There are sparse data to indicate the extent that macrolide-resistant Mycoplasma pneumoniae (MRMp) occurs in the United States or its clinical significance. Between 2015 and 2018, hospitals in 8 states collected and stored respiratory specimens that tested positive for M. pneumoniae and sent them to the University of Alabama at Birmingham, where real-time PCR was performed for detection of 23S rRNA mutations known to confer macrolide resistance. MRMp was detected in 27 of 360 specimens (7.5%).
KEYWORDS: Mycoplasma pneumoniae, antibiotic resistance, macrolide, surveillance
ABSTRACT
There are sparse data to indicate the extent that macrolide-resistant Mycoplasma pneumoniae (MRMp) occurs in the United States or its clinical significance. Between 2015 and 2018, hospitals in 8 states collected and stored respiratory specimens that tested positive for M. pneumoniae and sent them to the University of Alabama at Birmingham, where real-time PCR was performed for detection of 23S rRNA mutations known to confer macrolide resistance. MRMp was detected in 27 of 360 specimens (7.5%). MRMp prevalence was significantly higher in the South and East (18.3%) than in the West (2.1%). A2063G was the predominant 23S rRNA mutation detected. MICs for macrolide-susceptible M. pneumoniae (MSMp) were ≤0.008 μg/ml, whereas MICs for MRMp were 16 to 32 μg/ml. Patients with MRMp infection were more likely to have a history of immunodeficiency or malignancy. Otherwise, there were no other significant differences in the clinical features between patients infected with MRMp and those infected with MSMp, nor were there any differences in radiographic findings, hospitalization rates, viral coinfections, the mean duration of antimicrobial treatment, or clinical outcomes. There was no significant change in MRMp incidence over time or according to age, sex, race/ethnicity, or status as an inpatient or an outpatient. Patients with MRMp were more likely to have received a macrolide prior to presentation, and their treatment was more likely to have been changed to a fluoroquinolone after presentation. This is the first national surveillance program for M. pneumoniae in the United States. Additional surveillance is needed to assess the clinical significance of MRMp and to monitor changes in MRMp prevalence.
INTRODUCTION
Mycoplasma pneumoniae occurs endemically and epidemically worldwide. The Etiology of Pneumoniae in Community (EPIC) Study determined that M. pneumoniae was the most common bacterial pathogen detected in children with community-acquired pneumonia (CAP) (8%), and it was found in 2% of adult cases of CAP during periods of endemicity (1, 2). Up to 20 to 40% of cases of CAP in the general population and 70% in closed populations can be caused by M. pneumoniae during epidemic periods, which occur every few years (3). An estimated 2 million cases of M. pneumoniae pneumonia occur annually, resulting in about 100,000 hospitalizations of adults in the United States (4). However, due to the relatively mild nature of many M. pneumoniae infections, similarity in presentation to other causes of CAP, a lack of point-of-care diagnostic tests, and no requirement for reporting, this rate likely underestimates the true incidence. School-age children and adolescents are the most common age groups affected, but M. pneumoniae can cause infections of persons from infancy up through old age (4, 5). Coinfection with M. pneumoniae and other respiratory pathogens is common, but it is not known with certainty whether coinfection is related to the severity of illness (6). The lack of an organized surveillance program makes it difficult to assess the true impact of M. pneumoniae on public health in the United States.
Macrolides are the treatments of choice for infections due to M. pneumoniae. Macrolide-resistant M. pneumoniae (MRMp) appeared in Japan in the early 2000s, with resistance rates exceeding 90% within 10 years, and subsequently spread through Asia and eventually to Europe and North America (7, 8). In Europe, MRMp rates range from about 1% to 30% and vary from one country to another (9). Yamada et al. reported an MRMp prevalence of 8.2% in Missouri children in 2011 (10). Diaz et al. published results from case patients, small clusters, and outbreaks investigated by the CDC that occurred from 2006 to 2013 and found an overall 10% prevalence of MRMp (11). Zheng et al. reported that the prevalence of MRMp from several regions in the United States from 2012 to 2014 was 13.2% (12). Most cases of MRMp have been reported in children, but some have also been seen in adults (13, 14). Mutations in the 23S rRNA gene for the 50S ribosome that alter the affinity for all macrolides are responsible for the development of MRMp. While data from Asia and Europe strongly indicate that MRMp can be clinically significant and affect patient outcomes compared to those for patients with macrolide-susceptible M. pneumoniae (MSMp) infections (9, 13, 15–20), no studies that have adequately addressed the question of the clinical significance of MRMp have been conducted in the United States since the numbers of available clinical specimens and M. pneumoniae isolates have been so small. Thus, the debate about whether the treatment of MRMp infections warrants a change to another drug class has not been resolved.
In 2017, the U.S. Centers for Disease Control and Prevention (CDC) sponsored a national surveillance program designed to determine the prevalence of MRMp infections at the state, regional, and national levels; determine its clinical characteristics and clinical significance, as assessed by patient outcomes; and perform molecular strain typing to determine the mode of spread. This paper reports the epidemiology and clinical significance of MRMp in the United States between 2015 and 2018.
MATERIALS AND METHODS
Surveillance population.
Persons with respiratory tract infection who had undergone testing for M. pneumoniae at 8 medical centers selected to represent all geographic areas of the United States comprised the surveillance population. The following were the participating hospitals: Children’s of Alabama, Birmingham, AL; Children’s Mercy Hospital, Kansas City, MO; Seattle Children’s Hospital, Seattle, WA; Memorial Sloan Kettering Cancer Center, New York, NY; Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Children’s Hospital Los Angeles, Los Angeles, CA; Hackensack University Medical Center, Hackensack, NJ; and University of Texas Health Science Center, San Antonio, TX. There were no age restrictions, but most specimens were obtained from children since all but one of the clinical sites are children’s hospitals and children are the population most likely to have MRMp (9, 21). Specimens were obtained between January 2015 and September 2018, representing a 45-month time period.
Case definition.
A case of M. pneumoniae infection was defined if a specimen collected from a symptomatic patient was positive for M. pneumoniae by molecular testing at the clinical site using the BioFire FilmArray respiratory panel (BioFire Diagnostics, Salt Lake City, UT), the GenMark respiratory pathogen panel (GenMark Dx, Carlsbad, CA), or a University of Alabama at Birmingham (UAB) laboratory-developed real-time PCR (22). Each patient was counted only once if multiple specimens were received, and all duplicates were removed from the database.
