Abstract
Protists are pivotal components of marine ecosystems in terms of their high diversity, but protist communities have been poorly explored in benthic environments. Here, we investigated protist diversity and community assembly in surface sediments in the South China Sea (SCS) at a basin scale. Pyrosequencing of 18S rDNA was performed for a total of six samples taken from the surface seafloor at water depths ranging from 79 to 2,939 m. We found that Cercozoa was the dominant group, accounting for an average of 39.9% and 25.3% of the reads and operational taxonomic units (OTUs), respectively. The Cercozoa taxa were highly diverse, comprising 14 phylogenetic clades, six of which were affiliated with unknown groups belonging to Filosa and Endomyxa. Fungi were also an important group in both read‐ (18.1% on average) and OTU‐derived (9.3% on average) results. Moreover, the turnover patterns of the protist communities were differently explained by species sorting (53.3%), dispersal limitation (33.3%), mass effects (0%), and drift (13.3%). In summary, our findings show that the basin‐wide protist communities in the surface sediments of the SCS are primarily dominated by Cercozoa and are mainly assembled by species sorting and dispersal limitation.
Keywords: 18S rDNA, dispersal limitation, pyrosequencing, species sorting, UniFrac distance
Diversity of Cercozoa in surface sediments of the South China Sea.

1. INTRODUCTION
Protists constitute essential components of marine sediment systems (Orsi, 2018). Importantly, protists play diverse roles in maintaining benthic ecosystem functioning. For example, protists exert significant influences on bacterial communities via grazing effects in deep‐sea sediments and further alter the hydrocarbon‐degrading process (Beaudoin et al., 2016). Metabolically active protists are widely detected in the subsurface of sea floors (Edgcomb, Kysela, Teske, de Vera Gomez, & Sogin, 2002), in which protists maintain important biogeochemical cycles (Edgcomb et al., 2016). In addition, protists can dominate the biomass of benthic microbiomes (Bochdansky, Clouse, & Herndl, 2017) and persist at record depths (>1,500 m) below the seafloor of the Canterbury Basin (Ciobanu et al., 2014).
Protist diversity has been poorly investigated in marine sediments compared to planktonic systems (Cheung, Au, Chu, Kwan, & Wong, 2010; Christaki et al., 2014; Logares et al., 2014; Stoeck et al., 2010; de Vargas et al., 2015; Wu, Logares, Huang, & Hsieh, 2017). A few consensuses have been reached for planktonic protists, such as the dominance of parasite groups within Alveolata (Guillou et al., 2008; de Vargas et al., 2015). Moreover, it is well recognized that benthic protists are significantly different from planktonic groups (Chen, Pan, Yu, Yang, & Zhang, 2017; Cleary & Durbin, 2016; Coolen & Shtereva, 2009; Epstein & López‐García, 2008; Massana et al., 2015) and can even exhibit higher diversity than planktonic taxa (Chen et al., 2017; Forster et al., 2016). Furthermore, deep‐sea protists are much less studied (Pawlowski et al., 2011) relative to protists in coastal and shallow‐sea sediments (e.g., Gong et al., 2015; Massana et al., 2015; Chen et al., 2017).
Little is known about how protist communities are assembled in deep‐sea sediments from a metacommunity perspective (Leibold et al., 2004; Vellend, 2010). Petro, Starnawski, Schramm, and Kjeldsen (2017) proposed four major processes of microbial community assembly in marine sediments: selection (i.e., species sorting), dispersal, diversification, and drift. As the predominant process (Petro et al., 2017), species sorting may be imposed by sediment differences such as water depth, pressure, and the properties of sediment particles. Moreover, dispersal limitation (derived from low dispersal), rather than mass effects (representing high dispersal), accounts for the importance of microbial dispersal in marine sediments because the microbial dispersal is passive and largely limited at a large spatial scale (e.g., the basin scale). Diversification (i.e., speciation) is supposed to have little influence within a metacommunity with individual dispersal (Stegen et al., 2013). Drift (acting alone), resulting from stochastic changes in birth and death rates, can be the dominant mechanism in extremely uniform habitats, which is not the case in marine sediments (Jacob, Soltwedel, Boetius, & Ramette, 2013). Therefore, we hypothesized that compositional turnover in protist communities in marine sediments at a basin scale would be mainly governed by a combination of species sorting and dispersal limitation.
The South China Sea (SCS) is one of the largest marginal seas located in the western Pacific Ocean, but the protist diversity across the basin‐wide SCS sediments remains unclear. The SCS is characterized by a wide water depth range spanning over 5,000 m accompanied by distinct types of sediments (Liu et al., 2013). These sediments with contrasting characteristics have been shown to contribute to the compositional turnover in benthic microbial communities (Zhu, Tanabe, Yang, Zhang, & Sun, 2013). In addition, the semiclosed SCS is strongly influenced by the regulation of surface circulations by the East Asian monsoon system (Liu et al., 2002), which can also influence the seafloor microbial communities (Hamdan et al., 2013). This influence is partially due to seasonal monsoons that contribute to the transport of fluvial sediments in the SCS (Liu et al., 2016; Schroeder, Wiesner, & Liu, 2015).
The goal of this study was to investigate protist diversity and community assembly in surface sediments of the SCS. We investigated six sites (79–2,939 m depth) that represented common habitat types in the SCS seafloor and performed pyrosequencing of the V1–V2 region of 18S rDNA. We revealed the underlying processes that regulated community patterns of benthic protists using null model analysis and tested the hypothesis that species sorting and dispersal limitation are the two key driving forces. Overall, this study provides baseline information on the protist diversity and assembly in surface sediments of the SCS.
2. MATERIALS AND METHODS
2.1. Sample collection
A total of six sediment samples were collected from the surface seafloor using a grab sampler in the SCS during 28th April–21st May in 2010 (Figure 1). This sampling design included one station (ST76) from the shallow coast (water depth = 79 m) and five stations located in the deep basin (water depths >880 m) (Table 1). Surface sediment samples (0–20 cm) were immediately collected and stored at −20°C until further analyses. Hydrodynamic profiles (i.e., temperature and salinity with water depth) of the upper waters at each station were obtained with an SBE‐911 instrument (Sea‐Bird Electronics, USA).
Figure 1.
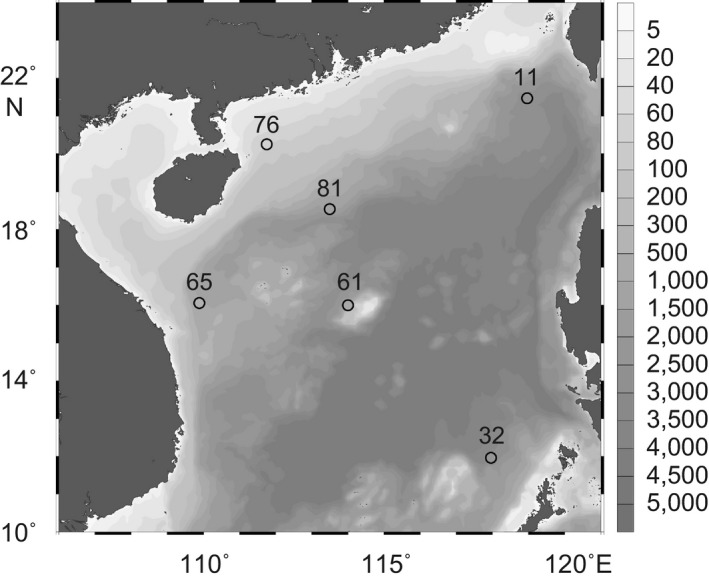
Locations of the six samples (circles) taken from surface sediments in the South China Sea. Gray contours represent bottom depths (m). The map was generated using Ocean Data View (Schlitzer, 2018)
Table 1.
Summary of sampling information (locations and water depths), sequencing results (the number of quality‐checked reads and observed operational taxonomic units, OTUs) and richness estimators based on an equal sequencing depth of 5,792 reads (Chao1 and Shannon indexes)
| Sample | Station | Depth (m) | Quality‐checked reads | OTUs | Chao1 | Shannon |
|---|---|---|---|---|---|---|
| ST11 | 11 | 2,801 | 13,620 | 370 | 461 | 4.42 |
| ST32 | 32 | 2,939 | 8,911 | 315 | 368 | 3.6 |
| ST61 | 61 | 1,250 | 14,022 | 317 | 371 | 4.21 |
| ST65 | 65 | 880 | 10,737 | 402 | 475 | 4.71 |
| ST76 | 76 | 79 | 5,792 | 341 | 474 | 4.26 |
| ST81 | 81 | 1,469 | 21,009 | 276 | 301 | 4.2 |
2.2. DNA extraction and pyrosequencing
For each sediment sample, the top 0–1 cm segment was used for molecular analyses. Total DNA was extracted using an UltraClean Soil DNA Isolation Kit (MO BIO Laboratories, USA) according to the manufacturer's instructions, during which samples were homogenized for 60 s at 4 m/s using a FastPrep‐24 instrument (MP Biomedicals, USA). The DNA extracts were quantified using a NanoDrop ND‐1000 spectrophotometer (Nanodrop Technologies, USA). PCR amplification was performed for the V1–V2 region of 18S rDNA (approximately 420 bp) using the primers SSU_F04 (5'‐GCTTGTCTCAAAGATTAAGCC‐3') and SSU_R22 (5'‐GCCTGCTGCCTTCCTTGGA‐3') (Bik et al., 2012). The PCR program consisted of an initial denaturation step at 95°C for 2 min; 30 cycles of 95°C for 30 s, 53°C for 30 s and 72°C for 30 s; and a final extension at 72°C for 5 min. The amplification products were then purified using an AxyPrep DNA Gel Extraction Kit (Axygen, USA). Pyrosequencing was carried out on a 454 GS FLX Titanium system (Roche, USA) following the manufacturer's instructions. Raw sequence data have been deposited in the Sequence Read Archive (NCBI) under accession number SRP083955.
2.3. Sequence processing
The pyrosequencing data were processed using the Quantitative Insights Into Microbial Ecology (QIIME v. 1.9.1) pipeline (Caporaso et al., 2010). Briefly, the quality of reads was checked using a 50‐bp sliding window and an average Phred threshold of 25, and short reads (<200 bp) were discarded. The remaining reads were run through DeNoiser (Reeder & Knight, 2010) to reduce pyrosequencing errors. The resulting sequences were grouped into operational taxonomic units (OTUs) using UCLUST (Edgar, 2010) with a minimum identity of 97%. The representative sequence per OTU was selected, and chimeras were checked using ChimeraSlayer (Haas et al., 2011). The assignment of the representative sequences was determined using the PR2 database (Guillou et al., 2013) with a BLAST E‐value of 10−6 and a minimum percent similarity of 90% (Zhang, Schwartz, Wagner, & Miller, 2000). Singletons (OTUs with only a single sequence in the entire data set) and OTUs with sequences detected in only a single sample were removed. Metazoans, as multicellular animals, were also removed because this study focused on single‐celled protists. Consequently, OTUs assigned to metazoans were removed from further analyses. OTU representative sequences were aligned using MAFFT with the FFT‐NS‐2 method (Katoh & Standley, 2013), and the resulting alignments were used to generate a phylogenetic tree with FastTree (Price, Dehal, & Arkin, 2009).
2.4. Phylogenetic analysis of Cercozoa
Considering the large percentage of Cercozoa sequences detected in sediment protist communities, we performed detailed phylogenetic analyses of the benthic Cercozoa. We carefully checked all representative sequences affiliated with the Cercozoa to ensure the performance of the phylogenetic analysis. The raw reads were generated from the orientation of the forward primer, while only sequences containing the accurate reverse primer (no mismatches) were retained in the subset of Cercozoa. All resulting sequences were aligned using MAFFT with the E‐INS‐i method, and the reverse primer was excluded. Each sequence was then manually checked using BLAST against the GenBank database. If a sequence had a similarity lower than 90% with the GenBank top hit and was rare (relative abundance <1% in all samples), we removed it from the data set. Reference sequences were added to perform phylogenetic analyses, and the whole sequences were aligned using the E‐INS‐i method. We manually trimmed positions with >95% gaps in each aligned column. A maximum‐likelihood phylogenetic tree was constructed using PhyML (Guindon et al., 2010) with 1,000 bootstraps and the GTR + G + I model.
