This report shows the whole-genome sequence of the multidrug-resistant Salmonella enterica subsp. enterica serovar Infantis strain FARPER-219. Antibiotic resistance genes are found mainly in the plasmid. Our findings show important genetic information that provides an understanding of the recent spread of this serotype in poultry.
ABSTRACT
This report shows the whole-genome sequence of the multidrug-resistant Salmonella enterica subsp. enterica serovar Infantis strain FARPER-219. Antibiotic resistance genes are found mainly in the plasmid. Our findings show important genetic information that provides an understanding of the recent spread of this serotype in poultry.
ANNOUNCEMENT
Salmonella enterica subsp. enterica serovar Infantis (S. Infantis) is an emerging serotype in poultry, reflected by an increased prevalence in poultry flocks, in broiler meat, and in human foodborne illness cases (1–4), producing a significant economic loss for the poultry industry (5). In several countries, this serotype has been reported to be associated with a high prevalence and antimicrobial resistance (6–9).
In Peru, similar data have been reported, showing that S. Infantis is the most prevalent serotype (91.43%) in chicken farms (10) and the third most frequently isolated strain from humans and food (11–13). However, there is limited information on full-genome sequences of S. Infantis, mainly isolated from poultry.
Here, we report the whole-genome sequence and the annotation of the FARPER-219 strain. It was isolated from a small farm in the southern region of Peru in 2017 from the liver and spleen of broiler chickens. The strain was identified as S. enterica using colony morphology and specific DNA PCR (14). Genomic DNA was extracted from bacterial culture (brain heart infusion [BHI] broth at 37°C overnight) using a phenol-chloroform protocol (15). Genome sequencing was performed with a 20-kb SMRTbell library (PacBio DNA/polymerase binding kit P6) on the PacBio RS II platform (PacBio DNA sequencing kit 4.0) using C4 chemistry with 8 single-molecule real-time (SMRT) cells (Macrogen, Inc., South Korea). Genome assembly was performed de novo using Hierarchical Genome Assembly Process (HGAP) version 3.0 (16) from the SMRT portal version 2.3, with default parameters, by Macrogen, Inc.
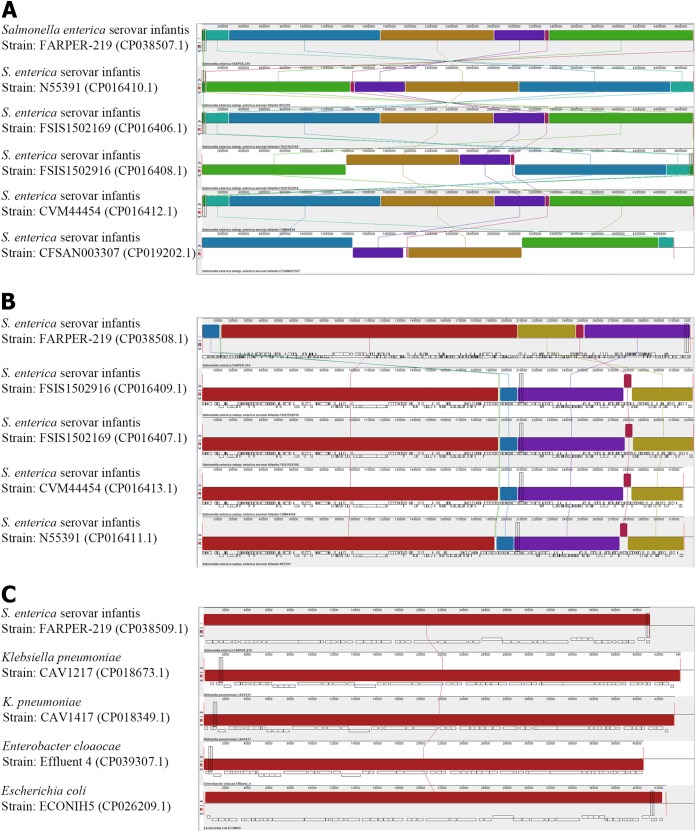
A total of 99,112 reads (average length, 7,478 bp; N50, 11,183 bp) were generated, and the assembled genome resulted in one closed circular chromosome of 4,727,696 bp (G+C content of 52.3%; coverage, 105×), including a circular plasmid of 320,892 bp (G+C content of 50.4%; coverage, 99×) and one linear contig of 41,193 bp (G+C content of 34.5%; coverage, 231×). The NCBI Prokaryotic Genome Automatic Annotation Pipeline version 4.7 (17) identified 4,717 genes, 4,598 coding DNA sequences (CDSs), and 119 RNA genes (22 rRNAs, 84 tRNAs, 1 transfer-messenger RNA [tmRNA], and 12 noncoding RNAs [ncRNAs]) on the chromosome; 369 genes, 368 CDSs, and 1 tRNA on the plasmid; and 55 genes and 55 CDSs on the linear contig. In order to corroborate the assemblies, we performed a multiple sequence alignment by Mauve version 20150226 (18) for synteny analysis (Fig. 1).