Microbiological specimens.
Nasopharyngeal or oropharyngeal swabs/aspirates, bronchoalveolar lavage (BAL) fluid, or sputum was an acceptable specimen. Most specimens were nasopharyngeal swabs, since those are the preferred specimen for the BioFire and GenMark panels.
Clinical data.
Investigators at the clinical sites utilized the REDCap (Research Electronic Data Capture) system, a browser-based software for clinical research databases (23), to transmit clinical data to UAB; the corresponding clinical specimens were also sent to UAB. Laboratory and clinical data without any identifiers were merged into an electronic database and used to assess the characteristics of MRMp and MSMp infections.
Laboratory testing.
Clinical specimens testing positive for M. pneumoniae were shipped to the Diagnostic Mycoplasma Laboratory at UAB from each respective clinical site and tested by real-time PCR (24) using a Roche LightCycler 480 instrument (Roche Diagnostics, Indianapolis, IN) to detect M. pneumoniae and to detect all point mutations in the 23S rRNA gene known to be associated with macrolide resistance in M. pneumoniae, based on the different melting points for the mutated nucleotide base pairs compared to those for the wild type (9, 24). Sanger sequencing using the same primers for PCR was performed to confirm and detect the point mutations in the 23S rRNA gene known to be associated with macrolide resistance. Specimens were also cultured to obtain M. pneumoniae isolates using the SP4 broth-to-agar method (25). Isolates obtained on SP4 agar were stored frozen at −80°C for further testing, as needed. The MICs of erythromycin, as a representative of the macrolide class, were determined for all M. pneumoniae isolates using the standardized methods and quality control procedures established by the Clinical and Laboratory Standards Institute (CLSI) (26).
Statistical analyses.
We estimated the overall crude prevalence of MRMp and calculated the respective 95% confidence intervals. The prevalence of MRMp was stratified by age group (≤18 years, 19 to 64 years, and 65+ years), by geographic location and region (West, Midwest, East), and by year of collection. Clinical data and outcomes for patients with MRMp infections were compared to those for patients with MSMp infections using the chi-square test, Student’s t test, or Fisher’s exact test, as appropriate. The clinical data collected included the symptoms at presentation, extrapulmonary manifestations, oxygenation status, respiratory findings, chest radiography, comorbidities, coinfections, and prior, current, and future antimicrobial use. Clinical outcomes included the duration of fever, hospitalization, and intensive care unit admission. Standard logistic regression techniques were used to explore the effects of MRMp, antimicrobial use, the presence of coinfections, and the extent of pulmonary manifestations and extrapulmonary manifestations on clinical outcomes. We used standard linear regression to explore the same effects on the length of stay (a dependent measure). The 95% confidence intervals and P values were calculated for all pertinent variables.
Human subject considerations.
Institutional review board approvals were obtained at UAB and all clinical test sites. Any identifying information was removed from specimens before shipment to UAB for testing. All clinical data without any identifiers were entered electronically into the REDCap system and linked to laboratory data only by the study numbers.
RESULTS
Specimen summary.
There were 371 specimens received at UAB. Among these, 367 were proven to be positive by PCR at UAB or by PCR testing at the Mycoplasma Laboratory at the CDC. Seven specimens were excluded from analysis because they were shown to be duplicates from the same patient, they were collected prior to 2015, or the PCR assay result or macrolide resistance was indeterminate. There were 360 specimens included in the final analysis, and of these 27 (7.5%; 95% confidence interval, 5.0% to 10.7%) contained MRMp. There were 339 (94.2%) specimens that were obtained from the upper respiratory tract as either nasopharyngeal or throat swab specimens, as nasopharyngeal swab specimens are the preferred specimen type for the BioFire system.
Demographics of the surveillance population.
The characteristics of the surveillance population are shown in Table 1. Three hundred six specimens were from persons who were younger than 19 years, while the remaining 54 specimens were from persons aged 19 years or older. There were no significant differences between patients with MRMp and MSMp infection with regard to any characteristic, including sex, race/ethnicity, age, or status as an inpatient or outpatient.
TABLE 1.
Demographics of study population
| Characteristic | Value for patients infected with: |
P value | |
|---|---|---|---|
| Macrolide-resistant isolates (n = 27) | Macrolide-susceptible isolates (n = 333) | ||
| No. (%) of patients by sex | |||
| Females | 14 (51.8) | 141 (42.5) | 0.34 |
| Males | 13 (48.2) | 191 (57.5) | |
| No. (%) of patients by race/ethnicity | |||
| White | 15 (55.6) | 151 (45.3) | 0.32 |
| Black | 2 (7.4) | 27 (8.1) | 0.99 |
| Hispanic | 1 (3.7) | 56 (16.8) | 0.09 |
| Asian/Other/unknown | 9 (33.3) | 99 (29.7) | 0.07 |
| Mean ± SD (range) age (yr) | 22.3 ± 22.8 (1–83) | 14 ± 18.5 (0–88) | 0.10 |
| No. (%) of patients: | |||
| Under age 19 yr (n = 306) | 21 (6.9) | 285 (93.1) | 0.27 |
| Age 19 yr or older (n = 54) | 6 (11.1) | 48 (88.9) | |
| No. (%) of patients by patient location | |||
| Inpatient | 9 (36) | 122 (39.4) | 0.74 |
| Outpatient (emergency room, clinic) | 16 (64) | 188 (60.6) | |
Prevalence and characteristics of MRMp.
MRMp prevalence rates stratified by geographic location and region are shown in Fig. 1. The overall prevalence of MRMp was 7.5% (27 of 360 specimens tested), but its occurrence was significantly higher in the southern and eastern states than on the West Coast (P = 0.00001). MRMp rates exceeding 20% were detected in Birmingham, AL, and Hackensack, NJ, whereas they were only 1.9% in Seattle, WA, and 2.8% in Los Angeles, CA. When stratified by year of specimen collection (Fig. 2), MRMp prevalence ranged from a low of 4.9% in 2015 to 10.2% in 2018, showing an increasing trend, but the change was not statistically significant (P = 0.27). The largest percentage of specimens was obtained from children 6 to 11 years of age (29.2%), an age group in which M. pneumoniae is particularly common. Overall, 83.9% of specimens were from persons under 19 years of age. The distribution of MRMp specimens among the various age groups ranged from 7% to 15%, but the differences among the groups were not significant (P = 0.18 to 0.99). The small number of MRMp specimens overall and particularly in adults made it impossible to judge whether MRMp is likely to occur in any age group.
