In an ongoing surge to unravel the tremendously complex signaling pathways even in simple biological systems, appropriate methods to conditionally control protein function in cells are scarce. Small molecule inhibitors for antagonizing the function of an enzyme often lack selectivity, thereby leading to the emergence of undesired systemic side effects. Moreover, for many proteins, specific inhibitors have not been discovered yet. In recent work, a research team led by Craig M. Crews and Erick M. Carreira introduces photoPROTACs to tackle the problem of selectivity by incorporating a photoswitchable unit in the molecular core of the proteolysis targeting chimera (PROTAC) for reversible ON and OFF switching of protein degradation.1
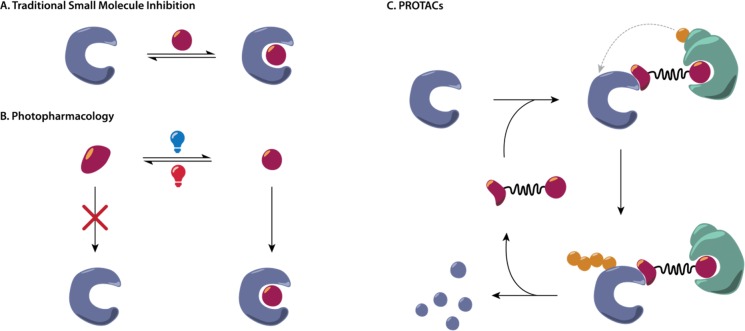
Cells use a sophisticated system to identify, label, separate, and eventually dump malfunctioning protein “waste”. Exploiting the complex natural machinery responsible for this “cleaning” process, specific degradation of proteins can be induced by PROTACs. They are able to link the protein of interest and an E3 ligase in a ternary complex, resulting in labeling of the protein through polyubiquitination for subsequent degradation via the proteasome (Figure 1c).2 This ability to regulate protein levels within a cell via proteolysis irrespective of any intracellular signaling context constitutes a powerful new tool in pharmacology. To diminish undesired side effects, recent approaches in pharmacology combine rather unspecific processes with an external light stimulus that offers high spatiotemporal control, for example, in photocaging3 and the use of inducible degrons.4 Unfortunately, in these cases only an irreversible release of the active agent has been achieved. In contrast, photopharmacology offers the advantage of a light-induced reversible isomerization of a photoswitchable drug from an inactive to an active state that interacts with the target enzyme selectively (Figure 1b).5
Figure 1.
(A) The concept of traditional small molecule inhibitors is based on stoichiometric binding to the active site of the protein to block their function. (B) Activation and deactivation of the inhibitor are achieved by light-controlled binding of one isomer only to modulate protein function. (C) The bifunctional PROTAC binds to the protein and E3 ligase to form a ternary complex. The proximity-induced ubiquitination by an E2 enzyme, which is mediated by the E3 ligase, eventually leads to a dissociation of the complex and proteasomal degradation of the tagged protein while reobtaining the PROTAC.
Covering a wide range for selective degradation of membrane proteins to nuclear hormone receptors, PROTACs have evolved as a powerful tool for the control of specific cellular protein levels. However, current approaches still lack selectivity. To overcome this challenge, the authors of this study bestowed photoswitchability on the PROTAC, allowing them to optically control cellular protein levels. The new photoPROTAC consists of a photoswitchable ortho-tetrafluoroazobenzene that links one ligand for binding to E3 ligase to another ligand for binding the protein of interest. Note that a closely related approach using regular, i.e. non-ortho-fluorinated, as well as cyclic azobenzene linkers has recently been disclosed by the Trauner and Pagano groups.6 Light-induced trans/cis-photoisomerization of the azobenzene linker causes a decrease in effective linker length, which is crucial for the active recruitment of E3 ligase to the protein of interest, giving rise to proximity-induced ubiquitination and subsequent degradation (Figure 2). Irradiation of the active trans-photoPROTAC with 530 nm green light generates the inactive cis-photoPROTAC with good conversion, i.e., 68% cis-content of the photostationary state (PSS). The cis-photoPROTAC, which is too short to engage both proteins in a ternary complex, is thermally stable for several days at 37 °C. Using 415 nm blue light, the trans-photoPROTAC is reobtained in almost quantitative with a PSS of 95% trans-isomer. Even though the rather inefficient OFF-switching of the active trans-isomer generates only 68% of the inactive form, no protein degradation was observed, suggesting a higher binding affinity of the cis-isomer as compared to the trans-isomer. Indeed, even a content of 35% of the inactive cis-photoPROTAC is sufficient for an effective suppression of BRD2 degradation.
