Table 3 for each specimen type overall and by symptom status. In females, prevalence was lowest (7.9%) in endocervical and urine samples from asymptomatic subjects and highest (11.6%) in urine samples and patient-collected vaginal specimens from symptomatic subjects. In males, prevalence was lowest in urine specimens (8.7%) from asymptomatic subjects and highest (12%) in urethral swab and urine specimens from symptomatic subjects. The overall prevalence of M. genitalium was similar for clinician-collected vaginal swabs, patient-collected vaginal swab, endocervical swab, and female urine specimens (10.2, 10.2, 10.1, and 10.2%, respectively) and for urethral swab, penile-meatal swab, and male urine specimens (10.6, 10.6, and 10.5%, respectively).
TABLE 3.
Clinical performance characteristics of the Aptima Mycoplasma genitalium assay in urogenital specimens from female and male subjects
| Specimen type and subject symptom statusa | No. of specimens | Prevalence (%) | Sensitivity (% [95% CI]) | Specificity (% 95% CI) | PPV (95% CI)b | NPV (95% CI)c | PLR (95% CI)d | NLR (95% CI)e |
|---|---|---|---|---|---|---|---|---|
| Clinician-collected vaginal swab | ||||||||
| Sym | 1,040 | 11.5 | 93.3 (87.4–96.6) | 97.6 (96.4–98.4) | 83.6 (77.3–88.8) | 99.1 (98.3–99.6) | 39.0 (26.2–60.8) | 0.07 (0.03–0.13) |
| Asym | 669 | 8.1 | 88.9 (77.8–94.8) | 98.7 (97.5–99.3) | 85.7 (75.8–92.9) | 99.0 (98.0–99.6) | 68.3 (35.6–148.8) | 0.11 (0.04–0.23) |
| Overall | 1,709 | 10.2 | 92.0 (86.9–95.1) | 98.0 (97.2–98.6) | 84.2 (79.1–88.6) | 99.1 (98.5–99.5) | 47.0 (33.4–68.8) | 0.08 (0.05–0.13) |
| Patient-collected vaginal swab | ||||||||
| Sym | 1,047 | 11.6 | 100 (96.9–100) | 98.1 (96.9–98.8) | 87.1 (81.1–91.9) | 100 (99.6–100) | 51.4 (32.7–86.5) | 0.00 (0.00–0.03) |
| Asym | 677 | 8.0 | 96.3 (87.5–99.0) | 99.0 (97.9–99.6) | 89.7 (80.4–95.7) | 99.7 (98.9–100) | 100 (47.4–258.2) | 0.04 (0.00–0.13) |
| Overall | 1,724 | 10.2 | 98.9 (95.9–99.7) | 98.5 (97.7–99.0) | 87.8 (83.1–91.7) | 99.9 (99.5–100) | 63.8 (43.4–97.9) | 0.01 (0.00–0.04) |
| Endocervical swab | ||||||||
| Sym | 1,046 | 11.5 | 84.2 (76.6–89.6) | 98.2 (97.1–98.9) | 85.6 (79.1–90.8) | 98.0 (97.0–98.7) | 45.9 (29.1–76.4) | 0.16 (0.1–0.24) |
| Asym | 669 | 7.9 | 75.5 (62.4–85.1) | 98.5 (97.2–99.2) | 81.6 (70.3–90.2) | 97.9 (96.8–98.8) | 51.7 (27.5–107.2) | 0.25 (0.14–0.39) |
| Overall | 1,715 | 10.1 | 81.5 (75.1–86.6) | 98.3 (97.5–98.8) | 84.4 (78.9–89.1) | 97.9 (97.2–98.5) | 48.3 (33.3–72.7) | 0.19 (0.13–0.25) |
| Female urine | ||||||||
| Sym | 1,051 | 11.6 | 79.5 (71.5–85.7) | 98.4 (97.4–99.0) | 86.6 (80.0–91.8) | 97.3 (96.3–98.2) | 49.2 (30.4–85.7) | 0.21 (0.14–0.29) |
| Asym | 682 | 7.9 | 74.1 (61.1–83.9) | 99.8 (99.1–100) | 97.6 (88.7–99.9) | 97.8 (96.7–98.7) | 465.2 (91.6–15,195.2) | 0.26 (0.15–0.4) |
| Overall | 1,733 | 10.2 | 77.8 (71.1–83.3) | 99.0 (98.3–99.4) | 89.5 (84.3–93.6) | 97.5 (96.8–98.2) | 75.8 (47.5–128.6) | 0.22 (0.17–0.29) |
| Male urethral swab | ||||||||
| Sym | 866 | 12.0 | 98.1 (93.3–99.5) | 99.9 (99.3–100) | 99.0 (94.9–100) | 99.7 (99.1–100) | 747.4 (136.8–27,947.9) | 0.02 (0.00–0.07) |
| Asym | 697 | 8.8 | 98.4 (91.3–99.7) | 99.2 (98.2–99.7) | 92.3 (84.0–97.3) | 99.8 (99.2–100) | 125.1 (54.7–369.0) | 0.02 (0.00–0.09) |
| Overall | 1,563 | 10.6 | 98.2 (94.8–99.4) | 99.6 (99.1–99.8) | 96.4 (92.7–98.6) | 99.8 (99.4–100) | 228.8 (106.8–605.2) | 0.02 (0.00–0.05) |
| Penile meatal swab | ||||||||
| Sym | 865 | 11.9 | 89.3 (81.9–93.9) | 97.8 (96.5–98.6) | 84.4 (77.5–90.0) | 98.5 (97.6–99.2) | 40.0 (25.4–66.7) | 0.11 (0.06–0.19) |
