Abstract
Biofilms are microbial communities embedded in extracellular matrix. Exopolysaccharide Psl (ePsl) is a key biofilm matrix component that initiates attachment, maintains biofilms architecture, and protects bacteria within biofilms of Pseudomonas aeruginosa, an opportunistic pathogen. There are at least 12 Psl proteins involved in the biosynthesis of this exopolysaccharide. However, it remains unclear about the function of each Psl protein and how these proteins work together during the biosynthesis of ePsl. PslG has been characterized as a degrader of ePsl in extracellular or periplasm and PslD is predicted to be a transporter. In this study, we found that PslG and its glycoside hydrolytic activity were also involved in the biosynthesis of ePsl. PslG localized mainly in the inner membrane and some in the periplasm. The inner membrane association of PslG was critical for the biosynthesis of ePsl. The expression of PslA, PslD, and PslE helped PslG remain in the inner membrane. The bacterial two‐hybrid results suggested that PslE could interacted with either PslA, PslD, or PslG. The strongest interaction was found between PslE and PslD. Consistently, PslD was disabled to localize on the outer membrane in the ΔpslE strain, suggesting that the PslE‐PslD interaction affected the localization of PslD. Our results shed light on the assembly of ePsl biosynthesis machinery and suggested that the membrane‐associated PslG was a part of ePsl biosynthesis proteins complex.
Keywords: biofilm, exopolysaccharide Psl, glycosyl hydrolase, Pseudomonas aeruginosa
In this study, we shed light on the assembly of exopolysaccharide Psl (ePsl) biosynthesis machinery in Pseudomonas aeruginosa. We improve our understanding about the function of PslG, the interactions among Psl proteins and the effect of proteins interactions on the localization of Psl protein during ePsl biosynthesis .
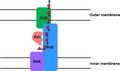
1. INTRODUCTION
Structured, surfaced‐associated communities of bacteria known as biofilms are prevalent in nature, industrial, and clinical settings (Costerton, Lewandowski, Caldwell, Korber, & Lappin‐Scott, 1995; Stoodley, Sauer, Davies, & Costerton, 2002). Biofilm matrix, which plays a key role in biofilm development, is extracellular substance secreted by biofilm bacteria. Although the component of biofilm matrix differs among species, it is generally composed of polysaccharides, proteins, and nucleic acids (Flemming & Wingender, 2010; Stoodley et al., 2002). The extracellular polysaccharides have a key role in biofilm matrix function because they promote attachment to surfaces and other cells, act as a scaffold to help maintain biofilm structure, and protect cells from antibiotics and host defenses (Häussler & Parsek, 2010; Stewart & Costerton, 2001; Stewart & Costerton, 2001). Although the importance of exopolysaccharide is widely accepted, the exact mechanism underlying their biosynthesis remains poorly understood. A better understanding of the molecular mechanisms of polysaccharide biosynthesis may provide strategies for the control of chronic infections and problems related to biofilm formation.
Pseudomonas aeruginosa is an opportunistic human pathogen that can cause life‐threatening infections in cystic fibrosis patients and individuals with compromised immune system (Govan & Deretic, 1996; Lyczak, Cannon, & Pier, 2000; Ramsey & Wozniak, 2005). P. aeruginosa is a model organism to study the process of biofilm development. There are at least three unique exopolysaccharides implicated in P. aeruginosa biofilm development, alginate, ePsl, and Pel (Branda, Vik, Friedman, & Kolter, 2005; Colvin et al., 2012; Ma, Jackson, Landry, Parsek, & Wozniak, 2006; Ramsey & Wozniak, 2005). Alginates are anionic exopolysaccharides composed of variable proportions of 1,4‐linked β‐ᴅ‐mannuronic acid and its C‐5 epimer α‐ʟ‐guluronic acid (Hay, Rehman, Ghafoor, & Rehm, 2010). Twelve proteins are required for the biosynthesis of alginate (Chitnis & Ohman, 1993; Franklin, Nivens, Weadge, & Howell, 2011). They have been characterized to elucidate the alginate biosynthetic mechanism, including polymerization, epimerization, acetylation, secretion, and regulation (Franklin et al., 2011; Moradali, Donati, Sims, Ghods, & Rehm, 2015; Rehman, Wang, Moradali, Hay, & Rehm, 2013). Pel is a positively charged polysaccharide composed of partially acetylated 1–4 glycosidic linkages of N‐acetylgalactosamine and N‐acetylglucosamine (Jennings et al., 2015). A seven‐gene operon (pelABCDEFG) is essential for Pel biosynthesis (Friedman & Kolter, 2004; Vasseur, Vallet‐Gely, Soscia, Genin, & Filloux, 2005). Structural and biochemical analyses have shed light on the understanding of Pel polymerization, deacetylation, and exportation (Colvin et al., 2013; Ghafoor, Jordens, & Rehm, 2013; Marmont et al., 2017; Whitney et al., 2012).
The ePsl is a neutral pentasaccharide repeat containing ᴅ‐mannose, ᴅ‐glucose, and ʟ‐rhamnose (Byrd et al., 2009). The polysaccharide synthesis locus (psl) contains 15 genes, 11 of which (pslACDEFGHIJKL) are required for ePsl biosynthesis (Byrd et al., 2009). However, the function of each Psl protein remains largely unknown. It has been reported that PslB is a bifunctional enzyme and is involved in sugar‐nucleotide precursor production for ePsl biosynthesis (Byrd et al., 2009; Lee, Chang, Venkatesan, & Peng, 2008). PslD is a secreted protein and may play a role in exopolysaccharide export (Campisano, Schroeder, Schemionek, Overhage, & Rehm, 2006). Our previous study (Yu et al., 2015) has demonstrated that PslG is an endoglycosidase mainly targeted ePsl and, the catalytic residues E165 and E276 are critical for the hydrolytic activity. PslG can degrade ePsl to prevent biofilm formation and disassemble existing biofilm when supplied exogenously. While whether PslG is involved in the biosynthesis of ePsl remains controversial. Byrd et al. (2009) considered PslG was required for the biosynthesis of ePsl. On the contrary, Baker et al. (2015) found that neither PslG nor its enzymatic activity appeared to be required for ePsl biosynthesis and biofilm formation. Strain PAO1ΔpslG constructed by Byrd et al. (2009) has deleted a cis‐acting element located in the 3’ of pslG that altered the translation of pslH (Baker et al., 2015), while, the ΔpslG strain constructed by Baker et al. (2015) is in the background of a psl overexpression strain PAO1ΔpelFPBAD psl rather than wild type PAO1.
Bioinformatic analyses suggest that ePsl biosynthesis mechanism resembles the biosynthesis of Escherichia coli group 1 capsular polysaccharides, with PslA, PslD, and PslE similar to WbaP, Wza, and Wzc, respectively (Franklin et al., 2011). It is proposed that biosynthesis and translocation of ePsl is temporally and spatially coupled by multiprotein complex. Nevertheless, there has not been any investigation about the interaction and localization of Psl proteins that involved in the ePsl biosynthesis.
In this study, we further investigate the role of PslG and its hydrolytic activity on the biosynthesis of ePsl in P. aeruginosa PAO1. Interactions among Psl proteins (PslA, PslD, PslG, and PslE) and their effects on the subcellular localization of Psl proteins have been examined. Our results shed light on the assembly of ePsl biosynthesis machinery.
2. MATERIALS AND METHODS
2.1. Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study are listed in Table 1. Unless indicated, E. coli strains were grown at 37°C in Luria Bertani Broth (LB, Becton Dickinson), P. aeruginosa stains at 37°C in LB without sodium chloride (LBNS) or Jensen's, a chemically defined medium (Jensen, Fecycz, & Campbell, 1980). L‐arabinose (Sigma) was used as inducer for genes transcribed from PBAD promoter in P. aeruginosa. Antibiotics for P. aeruginosa were added at the following concentrations: gentamicin 30 μg/ml; ampicillin 100 μg/ml; carbenicillin 300 μg/ml; chloramphenicol 25 μg/ml; tetracycline 12.5 μg/ml. Gentamicin at 15 μg/ml was used for E. coli. For Pseudomonas selection media, Irgasan at 25 μg/ml was used.
Table 1.