FIG 1.
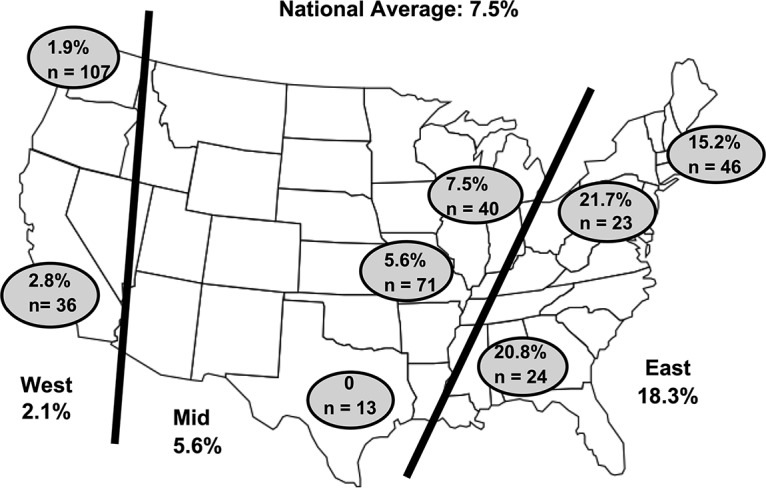
Geographic locations of hospitals participating in surveillance program. The figure shows the geographic locations of the 8 hospitals that provided specimens for the surveillance study and the relative prevalence of MRMp in each location. The national average was 7.5%, but there was considerable variation according to location, ranging from 1.9% in Seattle, WA, to 21.7% in Hackensack, NJ. The variation of MRMp prevalence in the western United States versus that in the eastern United States was significant (P = 0.00001).
FIG 2.
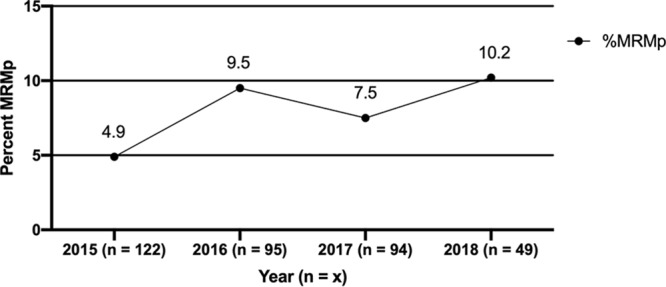
Prevalence of MRMp stratified by year of specimen collection. The figure demonstrates an increasing trend for MRMp prevalence over time, but this difference was not statistically significant (P = 0.27).
M. pneumoniae was successfully isolated from 249/360 specimens (69.2%). This included 15 isolates from specimens shown to have MRMp by PCR. The 234 isolates from MSMp-positive specimens had MICs ranging from ≤0.001 to 0.008 μg/ml, whereas the MICs for 15 MRMp isolates were 16 to 32 μg/ml. A2063G was the most common 23S rRNA mutation associated with macrolide resistance (n = 22), followed by A2064G (n = 4). One specimen had a mixture of A2063T and A2063G. These sequence position numbers are based on the M. pneumoniae numbering system.
Clinical features and outcomes.
Table 2 shows the clinical manifestations of persons with MRMp and MSMp at initial presentation and the occurrences of underlying conditions. Tables S2a and b in the supplemental material show these same data broken down by age into two groups: one consisting of persons aged less than 19 years and one consisting of persons aged 19 years or older. The most common clinical manifestations of persons with M. pneumoniae infection were cough, present in 68.9% of patients overall, followed by fever (60.5%), rhinorrhea (14.7%), and vomiting (10.8%). Other manifestations occurred in less than 10% of patients. There were no significant differences detected at initial presentation between patients with MRMp and patients with MSMp with regard to any clinical parameters, except that the respiratory rate was higher in the MSMp group in persons aged less than 19 years and rhonchi were detected more often on physical examination in persons with MRMp in the group aged 19 years or older. These minor findings are not believed to hold any clinical significance.
TABLE 2.
Clinical manifestations at initial presentation and underlying conditions
| Characteristica | Value for patients infected with: |
P value | |
|---|---|---|---|
| Macrolide-resistant isolates (n = 27) | Macrolide-susceptible isolates (n = 333) | ||
| No. (%) of patients with the following clinical manifestations at presentation: | |||
| Fever | 17 (62.9) | 201 (60.4) | 0.84 |
| Headache | 2 (7.4) | 18 (5.4) | 0.65 |
| Vomiting | 1 (3.7) | 38 (11.4) | 0.34 |
| Malaise | 0 (0.0) | 30 (9.0) | 0.14 |
| Rash | 0 (0.0) | 9 (2.7) | 0.99 |
| Diarrhea | 0 (0.0) | 20 (6.0) | 0.38 |
| Cough | 20 (74.1) | 228 (68.5) | 0.66 |
| Rhinorrhea | 1 (3.7) | 52 (15.6) | 0.15 |
| Dyspnea/shortness of breath | 2 (7.4) | 25 (7.5) | 0.99 |
| Sore throat | 1 (3.7) | 14 (4.2) | 0.99 |
| Lethargy/confusion | 1 (3.7) | 11 (3.3) | 0.99 |
| Mean ± SD (range) temp (°F) | 99.1 ± 1.6 (96.1–103.0) | 99.8 ± 1.9 (96.2–106.5) | 0.34 |
| Mean ± SD (range) respiratory rate (breaths per minute) | 24.5 ± 7.8 (12.0–44.0) | 30.1 ± 14.7 (14.0–124) | 0.003 |
| Mean ± SD (range) heart rate (beats per minute) | 114.2 ± 24.8 (63.0–169.0) | 119.0 ± 29.3 (32.0–222.0) | 0.33 |
| No. (%) of patients with the following HEENT findings: | |||
| Pharyngeal exudates | 0 (0.0) | 3 (0.9) | 0.99 |
| Rhinorrhea | 1 (3.7) | 37 (11.1) | 0.34 |
| Otitis media | 1 (3.7) | 13 (3.9) | 0.99 |
| Lymphadenopathy | 1 (3.7) | 6 (1.8) | 0.42 |
| Other | 1 (3.7) | 15 (4.5) | |
| No. (%) of patients with the following chest findings: | |||
| Rales | 2 (7.4) | 33 (9.9) | 0.99 |
| Rhonchi | 3 (11.1) | 14 (4.2) | 0.08 |
| Wheezing | 3 (11.1) | 27 (8.1) | 0.48 |
| Increased work of breathing | 1 (3.7) | 18 (5.4) | 0.99 |
| Other | 2 (7.4) | 43 (12.9) | |
| No. (%) of patients with the following underlying conditions: | |||
| Immune deficiency/malignancy | 9 (33.3) | 55 (16.5) | 0.03 |
| Congenital heart disease | 1 (3.7) | 20 (6.0) | 0.99 |
| Asthma | 3 (11.1) | 46 (13.8) | 1.0 |
| Diabetes mellitus | 2 (7.4) | 6 (1.8) | 0.11 |
| Atherosclerotic cardiovascular disease | 0 | 1 (0.3) | 0.99 |
| Cerebrovascular accident | 0 | 4 (1.2) | 0.99 |
| Neurologic disease | 0 | 15 (4.5) | 0.62 |
| Chronic kidney disease | 0 | 7 (2.1) | 0.99 |
| Heart failure/CHF | 0 | 3 (0.9) | 0.99 |
| Preterm birth | 0 | 17 (5.1) | 0.63 |
| Sickle cell anemia | 0 | 3 (0.9) | 0.99 |
| Other | 7 (25.9) | 79 (23.7) | 0.82 |
HEENT, head, eyes, ears, nose, and throat examination; CHF, congestive heart failure.
