Abstract
Objective
The helioxanthin derivative 4-(4-methoxyphenyl)thieno[2,3-b:5,4-c′]dipyridine-2-carboxamide (TH) is a low-molecular-weight compound that was identified through screening for osteogenic compounds that enhance the activity of mouse preosteoblastic MC3T3-E1 cells. In the present study, we found that TH suppressed osteoclast differentiation.
Methods
Using the hematopoietic stem cells of ddY mice, TH was added to the culture in the experimental group, and the number of osteoclasts was measured with rhodamine phalloidin staining and TRAP staining. In osteo assay, bone resorption area was compared by the von Kossa staining.
Results
Specifically, TH inhibited the cyclic guanosine monophosphate (cGMP)-degrading activity of phosphodiesterase (PDE), promoted nitric oxide (NO) production, and dose-dependently suppressed osteoclast differentiation in an osteoclast formation culture of mouse bone marrow cells. The NO-competitive guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) attenuated the suppressive activity of TH on osteoclast differentiation. Conclusion: Given the previously reported suppressive action of cGMP on osteoclastogenesis, our data suggest that TH negatively impacts osteoclast differentiation at least to some extent by stimulating NO production and inhibiting PDE activity, both of which lead to the upregulation of intracellular cGMP. This study supports the potential use of TH as a novel antiosteoporotic reagent that not only stimulates bone formation but also inhibits bone resorption.
Keywords: Bone resorption, Helioxanthin derivative, NO, Osteoclast, PDE inhibitor
1. Introduction
As the Japanese population ages, an increasing number of elderly people with osteoporosis and patients with metabolic diseases such as diabetes who do not experience sufficient bone repair are being reported. For intractable fractures that occur in such patients, developing a minimally invasive yet highly effective novel treatment method that promotes bone formation through regenerative medicine has become necessary [1], [2], [3]. The disadvantages of applying regenerative medicine through biologics or gene introduction include the expensive preparation of biologics and therapeutic genes as well as their unproven safety. To overcome these drawbacks, the use of low-molecular-weight compounds with the same effects is being considered [4].
Nitric oxide (NO) is believed to play a role of suppressing excessive osteoclast formation and bone resorption by self-regulation of osteoclast formation. Its pharmacological action involves the activation of guanylate cyclase (GC) by its NO-like action, followed by increasing cyclic guanosine monophosphate (cGMP) levels. As a consequence, cGMP-dependent protein kinase (PKG) is activated [5]. We previously identified a low-molecular-weight helioxanthin derivative (TH; 4-(4-methoxyphenyl)thieno[2,3-b:5,4-c′]dipyridine-2-carboxamide) that promotes osteoblast differentiation in vitro when combined with bone morphogenetic proteins (BMPs) [6]. TH was also shown to promote bone regeneration in a mouse model of cranial bone defects [7]. We recently demonstrated that NO is produced in the medium when nicorandil is metabolized, which has a suppressive effect on osteoclastic bone resorption. NO may be also produced from the nitrogen present in TH; accordingly, similar to nicorandil, TH may affect osteoclast differentiation via NO.
In addition to bone formation by osteoblasts, bone resorption by osteoclasts is a very important factor in bone tissue regeneration and the maintenance of bone mass. Indeed, as observed with the typical bisphosphonate alendronate, bone mass increases after being treated with compounds capable of inhibiting osteoclastic bone resorption [8]. Theophylline, a xanthine derivative, has been shown to inhibit phosphodiesterase (PDE) activity [9], and PDE inhibitors have been reported to inhibit osteoclast formation and bone resorption [10], [11].
When TH was loaded into calcium phosphate tetrapods and transplanted into rat femur bone defects in vivo, bone regeneration-inducing activity (osteogenesis-inducing activity) was promoted [12]. This result led us to hypothesize that TH inhibits bone resorption by osteoclasts as osteogenesis progresses. In this study, we examined the effect of a PDE inhibitor, TH on osteoclast differentiation in an in vitro osteoclast induction system consisting of hematopoietic stem cells derived from mice.
