Abstract
Purpose of review
Urine is the most useful of body fluids for biomarker research. Therefore, we have focused on urinary proteomics, using capillary electrophoresis coupled to mass spectrometry (CE-MS), to investigate kidney disease in recent years.
Recent Findings
Several urinary proteomics studies for the detection of various kidney diseases have indicated the potential of this approach aimed at diagnostic and prognostic assessment. Urinary protein biomarkers such as collagen fragments, serum albumin, alpha-1-antitrypsin, and uromodulin can help to explain the processes involved during disease progression.
Summary
Urinary proteomics has been used in several studies, in order to identify and validate biomarkers associated with different kidney diseases. These biomarkers, with improved sensitivity and specificity when compared to the current gold standards, provide a significant alternative for diagnosis and prognosis, as well as improving clinic decision making.
Keywords: urinary proteomics, kidney diseases, capillary electrophoresis-mass spectrometry, biomarkers
Introduction
Renal function is essential for maintaining body homeostasis by contributing to the control of blood composition, pressure, volume and pH (1). Proteomics, the analysis of the total protein content of a sample, is a field of research constantly evolving and during the last decade several techniques have been developed to analyze and characterize the proteome of samples from very different origins (2). Urinary proteomics has become an essential tool for the discovery of novel biomarkers in kidney disease. It has been shown to contribute to early diagnosis and clinical assessment, based on better insight into kidney disease and its development (3)*.
Current situation of clinical proteomics
In the last two decades, mass spectrometry (MS) has become one of the most prevalent techniques in the detection and characterization of proteins and peptides (4). Techniques such as two-dimensional gel electrophoresis (2-DE), liquid chromatography (LC), surface enhanced laser desorption/ionization (SELDI), and capillary electrophoresis (CE) coupled with MS have had a significant impact in biology and medicine. The advantages and disadvantages of these different proteomics approaches have been described in a number of reviews in detail (5–7). Although a large number of studies have been published on the use of proteomics for the identification of biomarkers for diseases, they often had significant shortcomings, e.g. absence of appropriate statistical assessment, insufficient power, or lack of validation in an independent cohort. Sparked by these observations, recommendations for biomarker research in the field of clinical proteomics were elaborated to improve the validity of biomarkers identified in these studies (8–10).
Recent urinary proteomic biomarker studies
Several clinical urinary proteomic studies in kidney diseases conforming to the above recommendations were recently (last five years) published. However, most of them are based on capillary electrophoresis coupled to mass spectrometry (CE-MS). In the next sections, these studies will be discussed in detail and are listed in Table 1, as well as depicted in Figure 1.
Table 1. Summary of recent publications for the diagnosis and prognosis of kidney diseases based on CE-MS.
In some studies sensitivity, specificity, or AUCs are not reported. *added value of proteome analysis with respect to the currently used parameters
| Kidney disease | Number of peptides | Performance | Accuracy | References |
|---|---|---|---|---|
| CKD | 273 | Diagnosis | Sensitivity: 86% Specificity: 100% AUC: 0.96 |
(11) |
| DN | Diagnosis | Sensitivity: 95% Specificity: 89% AUC: 0.96 |
(12) | |
| CKD | Prognosis | --- | (13) | |
| CKD | Prognosis | --- | (14) | |
| DN | Prognosis | AUC: 0.92 | (15) | |
| DN | Prognosis | --- | (16) | |
| CKD | Diagnosis (Prognosis) | Sensitivity: 95% Specificity: 100% AUC: 0.98 (AUC: 0.91*) |
(17) | |
| DN | Diagnosis | AUC: 0.95 | (18) | |
| CKD | Prognosis | AUC: 0.83* | (19) | |
| CKD | Prognosis | --- | (20) | |
| MGN | 311 | Diagnosis | AUC: 0.87 | (21) |
| FSGS | 287 | Diagnosis | AUC: 0.88 | |
| MCD | 291 | Diagnosis | AUC: 0.77 | |
| DN/HN | 619 | Diagnosis | AUC: 0.92 | |
| IgAN | 116 | Diagnosis | AUC: 0.82 | |
| Vasculitis | 509 | Diagnosis | AUC: 0.95 | |
| LN | 172 | Diagnosis | AUC: 0.82 | |
| ADPKD | 142 | Diagnosis | Sensitivity: 85% Specificity: 94% |
(22) |
| ADPKD | 20 | Prognosis | AUC: 0.83 | (23) |
| FIKD | 64 | Diagnosis | Sensitivity: 88% Specificity: 98% AUC: 0.97 |
(24) |
| PUV | 12 | Prognosis | Sensitivity: 88% Specificity: 95% |
(25) |
| UPJO | 51 | Prognosis | Sensitivity: 100% Specificity: 85% AUC: 0.92 |
(26) |
| Prognosis (age<1) | Sensitivity: 83% Specificity: 92% AUC: 0.88 |
(27) | ||
| VUR | 9 | Diagnosis | Sensitivity: 88% Specificity: 79% |
(28) |
Figure 1.
