SUMMARY
The whole human body receives rich sensory innervation with only one exception and that is the brain tissue. The orofacial region is hence no exception. The head region consequently receives a rich network of sensory nerves making it special because the two types of sensory fibres, visceral and somatic overlap, especially in the pharynx. Also, different pain syndromes that affect this region are rather specific in comparison to their presentation in other body regions. With this review article we wanted to show the detailed anatomy of the peripheral sensory pathways, because of its importance in everyday body functions (eating, drinking, speech) as well as the importance it has in pathological conditions (pain syndromes), in diagnostics and regional analgesia and anaesthesia.
Key words: orofacial region, trigeminal nerve, pain, sensory innervation
Introduction
The sensory innervation reaches almost all tissues except for the brain tissue making the orofacial region hence no exception. The head itself is a special region since it is often subject to different pain syndromes which do not resemble syndromes in other body regions. In this review we would like to point out the anatomical and functional uniqueness of the orofacial region and give some new insights into the importance of overlapping somatic and visceral afferent fibres for understanding pain syndromes, as well as for targeting these nerves in regional analgesia and anaesthesia. Central representation of sensory information will not be considered here.
Anatomy of the Trigeminal Nerve
The ophthalmic, maxillary and mandibular branches that constitute the trigeminal nerve make it the largest of cranial nerves.
Trigeminal sensory root (portio major, radix sensoria) is joined with smaller, motor root (portio minor, radix motoria) which travels together with the mandibular nerve (V3). All three sensory branches are the peripheral processes of the primary sensory neurons whose somata make the trigeminal ganglion (ganglion semilunare, Gasseri) located in the Meckel’s cave on the petrous part of the temporal bone. Functional imaging has confirmed a somatotopy in the organization of cells inside the ganglion with ophthalmic somata lying superomedially, mandibular cells posterolaterally, and maxillary cells in-between (1).
Central processes of these neurons form a large sensory trigeminal root and enter the brainstem at the mid-pontine level. Motor root that carries efferent fibres from the motoric trigeminal nucleus accompanies the mandibular nerve.
Within the sensory root, an approximate somatotopy has been confirmed with each division holding their own, although overlapping territory (2). Afferents from the nociceptors, mechanoreceptors and thermal receptors however intermingle making the separation of modalities impossible (3).
From the sensory root, most of the fibres pass posteriorly to form the spinal trigeminal tract that lies close to the spinal trigeminal nuclei and extends from the mid-pons to the second cervical level.
Within the spinal tract there is an approximate topography, with ophthalmic nerve fibres lying ventrally, maxillary nerve fibres intermediately and mandibular nerve fibres dorsally (4), (5) (Figure 1).
Fig. 1.
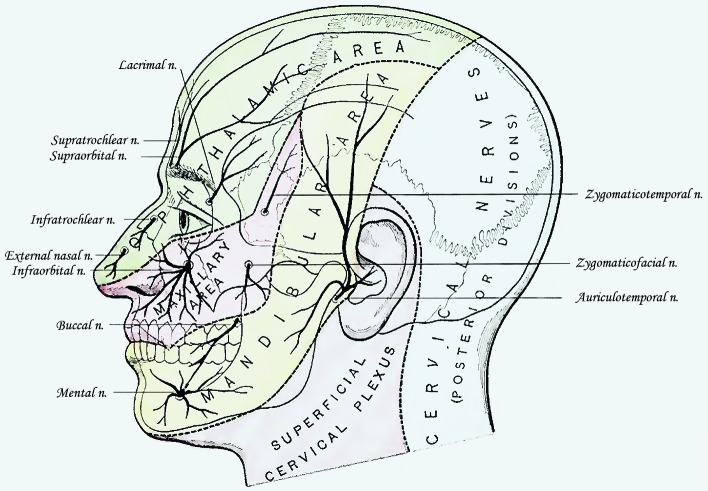
Cutaneous Innervation by the Trigeminal Nerve (Modified according to Gray’s Anatomy, 20th ed, 1918) Trigeminal nerve (V) is primarily responsible for giving sensory innervation to the skin, subcutaneous structures (including the periosteum), epithelium and dura of the orofacial region. The area of skin innervated roughly encloses the anterior half of the head following the line that goes somewhat in front of the posterior border of the mandibular ramus, and just anterior to the ear auricle. The trigeminal nerve also innervates the top of the head, mainly the skin of the parietal region. The posterior part of the skin of the head as well as the skin of the whole neck is innervated by the spinal nerves. Only in small skin areas the overlapping between spinal nerve innervation and trigeminal nerve innervation occurs where usually the trigeminal nerve itself prolongs its innervation area into the skin usually dominantly innervated by spinal nerves.