One-third of patients with MRMp had a history of immunodeficiency or malignancy (multiple myeloma, chronic lymphocytic leukemia, other leukemia, lymphoma, immunosuppressive therapy, or solid organ malignancy), while only 15% of patients with MSMp had such a history (9 [33.3%] patients with MRMp, 50 [15.0%] patients with MSMp; P = 0.03). However, this significant difference was not observed in persons aged 19 years or older. A variety of other underlying conditions, including diabetes mellitus, congenital heart disease, and preterm birth, were noted in the clinical records for some subjects, but there were no significant differences between patients with MRMp and patients with MSMp in terms of their occurrences.
There were 21 (77.8%) patients with MRMp and 216 (78.6%) patients with MSMp for whom a chest radiograph was obtained. Pneumonia was evident in 12 (44.4%) patients with MRMp and 113 (33.9%) patients with MSMp (P = 0.30). Interstitial infiltrate/airspace disease was evident in 4 (14.8%) patients with MRMp versus 57 (17.1%) patients with MSMp (P = 0.99). Thus, more than half of all patients had evidence of some type of lower respiratory infiltrate, but there were no differences in its occurrence or character between MRMp and MSMp.
Table 3 shows that there were no significant differences between patients with MRMp and patients with MSMp with regard to any medical complications, the requirement for hospitalization, the duration of hospitalization or fever, the requirement for intensive care unit (ICU) admission, or the administration of other treatments. Tables S3a and b show these data broken down into age groups, as described above for Tables S2 and b. While most M. pneumoniae infections are not of sufficient severity to warrant hospitalization (9), 13 (48.2%) patients with MRMp and 201 (60.5%) patients with MSMp included in this study were sick enough to be hospitalized. Furthermore, there were 2 (7.4%) patients with MRMp and 44 (13.2%) patients with MSMp that required ICU admission. These numbers were not significantly different (P = 0.19). Only one patient died.
TABLE 3.
Hospitalizations and outcomes
| Characteristic | Value for patients infected with: |
P value | |
|---|---|---|---|
| Macrolide-resistant isolates (n = 27) | Macrolide-susceptible isolates (n = 333) | ||
| No. (%) of patients with the following complications: | |||
| Encephalitis/encephalopathy | 0 | 3 (0.9) | 0.99 |
| Hematologic | 0 | 3 (0.9) | 0.99 |
| Dermatologic | 1 (3.7) | 0 | 0.08 |
| Rheumatologic | 0 | 1 (0.3) | 0.99 |
| Other | 0 | 7 (2.1) | 0.99 |
| No. (%) of patients with ICU admission | 2 (7.4) | 44 (13.2) | 0.19 |
| Mean ± SD (range) hospitalization duration (days) | 5.2 ± 4.5 (0–14) | 5.7 ± 11.5 (0–110) | 0.38 |
| Mean ± SD (range) duration of fever of >101°F (days) | 1.7 ± 2.0 (0–5) | 0.89 ± 1.22 (0–7) | 0.33 |
| No. (%) of patients hospitalized | |||
| Yes | 13 (48.2) | 201 (60.5) | 0.45 |
| No | 13 48.2) | 121 (36.5 | |
| Unknown | 1 (3.6) | 10 (3.0) | |
| No. (%) of patients with the following outcome of hospitalization: | |||
| Survived and discharged | 12 (44.4) | 205 (61.6) | |
| Died | 0 | 1 (0.3) | 0.99 |
| Unknown | 0 | 4 (1.2) | |
| No. (%) of patients administered the following other treatments: | |||
| O2 per nasal cannula | 3 (11.1) | 93 (27.9) | 0.07 |
| Extracorporeal membrane oxygenation | 0 | 1 (0.3) | 0.99 |
| CPAP/BIPAPa | 0 | 12 (3.6) | 0.61 |
| Mechanical ventilation | 0 | 13 (3.9) | 0.61 |
| Other | 2 (7.4) | 14 (4.2) | 0.34 |
CPAP/BIPAP, continuous positive airway pressure/bilevel positive airway pressure.
Antimicrobial treatment.
