Abstract
Background
Tetrabromobisphenol A (TBBPA), one of the most widely used brominated flame-retardants, is a representative persistent organic pollutants group. Studies on TBBPA toxicity have been conducted using various target cells; however, few studies have investigated TBBPA toxicity in bone cells. Therefore, this study investigated the in vitro effects of TBBPA on osteoclasts, a cell type involved in bone metabolism.
Methods
RAW264.7 cells were cultured in medium containing 50 ng/mL receptor activator of nuclear factor kappa B ligand (RANKL) and varying concentrations of TBBPA. To evaluate the effects of TBBPA on the differentiation and function of osteoclasts, osteoclast-specific gene expression, tartrate-resistant acid phosphatase (TRAP) activity, bone resorbing activity, mitochondrial membrane potential (MMP) and mitochondrial superoxide were measured.
Results
The presence of 20 μM TBBPA significantly increased TRAP activity in RANKL-stimulated RAW264.7 cells, the bone resorbing activity of osteoclasts, and the gene expression of Akt2, nuclear factor of activated T-cells cytoplasmic 1, and chloride channel voltage-sensitive 7. However, TBBPA treatment caused no change in the expression of carbonic anhydrase II, cathepsin K, osteopetrosis-associated transmembrane protein 1, Src, extracellular signal-related kinase, GAB2, c-Fos, or matrix metalloproteinase 9. Furthermore, 20 μM TBBPA caused a significant decrease in MMP and a significant increase in mitochondrial superoxide production.
Conclusion
This study suggests that TBBPA promotes osteoclast differentiation and activity. The mechanism of TBBPA-stimulated osteoclastogenesis might include increased expression of several genes involved in osteoclast differentiation and reactive oxygen species production.
Keywords: Mitochondrial Function, RAW264.7 Cells, Tetrabromobisphenol A, Osteoclastogenesis
Graphical Abstract
INTRODUCTION
Tetrabromobisphenol A (TBBPA) is the most commonly used brominated flame retardant, and is either physically added or chemically bonded to flammable products. TBBPA and its related compounds can leach into the environment and produce adverse effects on human health. Human exposure to TBBPA occurs by inhalation of dust, dermal contact, and ingestion.1,2,3 TBBPA has been found in commercial drinking water stored in reusable polycarbonate containers and in seafood.4 Furthermore, it accumulates in biologics, such as aquatic life and marine mammals, through the food chain.5 TBBPA commonly enters the human body through foods and has been detected in human blood, adipose tissue, and breast milk.6 TBBPA disrupts thyroid7 and estrogen hormones,8 and negatively affects the immune9 and nervous systems.10 TBBPA has agonistic effects on peroxisome proliferator-activated receptor γ, which is a key regulator of adipocyte differentiation and lipid and carbohydrate metabolism.11 It causes preadipocyte differentiation in TBBPA-treated 3T3-L1 cells,11 raises total cholesterol levels in blood, increases liver weight in pregnant mice exposed to TBBPA,12 and increases abnormal cytokine secretion in mice that ingested TBBPA.13 TBBPA and its debrominated congener accumulate at high levels in breast milk, and that debrominated congeners promote adipocyte differentiation.14
In our previous study, we showed that TBBPA inhibits osteoblast function and has detrimental effects on osteoblasts via a mechanism involving oxidative stress and mitochondrial dysfunction.15 Normal bone remodeling requires a homeostatic balance between the activities of bone-forming osteoblasts and bone-resorbing osteoclasts.16 Osteoclasts are responsible for the dissolution and absorption of bone, whereas osteoblasts are responsible for the synthesis and mineralization of bone. This homeostasis is compromised when osteoclast and osteoblast activities are disturbed.17 Excessive osteoclast formation causes pathological bone diseases such as rheumatoid arthritis, periodontal disease, and osteoporosis.18 Considering that normal bone remodeling requires tight coupling of bone resorption and bone formation, it is important to identify the effects of TBBPA on osteoclasts, as well as osteoblasts. However, no reports have investigated the effects of TBBPA on osteoclast differentiation. Therefore, this study evaluated the effects of TBBPA on receptor activator of nuclear factor kappa B ligand (RANKL)-induced osteoclast differentiation in RAW264.7 cells.
METHODS
Osteoclast differentiation of RAW264.7 cells
The RAW264.7 mouse macrophage cell line, a well-known in vitro model of osteoclastogenesis, maintains the capability to differentiate into mature osteoclast-like cells.19 RANKL-treated RAW264.7 cells produce high levels of osteoclast-specific markers, such as tartrate-resistant acid phosphatase (TRAP), which is commonly used to evaluate bone resorption.20
As in our previous study with RAW264.7 cell line, the RAW264.7 cell line was obtained from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; Gibco BRL, Grand Island, NY, USA) supplemented with antibiotics (100 U/mL penicillin A and 100 U/mL streptomycin) and 10% heat inactivated fetal bovine serum (FBS), and maintained at 37°C in 5% CO2 humidified air. The cells (2 × 104 cells/well) were seeded in 24-well plates and incubated to up to 70% confluence. For differentiation into osteoclasts, RAW264.7 cells were cultured in DMEM, containing 50 and 100 ng/mL RANKL (R&D Systems, Minneapolis, MN, USA) for 3 days.21 To examine the effects of TBBPA on osteoclast differentiation and activity, RAW264.7 cells were treated with varying concentrations of TBBPA (10, 20, and 40 µM), in the presence of RANKL for an additional 3 days.