Interestingly, the trigeminal tract in the brainstem does not receive just afferents from the trigeminal ganglion, but also from other nerves such as intermedius (VII), glossopharyngeal (IX), vagus (X), and nerves from the upper cervical segments. The trigeminal afferents themselves project to other brainstem regions (5) like to solitary tract and reticular formation. Projections that finish in the rostral pole of the solitary tract are mainly concerned with gustation, while the caudal pole is concerned with cardiovascular, respiratory and gastrointestinal functions. These trigeminal projections to the solitary tract are therefore thought to be important in the integration of the sensory activity from the oral cavity, pharynx, and oesophagus during mastication and swallowing.
Trigeminal Sensory Nuclear Complex
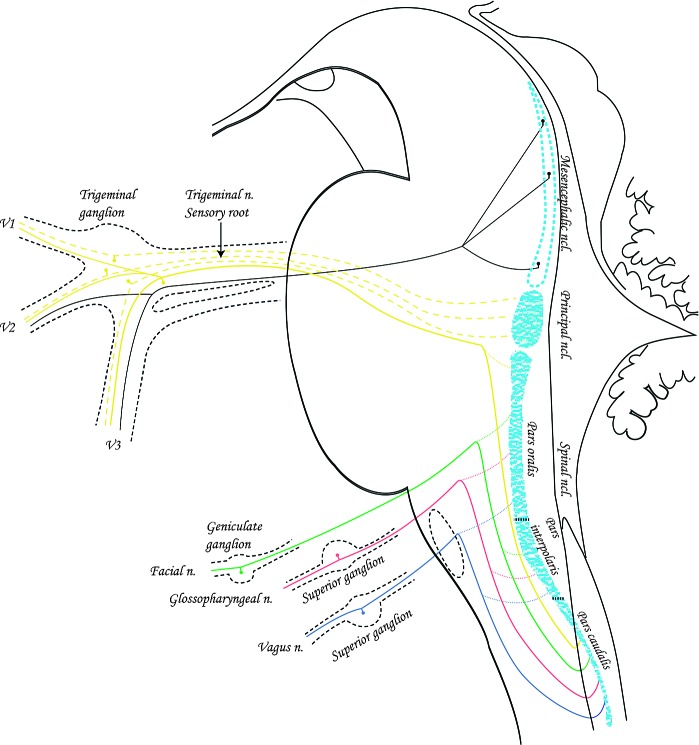
The trigeminal nuclear complex is a column of cell bodies of the secondary sensory neurons extending from the rostral pons to the second cervical level (5). The complex constitutes four nuclei: principal nucleus (or main sensory nucleus) and trigeminal spinal nucleus which consists of three sensory subnuclei: oralis, interpolaris, and caudalis. Studies of lesions that affect this area have indicated a somatotopic organization within this complex, with the ophthalmic division being positioned ventrally, maxillary centrally, and mandibular dorsally where the midline facial regions are represented medially, and more lateral skin regions represented laterally. It seems though that some facial regions have differences in central representation at different levels.
Principal nucleus with its position in the lateral pontine tegmentum is thought to correspond to the dorsal column nuclei which transmit discriminative tactile sensations from the face.
The caudal nucleus is the main site to provide for transmission of nociceptive information inside the brainstem. It corresponds both functionally and morphologically to the spinal dorsal horn. Neurons inside this nucleus have laminar distribution, and the superficial zone corresponds to the substantia gelatinosa of the spinal cord.
One of the most distressing clinical symptoms is pain from the trigeminal distribution. Nociceptive stimuli from the nerves VII, IX and X also converge to the spinal trigeminal nucleus. These cranial nerves do not carry only the afferents from the skin of the external acustic meatus and auricle, but also nociceptive information from the pharynx, pharyngotympanic tube and tympanic cavity.