| Asym | 689 | 8.9 | 86.9 (76.2–93.2) | 97.9 (96.5–98.8) | 80.3 (70.8–88.1) | 98.7 (97.7–99.4) | 42.0 (25.0–75.9) | 0.13 (0.06–0.24) |
| Overall | 1,554 | 10.6 | 88.4 (82.6–92.5) | 97.8 (96.9–98.5) | 82.9 (77.4–87.6) | 98.6 (97.9–99.1) | 40.9 (29.0–59.8) | 0.12 (0.07–0.18) |
| Male urine | ||||||||
| Sym | 866 | 12.0 | 89.4 (82.0–94.0) | 99.1 (98.1–99.6) | 93.0 (86.9–96.9) | 98.6 (97.6–99.3) | 97.3 (48.5–228.4) | 0.11 (0.06–0.18) |
| Asym | 693 | 8.7 | 93.3 (84.1–97.4) | 99.7 (98.9–99.9) | 96.6 (89.0–99.5) | 99.4 (98.5–99.8) | 295.4 (85.6–2,229.8) | 0.07 (0.02–0.16) |
| Overall | 1,559 | 10.5 | 90.9 (85.5–94.4) | 99.4 (98.8–99.7) | 94.3 (90.0–97.2) | 98.9 (98.3–99.4) | 140.8 (76.2–294.7) | 0.09 (0.05–0.15) |
Symptom status was determined based on subject-reported symptoms. Asym, asymptomatic; Sym, symptomatic.
PPV, positive predictive value.
NPV, negative predictive value.
PLR, positive likelihood ratio.
NLR, negative likelihood ratio.
Overall sensitivity of the investigational test for detection of M. genitalium-infected subjects was ≥90% for clinician- and patient-collected vaginal and male urethral swab specimens and male urine specimens, 88.4% for penile-meatal swab specimens, 81.5% for endocervical specimens, and 77.8% for female urine specimens. Overall specificity was ≥97.8% for all specimen types. The combination of investigational and reference assay M. genitalium results for all subjects with a conclusive PIS status and valid AMG assay results are shown in Tables S1 and S2 in the supplemental material for female and male urogenital specimens, respectively. Sensitivity and specificity estimates were similar in asymptomatic and symptomatic subjects for each specimen type. Assay performance was similar among races and ethnicities for each specimen type (Tables S3 and S4).
In the absence of results from FDA-approved assays for M. genitalium detection for performance comparison, positive (PLR) and negative (NLR) likelihood ratios were also calculated. By symptom status, estimates of the PLR in female specimen types ranged from 39.0 (95% CI, 26.2 to 60.8) for clinician-collected vaginal swab specimens from symptomatic subjects to 465.2 (95% CI, 91.6 to 15,195.2) for female urine specimens from asymptomatic subjects. For male specimens, PLR estimates ranged from 40.0 (95% CI, 25.4 to 66.7) for penile meatal swab specimens from symptomatic subjects to 747.4 (95% CI, 136.8 to 27,947.9) for urethral swab specimens from symptomatic subjects. For all specimen types, NLR ratios were less than 0.26. Together, these results demonstrated highly relevant and statistically significant increases in knowledge based on positive and negative AMG assay results in all specimen types.
To investigate the effect of the clinical specimen matrix on investigational assay performance for specimens other than the male urethral swab and self-collected vaginal swab, a comparison of AMG assay and Alt TMA assay results within the same specimen type was performed (Table 4). Infected specimen status was determined for these analyses using the same general rules as for the PIS, except that the infected status was determined based on the composite Alt TMA reference result for that specimen instead of in comparison to the patient-collected vaginal swab specimen (for women) or the urethral swab specimen (for men). Positive percent agreement (PPA) using the specimen infected status standard was >95% for all specimen types except female urine, for which the PPA was 94.6% in symptomatic subjects and 93.2% in asymptomatic subjects. Negative percent agreement (NPA) was >98% in all specimen types.
TABLE 4.