2.5. Statistical analysis
Rarefaction analyses were performed to examine the degree of sampling saturation. To compare the OTU richness among the six sediment samples, we calculated nonparametric richness estimators (Chao1 and Shannon indexes). Chao1 and Shannon indexes were estimated based on the standardized data of 5,792 sequences per sample using the vegan package (Oksanen et al., 2014). To compare community dissimilarities, we performed phylogenetically informed beta diversity analyses using the weighted UniFrac distance metric (Lozupone & Knight, 2005) implemented in the QIIME pipeline (based on a standardized OTU table of 5,792 sequences per sample). Principal coordinates analysis (PCoA) was conducted on the weighted UniFrac distances to display the results. To further examine community dissimilarities (i.e., weighted UniFrac distance) against water depth, a Mantel test was performed using the vegan package. However, we could not rule out that other unmeasured environmental factors might also be important in shaping these benthic protist communities.
Null model analysis was performed to estimate the relative importance of different ecological processes (i.e., species sorting, dispersal limitation, mass effects, and drift) using the framework of Stegen et al. (2013). First, the between‐community variation in βMNTD was calculated based on the rarified OTU table (5,792 sequences per sample) using the picante package (Kembel et al., 2010). The degree to which the observed βMNTD deviated from a null model expectation was quantified after 999 randomizations. Standardized effect sizes of βMNTD (i.e., βNTI) <−2 or >2 indicated that compositional differences between community pairs were driven by species sorting. Second, we calculated the Raup‐Crick dissimilarity metric (RCbray) for each community pair (999 null iterations) for cases of |βNTI| <2. RCbray values >+0.95, <−0.95, and between −0.95 and +0.95 were assumed to indicate the operation of dispersal limitation, mass effects, and drift, respectively. Statistical analyses were mainly conducted in R (R Core Team, 2018).
3. RESULTS
3.1. Water column environment
Vertical hydrographic profiles of the upper waters indicated that the sampling sites were characterized by low temperature (e.g., 5.8°C at a 796 m depth at ST65; Figure A1a) and high salinity (e.g., 34.5 psu at a 795 m depth at ST61; Figure A1b), except for the coastal site ST76 (21.7°C and 34.2 psu at a 61 m depth). However, detailed in situ environmental variables were unavailable for sediments.
3.2. Benthic diversity
Pyrosequencing recovered a total of 74,091 quality‐filtered reads (5,792–21,009 reads per sample) that were grouped into 269–408 OTUs per sample (Table 1). The rarefaction curves of the observed OTUs showed unsaturated sampling profiles for all six samples (Figure 2), indicating high diversity of benthic protists. ST81 and ST65 had the lowest and highest richness, respectively, based on an equal sequencing depth of 5,792 reads (Table 1).
Figure 2.
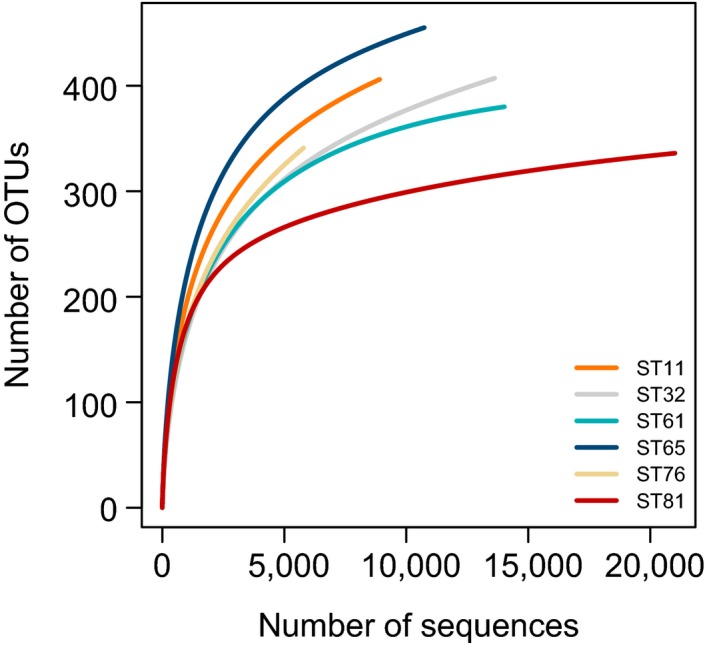
Rarefaction curves of observed OTUs for the six samples indicated by different colors. OTUs, operational taxonomic units
Based on the read‐based community patterns, Cercozoa was the most abundant group, accounting for proportions ranging from 27.3% (ST32) to 50.4% (ST61) (Figure 3a). Fungi were another abundant group, with an average proportion of 18.1% (Figure 3a). Remarkably, fungi comprised 38.3% of the total sequences at ST32 and were thus the most abundant group. Dinoflagellata also made substantial contributions ranging from 7.7% (ST81) to 24.1% (ST65). Radiolaria and stramenopiles_X had comparatively stable proportions across the six samples, showing an average of 7.6% and 6.7%, respectively. A few photosynthetic groups were retrieved, such as Cryptophyceae, Chlorophyta, Haptophyta, and Streptophyta. In particular, Cryptophyceae accounted for 2% of the sequences at ST65 (Figure 3a). Based on the OTU‐based community patterns, Cercozoa repeatedly appeared as the most abundant group, showing an average proportion of 25.3%, followed by Dinoflagellata (15.4%), stramenopiles_X (11.1%), and fungi (9.3%) (Figure 3b).
Figure 3.
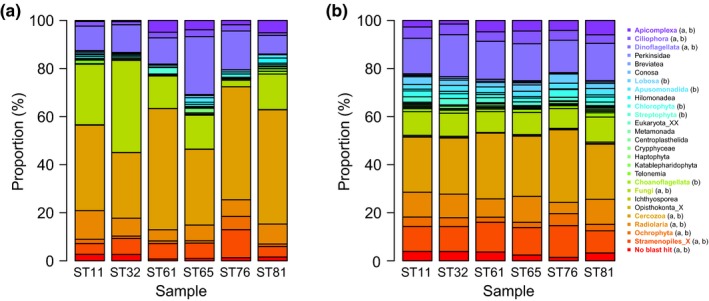
Taxonomic compositions (%) of reads (a) and OTUs (b). The groups showing an average contribution greater than 1% in the six samples are in bold and marked with corresponding colors in the bar plots, and the panel in which these groups are abundant (>1%) is indicated by the character in brackets. OTUs, operational taxonomic units
3.3. Cercozoa dominate benthic diversity
Sequences of Cercozoa were clustered into 180 OTUs belonging to 14 phylogenetic groups (Figure 4a), suggesting a striking diversity of benthic Cercozoa. Remarkably, a few phylogenetic groups belonged to unknown clades (e.g., Unknown Filosa Groups I, II, III, and IV; Unknown Endomyxa Groups I and II), indicating that they might be novel taxa. Ascetosporea, Euglyphida, and Thecofilosea, as the top 3 groups, contributed an average proportion of 32.1%, 11.4%, and 10%, respectively, to the total Cercozoa OTUs (Figure 4b and Table A1).
Figure 4.
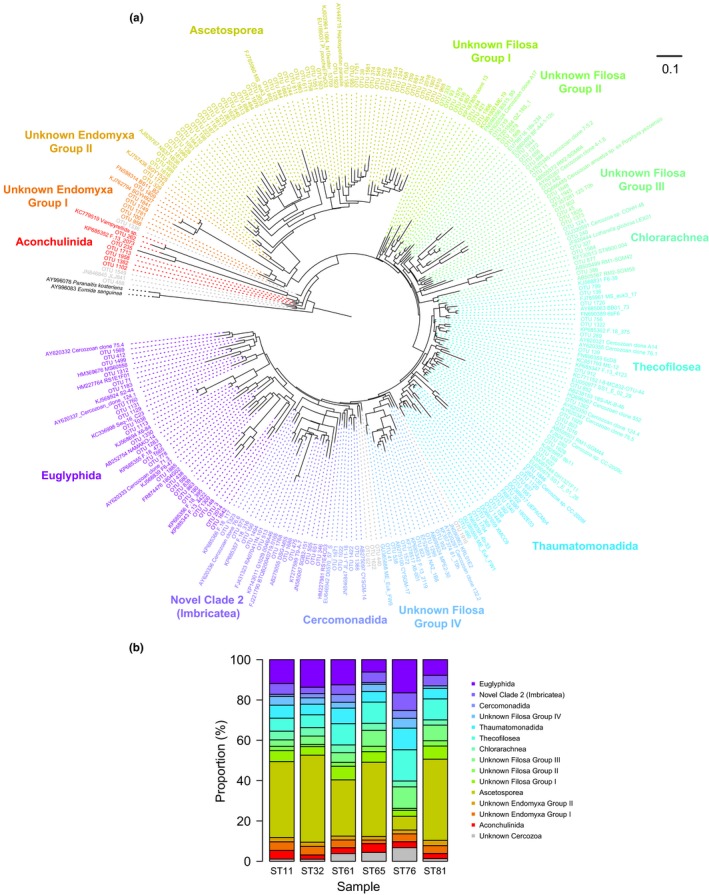
(a) Maximum‐likelihood tree inferred from 18S rDNA sequences of Cercozoa (376 positions) in surface sediments from the South China Sea. Taxa include the sequences obtained by pyrosequencing and reference sequences. Subgroups are color coded according to taxonomic assignments. The scale bar corresponds to 0.1 substitutions per base. (b) Relative contribution (%) of subclade OTUs to the total Cercozoa OTUs in each sample. OTUs, operational taxonomic units
3.4. Benthic community structure and assembly
Principal coordinates analysis plots using UniFrac dissimilarities showed that protist communities from different water depths were well separated (Figure 5), which suggested that water depth played an important role in shaping the benthic protist communities. Specifically, a linear regression using water depths and PCoA 1 values was significant and yielded an r 2 statistic of 0.77 (Pearson's coefficient, p < 0.05). This outcome that water depth shaped beta diversity was also supported by the result of the Mantel test, showing a significant correlation between water depths and the weighted UniFrac distances (r = 0.52; p < 0.05; permutations = 720).
Figure 5.
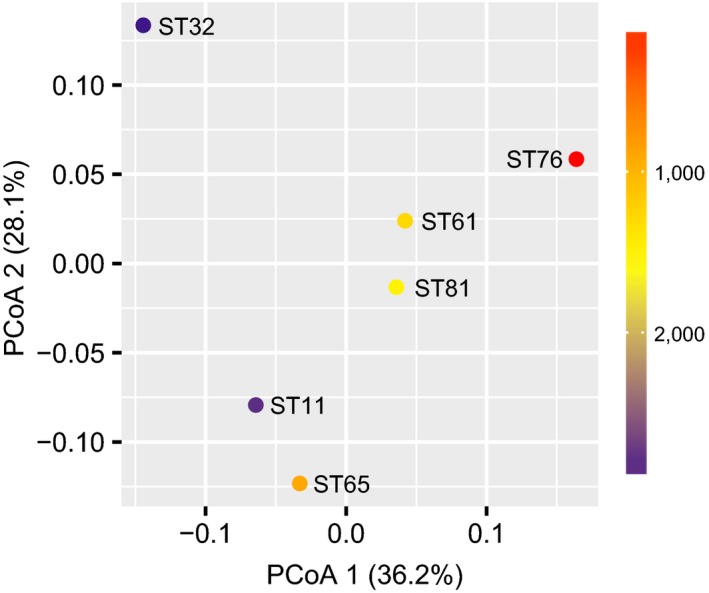
Principal coordinates analysis (PCoA) plots of the weighted UniFrac distance coupled with the water depth at each station (color coded in the heat map legend)
The results of the null model analysis showed that species sorting, dispersal limitation, mass effects, and drift accounted for 53.3%, 33.3%, 0%, and 13.3% of protist community pairs, respectively.