Overall, 306/360 (85%) of patients with a positive M. pneumoniae test at 1 of the 8 clinical sites received some type of antimicrobial following initial presentation. Table 4 shows a comparison of the antimicrobials received prior to and following initial presentation. These data are broken down by age group, as described above, in the corresponding Tables S4a and b. Macrolides (azithromycin or clarithromycin) were the most common drugs prescribed in 207/360 (57.5%) patients, followed by various beta-lactams. Interestingly, the proportion of patients with MSMp who received a macrolide (58.9%) was significantly higher than that of patients with MRMp (40.7%) (P = 0.007). There were 25.9% of MRMp patients and 30.9% of MSMp patients who received a macrolide as well as another type of antimicrobial following initial presentation (P = 0.67). The mean duration of antimicrobial treatment was 5 days for patients with MRMp infections and 4 days for patients with MSMp infections, reflecting the fact that a short treatment course with azithromycin was the predominant mode of therapy for both groups. Patients aged 19 years or younger with MRMp were significantly more likely to have received a macrolide at presentation than patients with MSMp (37% versus 9.6%; P = 0.003). Furthermore, the subsequent administration of fluoroquinolones, such as levofloxacin, ciprofloxacin, or moxifloxacin, was more common in patients with MRMp than in those with MSMp (18.5% versus 6.9%; P = 0.05). When fluoroquinolone treatment was stratified by age group, patients with MRMp aged 19 years and younger were significantly more likely to receive a fluoroquinolone than those with MSMp (19% versus 2%; P = 0.002), but there was no significant difference in fluoroquinolone use in persons 19 years of age or older (P = 0.65).
TABLE 4.
Antimicrobials given prior to and after presentation
| Drug(s) administered prior to presentation | No. (%) of infected patients receiving the indicated drug(s): |
P value | |
|---|---|---|---|
| Macrolide-resistant isolates (n = 27) | Macrolide-susceptible isolates (n = 333) | ||
| Any antimicrobial | 16 (59.3) | 154 (46.3) | 0.23 |
| Azithromycin or clarithromycin | 10 (37) | 32 (9.6) | 0.0003 |
| Amoxicillin, penicillin, or ampicillin-sulbactam | 5 (18.5) | 78 (23.4) | 0.64 |
| Fluoroquinolones | 1 (3.7) | 9 (2.7) | 0.55 |
| Other | 6 (22.2) | 59 (17.2) | 0.60 |
| Drugs after presentation | |||
| Any antimicrobial | 15 (55.6) | 291 (65.8) | 0.30 |
| Azithromycin or clarithromycin | 11 (40.7) | 196 (58.9) | 0.007 |
| Ceftriaxone | 6 (22.2) | 72 (21.6) | 0.99 |
| Ampicillin | 2 (7.4) | 28 (8.4) | 0.99 |
| Fluoroquinolones | 5 (18.5) | 23 (6.9) | 0.05 |
| Vancomycin | 1 (3.7) | 14 (4.2) | 0.99 |
| Clindamycin | 0 | 18 (5.4) | 0.38 |
| Other | 1 (3.7) | 5 (1.5) | 0.38 |
Coinfections.
The BioFire respiratory panel includes targets for several respiratory viruses as well as two other bacterial pathogens, Chlamydia pneumoniae and Bordetella pertussis, in addition to M. pneumoniae. Since this system was used to obtain M. pneumoniae-positive specimens for all hospitals except for Children’s of Alabama, it was possible to evaluate whether the presence of other respiratory pathogens had any effect on the severity or outcome of M. pneumoniae infections in 336 patients. As shown in Table 5, 93/243 (27.7%) patients had one or more concurrent viral infections. Only one patient in the MSMp group tested positive for C. pneumoniae, and there were no positive tests for B. pertussis. However, coinfections with various respiratory viruses were common in both the MRMp and the MSMp groups. Rhinoviruses and enteroviruses accounted for most coinfections, followed by influenza virus, parainfluenza virus, and adenovirus. Patients <19 years old who had viral coinfections were more likely to have rhinorrhea than patients in this age range without coinfections (rhinorrhea and coinfections, 25/98 [25.5%]; rhinorrhea and no coinfection, 28/262 [10.7%]; P = 0.0007). This difference was not seen in patients 19 years of age and older. Patients with MRMp who had underlying immunodeficiencies (8 of 12; 66.7%) were more likely than those with MSMp (26 of 96; 27.1%) to have coinfections with other pathogens (P = 0.009). When patients with M. pneumoniae and viral coinfections were compared with those without coinfections, there was no significant difference with regard to clinical parameters, as shown in Table 5, with the exceptions that patients under 19 of age years with viral coinfections had a significantly higher mean heart rate and those without coinfections had a significantly higher mean temperature at the time of presentation, These data are broken down according to age and are shown in Tables S5a and b.
TABLE 5.
Clinical significance of viral coinfections
| Characteristic | Value for patients with: |
P value | |
|---|---|---|---|
| Coinfections (n = 93) | No coinfections (n = 243) | ||
| Mean ± SD (range) duration of hospitalization (days) | 4.9 ± 6.6 (0–35) | 5.0 ± 9.24 (0–81) | 0.96 |
| Mean ± SD (range) temp (°F) | 99.2 ± 1.6 (96.1–103.6) | 99.9 ± 2.0 (96.2–106.5) | 0.005 |
| Mean ± SD (range) respiratory rate (breaths per minute) | 32.1 ± 14.5 (14–90) | 29.1 ± 14.6 (14–124) | 0.10 |
| Mean ± SD (range) heart rate (beats per minute) | 128.5 ± 31.1 (70–222) | 115.6 ± 27.7 (32–210) | 0.0003 |
| O2 per nasal cannula (%) | 22 (23.7) | 64 (26.3) | 0.67 |
| No. (%) of patients: | |||
| Hospitalized | 58 (62.4) | 141 (58.3) | 0.53 |
| Admitted to ICU | 12 (12.9) | 26 (10.7) | 0.57 |
DISCUSSION
MRMp has now been reported across the globe for almost 20 years and is presumably driven by antimicrobial pressure, related to the widespread use of macrolides, such as azithromycin, for the empirical treatment of respiratory infections in outpatients, for whom no microbiological diagnosis is attempted most of the time. According to one study that included patients of all ages, azithromycin was the single most widely prescribed antibiotic in the United States during 2011, with 54.1 million prescriptions, mainly for respiratory tract infections, being written (27). Thus, it is not surprising to see evidence of macrolide resistance in this country. The MRMp prevalence reported from the United States between 2006 and 2014 ranged from 8.2 to 13.2% (10–12). In this report, we have described the first national surveillance program for MRMp in the United States, finding an overall MRMp prevalence of 7.5% based on sampling 360 nonduplicate specimens from children and adults in 8 states across the country over a 45-month surveillance period. Even though the overall occurrence of MRMp was fairly low, there were significant differences in MRMp prevalence when different regions of the United States were compared, so reporting of a national MRMp prevalence is not necessarily predictive of what is happening at the local or regional levels. In the Midwest, MRMp rates ranged from 5.6% in Kansas City, MO, to 7.5% in Chicago, IL, similar to the national average. In contrast, MRMp was quite uncommon in Los Angeles, CA (2.8%), and Seattle, WA (1.9%), on the West Coast but was much higher in the Southeast in Birmingham, AL (20.8%), and in the Northeast in Hackensack, NJ (21.75%). Neighboring New York City reported an MRMp prevalence rate of 15.2%, which was double the national average. Our findings of significant differences in the prevalence of MRMp from west to east could possibly be due to regional differences in antibiotic use, thus affecting the degree of antimicrobial pressure driving resistance. Prescribing rates for antimicrobials have been reported to be higher in the South (931 prescriptions per 1,000 persons) than in the West (647 prescriptions per 1,000 persons) (P < 0.001), with this pattern being observed among all age groups (27). Data from another study of antibiotic prescriptions given for adults in ambulatory care demonstrated that antibiotics were prescribed for respiratory conditions for which they are rarely indicated during 38% of visits in the West, compared with 60% of visits in the South (28).