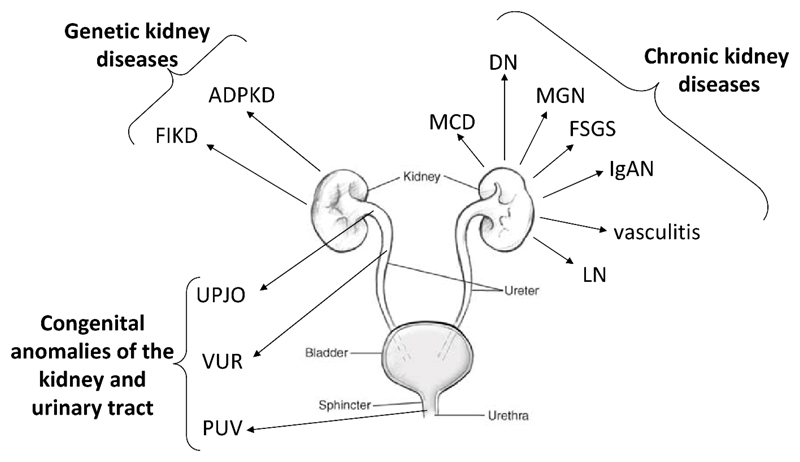
Overview of the different kidney diseases, which were successfully investigated in the context of clinical urinary proteome analysis. The arrows pointed out in which area of the renal system the disease appears. Abbreviations: ADPKD - autosomal dominant polycystic kidney disease; DN – diabetic nephropathy; FIKD – Fabry induced kidney disease; FSGS - focal segmental glomerulosclerosis; IgAN – IgA nephropathy; LN – lupus nephrithis; MCD – minimal changed disease; MGN - membranous glomerulonephritis; PUV - posterior urethral valves; UPJO - ureteropelvic junction obstruction; vasculitis - ANCA-associated vasculitis.
Chronic Kidney Diseases
Chronic kidney disease (CKD) is defined as the progressive loss of kidney function and a reduction in the glomerular filtration rate (GFR) which can result in end-stage renal disease (ESRD). At this stage, the patients require renal replacement therapies like dialysis or kidney transplantation (29). The most common causes of CKD are diabetic nephropathy (DN), hypertension, and glomerulonephritis (30). DN is caused by diabetes mellitus, a chronic metabolic disease, associated with cardiovascular and renal complications. CKD diagnosis is currently obtained by the detection of alterations in estimated glomerular filtration rate (eGFR) and/or albuminuria as indicators of renal dysfunction (29). However, eGFR has limited value in predicting risk of CKD progression unless substantially reduced. Instead (or together with eGFR) albuminuria is often used even though, in a non-negligible number of patients, renal disease progresses despite the absence of albuminuria (31).
Over the last five years CE-MS has been a frequently used proteomics approach to discover urinary biomarkers for the diagnosis and prognosis of CKD (see Figure 2). The basis for all these validation studies was a publication in 2010 (11). Using CE-MS, Good et al. were able to define 273 urinary peptide markers for CKD (named the “CKD273-classifier”) in a cohort of 379 healthy controls and 230 patients with CKD derived from different etiologies. These peptide markers were mostly different fragments of various collagens, blood proteins (e.g. serum albumin, α-1-antitrypsin) and specific kidney-derived proteins (e.g. uromodulin). In order to validate the defined biomarkers, Good et al. applied the CKD273-classifier to a set of 144 samples consisting of 34 controls and 110 patients with CKD, showing a sensitivity of 85% and specificity of 100% (AUC=0.96).
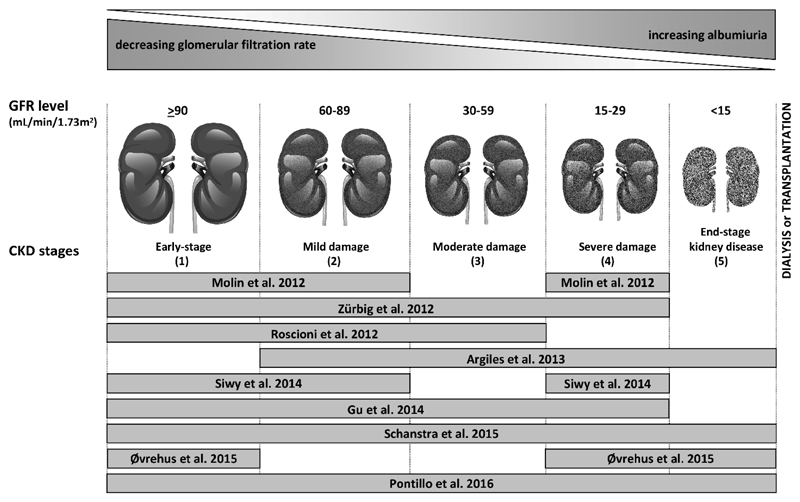
Figure 2.
