In February 2019, the Population Approach Group of Australia and New Zealand (PAGANZ) celebrated 20 years of providing education, mentoring, and support to the Australasian pharmacometrics community. This article provides insight into the history of this group and its operations to the present date.
The History of PAGANZ
In the 1980s, Nick Holford (Department of Pharmacology and Clinical Pharmacology, University of Auckland) presented pharmacokinetic (PK) and pharmacodynamic (PD) analyses using the “population” or “mixed effects” modeling approach at the Australasian Society of Clinical and Experimental Pharmacologists (ASCEP) meetings (in 1990, ASCEP became the Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT)). Few, if any, in the audience understood this approach, but interest grew in this methodology, with the potential to improve clinical pharmacology understanding and pharmacotherapeutics in patients in whom blood sampling for traditional PK studies was impractical.
Nick Holford agreed to run a course in population PK modeling using NONMEM, and in 1993 a meeting was held in Auckland, New Zealand. Six participants from Australia and a few Auckland researchers attended. The following topics were taught: Nonlinear mixed effects analysis; differences between naive‐pooled, two‐stage, and mixed‐effects approaches to data analysis; conceptualization of variability and its analysis; techniques for screening influential covariates; data file and NM‐TRAN control stream structures; model diagnostics; and the hands‐on running of NONMEM. At the conclusion of the course, attendees were offered the use of a computer system in Auckland until the centers established their own computing facilities.
Between 1993 and 1998, there were developments that significantly accelerated pharmacometric activity in Australia and New Zealand. Several emerging leaders took up positions in Australasian universities and hospitals following doctoral or postdoctoral training overseas and brought with them a wealth of experience in this area.
Australian health regulators also began showing interest. A Drug Information Association–sponsored meeting was convened in Canberra in 1997 at the request of the Australian Drug Evaluation Committee to discuss the role of population PKPD modeling in drug evaluation and development,1 with a particular interest in the registration of drugs in children. It was at this meeting that the names Population Approach New Zealand Australia Society (PANZAS) or Population Approach Group of Australia and New Zealand (PAGANZ) were discussed. Naming the meeting turned out to be controversial; should it be PANZAS or PAGANZ (i.e., should New Zealand or Australia be mentioned first)? PAGANZ won the day due to the larger number of Australians.
By the late 1990s, it was apparent that the pharmacometric community would benefit from having a local association. Major incentives were the tyranny of distance and cost associated with regularly attending northern hemisphere meetings. Local pharmacometricians grasped the initiative to establish PAGANZ. Meetings were held annually in an Australian or New Zealand city. In 1998 PAGANZ was born, with the witty acronym raising a few eyebrows elsewhere.
The First Meeting
The inaugural PAGANZ meeting was held in January 1999 at the Department of Pharmacology and Clinical Pharmacology, The University of Auckland. The format comprised two sections: a practical training module Population Analysis Workshop (PAWS) followed by the PAGANZ scientific program (see https://www.paganz.org/). In the early years, the meetings were run on “the smell of an oily rag,” with registration fees kept to a bare minimum. Most universities did not charge for the use of their facilities, which assisted greatly. Several national and international pharmacometric luminaries generously gave their time, often on several occasions. At the inaugural meeting, Dr Janet Wade presented a novel methodology for combined PKPD analysis, Dr Mats Karlsson presented on automated covariate models, Dr Nick Holford presented on disease progression modeling, Dr Diane Mould talked about problems in population PKPD modeling, and Dr Brian Anderson presented on paediatric pain management and paracetamol.
The Middle Years to Now
As attendance increased and the depth of knowledge of attendees grew, PAWS was split into beginners and an intermediate group. These have been running since 1999 and now have 1.5 days of content (Table 1 ). The “intermediate” PAWS was deliberately named as such, as Stuart Beal from University of California, San Francisco UCSF once said that nobody was smart enough to do an advanced NONMEM course. Increasing numbers of research students were encouraged to attend as well as clinicians, hospital scientists, and regulatory and industry personnel.