The strains and plasmids used in this study
| Strain or plasmid | Genotype and/or relevant characteristics | Source or reference |
|---|---|---|
| P. aeruginosa PAO1 series strains | ||
| P. aeruginosa PAO1 | Prototroph | Holloway (1955) |
| ΔpslG2 | In‐frame deletion of pslG | This study |
| ΔpslA | In‐frame deletion of pslA | Byrd et al. (2009) |
| ΔpslD | In‐frame deletion of pslD | Byrd et al. (2009) |
| ΔpslE | In‐frame deletion of pslE | Byrd et al. (2009) |
| WFPA800 | ePsl‐negative strain, psl operon promoter deletion mutant, ΔPpsl | Ma et al. (2006) |
| WFPA801 | ePsl‐overproduced strain, PBAD‐psl | Ma et al. (2006) |
| WFPA801ΔpslA | In‐frame deletion of pslA | This study |
| WFPA801ΔpslD | In‐frame deletion of pslD | This study |
| WFPA801ΔpslE | In‐frame deletion of pslE | This study |
| ΔpslG2::pslGE165Q + E276Q | pslG was replaced by the active site mutated pslG (E165Q + E276Q) | This study |
| ΔpslG2::pslG | pslG was knocked into the pslG deletion mutant | This study |
| E.coli strains | ||
| XL1‐Blue MRF’ kan | Reporter strain of BacterioMatch II Two‐Hybrid System | Zhang et al. (2009) |
| BL21(DE3) | F‐ ompT gal [dcm] [lon] hsdSB (rB‐mB‐; an E. coli B strain) with DE3, a λ prophage carrying T7 RNA polymerase gene | Novagen |
| Plasmids | ||
| pHERD20T | E. coli‐P. aeruginosa shuttle plasmid containing arabinose inducible PBAD promoter, Apr | Qiu, Damron, Mima, Schweizer, and Yu (2008) |
| pG | pHERD20T with pslG, Apr | Yu et al. (2015) |
| pGDM | pHERD20T with active sites mutated pslG (E165Q + E276Q), Apr | This study |
| pBT | Bait vector of BacterioMatch II Two‐Hybrid System, Cmr | Zhang et al. (2009) |
| pTRG | Target vector of BacterioMatch II Two‐Hybrid System, Tcr | Zhang et al. (2009) |
| pEX18Gm | Cloning vector, Gmr | Hoang, Karkhoff‐Schweizer, Kutchma, and Schweizer (1998) |
| pMA9 | pEX18Gm derived plasmid for replacing psl operon promoter with araC‐pBAD, Gmr | Ma et al. (2006) |
| pEX‐ΔpslG2 | pEX18Gm derived plasmid for pslG in‐frame deletion, Gmr | This study |
| pEX‐pslG | pEX18Gm derived plasmid for knocking in pslG into ΔpslG2, Gmr | This study |
| pEX‐pslGE165Q + E276Q | pEX18Gm derived plasmid for replacing pslG with pslGE165Q + E276Q, Gmr | This study |
| pGLO1‐pslG | pGLO1 derived plasmid for PslG31−442 purification, Apr | Yu et al. (2015) |
| pSadC‐GFP | C‐terminal Gfp‐tagged SadC expressed in pHERD20T, Apr | Zhu et al. (2016) |
2.2. Strain construction
The in‐frame pslG deletion mutant ΔpslG2 was constructed by an unmarked, nonpolar deletion strategy as previously described (Carter, Chen, & Lory, 2010). The native sequence located 17 bp upstream of the pslH start codon and the 24 bp downstream of the pslG start codon was retained. Flanking regions of pslG were obtained by overlapping PCR with primers UpPslG2‐F (CCGGAATTCCCTCTACCAGTTGAAGGCAC, italics denote the restriction enzyme sites), UpPslG2‐R (TTCACTCCCACAGATAGAGTCCCTTAC), and DwPslG2‐F (ACTCTATCTGTGGGAGTGAAGCCACC), DwPslG2‐R (CCCAAGCTTCGACGTTGTGCTCGGTGAG) and then cloned into suicide vector pEX18Gm at EcoRI and HindIII sites, generating plasmid pEX‐ΔpslG2. This plasmid was transformed into S17‐1 and subsequently transferred to P. aeruginosa by conjugation. For single recombination mutant selection, LBNS plates with 30 μg/ml gentamycin and 25 μg/ml irgasan were used; for double recombination mutant selection, LBNS plates containing 10% sucrose were used. The chromosomal point mutation strain ΔpslG2::pslGE165Q + E276Q was constructed with the similar method described above by using the allelic exchange plasmid pEX‐pslGE165Q + E276Q to knock in pslGE165Q + E276Q into ΔpslG2. The psl‐inducible strains WFPA801∆pslA, WFPA801∆pslD, and WFPA801∆pslE were constructed in accordance with WFPA801 (Ma et al., 2006). Briefly, plasmid pMA9 was transferred into deletion mutants ∆pslA, ∆pslD, and ∆pslE, respectively, and double‐crossover recombinants were selected.
2.3. Microtiter dish biofilm assay
In the biofilm attachment assay, 1/100 dilution of a saturated (overnight) culture in Jensen's media for P. aeruginosa was inoculated into glass tubes. When the OD600 reached 0.5, the culture was inoculated into 96‐well PVC microtiter dish (BD Falcon), and incubated at 30°C for 30 min. Then the planktonic and loosely adherent bacteria cells were washed off by rinsing the plate in water. The remaining surface‐attached cells were stained by 0.1% crystal violet, solubilized in 30% acetic acid, and finally measured (OD560) as described previously (Ma et al., 2006; O'Toole, 2011).
2.4. Antibody preparation
Anti‐PslG serum was made by Abmart company (Shanghai, China) by using purified PslG31‐442 and a 70 d standard protocol. The antiserum against PslG31‐442 was absorbed by using P. aeruginosa ΔpslG2 whole cell lysates. The absorption was performed at 4°C for 2 hr by mixing 2 μl anti‐PslG antisera, 60 μl ΔpslG2 cell lysate in 440 μl of PBST (140 mM NaCl, 2 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 0.005% Tween) containing 2% BSA and 10 mM EDTA, then centrifuged at 10,000 rpm for 15 min at 4°C. The supernatant was collected as the purified antiserum. Anti‐PslD antibody was made by epitope approach. A synthetic polypeptide (RRVALMREDSEG) corresponding to residues 174–185 of PslD was selected on the basis of an antigenic epitope analysis. The polypeptide was used to immunize rabbits to obtain the polyclonal antibody serum by Abmart (Shanghai, China).
2.5. ePsl immuno‐dot blotting and cell extract western blotting analysis
P. aeruginosa cell surface associated polysaccharide extracts were obtained from culture that equivalents approximately 4 OD600, and examined by immunoblotting using anti‐ePsl antiserum as previously described (Byrd et al., 2009). To induce the transcription of the pslG in the recombinant plasmid, arabinose was added to Jensen's media. The immunoblotting data were analyzed using Image Lab software.
Two milliliters of overnight culture (OD600 of ~2) grown in LBNS was harvested and resuspended in 100 μl Lysis buffer (50 mM potassium phosphate pH 7.8, 400 mM NaCl, 100 mM KCl, 10% glycerol, 0.5% Triton X‐100, 10 mM imidazole). Samples were frozen in liquid nitrogen and then thawed at 42°C, repeated 3 times to obtain the whole cell extracts. The equivalent amount of whole cell extracts was mixed with 2 × SDS‐PAGE sample buffer and boiled for 5 min. Proteins were separated by SDS‐PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane. The PslG or PslD protein was detected by incubating the membrane with primary antibody against the absorbed anti‐PslG antibody and the anti‐PslD antibody, respectively. RNA polymerase was detected using anti‐RNAp antibody (Abcam, shanghai China). The software Image Lab was used to analyze the immune‐blotting data.
2.6. Subcellular fractionation
Subcellular fractionation was adapted from a previously described procedure (Baker et al., 2015; Colvin et al., 2013; Liu & Walsh, 1990; Russell et al., 2011). Briefly, 1 L of P. aeruginosa culture grown overnight was harvested by centrifugation (5,000 rpm, 30 min, 4°C). The pellet was resuspended in 5 ml buffer I (0.2 M Tris‐HCl pH 8.0, 1 M sucrose, 1 mM EDTA, 1 mg/ml lysozyme) and incubated at room temperature for 5 min. Then 20 ml of ddH2O was gently added. The sample was placed on ice for 20 min, and then centrifuged at 45,000 rpm for 45 min at 4°C. The supernatant fraction was collected as periplasmic sample. The pellet was resuspended in 50 ml buffer II (10 mM Tris‐HCl pH 7.5, 5 mM EDTA, 1 mM DTT, 10 μg/ml DNase I), and then applied to sonication. Unlysed cells were removed by centrifugation (16,000 rpm, 20 min, 4°C). The supernatant was further centrifuged at 45,000 rpm for 2 hr at 4°C. The supernatant consisted of the cytoplasmic fraction, and the pellet contained the membrane fraction. The pellet was resuspended in 25 ml buffer III (50 mM Tris‐HCl pH 8.0, 2% (v/v) Triton X‐100, 10 mM MgCl2). The sample was centrifuged (35,000 rpm, 30 min, 4°C) and the resulting supernatant contained the inner membrane fraction while the pellet contained the outer membrane fraction. The pellet was washed in 50 ml buffer III twice, and centrifuged at 35,000 rpm for 30 min at 4°C. The samples were dissolved in SDS‐PAGE loading buffer and detected by western blotting using purified anti‐PslG antibody, anti‐PslD antibody, or anti‐Gfp antibody (Abcam, Shanghai China).