Schematic depiction of the reviewed studies evaluating the performance of the CKD273-classifier in diagnosis and prognosis of CKD according to disease stage. The bars shows the CKD stages of the in the study included patients. Figure adapted from Critselis et al. (35).
This CKD273-classifier was further validated in several independent studies with patients with CKD derived from different origins: The first study was to validate the diagnostic performance in patients suffering from type 2 diabetes. Molin et al. compared 137 urine samples (62 patients and 75 controls), based on CKD273-classifier (12). The validation resulted in a similar accuracy (AUC=0.96) as in the initial discovery study (11). Argilés et al. examined the CKD273-classifier based on a cohort of 53 patients with CKD at different stages, where the CKD273-classifier enabled prediction of ESRD or death (13). Gu et al. also confirmed the ability of the CDK273-classifier to predict progression of CKD in a cohort of 797 individuals. With an average follow-up time of 4.8 years, this validation effectively predicted development of renal dysfunction and cardiovascular complications (14). Zürbig et al. studied the prognosis of DN with the use of the CKD273-classifier in a longitudinal study of normoalbuminuric diabetic individuals (15). The authors were able to predict progression to macroalbuminuria over 5 years with an AUC of 0.93 superior to baseline albuminuria (AUC=0.67). Furthermore, the CKD273-classifier identified the progressors in 65% of the case subjects earlier than urinary albumin, the standard clinical test. On average the CKD273-classifier detected progression 1.5 years earlier than microalbuminuria. In a similar study with a follow-up over 3 years, Roscioni et al. evaluated the prediction of the transition from normo- to microalbuminuria and from micro- to macroalbuminuria (16). In a cohort of 44 patients with type 2 diabetes, the CKD273-classifier allowed assessment of early renal risk in diabetic patients. Recently, the CKD273-classifier was also validated in a cohort of 18 patients with CKD stage 4-5 (of whom six had hypertensive nephropathy) and 17 healthy controls, in order to compare the prevalence of CKD and progression beyond albuminuria (17)*. Special intention was paid to the diagnosis of hypertensive nephropathy, because it is surprisingly understudied, and proteomic analyses has never been performed (32). The results showed that the classifier performed equally well in patients with hypertensive nephropathy as in patients with other CKD causes.
A further validation of the classifier was obtained in assessing treatment. Using the CKD273-classifier, Andersen et al. analysed the effects of Irbesartan, an angiotensin receptor blocker, in a cohort of patients with type 2 diabetes with microalbuminuria (33). The scores of the classifier changed significantly after treatment with Irbesartan to values of more healthy individuals. Furthermore, the authors identified several peptides that showed significant change after 2 years with Irbesartan treatment in contrast to placebo. After Irbesartan treatment, an increase of collagen fragments showed the most significant association with DN. These results have led to the initiation of the PRIORITY study (34)* in order to evaluate the value of urinary proteomics in patient stratification for the presence of early signs of DN in an interventional trial. In this context, 165 type 2 diabetes patients were analysed with the CKD273-classifier assessing the benefits of stratifying patients for intervention with urinary proteomics (18).
Recent validation studies used much larger cohorts of individuals with different stages of CKD for the assessment of the prognostic potential of the CKD273-classifier: In a large cross-sectional multicenter cohort of 1990 individuals, including 522 with follow-up data, Schanstra et al. correlated the CKD273-classifier score with the estimated glomerular filtration rate (eGFR) and its decline (19)**. They validated that the CKD273-classifier performed significantly better in detecting and predicting progression of CKD than the current clinical standard, urinary albumin. The classifier was also more sensitive for identifying patients with rapidly progressing CKD. Compared with the combination of baseline eGFR and albuminuria (AUC=0.76), the addition of the CKD273-classifier significantly improved CKD risk prediction (AUC=0.83). In addition, when applying the Oxford Evidence-Based Medicine (EBM) and Strength of Recommendation Taxonomy (SORT) guidelines, additional evidence was obtained which supports the CKD273-classifier’s value in predicting CKD progression (35). In a recent study, the classifier and urinary albumin excretion was compared to predict the progression of CKD, involving 2672 patients at different CKD stages. The findings confirmed that the CKD273-classifier has a better performance at early stages of disease. On the other hand urinary albumin was a better predictor for progression at a late stage. In moderately advanced disease, these two tests had similar predictive abilities (20)**.