4. DISCUSSION
4.1. Diversity of benthic protists
First of all, our results uncovered the dominance of Cercozoa in protist communities of the surface sediments of the SCS (Figure 3). The dominance of Cercozoa suggests distinct microbial webs in surface sediments compared with planktonic ecosystems in the SCS, where protist communities are commonly dominated by Syndiniales (in pelagic waters) (Strassert et al., 2018; Wu, Huang, Liao, & Sun, 2014) and Radiolaria and Polycystinea (in bathypelagic waters) (Xu et al., 2017). In European coasts, the prevalence of Cercozoa generated a major difference in community composition between planktonic and benthic protists (Forster et al., 2016). However, Cercozoa failed to show dominance in estuarine sediments in Sydney Harbor (Chariton, Court, Hartley, Colloff, & Hardy, 2010) and the East China Sea (Jiang, Wang, Yu, & Liu, 2016). These disagreements support the idea that deep‐sea sediments harbor different protists than coastal and shallow‐sea sediments; thus, water depth can strongly influence benthic protist communities (Gong et al., 2015). However, it remains unclear whether the primer pair used in this study targeting the V1–V2 region, rather than the most often V4 and V9 regions, biases protist community patterns, which imposes potential effects on the dominance of Cercozoa.
A number of other groups, in addition to Cercozoa, made considerable contributions to the protist communities (Figure 3). Fungi are crucial players among marine benthos (Pasulka et al., 2016). Fungal species thrive and exhibit metabolic activities in subsurface sediments from the Peru Margin and the Peru Trench (Edgcomb, Beaudoin, Gast, Biddle, & Teske, 2011). Interestingly, some photosynthetic groups (e.g., Bacillariophyceae, Haptophyta, Prasinophyceae, and Dinophyceae) have been detected in benthic environments. Ubiquitous healthy Bacillariophyceae were recently reported in the deep sea (Agusti et al., 2015), where they may survive in resting stages (Piredda et al., 2017). Again, since DNA signatures were used in this study, we cannot rule out the possibility that these species were from the upper waters (Capo, Debroas, Arnaud, & Domaizon, 2015). Some studies based on rRNA sequencing confirm the existence of active protists in marine sediments (Bernhard et al., 2014). For example, Bacillariophyceae rRNA sequences can even be detected in subsurface sediments, suggesting that rRNA may be more stable than previously considered in benthic environments (Orsi, Biddle, & Edgcomb, 2013). In addition, Haptophyta and Prasinophyceae in fjord sediments germinate, indicating their long‐term survival in a resting stage in up to 40‐year‐old sediment layers (Ellegaard, Moestrup, Joest Andersen, & Lundholm, 2016). Haptophyta species with metabolic activity were also detected in surface sediments of the Black Sea (Coolen & Shtereva, 2009). Similarly, Dinophyceae cysts can even be germinated from 100‐year‐old sediment archives from the northern Baltic Sea (Kremp, Hinners, Klais, Leppänen, & Kallio, 2018).
4.2. Cercozoa dominate benthic diversity
This study detected a large number of Cercozoa OTUs (Figure 4), indicating a high diversity of ecological functions of Cercozoa (Bass & Cavalier‐Smith, 2004). For example, OTU262 is closely related to the predatory vampire amoebae (Berney et al., 2013), showing a similarity of 96% (Table A1). Several Ascetosporea OTUs are affiliated with 5 taxa with parasitical life styles (Sierra et al., 2016). Within these 5 taxa, the Paradinium poucheti isolate PaOi30 was isolated from the copepod host Oithona similis (Skovgaard & Daugbjerg, 2008) and the spot prawn Pandalus platyceros (Reece, Siddall, Stokes, & Burreson, 2004). In addition, several OTUs (e.g., OTU1648 and OTU1945) are closely related to a parasitical Cercozoan amoeba sp. (ex Porphyra yezoensis) belonging to the unknown Filosa Group III.
Notably, the top hits of Cercozoa OTUs originated from diverse habitats (Table A1), indicating that marine sediments are an outstanding reservoir of life. The majority of the OTUs were affiliated with taxa derived from benthos. For example, these representative taxa included A17 (unknown Filosa Group II) from the low‐tide sand of Vancouver Island (Bass & Cavalier‐Smith, 2004), RM1‐SGM42 (Chlorarachnea) from deep‐sea cold seep sediments (Takishita, Kakizoe, Yoshida, & Maruyama, 2010), JLJ‐11‐18 (Cercomonadida) from urban surface sediments, and NAMAKO‐14 (Euglyphida) from anoxic sediments (Takishita et al., 2007). Moreover, a set of planktonic species were included in the closest taxa, such as NS371B38 (Ascetosporea) from the 100 m water depth of the SCS (Yuan et al., 2004), BS15_B5 (unknown Filosa Group II) from the 2,593 m water depth surrounding chimneys (Sauvadet, Gobet, & Guillou, 2010), 1802E03 (Thaumatomonadida) from coastal water (Newbold et al., 2012), and RS1E4C03 (Novel Clade 2) from the Arraial do Cabo upwelling (Cury et al., 2011). These results support the idea that DNA from planktonic protists can be detected in marine sediments (Capo et al., 2015). Remarkably, unknown Filosa Group III is characterized by some taxa that were originally detected in forest soil (18s‐234) and anoxic slurries of an agricultural soil (125 T0h) (Chatzinotas, Schellenberger, Glaser, & Kolb, 2013). In contrast, unknown Filosa Group IV contains taxa from freshwaters, for example, KRL01E2 from Karla Lake, Greece (Oikonomou, Katsiapi, Karayanni, Moustaka‐Gouni, & Kormas, 2012), and MPE2‐30 from Hotoke‐Ike Lake, Antarctica (Nakai et al., 2012). The complexity of these closest retrieves indicates the existence of many potentially novel groups of protists in marine sediments.
4.3. Community assembly of benthic protists
Protist communities in the basin‐wide surface sediments of the SCS are mainly shaped by species sorting and dispersal limitation. This finding supports the idea that species sorting and dispersal limitation are the two key drivers of microbial community assembly in marine sediments (Petro et al., 2017). Moreover, the relative importance of species sorting indicates that benthic habitats are strongly different. Water depth may act as an important factor shaping benthic protist communities. The relationship between community dissimilarity and water depth agrees with the so‐called depth decay in marine sediments (Jacob et al., 2013). However, it should be noted that water depth may have been a proxy of a set of associated environmental variables that were unmeasured in this study. That is, benthic protist communities may be structured by something other than the water depth itself. Marine sediments represent extreme energy‐limited habitats in which species sorting can predominantly assemble benthic communities (Starnawski et al., 2017). In addition to abiotic conditions, biotic interactions can also influence benthic protist communities (i.e., top‐down controls). For example, benthic protists can impose significant grazing effects on bacterial community patterns and further influence hydrocarbon‐degrading processes in marine sediments (Beaudoin et al., 2016). This kind of driving force contributes to the relative importance of species sorting in protist communities because bacterial communities are also under selective pressure from local environments. However, the resting stage of some groups, such as Bacillariophyceae (Piredda et al., 2017), Haptophyta, Prasinophyceae (Ellegaard et al., 2016), and Dinophyceae (Kremp et al., 2018), may weaken species sorting because dormant taxa respond weakly to local environmental conditions.
The relative importance of dispersal limitation suggests that slow deep‐sea circulations (Wang, Xie, Qu, & Huang, 2011) contribute little to the dispersal of protists but generate an ecological barrier. It has been reported that benthic bacteria can show steeper distance‐decay curves than both surface‐sea and deep‐sea bacteria can (Zinger, Boetius, & Ramette, 2014). This difference may mainly result from the difference in the extent of dispersal potential of microorganisms between benthic and planktonic habitats. In contrast, Chen et al. (2017) showed that protist communities in intertidal sediments were strongly governed by spatial processes, potentially because the passive dispersal of protists contributed by water currents is very intense (i.e., mass effects) in shallow sediments relative to deep‐sea sediments. Again, disentangling protist communities can be obscured by the limitation that sedimentary DNA may be from numerous planktonic groups (Capo et al., 2015) that are not part of the indigenous and active protist community.
5. CONCLUSION
Our results provide baseline information on the diversity and community assembly of benthic protists in the subtropical‐tropical SCS. We show that the highly diverse Cercozoa group dominates the protist communities at the basin scale, and species sorting and dispersal limitation represent the two main forces that drive the community assembly of the benthic protists. Finally, we propose that more efforts, such as RNA‐based surveys, are needed to unveil the hidden diversity and function of protists in marine sediments.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
W.W. and B.H. conceived the study. W.W. collected sediment samples and conducted molecular laboratory work. W.W. and B.H. contributed to the data interpretation and the writing of the manuscript.
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
This work was also supported by the National Key Research and Development Program (2016YFA0601201). W.W. was supported by funds (09530‐18070002 and 42000‐18841200) provided by Sun Yat‐sen University. We thank H.M. at the South China Sea Institute of Oceanography (CAS) for the hydrographic data.
APPENDIX 1.
Table A1.