Table 1.
Intermediate PAWS workshop topics from 1999–2019
| Year | Workshop title | Workshop description |
|---|---|---|
| 2019 | PAWS at the joint ASCEPT‐PAGANZ 2019 meeting | Nlmixr workshop |
| TMDD | Target‐mediated drug disposition model, modeling, and applications in drug development | |
| CellML | Model definition using the mark‐up language CellML | |
| OpenCOR | Framework for simulation using CellML | |
| 2018 | STAN | Model estimation and Bayesian inference using Stan |
| DDMoRe | Overview of the Drug Disease Model Resources (DDMoRe) project | |
| 2017 | R and Shiny | Model building and evaluation using R and Shiny apps |
| Receptor theory | Introduction to multistate receptor theory and biased ligands | |
| 2016 | No PAGANZ meeting | PAGANZ Inc. hosted WCoP2016 (www.wcop2016.org) |
| 2015 | PBPK | Physiologically‐based pharmacokinetic model definition, estimation, and evaluation using Berkeley Madonna |
| TTE | Time‐to‐event model development and evaluation | |
| 2014 | Phoenix | Model definition, estimation, and evaluation using Phoenix NLME |
| QT interval | Population PKPD modelling of the QT interval using Phoenix NLME and NM‐TRAN | |
| Lifespan models | An introduction to lifespan‐based indirect drug response models | |
| 2013 | Optimal design | Application of population optimal design in drug development, preclinical and clinical trials |
| Categorical data | Development and evaluation of population models of categorical data | |
| 2012 | Transformation | Transformation of parameter and residual error distributions |
| Trial Simulator | Clinical trial and adaptive simulation | |
| Anti‐infectives | Population PKPD modelling of antibiotics including bacterial growth, resistance, and killing | |
| 2011 | Missing data | Modeling of left‐censored PK data and missing covariates |
| Biological systems | Large‐system simulation using the CellML mark‐up language | |
| Optimal design | Model discrimination using optimal design and introduction to DT optimality | |
| 2010 | VPC | The use and interpretation of visual predictive check plots in model evaluation |
| Monolix | Population PKPD modelling using Monolix and NONMEM | |
| 2009 | WinBUGS | Introduction to Bayesian statistics and WinBUGS |
| TTE | Time‐to‐event model development and evaluation using NONMEM and WinBUGS | |
| 2008 | Model Diagnostics | Empirical Bayes estimates, shrinkage, and residual error using PsN and Xpose for model diagnosis |
| Nonparametric | Semiparametric and nonparametric estimation in NONMEM VI | |
| 2007 | Monolix | An introduction to population PKPD modelling using Monolix |
| TTE | Time‐to‐event model development and evaluation | |
| 2006 | Discrete Data | Modelling non‐continuous data in NONMEM |
| Pediatrics | PKPD model development in pediatrics | |
| Optimal Design | Optimal design in drug development and postmarketing clinical trials | |
| 2005 | PD | Introduction to immediate effect models, effect compartment, and turnover models |
| PKPD | Simultaneous vs. sequential PKPD modelling | |
| 2004 | NONMEM | The use of “advanced” features of NONMEM including OMEGA blocks and baseline effect parameter |
| 2003 | NONMEM | The use of “advanced” features of NONMEM including logistic regression, differential equations, and bootstrapping |
| 2002 | PAGANZA | Meeting in South Africa |
| 2002 | CTS | Introduction to clinical trial simulation including design and simulation plans |
| Pharmacoeconomic models | Introduction to pharmacoeconomic models | |
| 2001 | Bayesian statistics | Introduction to Bayesian statistics and PKBUGS. Also, FOCE, EBEs, and the PRIOR subroutine in NONMEM |
| 2000 | PK and PKPD | Introduction to population PK and PKPD model development |
| 1999 | No PAWS at the first meeting | – |
ASCEPT, Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists; CTS, clinical trial design; DT, optimality criteria; EBEs, empirical Bayes estimates; FOCE, first order conditional estimation; NLME, nonlinear mixed effects; PAWS, Population Analysis Workshop; PBPK, physiologically‐based pharmacokinetic; PD, pharmacodynamic; PK, pharmacokinetic; TMDD, target‐mediated drug disposition; VPC, visual predictive checks; WCoP2016, World Conference of Pharmacometrics 2016.