In spite of its invasive nature and risks, kidney biopsy is currently required for precise diagnosis of many chronic kidney diseases (CKDs). Therefore, specific biomarkers for different types of CKD, such as ANCA-associated vasculitis, IgAN, and DN, were defined using urinary proteome analysis. The value of CE-MS-based proteomics to differentiate types of CKD etiologies was demonstrated, but mostly in small patient populations (36–39). A recent study from Siwy et al. (21)** was designed with the aim of identifying specific urinary peptide markers for main types of CKD using datasets from a large cohort of 1180 individuals with CKD. For seven different types of CKD (primary focal segmental glomerulosclerosis, IgA nephropathy, minimal-change disease, membranous nephropathy, diabetic and hypertensive nephropathy, lupus nephritis, and vasculitis-induced kidney disease), several potential urinary biomarker peptides (ranging from 116 to 619 peptides, see Table 1) were defined and combined into classifiers specific for each CKD. These classifiers were validated in an independent cohort and showed good to excellent accuracy for discrimination of one CKD etiology from the other (AUCs ranged from 0.77 to 0.95).
Polycystic kidney disease
The most common genetic disorder in kidney disease (1:400 and 1:1000 individuals) is autosomal dominant polycystic kidney disease (ADPKD), a mutation in PKD1 (85% of cases) and PKD2 (15% of cases), results in cyst formation and loss of renal function (40;41). Kistler et al. (22) performed CE-MS analysis to identify peptide markers for ADPKD. Urine samples from 41 ADPKD patients and 189 healthy controls were analyzed leading to the identification of 142 consistent peptide biomarkers associated with ADPKD. Combined in a panel, these markers classified an independent validation cohort of 251 ADPKD patients from five different centres and 86 healthy controls with 84.5% of sensitivity and 94.2% of specificity (22). As in the CKD273 classifier, most of the urinary biomarkers were fragments of collagen, which may indicate changes in extracellular matrix during cyst formation. In a recent study involving 221 ADPKD patients aged 15-46 years and followed-up for 10-13 years, urinary proteome analysis using CE-MS generated a 20 peptide-based classifier allowing prediction of progression risk in ADPKD patients to ESRD (23)**. Prediction of the proteases involved in generation of these peptides, was performed suggesting modification of matrix metalloproteinases and cathepsin activity in ADPKD patients progressing to ESRD.
Fabry induced kidney disease
Fabry disease is a rare X-linked lysosomal inherited disorder characterized by deficient enzymatic activity of α-galactosidase A (GLA) (42), which can cause a wide range of systemic symptoms (like pain, kidney involvement, and cardiac manifestations). Fabry induced kidney disease (FIKD) is a glomerular disease, which was investigated by Kistler et al. (24). Urine samples from 35 treatment-naive female Fabry patients and 89 age-matched healthy controls were collected and analysed by CE-MS. A classifier was established based on 64 urinary peptides for FIKD diagnosis, with high specificity (97.8%) and sensitivity (88.2%) in an independent cohort composed by 17 treatment-naive Fabry patients and 45 controls. The most common of the sequenced peptides were collagen and uromodulin fragments. Interestingly, the up-regulated collagen fragments exhibited one of two characteristic C-terminal motivs, PPG or PGP. Furthermore, the uromodulin fragments (all were C-terminal fragments) were also up-regulated as in another recent urinary proteomic study of Fabry disease (43).
Congenital anomalies of the kidney and urinary tract
Congenital anomalies of the kidney and urinary tract (CAKUT) constitute approximately 20-30% of all anomalies identified in the prenatal period (44). Defects can be bilateral or unilateral, and different defects often coexist. Because CAKUT plays a causative role in 30-50% of cases of ESRD in children (45), it is important to diagnose these anomalies and initiate therapy to minimize renal damage, preventing or delaying the onset of ESRD, and providing supportive care to avoid complications of ESRD. CAKUT manifest as structural abnormalities of the kidney, including obstructive uropathy, renal dysplasia and urinary tract malformations (46). CAKUT displays an extensive spectrum of prenatal and postnatal outcomes alternating from death in utero to normal postnatal renal function (47).