TRAP activity assay
To investigate RAW264.7 cell osteoclastogenesis via RANK-RANKL signaling, TRAP activity was measured in RAW264.7 cells stimulated with 0, 50, and 100 ng/mL RANKL using the Acid Phosphatase Assay kit (BioVision Inc., Milpitas, CA, USA). The kit uses p-nitrophenyl phosphate as a phosphatase substrate, which turns yellow (λmax = 405 nm) when dephosphorylated by acid phosphatase. After removing the medium, the cultured cells were gently washed twice with cold phosphate-buffered saline (PBS) and lysed with 0.05% Triton-X100 at 4°C. The total protein content in the cell lysates was measured using Bio-Rad protein assay reagents (Bio-Rad Laboratories, Hercules, CA, USA), and TRAP activity was expressed according to the protein level. To confirm the generation of multinucleated osteoclasts, cells were fixed with 3.7% formalin (Sigma, St. Louis, MO, USA), permeablized with 0.1% Triton X-100 and finally stained for TRAP with the leukocyte acid phosphatase kit (Sigma) as in our previous study.22 The images of TRAP-positive cells were captured under the inverted microscope (Olympus, Tokyo, Japan).
Cytotoxicity analyses
To examine the effects of TBBPA treatment on cell viability during RANKL stimulation, RAW264.7 cells were treated with 10, 20, and 40 µM TBBPA in the presence of 50 ng/mL RANKL. The cytotoxic effects of TBBPA were detected using the WST-1 assay (Roche Diagnostics, Mannheim, Germany). The WST-1 cell assay is a colorimetric assay, based on the cleavage of a tetrazolium salt WST-1 to formazan, by cellular mitochondrial dehydrogenases in viable cells. The larger the number of viable cells, the greater the amount of formazan product produced, following the addition of WST-1. RAW 264.7 cells were cultured in DMEM containing antibiotics and 10% heat-inactivated FBS, and maintained at 37°C in 5% CO2 humidified air. Next, the cells were cultured in DMEM containing 50 ng/mL RANKL and varying concentrations of TBBPA (10, 20, and 40 µM), WST-1 solution (10%) was added to each well and mixed gently. After a 3 hours incubation at 37°C in an atmosphere containing 5% CO2, the absorbance at 450 nm was measured using a microplate reader versus a 650 nm reference as in our previous study.22
RNA extraction and quantitative polymerase chain reaction (qPCR)
Gene expression was detected by quantitative real-time polymerase chain reaction (PCR). The expressions of Akt2, nuclear factor of activated T-cells cytoplasmic 1 (NFATc1), chloride channel voltage-sensitive 7 (CLCN7), carbonic anhydrase II (CAII), cathepsin K (CTK), osteopetrosis-associated transmembrane protein 1 (OSTM1), Src, extracellular signal-related kinase (ERK), Grb-2-associated binder 2 (GAB2), c-Fos, and matrix metalloproteinases (MMP9) genes were measured. Akt2, NFATc1, Src, extracellular-related kinase (ERK), GAB2, and c-Fos genes are associated with osteoclastogenesis. CLCN7, CAII, CTK, OSTM1, and MMP9 genes are associated with enzymes involved in bone resorption by mature osteoclasts. Total RNA was isolated from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). After isolation, RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA, USA). Complementary DNAs (cDNAs) were synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics) and stored at −70°C until further processing. All procedures were followed according to the manufacturer's instructions. The qPCR was performed to verify the differential expression of selected genes using the Roche LightCycler 480 system (Roche Diagnostics) and the Taqman method using the Roche Universal ProbeLibrary (UPL) kit. Relative gene expression was determined by employing the comparative CT method. All reactions were performed in a total volume of 20 µL containing 10.0 µL 2× UPL master mix, 1.0 µL 5′ primer (10 pmol/μL), 1.0 µL 3′ primer (10 pmol/mL), 0.2 µL UPL probe, 1.0 µL cDNA, and 6.8 µL sterile water. The thermal cycling conditions for PCR were initial denaturation for 10 min at 95°C, followed by 40 cycles of 94°C for 10 seconds and 60°C for 30 seconds. The primers summarized in Table 1 were designed using the Roche ProbeFinder assay tool. For the qPCR analyses, duplicate PCR reactions were performed for each cDNA. Negative controls (without template) were included in the PCR reaction to ensure specific amplification as in a previous study.23 Then qPCR was conducted using the Roche LightCycler 480 system (Roche Diagnostics) with 1.0 μg RNA with A260:A280 ratios > 1.8, as confirmed by the 2100 Bioanalyzer (Agilent). The values obtained from each sample were normalized to hypoxanthine guanine phosphoribosyl transferase expression. Expression levels of each gene in all experimental groups were compared to the expression levels of the control group.