Warfarin PKPD data, described in two publications by O'Reilly et al.2, 3 in the 1960s, were introduced to the intermediate PAWS at the 2005 Brisbane meeting and remains the mainstay example for teaching. The PAWS for beginners is often used as introductory training for postgraduate students. Recently, an introduction to R has been included, thus exposing the next generation to an important tool for reproducible research.
A global outreach with other population approach organizations started with joint meetings of PAGANZ with a group in South Africa (PAGANZA 2002) and Japan (PAGANZ‐PAGJA 2006). PAGANZ was hosted in 2007 at The National University of Singapore. Population Approach Group India (PAGIN) started in 2008 with support of PAGANZ members, and the International Society of Pharmacometrics (ISoP), a sponsor of the PAGANZ meetings since 2012. In total, PAGANZ has attracted 456 participants during the past 10 years with a broad global reach (Figure 1 a).
Figure 1.
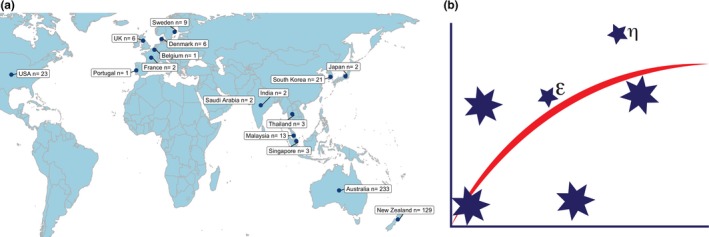
Population Approach Group of Australia and New Zealand (PAGANZ) over time. The (a) number of participants at PAGANZ meetings by country of origin during the last 10 years and (b) the PAGANZ logo.
PAGANZ hosted the Second World Conference of Pharmacometrics (WCoP) in Brisbane in 2016, and PAGANZ members represented the Oceania region on the WCoP executive committee. To promote pharmacometrics to the wider scientific community and as an acknowledgement of past support from ASCEPT, a joint ASCEPT‐PAGANZ meeting will be held in November 2019.
PAGANZ members have supported the pharmacometrics community through the development of tools and center of research, including MKmodel, Wings for NONMEM, SeBaGEN, WinPOPT, TCIWorks, NextDose, The Australian Centre for Paediatric Pharmacokinetic, and The Australian Centre for Pharmacometrics.
PAGANZ Structure and Culture
PAGANZ Inc. was established as an incorporated society in 2012, thus allowing PAGANZ to operate as a legal entity. Nick Holford was the inaugural president. Annual general meetings are held at each PAGANZ meeting. A logo (Figure 1 b) was created based on the Southern Cross constellation, recognizing the geographic location of the society. A rectangular hyperbola similar to an Emax PD model is superimposed on the constellation. Two smaller actual stars are named eta (η) and epsilon (ϵ), each having a specific meaning within the modeling and the population approach (ϵ, distance from line is an estimate of residual error; η, separation indicates between people (or star) variability). Membership of the society is free upon registration via the PAGANZ website (https://www.paganz.org/join).