Posterior urethral valves (PUV), the prototypic bilateral CAKUT is an obstructing membrane in the posterior male urethra. Klein et al. (25) studied the fetal urinary peptidome with CE-MS in a cohort of 28 patients with PUV. They identified 26 fetal urinary peptides associated with fetuses with PUV displaying early ESRD. Twelve of these peptides were combined in a model (12PUV-classifier). This classifier allowed correct prediction of postnatal renal function with 88% sensitivity and 95% specificity, in an independent blinded cohort of 38 PUV patients. Collagen fragments were the main constituents of the classifier, and in contrast to the collagen fragments associated with CKD in postnatal urine in adults, the abundance of collagen fragments, was increased in fetal urine of patients with PUV displaying severe ESRD (47).
Ureteropelvic junction obstruction (UPJO) is a frequent cause of congenital obstructive nephropathy and is characterized by a stenosis between the ureter and the kidney, inducing accumulation of urine in renal pelvis and calyces, called hydronephrosis (48;49). In severe cases this condition is treated surgically. However, in the milder UPJO cases (often) invasive surveillance is necessary during the first years of life to determine whether surgery is necessary. Urinary proteome analysis was employed by Decramer et al. in 2006 to determine the presence of urinary markers that could predict the progression of UPJO at an early stage (26). They identified 51 markers that combined in a classifier predicting progression of UPJO with the need of surgical intervention several months in advance. Drube et al. (27) validated this classifier in an independent study with 27 pediatric patients. In 19 children <1 year old, the model for UPJO showed 83% sensitivity and 92% specificity. On the other hand, in older patients, the analysis yielded a sensitivity and specificity of 20% and 66%, respectively. This suggests that classifiers should be validated within their context of use, in this case detection of severe UPJO before the age of 1 year. Bandin et al. (50) used urinary proteome analysis to suggest that early surgery in UPJO might be beneficial compared to classical conservative clinical surveillance of the disease. At 5-year follow-up urinary proteomes were similar between patients with early surgical correction of UPJO and age matched controls. In contrast, urinary proteomes differed significantly between conservatively followed patients and controls. Analyses of the proteome differences suggested ongoing renal or ureteral remodeling in the conservatively followed patients that was not clinically visible.
High-grade vesicoureteral reflux (VUR) is described by an abnormal condition of flow of the urine from the bladder to the kidney during micturition. This condition is a risk factor for impaired renal function, renal scarring and arterial hypertension, although is not a frequent condition in children. Current diagnosis requires an invasive and highly uncomfortable method - voiding cystourethrography (VCUG). Drube et al. studied a cohort of 73 children with the use of CE-MS analysis (28). In this case-control study, a VUR-classifier was established in 18 patients with primary VUR grade IV or V, distinguishing these from 19 patients without VUR. This VUR-classifier was independently validated in a blinded cohort of 17 patients with VUR grade IV or V and 19 patients without VUR with a sensitivity of 88% and a specificity of 79%. Five of the urinary peptides of the classifier were sequenced. Three were fragments of collagen alpha-1 (I) chain and the other two were fragments of sodium/ potassium-transporting ATPase and of CD99 antigen.
Conclusion
Urinary proteomics using CE-MS has identified a number of peptide classifiers that can become an important alternative for diagnosis and prognosis of kidney disease compared to the current, often poorly performing, invasive clinical tests. Several urinary biomarkers were identified including fragments from different collagens, serum albumin, alpha-1-antitrypsin and uromodulin which could also provide more information regarding the patho-physiology of kidney disease and ongoing disease progression. In the near future these different biomarker classifiers could be applied in clinical trials as well as in clinical practice, with the aim of improving the benefits for the patients with respect to early intervention or stratification.
Key points.
The urinary proteomic classifier, CKD273, was validated in several independent studies to be significantly associated with chronic kidney disease (CKD) and to enable detection of CKD at very early stages of the disease superior to albuminuria.
First multicentre interventional trial with the use of urinary proteomic biomarkers is used to target a preventive and therapeutic approach in clinical practice (stratified medicine).
Differential diagnosis of CKD subtypes with the use of proteome analysis, in contrast to biopsy, offers the possibility of being applied early in the course of the disease when the benefit of intervention is optimal and of being repeated without any risk for the patient.
Urinary proteome analysis can also be applied for the diagnosis and prognosis of other kidney diseases, like autosomal dominant polycystic kidney disease or ureteropelvic junction obstruction.
Acknowledgment
H.M. and P.Z. were supported in part by the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 608332 (iMODECKD), grant agreement 305608 (EURenOmics), and grant agreement 603288 (SysVasc). Furthermore, P.M. received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement no. 642937 (RENALTRACT).