Our initial plans were to evaluate at least 350 specimens divided equally among 8 hospitals to get a true overall picture of MRMp across the United States. However, due to differences in the availability of positive samples, Seattle contributed the largest number of specimens (n = 107), which represented 29.7% of the total of 360 specimens tested. The very low MRMp rate in Seattle (1.9%) undoubtedly caused the overall national rate to be much lower than expected. If the data from Seattle were excluded, then we would have 25 MRMp out of a total 253 specimens, yielding a national prevalence or overall rate for MRMp of 9.9%.
Studies from other countries have reported that persons infected with MRMp who receive macrolides may experience a longer febrile period and may require extended antibiotic therapy or a change to another drug class compared with those infected with MSMp (9, 15, 16, 18–20, 29, 30). Prior to the present investigation, there have been no data from the United States to confirm whether MRMp is clinically significant. We found that that the characteristics of our study population by gender, race/ethnicity, age, underlying conditions, and severity of illness, based on whether or not hospitalization was necessary, were comparable between persons with MRMp infections and persons with MSMp infections. There were no significant differences when comparing persons with infections caused by MRMp with persons with infections caused by MSMp with respect to clinical presentation, radiographic findings, complications, duration of fever, requirement for hospitalization, intensive care unit admission, duration of hospitalization, or duration of antimicrobial use. A modest increase in MRMp prevalence was observed over the duration of the surveillance period, but this increase was not statistically significant. Our finding of no apparent difference in the clinical or radiographic presentation of patients with MRMp versus MSMp infections is consistent with what has been reported by others, so it is impossible to predict who may be harboring resistant organisms (13, 15, 16, 31–33). However, the lack of clinical significance of MRMp in terms of patient outcome was initially surprising, considering what has been reported primarily from Japan and China (13, 15, 16, 20, 30, 34). However, in those countries, the prevalence of MRMp is extremely high, often more than 90% (9), so it is possible to evaluate large numbers of patients with MRMp in those countries. An important limitation of our study is that only 27 patients with MRMp were identified. Determination as to whether or not MRMp infections are truly clinically significant in the United States will require additional surveillance data on larger numbers of persons.
Patients with MRMp were more likely than those with MSMp to have underlying immunodeficiencies or malignancies, and this could be related to a greater likelihood that patients with MRMp receive macrolide antibiotics on multiple occasions prior to presentation because they more frequently have illnesses than otherwise healthy persons. Persons with MRMp were more likely to have received macrolides prior to presentation than persons with MSMp. Prior exposure to macrolides has been associated with the development of macrolide resistance in previous studies (35–37). This observation could possibly be related to the fact that they had previously received a macrolide and were persistently ill or had relapsed and the physician decided to choose a drug from an alternative drug class. Even though we were unable to document any clinical significance of MRMp, presumably because of the small numbers, the investigators in Birmingham have had clinical experience with children with severe infections due to MRMp that required a switch to fluoroquinolones to resolve the infection (38). The fact that MRMp rates exceeded 20% in Birmingham, AL, and Hackensack, NJ, makes it important to carefully monitor patients treated with macrolides for suspected or proven M. pneumoniae infection and be ready to change to a drug from another drug class if there is no prompt clinical response.
Although we were unable to demonstrate any significant differences between patients with MRMp and patients with MSMp in terms of the duration of antimicrobial treatment or treatment with more than one antimicrobial, we found a significant association of fluoroquinolone administration in persons 18 years of age and under with MRMp but not in those with MSMp, thus suggesting that there were treatment failures since fluoroquinolones are not recommended as first-line drugs for respiratory infections in children. The clinicians caring for these patients had no way to know that they might be dealing with MRMp infections, unless there was a lack of a clinical response to the first-line macrolide treatment. The UAB Diagnostic Mycoplasma Laboratory's practice of performing a reflexive test for the detection of mutations conferring macrolide resistance by real-time PCR (24) is one practical means for guiding antimicrobial management that can be quite useful in locations where there is a high prevalence of MRMp. At present, there are no FDA-approved commercial molecular-based tests that can detect MRMp on a rapid basis.
One-third of all patients included in this study were hospitalized, and more than half had evidence of pneumonia, an interstitial infiltrate, or airspace disease on chest radiographs. Thus, these patients were likely sicker than those in the general population who present with M. pneumoniae infections in an ambulatory care setting, who are seldom hospitalized and who may not have pneumonia (9). This bias toward sicker patients is likely because those who are not as ill are less likely to have an expensive molecular-based test performed to obtain a microbiological diagnosis. Whether this selection bias had any effect on MRMp prevalence is not known, but it appears unlikely, since we observed no differences in clinical severity at presentation between patients with MRMp and patients with MSMp.