Specimen-specific agreement of the Aptima Mycoplasma genitalium assay in urogenital specimens from female and male subjects
| Specimen type and subject symptom statusa | No. of specimens | Comparison of assay results (no.) |
Positive % agreement (95% CI) | Negative % agreement (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Aptima positive |
Aptima negative |
||||||
| Reference positive | Reference negative | Reference negative | Reference positive | ||||
| Clinician-collected vaginal swab | |||||||
| Sym | 1,050 | 123 | 12 | 913 | 2 | 98.4 (94.4–99.6) | 98.7 (97.7–99.3) |
| Asym | 679 | 52 | 5 | 621 | 1 | 98.1 (90.1–99.7) | 99.2 (98.1–99.7) |
| Overall | 1,729 | 175 | 17 | 1,534 | 3 | 98.3 (95.2–99.4) | 98.9 (98.3–99.3) |
| Patient-collected vaginal swab | |||||||
| Sym | 1,047 | 121 | 18 | 908 | 0 | 100 (96.9–100) | 98.1 (96.9–98.8) |
| Asym | 677 | 52 | 6 | 617 | 2 | 96.3 (87.5–99.0) | 99.0 (97.9–99.6) |
| Overall | 1,724 | 173 | 24 | 1,525 | 2 | 98.9 (95.9–99.7) | 98.5 (97.7–99.0) |
| Endocervical swab | |||||||
| Sym | 1,057 | 115 | 4 | 935 | 3 | 97.5 (92.8–99.1) | 99.6 (98.9–99.8) |
| Asym | 677 | 48 | 3 | 624 | 2 | 96.0 (86.5–98.9) | 99.5 (98.6–99.8) |
| Overall | 1,734 | 163 | 7 | 1,559 | 5 | 97.0 (93.2–98.7) | 99.6 (99.1–99.8) |
| Female urine | |||||||
| Sym | 1,074 | 106 | 7 | 955 | 6 | 94.6 (88.8–97.5) | 99.3 (98.5–99.6) |
| Asym | 700 | 41 | 2 | 654 | 3 | 93.2 (81.8–97.7) | 99.7 (98.9–99.9) |
| Overall | 1,774 | 147 | 9 | 1,609 | 9 | 94.2 (89.4–96.9) | 99.4 (98.9–99.7) |
| Male urethral swab | |||||||
| Sym | 866 | 102 | 1 | 761 | 2 | 98.1 (93.3–99.5) | 99.9 (99.3–100) |
| Asym | 697 | 60 | 5 | 631 | 1 | 98.4 (91.3–99.7) | 99.2 (98.2–99.7) |
| Overall | 1,563 | 162 | 6 | 1,392 | 3 | 98.2 (94.8–99.4) | 99.6 (99.1–99.8) |
| Penile meatal swab | |||||||
| Sym | 870 | 101 | 8 | 756 | 5 | 95.3 (89.4–98.0) | 99.0 (97.9–99.5) |
| Asym | 693 | 61 | 6 | 623 | 3 | 95.3 (87.1–98.4) | 99.0 (97.9–99.6) |
| Overall | 1,563 | 162 | 14 | 1,379 | 8 | 95.3 (91.0–97.6) | 99.0 (98.3–99.4) |
| Male urine | |||||||
| Sym | 874 | 99 | 2 | 770 | 3 | 97.1 (91.7–99.0) | 99.7 (99.1–99.9) |
| Asym | 704 | 60 | 0 | 643 | 1 | 98.4 (91.3–99.7) | 100 (99.4–100) |
| Overall | 1,578 | 159 | 2 | 1,413 | 4 | 97.5 (93.9–99.0) | 99.9 (99.5–100) |
Symptom status was determined based on subject-reported symptoms. Asym, asymptomatic; Sym, symptomatic.
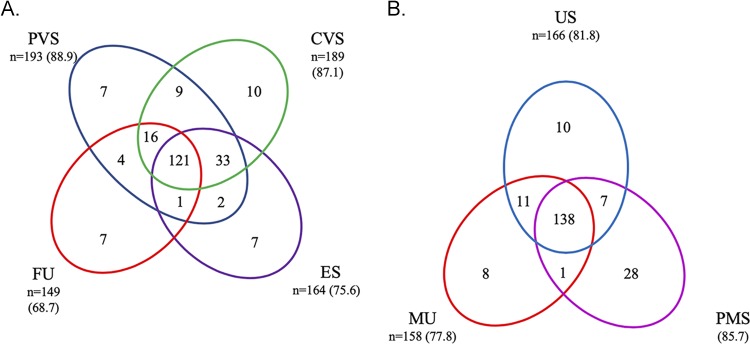
Figure 2 depicts the distribution of female and male urogenital specimens with unique and shared positive AMG assay results. The majority of subjects were positive for M. genitalium in more than one specimen type (85.7% for female specimens; 77.3% for male specimens). However, for both female and male subjects, a minority of subjects had positive AMG assay results in only one sample type (e.g., 7/193 patient-collected vaginal swab specimens; 28/174 penile meatal swab specimens). Most subjects had positive AMG results for two or more specimen types; 121 (55.8%) females and 138 (68%) males reported positive AMG results in all specimen types assessed.