Summary of operational taxonomic unit (OTU; 97% similarity level) assignments of the benthic Cercozoa recovered in this study. For each OTU, we provide the closest relative in GenBank with the accession number (20‐Mar‐2016 database), sequence percent similarity, BLAST score, query/subject ratio, relative abundance (%) in each sample, and taxonomy
| Clone | Closest relative | GenBank Accession | Similarity (%) | BLAST score | Query/Subject | ST11 | ST32 | ST61 | ST65 | ST76 | ST81 | Taxonomy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OTU1102 | Uncultured eukaryote band JLJ‐8‐41 | JN846845 | 93 | 540 | 343/368 | 0.29 | 0.16 | 0.06 | 0.42 | 0.10 | 0.22 | Aconchulinida |
| OTU1382 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 92 | 527 | 343/371 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | Aconchulinida |
| OTU1713 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 91 | 503 | 337/369 | 0.04 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | Aconchulinida |
| OTU1958 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 93 | 536 | 343/369 | 0.02 | 0.00 | 0.00 | 0.19 | 0.17 | 0.00 | Aconchulinida |
| OTU235 | Uncultured Rhizaria clone F.13_2073 | KP685352 | 90 | 473 | 335/371 | 0.00 | 0.02 | 0.45 | 0.05 | 0.00 | 0.00 | Aconchulinida |
| OTU262 | Vampyrellida sp. KibAr | KC779519 | 96 | 604 | 355/368 | 0.00 | 0.00 | 0.31 | 0.01 | 0.00 | 0.23 | Aconchulinida |
| OTU1091 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 90 | 477 | 331/366 | 0.41 | 0.19 | 0.04 | 0.01 | 0.00 | 0.17 | Ascetosporea |
| OTU1110 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 91 | 490 | 333/365 | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 | 0.01 | Ascetosporea |
| OTU1156 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 96 | 597 | 350/363 | 2.52 | 3.27 | 0.33 | 0.88 | 0.03 | 2.64 | Ascetosporea |
| OTU1161 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 92 | 508 | 339/369 | 0.00 | 0.00 | 0.00 | 1.50 | 0.00 | 0.01 | Ascetosporea |
| OTU1187 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 94 | 555 | 344/365 | 0.35 | 0.64 | 0.00 | 0.21 | 0.00 | 0.01 | Ascetosporea |
| OTU1244 | Uncultured eukaryote clone 1084_ts10water_13709 | KJ003964 | 90 | 459 | 328/366 | 0.11 | 1.73 | 0.09 | 2.64 | 0.00 | 0.06 | Ascetosporea |
| OTU1247 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 94 | 542 | 340/363 | 2.89 | 2.51 | 0.40 | 1.97 | 1.00 | 2.64 | Ascetosporea |
| OTU125 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 93 | 532 | 340/365 | 0.01 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | Ascetosporea |
| OTU1278 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 91 | 484 | 333/366 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU128 | Uncultured eukaryote clone 1084_ts10water_13709 | KJ003964 | 91 | 484 | 329/362 | 0.08 | 0.01 | 0.01 | 0.10 | 0.00 | 0.01 | Ascetosporea |
| OTU134 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 91 | 494 | 335/367 | 0.07 | 0.19 | 0.07 | 0.03 | 0.00 | 0.00 | Ascetosporea |
| OTU1513 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 91 | 486 | 332/365 | 0.52 | 0.34 | 2.61 | 0.01 | 0.00 | 0.07 | Ascetosporea |
| OTU1514 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 93 | 529 | 338/363 | 0.04 | 0.04 | 0.01 | 0.00 | 0.00 | 0.03 | Ascetosporea |
| OTU1551 | Paradinium poucheti isolate PaOi30 | EU189031 | 92 | 518 | 343/373 | 0.32 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | Ascetosporea |
| OTU1581 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 94 | 553 | 342/363 | 0.05 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU1610 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 93 | 520 | 336/363 | 0.05 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU1616 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 90 | 477 | 332/367 | 0.03 | 2.65 | 1.11 | 0.32 | 9.63 | 0.01 | Ascetosporea |
| OTU1625 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 92 | 514 | 337/365 | 0.02 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU1751 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 94 | 553 | 342/363 | 0.09 | 0.19 | 0.02 | 0.07 | 0.00 | 0.30 | Ascetosporea |
| OTU1758 | Uncultured eukaryote clone 1084_ts10water_13709 | KJ003964 | 90 | 466 | 327/363 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU1807 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 91 | 484 | 332/366 | 0.00 | 0.01 | 0.00 | 0.05 | 0.00 | 0.00 | Ascetosporea |
| OTU1814 | Uncultured marine eukaryote clone NS371B38 | AJ829787 | 95 | 577 | 354/374 | 0.00 | 0.03 | 0.01 | 0.01 | 0.00 | 0.00 | Ascetosporea |
| OTU1817 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 93 | 525 | 339/365 | 0.00 | 0.01 | 0.09 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU1863 | Uncultured eukaryote clone 1084_ts10water_13709 | KJ003964 | 91 | 490 | 333/365 | 0.00 | 0.01 | 0.00 | 0.04 | 0.00 | 0.00 | Ascetosporea |
| OTU1882 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 90 | 475 | 331/366 | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | 0.00 | Ascetosporea |
| OTU1923 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 95 | 571 | 347/365 | 0.46 | 0.24 | 0.32 | 0.28 | 0.00 | 0.23 | Ascetosporea |
| OTU1943 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 93 | 532 | 342/367 | 0.32 | 0.17 | 0.00 | 0.27 | 0.00 | 0.01 | Ascetosporea |
| OTU1976 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 93 | 536 | 342/367 | 0.00 | 0.01 | 0.00 | 0.04 | 0.00 | 0.00 | Ascetosporea |
| OTU198 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 94 | 549 | 343/365 | 0.00 | 0.08 | 0.00 | 0.07 | 0.00 | 0.75 | Ascetosporea |
| OTU1980 | Uncultured marine eukaryote clone NS371B38 | AJ829787 | 98 | 636 | 364/373 | 0.10 | 0.94 | 0.39 | 0.29 | 0.07 | 0.13 | Ascetosporea |
| OTU2018 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 91 | 497 | 332/363 | 0.12 | 0.01 | 0.00 | 0.04 | 0.00 | 0.00 | Ascetosporea |
| OTU2032 | Uncultured marine eukaryote clone NS371B38 | AJ829787 | 95 | 584 | 353/371 | 0.00 | 0.02 | 0.00 | 0.02 | 0.00 | 0.00 | Ascetosporea |
| OTU2033 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 97 | 619 | 355/365 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU223 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 93 | 529 | 339/365 | 0.00 | 0.01 | 0.01 | 0.07 | 0.00 | 0.00 | Ascetosporea |
| OTU258 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 92 | 503 | 335/365 | 0.09 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU261 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 89 | 451 | 326/365 | 0.00 | 0.00 | 0.11 | 1.94 | 0.00 | 0.05 | Ascetosporea |
| OTU374 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 94 | 547 | 341/363 | 0.25 | 0.02 | 0.00 | 0.07 | 0.00 | 0.00 | Ascetosporea |
| OTU38 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 93 | 538 | 339/363 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.19 | Ascetosporea |
| OTU399 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 90 | 473 | 332/368 | 0.00 | 0.00 | 0.01 | 0.20 | 0.09 | 0.07 | Ascetosporea |
| OTU484 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 92 | 497 | 334/365 | 0.04 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | Ascetosporea |
| OTU503 | Paradinium poucheti isolate PaOi30 | EU189031 | 91 | 507 | 341/373 | 0.04 | 0.00 | 0.00 | 0.13 | 0.03 | 0.10 | Ascetosporea |
| OTU540 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 93 | 531 | 340/365 | 0.00 | 0.06 | 0.02 | 0.03 | 0.00 | 0.00 | Ascetosporea |
| OTU549 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 91 | 481 | 337/372 | 0.29 | 0.12 | 0.00 | 0.13 | 0.00 | 0.07 | Ascetosporea |
| OTU611 | Uncultured eukaryote clone 1084_ts10water_13709 | KJ003964 | 90 | 468 | 328/364 | 0.01 | 0.03 | 0.00 | 0.00 | 0.00 | 0.04 | Ascetosporea |
| OTU702 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 93 | 520 | 338/365 | 0.01 | 0.16 | 0.00 | 0.02 | 0.00 | 0.00 | Ascetosporea |
| OTU709 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 94 | 542 | 340/363 | 0.00 | 0.06 | 0.06 | 0.06 | 0.00 | 0.00 | Ascetosporea |
| OTU768 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 94 | 544 | 341/364 | 0.05 | 0.04 | 0.04 | 0.01 | 0.00 | 0.00 | Ascetosporea |
| OTU802 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 95 | 569 | 347/365 | 0.01 | 0.08 | 0.00 | 0.10 | 0.02 | 0.08 | Ascetosporea |
| OTU811 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 90 | 466 | 328/365 | 0.00 | 4.70 | 0.19 | 0.12 | 0.00 | 0.00 | Ascetosporea |
| OTU844 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 93 | 540 | 344/368 | 0.01 | 0.08 | 0.00 | 0.01 | 0.00 | 0.00 | Ascetosporea |
| OTU886 | Uncultured eukaryote band MS_euk3_18 | FJ785960 | 92 | 516 | 338/366 | 0.26 | 3.10 | 0.64 | 0.28 | 0.00 | 0.41 | Ascetosporea |
| OTU891 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 91 | 497 | 332/363 | 0.00 | 0.20 | 0.02 | 0.02 | 0.00 | 0.02 | Ascetosporea |
| OTU950 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 94 | 558 | 343/363 | 0.10 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU965 | Haplosporidian parasite of Pandalus platyceros clone 3 | AY449715 | 91 | 492 | 331/363 | 0.02 | 0.08 | 0.04 | 0.00 | 0.00 | 0.00 | Ascetosporea |