2. Materials and methods
2.1. Cells and materials
ddY mice were purchased from SHIMIZU Laboratory Supplies Co., Ltd. (Kyoto, Japan). Experiments were conducted according to the Animal Experiment Implementation Guidelines of Osaka Dental University (Approval number: 18-02014). Macrophage colony-stimulating factor 1 (CSF-1/M-CSF) and soluble NfϰB ligand (sRANKL) were purchased from PeproTech (Rocky Hill, NJ, USA). 1H-[1,2,4]oxadiazolo[4,3a]quinoxalin-1-one (ODQ) was purchased from Cayman Chemical (Ann Arbor, MI, USA), and l-NAME was purchased from Dojindo Molecular Technologies (Kumamoto, Japan). TH was synthesized by Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
2.2. Osteoclast formation
Following the method of Emori et al. [13], hematopoietic stem cells were excised from both ends of the femurs and tibiae of 5- to 8-week-old ddY mice, and the bone marrow fluid was extruded using a 27-G Myjector syringe (Terumo, Tokyo, Japan). Whole bone marrow cells were collected and suspended in α-minimal essential medium (α-MEM, pH 7.0; Wako Pure Chemical Industries Ltd., Osaka) containing 10% fetal bovine serum (FBS, SAFC Biosciences Inc., Lenexa, KS), 1% GlutaMAX (Invitrogen Corporation, Carlsbad, CA), and a penicillin-streptomycin Mixed Solution (Nacalai Tesque Inc., Kyoto, Japan). Each cell suspension was passed through Sephadex G10 (GE Healthcare UK Ltd., Buckinghamshire, UK) packed in disposable Econo-Pac chromatography columns (Bio-Rad, CA, USA) and collected. To remove erythrocytes, each centrifuged cell pellet was suspended in a 0.83% NH4Cl-Tris-HCl (pH 7.4) buffer solution and allowed to stand on ice for 20 min. The cells were centrifuged again, resuspended in α-MEM and seeded in 96-well plastic culture plates at 5 × 104 cells per well. After 25 ng/ml M-CSF and 100 ng/ml RANKL were added to the medium, TH was added to the wells of the experimental group at concentrations of 1 μM, 2 μM, 5 μM, and 10 μM; a control group was also prepared with no additive. Both groups were cultured for 6 days at 37 °C under 5% CO2 and 95% air. The medium was exchanged every 3 days.
2.3. Rhodamine-phalloidin staining and tartrate-resistant acid phosphatase (TRAP) staining
After culturing was complete, the cells were fixed with 4% paraformaldehyde. A solution of 0.1% Triton X was added to the fixed cells and was replaced with a rhodamine-phalloidin solution after 5 min; the samples were then allowed to stand for 30 min in the dark to stain F-actin. The numbers of osteoclasts with fluorescent rings were determined using a confocal laser microscope (LSM700, Carl Zeiss, Germany). TRAP staining was performed by adding TRAP solution and incubating the osteoclasts at 37 °C for 15 min. The numbers of cells with 3 or more nuclei were measured under an optical microscope.
2.4. Evaluation of bone resorption
Using the same procedures and conditions as those used in the osteoclastogenesis experiment, cells were seeded in hydroxylapatite-coated 1x8 Osteo Assay Stripwell culture dishes (CORNING, USA). After 8 days of culturing, the cells were fixed with a 1 M ammonium chloride aqueous solution. To perform von Kossa staining, a 5% silver nitrate solution was added, and the samples were exposed to light irradiation for at least 60 min. Five percent sodium thiosulfate was added, turning the hydroxylapatite black. Regions with bone resorption were observed, quantified and evaluated under an optical microscope. The area of the bone resorption region was determined using Adobe Photoshop CC 2014 (Adobe Systems, USA). Bone resorption evaluation was also performed for the NO inhibition recovery experiment with ODQ.
2.5. NO measurement
The culture supernatants of the 2 μMTH group, the 5 μMTH group, and the control group with no additive were collected after 0, 3, and 6 days. To measure the amount of NO present in the culture supernatant, an NO2/NO3 assay kit (Dojindo Molecular Technologies, Kumamoto, Japan) was used according to the Griess method. A microplate reader (Spectra Max Pro, Molecular Devices, Tokyo, Japan) was used to measure the absorbance. The control group and the nitric-oxide synthase (NOS) inhibitor l-NAME-added group were cultured separately to confirm the differentiation status of osteoclasts and to determine whether endogenous NO production affected osteoclast differentiation. The cells were seeded in 96-well plastic culture plates at 5 × 104 cells per well in α-MEM containing 10% FBS, 25 ng/ml M-CSF, and 100 ng/ml RANKL. In the osteoclastogenesis experiment, the cells were divided into a NOS inhibitor l-NAME group and a control group and were cultured for 6 days at 37 °C under 5% CO2 and 95% air. The medium was exchanged every 3 days.
2.6. PDE activity measurement
Experiments were conducted using 96-well plastic culture plates with cAMP or cGMP as a substrate, and PDE activity was measured using a cyclic nucleotide phosphodiesterase assay kit (Enzo Life Sciences, NY, USA). Substrates were added to the brain-derived PDE and 5′-nucleotidase, and the samples were incubated for 30 min at 30 °C separately as follows: a control group with no additive, an IBMX group (positive control for PDE inhibition), a 1 μMTH group, a 2 μMTH group, a 5 μMTH group, and a 10 μMTH group. Thereafter, BIOMOL GREEN was added, and the mixtures were stirred gently and incubated at room temperature for 20 min. Finally, the absorbance was measured at 620 nm using a microplate reader (Spectra Max Pro, Molecular Devices, Tokyo, Japan).