Even though visceral afferent fibres, which developmentally have origin from the inner parts of the pharyngeal arches and endoderm, project primarily to the solitary nucleus, in some areas the mucosae of the root of the tongue, palate, roof of the pharynx, deep part of the nasal cavity, auditory tube and tympanic cavity receive afferent innervation which project to the trigeminal spinal nuclei.
It is likely that this does not include only nociceptive stimuli but also mechanoreceptive and chemoreceptive fibres. The aforementioned areas seem to represent a transitional zone with two types of afferent innervation.
The majority of nociceptive projections to the other two nuclei (oralis and interpolaris) enter only secondary from the nucleus caudalis. The transitional zone between the nucleus interpolaris and caudalis is an area of neurons which process deep orofacial pain (Figure 2).
Fig.2.
Sensory Nuclei in the Brainstem (Modified according to Gray’s Anatomy, 20th ed, 1918) The figure shows sensory ganglia, sensory nerves (V, VII, IX, X) and their central processes that end in the nuclei of the brain stem.
Trigeminal mesencephalic nucleus is a unique sensory structure which contains cell bodies of primary afferent proprioceptors from masticatory muscles and periodontium. These cells project to the motor trigeminal nucleus forming a monosynaptic reflex arc.
Somatosensory Innervation
Branches of the ophthalmic nerve (V1) give sensory innervation to the skin of the forehead, top of the head (vertex) (nervus frontalis), nose and upper eyelid (nervus nasociliaris). The boundaries of this innervation reach from the lateral and medial corner of the eye (canthus) up above the temporal region, and posteriorly it innervates the top of the head all the way to the occipital region.
Branches of the ophthalmic nerve innervate the mucosa of the upper eyelid (nervus nasociliaris), upper periorbit, lacrimal gland (nervus lacrimalis), the whole eye bulb (nervi ciliares brevi et longi), dura of the anterior cranial fossa, dura of the calvaria, falx and upper part of the tentorium. The mucosa of the roof and anterior part of the nasal cavity, mucosa of the frontal, ethmoid and sphenoid sinuses are completely innervated by the branches of ethmoid nerves (nervus nasociliaris).
Maxillary nerve branches give sensory innervation to the skin of the middle part of the face covering the maxilla (nervus infraorbitalis), zygomatic bone and anterior upper part of the temporal region (nervus zygomaticus) up to the lateral corner of the eye. The skin of the nose is supplied only to the bottom of the lateral sides of the nose while the nasal cavity receives maxillary branches for posterior lateral sides of the nose all the way to the orifice of the pharyngotympanic tube, parts of the septum (rami nasales), and mucosae of hard palate (nervus palatinus major et minor), lower eyelid and upper lip. By giving superior dental plexus the maxillary nerve innervates also the mucosae of maxillary sinus, gingiva over maxilla (rami gingivales), and upper teeth (rami dentales superiores).
Branches of the mandibular nerve (V3) innervate the skin of the face from the lateral angle of the mouth to the lower angle of mandible, skin of the cheeks (nervus buccalis), posteriorly the majority of the skin covering mandibular ramus, temporomandibular joint, anterior lower part of the temporal region, upper half of the ear auricle (nervus auriculotemporalis), chin (nervus mentalis) and lower lip. It also gives sensory innervation to the oral mucosa of the inner side of the cheeks, mucosa of the lower lip, teeth of the lower jaw (nervus alveolaris inferior) and their vestibular gingiva, tongue (except for the taste buds) (nervus lingualis), and it reaches posteriorly to the terminal groove and tonsillar fossa where the palatine tonsil is situated.
Besides providing visceroafferent innervation to the orofacial region glossopharyngeal nerve and vagus also give substantial number of general somatic afferent fibres which innervate external acustic meatus, tympanic cavity, palate, root of the tongue and epiglottis. In this region we can also find anastomoses among trigeminal branches with these nerves, e.g. the auriculotemporal nerve branch that innervates the skin of the auricle anastomoses with the auricular branch of vagus that through the mastoid canaliculus enters the external acustic meatus. This auricular branch of vagus is composed of peripheral branches of pseudounipolar neurons situated in the superior ganglion of vagus nerve just before exiting the jugular foramen (jugular ganglion) while central branches project to the brainstem in the spinal trigeminal nucleus making the sensation a general somatic one. Superior jugular ganglion neurons send their axons to the dura of the posterior cranial fossa and likely to the epiglottic area.