| OTU1022 | Uncultured marine eukaryote clone I‐8‐MC832‐OTU‐44 | KC771152 | 94 | 564 | 348/369 | 0.00 | 0.00 | 0.13 | 0.09 | 0.07 | 0.00 | Cercomonadida |
| OTU1366 | Uncultured eukaryote clone CYSGM‐14 | AB275097 | 96 | 599 | 352/365 | 0.00 | 0.03 | 0.14 | 0.00 | 0.03 | 0.00 | Cercomonadida |
| OTU1422 | Uncultured eukaryote band JLJ‐11‐18 | JN846847 | 97 | 627 | 360/370 | 0.00 | 0.15 | 0.18 | 0.00 | 0.07 | 0.00 | Cercomonadida |
| OTU1671 | Uncultured marine picoeukaryote clone 1802000 | FR874390 | 95 | 575 | 351/370 | 0.01 | 0.00 | 1.16 | 0.00 | 0.24 | 0.00 | Cercomonadida |
| OTU1241 | Uncultured eukaryote clone 18s2‐34 | EU798716 | 95 | 571 | 351/371 | 0.03 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | Chlorarachnea |
| OTU1386 | Uncultured eukaryote clone RM2‐SGM59 | AB505567 | 99 | 662 | 362/364 | 0.07 | 0.09 | 0.03 | 0.02 | 0.03 | 0.30 | Chlorarachnea |
| OTU1584 | Uncultured eukaryote clone ST8900.004 | KF130513 | 98 | 617 | 346/352 | 0.00 | 0.08 | 0.01 | 0.07 | 0.00 | 0.00 | Chlorarachnea |
| OTU240 | Uncultured eukaryote clone T0h‐125 | KF357281 | 92 | 521 | 341/370 | 0.00 | 0.00 | 0.00 | 0.05 | 0.02 | 0.00 | Chlorarachnea |
| OTU327 | Lotharella globosa strain LEX01 | JF826444 | 91 | 473 | 321/352 | 0.07 | 0.20 | 0.09 | 1.14 | 0.00 | 0.56 | Chlorarachnea |
| OTU877 | Uncultured eukaryote clone RM1‐SGM42 | AB505499 | 92 | 521 | 351/381 | 0.04 | 0.11 | 0.09 | 0.00 | 0.00 | 0.00 | Chlorarachnea |
| OTU1038 | Uncultured eukaryote clone Seq‐16_C23 | KC336998 | 99 | 662 | 360/361 | 0.01 | 0.00 | 0.13 | 0.29 | 1.00 | 0.28 | Euglyphida |
| OTU1113 | Uncultured eukaryote clone Seq‐16_C23 | KC336998 | 96 | 593 | 349/362 | 0.00 | 0.00 | 0.00 | 0.01 | 0.17 | 0.00 | Euglyphida |
| OTU1129 | Uncultured cercozoan clone 12‐4.1 | AY620337 | 92 | 514 | 343/372 | 0.00 | 0.03 | 0.04 | 0.00 | 0.00 | 0.00 | Euglyphida |
| OTU1131 | Uncultured cercozoan clone 12‐4.1 | AY620337 | 94 | 568 | 352/373 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.04 | Euglyphida |
| OTU1183 | Uncultured eukaryote clone S2‐44 | KJ568924 | 91 | 486 | 336/370 | 0.00 | 0.01 | 0.00 | 0.00 | 0.16 | 0.00 | Euglyphida |
| OTU1283 | Uncultured eukaryote clone NAMAKO‐14 | AB252754 | 99 | 652 | 353/356 | 0.00 | 0.01 | 0.00 | 0.12 | 0.02 | 0.18 | Euglyphida |
| OTU1300 | Uncultured cercozoan clone F.18_395 | KP685356 | 93 | 538 | 342/366 | 0.00 | 0.03 | 0.02 | 0.00 | 0.00 | 0.00 | Euglyphida |
| OTU1312 | Uncultured eukaryote clone MS605‐58 | HM369676 | 92 | 523 | 341/369 | 0.08 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | Euglyphida |
| OTU1330 | Uncultured eukaryote clone X6‐84 | KJ568694 | 99 | 638 | 355/360 | 0.05 | 0.17 | 0.00 | 0.01 | 0.00 | 0.00 | Euglyphida |
| OTU148 | Uncultured eukaryote clone 18S‐AK‐B‐46 | AB238153 | 93 | 532 | 342/368 | 0.00 | 0.06 | 0.02 | 0.00 | 0.00 | 0.00 | Euglyphida |
| OTU1499 | Cercomonadida clone D0570_57_S | EU646942 | 96 | 595 | 355/370 | 0.00 | 0.00 | 0.00 | 0.03 | 0.10 | 0.00 | Euglyphida |
| OTU1569 | Uncultured cercozoan clone 7‐5.4 | AY620332 | 95 | 580 | 350/368 | 0.01 | 0.00 | 1.72 | 0.00 | 0.02 | 0.00 | Euglyphida |
| OTU1642 | Uncultured cercozoan clone 7‐1.3 | AY620333 | 91 | 492 | 337/370 | 0.02 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | Euglyphida |
| OTU1665 | Uncultured cercozoan clone F.18_473 | KP685355 | 94 | 556 | 350/373 | 0.07 | 0.00 | 0.01 | 0.00 | 0.02 | 0.00 | Euglyphida |
| OTU1760 | Uncultured cercozoan clone 12‐4.1 | AY620337 | 99 | 675 | 366/367 | 0.00 | 0.00 | 0.00 | 0.01 | 0.47 | 0.00 | Euglyphida |
| OTU180 | Uncultured cercozoan clone F.13_4422 | KP685343 | 92 | 503 | 339/370 | 0.00 | 0.00 | 0.08 | 0.00 | 0.02 | 0.00 | Euglyphida |
| OTU1885 | Uncultured eukaryote clone F6‐47 | KJ568839 | 96 | 593 | 535/368 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.11 | Euglyphida |
| OTU2014 | Uncultured cercozoan clone DH1321F11 | KM067448 | 91 | 486 | 334/369 | 0.00 | 0.01 | 0.03 | 0.00 | 0.00 | 0.00 | Euglyphida |
| OTU3 | Uncultured eukaryote clone X6‐001 | KF378517 | 91 | 486 | 338/373 | 0.23 | 0.19 | 0.31 | 0.00 | 0.00 | 0.00 | Euglyphida |
| OTU412 | Uncultured cercozoan clone 7‐5.4 | AY620332 | 98 | 628 | 359/368 | 0.40 | 0.00 | 0.48 | 0.00 | 0.07 | 0.00 | Euglyphida |
| OTU438 | Uncultured marine picoeukaryote clone 1804G02 | FR874476 | 95 | 568 | 348/368 | 0.51 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | Euglyphida |
| OTU580 | Uncultured eukaryote clone G1029.0046A46 | KP143011 | 92 | 518 | 340/369 | 0.03 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | Euglyphida |
| OTU71 | Uncultured eukaryote clone RS1E1F01 | HM227764 | 99 | 643 | 358/363 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.10 | Euglyphida |
| OTU778 | Uncultured cercozoan clone F.18_473 | KP685355 | 94 | 564 | 351/372 | 0.00 | 0.01 | 0.24 | 0.00 | 0.00 | 0.00 | Euglyphida |
| OTU839 | Uncultured cercozoan clone 13‐2.2 | AY620297 | 92 | 508 | 339/369 | 0.00 | 0.01 | 0.07 | 0.00 | 0.02 | 0.00 | Euglyphida |
| OTU863 | Uncultured cercozoan clone F.18_395 | KP685356 | 94 | 547 | 346/369 | 0.90 | 0.02 | 1.05 | 0.44 | 0.17 | 1.02 | Euglyphida |
| OTU1323 | Uncultured cercozoan clone F.18_117 | KP685366 | 99 | 673 | 366/367 | 0.01 | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | Noveb Clade 2 (Imbricatea) |
| OTU1494 | Uncultured eukaryote clone G1029.0046A46 | KP143011 | 94 | 562 | 347/368 | 0.00 | 0.00 | 0.00 | 0.09 | 0.21 | 0.00 | Noveb Clade 2 (Imbricatea) |
| OTU150 | Uncultured cercozoan clone F.18_316 | KP685357 | 97 | 610 | 354/365 | 0.00 | 0.00 | 0.02 | 0.03 | 0.47 | 0.00 | Noveb Clade 2 (Imbricatea) |
| OTU1688 | Uncultured eukaryote clone DSGM‐55 | AB275055 | 99 | 667 | 363/364 | 0.02 | 0.01 | 0.12 | 0.11 | 4.18 | 0.18 | Noveb Clade 2 (Imbricatea) |
| OTU1768 | Uncultured marine eukaryote clone BTQB20040719.0165 | FJ221790 | 98 | 628 | 354/361 | 0.01 | 0.03 | 0.02 | 0.16 | 0.02 | 0.00 | Noveb Clade 2 (Imbricatea) |
| OTU346 | Uncultured eukaryote clone RS1E4C03 | HM227981 | 92 | 523 | 343/371 | 0.00 | 0.00 | 0.01 | 0.00 | 0.26 | 0.00 | Noveb Clade 2 (Imbricatea) |
| OTU475 | Uncultured marine eukaryote clone SGB2‐151 | JN585087 | 97 | 606 | 353/365 | 0.00 | 0.00 | 0.00 | 0.20 | 0.36 | 0.03 | Noveb Clade 2 (Imbricatea) |
| OTU505 | Uncultured marine eukaryote clone SGB2‐151 | JN585087 | 94 | 544 | 346/370 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.23 | Noveb Clade 2 (Imbricatea) |
| OTU513 | Uncultured marine cercozoan clone RA070411N.101 | FJ431323 | 95 | 568 | 350/370 | 0.00 | 0.00 | 0.00 | 0.03 | 0.12 | 0.00 | Noveb Clade 2 (Imbricatea) |
| OTU573 | Uncultured cercozoan clone 7‐6.6 | AY620336 | 94 | 560 | 346/367 | 0.01 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | Noveb Clade 2 (Imbricatea) |
| OTU651 | Uncultured eukaryote clone T9‐A‐7 | KT277599 | 91 | 486 | 336/371 | 0.00 | 0.04 | 0.00 | 0.00 | 0.02 | 0.00 | Noveb Clade 2 (Imbricatea) |
| OTU1120 | Cercozoa sp. CC‐2009f | FJ824130 | 93 | 538 | 344/370 | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 | 0.09 | Thaumatomonadida |
| OTU1340 | Uncultured marine picoeukaryote clone 1802E03 | FR874390 | 96 | 593 | 354/370 | 0.00 | 0.02 | 0.00 | 0.00 | 0.09 | 0.00 | Thaumatomonadida |
| OTU1448 | Uncultured eukaryotic clone XMCC9 | DQ667659 | 95 | 580 | 352/370 | 0.00 | 0.00 | 0.15 | 0.00 | 0.03 | 0.00 | Thaumatomonadida |
| OTU158 | Uncultured marine eukaryote clone UEPACMp4 | DQ369017 | 96 | 597 | 356/371 | 0.01 | 0.01 | 0.00 | 0.00 | 2.66 | 0.00 | Thaumatomonadida |
| OTU1704 | Uncultured cercozoan clone 4b‐33 | FN690391 | 98 | 634 | 363/372 | 2.40 | 1.36 | 2.41 | 2.85 | 0.26 | 5.26 | Thaumatomonadida |
| OTU1846 | Uncultured Rhizaria clone F.13_4123 | KP685347 | 95 | 577 | 350/369 | 0.00 | 0.00 | 0.00 | 0.08 | 0.02 | 0.00 | Thaumatomonadida |
| OTU1951 | Uncultured marine picoeukaryote clone 1802E03 | FR874390 | 96 | 590 | 353/369 | 0.00 | 0.00 | 0.08 | 0.00 | 0.07 | 0.00 | Thaumatomonadida |
| OTU302 | Uncultured marine picoeukaryote clone 1802E03 | FR874390 | 100 | 682 | 369/369 | 0.18 | 0.01 | 8.73 | 0.33 | 0.31 | 0.18 | Thaumatomonadida |
| OTU362 | Cercomonadida clone D0570_57_S | EU646942 | 92 | 507 | 337/368 | 0.08 | 0.00 | 0.01 | 0.01 | 0.02 | 0.00 | Thaumatomonadida |
| OTU369 | Uncultured cercozoan clone 4b‐33 | FN690391 | 97 | 621 | 363/375 | 0.08 | 0.01 | 0.00 | 0.02 | 0.02 | 0.02 | Thaumatomonadida |
| OTU490 | Uncultured marine eukaryote clone ME_Euk_FW1 | GU385595 | 97 | 608 | 356/368 | 0.01 | 0.00 | 0.10 | 0.01 | 1.52 | 0.00 | Thaumatomonadida |
| OTU637 | Cercozoa sp. CC‐2009f | FJ824130 | 93 | 544 | 346/371 | 0.00 | 0.00 | 1.83 | 0.00 | 0.05 | 0.00 | Thaumatomonadida |
| OTU1016 | Uncultured eukaryote clone RM1‐SGM44 | AB505501 | 96 | 595 | 351/365 | 0.00 | 0.04 | 0.00 | 0.00 | 0.03 | 0.00 | Thecofilosea |
| OTU1322 | Uncultured Rhizaria clone F.18_375 | KP685362 | 99 | 658 | 365/369 | 0.60 | 1.25 | 3.62 | 1.15 | 9.65 | 7.87 | Thecofilosea |
| OTU136 | Uncultured eukaryote band MS_euk3_17 | FJ785961 | 96 | 604 | 356/370 | 0.00 | 0.00 | 0.00 | 0.12 | 0.07 | 0.00 | Thecofilosea |