FIG 1.
Synteny between the FARPER-219 genome and similar genomes. Pairwise alignments were generated with progressiveMauve. Accession numbers are indicated on the left. Colored boxes indicate synteny regions that aligned to other genomes, and colored lines connect regions. (A) Chromosome; (B) plasmid; and (C) lineal contig.
The multilocus sequence typing (MLST) profile of the genome (sequence type 32 [ST-32]) and the plasmid (IncI1) was performed using MLST version 2.0 (19) and pMLST version 2.0 (20), respectively. Using ResFinder version 3.1 (21), a total of 9 genes associated with antimicrobial resistance were found in the plasmid, namely, aac(3)-IV, aph(4)-Ia, and aadA1, which confer aminoglycoside resistance; blaCTX-M-65, which confers beta-lactam resistance; fosA3, which confers fosfomycin resistance; floR, which confers phenicol resistance; sul1, which confers sulfonamide resistance; tet(A), which confers tetracycline resistance; and dfrA14, which confers trimethoprim resistance. Only one antimicrobial resistance gene was found in the chromosome, namely, aac(6′)-Iaa, which confers aminoglycoside resistance.
The similarity of the strain FARPER-219 to other strains was measured by BLASTn analysis of the 16S rRNA gene, and genomic average nucleotide identity (ANI) was calculated with ANI Calculator (22). FARPER-219 showed 99.96% ANI and 100% 16S pairwise identity with S. Infantis FSIS1502916 (GenBank accession number CP016408), isolated from ground chicken (United States) (23), suggesting a monophyletic lineage. BLASTn analysis of the linear contig indicated 97.66% identity and 92% coverage (nucleotide database) with two extrachromosomal segments of Klebsiella pneumoniae, namely, lineal (GenBank accession number CP018673) and circular (GenBank accession number CP018349) segments.
This study provides important genome information to facilitate molecular studies of S. Infantis in the poultry farms and to establish a basis on which to improve epidemiological surveillance of this important serovar. In addition, future studies are needed to clarify the presence of the extrachromosomal segment of Klebsiella pneumoniae in S. Infantis, as it had not been previously reported.
Data availability.
The genome sequence of FARPER-219 has been deposited in the GenBank database under accession numbers CP038507, CP038508, and CP038509. Raw data are available in BioSample accession number SAMN11252736 and SRA run number SRR8903487.
ACKNOWLEDGMENTS
We thank Elmer Delgado for his excellent technical assistance.