2.7. Protein expression and purification
PslG31‐442 was expressed and purified as previously described (Yu et al., 2015). The first 30 residues of PslG were truncated because they were predicted to be a signal peptide by the Signal P4.1 server. Briefly, E. coli BL21 (DE3) carried pGLO1‐pslG was grown in 1 L LB containing 100 μg/ml ampicillin at 37°C. When the OD600 of the culture reached 0.5–0.8, protein expression was induced overnight with 0.1 mM isopropyl β‐D‐thiogalactopyranoside at 22 ºC. Bacteria cells were harvest by centrifugation at 4,000 rpm for 30 min at 4 ºC and resuspended in buffer A (25 mM Tris‐HCl, pH 8.0, 200 mM NaCl, 60 mM imidazole). The bacterial suspension was lysed by sonication and centrifuged at 16,000 rpm for 30 min at 4 ºC. The supernatant was applied to a nickel affinity column (Chelating Sepharose Fast Flow, GE Healthcare), and washed with three column volumes of binding buffer to remove the non‐specific proteins. The expressed protein was eluted with buffer B (25 mM Tris‐HCl, pH 8.0, 200 mM NaCl, 250 mM imidazole). The eluted fraction containing the protein was purified by size‐exclusion chromatography (Superdex 200 10/300 GL, GE Healthcare) with buffer C (10 mM Tris‐HCl, pH 8.0, 100 mM NaCl, 5% (v/v) glycerol). The purified PslD was a gift from prof. Lichuan Gu.
2.8. Bacterial two‐hybrid system
Bacterial two‐hybrid experiments were conducted as described (Zhang et al., 2009). PCR fragments corresponding to pslA, pslD, pslE, and pslG were cloned into the pBT and pTRG vectors. The DNA region containing the signal peptide domain of PslG (PslG1‐45) and DNA region without the signal peptide domain of PslG (PslG31‐442) were amplified by PCR using genomic DNA isolated from P. aeruginosa PAO1. All fusion proteins were confirmed by DNA sequencing. A hisB mutant E. coli strain XL1‐Blue MRF’ Kan, transformed with the pBT‐ and pTRG‐derived plasmids, was used as reporter strain to screen for positive interactions. Detection of protein‐protein interactions is based on transcriptional activation of the HIS3 reporter gene, which allows the reporter strain to grow on the M9+ His‐dropout Broth (containing 25 μg/ml chloramphenicol and 12.5 μg/ml tetracycline) plate supplemented with 5 mM 3‐amino‐1,2,4‐triazole (3‐AT), a competitive inhibitor of His3 enzyme. pTRG vector carrying warA and pBT vector carrying sadC fragment were transformed into E. coli XL1‐Blue MRF’ Kan and used as a positive control. Positives were verified by using the aadA gene, which confers streptomycin resistance, as a second reporter. Cells harboring weaker interactors grew more slowly, requiring longer incubation time for colony development.
2.9. Statistical analyses
All the experiments were performed in at least three triplicates. The results are presented as the mean ± SD. Student's t‐tests were used to evaluate significance.
3. RESULTS
3.1. PslG and its glycoside hydrolytic activity are involved in the biosynthesis of ePsl in P. aeruginosa
Our previous data indicated that overproduced PslG in wild type strain PAO1 reduced the production of ePsl and biofilm biomass, yet overproduced catalytically inactive PslGE165Q + E276Q did not affect the ePsl production and slightly increased biofilm biomass (Yu et al., 2015). These results suggested that PslG might be involved in the biosynthesis of ePsl. To further investigate the role of PslG in ePsl biosynthesis, we constructed an unmarked, non‐polar pslG deletion mutant in the PAO1 background named ΔpslG2. The immune‐dot blotting showed that the ePsl production of ΔpslG2 declined up to 80% compared to PAO1 (Figure 1a). We further examined the initial attachment ability of ΔpslG2 in a microtiter dish because ePsl level impacts bacterial surface‐attachment dramatically. The ΔpslG2 mutant showed attachment similar to the ePsl‐negative strain WFPA800 (Figure 1b). Flagellum and type IV pili (T4P) also influence the initial attachment of P. aeruginosa (Klausen et al., 2003; O'Toole & Kolter, 1998). Therefore, we evaluated the flagellum‐mediated swimming motility and the T4P‐mediated twitching motility, the ΔpslG2 mutant showed similar levels of swimming and twitching motilities as wild type strain PAO1 (Appendix Figure AA1), indicating the normal function of flagellum and T4P in ΔpslG2. The biofilm biomass of ΔpslG2 was slightly higher than WFPA800 in a 2‐hr biofilm assay (Appendix Figure AA2), indicating the ePsl synthesized from ΔpslG2 is functional. These results further suggest that PslG is involved in ePsl biosynthesis.
Figure 1.
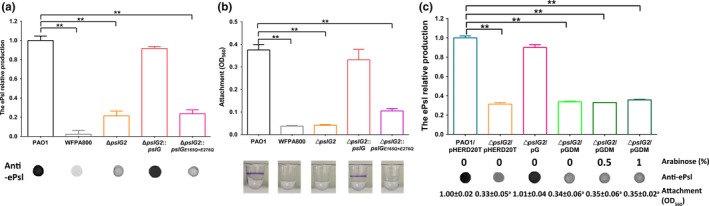
The contribution of PslG and its glycoside hydrolytic activity on the production of ePsl and initial attachment of P. aeruginosa. (a) The relative ePsl production of PAO1, ePsl‐negative strain WFPA800, the pslG in‐frame deletion mutant ΔpslG2, ΔpslG2::pslG, and the PslG catalytic residues mutant ΔpslG2::pslGE165Q + E276Q. The amount of ePsl is normalized to the level of PAO1. The corresponding anti‐ePsl immune‐dot blot is shown under each bar. (b) Shown is the corresponding initial attachment of the five strains. Values are means from two independent experiments, each with three replicates. The image under each bar is a representative microtiter dish well from corresponding crystal violet biofilm assay. (C) The ePsl production of ∆pslG2 that complemented by plasmid expressing wild type PslG (pG) or PslGE165Q + E276Q (pGDM). The amount of ePsl is normalized to the level of PAO1/pHERD20T. The corresponding anti‐ePsl immune‐dot blot and arabinose concentration are listed below each bar. The corresponding value of attachment assay for each strain shown under is normalized to the level of PAO1/pHERD20T, the superscript letter “a” indicates a significant difference compared to PAO1/pHERD20T of p < 0.01, as determined by Student's t test. **p < 0.01, Student's t test
We then further investigated whether the glycoside hydrolytic activity of PslG is important for ePsl production. We constructed a chromosomal site‐mutation strain ΔpslG2::pslGE165Q + E276Q with E165Q and E276Q mutation within PslG. This pslG mutant strain showed little ePsl production as that of ΔpslG2 mutant (Figure 1a). Although the attachment ability of ΔpslG2::pslGE165Q + E276Q was higher than ΔpslG2, it was still significantly less than that of PAO1 (fourfold lower than PAO1, Figure 1b). The ePsl production of ΔpslG2 could be restored by a baseline level expression of PslG (grown without inducer arabinose) from the plasmid pG (PslG was cloned in pHERD20T, Table 1), but it could not be restored by plasmid pGDM (PslGE165Q + E276Q in pHERD20T), regardless of the inducer level applied (0%, 0.5%, or 1%) (Figure 1c). The corresponding attachment was also consistent with the ePsl production (Figure 1c, the value shown under each column). These results suggested the importance of PslG glycoside hydrolytic activity in ePsl production and implied that the hydrolytic activity was not only required for degradation of ePsl, but also involved in the biosynthesis of ePsl. Taken together, these results suggested that the PslG and its hydrolytic activity contributed on ePsl production and initial attachment in PAO1.