Table 1. Primer sequences.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| Akt2 | 5′-CGA CCC AAC ACC TTT GTC A-3′ | 5′-GAT AGC CCG CAT CCA CTC T-3′ |
| CAII | 5′-GGG GAT ACA GCA AGC ACA AC-3′ | 5′-GAC TGC CGG TCT CCA TTG-3′ |
| Cathepsin K | 5′-CGA AAA GAG CCT AGC GAA CA-3′ | 5′-TGG GTA GCA GCA GAA ACT TG-3′ |
| c-Fos | 5′-CAG CCT TTC CTA CTA CCA TTC C-3′ | 5′-ACA GAT CTG CGC AAA AGT CC-3′ |
| CLCN7 | 5′-TCG GAC AGA TGA ACA ACG TG-3′ | 5′-GGT GTG AGG AGG ATC GAC TT-3′ |
| ERK1 | 5′-TGG AAG CCA TGA GAG ATG TTT-3′ | 5′-GCT CAG CTG CTG GCT TTT A-3′ |
| GAB2 | 5′-AGA TCT GCG GCT TCA ATC AG-3′ | 5′-GAC TGG CTG AAG AAA GGT TCC-3′ |
| HPRT | 5′-TCC TCC TCA GAC CGC TTT T-3′ | 5′-CCT GGT TCA TCA TCG CTA ATC-3′ |
| MMP9 | 5′-ACG ACA TAG ACG GCA TCC A-3′ | 5′-GCT GTG GTT CAG TTG TGG TG-3′ |
| NFATc1 | 5′-TCC AAA GTC ATT TTC GTG GA-3′ | 5′-CTT TGC TTC CAT CTC CCA GA-3′ |
| OSTM1 | 5′-GGT CTC TGA GTT TTT CAA CAG CA-3′ | 5′-CCT CAC CAT TGT TTG TTA GGC-3′ |
| Src | 5′-CTT CGG AGA GGT GTG GAT G-3′ | 5′-GTG CCT GGG TTC AGA GTT TT-3′ |
Osteoclast bone-resorbing activity
The OsteoLyse™ Assay Kit (Lonza, Walkersville, MD, USA) was used to further examine whether TBBPA affected the ability of mature osteoclasts to resorb bone. This kit, which was used in a previous study,22 provides an easy-to-use protocol for quantitatively measuring in vitro osteoclast-mediated bone resorption in a high-throughput format. The assay directly measures the release of europium-labeled collagen fragments in osteoclast cell culture supernatant via time-resolved fluorescence, which indicates their resorption activity levels. Osteoclasts were seeded onto OsteoLyse™ plates and incubated until they reached up to 70% confluence. Next, the cells were cultured in DMEM containing 50 ng/mL RANKL with various concentrations of TBBPA for 3 days, followed by RANKL treatment for an additional 3 days. Quantification of bone resorption by osteoclasts in vitro was measured using the OsteoLyse™ Assay Kit (Lonza) according to the manufacturer's instructions.24
Resorption pit assay
The resorptive function of mature osteoclast cells was analyzed on Osteologic Plates (BD BioCoat Osteologic Bone Cell Culture System, BD Biosciences, San Jose, CA, USA). The cells were cultured in DMEM containing 50ng/mL RANKL with TBBPA for 3 days, followed by RANKL treatment for an additional 3 days. After the culture, osteoclasts were removed with 1 N NaOH for 20 minutes, and the slices were washed twice with PBS and resorption pits were stained with Mayer's haematoxylin (Sigma) for 30 seconds. Finally, bovine bone slices were transferred onto glass slides, mounted with glycerol, covered with glass cover slips and observed under the light microscope (Olympus).
Determination of mitochondrial membrane potential (MMP)
To test whether mitochondrial dysfunction was involved in the TBBPA-induced increase in osteoclastogenesis in RAW264.7 cells, MMP was analyzed in cells treated with TBBPA (10 and 20 µM). The JC-1 MMP Assay Kit (Cayman Chemical Co., Ann Arbor, MI, USA) was used to assess the changes in MMP in cells as in our previous study.24 JC-1 is a lipophilic and cationic dye that permeates plasma and mitochondrial membranes. The dye fluoresces red when it aggregates in healthy mitochondria with a high membrane potential, whereas it appears in a monomeric form and fluoresces green in mitochondria with a diminished membrane potential. Cells treated with 50 ng/mL RANKL and TBBPA (10 and 20 µM) were incubated with MMP-sensitive fluorescent dye JC-1 for 20 min at 37°C and washed twice in PBS; red (excitation, 550 nm; emission, 600 nm) and green (excitation, 485 nm; emission, 535 nm) fluorescence were measured using a fluorescence microplate reader (Molecular Devices, Downingtown, PA, USA). Mitochondrial depolarization (i.e., loss of MMP) manifests as a decrease in the red/green fluorescence ratio.22,24
Measurement of mitochondrial superoxide
To determine whether TBBPA regulates mitochondrial reactive oxygen species (ROS) accumulation in osteoclasts, mitochondrial superoxide levels were measured using the MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen Molecular Probes, Eugene, OR, USA). MitoSOX™ Red (Ex/Em = 510/580 nm) is a fluorogenic dye used for the highly selective detection of superoxide in the mitochondria of cells. Cells were incubated with 2 μM MitoSOX™ Red at 37°C for 20 minutes according to the manufacturer's instructions. After the cells had been washed, MitoSOX™ Red fluorescence was detected.21
Statistical analyses
Results were expressed as the mean ± standard error of the mean. Statistical significance was determined by analysis of variance, and Dunnett's t-test was used as post-hoc analysis because homogeneity of variance was not assumed. Statistical analyses were carried out using PASW software, version 20.0 (IBM Co., Armonk, NY, USA). A P < 0.05 was considered to indicate statistical significance.