FIG 2.
Joint distribution of Aptima Mycoplasma genitalium assay positive results in clinical specimens from female (n = 217) (A) and male (n = 203) (B) subjects. Specimen category values are the number (percent) of test-positive subjects for each specimen type. CVS, clinician-collected vaginal swab; ES, endocervical swab; FU, female urine; MU, male urine; PMS, penile meatal swab; PVS, patient-collected vaginal swab; US, male urethral swab.
Receiver operating characteristic (ROC) curve analysis was performed on the female and male urogenital specimens (Fig. 3). The area under the curve (AUC) estimates ranged from 88.8 for female urine specimens to 98.9 for patient-collected vaginal swab specimens and from 94.5 for male penile meatal swab specimens to 99.4 for male urethral swab specimens.
FIG 3.
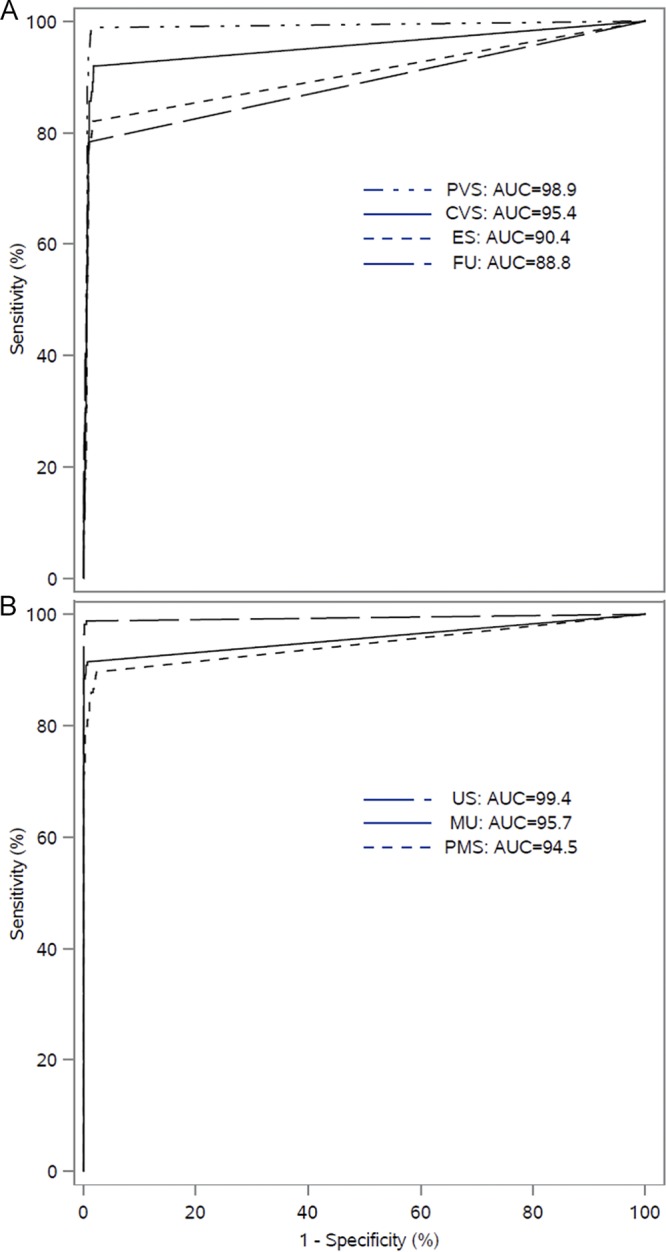
ROC curve analysis of female (A) and male (B) clinical specimen types for detection of M. genitalium-infected subjects. CVS, clinician-collected vaginal swab; ES, endocervical swab; FU, female urine; MU, male urine; PMS, penile meatal swab; PVS, patient-collected vaginal swab; US, male urethral swab.
DISCUSSION
This study reports the results of a prospective multicenter clinical performance evaluation of the Aptima Mycoplasma genitalium assay for detection of M. genitalium. These results provide clinical efficacy evidence for the first IVD NAAT for M. genitalium detection in the United States. Following completion of this study, the assay received FDA clearance (510k# DEN180047) for the detection of M. genitalium in patient- and clinician-collected urogenital specimens from symptomatic and asymptomatic subjects, including minors. The FDA clearance will now allow laboratories to test for M. genitalium in a wide variety of urogenital specimens without having to develop and validate laboratory-developed tests (30) and will allow clinicians to easily detect this pathogen in their patients.