3.2. Inner membrane fraction of PslG is critical for the biosynthesis of ePsl
The results of Baker et al. (2015) indicated that PslG could localize to both the inner membrane and the periplasm. We further investigated whether the subcellular localization of PslG is important for ePsl biosynthesis. We first detected the localization of PslG in the wild type strain PAO1 by anti‐PslG antibody, PslG was found in the inner membrane fraction, little in the periplasmic fraction (Figure 2a). No band was detected in all fractions from ΔpslG2 strain (Figure 2a), indicating a PslG‐specific detection. We also determined the PslG localization in the psl‐inducible strain WFPA801, which produced high amount of ePsl with arabinose as the inducer. WFPA801 showed a strong PslG band in the inner membrane, a weak band in the periplasmic fraction (3‐fold lower than IM band, Figure 2a) while grown with 1% arabinose. The molecular weight (MW) of protein band detected in the periplasm was similar to the purified protein PslG31‐442, indicating that it was a PslG without signal peptide, yet the band detected on inner membrane had a MW of full length PslG. The previous publication showed that SadC was localized in the inner membrane (Zhu et al., 2016). Therefore, we have transferred a plasmid pSadC‐GFP (carrying the sadC‐gfp gene, Table 1) into all tested strains in order to use the SadC‐Gfp as a loading control for membrane fraction. In addition, RNA polymerase was used as a loading control for the cytoplasmic fraction. The results of loading controls indicated that the same amount of cell fractions was loaded for each experiment, and each fraction was well separated.
Figure 2.
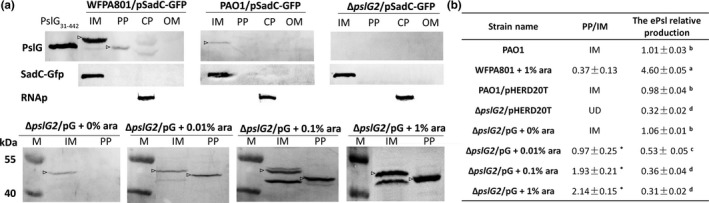
The subcellular localization of PslG and its effect on the biosynthesis of ePsl. (a) Western blotting of the inner membrane (IM), periplasm (PP), cytoplasm (CP), and outer membrane (OM) fractions are shown for PAO1/pSadC‐GFP grown with 1% arabinose, ePsl‐inducible strain WFPA801/pSadC‐GFP grown with 1% arabinose, ΔpslG2, and ΔpslG2/pG grown with different concentrations of arabinose. Subcellular fractions were probed for PslG, SadC‐Gfp (inner membrane protein, IM), or RNA polymerase (cytoplasmic protein, CP). M: marker. PslG31‐442: purified PslG protein loading as the positive control. Arrows indicate protein bands detected by anti‐PslG with right molecular weight. (b) A list of the ratios of PslG localized in periplasm to inner membrane, and the corresponding ePsl production of all tested strains. The amount of ePsl is normalized to the level of PAO1. IM: PslG is mainly detected in the inner membrane. UD: undetectable. Means and SD from triplicate experiments are shown. “*” indicates a significant difference compared to WFPA801 of p < 0.05, as determined by Student's t test. Different superscript letters (a, b, c, d) show significant differences compared to each other at p < 0.01, Student's t test
We then further studied whether the expression level of PslG affected its localization. The pG could restore ePsl production of ΔpslG2 to the level of PAO1 at a baseline level expression of PslG (grown without arabinose) as shown in Figure 1c. While induced with 0.01% and 0.1% arabinose, the ePsl production of ΔpslG2/pG was decreased by 47% and 64%, respectively (Figure 2b). A total of 0.1% or 1% arabinose induction decreased the ePsl production of ΔpslG2/pG to the level of negative control ΔpslG2/pHERD20T (Figure 2b). Accordingly, PslG was detected mainly in the inner membrane fraction of ΔpslG2/pG without arabinose (Figure 2a), and the band intensity was similar to that of PAO1. PslG was detected both in the periplasm and inner membrane of ΔpslG2/pG inducing with 0.01%, 0.1%, and 1% arabinose (indicated by arrow, Figure 2a). Bands with lower MW in the inner membrane might be partially degraded PslG, which was only found in the PslG‐overexpressed samples (ΔpslG2/pG with either 0.1% or 1% arabinose). More PslG was detected in the periplasm of ΔpslG2/pG when induced with higher concentration of arabinose (Figure 2a). This suggested that overexpression of PslG led to more PslG releasing to the periplasm. Therefore, we calculated the ratio of PslG in periplasm to inner membrane (Figure 2b, PP/IM). In the ePsl‐inducible strain WFPA801, the transcription of entire psl locus was induced by arabinose, its PP/IM value of PslG was 0.37 with 1% arabinose (Figure 2b). For ΔpslG2/pG, arabinose only induced the expression of PslG, there was more PslG localized in the periplasm, the PP/IM value of PslG was 0.97, 1.93, and 2.14 while induced with 0.01%, 0.1%, and 1% arabinose, respectively (Figure 2b). These data suggest that some Psl proteins might help PslG stay in the inner membrane. In addition, WFPA801 with 1% arabinose produced large amount of ePsl. However, ΔpslG2/pG produced a little ePsl when induced with 0.01%, 0.1%, and 1% arabinose (Figure 2b). The ePsl production of ΔpslG2/pG was reduced and the PP/IM value of PslG was elevated while increasing the concentration of arabinose (Figure 2b). These results suggested that PslG localized in the inner membrane was important for the biosynthesis of ePsl and the ratio of PslG in the periplasm to inner membrane determined the amount of ePsl in extracellular.
3.3. The localization of PslG is affected by PslA, PslD, and PslE
To figure out any Psl protein affecting the localization of PslG, we focused on proteins PslA, PslD, and PslE, which were predicted to be localized on the inner membrane and possessed periplasmic domains (Franklin et al., 2011). WFPA801ΔpslA, WFPA801ΔpslD, and WFPA801ΔpslE containing the plasmid pSadC‐GFP were constructed to examine the effect of Psl proteins on the localization of PslG. Western blot results showed that more PslG localized in the periplasm than in the inner membrane in above PslA, PslD, or PslE‐deleted strains (Figure 3a). The ratio of PslG in periplasm to inner membrane was 1.46, 1.77, and 1.42 in PslA, PslD, and PslE mutants (Figure 3b), indicating that these three proteins are important to maintain PslG in the inner membrane.
Figure 3.
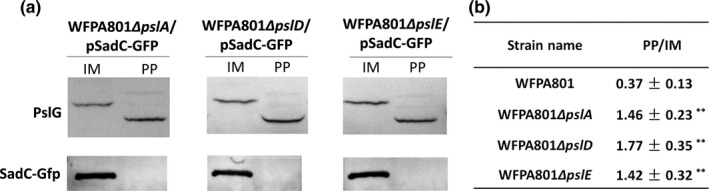
The localization of PslG in the ΔpslA, ΔpslD, and ΔpslE mutants. (a) PslG subcellular localization in pslA, pslD, and pslE in‐frame deletion mutants in the background of WFPA801 containing pSadC‐GFP, respectively. SadC‐Gfp is shown as the inner membrane loading control. (b) The ratio of PslG in periplasm to inner membrane of the three mutants and WFPA801. All strains were grown with 1% arabinose. Means and SD from triplicate experiments are shown. “**” indicates a significant difference compared to WFPA801 of p < 0.01, as determined by Student's t test
3.4. Protein‐protein interaction among PslE with PslA, PslD, and PslG
We utilized bacterial two‐hybrid system to determine whether there are direct interactions among PslA, PslD, PslE, and PslG (Table 2). pBT and pTRG were empty vectors used as negative control. The interaction of SadC and WarA was used as positive control (McCarthy et al., 2017).The results suggested that there was a direct interaction among PslE with PslG, PslD, or PslA. PslE and PslD showed the strongest interaction (Table 2). We did not detect direct interactions between PslG with either PslA or PslD although they both affected the localization of PslG (Figure 3). These results suggested that PslA and PslD might affect PslG localization through PslE or bacterial two‐hybrid system might not be a best way to detect PslA‐PslG and PslD‐PslG interactions.
Table 2.