Figure 2.
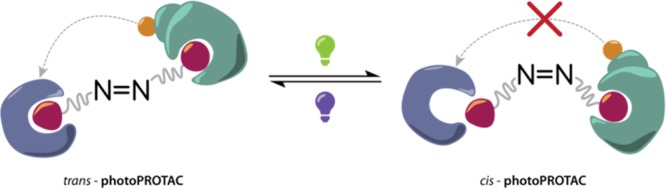
Replacement of the linear polyether linker by a photoswitchable azobenzene allows for reversible control over the topological distance between both protein ligands, in which the cis-photoPROTAC is too short to reach the binding pocket of the second binding partner.
In order to obtain a thermally bistable photoswitch, the para-substitution pattern of the ortho-tetrafluoroazobenzene moiety was slightly changed to a “pull–pull” system in contrast to the original “push–pull” substitution in BET protein degrader ARV-771. As a consequence of the reversed amide bond, a different selectivity of trans-photoPROTAC toward BRD2 over BRD4 is observed, whereas ARV-771 induces degradation of both proteins. Incubation of the inactive cis-photoPROTAC during irradiation with 415 nm blue light causes a significant BRD2 degradation and vice versa proving the dynamic switching between an active and inactive state in a biological environment.
The combination of specific protein degradation with a light stimulus for selective on-site activation is an extraordinarily modular approach for studying signaling pathways that are yet insufficiently understood. Obviously, future work focusing on activation with NIR light is a significant and essential step in the promising field of photoswitchable PROTACs. By influencing the cellular level of the protein by proteolysis, PROTACs inhibit the activity of their target unlike conventional drugs. This mechanistic difference leads to catalytic degradation of the target protein. Moreover, previously undruggable proteins can potentially be explored. The latter demands a special solution for local activation to avoid undesired systemic toxicity while paving the way for new types of precision therapeutics.
The authors declare no competing financial interest.
A sentence and reference have been added to this paper from it’s original posting of October 3, 2019.
References
- Pfaff; et al. ACS Cent. Sci. 2019, 10.1021/acscentsci.9b00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A. C.; Crews C. M. Nat. Rev. Drug Discovery 2017, 16, 101–114. 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour Q.; Li C.; Plamont M. A.; Billon-Denis E.; Aujard I.; Le Saux T.; Jullien L.; Gautier A. ACS Chem. Biol. 2015, 10, 1643–1647. 10.1021/acschembio.5b00069. [DOI] [PubMed] [Google Scholar]
- Bonger K. M.; Rakhit R.; Payumo A. Y.; Chen J. K.; Wandless T. J. ACS Chem. Biol. 2014, 9, 111–115. 10.1021/cb400755b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch M. M.; Hansen M. J.; Van Dam G. M.; Szymanski W.; Feringa B. Angew. Chem., Int. Ed. 2016, 55, 10978–10999. 10.1002/anie.201601931. [DOI] [PubMed] [Google Scholar]
- Reynders M.; Matsuura B.; Bérouti M.; Simoneschi D.; Marzio A.; Pagano M.; Trauner D.. PHOTACs Enable Optical Control of Protein Degradation. ChemRxiv 2019, preprint, 10.26434/chemrxiv.8206688. [DOI] [PMC free article] [PubMed] [Google Scholar]