Footnotes
Conflict of interest
H.M. is cofounder and a shareholder of mosaiques diagnostics GmbH. P.M. and P.Z. are employees of mosaiques diagnostics GmbH.
References
* of special interest:
** of outstanding interest:
- (1).Donato V, Lacquaniti A, Cernaro V, et al. From water to aquaretics: a legendary route. Cell Physiol Biochem. 2014;33(5):1369–88. doi: 10.1159/000358704. [DOI] [PubMed] [Google Scholar]
- (2).Rodriguez-Suarez E, Siwy J, Zurbig P, et al. Urine as a source for clinical proteome analysis: From discovery to clinical application. Biochim Biophys Acta. 2013 Jul 2; doi: 10.1016/j.bbapap.2013.06.016. [DOI] [PubMed] [Google Scholar]
- (3).Mischak H, Delles C, Vlahou A, et al. Proteomic biomarkers in kidney disease: issues in development and implementation. Nat Rev Nephrol. 2015 Apr;11(4):221–32. doi: 10.1038/nrneph.2014.247. [* This Review describes the current status of proteomic and protein biomarkers in the context of kidney diseases and also includes an overview of protein-based biomarker candidates that are undergoing development for use in nephrology, focusing on those with the greatest potential for clinical implementation. It is an excellent guidance for the design of future proteomic studies.] [DOI] [PubMed] [Google Scholar]
- (4).Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003 Mar 13;422(6928):198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- (5).Decramer S, Gonzalez de PA, Breuil B, et al. Urine in clinical proteomics. Mol Cell Proteomics. 2008 Oct;7(10):1850–62. doi: 10.1074/mcp.R800001-MCP200. [DOI] [PubMed] [Google Scholar]
- (6).Fliser D, Novak J, Thongboonkerd V, et al. Advances in urinary proteome analysis and biomarker discovery. J Am Soc Nephrol. 2007;18(4):1057–71. doi: 10.1681/ASN.2006090956. [DOI] [PubMed] [Google Scholar]
- (7).Kolch W, Mischak H, Pitt AR. The molecular make-up of a tumour: proteomics in cancer research. Clin Sci (Lond) 2005 May;108(5):369–83. doi: 10.1042/CS20050006. [DOI] [PubMed] [Google Scholar]
- (8).Mischak H, Apweiler R, Banks RE, et al. Clinical proteomics: a need to define the field and to begin to set adequate standards. Proteomics Clin Appl. 2007;1:148–56. doi: 10.1002/prca.200600771. [DOI] [PubMed] [Google Scholar]
- (9).Mischak H, Allmaier G, Apweiler R, et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med. 2010 Aug 25;2(46):46ps42. doi: 10.1126/scitranslmed.3001249. [DOI] [PubMed] [Google Scholar]
- (10).Mischak H, Vlahou A, Righetti PG, et al. Putting value in biomarker research and reporting. J Proteomics. 2014 Jan 16;96:A1–A3. doi: 10.1016/j.jprot.2013.12.002. [DOI] [PubMed] [Google Scholar]
- (11).Good DM, Zürbig P, Argiles A, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010 Nov;9(11):2424–37. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Molin L, Seraglia R, Lapolla A, et al. A comparison between MALDI-MS and CE-MS data for biomarker assessment in chronic kidney diseases. J Proteomics. 2012 Oct 22;75(18):5888–97. doi: 10.1016/j.jprot.2012.07.024. [DOI] [PubMed] [Google Scholar]
- (13).Argiles A, Siwy J, Duranton F, et al. CKD273, a New Proteomics Classifier Assessing CKD and Its Prognosis. PLoS One. 2013;8(5):e62837. doi: 10.1371/journal.pone.0062837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Gu YM, Thijs L, Liu YP, et al. The urinary proteome as correlate and predictor of renal function in a population study. Nephrol Dial Transplant. 2014 Jun 30; doi: 10.1093/ndt/gfu234. [DOI] [PubMed] [Google Scholar]
- (15).Zürbig P, Jerums G, Hovind P, et al. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes. 2012 Aug 7;61(12):3304–13. doi: 10.2337/db12-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Roscioni SS, de ZD, Hellemons ME, et al. A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia. 2012 Oct 20;56(2):259–67. doi: 10.1007/s00125-012-2755-2. [DOI] [PubMed] [Google Scholar]