The results of this study are in concert with previous research study results that have highlighted the accuracy of this assay (25, 29–32). The prevalence of M. genitalium observed in this study in symptomatic and asymptomatic persons is consistent with that previously reported for the various types of specimens used for detection in males and females (9, 15–17, 26, 31). The performance of the assay in multiple specimen types should allow clinicians to choose the specimen type most appropriate for individual patient management. For females, the vaginal swab had the best performance, with self-collected vaginal swabs having higher sensitivity (98.9%) than clinician-collection vaginal swabs (92%). Female urine and endocervical swabs showed somewhat lower sensitivity (81.5% and 77.8%, respectively), consistent with previous reports (25, 36); however, as with the vaginal swab specimens, these two specimen types had positive and negative likelihood ratios significantly different from unity, indicating high probabilities for diagnostic accuracy using these two specimen types. In males, the urethral swab demonstrated high sensitivity of 98.2%, and detection rates of infections using male urine and the self-collected penile-meatal swab were similar, giving clinicians options for sampling male patients. From the ROC analysis, all female and male specimen types had AUC values greater than 88%.
To assess the effect of anatomic site-specific infection on sensitivity and specificity estimates determined using the PIS standard, a comparison of AMG assay and Alt TMA assay results within the same specimen type was performed. Positive percent agreement with the specimen-infected status was >95% for all specimen types except female urine, for which the PPA was 94.6% in symptomatic subjects and 93.2% in asymptomatic subjects. Negative percent agreement was >98% for all specimen types. This suggests the somewhat lower diagnostic value (PLR and NLR) and/or diagnostic accuracy (sensitivity and specificity) estimates associated with some specimen types such as female urine may be due to anatomic site-specific infections (e.g., urinary tract-positive/genital tract-negative) rather than specimen matrix effects on assay performance.
While there is little debate that M. genitalium is sexually transmitted, there is wide discrepancy in the prevalence detected in the general population compared to the prevalence in patients seeking care from many types of clinics. In lower-risk populations, an M. genitalium prevalence of approximately 1% to 3% has been reported in both men and women (38, 39). In higher-risk populations attending STI clinics, prevalences of 9% to 24% in men and 11% to 16% in women have been reported (13–16). The debate is whether asymptomatic infection with M. genitalium is associated with disease and the future development of adverse sequelae. In this AMES study, the prevalences in symptomatic males and females were 12% and 11.6%, respectively, whereas, in asymptomatic persons the prevalences were 8.8% and 7.9%, respectively, demonstrating a prevalence not very different from the prevalence of chlamydia in symptomatic women seen in many health care settings. There are many observational reports that disease manifestations of persistent urethritis (25), cervicitis (16, 17, 40–42), and even pelvic inflammatory disease (PID) and other adverse reproductive sequelae (17, 43, 44) are associated with M. genitalium detection in males and females. An unmet need for understanding the public health significance of infection with M. genitalium includes prospective trials that demonstrate that screening and treating asymptomatic persons prevents adverse reproductive sequelae in women and persistent urethritis and sequelae in asymptomatic men although such studies are likely to be costly.
A major concern with the increasing use of diagnostic testing and treatment of infected patients is the high antibiotic resistance of M. genitalium to azithromycin, the first-line antibiotic used to treat urogenital infections (22, 23, 31, 33). Macrolide resistance rates of greater than 40% are common worldwide and appear to be increasing; this is especially important for the treatment of PID caused by M. genitalium, where standard syndromic treatment may fail (45–48). It will be important to incorporate antibiotic resistance detection for macrolides and other antibiotic classes into future screening algorithms for M. genitalium as part of the larger antibiotic stewardship efforts needed for the clinical management of all STIs.
Our study has limitations. We did not collect oropharyngeal or rectal specimens, potentially important sources of M. genitalium infection and transmission, and we do not have coinfection data for other STIs. Additionally, there is no information about the antibiotic resistance status of M. genitalium-positive subjects, which is an important clinical consideration (31, 46–48). We also lack extensive history on the sexual orientation of subjects and their sex partners, HIV status, or other risk factors. However, this study was not designed to address those questions.
Strengths of this study include adherence to cGCP regulations to enroll a large, diverse cohort from six geographic areas of the United States, including 15 states and the District of Columbia and representing patients attending multiple clinical practice types. In addition, multiple sample types were collected from each patient, providing clinicians options for patient management. Finally, the composite reference standard used in this study consisted of a consensus result from three validated TMA assays targeting M. genitalium rRNAs, eliminating potential bias due to differences in sensitivities between the investigational test and reference assays (34).
In summary, we now have an FDA-cleared IVD assay that can be used to detect M. genitalium urogenital infections in men and women using cervical, vaginal, urethral, penile-meatal, and urine specimens. Future research will be required to further define the pathogenicity of M. genitalium, the best treatment algorithms, and its significance when detected in asymptomatic persons.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients for their participation in this study.