| OTU1362 | Uncultured cercozoan clone 552 | HQ696567 | 93 | 534 | 344/370 | 0.00 | 0.00 | 0.04 | 0.06 | 0.00 | 0.00 | Thecofilosea |
| OTU139 | Uncultured cercozoan clone 6c‐D8 | FN690359 | 99 | 658 | 364/368 | 0.11 | 0.04 | 0.18 | 0.01 | 0.35 | 0.08 | Thecofilosea |
| OTU1628 | Uncultured eukaryote clone 8b11 | KJ925997 | 98 | 649 | 363/369 | 0.00 | 0.00 | 0.16 | 0.00 | 0.03 | 0.00 | Thecofilosea |
| OTU168 | Uncultured eukaryote clone SS1_E_01_26 | EU050975 | 98 | 630 | 359/368 | 0.00 | 0.00 | 0.01 | 0.00 | 0.02 | 0.00 | Thecofilosea |
| OTU1726 | Uncultured eukaryote clone BB01_73 | AY885063 | 98 | 651 | 366/372 | 0.00 | 0.00 | 0.00 | 0.01 | 0.05 | 0.00 | Thecofilosea |
| OTU1929 | Uncultured cercozoan clone 7‐5.5 | AY620347 | 97 | 617 | 359/371 | 0.08 | 0.01 | 0.00 | 0.04 | 0.02 | 0.00 | Thecofilosea |
| OTU280 | Cercozoa sp. CC‐2009c | FJ824127 | 98 | 638 | 363/371 | 0.00 | 0.00 | 0.16 | 0.06 | 0.05 | 0.07 | Thecofilosea |
| OTU289 | Uncultured cercozoan clone A14 | AY620321 | 97 | 621 | 358/369 | 0.00 | 0.00 | 0.03 | 0.00 | 0.02 | 0.00 | Thecofilosea |
| OTU622 | Uncultured eukaryote clone SS1_E_01_26 | EU050975 | 99 | 652 | 364/369 | 1.06 | 1.66 | 13.25 | 2.53 | 2.33 | 15.02 | Thecofilosea |
| OTU756 | Uncultured cercozoan clone 6b‐F6 | FN690389 | 98 | 640 | 364/372 | 0.00 | 0.00 | 0.02 | 0.04 | 0.07 | 0.00 | Thecofilosea |
| OTU799 | Uncultured eukaryote band MS_euk3_17 | FJ785961 | 98 | 643 | 362/369 | 0.00 | 0.00 | 0.04 | 0.07 | 0.29 | 0.00 | Thecofilosea |
| OTU803 | Uncultured cercozoan clone 12‐4.4 | AY620350 | 97 | 621 | 359/370 | 0.01 | 0.00 | 0.02 | 0.16 | 0.03 | 0.12 | Thecofilosea |
| OTU852 | Uncultured eukaryote clone SS1_E_02_29 | EU050977 | 99 | 656 | 366/371 | 0.01 | 0.01 | 0.00 | 0.00 | 0.14 | 0.00 | Thecofilosea |
| OTU912 | Uncultured Rhizaria clone F.13_4123 | KP685347 | 97 | 614 | 357/369 | 0.00 | 0.00 | 0.00 | 0.14 | 0.02 | 0.00 | Thecofilosea |
| OTU1575 | Uncultured cercozoan clone BS15_B5 | FN598356 | 92 | 516 | 340/370 | 0.00 | 0.01 | 0.03 | 0.20 | 0.00 | 0.00 | Unknown Filosa Group I |
| OTU1906 | Uncultured microeukaryote clone ME‐19 | KC851800 | 99 | 665 | 364/366 | 0.07 | 0.06 | 0.03 | 0.07 | 0.19 | 0.20 | Unknown Filosa Group I |
| OTU244 | Uncultured eukaryote clone 13 | KP187808 | 95 | 580 | 351/369 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | Unknown Filosa Group I |
| OTU333 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 92 | 525 | 345/374 | 0.26 | 0.10 | 0.05 | 0.02 | 0.02 | 0.06 | Unknown Filosa Group I |
| OTU421 | Uncultured eukaryote clone 13 | KP187808 | 91 | 505 | 345/378 | 0.01 | 0.00 | 0.03 | 0.04 | 0.00 | 0.00 | Unknown Filosa Group I |
| OTU616 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 95 | 586 | 353/370 | 0.00 | 0.00 | 0.05 | 0.07 | 0.02 | 0.00 | Unknown Filosa Group I |
| OTU857 | Uncultured eukaryote clone RM2‐SGM59 | AB505567 | 92 | 505 | 339/370 | 0.11 | 0.00 | 0.02 | 0.00 | 0.00 | 0.22 | Unknown Filosa Group I |
| OTU93 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 92 | 514 | 341/371 | 0.12 | 0.02 | 0.01 | 0.00 | 0.00 | 0.15 | Unknown Filosa Group I |
| OTU1578 | Uncultured cercozoan clone A17 | AY620324 | 93 | 534 | 346/374 | 0.01 | 0.04 | 0.02 | 0.02 | 0.14 | 0.00 | Unknown Filosa Group II |
| OTU219 | Uncultured eukaryote clone QZ.18S_1 | KT201564 | 97 | 627 | 362/373 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.04 | Unknown Filosa Group II |
| OTU569 | Uncultured cercozoan clone A17 | AY620324 | 94 | 571 | 352/373 | 0.03 | 0.00 | 0.16 | 0.01 | 0.00 | 0.06 | Unknown Filosa Group II |
| OTU11 | Uncultured eukaryote clone RM2‐SGM64 | AB505572 | 99 | 656 | 362/365 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.10 | Unknown Filosa Group III |
| OTU1372 | Uncultured eukaryote clone T0h‐125 | KF357281 | 92 | 514 | 342/373 | 0.00 | 0.00 | 0.01 | 0.00 | 0.35 | 0.00 | Unknown Filosa Group III |
| OTU1373 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 94 | 566 | 349/370 | 0.00 | 0.00 | 0.00 | 0.02 | 0.21 | 0.01 | Unknown Filosa Group III |
| OTU1648 | Cercozoan amoeba sp. ex Porpyra yezoensis | DQ666485 | 90 | 490 | 341/377 | 0.00 | 0.00 | 0.00 | 0.03 | 0.10 | 0.00 | Unknown Filosa Group III |
| OTU1826 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 94 | 566 | 350/371 | 0.24 | 0.09 | 0.06 | 0.29 | 0.16 | 0.44 | Unknown Filosa Group III |
| OTU1944 | Uncultured cercozoan clone 4‐1.8 | AY620267 | 96 | 593 | 355/371 | 0.00 | 0.00 | 0.03 | 0.08 | 0.03 | 0.06 | Unknown Filosa Group III |
| OTU1945 | Cercozoan amoeba sp. ex Porpyra yezoensis | DQ666485 | 90 | 486 | 340/376 | 0.03 | 0.00 | 0.04 | 0.11 | 0.28 | 0.00 | Unknown Filosa Group III |
| OTU442 | Uncultured cercozoan clone 4‐1.8 | AY620267 | 90 | 475 | 334/371 | 0.00 | 0.02 | 0.00 | 0.00 | 0.02 | 0.00 | Unknown Filosa Group III |
| OTU45 | Uncultured eukaryote clone BF‐A4‐1‐12c | EU860499 | 95 | 573 | 351/371 | 0.00 | 0.01 | 0.00 | 0.10 | 0.05 | 0.00 | Unknown Filosa Group III |
| OTU658 | Uncultured eukaryote clone 125 | KF357281 | 90 | 486 | 337/373 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.11 | Unknown Filosa Group III |
| OTU884 | Uncultured cercozoan clone 7‐5.2 | AY620346 | 95 | 588 | 353/370 | 0.00 | 0.00 | 0.01 | 0.00 | 0.03 | 0.00 | Unknown Filosa Group III |
| OTU902 | Uncultured eukaryote clone T0h‐125 | KF357281 | 96 | 612 | 357/370 | 0.01 | 0.01 | 0.00 | 0.03 | 0.02 | 0.01 | Unknown Filosa Group III |
| OTU1299 | Uncultured marine eukaryote clone NA2_1B8 | EF526891 | 98 | 652 | 366/372 | 0.44 | 0.24 | 0.63 | 0.00 | 0.50 | 0.00 | Unknown Filosa Group IV |
| OTU1572 | Uncultured eukaryotic clone CYSGM‐17 | AB275100 | 99 | 649 | 362/367 | 0.01 | 0.35 | 0.00 | 0.01 | 0.17 | 0.00 | Unknown Filosa Group IV |
| OTU41 | Uncultured marine eukaryote clone ME_Euk_FW9 | GU385688 | 97 | 625 | 359/369 | 0.25 | 0.00 | 0.01 | 0.02 | 0.02 | 0.00 | Unknown Filosa Group IV |
| OTU538 | Uncultured eukaryote clone MPE2‐30 | AB695524 | 95 | 586 | 356/374 | 0.00 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 | Unknown Filosa Group IV |
| OTU823 | Uncultured Rhizaria clone F.13_2119 | KP685342 | 96 | 608 | 357/371 | 0.00 | 0.00 | 0.08 | 0.00 | 0.07 | 0.00 | Unknown Filosa Group IV |
| OTU952 | Uncultured eukaryote clone T0h‐81 | KF357374 | 94 | 558 | 349/371 | 0.04 | 0.01 | 0.00 | 0.07 | 0.00 | 0.00 | Unknown Filosa Group IV |
| OTU1001 | Cercozoa sp. COHH 48 | GU320591 | 91 | 486 | 336/371 | 0.00 | 0.00 | 0.06 | 0.00 | 0.05 | 0.00 | Unknown Endomyxa Group I |
| OTU1409 | Uncultured marine cercozoan clone BS11_B2 | FN598314 | 99 | 676 | 370/372 | 0.20 | 0.76 | 0.24 | 0.35 | 0.02 | 0.16 | Unknown Endomyxa Group I |
| OTU1749 | Uncultured marine cercozoan clone BS11_B2 | FN598314 | 93 | 542 | 346/372 | 0.54 | 0.39 | 0.08 | 0.76 | 0.00 | 0.32 | Unknown Endomyxa Group I |
| OTU1781 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 91 | 503 | 338/370 | 0.00 | 0.09 | 0.00 | 0.00 | 0.02 | 0.00 | Unknown Endomyxa Group I |
| OTU1841 | Uncultured eukaryote clone SGYH927 | KJ762794 | 99 | 675 | 371/374 | 0.07 | 0.13 | 0.05 | 0.00 | 0.00 | 0.06 | Unknown Endomyxa Group I |
| OTU958 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 92 | 521 | 347/377 | 0.04 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | Unknown Endomyxa Group I |
| OTU1775 | Uncultured eukaryote clone SGYY525 | KJ757438 | 97 | 612 | 356/368 | 1.56 | 7.45 | 2.31 | 4.08 | 4.45 | 2.74 | Unknown Endomyxa Group II |
| OTU329 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 89 | 464 | 332/371 | 1.87 | 3.58 | 1.40 | 0.61 | 0.07 | 2.29 | Unknown Endomyxa Group II |
| OTU1336 | Uncultured marine cercozoan clone BS15_B5 | FN598356 | 93 | 540 | 344/369 | 0.01 | 0.00 | 0.02 | 0.01 | 0.16 | 0.00 | Unknown Cercozoa |
| OTU1549 | Uncultured eukaryote band JLJ‐8‐41 | JN846845 | 94 | 549 | 345/368 | 0.00 | 0.00 | 0.00 | 0.23 | 0.36 | 0.13 | Unknown Cercozoa |
| OTU1622 | Uncultured cercozoan clone 7‐6.1 | AY620355 | 93 | 538 | 344/369 | 0.00 | 0.00 | 0.02 | 0.05 | 0.05 | 0.00 | Unknown Cercozoa |
| OTU1910 | Uncultured eukaryote clone KRL01E2 | JN090862 | 94 | 555 | 351/375 | 0.00 | 0.00 | 0.07 | 0.00 | 0.02 | 0.00 | Unknown Cercozoa |
| OTU440 | Uncultured microeukaryote clone ME‐12 | KC851793 | 96 | 599 | 355/370 | 0.00 | 0.08 | 0.12 | 0.00 | 0.22 | 0.00 | Unknown Cercozoa |
| OTU488 | Uncultured eukaryote band JLJ‐8‐41 | JN846845 | 96 | 586 | 352/368 | 0.00 | 0.00 | 0.00 | 0.05 | 0.62 | 0.00 | Unknown Cercozoa |
| OTU621 | Uncultured eukaryote clone F6‐39 | KJ568831 | 95 | 580 | 354/373 | 0.00 | 0.00 | 0.00 | 0.01 | 0.14 | 0.00 | Unknown Cercozoa |
Figure A1.
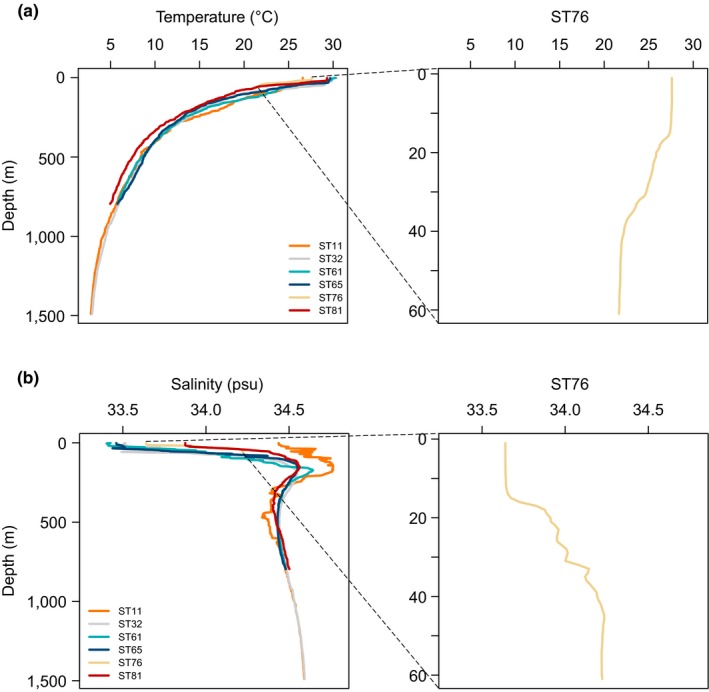
Temperature (a) and salinity (b) profiles of the upper waters of the sampling sites. Vertical profiles are based on the records at a water depth of 1,489, 1,489,795, 796, 61, and 796 m at ST11, ST32, ST61, ST65, ST76, and ST81, respectively
Wu W, Huang B. Protist diversity and community assembly in surface sediments of the South China Sea. MicrobiologyOpen. 2019;8:e891 10.1002/mbo3.891
Funding information
This was supported by National Key Research and Development Program (grant/award number: “2016YFA0601201”).