Even though they are not abundant, the somatoafferents that join facial nerve and project from the geniculate ganglion to the spinal trigeminal nucleus innervate a small area behind the ear auricle.
Special somatic afferent fibres are found in the vestibulocochlear nerve that has two ganglia (ganglion spirale cochleae et ganglion vestibulare) that possess bipolar neurons.
Viscerosensory Innervation
General visceral afferent fibres to the orofacial region are carried in whole by the glossopharyngeal nerve and vagus. These peripheral branches that innervate predominantly the pharynx and larynx have the majority of the somata in the bigger inferior ganglia. They project to the lower part of the solitary nucleus, from where the central projections finish in cortical areas which process general visceral sensations and take part in pharyngeal reflex actions (swallowing, throwing up, and coughing) (6).
As for the epiglottis it is still not completely clear from which ganglion its peripheral branches come from, although it is assumed that they are from the inferior vagus ganglion. The epiglottic taste buds, on the other hand, have their pseudounipolar somata in the inferior vagus ganglion which, together with the projections from the taste buds on the root of the tongue (inferior glossopharyngeal ganglion) and from the anterior 2/3 of the tongue (geniculate ganglion of VII), project to the gustatory part of the solitary nucleus (its upper 1/3).
Neurons from the inferior glossopharyngeal ganglion also send their axons to the mucosa of the root of the tongue, soft palate mucosa, and roof of the pharynx, to tonsils and tonsillar fossae as well as to the mucosae of pharyngotympanic tubes, middle ear and project to the lower 2/3 of the solitary nucleus. Since these mucous membranes are innervated also by neurons from the superior ganglion, which project to the spinal trigeminal nucleus, it is understandable that a stimulus from these areas could be processed as a somatic one.
It is still not clear whether general somatic nerves that reach mucosae in the middle ear, pharyngotympanic tube, upper part of the pharynx and root of the tongue have their innervation areas separated from the areas innervated by general visceral sensory fibres, or the innervation areas and receptors of these two types of fibres overlap. Superior, smaller ganglia of both glossopharyngeal nerve and vagus dominantly contain bodies of somatic afferent neurons while the inferior, bigger ganglia contain visceral afferent neurons. A vast number of neurons also transmit signals from chemoreceptors and pressure receptors located around the bifurcation of common carotid artery (IX) and aortal arch and its branches (X).
The pseudounipolar neurons in the geniculate ganglion (sensory ganglion of the facial nerve) project to both portions of the solitary nucleus. The majority of neurons that project to the rostral portion carry special visceral fibres from the taste buds of the anterior 2/3 of the tongue and palate, while the rest of neurons project to caudal portion of the solitary nucleus carrying general visceral fibres for the innervation of tympanic cavity (tympanic communicating branch) and oropharyngeal area around palatine tonsil (these follow parasympathetic fibres of the greater petrosal nerve).
Interestingly, the pseudounipolar neurons could also be found inside the autonomic ganglia and it is thought they only carry sensory information for autonomic system functions (7).
Visceral afferents that follow sympathetic and parasympathetic fibres and transmit pain stimuli as well as other sensory information mainly have their somata in the sensory ganglia (parasympathetics in sensory ganglia of VII, IX, X). Sympathetic fibres for the head come wrapped around arteries (carotid plexus). Afferents that follow them probably have their somata in the trigeminal ganglia, while sympathetics around arteries in the neck, skull base and vertebral artery branches carry afferent fibres probably from the spinal nerves in the cervical plexus. The same sensory fibres innervate pia and arachnoid while the brain parenchima does not receive sensory innervation.
Afferent Innervation of the Muscles: Proprioception and Pain Transmission
Afferent innervation of the striated muscles of head and neck is still not completely understood. Today it is generally accepted that only muscles of the upper part of the pharynx are devoid of the main type of proprioceptors, muscle spindles, but are innervated by different kinds of mechanoreceptors, which presumably work as proprioceptors (8). Unlike them, the rest of proprioceptive pseudounipolar neurons project to mesencephalic nucleus which is a unique example of primary neurons inside a sensory nucleus in the brain stem. Their peripheral branches reach masticatory muscles and other muscles that are innervated by the trigeminal nerve and by whose branch they reach their final destination muscle (V3).