- (17).Ovrehus MA, Zurbig P, Vikse BE, et al. Urinary proteomics in chronic kidney disease: diagnosis and risk of progression beyond albuminuria. Clin Proteomics. 2015;12(1):21. doi: 10.1186/s12014-015-9092-7. [* Beside the general validation of the CKD273-classifier, this study also pointed out the utilisation of the classifier especially in patients with hypertensive nephropathy for the diagnosis and prognosis of CKD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Siwy J, Schanstra JP, Argiles A, et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transplant. 2014 Mar 2;29(8):1563–70. doi: 10.1093/ndt/gfu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Schanstra JP, Zurbig P, Alkhalaf A, et al. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol. 2015 Jan 14;26:1999–2010. doi: 10.1681/ASN.2014050423. [** The study of a large cross-sectional multicenter cohort of 1990 individuals validated that CKD273-classifier performed significantly better in detecting and predicting progression of CKD than urinary albumin. The classifier was also more sensitive for identifying patients with rapidly progressing CKD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Pontillo C, Jacobs L, Staessen J, et al. A urinary proteome-based classifier for the early detection of decline in glomerular filtration. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw239. in press. [** This study is a multicentre, large-scale validation of the CKD273-classifier. Here, the prognostic ability of the classifier versus albuminuria was tested at different disease stages. The results suggest that the CKD273-classifier allow the detection of progressive disease at early CKD stages, at which therapeutic intervention is likely more effective.] [DOI] [PubMed] [Google Scholar]
- (21).Siwy J, Zürbig P, Argiles A, et al. Non-invasive diagnosis of chronic kidney diseases using urinary proteome analysis. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw337. in press. [** In this study urinary proteome analysis clearly differentiated various types of CKD. In contrast to kidney biopsy, this offers the possibility of being applied early in the course of the disease when the benefit of intervention is optimal and of being repeated without any risk for the patient and, thus, can be used to monitor treatment response.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kistler AD, Serra AL, Siwy J, et al. Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS One. 2013;8(1):e53016. doi: 10.1371/journal.pone.0053016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Pejchinovski M, Siwy J, Metzger J, et al. Urine peptidome analysis predicts risk for end stage renal disease and reveals proteolytic pathways involved in autosomal dominant polycystic kidney disease progression. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw243. in press. [** In this study, a classifier was developed based on urinary proteome analysis that predicts accurately relevant clinical outcomes in ADPKD patients, especially in young patients.] [DOI] [PubMed] [Google Scholar]
- (24).Kistler AD, Siwy J, Breunig F, et al. A distinct urinary biomarker pattern characteristic of female Fabry patients that mirrors response to enzyme replacement therapy. PLoS ONE. 2011;6(6):e20534. doi: 10.1371/journal.pone.0020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Klein J, Lacroix C, Caubet C, et al. Fetal urinary peptides to predict postnatal outcome of renal disease in fetuses with posterior urethral valves (PUV) Sci Transl Med. 2013 Aug 14;5(198):198ra106. doi: 10.1126/scitranslmed.3005807. [DOI] [PubMed] [Google Scholar]
- (26).Decramer S, Wittke S, Mischak H, et al. Predicting the clinical outcome of congenital unilateral ureteropelvic junction obstruction in newborn by urinary proteome analysis. Nat Med. 2006 Mar 19;12(4):398–400. doi: 10.1038/nm1384. [DOI] [PubMed] [Google Scholar]
- (27).Drube J, Zurbig P, Schiffer E, et al. Urinary proteome analysis identifies infants but not older children requiring pyeloplasty. Pediatr Nephrol. 2010 Sep;25(9):1673–8. doi: 10.1007/s00467-010-1455-8. [DOI] [PubMed] [Google Scholar]
- (28).Drube J, Schiffer E, Lau E, et al. Urinary proteome analysis to exclude severe vesicoureteral reflux. Pediatrics. 2012 Feb;129(2):e356–e363. doi: 10.1542/peds.2010-3467. [DOI] [PubMed] [Google Scholar]
- (29).Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005 Jun;67(6):2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- (30).Alebiosu CO, Ayodele OE. The global burden of chronic kidney disease and the way forward. Ethn Dis. 2005;15(3):418–23. [PubMed] [Google Scholar]