RESULTS
Effects of TBBPA on RANKL-induced osteoclast differentiation
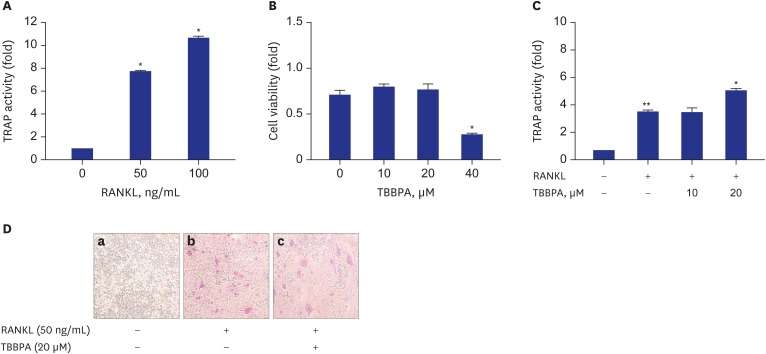
Incubation with RANKL (50 and 100 ng/mL) for 3 days significantly increased TRAP activity compared to control, indicating osteoclastogenesis of RAW264.7 cells (Fig. 1A). As shown in Fig. 1B, TBBPA at ≤ 20 µM had no effect on cell viability after 3 days. But, cell viability was significantly decreased by 40 µM TBBPA, compared to the control. This suggests that TBBPA could induce RAW264.7 cellular toxicity at concentrations ≥ 40 µM TBBPA. Therefore, further experiments were conducted at non-toxic TBBPA concentrations (≤ 20 µM). In 50 ng/mL RANKL-stimulated RAW264.7 cells, TRAP activity was measured at 0, 10, and 20 µM TBBPA. There was no difference in TRAP activity between differentiated control cells and 10 µM TBBPA treated cells. But, TRAP activity was increased significantly at a concentration of 20 µM TBBPA, compared to differentiated control cells (Fig. 1C), suggesting that 20 µM TBBPA treatment could promote the osteoclastogenesis by RANK-RANKL signaling. The microscopic photographs are shown in Fig. 1D, and it is evident that RANKL-stimulated RAW264.7 cells differentiated into mature TRAP-positive multinucleated cells. The treatment with 20 μM TBBPA increased RANKL-induced formation of TRAP-positive cells.
Fig. 1. Effects of TBBPA on RANKL-induced osteoclast differentiation in RAW264.7 cells. RAW264.7 cells were seeded in 24-well plates and incubated until reaching up to 70% confluence. Then, the cells were cultured in Dulbecco's Modified Eagle Medium containing 50 ng/mL RANKL and various concentrations of TBBPA for 3 days. (A, C) TRAP activity, (B) cell viability. (D) Photograph of TRAP staining (100×) (a, Vehicle; b, RANKL [50 ng/mL]; c, RANKL [50 ng/mL] + 20 μM TBBPA).
TBBPA = tetrabromobisphenol A, RANKL = receptor activator of nuclear factor kappa B ligand, TRAP = tartrate-resistant acid phosphatase.
*P < 0.05 vs. differentiated control cells; **P < 0.05 vs. undifferentiated cells.
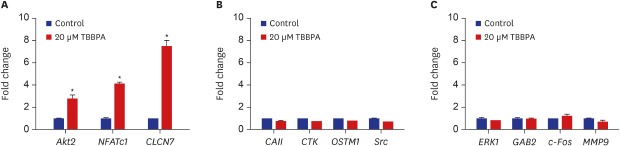
Effects of TBBPA on osteoclast-specific gene expression
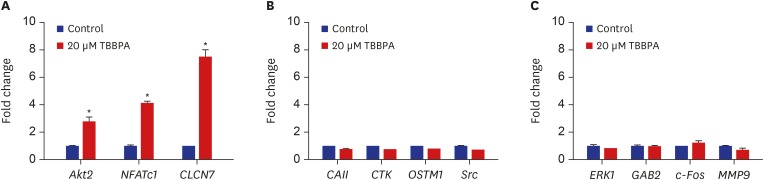
As shown in Fig. 2, the expression of Akt2, NFATc1, and CLCN7 genes were significantly increased by 20 µM TBBPA. Meanwhile, 20 µM TBBPA had no effect on the expression of CAII, CTK, OSTM1, Src, ERK, GAB2, c-Fos, and MMP9 genes. These results revealed increased expression of several genes involved in osteoclast differentiation and function.
Fig. 2. Effects of TBBPA on the expression of key signaling molecules involved in osteoclastogenesis. RAW264.7 cells were cultured in Dulbecco's Modified Eagle Medium containing 50 ng/mL receptor activator of nuclear factor kappa B ligand and 20 μM TBBPA for 3 days. Gene expression was detected by quantitative real-time polymerase chain reaction.
TBBPA = tetrabromobisphenol A, NFATc1 = nuclear factor of activated T-cells cytoplasmic 1, CLCN7 = chloride channel voltage-sensitive 7, CAII = carbonic anhydrase II, OSTM1 = osteopetrosis-associated transmembrane protein 1, ERK = extracellular-related kinase, GAB2 = Grb-2-associated binder 2, MMP-9 = matrix metallopeptidase 9.
*P < 0.05 vs. control cells.