DATA ACCESSIBILITY
The raw sequence data were deposited in the Sequence Read Archive (NCBI) under the accession number SRP083955.
REFERENCES
- Agusti, S. , González‐Gordillo, J. I. , Vaqué, D. , Estrada, M. , Cerezo, M. I. , Salazar, G. , … Duarte, C. M. (2015). Ubiquitous healthy diatoms in the deep sea confirm deep carbon injection by the biological pump. Nature Communications, 6, 7608 10.1038/ncomms8608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, D. , & Cavalier‐Smith, T. (2004). Phylum‐specific environmental DNA analysis reveals remarkably high global biodiversity of Cercozoa (Protozoa). International Journal of Systematic and Evolutionary Microbiology, 54, 2393–2404. 10.1099/ijs.0.63229-0 [DOI] [PubMed] [Google Scholar]
- Beaudoin, D. J. , Carmichael, C. A. , Nelson, R. K. , Reddy, C. M. , Teske, A. P. , & Edgcomb, V. P. (2016). Impact of protists on a hydrocarbon‐degrading bacterial community from deep‐sea Gulf of Mexico sediments: A microcosm study. Deep‐Sea Research II, 129, 350–359. 10.1016/j.dsr2.2014.01.007 [DOI] [Google Scholar]
- Berney, C. , Romac, S. , Mahé, F. , Santini, S. , Siano, R. , & Bass, D. (2013). Vampires in the oceans: Predatory cercozoan amoebae in marine habitats. The ISME Journal, 7, 2387–2399. 10.1038/ismej.2013.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard, J. M. , Kormas, K. , Pachiadaki, M. G. , Rocke, E. , Beaudoin, D. J. , Morrison, C. , … Edgcomb, V. P. (2014). Benthic protists and fungi of Mediterranean deep hypsersaline anoxic basin redoxcline sediments. Frontiers in Microbiology, 5, 605 10.3389/fmicb.2014.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bik, H. M. , Sung, W. , De Ley, P. , Baldwin, J. G. , Sharma, J. , Rocha‐Olivares, A. , & Thomas, W. K. (2012). Metagenetic community analysis of microbial eukaryotes illuminates biogeographic patterns in deep‐sea and shallow water sediments. Molecular Ecology, 21, 1048–1059. 10.1111/j.1365-294X.2011.05297.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochdansky, A. B. , Clouse, M. A. , & Herndl, G. J. (2017). Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. The ISME Journal, 11, 362–373. 10.1038/ismej.2016.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo, E. , Debroas, D. , Arnaud, F. , & Domaizon, I. (2015). Is planktonic diversity well recorded in sedimentary DNA? Toward the reconstruction of past protistan diversity. Microbial Ecology, 70, 865–875. 10.1007/s00248-015-0627-2 [DOI] [PubMed] [Google Scholar]
- Caporaso, J. G. , Kuczynski, J. , Stombaugh, J. , Bittinger, K. , Bushman, F. D. , Costello, E. K. , … Knight, R. (2010). QIIME allows analysis of high‐throughput community sequencing data. Nature Methods, 7, 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chariton, A. A. , Court, L. N. , Hartley, D. M. , Colloff, M. J. , & Hardy, C. M. (2010). Ecological assessment of estuarine sediments by pyrosequencing eukaryotic ribosomal DNA. Frontiers in Ecology and the Environment, 8, 233–238. 10.1890/090115 [DOI] [Google Scholar]
- Chatzinotas, A. , Schellenberger, S. , Glaser, K. , & Kolb, S. (2013). Assimilation of cellulose‐derived carbon by protists in oxic and anoxic slurries of an aerated soil. Applied and Environmental Microbiology, 79, 5777–5781. 10.1128/aem.01598-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Pan, Y. , Yu, L. , Yang, J. , & Zhang, W. (2017). Patterns and processes in marine protist community biogeography from Xiamen coastal waters and intertidal sediments, Southeast China. Frontiers in Microbiology, 8, 1912 10.3389/fmicb.2017.01912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, M. K. , Au, C. H. , Chu, K. H. , Kwan, H. S. , & Wong, C. K. (2010). Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. The ISME Journal, 4, 1053–1059. 10.1038/ismej.2010.26 [DOI] [PubMed] [Google Scholar]
- Christaki, U. , Kormas, K. A. , Genitsaris, S. , Georges, C. , Sime‐Ngando, T. , Viscogliosi, E. , & Monchy, S. (2014). Winter‐summer succession of unicellular eukaryotes in a meso‐eutrophic coastal system. Microbial Ecology, 67, 13–23. 10.1007/s00248-013-0290-4 [DOI] [PubMed] [Google Scholar]
- Ciobanu, M.‐C. , Burgaud, G. , Dufresne, A. , Breuker, A. , Rédou, V. , Ben Maamar, S. , … Alain, K. (2014). Microorganisms persist at record depths in the subseafloor of the Canterbury Basin. The ISME Journal, 8, 1370–1380. 10.1038/ismej.2013.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary, A. C. , & Durbin, E. G. (2016). Unexpected prevalence of parasite 18S rDNA sequences in winter among Antarctic marine protists. Journal of Plankton Research, 38, 401–417. 10.1093/plankt/fbw005 [DOI] [Google Scholar]
- Coolen, M. J. L. , & Shtereva, G. (2009). Vertical distribution of metabolically active eukaryotes in the water column and sediments of the Black Sea. FEMS Microbiology Ecology, 70, 525–539. 10.1111/j.1574-6941.2009.00756.x [DOI] [PubMed] [Google Scholar]
- Cury, J. C. , Araujo, F. V. , Coelho‐Souza, S. A. , Peixoto, R. S. , Oliveira, J. A. L. , Santos, H. F. , … Rosado, A. S. (2011). Microbial diversity of a Brazilian coastal region influenced by an upwelling system and anthropogenic activity. PLoS ONE, 6, e16553 10.1371/journal.pone.0016553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vargas, C. , Audic, S. , Henry, N. , Decelle, J. , Mahe, F. , Logares, R. , … Velayoudon, D. (2015). Eukaryotic plankton diversity in the sunlit ocean. Science, 348, 1261605–1261605. 10.1126/science.1261605 [DOI] [PubMed] [Google Scholar]
- Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics, 26, 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Edgcomb, V. P. , Beaudoin, D. , Gast, R. , Biddle, J. F. , & Teske, A. (2011). Marine subsurface eukaryotes: The fungal majority. Environmental Microbiology, 13, 172–183. 10.1111/j.1462-2920.2010.02318.x [DOI] [PubMed] [Google Scholar]
- Edgcomb, V. P. , Kysela, D. T. , Teske, A. , de Vera Gomez, A. , & Sogin, M. L. (2002). Benthic eukaryotic diversity in the Guaymas Basin hydrothermal vent environment. Proceedings of the National Academy of Sciences of the United States of America, 99, 7658–7662. 10.1073/pnas.062186399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgcomb, V. P. , Pachiadaki, M. G. , Mara, P. , Kormas, K. A. , Leadbetter, E. R. , & Bernhard, J. M. (2016). Gene expression profiling of microbial activities and interactions in sediments under haloclines of E. Mediterranean deep hypersaline anoxic basins. The ISME Journal, 10, 2643–2657. 10.1038/ismej.2016.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegaard, M. , Moestrup, Ø. , Joest Andersen, T. , & Lundholm, N. (2016). Long‐term survival of haptophyte and prasinophyte resting stages in marine sediment. European Journal of Phycology, 51, 328–337. 10.1080/09670262.2016.1161243 [DOI] [Google Scholar]
- Epstein, S. , & López‐García, P. (2008). “Missing” protists: A molecular prospective. Biodiversity and Conservation, 17, 261–276. 10.1007/s10531-007-9250-y [DOI] [Google Scholar]
- Forster, D. , Dunthorn, M. , Mahé, F. , Dolan, J. R. , Audic, S. , Bass, D. , … Stoeck, T. (2016). Benthic protists: The under‐charted majority. FEMS Microbiology Ecology, 92, fiw120 10.1093/femsec/fiw120 [DOI] [PubMed] [Google Scholar]
- Gong, J. , Shi, F. , Ma, B. , Dong, J. , Pachiadaki, M. , Zhang, X. , & Edgcomb, V. P. (2015). Depth shapes α‐ and β‐diversities of microbial eukaryotes in surficial sediments of coastal ecosystems. Environmental Microbiology, 17, 3722–3737. 10.1111/1462-2920.12763 [DOI] [PubMed] [Google Scholar]
- Guillou, L. , Bachar, D. , Audic, S. , Bass, D. , Berney, C. , Bittner, L. , … Christen, R. (2013). The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote small sub‐unit rRNA sequences with curated taxonomy. Nucleic Acids Research, 41, D597–D604. 10.1093/nar/gks1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou, L. , Viprey, M. , Chambouvet, A. , Welsh, R. M. , Kirkham, A. R. , Massana, R. , … Worden, A. Z. (2008). Widespread occurrence and genetic diversity of marine parasitoids belonging to Syndiniales (Alveolata). Environmental Microbiology, 10, 3349–3365. 10.1111/j.1462-2920.2008.01731.x [DOI] [PubMed] [Google Scholar]
- Guindon, S. , Dufayard, J.‐F. , Lefort, V. , Anisimova, M. , Hordijk, W. , & Gascuel, O. (2010). New algorithms and methods to estimate maximum‐likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology, 59, 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- Haas, B. J. , Gevers, D. , Earl, A. M. , Feldgarden, M. , Ward, D. V. , Giannoukos, G. , … Birren, B. W. (2011). Chimeric 16S rRNA sequence formation and detection in Sanger and 454‐pyrosequenced PCR amplicons. Genome Research, 21, 494–504. 10.1101/gr.112730.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan, L. J. , Coffin, R. B. , Sikaroodi, M. , Greinert, J. , Treude, T. , & Gillevet, P. M. (2013). Ocean currents shape the microbiome of Arctic marine sediments. The ISME Journal, 7, 685–696. 10.1038/ismej.2012.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob, M. , Soltwedel, T. , Boetius, A. , & Ramette, A. (2013). Biogeography of deep‐sea benthic bacteria at regional scale (LTER HAUSGARTEN, Fram Strait, Arctic). PLoS ONE, 8, e72779 10.1371/journal.pone.0072779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, R. , Wang, J. , Yu, K. , & Liu, M. (2016). Micro‐eukaryotic diversity in the surface layer of sediments from the East China Sea. Evolutionary Ecology Research, 17, 125–140. [Google Scholar]
- Katoh, K. , & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution, 30, 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel, S. W. , Cowan, P. D. , Helmus, M. R. , Cornwell, W. K. , Morlon, H. , Ackerly, D. D. , … Webb, C. O. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics, 26, 1463–1464. 10.1093/bioinformatics/btq166 [DOI] [PubMed] [Google Scholar]
- Kremp, A. , Hinners, J. , Klais, R. , Leppänen, A. , & Kallio, A. (2018). Patterns of vertical cyst distribution and survival in 100‐year‐old sediment archives of three spring dinoflagellate species from the Northern Baltic Sea. European Journal of Phycology, 53, 135–145. 10.1080/09670262.2017.1386330 [DOI] [Google Scholar]
- Leibold, M. A. , Holyoak, M. , Mouquet, N. , Amarasekare, P. , Chase, J. M. , Hoopes, M. F. , … Gonzalez, A. (2004). The metacommunity concept: A framework for multi‐scale community ecology. Ecology Letters, 7, 601–613. 10.1111/j.1461-0248.2004.00608.x [DOI] [Google Scholar]