Protein interactions among PslA, PslD, PslG, and PslE
| a E. coli strain containing | pBT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SadC | none | PslA | PslG | PslG1−45 | PslG3 1−442 | PslE | PslD | ||
| pTRG | WarA | + | ND | ND | ND | ND | ND | ND | ND |
| none | ND | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| PslA | ND | ‐ | ‐ | ‐ | ‐ | ‐ | ++ | ‐ | |
| PslG | ND | ‐ | ‐ | ‐ | ‐ | ‐ | + | ‐ | |
| PslG1−45 | ND | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| PslG3 1−442 | ND | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| PslD | ND | ‐ | ‐ | ‐ | ‐ | ‐ | +++ | ‐ | |
| PslE | ND | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | + | |
Proteins expressed from bait vector pBT were listed in a row, proteins expressed from target vector pTRG were listed in a vertical column. The interaction of SadC and WarA was used as positive control. Protein interactions in the E. coli strain XL1‐Blue MRF’ Kan were detected by the ability of the cells to grow on the M9+ His‐dropout Broth plate supplemented with 5 mM 3‐AT and 12.5 μg/ml streptomycin. The strength of interaction was based on the growth rate of cells on the plate. ‐: no interaction. +: weak interaction. ++: moderate interaction. +++: strong interaction. ND: not determined.
To know whether the full length of PslG is necessary for the interaction with other Psl proteins, we detected the interaction of the N‐terminal 45 amino acid residues of PslG (PslG1‐45, contained the entire signal peptide domain) or PslG31‐442 (contained only the soluble domain of PslG) with PslA, PslD, or PslE (Table 2). No interactions were found for either PslG1‐45 or PslG31‐442 with these three Psl proteins. These results suggested that the interaction with PslE required a full length PslG.
PslE‐PslD showed the strongest interaction, thus we further asked whether PslE can affect the localization of PslD. To detect PslD, we made an anti‐PslD antibody by an antigenic epitope approach. This antibody was first examined for its specificity by western blotting against the whole cell extracts of PAO1 and WFPA801 as positive controls, WFPA800 and ΔpslD strain as negative controls (Figure 4a, arrows indicated the bands of PslD protein). Then this anti‐PslD antibody was used for the detection of PslD. We first examined the PslD in the whole cell extract from PAO1, ΔpslE, ΔpslA, ΔpslD, and ΔpslG2 strains. RNA polymerase was used as a loading control (Figure 4b). The whole cell extract of wild type PAO1 had more PslD than that of the three mutant strains (Figure 4b). We then investigate PslD's localization. PslD was found to be enriched in both the inner membrane fraction and outer membrane fraction in PAO1 (Figure 4c). Then we extracted membrane fractions of ΔpslE, ΔpslA, and ΔpslG2. PslD was detected only in the inner membrane but not in outer membrane in the absence of PslE (Figure 4c), while deletion of pslA or pslG had no influence on PslD localization (Figure 4c). These results were consistent with the results of proteins interactions (Table 2), suggesting that PslE might help PslD to span to the outer membrane by direct PslE‐PslD interaction.
Figure 4.
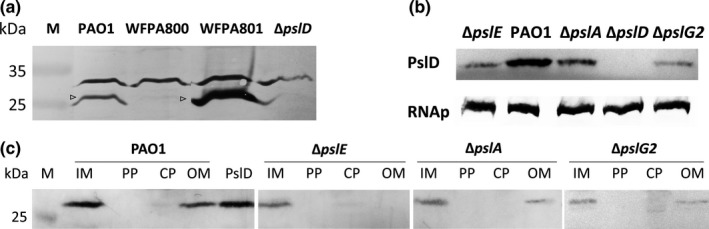
The effect of PslE on the localization of PslD. (a) Western blotting analysis of whole cell extracts of PAO1, WFPA800, WFPA801 grown with 1% arabinose, and ΔpslD using anti‐PslD antibody. The molecular weight of PslD is 27.9 kDa. Arrows indicate the bands of PslD protein. (b) Western blotting analysis of whole cell extracts of ΔpslE, PAO1, ΔpslA, ΔpslD, and ΔpslG2 using anti‐PslD and anti‐RNA polymerase (RNAp) antibody, respectively. (C) Identification of PslD in the IM, PP, CP, and OM fractions from PAO1 and its derived ΔpslA, ΔpslE, and ΔpslG2 mutants by western blotting analysis with anti‐PslD antibody. PslD: purified PslD protein loading as the positive control. M: marker
4. DISCUSSION
The ePsl is a key biofilm matrix component of the life‐threaten pathogen P. aeruginosa. It promotes bacteria cell‐cell and cell‐surface interaction by acting as a “molecular glue” (Ma et al., 2009, 2006); it forms a fiber‐like matrix to protect bacteria from antibiotics and phagocytic cells (Billings et al., 2013; Mishra et al., 2012); and it can function as a signal to stimulate biofilm formation (Irie et al., 2012). However, the molecular mechanism of ePsl biosynthesis remains unknown. In this study, we focused on the role of glycoside hydrolase PslG in the biosynthesis of ePsl. We investigated the protein interactions of PslA, PslD, PslE, and PslG and examined the effects of protein interactions on protein localization of PslD and PslG. Our data suggested that the membrane‐associated PslG was a part of ePsl biosynthesis machinery and the Psl proteins interactions might control the release of PslG into the periplasmic space.
Glycoside hydrolases are common in many bacterial exopolysaccharide biosynthesis operons, such as PssZ in Listeria monocytogenes (Koseoglu et al., 2015), PgaB and BcsZ in E. coli (Mazur & Zimmer, 2011; Wang, Preston, & Romeo, 2004), and WssD and AlgL in Pseudomonas fluorescence (Bakkevig et al., 2005; Spiers, Bohannon, Gehrig, & Rainey, 2003). Our previous study demonstrated the structure of glycoside hydrolase PslG and its effects on biofilm when applied exogenously (Yu et al., 2015), while little is known about its function in the process of ePsl biosynthesis. Baker et al. (2015) had studied the role of pslG in a psl overexpression strain PAO1ΔpelFPBAD psl. They concluded that pslG had no involvement in the biosynthesis of ePsl. However, in a psl overexpression system, only a huge change on ePsl production could be find. Therefore, to determine the role of PslG and its endoglycosidase activity in the biosynthesis of ePsl in P. aeruginosa PAO1, we constructed strain ΔpslG2 and ΔpslG2::pslGE165Q + E276Q, and found that PslG and its hydrolytic activity were important for initial attachment and ePsl production. Monday and Schiller (1996) and Penaloza Vazquez, (1997) considered AlgL functions as the integral component in the alginate biosynthesis complex and lacking of algL resulted in less alginate production. Here lacking of pslG decreased ePsl production, suggesting PslG serves as the integral component in the ePsl biosynthesis complex. The ePsl production of ΔpslG2 could not be restored by PslGE165Q + E276Q, indicating the hydrolytic activity of PslG is critical for optimal ePsl biosynthesis, similar to the cellulose degrading enzyme, BcsZ (Mazur & Zimmer, 2011). Though the differences in ePsl production between WFPA800, ΔpslG2, and ΔpslG2::pslGE165Q + E276Q were not enough to make significant differences in a 30 min attachment assay, the differences of biofilm biomass could be found in a biofilm assay post 2 hr incubation (Appendix Figure AA2), in which the biofilm biomass of ΔpslG2, and ΔpslG2::pslGE165Q + E276Q were slightly higher than WFPA800, suggesting the ePsl synthesized from pslG mutants is functional.
PslG localizes in the inner membrane and periplasm (Baker et al., 2015). We are interested in whether the specific localization of PslG plays different role in the biosynthesis of ePsl. We found PslG in wild type PAO1 mainly localized in the inner membrane. When PslG was overexpressed alone, more PslG localized in the periplasm with a decrease in ePsl production. These results suggest inner membrane association of PslG helps synthesize ePsl polymer, while PslG in the periplasm may degrade ePsl polymer randomly.
As the localization of PslG is critical to ePsl production, we have further investigated other Psl proteins that might modulate the localization of PslG. We focus on the predicted periplasmic proteins (PslA, PslD, and PslE) that may interact with PslG in the ePsl assembly apparatus. We found that more PslG localized to the periplasm in the absence of PslA, PslD, or PslE. Interaction of PslE with PslG was further confirmed via bacterial two‐hybrid assay. These results suggested that the membrane‐associated PslG was a part of ePsl biosynthesis machinery, in which PslA, PslD, and PslE might help control or delay the release of PslG into periplasmic space. Our data have also shown that the hydrolytic activity of PslG is important for the synthesis of ePsl, implying that the ePsl biosynthesis machinery may allow PslG in an optimal localization to control the degradation of ePsl polymer at certain length (Figure 5).
Figure 5.
