LETTER
The kinetics of anti-Zika virus (ZIKV) antibodies after acute ZIKV infection is not well known (1, 2), especially in areas where different flaviviruses circulate (3). The objective of this study was to describe the kinetics of anti-ZIKV antibodies in women in whom an acute ZIKV infection was diagnosed during pregnancy. Within a cohort of pregnant women living in Guadeloupe, France, and exposed to ZIKV during the 2016 Zika outbreak (4), we identified 65 women who presented with an acute, symptomatic, PCR-confirmed (RealStar Zika virus reverse transcription-PCR [RT-PCR] kit 1.0; Altona Diagnostics) ZIKV infection at various times of their pregnancy with a known date of the first Zika symptoms. Serum samples obtained at delivery in all women and at various interim time points between acute ZIKV infection and delivery in 20 women (23 samples) were tested for anti-ZIKV antibodies. The 88 serum samples were batch processed using the commercially available Euroimmun enzyme-linked immunosorbent assay (ELISA) (5, 6) and a virus neutralization test (VNT) that was performed at the French National Reference Center for Arboviruses (7) in order to detect anti-ZIKV IgM and IgG antibodies and confirm the presence of anti-ZIKV neutralizing antibodies, respectively. Moreover, a dengue virus (DENV) ELISA was performed on all samples.
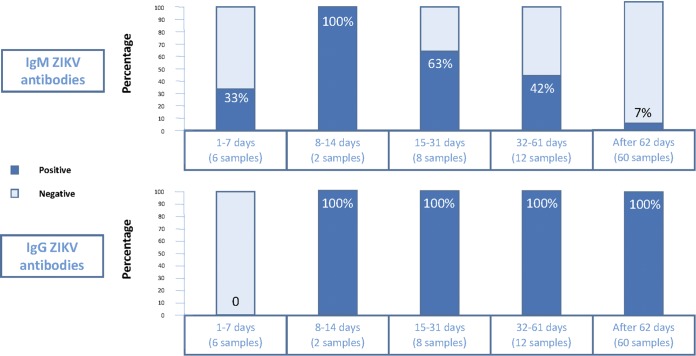
The patients’ mean age was 30 years. The time between first symptoms of ZIKV infection and delivery ranged from 17 to 229 days. The mean times between ZIKV infection and delivery were 197, 119, and 50 days for women who had acute ZIKV infection during the 1st (n = 14), 2nd (n = 35), and 3rd (n = 16) trimesters of pregnancy, respectively. DENV serology was positive in all women. ZIKV serology on delivery samples was positive in 65/65 (100%; one-sided 97.5% confidence interval [CI], 94.4% to 100%) women by both the IgG ELISA and VNT assay. IgM anti-ZIKV antibodies were detected as early as 2 days after the first symptom and progressively faded away over a few weeks. They were detected on delivery samples in only 5/65 (8%) women, in whom the time intervals between acute ZIKV infection and sampling were 17, 27, 36, 38, and 142 days. IgG anti-ZIKV antibodies were negative in all 6 interim samples that had been drawn within 7 days of the first symptom. They were detected from day 13 and remained positive afterwards. The kinetics of anti-ZIKV antibodies is summarized in Fig. 1.
FIG 1.
Kinetics of anti-ZIKV antibodies in the 88 samples tested in 65 pregnant women. Note that the time intervals are between day of first Zika symptoms and the day of blood sampling. Five women delivered within 2 months of acute ZIKV infection, which explains why only 60 samples were available in the interval “After 62 days.”
The main finding of this study is that with the Euroimmun assay, IgG anti-ZIKV antibodies were detected as early as the second week after acute ZIKV infection and remained detectable until delivery in all women.
The strengths of this study are 2-fold, as follows: (i) the kinetics of antibodies could be established because the date of acute ZIKV infection was ascertained by the combination of consistent clinical symptoms and concomitant positive nucleic acid testing, and (ii) the antibodies detected by the Euroimmun ELISA were specific to ZIKV, as evidenced by the results of a seroneutralization assay. The main limitation of this study results from the small number of serum samples that were drawn between acute infection and delivery. However, these numbers were in the same range as those in two comparable studies that showed results similar to ours regarding the kinetics of anti-ZIKV IgG antibodies (1, 2).