The members of the AMES Study Group include the following: Anitra Beasley (Planned Parenthood Gulf Coast), Steven Chavoustie (Segal Institute for Clinical Research, Healthcare Clinical Data, Inc.), Douglas Denham (Clinical Trials of Texas, Inc.), Julie Dombrowski (University of Washington), Michael Dunn (Quality Clinical Research, Inc.), Christopher Emery (Indiana University), Charlotte A. Gaydos (Johns Hopkins University), Wayne Harper (Wake Research Associate, LLC), Edward W. Hook III (University of Alabama at Birmingham), Christopher Jones (Cooper University), Clifford Kinder (AIDS Healthcare Foundation—Miami), Jeffrey D. Klausner (University of California Los Angeles, AIDS Healthcare Foundation—Los Angeles), Rebecca A. Lillis (Louisiana State University Health Sciences Center), Michael Lyons (University of Cincinnati), Lisa E. Manhart (University of Washington), Joseph Miller (Henry Ford Hospital), Mobeen Rathore (University of Florida), Robert Shesser (George Washington University), Dane Shipp (Wake Medical Center for Clinical Research), Timothy Spurrell (Planned Parenthood South New England), Stephanie N. Taylor (Louisiana State University Health Sciences Center), Wayne Trout (Ohio State University), Kimberly Ann Workowski (Emory University), and David Yamane (George Washington University).
C.A.G. received University research funds from Hologic, Inc., for this study and acknowledges receipt of Mycoplasma genitalium test research kits from Hologic, Inc., for other studies. L.E.M. received honoraria, reagents, and test kits for diagnostic assays from Hologic, Inc. S.N.T. received research funds from Abbott, Becton, Dickinson, Binx, Hologic, Inc., and Roche. J.D.K. received donated research supplies from Hologic, Inc. C.V.R., M.L., B.M., and D.K.G. are scientists employed by Hologic. Inc., the study sponsor and the manufacturer of the diagnostic tests used in this study.
R.A.L. and E.W.H. have no conflicts of interest to report.
This study was funded by Hologic, Inc. C.A.G. was also funded by U54 EB007958 from the National Institute of Biomedical Imaging and Bioengineering, NIH, and by U-01068613 from the National Institute of Allergy and Infectious Diseases, NIH. Hologic, Inc., was involved in the study design, data interpretation, and the decision to submit for publication in conjunction with the authors.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01125-19.
REFERENCES
- 1.Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA, Fleischmann RD, Bult CJ, Kerlavage AR, Sutton G, Kelley JM, Fritchman RD, Weidman JF, Small KV, Sandusky M, Fuhrmann J, Nguyen D, Utterback TR, Saudek DM, Phillips CA, Merrick JM, Tomb JF, Dougherty BA, Bott KF, Hu PC, Lucier TS, Peterson SN, Smith HO, Hutchison CA III, Venter JC. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 2.Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, Hutchison CA III, Smith HO, Venter JC. 2006. Essential genes of a minimal bacterium. Proc Natl Acad Sci U S A 103:425–430. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tully JG, Taylor-Robinson D, Cole RM, Rose DL. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet 1:1288–1291. doi: 10.1016/s0140-6736(81)92461-2. [DOI] [PubMed] [Google Scholar]
- 4.Hamasuna R, Osada Y, Jensen JS. 2007. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with Vero cells. J Clin Microbiol 45:847–850. doi: 10.1128/JCM.02056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deguchi T, Yoshida T, Yokoi S, Ito M, Tamaki M, Ishiko H, Maeda S. 2002. Longitudinal quantitative detection by real-time PCR of Mycoplasma genitalium in first-pass urine of men with recurrent nongonococcal urethritis. J Clin Microbiol 40:3854–3856. doi: 10.1128/jcm.40.10.3854-3856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen JS, Uldum SA, Søndergård-Andersen J, Vuust J, Lind K. 1991. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol 29:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen JS, Borre MB, Dohn B. 2003. Detection of Mycoplasma genitalium by PCR amplification of the 16S rRNA gene. J Clin Microbiol 41:261–266. doi: 10.1128/jcm.41.1.261-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen JS, Björnelius E, Dohn B, Lidbrink P. 2004. Use of TaqMan 5 nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol 42:683–692. doi: 10.1128/JCM.42.2.683-692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horner PJ, Martin DH. 2017. Mycoplasma genitalium infection in men. J Infect Dis 216(Suppl 2):S396–S405. doi: 10.1093/infdis/jix145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen JS, Hansen HT, Lind K. 1996. Isolation of Mycoplasma genitalium strains from the male urethra. J Clin Microbiol 34:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis SC, Kent CK, Klausner JD, Rauch L, Kohn R, Hardick A, Gaydos CA. 2008. Prevalence of rectal Trichomonas vaginalis and Mycoplasma genitalium in male patients at the San Francisco STD clinic, 2005–2006. Sex Transm Dis 35:797–800. doi: 10.1097/OLQ.0b013e318177ec39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soni S, Alexander S, Verlander N, Saunders P, Richardson D, Fisher M, Ison C. 2010. The prevalence of urethral and rectal Mycoplasma genitalium and its associations in men who have sex with men attending a genitourinary medicine clinic. Sex Transm Infect 86:21–24. doi: 10.1136/sti.2009.038190. [DOI] [PubMed] [Google Scholar]