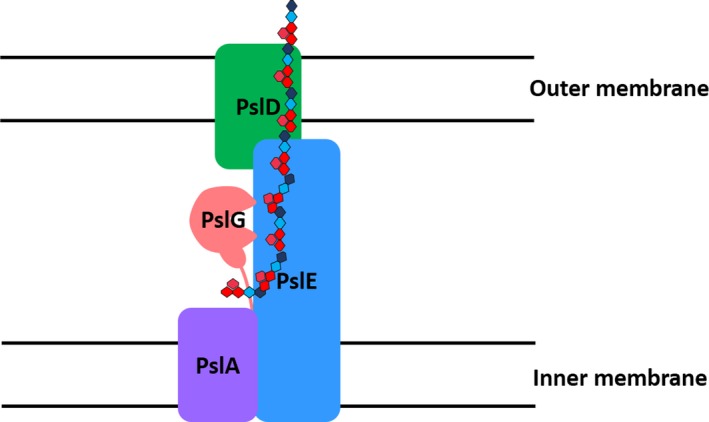
A schematic view of Psl proteins in the ePsl biosynthesis machinery. Membrane associated PslG is a part of the ePsl biosynthesis machinery, in which PslA, PslD, and PslE allow PslG in an optimal localization to control the degradation of ePsl polymer at certain length. PslE interacts with PslA, PslD, and PslG, it helps PslD to localize in the outer membrane to export the ePsl
The structures and functions of PslA, PslD, and PslE have not been experimentally determined. PslA might likely play a similar role to WbaP in providing a site for the assembly of the oligosaccharide repeating unit onto the isoprenoid lipid at the cytoplasmic face of the inner membrane (Franklin et al., 2011; Whitfield, 2006). PslE has characteristic domains of a Wzz (or Wzc) homolog and is therefore predicted to act as the polysaccharide co‐polymerase (PCP) component in this system (Franklin et al., 2011; Larue, Kimber, Ford, & Whitfield, 2009). The periplasmic domain of PCPs is proposed to affect polysaccharide chain length (Tocilj et al., 2008) and is thought to form critical interactions with the CPS/EPS export component thereby completing a complex that facilitates transfer of the polymer through the periplasm (Cuthbertson, Mainprize, Naismith, & Whitfield, 2009). PslD is predicted to be the polysaccharide exporter with structural similarity to the E. coli K30 capsule translocase, Wza, an integral outer membrane lipoprotein (Dong et al., 2006; Franklin et al., 2011). Predicted PslD 3‐dimensional structure (Appendix Figure AA3) has indicated that most of PslD can be structurally modeled onto Wza (PDB ID 2J58), but there is a clear difference, PslD appears to lack the outer membrane barrel and large periplasmic domain. Therefore, it is difficult to understand how the Psl polymer is translocated across the outer membrane. In this study, we found that PslD had a strong interaction with PslE and it could not localize to the outer membrane without PslE, which suggest that PslE, the Wzc homolog, interacts with PslD and helps PslD localize to the outer membrane. In addition, our data also suggest PslE is likely to act as the periplasmic scaffold and recruit proteins to form a polysaccharide biosynthetic complex because PslE can interact with PslA, PslD, and PslG (Table 2, Figure 5). More PslD was detected in PAO1 than in pslA, pslE, or pslG deletion mutant, implying that PslD integrated into the ePsl biosynthetic complex is more stable than free PslD.
To the best of our knowledge, this is the first study to investigate the connection between protein interactions and their localizations during ePsl biosynthesis of P. aeruginosa. Our data showed the glycoside hydrolase PslG and its hydrolytic activity were important to ePsl production of P. aeruginosa. The inner membrane association of PslG might be involved in the biosynthesis of ePsl, while PslG localized in the periplasm may degrade ePsl. We have experimentally proved the PslE interacted with PslA, PslD, and PslG in vivo. All the three proteins, PslA, PslD, and PslE, had an impact on PslG localization, which was critical to ePsl biosynthesis. PslE helped PslD localize the outer membrane, these two proteins might form a complex to help transport Psl across the outer membrane. In summary, we have shown in this study that ePsl biosynthesis is a complex processing with dynamic protein‐protein interactions, leading to the assembly of ePsl biosynthesis machinery.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
H.W., D.W., and L.Z.M. conceived and designed experiments, and contributed to the writing of the manuscript. H.W. and M.T. conducted experiments.
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
We thank Prof. Lichuan Gu at the Shandong University for providing purified PslD protein; Dr. Shiwei Wang and Dr. Qing Wei at the Institute of Microbiology, Chinese Academy of Sciences for critical reading of the manuscript. This work was supported by the National Natural Science Foundation of China to L.M. (31570126), and the National Basic Research Program of China (973 Program 2015CB150602).
APPENDIX 1.
Figure A1.
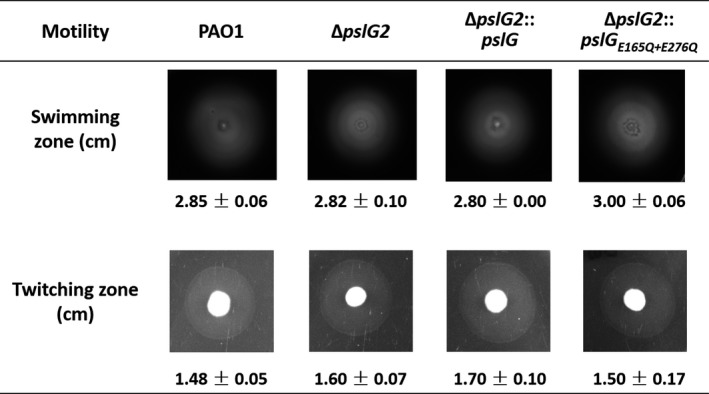
The flagella mediated swimming motility and type IV pili mediated twitching motility were tested for PAO1 and its derived pslG mutants. Diameters of the zones were averaged from triplicate experiments
Figure A2.
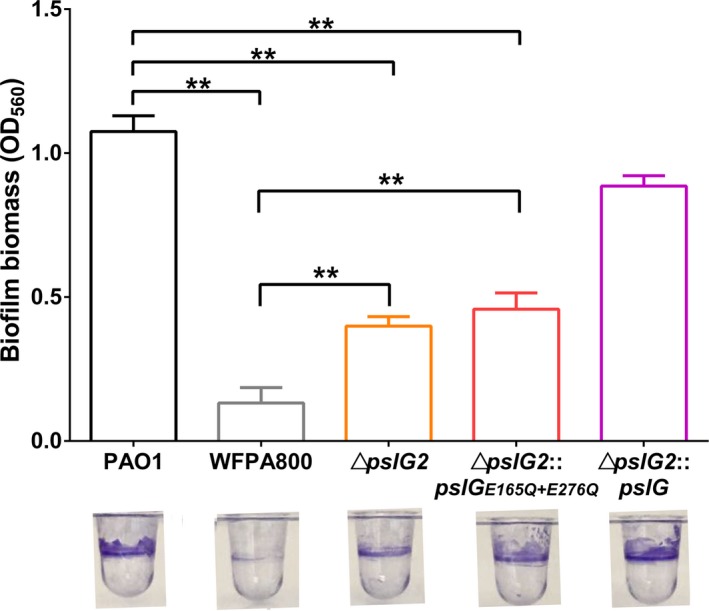
The results of 2‐hr biofilm assay of PAO1, WFPA800, ΔpslG2, ΔpslG2::pslG, and ΔpslG2::pslGE165Q + E276Q. Means and SD from triplicate repeats are shown. **p < 0.01, Student's t test
Figure A3.
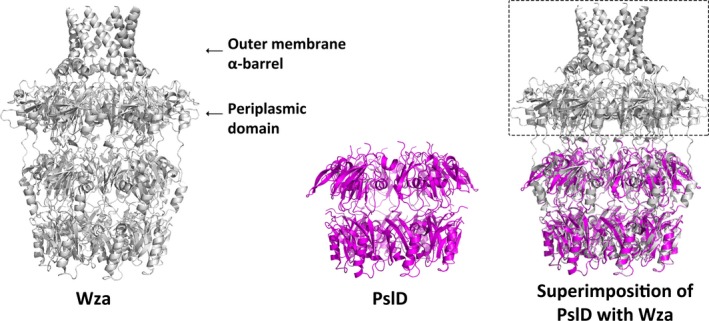
The schematic view of predicted PslD 3‐D structure and its comparison with Wza (PDB ID 2J58). Structures are shown in cartoon representation. The structure of Wza is shown in gray, and predicted PslD is shown in magenta, respectively. Dotted box indicates the domain lacking in the structure of PslD
Wu H, Wang D, Tang M, Ma LZ. The advance of assembly of exopolysaccharide Psl biosynthesis machinery in Pseudomonas aeruginosa . MicrobiologyOpen. 2019;8:e857 10.1002/mbo3.857
DATA ACCESSIBILITY
All data are provided in full in this paper.