The effects of TBBPA on the bone-resorbing activity of RANKL-induced osteoclasts
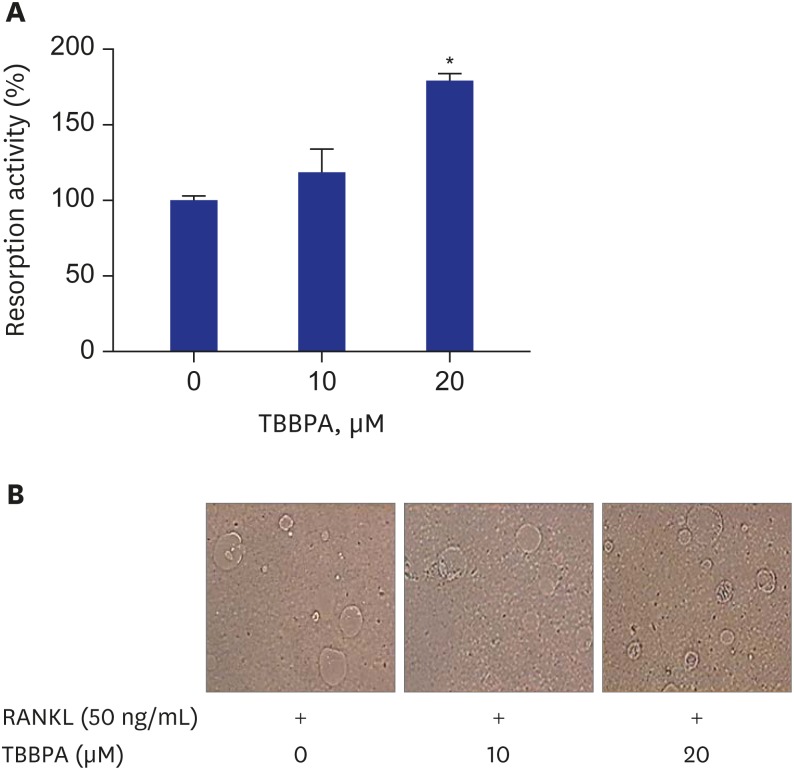
There was no difference in bone-resorbing activity of RANKL-induced osteoclasts between control and 10 µM TBBPA treated cells. But, treatment with 20 μM TBBPA significantly increased bone-resorbing activity of RANKL-induced osteoclasts, compared to control, suggesting that 20 µM TBBPA affected the ability of mature osteoclasts to resorb bone (Fig. 3A). Fig. 3B demonstrated that the area of resorption pit formed by RANKL-stimulated RAW264.7 cells was increased in the presence of 20 μM TBBPA.
Fig. 3. Effects of TBBPA on osteoclast bone resorbing activity and resorbed pits formation in RANKL-induced osteoclast differentiation. RAW264.7 cells were cultured in Dulbecco's Modified Eagle Medium containing 50 ng/mL RANKL and TBBPA (10 and 20 μM) for 3 days, followed by RANKL treatment for an additional 3 days. (A) Osteoclast bone resorbing activity was measured using an OsteoLyse™ assay kit. (B) Photograph of resorbed pits formation (400×).
TBBPA = tetrabromobisphenol A, RANKL = receptor activator of nuclear factor kappa B ligand.
*P < 0.05 vs. control.
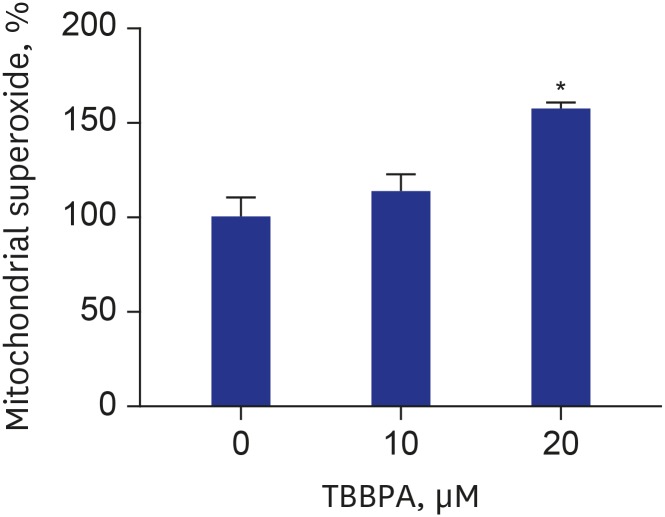
Effects of TBBPA on mitochondrial function and ROS production in osteoclasts
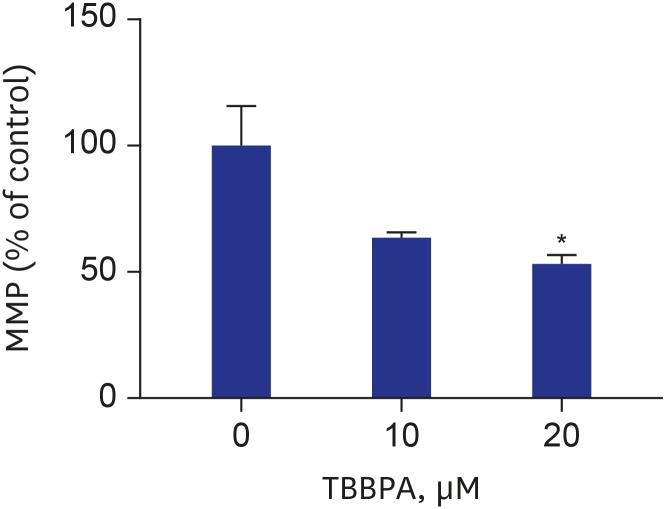
MMP was decreased in cells treated with TBBPA at concentrations of 10 and 20 µM compared to the control group (Fig. 4). The difference was statistically significant only at 20 µM TBBPA. Cells treated with 20 µM TBBPA demonstrated significantly higher levels of MitoSOX Red fluorescence, indicating that TBBPA increased the superoxide accumulation in osteoclast mitochondria. There was no difference of mitochondrial superoxide between 10 µM TBBPA and control. These results suggest that TBBPA could induce mitochondrial dysfunction and ROS production in osteoclast and osteoclast precursors (Fig. 5).