We declare no competing interests.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Pate M, Mičunovič J, Golob M, Vestby LK, Ocepek M. 2019. Salmonella Infantis in broiler flocks in Slovenia: the prevalence of multidrug resistant strains with high genetic homogeneity and low biofilm-forming ability. Biomed Res Int 2019:4981463–4981413. doi: 10.1155/2019/4981463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2018. Outbreak of multidrug-resistant Salmonella infections linked to raw chicken products. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/salmonella/infantis-10-18/index.html. [Google Scholar]
- 3.Antunes P, Mourão J, Campos J, Peixe L. 2016. Salmonellosis: the role of poultry meat. Clin Microbiol Infect 22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 4.European Food Safety Authority, European Centre for Disease Prevention and Control. 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J 13:4329. doi: 10.2903/j.efsa.2015.4329. [DOI] [Google Scholar]
- 5.Nógrády N, Tóth Á, Kostyák Á, Pászti J, Nagy B. 2007. Emergence of multidrug-resistant clones of Salmonella Infantis in broiler chickens and humans in Hungary. J Antimicrob Chemother 60:645–648. doi: 10.1093/jac/dkm249. [DOI] [PubMed] [Google Scholar]
- 6.Wilk T, Szabó M, Szmolka A, Kiss J, Olasz F, Nagy B. 2017. Genome sequences of Salmonella enterica subsp. enterica serovar Infantis strains from Hungary representing two peak incidence periods in three decades. Genome Announc 5:e01735-16. doi: 10.1128/genomeA.01735-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinueza-Burgos C, Cevallos M, Ron-Garrido L, Bertrand S, De Zutter L. 2016. Prevalence and diversity of Salmonella serotypes in Ecuadorian broilers at slaughter age. PLoS One 11:e0159567. doi: 10.1371/journal.pone.0159567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olasz F, Nagy T, Szabó M, Kiss J, Szmolka A, Barta E, van Tonder A, Thomson N, Barrow P, Nagy B. 2015. Genome sequences of three Salmonella enterica subsp. enterica serovar Infantis strains from healthy broiler chicks in Hungary and in the United Kingdom. Genome Announc 3:e01468-14. doi: 10.1128/genomeA.01468-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voss-Rech D, Vaz CSL, Alves L, Coldebella A, Leão JA, Rodrigues DP, Back A. 2015. A temporal study of Salmonella enterica serotypes from broiler farms in Brazil. Poult Sci 94:433–441. doi: 10.3382/ps/peu081. [DOI] [PubMed] [Google Scholar]
- 10.Valderrama W, Pastor J, Mantilla Salazar J, Ortiz M. 2014. Estudio de prevalencia de serotipos de Salmonella en granjas avícolas tecnificadas en el Perú. Servicio Nacional de Sanidad Agraria, La Molina, Peru. [Google Scholar]
- 11.Quino W, Hurtado CV, Escalante-Maldonado O, Flores-León D, Mestanza O, Vences-Rosales F, Zamudio ML, Gavilán RG. 2019. Multidrogorresistencia de Salmonella Infantis en Perú: un estudio mediante secuenciamiento de nueva generación. Rev Peru Med Exp Salud Publica 36:37–45. doi: 10.17843/rpmesp.2019.361.3934. [DOI] [PubMed] [Google Scholar]
- 12.Iriarte A, Giner-Lamia J, Silva C, Betancor L, Astocondor L, Cestero JJ, Ochoa T, García C, Puente JL, Chabalgoity JA, The Salmolber CYTED Network , García-del Portillo F. 2017. Draft genome sequence of Salmonella enterica subsp. enterica serovar Infantis strain SPE101, isolated from a chronic human infection. Genome Announc 5:e00679-17. doi: 10.1128/genomeA.00679-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamudio ML, Meza A, Bailón H, Martínez-Urtaza J, Campos J. 2011. Experiencias en la vigilancia epidemiológica de agentes patógenos transmitidos por alimentos a través de electroforesis en campo pulsado (PFGE) en el Perú. Rev Peru Med Exp Salud Publica 28:128–135. doi: 10.1590/S1726-46342011000100020. [DOI] [PubMed] [Google Scholar]
- 14.Rahn K, De Grandis SA, Clarke RC, McEwen SA, Galán JE, Ginocchio C, Curtiss R, Gyles CL. 1992. Amplification of an invA gene sequence of Salmonella Typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probes 6:271–279. doi: 10.1016/0890-8508(92)90002-F. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 16.Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 17.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. 2016. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res 44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darling ACE, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Pontén T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carattoli A, Zankari E, Garciá-Fernández A, Larsen MV, Lund O, Villa L, Aarestrup FM, Hasman H. 2014. In silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. 2017. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 23.Tate H, Folster JP, Hsu C-H, Chen J, Hoffmann M, Li C, Morales C, Tyson GH, Mukherjee S, Brown AC, Green A, Wilson W, Dessai U, Abbott J, Joseph L, Haro J, Ayers S, McDermott PF, Zhao S. 2017. Comparative analysis of extended-spectrum-β-lactamase CTX-M-65-producing Salmonella enterica serovar Infantis isolates from humans, food animals, and retail chickens in the United States. Antimicrob Agents Chemother 61:e00488-17. doi: 10.1128/AAC.00488-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genome sequence of FARPER-219 has been deposited in the GenBank database under accession numbers CP038507, CP038508, and CP038509. Raw data are available in BioSample accession number SAMN11252736 and SRA run number SRR8903487.