The data are more inclined to the possibility that it is about anastomoses between the trigeminal nerve and nerves that innervate external ocular muscles that are formed in the area around cavernous sinus. Some research indicate that proprioceptive fibres come straight from sensory nerves, i.e. from the ophthalmic nerve branches for the external eye muscles or from the lingual nerve for the tongue muscles, while others point to possible anastomoses of the lower cranial nerves (XI, XII) with spinal nerves. Proprioceptive information from the muscles of the pharynx and larynx probably come from glossopharyngeal nerve and vagus. Both of these nerves anastomose with both trigeminal branches and spinal nerves making them both a possible source of proprioceptive fibres.
Autonomic Innervation
Sympathetic and parasympathetic nerve fibres control smooth muscles and glands.
The autonomic efferent fibres make a purely motor part of the autonomic system. Even though they contain no sensory neurons they are thought to have an indirect role in painful sensations by regulating regional blood flow and interacting directly with sensory nerves especially with nerve injury that is known as sympathetically maintained pain (9).
In the orofacial region they control lacrimation, salivation, sweating, vascular smooth muscle tone, mucosal secretion, thermoregulation and intraocular smooth muscles. In some of those functions both sympathetic and parasympathetic fibres control the function, e.g. in iris muscles while the rest get only sympathetic innervation (blood vessels). New evidence has indicated that parasympathetic efferents could be found in the blood vessels of the skin and in the head (10), (11) and that they could have a role in migraine (12). It seems that these fibres begin to sprout after partial nerve injury. Following these indications it is possible that parasympathetic innervation sprouting may contribute to trigeminal neuropathic pain (11), (13).
The origins of the sympathetic nerves for the head are in the intermediolateral horn cells of the upper thoracic segments of the spinal cord. The neurons then ascend the sympathetic chain and synapse in the upper cervical ganglion. All the fibres for the head are hence postganglionic sympathetic fibres that reach their targets by the way of arteries.
The parasympathetic fibres for the head originate from the parasympathetic brainstem nuclei and exit the brainstem following the nerves III, VII, IX, and X. The oculomotor nerve carries preganglionic parasympathetic fibres from the Edinger-Westfal nucleus and they synapse in the ciliary ganglion. The postganglionic fibres reach the iris and innervate the pupillary sphincter and ciliary muscle of the eye.
The facial nerve carries preganglionic parasympathetic fibres from the lacrimal and superior salivatory nuclei and synapse in the pterygopalatine and submandibular ganglia from which the postganglionic fibres reach the lacrimal gland, submandibular and sublingual salivatory glands and nasal mucous glands.
The glossopharyngeal nerve carries the preganglionic parasympathetic fibres from the inferior salivatory nucleus which synapse in the otic ganglion and send their postganglionic fibres to the parotid gland. The vagus carries its preganglionic parasympathetic fibres from the dorsal nucleus of the vagus nerve. These nerves innervate the minority structures in the neck while they give the majority of their fibres to the structures in the thorax and abdomen where they synapse in ganglia near the target organs.
Besides the innervation provided to structures of the head and following the nerves III, VII, IX and X, the cranial parasympathetic fibres also control many trigemino-parasympathetic reflexes.
A well described group of primary headaches present with autonomic symptoms (cluster headache, paroxysmal hemicranias, short-lasting, unilateral neuralgiform headache with conjunctival injection and tearing) which include lacrimation, ptosis, nasal congestion and eyelid edema (14). The pain from these headaches is transferred by the afferents of the ophthalmic branch of the trigeminal nerve and the autonomic symptoms are due to activation of the cranial parasympathetic fibres within the facial nerve.
This implies a connection on the level of brainstem between the trigeminal sensory nuclei and autonomic nuclei.
It has been shown that a stimulation of nasal sensory branches of the trigeminal nerve could elicit a reflexive decrease in heart rate and arterial blood pressure (15)suggesting that even a peripheral stimulus could be enough to lead to this reflex.