Fig. 4. Effects of TBBPA on MMP in osteoclasts. RAW264.7 cells were seeded in black 96-well plates and incubated until reaching up to 70% confluence. Next, the cells were cultured in Dulbecco's Modified Eagle Medium containing 50 ng/mL receptor activator of nuclear factor kappa B ligand and TBBPA (10 and 20 μM) for 3 days. The MMP was analyzed using an established method involving fluorescence staining with JC-1.
TBBPA = tetrabromobisphenol A, MMP = mitochondrial membrane potential.
*P < 0.05 vs. control.
Fig. 5. Effects of TBBPA on mitochondrial superoxide production in osteoclasts. RAW264.7 cells were seeded in black 96-well plate and incubated until reaching up to 70% confluence. Then, the cells were cultured in Dulbecco's Modified Eagle Medium containing 50 ng/mL receptor activator of nuclear factor kappa B ligand and TBBPA (10 and 20 μM) for 3 days. The mitochondrial superoxide was analyzed using an established method involving fluorescence staining with MitoSOXTM Red.
TBBPA = tetrabromobisphenol A.
*P < 0.05 vs. control.
DISCUSSION
To investigate the effects of TBBPA on osteoclast differentiation, we examined TBBPA-treated osteoclast-like cells by measuring the activity of TRAP, a marker enzyme of osteoclasts. TBBPA promoted TRAP activity in RANKL-stimulated RAW264.7 cells. Bone resorption activity was also increased in the presence of TBBPA. These results indicate that TBBPA promotes both osteoclast differentiation and function.
The activation of osteoclasts is regulated by various molecular signals.25 RANKL-RANK signaling is critical in osteoclastogenesis. RANKL to RANK signaling activates three pathways via TRAF6: mitogen activated protein kinase (MAPKs) family members, the NF-κB pathway, and the Src kinase pathway. MAPKs pathway involves p38-MAPKα, β, γ and δ, c-JUN N-terminal kinases (JNK1, 2 and 3), and extracellular signal-regulated kinases (ERK1 and ERK2).26 c-Fos, AP-1 transcription factors, and MITF were activated in osteoclast precursors by MAPKs pathway, to stimulate osteoclast differentiation.27 NF-κB pathway induces NFATc1 transcription, a key factor in osteoclastogenesis.28 RANKL to RANK signaling also activates the PI3K/Akt pathway through TRAF6 and Src kinase.29 Furthermore, RANKL-RANK signaling induces activation of NFATc1 in osteoclast precursor cells, and NFATc1 activation is achieved by calcium signaling and costimulatory signaling.30 RANK does not seem to initiate calcium signaling directly.
In this study, the gene expressions of Akt2, NFATc1, CLCN7, CAII, CTK, OSTM1, Src, ERK1, GAB2, c-Fos, and MM9 were evaluated. This study analyzed osteoclastogenesis through RANKL-RANK signaling, and ERK1 and c-Fos expressions involved in the MAPKs pathway. NFATc1 expression is involved in the NF-κB pathway, whereas Akt2, Src, and GAB2 expressions are involved in Src kinase pathway. CLCN7, CAII, CTK, and MM9 expressions are associated with enzymes secreted from mature osteoclast. OSTM1 is required for osteoclast differentiation.31 This study revealed that TBBPA increased the expression of Akt2, NFATc1, and CLCN7.
There are three Akt family members: Akt1, Akt2, and Akt3. Akt1 and Akt2, but not Akt3, are abundantly expressed in both osteoblasts and osteoclasts.32 Several studies were reported that knockdown of Akt1 and Akt2 inhibited osteoclast differentiation and bone development.33,34 In this study, TBBPA did not increase Src and GAB2 expression, but increased Akt2 expression in RANKL-RANK mediated Src kinase pathways. This result suggests that TBBPA affected the downstream of PI3K and increased Akt2 expression. But, additional proteomics studies are needed to determine whether increased Akt expression leads to activity. The increased NFATc1 expression suggests that TBBPA could activate NF-κB pathways in RANKL-RANK signaling. NFATc1-deficient embryonic stem cells fail to differentiate into osteoclasts in response to RANKL.35 CLCN7 is a chloride channel localized to late endosomes, lysosomes, and the ruffled border in osteoclasts where it acts with the vacuolar H+-ATPase to acidify the resorptive space. CLCN7 is necessary for bone mineral solubilization and digestion of the organic bone matrix by acid proteases.36 The loss or inactivation of CLCN7 causes osteopetrosis as well as neurodegeneration and severe lysosomal storage disease.37 Increased CLCN7 expression may be linked to bone resorption activity. TBBPA stimulated both differentiation and activity of osteoclasts in this study.
This study showed that TBBPA decreased MMP and increased mitochondrial superoxide production. In a previous study, ROS-mediated signaling pathways induced NFATc1 activation through the NF-κB pathway in early osteoclast differentiation.38 This result is supported by evidence that antioxidants decrease NF-κB protein expression when bone marrow macrophages were treated with RANKL.39 ROS activate MAPKs pathways (JNK, ERK, and p38) and the NF-κB pathway in a dose-dependent manner.40 ROS increases intracellular calcium levels from endoplasmic reticulum, and maintains calcium oscillations and downstream signaling pathways. Furthermore, ROS induce NFATc1 auto-amplification and increase transcription and osteoclast-specific genes in late osteoclast differentiation.38 Thus, TBBPA potentially stimulates early and late osteoclast differentiation by production of intra-mitochondrial ROS, such as superoxide anions. Intra-mitochondrial ROS could lower the MMP to induce a retrograde signaling pathway, resulting in osteoclastogenesis in addition to mitochondrial dysfunction.41
These experimental results have limited application to humans. First, a one-time exposure to high-dose TBBPA rarely occurs. TBBPA induces toxicity in humans after long-term exposure to small amounts and its subsequent accumulation in the body.42 Thus, it is difficult to generalize the changes caused by a one-time TBBPA exposure to cells in vitro. Second, it has been suggested recently that persistent organic pollutants (POPs) are toxic to various tissues through multiple cellular responses in the form of a mixture rather than alone.43,44 However, as basic experiments investigating the effect of POPs on bone metabolism have yet to be conducted at the cellular level, this study potentially provides a basis for analyzing those effects in osteoporosis and several metabolic bone diseases in the future. A study of proteomics should be conducted to determine whether the increased gene expression identified in this study actually results in functional proteins.