2.7. NO inhibition recovery through ODQ
ODQ was dissolved in dimethylsulfoxide (DMSO). The cells were cultured in α-MEM containing 10% FBS, 25 ng/ml M-CSF, and 100 ng/ml RANKL and were seeded in 96-well plastic culture plates at 5 × 104 cells per well. In the experiment, a TH concentration of 5 μM, which corresponded to the IC50 in the osteoclastogenesis experiment, was selected. A group with 10 μM ODQ added and a control group with 5 μMTH alone were cultured separately at 37 °C under 5% CO2 and 95% air for 6 days. The medium was exchanged every 3 days. The differentiation status of the osteoclasts was observed using rhodamine-phalloidin and TRAP staining.
2.8. Statistical analysis
The data are expressed as the mean ± standard error of three independent experiments, with six times in each experiment (N = 6). P values of <0.05 were considered to indicate statistically significant differences. To determine the statistical significance of differences among multiple groups, Bonferroni's correction test was performed through one-way ANOVA using IBM SPSS ver. 16.
3. Results
3.1. Suppression of osteoclast differentiation by TH
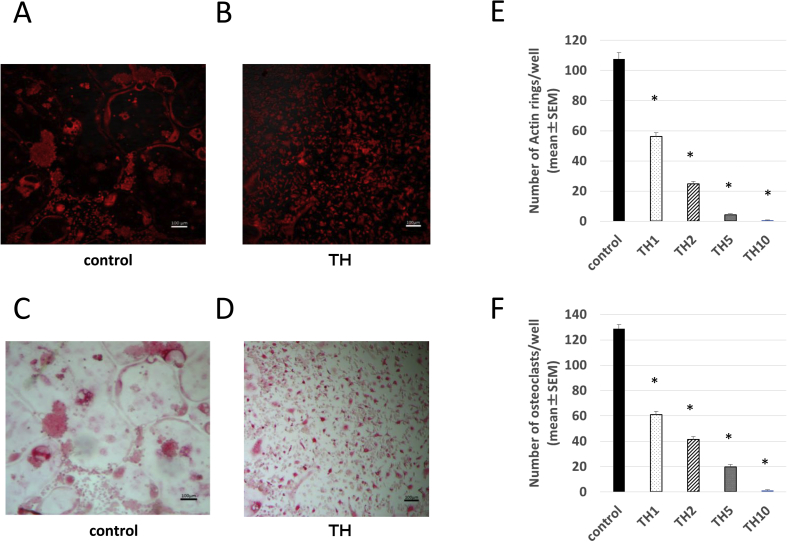
We first examined the impact of TH on osteoclast differentiation in osteoclast formation cultures. After rhodamine-phalloidin staining, a large ring of F-actin was observed in the control group activated osteoclasts (Fig. 1A); in the TH groups, no such enlargement tendency was observed (Fig. 1B). The formation of large, multinucleated osteoclasts was observed in the control group via TRAP staining (Fig. 1C); conversely, in the TH groups, the undifferentiated osteoclasts appeared very small (Fig. 1D). The control group had 107.67 ± 4.148 osteoclasts with F-actin rings, while 56.00 ± 2.819 were observed in the 1 μMTH group, 24.83 ± 1.588 in the 2 μMTH group, 4.17 ± 0.796 in the 5 μMTH group, and 0.67 ± 0.304 in the 10 μMTH group. Through TRAP staining, 128.83 ± 3.328 TRAP-positive osteoclasts were present in the control group, while 60.83 ± 2.618 were observed in the 1 μMTH group, 41.17 ± 2.443 in the 2 μMTH group, 19.83 ± 1.964 in the 5 μMTH group, and 1.17 ± 0.495 in the 10 μMTH group. Thus, the number of osteoclasts was decreased in a TH concentration-dependent manner (Fig. 1E and F). Although the differentiation of cells into osteoclasts was significantly suppressed by TH, a large number of undifferentiated cells were present. This result suggests that TH is not highly cytotoxic to osteoclasts or their progenitors.
Fig. 1.
Effect of TH on osteoclast differentiation. (A and B) Rhodamine-phalloidin staining of control cells (A) and cells treated with 5 μMTH (B). Bars, 100 μm. (C and D) TRAP staining of control cells (C) and cells treated with 5 μMTH (D). Bars, 100 μm. (E) Number of cells with F-actin rings visualized by rhodamine-phalloidin staining in the control, 1 μM TH (TH1), 2 μMTH (TH2), 5 μMTH (TH5) and 10 μMTH (TH10) groups. P < 0.05 vs. control group. (F) Number of TRAP-positive cells in the control, 1 μMTH (TH1), 2 μMTH (TH2), 5 μMTH (TH5) and 10 μMTH (TH10) groups. P < 0.05 vs. control group.