REFERENCES
- Baker, P. , Whitfield, G. B. , Hill, P. J. , Little, D. J. , Pestrak, M. J. , Robinson, H. , … Howell, P. L. (2015). Characterization of the Pseudomonas aeruginosa glycoside hydrolase PslG reveals that its levels are critical for Psl polysaccharide biosynthesis and biofilm formation. Journal of Biological Chemistry, 290, 28374–28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkevig, K. , Sletta, H. , Gimmestad, M. , Aune, R. , Ertesvag, H. , Degnes, K. , … Valla, S. (2005). Role of the Pseudomonas fluorescens alginate lyase (AlgL) in clearing the periplasm of alginates not exported to the extracellular environment. Journal of Bacteriology, 187, 8375–8384. 10.1128/jb.187.24.8375-8384.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings, N. , Millan, M. R. , Caldara, M. , Rusconi, R. , Tarasova, Y. , Stocker, R. , & Ribbeck, K. (2013). The extracellular matrix component Psl provides fast‐acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Path, 9, e1003526 10.1371/journal.ppat.1003526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda, S. S. , Vik, A. , Friedman, L. , & Kolter, R. (2005). Biofilms: The matrix revisited. Trends in Microbiology, 13, 20–26. 10.1016/j.tim.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Byrd, M. S. , Sadovskaya, I. , Vinogradov, E. , Lu, H. , Sprinkle, A. B. , Richardson, S. H. , … Wozniak, D. J. (2009). Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Molecular Microbiology, 73, 622–638. 10.1111/j.1365-2958.2009.06795.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisano, A. , Schroeder, C. , Schemionek, M. , Overhage, J. , & Rehm, B. H. (2006). PslD is a secreted protein required for biofilm formation by Pseudomonas aeruginosa . Applied and Environmental Microbiology, 72, 3066–3068. 10.1128/aem.72.4.3066-3068.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, M. Q. , Chen, J. S. , & Lory, S. (2010). The Pseudomonas aeruginosa pathogenicity island PAPI‐1 is transferred via a novel type IV pilus. Journal of Bacteriology, 192, 3249–3258. 10.1128/jb.00041-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis, C. E. , & Ohman, D. E. (1993). Genetic analysis of the alginate biosynthetic gene cluster of Pseudomonas aeruginosa shows evidence of an operonic structure. Molecular Microbiology, 8, 583–593. 10.1111/j.1365-2958.1993.tb01602.x [DOI] [PubMed] [Google Scholar]
- Colvin, K. M. , Alnabelseya, N. , Baker, P. , Whitney, J. C. , Howell, P. L. , & Parsek, M. R. (2013). PelA deacetylase activity is required for Pel polysaccharide synthesis in Pseudomonas aeruginosa . Journal of Bacteriology, 195, 2329–2339. 10.1128/jb.02150-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin, K. M. , Irie, Y. , Tart, C. S. , Urbano, R. , Whitney, J. C. , Ryder, C. , … Parsek, M. R. (2012). The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environmental Microbiology, 14, 1913–1928. 10.1111/j.1462-2920.2011.02657.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton, J. W. , Lewandowski, Z. , Caldwell, D. E. , Korber, D. R. , & Lappin‐Scott, H. M. (1995). Microbial Biofilms. Annual Reviews in Microbiology, 49, 711–745. 10.1146/annurev.mi.49.100195.003431 [DOI] [PubMed] [Google Scholar]
- Cuthbertson, L. , Mainprize, I. L. , Naismith, J. H. , & Whitfield, C. (2009). Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram‐negative bacteria. Microbiology and Molecular Biology Reviews, 73, 155–177. 10.1128/mmbr.00024-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, C. J. , Beis, K. , Nesper, J. , Brunkan‐Lamontagne, A. L. , Clarke, B. R. , Whitfield, C. , & Naismith, J. H. (2006). Wza the translocon for E‐coli capsular polysaccharides defines a new class of membrane protein. Nature, 444, 226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming, H. C. , & Wingender, J. (2010). The biofilm matrix. Nature Reviews Microbiology, 8, 623–633. [DOI] [PubMed] [Google Scholar]
- Franklin, M. J. , Nivens, D. E. , Weadge, J. T. , & Howell, P. L. (2011). Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Frontiers in Microbiology, 2, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, L. , & Kolter, R. (2004). Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Molecular Microbiology, 51, 675–690. 10.1046/j.1365-2958.2003.03877.x [DOI] [PubMed] [Google Scholar]
- Ghafoor, A. , Jordens, Z. , & Rehm, B. H. A. (2013). Role of PelF in Pel polysaccharide biosynthesis in Pseudomonas aeruginosa . Applied and Environmental Microbiology, 79, 2968–2978. 10.1128/aem.03666-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan, J. R. , & Deretic, V. (1996). Microbial pathogenesis in cystic fibrosis: Mucoid Pseudomonas aeruginosa and Burkholderia cepacia . Microbiological Reviews, 60, 539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussler, S. , & Parsek, M. R. (2010). Biofilms 2009: New perspectives at the heart of surface‐associated microbial communities. Journal of Bacteriology, 192, 2941–2949. 10.1128/jb.00332-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, I. D. , Rehman, Z. U. , Ghafoor, A. , & Rehm, B. H. A. (2010). Bacterial biosynthesis of alginates. Journal of Chemical Technology and Biotechnology, 85, 752–759. 10.1002/jctb.2372 [DOI] [Google Scholar]
- Hoang, T. T. , Karkhoff‐Schweizer, R. R. , Kutchma, A. J. , & Schweizer, H. P. (1998). A broad‐host‐range Flp‐FRT recombination system for site‐specific excision of chromosomally‐located DNA sequences: Application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene, 212, 77–86. 10.1016/s0378-1119(98)00130-9 [DOI] [PubMed] [Google Scholar]
- Holloway, B. W. (1955). Genetic recombination in Pseudomonas aeruginosa . Journal of General Microbiology, 13, 572–581. 10.1099/00221287-13-3-572 [DOI] [PubMed] [Google Scholar]
- Irie, Y. , Borlee, B. R. , O'Connor, J. R. , Hill, P. J. , Harwood, C. S. , Wozniak, D. J. , & Parsek, M. R. (2012). Self‐produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa . Proceedings of the National Academy of Sciences of the United States of America, 109, 20632–20636. 10.1073/pnas.1217993109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings, L. K. , Storek, K. M. , Ledvina, H. E. , Coulon, C. , Marmont, L. S. , Sadovskaya, I. , … Parsek, M. R. (2015). Pel is a cationic exopolysaccharide that cross‐links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proceedings of the National Academy of Sciences of the United States of America, 112, 11353–11358. 10.1073/pnas.1503058112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, S. E. , Fecycz, I. T. , & Campbell, J. N. (1980). Nutritional factors controlling exocellular protease production by Pseudomonas‐aeruginosa . Journal of Bacteriology, 144, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen, M. , Heydorn, A. , Ragas, P. , Lambertsen, L. , Aaes‐Jorgensen, A. , Molin, S. , & Tolker‐Nielsen, T. (2003). Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Molecular Microbiology, 48, 1511–1524. 10.1046/j.1365-2958.2003.03525.x [DOI] [PubMed] [Google Scholar]
- Koseoglu, V. K. , Heiss, C. , Azadi, P. , Topchiy, E. , Guvener, Z. T. , Lehmann, T. E. , … Gomelsky, M. (2015). Listeria monocytogenes exopolysaccharide: Origin, structure, biosynthetic machinery and c‐di‐GMP‐dependent regulation. Molecular Microbiology, 96, 728–743. 10.1111/mmi.12966 [DOI] [PubMed] [Google Scholar]
- Larue, K. , Kimber, M. S. , Ford, R. , & Whitfield, C. (2009). Biochemical and structural analysis of bacterial O‐antigen chain length regulator proteins reveals a conserved quaternary structure. Journal of Biological Chemistry, 284, 7395–7403. 10.1074/jbc.m809068200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. J. , Chang, H. Y. , Venkatesan, N. , & Peng, H. L. (2008). Identification of amino acid residues important for the phosphomannose isomerase activity of PslB in Pseudomonas aeruginosa PAO1. FEBS Letters, 582, 3479–3483. 10.1016/j.febslet.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Liu, J. , & Walsh, C. T. (1990). Peptidyl‐prolyl cis‐trans‐isomerase from Escherichia coli: A periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proceedings of the National Academy of Sciences of the United States of America, 87, 4028–4032. 10.1073/pnas.87.11.4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyczak, J. B. , Cannon, C. L. , & Pier, G. B. (2000). Establishment of Pseudomonas aeruginosa infection: Lessons from a versatile opportunist. Microbes and Infections, 2, 1051–1060. 10.1016/s1286-4579(00)01259-4 [DOI] [PubMed] [Google Scholar]