In conclusion, this study showed that TBBPA could promote osteoclast differentiation and activity. TBBPA increased the expression of several genes involved in osteoclast differentiation. Furthermore, TBBPA induced mitochondrial dysfunction and ROS production in osteoclasts. These results possibly explain the mechanism by which TBBPA contributes to increased osteoclastogenesis.
Footnotes
Funding: This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare (KHIDI), Republic of Korea (grant No: HI14C2700).
Disclosure: The authors have no conflicts of interest to disclose.
- Conceptualization: Oh S, Kim DY, Chon S.
- Data curation: Park SY, Choi EM, Suh KS.
- Formal analysis: Park SY, Choi EM, Suh KS.
- Funding acquisition: Chon S.
- Investigation: Park SY, Choi EM, Suh KS, Chon S.
- Methodology: Park SY, Choi EM, Suh KS, Chon S.
- Validation: Park SY, Choi EM, Suh KS, Kim HS, Chin SO, Rhee SY, Kim DY, Oh S, Chon S.
- Writing - original draft: Park SY, Choi EM.
- Writing - review & editing: Park SY, Choi EM, Suh KS, Kim HS, Chin SO, Rhee SY, Kim DY, Oh S, Chon S.
References
- 1.Jarosiewicz M, Bukowska B. Tetrabromobisphenol A - toxicity, environmental and occupational exposures. Med Pr. 2017;68(1):121–134. doi: 10.13075/mp.5893.00491. [DOI] [PubMed] [Google Scholar]
- 2.Liu A, Zhao Z, Qu G, Shen Z, Shi J, Jiang G. Transformation/degradation of tetrabromobisphenol A and its derivatives: a review of the metabolism and metabolites. Environ Pollut. 2018;243(Pt B):1141–1153. doi: 10.1016/j.envpol.2018.09.068. [DOI] [PubMed] [Google Scholar]
- 3.McAvoy DC, Pittinger CA, Willis AM. Biotransformation of tetrabromobisphenol A (TBBPA) in anaerobic digester sludge, soils, and freshwater sediments. Ecotoxicol Environ Saf. 2016;131:143–150. doi: 10.1016/j.ecoenv.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Peterman PH, Orazio CE, Gale RW, editors. Detection of tetrabromobisphenol A and formation of brominated 13C-bisphenol A's in commercial drinking water stored in reusable polycarbonate containers; 219th ACS National Meeting; 2000 Mar 26−30; San Francisco, CA. Washington, D.C.: American Chemical Society Division of Environmental Chemistry; 2000. Mar, p. 431. [Google Scholar]
- 5.Johnson-Restrepo B, Adams DH, Kannan K. Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere. 2008;70(11):1935–1944. doi: 10.1016/j.chemosphere.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kim UJ, Oh JE. Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant-mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environ Pollut. 2014;184:193–200. doi: 10.1016/j.envpol.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura S, Jinno N, Ohta S, Kuroki H, Fujimoto N. Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem Biophys Res Commun. 2002;293(1):554–559. doi: 10.1016/S0006-291X(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 8.Gosavi RA, Knudsen GA, Birnbaum LS, Pedersen LC. Mimicking of estradiol binding by flame retardants and their metabolites: a crystallographic analysis. Environ Health Perspect. 2013;121(10):1194–1199. doi: 10.1289/ehp.1306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cato A, Celada L, Kibakaya EC, Simmons N, Whalen MM. Brominated flame retardants, tetrabromobisphenol A and hexabromocyclododecane, activate mitogen-activated protein kinases (MAPKs) in human natural killer cells. Cell Biol Toxicol. 2014;30(6):345–360. doi: 10.1007/s10565-014-9289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wojtowicz AK, Szychowski KA, Kajta M. PPAR-γ agonist GW1929 but not antagonist GW9662 reduces TBBPA-induced neurotoxicity in primary neocortical cells. Neurotox Res. 2014;25(3):311–322. doi: 10.1007/s12640-013-9434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, et al. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect. 2011;119(9):1227–1232. doi: 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tada Y, Fujitani T, Yano N, Takahashi H, Yuzawa K, Ando H, et al. Effects of tetrabromobisphenol A, brominated flame retardant, in ICR mice after prenatal and postnatal exposure. Food Chem Toxicol. 2006;44(8):1408–1413. doi: 10.1016/j.fct.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe W, Shimizu T, Sawamura R, Hino A, Konno K, Hirose A, et al. Effects of tetrabromobisphenol A, a brominated flame retardant, on the immune response to respiratory syncytial virus infection in mice. Int Immunopharmacol. 2010;10(4):393–397. doi: 10.1016/j.intimp.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama E, Kakutani H, Nakao T, Motomura Y, Takano Y, Sorakubo R, et al. Facilitation of adipocyte differentiation of 3T3-L1 cells by debrominated tetrabromobisphenol A compounds detected in Japanese breast milk. Environ Res. 2015;140:157–164. doi: 10.1016/j.envres.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Choi EM, Suh KS, Rhee SY, Oh S, Kim SW, Pak YK, et al. Exposure to tetrabromobisphenol A induces cellular dysfunction in osteoblastic MC3T3-E1 cells. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2017;52(6):561–570. doi: 10.1080/10934529.2017.1284435. [DOI] [PubMed] [Google Scholar]