3.2. NO production in the medium by TH
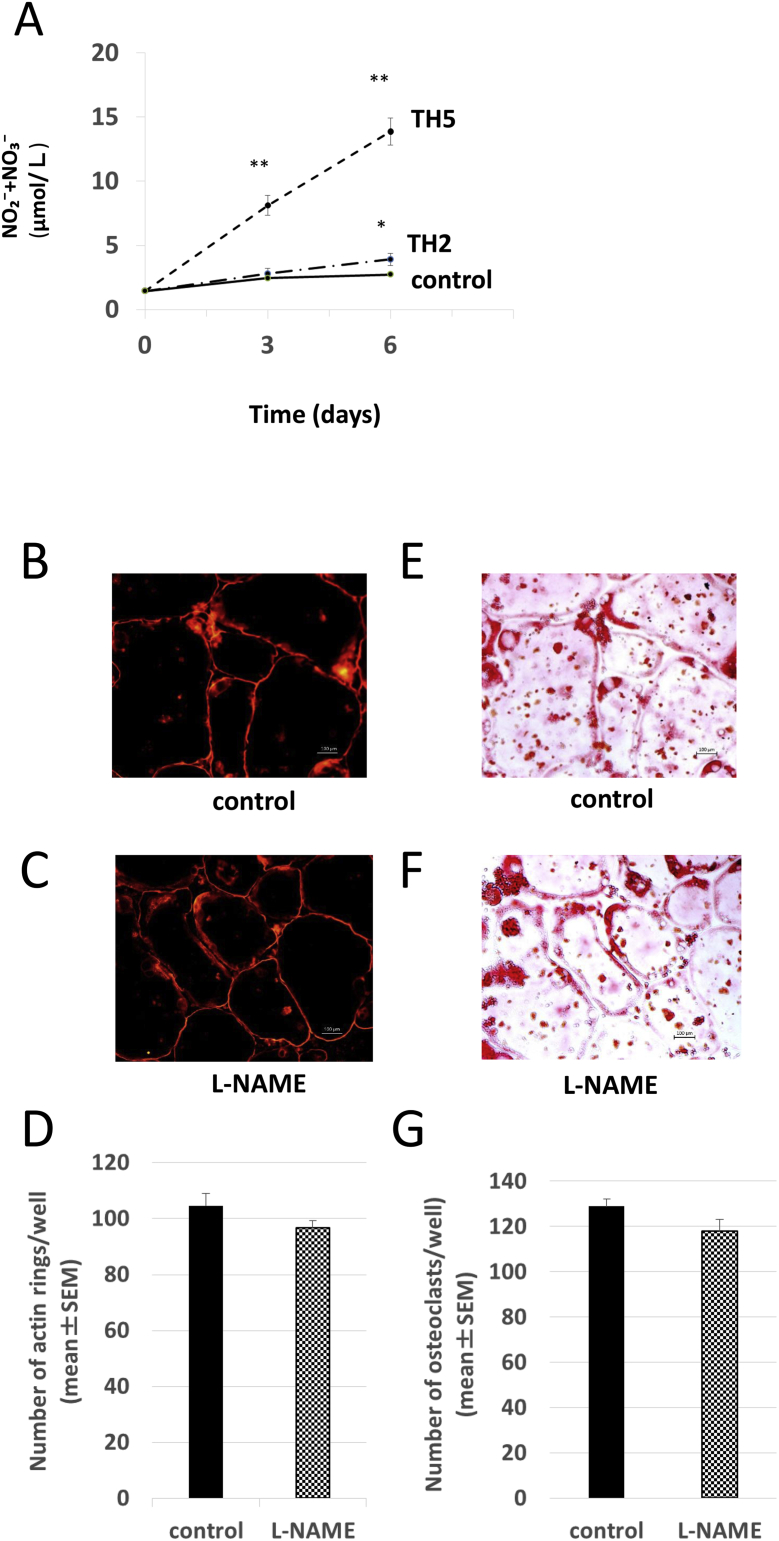
We next examined whether TH induced NO production in an osteoclast formation culture of mouse bone marrow cells. In the control group, only a trace amount of NO was produced, ranging from 1.443 ± 0.036 μmol/L on day 0–2.46 ± 0.107 μmol/L on day 3 and 2.74 ± 0.076 μmol/L on day 6, and it appeared to be due to endogenous NO production. However, a significant increase in NO production occurred in a time-dependent manner in cultures that were treated with TH at 2 μM and 5 μM. After the addition of 2 μMTH, the NO concentration was 2.80 ± 0.424 μmol/L on day 3 and 3.92 ± 0.369 μmol/L on day 6; at 5 μM, it was 8.124 ± 0.775 μmol/L on day 3 and 13.87 ± 0.672 μmol/L on day 6 (Fig. 2A).
Fig. 2.