- Ma, L. , Conover, M. , Lu, H. , Parsek, M. R. , Bayles, K. , & Wozniak, D. J. (2009). Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Path, 5, e1000354 10.1371/journal.ppat.1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Jackson, K. D. , Landry, R. M. , Parsek, M. R. , & Wozniak, D. J. (2006). Analysis of Pseudomonas aeruginosa conditional Psl variants reveals roles for the Psl polysaccharide in adhesion and maintaining biofilm structure postattachment. Journal of Bacteriology, 188, 8213–8221. 10.1128/jb.01202-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmont, L. S. , Rich, J. D. , Whitney, J. C. , Whitfield, G. B. , Almblad, H. , Robinson, H. , … Howell, P. L. (2017). Oligomeric lipoprotein PelC guides Pel polysaccharide export across the outer membrane of Pseudomonas aeruginosa . Proceedings of the National Academy of Sciences of the United States of America, 114, 2892–2897. 10.1073/pnas.1613606114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur, O. , & Zimmer, J. (2011). Apo‐ and cellopentaose‐bound structures of the bacterial cellulose synthase subunit BcsZ. Journal of Biological Chemistry, 286, 17601–17606. 10.1074/jbc.m111.227660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, R. R. , Mazon‐Moya, M. J. , Moscoso, J. A. , Hao, Y. A. , Lam, J. S. , Bordi, C. , … Filloux, A. (2017). Cyclic‐di‐GMP regulates lipopolysaccharide modification and contributes to Pseudomonas aeruginosa immune evasion. Nature, Microbiology, 2 10.1038/nmicrobiol.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, M. , Byrd, M. S. , Sergeant, S. , Azad, A. K. , Parsek, M. R. , McPhail, L. , … Wozniak, D. J. (2012). Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement‐mediated opsonization. Cellular Microbiology, 14, 95–106. 10.1111/j.1462-5822.2011.01704.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monday, S. R. , & Schiller, N. L. (1996). Alginate synthesis in Pseudomonas aeruginosa: The role of AlgL (alginate lyase) and AlgX. Journal of Bacteriology, 178, 625–632. 10.1128/jb.178.3.625-632.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradali, M. F. , Donati, I. , Sims, I. M. , Ghods, S. , & Rehm, B. H. (2015). Alginate polymerization and modification are linked in Pseudomonas aeruginosa . MBio, 6, e00453–e00515. 10.1128/mbio.00453-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole, G. A. (2011). Microtiter dish biofilm formation assay. Jove‐Journal of. Visualized Experiments, 47 10.3791/2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole, G. A. , & Kolter, R. (1998). Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Molecular Microbiology, 30, 295–304. 10.1046/j.1365-2958.1998.01062.x [DOI] [PubMed] [Google Scholar]
- Penaloza Vazquez, A., Kidambi, S. P., Chakrabarty, A. M., & … C. L. (1997). Characterization of the alginate biosynthetic gene cluster in Pseudomonas syringae pv syringae. Journal of Bacteriology, 179, 4464–4472. 10.1128/jb.179.14.4464-4472.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, D. , Damron, F. H. , Mima, T. , Schweizer, H. P. , & Yu, H. D. (2008). PBAD‐based shuttle vectors for functional analysis of toxic and highly regulated genes in Pseudomonas and Burkholderia spp. and other bacteria. Applied and Environmental Microbiology, 74, 7422–7426. 10.1128/aem.01369-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey, D. M. , & Wozniak, D. J. (2005). Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Molecular Microbiology, 56, 309–322. 10.1111/j.1365-2958.2005.04552.x [DOI] [PubMed] [Google Scholar]
- Rehman, Z. U. , Wang, Y. J. , Moradali, M. F. , Hay, I. D. , & Rehm, B. H. A. (2013). Insights into the assembly of the alginate biosynthesis machinery in Pseudomonas aeruginosa . Applied and Environmental Microbiology, 79, 3264–3272. 10.1128/aem.00460-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, A. B. , Hood, R. D. , Bui, N. K. , Leroux, M. , Vollmer, W. , & Mougous, J. D. (2011). Type VI secretion delivers bacteriolytic effectors to target cells. Nature, 475, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers, A. J. , Bohannon, J. , Gehrig, S. M. , & Rainey, P. B. (2003). Biofilm formation at the air‐liquid interface by the Pseudomonas fluorescens SBW25 wrinkly spreader requires an acetylated form of cellulose. Molecular Microbiology, 50, 15–27. 10.1046/j.1365-2958.2003.03670.x [DOI] [PubMed] [Google Scholar]
- Stewart, P. S. , & Costerton, J. W. (2001). Antibiotic resistance of bacteria in biofilms. The Lancet, 358, 135–138. [DOI] [PubMed] [Google Scholar]
- Stoodley, P. , Sauer, K. , Davies, D. , & Costerton, J. W. (2002). Biofilms as complex differentiated communities. Annual Reviews in Microbiology, 56, 187–209. 10.1146/annurev.micro.56.012302.160705 [DOI] [PubMed] [Google Scholar]
- Tocilj, A. , Munger, C. , Proteau, A. , Morona, R. , Purins, L. , Ajamian, E. , … Cygler, M. (2008). Bacterial polysaccharide co‐polymerases share a common framework for control of polymer length. Nature Structural & Molecular Biology, 15, 130–138. 10.1038/nsmb.1374 [DOI] [PubMed] [Google Scholar]
- Vasseur, P. , Vallet‐Gely, I. , Soscia, C. , Genin, S. , & Filloux, A. (2005). The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology, 151, 985–997. 10.1099/mic.0.27410-0 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Preston, J. F. , & Romeo, T. (2004). The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. Journal of Bacteriology, 186, 2724–2734. 10.1128/jb.186.9.2724-2734.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield, C. (2006). Biosynthesis and assembly of capsular polysaccharides in Escherichia coli . Annual Review of Biochemistry, 75, 39–68. 10.1146/annurev.biochem.75.103004.142545 [DOI] [PubMed] [Google Scholar]
- Whitney, J. C. , Colvin, K. M. , Marmont, L. S. , Robinson, H. , Parsek, M. R. , & Howell, P. L. (2012). Structure of the cytoplasmic region of PelD, a degenerate diguanylate cyclase receptor that regulates exopolysaccharide production in Pseudomonas aeruginosa . Journal of Biological Chemistry, 287, 23582–23593. 10.1074/jbc.m112.375378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S. , Su, T. , Wu, H. , Liu, S. , Wang, D. , Zhao, T. , … Ma, L. Z. (2015). PslG, a self‐produced glycosyl hydrolase, triggers biofilm disassembly by disrupting exopolysaccharide matrix. Cell Research, 25, 1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S. , Wei, Q. , Zhao, T. , Guo, Y. , & Ma, L. Z. (2016). A survival strategy for Pseudomonas aeruginosa that uses exopolysaccharides to sequester and store iron to stimulate Psl‐dependent biofilm formation. Applied and Environmental Microbiology, 82, 6403–6413. 10.1128/aem.01307-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Zhang, L. , Liu, Y. , Yang, S. F. , Gao, C. H. , Gong, H. C. , … He, Z. G. (2009). Archaeal eukaryote‐like Orc1/Cdc6 initiators physically interact with DNA polymerase B1 and regulate its functions (vol 106, pp. 7792, 2009). Proceedings of the National Academy of Sciences of the United States of America, 106, 15091‐15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, B. , Liu, C. L. , Liu, S. H. , Cong, H. J. , Chen, Y. H. , Gu, L. C. , & Ma, L. Y. Z. (2016). Membrane association of SadC enhances its diguanylate cyclase activity to control exopolysaccharides synthesis and biofilm formation in Pseudomonas aeruginosa . Environmental Microbiology, 18, 3440–3452. 10.1111/1462-2920.13263 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are provided in full in this paper.