- 13.Manhart LE. 2013. Mycoplasma genitalium, an emergent sexually transmitted disease? Infect Dis Clin North Am 27:779–792. doi: 10.1016/j.idc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. 2007. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health 97:1118–1125. doi: 10.2105/AJPH.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. 2009. Mycoplasma genitalium compared to chlamydia, gonorrhoea and trichomonas as an aetiological agent of urethritis in men attending STD clinics. Sex Transm Infect 85:438–440. doi: 10.1136/sti.2008.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. 2009. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis 36:598–606. doi: 10.1097/OLQ.0b013e3181b01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lis R, Rowhani-Rahbar A, Manhart LE. 2015. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 61:418–426. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 18.McGowin CL, Anderson-Smits C. 2011. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog 7:e1001324. doi: 10.1371/journal.ppat.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munson E, Wenten D, Jhansale S, Schuknecht MK, Pantuso N, Gerritts J, Steward A, Munson KL, Napierala M, Hamer D. 2017. Expansion of comprehensive screening of male sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecular assessment: a retrospective analysis. J Clin Microbiol 55:321–325. doi: 10.1128/JCM.01625-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakashima K, Shigehara K, Kawaguchi S, Wakatsuki A, Kobori Y, Nakashima K, Ishii Y, Shimamura M, Sasagawa T, Kitagawa Y, Mizokami A, Namiki M. 2014. Prevalence of human papillomavirus infection in the oropharynx and urine among sexually active men: a comparative study of infection by papillomavirus and other organisms, including Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma spp., and Ureaplasma spp. BMC Infect Dis 14:43. doi: 10.1186/1471-2334-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Napierala Mavedzenge S, Weiss HA. 2009. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 23:611–620. doi: 10.1097/QAD.0b013e328323da3e. [DOI] [PubMed] [Google Scholar]
- 22.Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. 2008. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis 47:1546–1553. doi: 10.1086/593188. [DOI] [PubMed] [Google Scholar]
- 23.Hokynar K, Hiltunen-Back E, Mannonen L, Puolakkainen M. 2018. Prevalence of Mycoplasma genitalium and mutations associated with macrolide and fluoroquinolone resistance in Finland. Int J STD AIDS 29:904–907. doi: 10.1177/0956462418764482. [DOI] [PubMed] [Google Scholar]
- 24.Taylor-Robinson D, Jensen JS. 2011. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardick J, Giles J, Hardick A, Hsieh Y-H, Quinn T, Gaydos C. 2006. Performance of the Gen-Probe transcription-mediated amplification research assay compared to that of a multitarget real-time PCR for Mycoplasma genitalium detection. J Clin Microbiol 44:1236–1240. doi: 10.1128/JCM.44.4.1236-1240.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munson E, Bykowski H, Munson KL, Napierala M, Reiss PJ, Schell RF, Hryciuk JE. 2016. Clinical laboratory assessment of Mycoplasma genitalium transcription-mediated amplification using primary female urogenital specimens. J Clin Microbiol 54:432–438. doi: 10.1128/JCM.02463-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wroblewski J, Manhart LE, Dickey K, Hudspeth M, Totten PA. 2006. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J Clin Microbiol 44:3306–3312. doi: 10.1128/JCM.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabrizi SN, Costa AM, Su J, Lowe P, Bradshaw CS, Fairley CK, Garland SM. 2016. Evaluation of the Hologic Panther transcription-mediated amplification assay for detection of Mycoplasma genitalium. J Clin Microbiol 54:2201–2203. doi: 10.1128/JCM.01038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unemo M, Salado-Rasmussen K, Hansen M, Olsen AO, Falk M, Golparian D, Aasterød M, Ringlander J, Nilsson CS, Sundqvist M, Schønning K, Moi H, Westh H, Jensen JS. 2018. Clinical and analytical evaluation of the new Aptima Mycoplasma genitalium assay, with data on M. genitalium prevalence and antimicrobial resistance in M. genitalium in Denmark, Norway and Sweden in 2016. Clin Microbiol Infect 24:533–539. doi: 10.1016/j.cmi.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Gaydos C. 2017. Mycoplasma genitalium: accurate diagnosis is necessary for adequate treatment. J Infect Dis 16(Suppl 2):S406–S411. doi: 10.1093/infdis/jix104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Getman D, Jiang A, O'Donnell M, Cohen S. 2016. Mycoplasma genitalium, prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 54:2278–2283. doi: 10.1128/JCM.01053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hologic, Inc. 2015. Aptima Mycoplasma genitalium assay package insert. Document AW-14170–001, Rev002 Hologic, Inc., San Diego, CA. [Google Scholar]