Nitric oxide (NO) production in osteoclast formation cultures treated with or without TH (A) and the effects of a nitric-oxide synthase (NOS) inhibitor on osteoclast formation (B–G). (A) The amount of NO in culture supernatants collected after 0, 3, and 6 days of osteoclast formation culture was measured. TH2, 2 μMTH; TH5, 5 μM TH. *P < 0.05 vs. control group; **P < 0.01 vs. control group. (B and C) Rhodamine-phalloidin staining of control cells (B) and NOS inhibitor l-NAME-treated cells (200 μM) (C). Staining was performed after 6 days of osteoclast formation culture without TH. Bars, 100 μm. (D) Number of cells with F-actin rings visualized by rhodamine-phalloidin staining in the control and l-NAME groups. (E and F) TRAP staining of control cells (E) and l-NAME-treated cells (200 μM) (F). Staining was performed after 6 days of osteoclast formation culture without TH. Bars, 100 μm. (G) Number of TRAP-positive cells in the control and l-NAME group cultures.
To understand the relationship between endogenously produced NO and osteoclast formation, we tested the effects of the NOS inhibitor l-NAME in an osteoclast formation culture. The results of rhodamine-phalloidin staining (Fig. 2B–D) showed no significant differences in either the size or number of differentiated osteoclasts between the control group and the l-NAME group. In the TRAP staining results (Fig. 2E–G), the size and number of TRAP-positive osteoclasts were also not significantly different between the control group and the l-NAME group. These results suggested that endogenous NO had no effect on osteoclast differentiation. NO may be also produced from the nitrogen present in TH; accordingly, similar to nicorandil.
3.3. PDE inhibitory action by TH
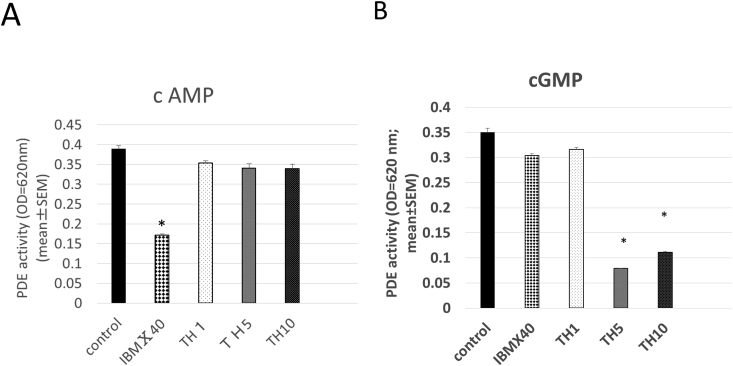
Theophylline, a xanthine derivative, has been shown to possess PDE inhibitory activity that suppresses osteoclast bone resorption [10]. Therefore, the PDE inhibitory activity of the xanthine derivative TH was investigated. When cAMP was used as a substrate, IBMX significantly inhibited PDE activity,while TH did not change (Fig.3A). When cGMP was used as a substrate, 5 and 10 μMTH significantly suppressed PDE activity compared to that of the control, while IBMX and 1 μMTH did not (Fig. 3B). No significant differences between the 1 μMTH group and the control group were observed. Thus, the inhibitory effect of TH on PDE was dependent on its concentration.
Fig. 3.
Effect of TH on phosphodiesterase (PDE) activity. PDE activity was measured in the presence or absence of TH using cGMP as the substrate. IBMX (40 μM) was used as a positive control. Various concentrations of TH were tested: TH1, 1 μMTH; TH5, 5 μMTH; and TH10, 10 μM TH. *P < 0.01 vs. control group.