- 16.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285(33):25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6(1):121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92(10):860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999;96(7):3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igarashi Y, Lee MY, Matsuzaki S. Acid phosphatases as markers of bone metabolism. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781(1-2):345–358. doi: 10.1016/s1570-0232(02)00431-2. [DOI] [PubMed] [Google Scholar]
- 21.Suh KS, Chon S, Jung WW, Choi EM. Effect of bergenin on RANKL-induced osteoclast differentiation in the presence of methylglyoxal. Toxicol In Vitro. 2019;61:104613. doi: 10.1016/j.tiv.2019.104613. [DOI] [PubMed] [Google Scholar]
- 22.Suh KS, Chon S, Jung WW, Choi EM. Crocin attenuates methylglyoxal-induced osteoclast dysfunction by regulating glyoxalase, oxidative stress, and mitochondrial function. Food Chem Toxicol. 2019;124:367–373. doi: 10.1016/j.fct.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Suh KS, Ko A, Sul D, Choi D, Lee SK, et al. The flavonoid glabridin attenuates 2-deoxy-D-ribose-induced oxidative damage and cellular dysfunction in MC3T3-E1 osteoblastic cells. Int J Mol Med. 2013;31(1):243–251. doi: 10.3892/ijmm.2012.1172. [DOI] [PubMed] [Google Scholar]
- 24.Suh KS, Chon S, Jung WW, Choi EM. Effects of methylglyoxal on RANKL-induced osteoclast differentiation in RAW264.7 cells. Chem Biol Interact. 2018;296:18–25. doi: 10.1016/j.cbi.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Reuter S, Prasad S, Phromnoi K, Kannappan R, Yadav VR, Aggarwal BB. Embelin suppresses osteoclastogenesis induced by receptor activator of NF-κB ligand and tumor cells in vitro through inhibition of the NF-κB cell signaling pathway. Mol Cancer Res. 2010;8(10):1425–1436. doi: 10.1158/1541-7786.MCR-10-0141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Mizukami J, Takaesu G, Akatsuka H, Sakurai H, Ninomiya-Tsuji J, Matsumoto K, et al. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol Cell Biol. 2002;22(4):992–1000. doi: 10.1128/MCB.22.4.992-1000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto A, Miyazaki T, Kadono Y, Takayanagi H, Miura T, Nishina H, et al. Possible involvement of IkappaB kinase 2 and MKK7 in osteoclastogenesis induced by receptor activator of nuclear factor kappaB ligand. J Bone Miner Res. 2002;17(4):612–621. doi: 10.1359/jbmr.2002.17.4.612. [DOI] [PubMed] [Google Scholar]
- 28.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202(9):1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4(6):1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 30.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 31.Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature. 2006;440(7081):220–223. doi: 10.1038/nature04535. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura N, Kugimiya F, Oshima Y, Ohba S, Ikeda T, Saito T, et al. Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS One. 2007;2(10):e1058. doi: 10.1371/journal.pone.0001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17(11):1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem. 2005;280(5):3583–3589. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- 35.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Rousselle AV, Heymann D. Osteoclastic acidification pathways during bone resorption. Bone. 2002;30(4):533–540. doi: 10.1016/s8756-3282(02)00672-5. [DOI] [PubMed] [Google Scholar]
- 37.Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, et al. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 2005;24(5):1079–1091. doi: 10.1038/sj.emboj.7600576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33(4):359–370. doi: 10.1007/s00774-015-0656-4. [DOI] [PubMed] [Google Scholar]
- 39.Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, et al. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res. 2004;301(2):119–127. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106(3):852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan S, Koenigstein A, Joseph J, Sun L, Kalyanaraman B, Zaidi M, et al. Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann N Y Acad Sci. 2010;1192(1):245–252. doi: 10.1111/j.1749-6632.2009.05377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colnot T, Kacew S, Dekant W. Mammalian toxicology and human exposures to the flame retardant 2,2′,6,6′-tetrabromo-4,4′-isopropylidenediphenol (TBBPA): implications for risk assessment. Arch Toxicol. 2014;88(3):553–573. doi: 10.1007/s00204-013-1180-8. [DOI] [PubMed] [Google Scholar]
- 43.Hansen KE, Johanson SM, Steppeler C, Sødring M, Østby GC, Berntsen HF, et al. A mixture of persistent organic pollutants (POPs) and azoxymethane (AOM) show potential synergistic effects on intestinal tumorigenesis in the A/J Min/+ mouse model. Chemosphere. 2019;214:534–542. doi: 10.1016/j.chemosphere.2018.09.126. [DOI] [PubMed] [Google Scholar]
- 44.Khezri A, Lindeman B, Krogenæs AK, Berntsen HF, Zimmer KE, Ropstad E. Maternal exposure to a mixture of persistent organic pollutants (POPs) affects testis histology, epididymal sperm count and induces sperm DNA fragmentation in mice. Toxicol Appl Pharmacol. 2017;329:301–308. doi: 10.1016/j.taap.2017.06.019. [DOI] [PubMed] [Google Scholar]