Altogether, the pragmatic interpretation of our findings is that the absence of IgG anti-ZIKV antibodies at delivery appears to be a strong indicator of the absence of ZIKV infection during pregnancy, information that is quite useful to inform pregnant women on the potential risks for their neonates.
ACKNOWLEDGMENTS
We thank the women who participated in this study and acknowledge their altruism. We acknowledge all actors (physicians, midwives, clinical research assistants, health officers, and epidemiologists) who joined their efforts to help conduct this study. We are grateful to Joelle Colat-Peyron for handling serology testing at Karubiotec.
This study was funded by the French Ministry of Health (Soutien Exceptionnel à la Recherche et à l’Innovation) and by the European Union’s Horizon 2020 Research and Innovation Programme under ZIKAlliance grant agreement no. 734548.
REFERENCES
- 1.Pasquier C, Joguet G, Mengelle C, Chapuy-Regaud S, Pavili L, Prisant N, Izopet J, Bujan L, Mansuy JM. 2018. Kinetics of anti-ZIKV antibodies after Zika infection using two commercial enzyme-linked immunoassays. Diagn Microbiol Infect Dis 90:26–30. doi: 10.1016/j.diagmicrobio.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Lustig Y, Zelena H, Venturi G, van Esbroeck M, Rothe C, Perret C, Koren R, Katz-Likvornik S, Mendelson E, Schwartz E. 2017. Sensitivity and kinetics of an NS1-based Zika virus enzyme-linked immunosorbent assay in Zika virus-infected travelers from Israel, the Czech Republic, Italy, Belgium, Germany, and Chile. J Clin Microbiol 55:1894–1901. doi: 10.1128/JCM.00346-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matheus S, Talla C, Labeau B, de Laval F, Briolant S, Berthelot L, Vray M, Rousset D. 2019. Performance of 2 commercial serologic tests for diagnosing Zika virus infection. Emerg Infect Dis 25:1153–1160. doi: 10.3201/eid2506.180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoen B, Schaub B, Funk AL, Ardillon V, Boullard M, Cabié A, Callier C, Carles G, Cassadou S, Césaire R, Douine M, Herrmann-Storck C, Kadhel P, Laouénan C, Madec Y, Monthieux A, Nacher M, Najioullah F, Rousset D, Ryan C, Schepers K, Stegmann-Planchard S, Tressières B, Voluménie JL, Yassinguezo S, Janky E, Fontanet A. 2018. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med 378:985–994. doi: 10.1056/NEJMoa1709481. [DOI] [PubMed] [Google Scholar]
- 5.Steinhagen K, Probst C, Radzimski C, Schmidt-Chanasit J, Emmerich P, van Esbroeck M, Schinkel J, Grobusch MP, Goorhuis A, Warnecke JM, Lattwein E, Komorowski L, Deerberg A, Saschenbrecker S, Stöcker W, Schlumberger W. 2016. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill 21:30426 https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2016.21.50.30426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.L’Huillier AG, Hamid-Allie A, Kristjanson E, Papageorgiou L, Hung S, Wong CF, Stein DR, Olsha R, Goneau LW, Dimitrova K, Drebot M, Safronetz D, Gubbay JB. 2017. Evaluation of Euroimmun anti-Zika virus IgM and IgG enzyme-linked immunosorbent assays for Zika virus serologic testing. J Clin Microbiol 55:2462–2471. doi: 10.1128/JCM.00442-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurtop E, Villarroel PMS, Pastorino B, Ninove L, Drexler JF, Roca Y, Gake B, Dubot-Peres A, Grard G, Peyrefitte C, Priet S, de Lamballerie X, Gallian P. 2018. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol J 15:192–1105. doi: 10.1186/s12985-018-1105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]