3.4. Attenuation of the effects of TH on osteoclast differentiation by the NO pathway inhibitor ODQ
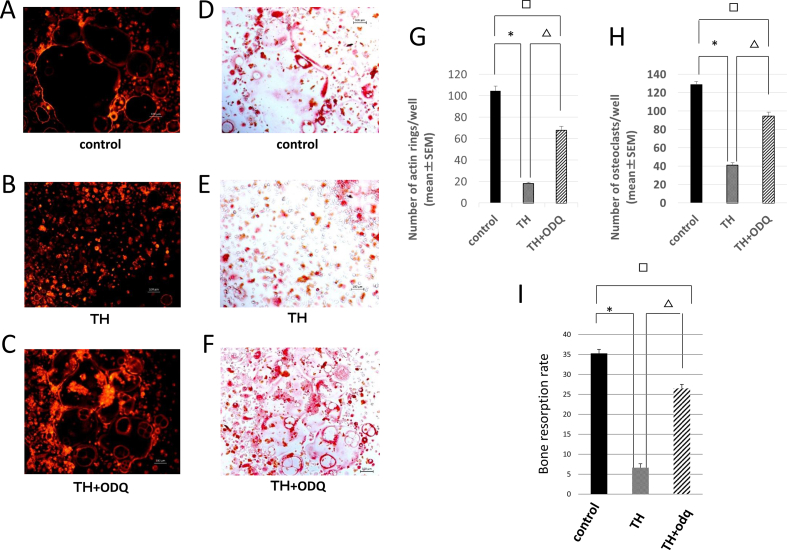
Lastly, to investigate NO pathway involvement in the mechanism by which TH affects osteoclast differentiation, we added the NO-competitive guanylyl cyclase inhibitor ODQ to cultures in the presence of TH. The rhodamine-phalloidin staining results showed that the control group contained active osteoclasts with large F-actin rings (Fig. 4A), whereas this enlargement tendency was markedly decreased in the 5 μMTH group (Fig. 4B). Although large F-actin rings were apparent in the ODQ group, they were clearly smaller in size and were incompletely shaped compared to those in the control group (Fig. 4C). TRAP staining revealed the presence of huge, multinucleated osteoclasts in the control group (Fig. 4D), whereas the 5 μMTH group contained small, undifferentiated osteoclasts (Fig. 4E). In the ODQ group, the inhibitory action of TH was attenuated; although large multinucleated osteoclasts were present, they were smaller than those in the control group, and their shapes were incomplete (Fig. 4F).
Fig. 4.
Effect of the NO pathway inhibitor ODQ on the TH-mediated suppression of osteoclast differentiation. (A–C) Rhodamine-phalloidin staining of control cells (A), cells treated with 5 μMTH (B), and cells treated with 5 μMTH and 10 μM ODQ. Bars, 100 μm. (D–F) TRAP staining of control cells (A), cells treated with 5 μMTH (B), and cells treated with 5 μMTH and 10 μM ODQ. Bars, 100 μm. (G) Number of cells with F-actin rings visualized by rhodamine-phalloidin staining in the control, 5 μMTH (TH) and 5 μMTH+10 μM ODQ (TH + ODQ) groups. *P < 0.01 control group vs. TH5; △P < 0.01 TH5 vs. TH + ODQ; □P < 0.01 control group vs. TH + ODQ. (H) Number of TRAP-positive cells in the control, 5 μMTH (TH) and 5 μMTH+10 μM ODQ (TH + ODQ) groups. *P < 0.01 control group vs. TH5; △P < 0.01 TH vs. TH + ODQ; P < 0.01 control group vs. TH + ODQ. (I) Bone resorption ability of osteoclasts cultured on hydroxylapatite (control) with 5 μMTH (TH5) and with 5 μMTH and 10 μM ODQ (TH + ODQ). △P < 0.01 TH + ODQ vs. TH; □P < 0.01 control group vs. TH.
The number of osteoclasts with F-actin rings decreased by 82.5% in the 5 μMTH group and by 33.2% in the ODQ group compared to that of the control group (Fig. 4G). TRAP staining revealed that the number of osteoclasts decreased by 68.0% in the 5 μMTH group and by 26.6% in the ODQ group compared to that of the control group (Fig. 4H). The bone resorption rate was 35.27 ± 2.519% in the control group, 6.62 ± 0.681% in the 5 μMTH group, and 26.50 ± 4.382% in the ODQ group (Fig. 4I). These data suggest that ODQ attenuates the suppressive activity of TH on osteoclast differentiation.
4. Discussion
Xanthine derivatives, organic compounds that are formed from purine bases, are found in most body tissues and fluids [14]. They are used as symptom-relieving drugs in bronchial asthma because of their mild stimulant action and bronchodilator effect [15]. In recent years, through the screening of low-molecular-weight osteogenic compounds using compound libraries, the helioxanthin derivative TH was found to possess bone regeneration-inducing (bone formation) activity [6], [11]. Furthermore, TH has been shown to promote bone formation in a cranial bone defect mouse model [7]. Conversely, the effects of TH on osteoclastic bone resorption remain unknown. Hence, in this study, we examined the effects of TH on osteoclast differentiation using hematopoietic stem cells from mice. We found that TH treatment decreased the formation of osteoclasts, TRAP-positive multinucleated cells and actin rings in a dose-dependent manner. The addition of TH reduced the area in which hydroxylapatite was absorbed by osteoclasts in vitro. In particular, the observation that the number of activated osteoclasts decreased markedly after TH treatment suggests that TH has a marked suppressive effect on the late differentiation of osteoclasts. These results further suggest that, aside from its previously reported osteogenesis-promoting action, TH also suppresses bone resorption by inhibiting osteoclastic differentiation.