- (31).Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009 Jan;55(1):24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- (32).Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011 Jan;57(1 Suppl 1):A8, e1–A8,526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- (33).Andersen S, Mischak H, Zürbig P, et al. Urinary proteome analysis enables assessment of renoprotective treatment in type 2 diabetic patients with microalbuminuria. BMC Nephrol. 2010 Nov 1;11(1):29. doi: 10.1186/1471-2369-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Lindhardt M, Persson F, Currie G, et al. Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention Of early diabetic nephRopathy in TYpe 2 diabetic patients with normoalbuminuria (PRIORITY): essential study design and rationale of a randomised clinical multicentre trial. BMJ Open. 2016;6(3):e010310. doi: 10.1136/bmjopen-2015-010310. [* This article describes the study design and background of the first prospective multicentre clinical trial (PRIORITY) with the aim to confirm performance of CKD273 and to test the ability of spironolactone to delay progression of early diabetic nephropathy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Critselis E, Heerspink HJ. Utility of the CKD273 peptide classifier in predicting chronic kidney disease progression: A systematic review of the current evidence. Nephrol Dial Transplant. 2014;31(2):249–54. doi: 10.1093/ndt/gfv062. [DOI] [PubMed] [Google Scholar]
- (36).Haubitz M, Wittke S, Weissinger EM, et al. Urine protein patterns can serve as diagnostic tools in patients with IgA nephropathy. Kidney Int. 2005 Jun;67(6):2313–20. doi: 10.1111/j.1523-1755.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- (37).Rossing K, Mischak H, Dakna M, et al. Urinary proteomics in diabetes and CKD. J Am Soc Nephrol. 2008 Apr 30;19(7):1283–90. doi: 10.1681/ASN.2007091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Weissinger EM, Wittke S, Kaiser T, et al. Proteomic patterns established with capillary electrophoresis and mass spectrometry for diagnostic purposes. Kidney Int. 2004 Jun;65(6):2426–34. doi: 10.1111/j.1523-1755.2004.00659.x. [DOI] [PubMed] [Google Scholar]
- (39).Julian BA, Wittke S, Novak J, et al. Electrophoretic methods for analysis of urinary polypeptides in IgA-associated renal diseases. Electrophoresis. 2007 Dec;28(23):4469–83. doi: 10.1002/elps.200700237. [DOI] [PubMed] [Google Scholar]
- (40).Chang MY, Ong AC. New treatments for autosomal dominant polycystic kidney disease. Br J Clin Pharmacol. 2013 Oct;76(4):524–35. doi: 10.1111/bcp.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010 Apr;6(4):197–206. doi: 10.1038/nrneph.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Waldek S, Feriozzi S. Fabry nephropathy: a review - how can we optimize the management of Fabry nephropathy? BMC Nephrol. 2014;15:72. doi: 10.1186/1471-2369-15-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Matafora V, Cuccurullo M, Beneduci A, et al. Early markers of Fabry disease revealed by proteomics. Mol Biosyst. 2015 Jun;11(6):1543–51. doi: 10.1039/c4mb00707g. [DOI] [PubMed] [Google Scholar]
- (44).Queisser-Luft A, Stolz G, Wiesel A, et al. Malformations in newborn: results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990-1998) Arch Gynecol Obstet. 2002 Jul;266(3):163–7. doi: 10.1007/s00404-001-0265-4. [DOI] [PubMed] [Google Scholar]
- (45).Seikaly MG, Ho PL, Emmett L, et al. Chronic renal insufficiency in children: the 2001 Annual Report of the NAPRTCS. Pediatr Nephrol. 2003 Aug;18(8):796–804. doi: 10.1007/s00467-003-1158-5. [DOI] [PubMed] [Google Scholar]
- (46).Holmes N, Harrison MR, Baskin LS. Fetal surgery for posterior urethral valves: long-term postnatal outcomes. Pediatrics. 2001 Jul;108(1):E7. doi: 10.1542/peds.108.1.e7. [DOI] [PubMed] [Google Scholar]
- (47).Klein J, Buffin-Meyer B, Mullen W, et al. Clinical proteomics in obstetrics and neonatology. Expert Rev Proteomics. 2014 Feb;11(1):75–89. doi: 10.1586/14789450.2014.872564. [DOI] [PubMed] [Google Scholar]
- (48).Caubet C, Lacroix C, Decramer S, et al. Advances in urinary proteome analysis and biomarker discovery in pediatric renal disease. Pediatr Nephrol. 2010 Jan;25(1):27–35. doi: 10.1007/s00467-009-1251-5. [DOI] [PubMed] [Google Scholar]
- (49).Pejchinovski M, Hrnjez D, Ramirez-Torres A, et al. Capillary zone electrophoresis online coupled to mass spectrometry: A perspective application for clinical proteomics. Proteomics Clin Appl. 2015 Jun;9(5–6):453–68. doi: 10.1002/prca.201400113. [DOI] [PubMed] [Google Scholar]
- (50).Bandin F, Siwy J, Breuil B, et al. Urinary Proteome Analysis at 5-Year Followup of Patients With Nonoperated Ureteropelvic Junction Obstruction Suggests Ongoing Kidney Remodeling. J Urol. 2012 Jan 18;187(3):1006–11. doi: 10.1016/j.juro.2011.10.169. [DOI] [PubMed] [Google Scholar]