- Liu, J. , Xiang, R. , Chen, Z. , Chen, M. , Yan, W. , Zhang, L. , & Chen, H. (2013). Sources, transport and deposition of surface sediments from the South China Sea. Deep Sea Research Part I: Oceanographic Research Papers, 71, 92–102. 10.1016/j.dsr.2012.09.006 [DOI] [Google Scholar]
- Liu, K.‐K. , Chao, S.‐Y. , Shaw, P.‐T. , Gong, G.‐C. , Chen, C.‐C. , & Tang, T. Y. (2002). Monsoon‐forced chlorophyll distribution and primary production in the South China Sea: Observations and a numerical study. Deep‐Sea Research I, 49, 1387–1412. 10.1016/S0967-0637(02)00035-3 [DOI] [Google Scholar]
- Liu, Z. , Zhao, Y. , Colin, C. , Stattegger, K. , Wiesner, M. G. , Huh, C.‐A. , … Li, Y. (2016). Source‐to‐sink transport processes of fluvial sediments in the South China Sea. Earth‐Science Reviews, 153, 238–273. 10.1016/j.earscirev.2015.08.005 [DOI] [Google Scholar]
- Logares, R. , Audic, S. , Bass, D. , Bittner, L. , Boutte, C. , Christen, R. , … Massana, R. (2014). Patterns of rare and abundant marine microbial eukaryotes. Current Biology, 24, 813–821. 10.1016/j.cub.2014.02.050 [DOI] [PubMed] [Google Scholar]
- Lozupone, C. , & Knight, R. (2005). UniFrac: A new phylogenetic method for comparing microbial communities. Applied and Environmental Microbiology, 71, 8228–8235. 10.1128/aem.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massana, R. , Gobet, A. , Audic, S. , Bass, D. , Bittner, L. , Boutte, C. , … de Vargas, C. (2015). Marine protist diversity in European coastal waters and sediments as revealed by high‐throughput sequencing. Environmental Microbiology, 17, 4035–4049. 10.1111/1462-2920.12955 [DOI] [PubMed] [Google Scholar]
- Nakai, R. , Abe, T. , Baba, T. , Imura, S. , Kagoshima, H. , Kanda, H. , … Naganuma, T. (2012). Eukaryotic phylotypes in aquatic moss pillars inhabiting a freshwater lake in East Antarctica, based on 18S rRNA gene analysis. Polar Biology, 35, 1495–1504. 10.1007/s00300-012-1188-1 [DOI] [Google Scholar]
- Newbold, L. K. , Oliver, A. E. , Booth, T. , Tiwari, B. , DeSantis, T. , Maguire, M. , … Whiteley, A. S. (2012). The response of marine picoplankton to ocean acidification. Environmental Microbiology, 14, 2293–2307. 10.1111/j.1462-2920.2012.02762.x [DOI] [PubMed] [Google Scholar]
- Oikonomou, A. , Katsiapi, M. , Karayanni, H. , Moustaka‐Gouni, M. , & Kormas, K. A. (2012). Plankton microorganisms coinciding with two consecutive mass fish kills in a newly reconstructed lake. The Scientific World Journal, 2012, 504135 10.1100/2012/504135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Kindt, R. , Legendre, P. , Minchin, P. R. , O'Hara, R. B. , … Wagner, H. (2014). Vegan: community ecology package. R package version 2.2‐0. http://CRAN.R-project.org/package=vegan/
- Orsi, W. D. (2018). Ecology and evolution of seafloor and subseafloor microbial communities. Nature Reviews Microbiology, 16, 671–683. 10.1038/s41579-018-0046-8 [DOI] [PubMed] [Google Scholar]
- Orsi, W. , Biddle, J. F. , & Edgcomb, V. (2013). Deep sequencing of subseafloor eukaryotic rRNA reveals active Fungi across marine subsurface provinces. PLoS ONE, 8, e56335 10.1371/journal.pone.0056335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasulka, A. L. , Levin, L. A. , Steele, J. A. , Case, D. H. , Landry, M. R. , & Orphan, V. J. (2016). Microbial eukaryotic distributions and diversity patterns in a deep‐sea methane seep ecosystem. Environmental Microbiology, 18, 3022–3043. 10.1111/1462-2920.13185 [DOI] [PubMed] [Google Scholar]
- Pawlowski, J. , Christen, R. , Lecroq, B. , Bachar, D. , Shahbazkia, H. R. , Amaral‐Zettler, L. , & Guillou, L. (2011). Eukaryotic richness in the abyss: Insights from pyrotag sequencing. PLoS ONE, 6, e18169 10.1371/journal.pone.0018169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro, C. , Starnawski, P. , Schramm, A. , & Kjeldsen, K. (2017). Microbial community assembly in marine sediments. Aquatic Microbial Ecology, 79, 177–195. 10.3354/ame01826 [DOI] [Google Scholar]
- Piredda, R. , Sarno, D. , Lange, C. B. , Tomasino, M. P. , Zingone, A. , & Montresor, M. (2017). Diatom resting stages in surface sediments: A pilot study comparing next generation sequencing and serial dilution cultures. Cryptogamie, Algologie, 38, 31–46. 10.7872/crya/v38.iss1.2017.31 [DOI] [Google Scholar]
- Price, M. N. , Dehal, P. S. , & Arkin, A. P. (2009). FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Molecular Biology and Evolution, 26, 1641–1650. 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org/ [Google Scholar]
- Reece, K. S. , Siddall, M. E. , Stokes, N. A. , & Burreson, E. M. (2004). Molecular phylogeny of the Haplosporidia based on two independent gene sequences. Journal of Parasitology, 90, 1111–1122. 10.1645/GE-102R [DOI] [PubMed] [Google Scholar]
- Reeder, J. , & Knight, R. (2010). Rapidly denoising pyrosequencing amplicon reads by exploiting rank‐abundance distributions. Nature Methods, 7, 668–669. 10.1038/nmeth0910-668b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvadet, A.‐L. , Gobet, A. , & Guillou, L. (2010). Comparative analysis between protist communities from the deep‐sea pelagic ecosystem and specific deep hydrothermal habitats. Environmental Microbiology, 12, 2946–2964. 10.1111/j.1462-2920.2010.02272.x [DOI] [PubMed] [Google Scholar]
- Schlitzer, R. (2018). Ocena data view. http://odv.awi.de/
- Schroeder, A. , Wiesner, M. G. , & Liu, Z. (2015). Fluxes of clay minerals in the South China Sea. Earth and Planetary Science Letters, 430, 30–42. 10.1016/j.epsl.2015.08.001 [DOI] [Google Scholar]
- Sierra, R. , Cañas‐Duarte, S. J. , Burki, F. , Schwelm, A. , Fogelqvist, J. , Dixelius, C. , … Pawlowski, J. (2016). Evolutionary origins of rhizarian parasites. Molecular Biology and Evolution, 33, 980–983. 10.1093/molbev/msv340 [DOI] [PubMed] [Google Scholar]
- Skovgaard, A. , & Daugbjerg, N. (2008). Identity and systematic position of Paradinium poucheti and other Paradinium‐like parasites of marine copepods based on morphology and nuclear‐encoded SSU rDNA. Protist, 159, 401–413. 10.1016/j.protis.2008.02.003 [DOI] [PubMed] [Google Scholar]
- Starnawski, P. , Bataillon, T. , Ettema, T. J. G. , Jochum, L. M. , Schreiber, L. , Chen, X. , … Kjeldsen, K. U. (2017). Microbial community assembly and evolution in subseafloor sediment. Proceedings of the National Academy of Sciences of the United States of America, 114, 2940–2945. 10.1073/pnas.1614190114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen, J. C. , Lin, X. , Fredrickson, J. K. , Chen, X. , Kennedy, D. W. , Murray, C. J. , … Konopka, A. (2013). Quantifying community assembly processes and identifying features that impose them. The ISME Journal, 7, 2069–2079. 10.1038/ismej.2013.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeck, T. , Bass, D. , Nebel, M. , Christen, R. , Jones, M. D. M. , Breiner, H.‐W. , & Richards, T. A. (2010). Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Molecular Ecology, 19, 21–31. 10.1111/j.1365-294X.2009.04480.x [DOI] [PubMed] [Google Scholar]
- Strassert, J. F. H. , Karnkowska, A. , Hehenberger, E. , del Campo, J. , Kolisko, M. , Okamoto, N. , … Keeling, P. J. (2018). Single cell genomics of uncultured marine alveolates shows paraphyly of basal dinoflagellates. The ISME Journal, 12, 304–308. 10.1038/ismej.2017.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takishita, K. , Kakizoe, N. , Yoshida, T. , & Maruyama, T. (2010). Molecular evidence that phylogenetically diverged ciliates are active in microbial mats of deep‐sea cold‐seep sediment. Journal of Eukaryotic Microbiology, 57, 76–86. 10.1111/j.1550-7408.2009.00457.x [DOI] [PubMed] [Google Scholar]
- Takishita, K. , Tsuchiya, M. , Kawato, M. , Oguri, K. , Kitazato, H. , & Maruyama, T. (2007). Genetic diversity of microbial eukaryotes in anoxic sediment of the saline meromictic lake Namako‐ike (Japan): On the detection of anaerobic or anoxic‐tolerant lineages of eukaryotes. Protist, 158, 51–64. 10.1016/j.protis.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Vellend, M. (2010). Conceptual synthesis in community ecology. The Quarterly Review of Biology, 85, 183–206. 10.1086/652373 [DOI] [PubMed] [Google Scholar]
- Wang, G. , Xie, S.‐P. , Qu, T. , & Huang, R. X. (2011). Deep South China Sea circulation. Geophysical Research Letters, 38, L05601 10.1029/2010GL046626 [DOI] [Google Scholar]
- Wu, W. , Huang, B. , Liao, Y. , & Sun, P. (2014). Picoeukaryotic diversity and distribution in the subtropical‐tropical South China Sea. FEMS Microbiology Ecology, 89, 563–579. 10.1111/1574-6941.12357 [DOI] [PubMed] [Google Scholar]
- Wu, W. , Logares, R. , Huang, B. , & Hsieh, C. (2017). Abundant and rare picoeukaryotic sub‐communities present contrasting patterns in the epipelagic waters of marginal seas in the northwestern Pacific Ocean. Environmental Microbiology, 19, 287–300. 10.1111/1462-2920.13606 [DOI] [PubMed] [Google Scholar]
- Xu, D. , Li, R. , Hu, C. , Sun, P. , Jiao, N. , & Warren, A. (2017). Microbial eukaryote diversity and activity in the water column of the South China Sea based on DNA and RNA high throughput sequencing. Frontiers in Microbiology, 8, 1121 10.3389/fmicb.2017.01121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J. , Chen, M.‐Y. , Shao, P. , Zhou, H. , Chen, Y.‐Q. , & Qu, L.‐H. (2004). Genetic diversity of small eukaryotes from the coastal waters of Nansha Islands in China. FEMS Microbiology Letters, 240, 163–170. 10.1016/j.femsle.2004.09.030 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Schwartz, S. , Wagner, L. , & Miller, W. (2000). A greedy algorithm for aligning DNA sequences. Journal of Computational Biology, 7, 203–214. 10.1089/10665270050081478 [DOI] [PubMed] [Google Scholar]
- Zhu, D. , Tanabe, S.‐H. , Yang, C. , Zhang, W. , & Sun, J. (2013). Bacterial community composition of South China Sea sediments through pyrosequencing‐based analysis of 16S rRNA genes. PLoS ONE, 8, e78501 10.1371/journal.pone.0078501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger, L. , Boetius, A. , & Ramette, A. (2014). Bacterial taxa‐area and distance‐decay relationships in marine environments. Molecular Ecology, 23, 954–964. 10.1111/mec.12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw sequence data were deposited in the Sequence Read Archive (NCBI) under the accession number SRP083955.