Detection of MRMp by PCR was confirmed in every case by the high MICs of erythromycin (16 to 32 μg/ml) and the presence of mutations in 23S rRNA. A2063G was the most common mutation, and this is consistent with the findings of other studies (9). The fact that real-time PCR performed on the Roche LightCycler instrument accurately detects MRMp makes it unnecessary to attempt culture to obtain phenotypic MICs of erythromycin or other macrolides for surveillance or diagnostic purposes. This makes it easier for public health laboratories or other health care entities to perform susceptibility testing or focused community surveillance for MRMp, if needed. Once commercial tests that incorporate testing for mutations conferring macrolide resistance become available, focused surveillance will become even easier.
An additional aspect addressed in this surveillance project was the occurrence of viral coinfections in patients with M. pneumoniae infections detected by the BioFire FilmArray respiratory panel. Slightly more than one-fourth of all specimens that were positive for M. pneumoniae also had one or more viruses detected, with rhinoviruses being the most common agents detected. Using a limited set of clinical parameters for comparison, we were unable to identify any significant effect of viral infection on the clinical course of adults with concurrent M. pneumoniae infection, but children with M. pneumoniae infection and a viral coinfection were more likely to present with rhinorrhea than children without a viral coinfection. Other publications have addressed the issue of M. pneumoniae and viral coinfections, and at least one found that viral coinfections can lead to a greater severity of M. pneumoniae infection. However, the different methodologies used for microbial detection make it difficult to compare the results among the studies.
In summary, we have reported the first data from a national surveillance program for macrolide resistance in an important respiratory pathogen, M. pneumoniae, and determined that while the overall prevalence of macrolide resistance is low, significant regional differences occur. Patients with MRMp were more likely to have previously received macrolides and were more likely to have been treated with a fluoroquinolone after presentation, but the numbers of patients studied were insufficient to determine any specific clinical significance of infection with MRMp on the outcomes of infection. Additional surveillance is required to determine the clinical significance of MRMp, whether the rate of this resistance is changing over time, and whether there are other local or regional difference in prevalence that were not readily apparent by testing specimens from only 8 sites. Important information about the molecular epidemiology and spread of MRMp was obtained from molecular typing, and this information will be reported in a separate publication focused on this particular aspect of MRMp.
Supplementary Material
ACKNOWLEDGMENTS
Financial support was provided by U.S. Centers for Disease Control and Prevention contract 200-2017-96217 to K. B. Waites. This study was supported in part by NIH/NCI Cancer Center support grant P30 CA008748 to Y.-W. Tang.
Gracie Ernstberger conducted the chart review and data entry at UAB.
We thank Melanie Fecanin, Amanda Adler, Neena Kanwar, Georgann Meredith, Krupa Jani, Tracy McMillen, and Juan Lopez for technical assistance. The support of the administrative and scientific staff at the CDC for their assistance in financial administration for this project as well as guidance in study design and data analysis is gratefully acknowledged. Specifically, we thank Stacey Spivey-Blackford, Jonas Winchell, Maureen Diaz, Alvaro Benitez, and Miwako Kobayashi.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00968-19.
REFERENCES
- 1.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, Zhu Y, Patel A, Hymas W, Chappell JD, Kaufman RA, Kan JH, Dansie D, Lenny N, Hillyard DR, Haynes LM, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, Wunderink RG, Edwards KM, Pavia AT, McCullers JA, Finelli L, CDC EPIC Study Team . 2015. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L, CDC EPIC Study Team . 2015. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs E, Ehrhardt I, Dumke R. 2015. New insights in the outbreak pattern of Mycoplasma pneumoniae. Int J Med Microbiol 305:705–708. doi: 10.1016/j.ijmm.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Winchell JM. 2013. Mycoplasma pneumoniae—a national public health perspective. Curr Pediatr Rev 9:324–333. doi: 10.2174/15733963113099990009. [DOI] [Google Scholar]
- 5.Gadsby NJ, Reynolds AJ, McMenamin J, Gunson RN, McDonagh S, Molyneaux PJ, Yirrell DL, Templeton KE. 2012. Increased reports of Mycoplasma pneumoniae from laboratories in Scotland in 2010 and 2011—impact of the epidemic in infants. Euro Surveill 17(10):pii=20110 10.2807/ese.17.10.20110-en. [DOI] [PubMed] [Google Scholar]
- 6.Mandell LA. 2015. Community-acquired pneumonia: an overview. Postgrad Med 127:607–615. doi: 10.1080/00325481.2015.1074030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki N, Narita M, Yamada S, Izumikawa K, Umetsu M, Kenri T, Sasaki Y, Arakawa Y, Sasaki T. 2001. Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol Immunol 45:617–620. doi: 10.1111/j.1348-0421.2001.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 8.Waites KB, Lysynyansky I, Bebear CM. 2014. Emerging antimicrobial resistance in mycoplasmas of humans and animals, p 289–322. In Browning G, Citti C (ed), Mollicutes molecular biology and pathogenesis. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 9.Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. 2017. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev 30:747–809. doi: 10.1128/CMR.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada M, Buller R, Bledsoe S, Storch GA. 2011. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 31:409–500. doi: 10.1097/INF.0b013e318247f3e0. [DOI] [PubMed] [Google Scholar]