The primary goal of PAGANZ is to encourage the use and development of pharmacometrics in Australia and New Zealand. The culture is informal, supportive, and diverse. All society members have the opportunity to present their work and gain feedback. Although some presentations are works in progress, others are world‐class contributions. In 2012 and in collaboration with ISoP, PAGANZ established the ISoP lecture and two student prizes. The Nick Holford Prize honors students who excel in clinical pharmacology research that applies pharmacometric methods to improve human health and includes an oral presentation at the following ASCEPT conference. The ISoP Student Prize for Pharmacometrics honors students who excel in their research using pharmacometric methods and includes an ISoP membership. PAGANZ subsidizes the attendance of presenting students. PAGANZ has a formalized policy on student support, with establishment of a formal co‐opting process of members into the executive to facilitate the development of early career researchers. A student careers evening in association with the PAGANZ meeting was initiated in 2017 as a direct result of student interest.
Over the years, numerous pharmacometrics groups have organized and hosted the meeting, shared their knowledge, and contributed hours of dedicated effort on preparing material and teaching the PAWS participants as all of them care deeply about the society and the supportive community.
In 2018, PAGANZ received not‐for‐profit status after being recognized by the New Zealand Royal Society Te Aparangi as being primarily established to promote and encourage scientific research.
Twenty Years Later
In February 2019, PAGANZ celebrated its 20‐year anniversary at the Grafton (Medical School) Campus of The University of Auckland, the same location as the inaugural meeting. The intermediate workshop was on target‐mediated drug disposition using monoclonal antibodies as case studies for nonlinear PK, followed by a workshop on the CellML language used with the OpenCOR interface for quantitative systems pharmacology modeling, building on a previous PAGANZ workshop some 8 years previously. The scientific program saw the return of most of the original 1999 meeting speakers and provided an opportunity to reflect on progress made during the past two decades. It was demonstrated that the question of how best to analyze combined PKPD data remains relevant today, particularly where the changing state of patients and their disease is considered. An update on covariate modeling methodologies was given as well as an update on pain medication dosing in children.
The 54 registered attendees at the 20th meeting comprised academics and industry and health professionals, including 23 students and 25 of the 71 PAGANZ society members. In 2019, the society financially supported the attendance of 17 students (30% of the conference attendees) through free registrations for both workshops and scientific programs.
PAGANZ now and in the future
Over the years, PAGANZ has been supported by many individuals, too numerous to name, as well as organizations such as ISoP, ASCEPT, ICON, Certara, ModelAnswers, PKPDRX Ltd., and local host universities. Without the efforts of so many individuals over the years, PAGANZ could have not grown to what it is today, and they all have our deep gratitude. Going forward, PAGANZ will maintain its commitment to encouraging the application and development of pharmacometrics in Australia and New Zealand.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
Acknowledgments
On behalf of the PAGANZ Inc. committee, the authors thank the participants, the speakers, the moderators, and the conference organizers and hosts as well as those who provided financial and in‐kind support to make PAGANZ meetings possible during the past 20 years. We welcome anyone interested in participating, promoting, and contributing to expand the pharmacometrics network in Australasia and Oceania. Stefanie Hennig was supported by a fellowship as senior researcher by the Alexander von Humboldt Foundation, Bonn, Germany.
This article reflects the opinion and historical recollection of the authors only. The corresponding author takes final responsibility for the submitted manuscript. The concept is based on a history of PAGANZ compiled originally by N.H. and B.G.C. (https://www.paganz.org/history/).
References
- 1. Tett, S.E. , Holford, N.H.G. & McLachlan, A.J. Population pharmacokinetics and pharmacodynamics: an underutilized resource. Drug Inf. J. 32, 693–710 (1998). [Google Scholar]
- 2. O'Reilly, R.A. & Aggeler, P.M. Studies on coumarin anticoagulant drugs. Initiation of warfarin therapy without a loading dose. Circulation 38, 169–177 (1968). [DOI] [PubMed] [Google Scholar]
- 3. O'Reilly, R.A. , Aggeler, P.M. & Leong, L.S. Studies of the coumarin anticoagulant drugs: the pharmacodynamics of warfarin in man. J. Clin. Invest. 42, 1542–1551 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]