Clinical Implications
The orofacial region is a very important region included in many daily activities (eating, drinking, and speech production) and with its rich sensory innervation makes the pain experience very unpleasant and often exhausting (16). Although the perception of pain is highly subjective and a subject to a lot of individual variation, three main types of pain are described in this region: normal (nociceptive), inflammatory and neuropathic pain (9). The nociceptive pain is felt when thinly myelinated (Aδ) and/or unmyelinated (C) afferent fibres reach conscious cortical level. Its role is mainly protective and the painful sensation matches the noxious stimulus (burning your tongue). An inflammatory pain that is felt as a constant pain or tenderness is usually provoked during infections, minor tissue injuries and burns. In these situations the nociceptor endings are assumed to increasingly respond (peripheral sensitization) to the chemical inflammatory mediators that are released in the injured tissue. The result is a nociceptive response at substantially lower threshold. A neuropathic pain results from an injury or disease of nerves or central nervous system. There is a considerable overlapping between the neuropathic and inflammatory pain since there is usually some spontaneous pain and hypersensibility also present in neuropathic pain, although it is generally considered that neuropathic pain is a result of the inflammation in a major nerve trunk (neuritis) (17).
The pain syndromes in the orofacial region are often hard to put in any of the above basic classifications since clinical presentations often overlap and share common characteristics.
The importance of knowing the peripheral sensory pathways in the orofacial region lies especially in knowing where to target the peripheral nerves for regional anaesthetic procedures in office procedures (18) (laceration repairs, foreign body removals, suturing, wound debridement, etc.), or its usage in more invasive procedures in managing acute and chronic pain situations which include gamma knife radiosurgery in seizures, tremors, rigidity of Parkinson’s disease, intractable pain, and certain neuroses (19).
When considering local anaesthesia from neuroanatomical view it is important to have in mind that concentrations of local anaesthetic needed for a sympathetic blockade are below that required for sensory and motor loss. Thus, a selective block that involves only unmyelinated C fibres and A delta fibres without effecting motor fibres (function) are possible.
Trigeminal block. In the ophthalmic nerve distribution, the possible targets are blocks to supratrochlear and supraorbital nerves which are especially useful during forehead surgical procedures, although they also might provide relief of neuralgias and headaches as well. Ciliary ganglion is usually a target in retrobulbar block.
The maxillary and mandibular nerves are usually targeted in dental procedures. The infraorbital nerve (V2) could be blocked by an extraoral approach in surgical procedures that require anaesthetizing the lower lid, lateral inferior portion of the nose and vestibule, and the upper lip and mucosa.
Nasopalatine block provides soft tissue palatal anaesthesia or to supplement anaesthesia of the nasal cavity. Pterygopalatine ganglion as a site of passage of many branches of trigeminal nerve, facial nerve, carotid plexus and superior cervical ganglion can be blocked either transnasally or through nasal pharynx. Trigeminal ganglion can be reached through the foramen ovale if all three trigeminal divisions need to be anaesthetized. The approach is used for treatment of chronic trigeminal neuralgia or intractable cancer pain in patients with refractory to aggressive pharmacological treatment. Longer nerve block duration can be achieved using also radiofrequency or neurolytic ganglionotomy.
Glossopharyngeal block.Targeting the ninth cranial nerve should be done with utmost care since it runs in close proximity to the vagus, accessory nerve, and sympathetic trunk. It is mainly used for treating glossopharyngeal neuralgias.
Sympathetic block. In diagnosing and management of some complex chronic pain conditions stellate (cervicothoracic) ganglion blockade should be considered.