- 11.Diaz MH, Benitez AJ, Winchell JM. 2015. Investigations of Mycoplasma pneumoniae infections in the United States: trends in molecular typing and macrolide resistance from 2006 to 2013. J Clin Microbiol 53:124–130. doi: 10.1128/JCM.02597-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng X, Lee S, Selvarangan R, Qin X, Tang YW, Stiles J, Hong T, Todd K, Ratliff AE, Crabb DM, Xiao L, Atkinson TP, Waites KB. 2015. Macrolide-resistant Mycoplasma pneumoniae, United States. Emerg Infect Dis 21:1470–1472. doi: 10.3201/eid2108.150273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao B, Zhao CJ, Yin YD, Zhao F, Song SF, Bai L, Zhang JZ, Liu YM, Zhang YY, Wang H, Wang C. 2010. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis 51:189–194. doi: 10.1086/653535. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Li X, Chen X, Luo F, Pan C, Zheng X, Tan F. 2015. Macrolide-resistant Mycoplasma pneumoniae in adults in Zhejiang, China. Antimicrob Agents Chemother 59:1048–1051. doi: 10.1128/AAC.04308-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki S, Yamazaki T, Narita M, Okazaki N, Suzuki I, Andoh T, Matsuoka M, Kenri T, Arakawa Y, Sasaki T. 2006. Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae. Antimicrob Agents Chemother 50:709–712. doi: 10.1128/AAC.50.2.709-712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsubara K, Morozumi M, Okada T, Matsushima T, Komiyama O, Shoji M, Ebihara T, Ubukata K, Sato Y, Akita H, Sunakawa K, Iwata S. 2009. A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J Infect Chemother 15:380–383. doi: 10.1007/s10156-009-0715-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhao F, Liu G, Wu J, Cao B, Tao X, He L, Meng F, Zhu L, Lv M, Yin Y, Zhang J. 2013. Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother 57:1521–1523. doi: 10.1128/AAC.02060-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardinale F, Chironna M, Chinellato I, Principi N, Esposito S. 2013. Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J Clin Microbiol 51:723–724. doi: 10.1128/JCM.02840-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo SJ, Kim HB, Choi SH, Lee SO, Kim SH, Hong SB, Sung H, Kim MN. 2012. Differences in the frequency of 23S rRNA gene mutations in Mycoplasma pneumoniae between children and adults with community-acquired pneumonia: clinical impact of mutations conferring macrolide resistance. Antimicrob Agents Chemother 56:6393–6396. doi: 10.1128/AAC.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Z, Zheng Y, Deng J, Ma X, Liu H. 2014. Characterization of macrolide resistance of Mycoplasma pneumoniae in children in Shenzhen, China. Pediatr Pulmonol 49:695–700. doi: 10.1002/ppul.22851. [DOI] [PubMed] [Google Scholar]
- 21.Holzman RS, Simberkoff MS. 2015. Chapter 185, Mycoplasma pneumoniae and atypical pneumonia In Bennett JE, Dolin R, Blazer M (ed), Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 8th ed. Elsevier Saunders, Philadelphia, PA. [Google Scholar]
- 22.Dumke R, Schurwanz N, Lenz M, Schuppler M, Luck C, Jacobs E. 2007. Sensitive detection of Mycoplasma pneumoniae in human respiratory tract samples by optimized real-time PCR approach. J Clin Microbiol 45:2726–2730. doi: 10.1128/JCM.00321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Atkinson TP, Hagood J, Makris C, Duffy LB, Waites KB. 2009. Emerging macrolide resistance in Mycoplasma pneumoniae in children: detection and characterization of resistant isolates. Pediatr Infect Dis J 28:693–696. doi: 10.1097/INF.0b013e31819e3f7a. [DOI] [PubMed] [Google Scholar]
- 25.Waites KB, Duffy LB, Schwartz S, Talkington DF. 2010. Mycoplasma and ureaplasma, p 3.15.11–13.15.17. In Garcia L. (ed), Clinical microbiology procedures handbook, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. 2011. Methods for antimicrobial susceptibility testing of human mycoplasmas. Approved guideline. CLSI document M43-A Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 27.Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH Jr, Schrag SJ. 2015. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 60:1308–1316. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. 2014. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 69:234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 29.Wu HM, Wong KS, Huang YC, Lai SH, Tsao KC, Lin YJ, Lin TY. 2013. Macrolide-resistant Mycoplasma pneumoniae in children in Taiwan. J Infect Chemother 19:782–786. doi: 10.1007/s10156-012-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai Y, Miyashita N, Yamaguchi T, Saitoh A, Kondoh E, Fujimoto H, Teranishi H, Inoue M, Wakabayashi T, Akaike H, Ogita S, Kawasaki K, Terada K, Kishi F, Ouchi K. 2012. Clinical efficacy of macrolide antibiotics against genetically determined macrolide-resistant Mycoplasma pneumoniae pneumonia in paediatric patients. Respirology 17:354–362. doi: 10.1111/j.1440-1843.2011.02102.x. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka M, Narita M, Okazaki N, Ohya H, Yamazaki T, Ouchi K, Suzuki I, Andoh T, Kenri T, Sasaki Y, Horino A, Shintani M, Arakawa Y, Sasaki T. 2004. Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob Agents Chemother 48:4624–4630. doi: 10.1128/AAC.48.12.4624-4630.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu PS, Chang LY, Lin HC, Chi H, Hsieh YC, Huang YC, Liu CC, Huang YC, Huang LM. 2013. Epidemiology and clinical manifestations of children with macrolide-resistant Mycoplasma pneumoniae pneumonia in Taiwan. Pediatr Pulmonol 48:904–911. doi: 10.1002/ppul.22706. [DOI] [PubMed] [Google Scholar]
- 33.Morozumi M, Iwata S, Hasegawa K, Chiba N, Takayanagi R, Matsubara K, Nakayama E, Sunakawa K, Ubukata K. 2008. Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother 52:348–350. doi: 10.1128/AAC.00779-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Zhang Y, Sheng Y, Zhang L, Shen Z, Chen Z. 2014. More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother 58:1034–1038. doi: 10.1128/AAC.01806-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki Y, Shimotai Y, Itagaki T, Seto J, Ikeda T, Yahagi K, Mizuta K, Hongo S, Matsuzaki Y. 2017. Development of macrolide resistance-associated mutations after macrolide treatment in children infected with Mycoplasma pneumoniae. J Med Microbiol 66:1531–1538. doi: 10.1099/jmm.0.000582. [DOI] [PubMed] [Google Scholar]
- 36.Dumke R, Stolz S, Jacobs E, Juretzek T. 2014. Molecular characterization of macrolide resistance of a Mycoplasma pneumoniae strain that developed during therapy of a patient with pneumonia. Int J Infect Dis 29:197–199. doi: 10.1016/j.ijid.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Hantz S, Garnier F, Peuchant O, Menetrey C, Charron A, Ploy MC, Bebear C, Pereyre S. 2012. Multilocus variable-number tandem-repeat analysis-confirmed emergence of a macrolide resistance-associated mutation in Mycoplasma pneumoniae during macrolide therapy for interstitial pneumonia in an immunocompromised child. J Clin Microbiol 50:3402–3405. doi: 10.1128/JCM.01248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atkinson TP, Boppana S, Theos A, Clements LS, Xiao L, Waites K. 2011. Stevens-Johnson syndrome in a boy with macrolide-resistant Mycoplasma pneumoniae pneumonia. Pediatrics 127:e1605–e1609. doi: 10.1542/peds.2010-2624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