- 33.Le Roy C, Pereyre S, Hénin N, Bébéar C. 2017. French prospective clinical evaluation of the Aptima Mycoplasma genitalium CE-IVD Assay and macrolide resistance detection using three distinct assays. J Clin Microbiol 55:3194–3200. doi: 10.1128/JCM.00579-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkconnell B, Weinbaum B, Santos K, Le Nguyen T, Astete S, Wood G, Totten PA, Getman DK. 2019. Design and validation of transcription-mediated amplification nucleic acid amplification tests for Mycoplasma genitalium. J Clin Microbiol 57:e00264-19. doi: 10.1128/JCM.00264-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondratovich M. 2008. Comparing two medical tests when results of reference standard are unavailable for those negative via both tests. J Biopharm Stat 18:145–166. doi: 10.1080/10543400701668308. [DOI] [PubMed] [Google Scholar]
- 36.Lillis RA, Nsuami MJ, Myers L, Martin DH. 2011. Utility of urine, vaginal, cervical, and rectal specimens for detection of Mycoplasma genitalium in women. J Clin Microbiol 49:1990–1992. doi: 10.1128/JCM.00129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dize L, Barnes P, Barnes M, Hsieh H-H, Marsiglia V, Duncan D, Hardick J, Gaydos CA. 2016. Performance of self-collected penile-meatal swabs compared to clinician-collected urethral swabs for the detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium by nucleic acid amplification assays. Diagn Microbiol Infect Dis 86:131–135. doi: 10.1016/j.diagmicrobio.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen B, Sokolowski I, Østergaard L, Møller JK, Olesen F, Jensen JS. 2006. Mycoplasma genitalium: prevalence and behavioural risk factors in the general population. Sex Transm Infect 83:237–241. doi: 10.1136/sti.2006.022970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oakeshott P, Aghaizu A, Hay P, Reid F, Kerry S, Atherton H, Simms I, Taylor-Robinson D, Dohn B, Jensen JS. 2010. Is Mycoplasma genitalium in women the “new chlamydia?” A community-based prospective cohort study. Clin Infect Dis 51:1160–1166. doi: 10.1086/656739. [DOI] [PubMed] [Google Scholar]
- 40.Mobley VL, Hobbs MM, Lau K, Weinbaum BS, Getman DK, Seña AC. 2012. Mycoplasma genitalium infection in women attending a sexually transmitted infection clinic: diagnostic specimen type, coinfections, and predictors. Sex Transm Dis 39:706–709. doi: 10.1097/OLQ.0b013e318255de03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anagrius C, Loré B, Jensen JS. 2005. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect 81:458–462. doi: 10.1136/sti.2004.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falk L, Fredlund H, Jensen JS. 2005. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect 81:73–78. doi: 10.1136/sti.2004.010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haggerty CL, Totten PA, Astete SG, Lee S, Hoferka SL, Kelsey SF, Ness RB. 2008. Failure of cefoxitin and doxycycline to eradicate endometrial Mycoplasma genitalium and the consequence for clinical cure of pelvic inflammatory disease. Sex Transm Infect 84:338–342. doi: 10.1136/sti.2008.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiesenfeld HC, Manhart LE. 2017. Mycoplasma genitalium in women: current knowledge and research priorities for this recently emerged pathogen. J Infect Dis 216(Suppl 2):S389–S395. doi: 10.1093/infdis/jix198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latimer RL, Read TRH, Vodstrcil LA, Goller JL, Ong JJ, Fairley CK, Hocking JS, Bradshaw CS. 2019. Clinical features and therapeutic response in women meeting criteria for presumptive treatment for pelvic inflammatory disease associated with Mycoplasma genitalium. Sex Transm Dis 46:73–79. doi: 10.1097/OLQ.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 46.Bissessor M, Tabrizi SN, Twin J, Abdo H, Fairley CK, Chen MY, Vodstrcil LA, Jensen JS, Hocking JS, Garland SM, Bradshaw CS. 2015. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 60:1228–1236. doi: 10.1093/cid/ciu1162. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Le WJ, Li S, Cao YP, Su XH. 2017. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 28:1106–1114. doi: 10.1177/0956462416688562. [DOI] [PubMed] [Google Scholar]
- 48.Read TRH, Fairley CK, Murray GL, Jensen JS, Danielewski J, Worthington K, Doyle M, Mokany E, Tan L, Chow EPF, Garland SM, Bradshaw CS. 2018. Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: a prospective evaluation. Clin Infect Dis 2019:554–560. doi: 10.1093/cid/ciy477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.