Acknowledgement
This work was supported by the Scientific Centre of Excellence for Basic, Clinical and Translational Neuroscience (project “Experimental and clinical research of hypoxic-ischemic damage in perinatal and adult brain”; GA KK01.1.1.01.0007 funded by the European Union through the European Regional Development Fund)
References
- 1.Borsook D, DaSilva AF, Ploghaus A, Becerra L. Specific and somatotopic functional magnetic resonance imaging activation in the trigeminal ganglion by brush and noxious heat. J Neurosci. 2003;23(21):7897–903. 10.1523/JNEUROSCI.23-21-07897.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelletier VA, Poulos DA, Lende RA. Functional localization in the trigeminal root. J Neurosurg. 1974;40(4):504–13. 10.3171/jns.1974.40.4.0504 [DOI] [PubMed] [Google Scholar]
- 3.Waite PME, Ashwell KWS. Chapter 31 - Trigeminal Sensory System. 2012. [Google Scholar]
- 4.Capra NF, Dessem D. Central connections of trigeminal primary afferent neurons: topographical and functional considerations. Crit Rev Oral Biol Med. 1992;4(1):1–52. 10.1177/10454411920040010101 [DOI] [PubMed] [Google Scholar]
- 5.Usunoff KG, Marani E, Schoen JH. The trigeminal system in man. Adv Anat Embryol Cell Biol. 1997;136:I–X, 1–126. 10.1007/978-3-642-60779-0 [DOI] [PubMed] [Google Scholar]
- 6.Nieuwenhuys R, Voogd J. HuijzenCv, The human central nervous system. 2008, Berlin; New York: Springer. [Google Scholar]
- 7.Jänig W, Integrative Action of the Autonomic Nervous System Neurobiology of Homeostasis. 2006, Cambridge: Cambridge University Press. [Google Scholar]
- 8.de Carlos F, Cobo J, Macias E, Feito J, Cobo T. Calavia MG i sur. The sensory innervation of the human pharynx: searching for mechanoreceptors. Anat Rec (Hoboken). 2013;296(11):1735–46. 10.1002/ar.22792 [DOI] [PubMed] [Google Scholar]
- 9.Tal M, Devor M. Anatomy and neurophysiology of orofacial pain-Chapter 2. 2008. [Google Scholar]
- 10.Kaji A, Maeda T, Watanabe S. Parasympathetic innervation of cutaneous blood vessels examined by retrograde tracing in the rat lower lip. J Auton Nerv Syst. 1991;32(2):153–8. 10.1016/0165-1838(91)90065-B [DOI] [PubMed] [Google Scholar]
- 11.Ramien M, Ruocco I, Cuello AC, St-Louis M, Ribeiro-Da-Silva A. Parasympathetic nerve fibres invade the upper dermis following sensory denervation of the rat lower lip skin. J Comp Neurol. 2004;469(1):83–95. 10.1002/cne.10998 [DOI] [PubMed] [Google Scholar]
- 12.Yarnitsky D, Goor-Aryeh I, Bajwa ZH, Ransil BI, Cutrer FM. Sottile A i sur. 2003 Wolff Award: Possible parasympathetic contributions to peripheral and central sensitization during migraine. Headache. 2003;43(7):704–14. 10.1046/j.1526-4610.2003.03127.x [DOI] [PubMed] [Google Scholar]
- 13.Grelik C, Bennett GJ, Ribeiro-da-Silva A. Autonomic fibre sprouting and changes in nociceptive sensory innervation in the rat lower lip skin following chronic constriction injury. Eur J Neurosci. 2005;21(9):2475–87. 10.1111/j.1460-9568.2005.04089.x [DOI] [PubMed] [Google Scholar]
- 14.May A. Update on the diagnosis and management of trigemino-autonomic headaches. J Neurol. 2006;253(12):1525–32. 10.1007/s00415-006-0303-2 [DOI] [PubMed] [Google Scholar]
- 15.Schaller BJ, Filis A, Buchfelder M. Trigemino-cardiac reflex in humans initiated by peripheral stimulation during neurosurgical skull-base operations. Its first description. Acta Neurochir (Wien). 2008;150(7):715–7, discussion 717–8. 10.1007/s00701-008-1602-1 [DOI] [PubMed] [Google Scholar]
- 16.Sessle BJ, Baad-Hansen L, Svensson P. Orofacial Pain. 2010:258-266.
- 17.Eliav E, Herzberg U, Ruda MA, Bennett GJ. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. Pain. 1999;83(2):169–82. 10.1016/S0304-3959(99)00102-5 [DOI] [PubMed] [Google Scholar]
- 18.Salam GA. Regional anesthesia for office procedures: part I. Head and neck surgeries. Am Fam Physician. 2004;69(3):585–90. [PubMed] [Google Scholar]
- 19.Rosenberg M, Phero JC. Regional anesthesia and invasive techniques to manage head and neck pain. Otolaryngol Clin North Am. 2003;36(6):1201–19. 10.1016/S0030-6665(03)00134-8 [DOI] [PubMed] [Google Scholar]