Previously, using the mouse osteoblast cell line MC3T3-E1, we proposed that TH possesses BMP-dependent osteogenic activity [6]. Alternatively, this study revealed that TH treatment increases the NO concentration in the medium in a time-dependent manner. In vivo, NO synthesis is mediated by NOS, which can be classified into constitutive NOS (cNOS, NOS that is always present in cells at constant levels) and induced NOS (iNOS, NOS that is induced by inflammation and stress). MacIntyre et al. [16] experimentally demonstrated that NO-generating agents inhibit the activity of osteoclasts isolated from rats and decrease the extent of areas with bone resorption. They hypothesized that the effects of NO-generating agents were not mediated by cGMP production but rather by the direct action of NO on the cytoskeleton or through its activity on kinases or other proteins such as kinesin. Kasten et al. [17] showed that NO is directly involved in osteoclast activity; thereafter, Iwaki et al. [18] demonstrated that NO suppresses the differentiation of osteoclasts and thereby suppresses bone resorption.
Although the mechanism underlying the inhibitory action of NO on osteoclast formation is still largely unknown, Yaroslavskiy et al. [19] proposed a model that may explain its action. According to their model, cGMP-dependent protein kinase (PKG) inhibits osteoclast differentiation. The link between NO and PKG must then be considered: PKG is activated by cGMP, which is generated by the NO-reactive enzyme guanylate cyclase, using GTP as a substrate. When NO is generated, it activates guanylate cyclase, producing cGMP, which passes through the cell membrane and leads to PKG activation via cGMP. In this study, endogenous NO did not affect osteoclast differentiation, but TH-derived NO suppressed osteoclast differentiation. The NO-competitive guanylate cyclase inhibitor ODQ restored the inhibition of osteoclast differentiation caused by TH. These results suggest that osteoclast differentiation was inhibited at least partly by TH-derived NO.
As studies of triple NOS knockout (KO) mice have revealed that NO promotes osteogenesis, our results also suggest that TH may simultaneously promote bone formation and suppress bone resorption via NO. The regulation of bone resorption by osteoclasts is important for inducing new bone formation after treatment with the bone filler beta-tricalcium phosphate (TCP) [20]. Due to its suppressive effect on bone resorption as well as its impacts on osteogenesis, TH may induce a considerable increase in bone mass [12]. The regulation of osteoblast and osteoclast activity has also been reported to be affected by the exposure of cells to hydroxylapatite coatings [21]. Via the same mechanism of action, TH may regulate the promotion of osteoblast bone formation and suppress osteoclast bone resorption in different ways.
Xanthine derivatives inhibit the hydrolase activity of PDE [11], [22]. Since cGMP is one of the main target molecules of PDE, the intracellular cGMP concentrations are expected to be maintained during treatment with xanthine derivatives. Indeed, TH inhibited the activity of PDE on cGMP in this study, suggesting that TH maintains intracellular cGMP concentrations by directly inhibiting PDE and that this action also contributes to the suppression of osteoclast differentiation. Given that the PDE inhibitory agent rolipram enhances prostaglandin activity and promotes bone formation, while the cGMP-specific PDE5 inhibitor zaprinast can inhibit osteoclast formation [5]. TH may also promote bone formation partially through PDEs inhibition as well.
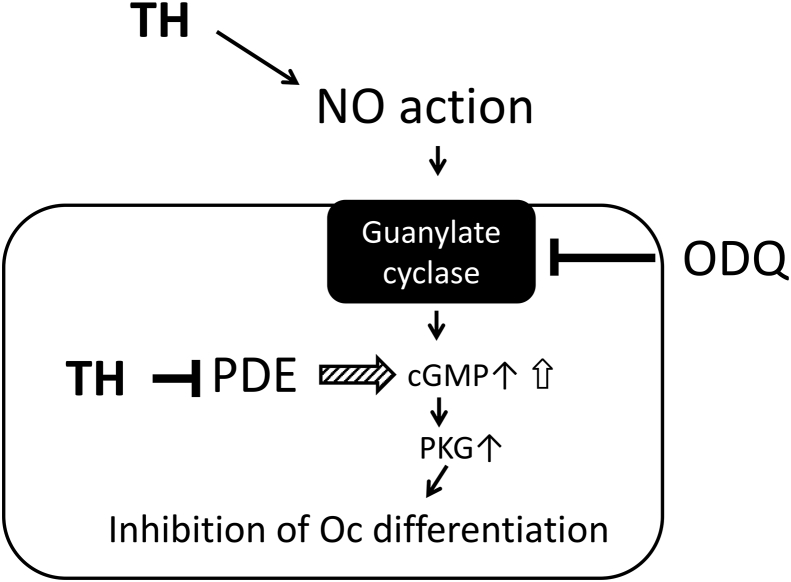
Collectively, TH is likely to suppress osteoclast differentiation at least partially through two different mechanisms: the direct inhibition of PDE and the promotion of NO synthesis through the metabolism of TH. Both of these mechanisms may lead to the upregulation of intracellular cGMP; because each mechanism is further enhanced by the other, the inhibitory effects of low concentrations of TH on osteoclast differentiation may persist for a long time (Fig. 5).
Fig. 5.
Diagram of the hypothetical mechanism underlying the suppressive effect of TH on osteoclast differentiation.
5. Conclusions
This study demonstrated that TH exerts a suppressive effect on osteoclast differentiation, supporting its potential use as a novel antiosteoporotic agent that not only stimulates bone formation but also inhibits bone resorption. The beneficial effects of TH on bone metabolism may also elucidate the mechanisms underlying its bone regeneration-inducing action in terms of the regulation of both osteoblast and osteoclast activities.
Funding
This study was supported by a grant from the Ministry of Education, Culture, Sports, Science and Scientific Research Basic Research C (No. 25462899).
Conflicts of interest
The authors declare no conflicts of interest in association with the present study.
Authors contributions
Hitoshi Amano designed the study and performed the experiments; Futoshi Iwaki performed the experiments, analyzed the data, and wrote the manuscript. And Meiko Oki Kazuhiro Aoki and Shinsuke Ohba provided critical advices to the manuscript and edited it.
Acknowledgments
We thank Masakazu Inubushi and Prof. Kiyoshi Ohura for their critical reading of the manuscript and helpful suggestions.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2019.08.007.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Giannoudis P., Tzioupis C., Almalki T., Buckley R. Fracture healing in osteoporotic fractures: is it really different? A basic science perspective. Injury. 2007;38:S90–S99. doi: 10.1016/j.injury.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Emara K.M., Diab R.A., Emara A.K. Recent biological trends in management of fracture non-union. World J Orthop. 2015;6:623–628. doi: 10.5312/wjo.v6.i8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz A.V., Sellmeyer D.E. Diabetes, fracture, and bone fragility. Curr Osteoporos Rep. 2007;5:105–111. doi: 10.1007/s11914-007-0025-x. [DOI] [PubMed] [Google Scholar]
- 4.Lo K.W., Ashe K.M., Kan H.M., Laurencin C.T. The role of small molecules in musculoskeletal regeneration. Regen Med. 2012;7:535–549. doi: 10.2217/rme.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holliday L.S., Dean A.D., Lin R.H., Greenwald J.E., Gluck S.L. Low NO concentrations inhibit osteoclast formation in mouse marrow cultures by cGMP-dependent mechanism. Am J Physiol. 1997;272:F283–F291. doi: 10.1152/ajprenal.1997.272.3.F283. [DOI] [PubMed] [Google Scholar]
- 6.Ohba S., Nakajima K., Komiyama Y., Kugimiya F., Igawa K., Itaka K. A novel osteogenic helioxanthin-derivative acts in a BMP-dependent manner. Biochem Biophys Res Commun. 2007;357:854–860. doi: 10.1016/j.bbrc.2007.03.173. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima K., Komiyama Y., Hojo H., Ohba S., Yano F., Nishikawa N. Enhancement of bone formation ex vivo and in vivo by a helioxanthin-derivative. Biochem Biophys Res Commun. 2010;395:502–508. doi: 10.1016/j.bbrc.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Liberman U.A., Weiss S.R., Broll J., Minne H.W., Quan H., Bell N.H. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase iii osteoporosis treatment study group. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 9.Essayan D.M. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol. 2001;108:671–680. doi: 10.1067/mai.2001.119555. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto K., Nishioka T., Waki Y., Nomura M., Katsuta H., Yokogawa K. Phosphodiesterase 4 inhibitor rolipram potentiates the inhibitory effect of calcitonin on osteoclastogenesis. J Bone Miner Metab. 2006;24:260–265. doi: 10.1007/s00774-006-0682-3. [DOI] [PubMed] [Google Scholar]
- 11.Liou S.F., Hsu J.H., Lin I.L., Ho M.L., Hsu P.C., Chen L.W. KMUP-1 suppresses RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss: roles of MAPKs, Akt, NF-κB and calcium/calcineurin/NFATc1 pathways. PLoS One. 2013;8(2013) doi: 10.1371/journal.pone.0069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda Y., Hojo H., Shimohata N., Choi S., Yamamoto K., Takato T. Bone healing by sterilizable calcium phosphate tetrapods eluting osteogenic molecules. Biomaterials. 2013;34:5530–5537. doi: 10.1016/j.biomaterials.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 13.Emori H., Iwai S., Ryu K., Amano H., Sambe T., Kobayashi T. A new method for measuring osteoclast formation by electrical impedance. J Pharmacol Sci. 2015;128:87–91. doi: 10.1016/j.jphs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Yasui K., Komiyama A. New clinical applications of xanthine derivatives: modulatory actions on leukocyte survival and function. Int J Hematol. 2001;73:87–92. doi: 10.1007/BF02981908. [DOI] [PubMed] [Google Scholar]
- 15.Barnes P.J. Theophylline in chronic obstructive pulmonary disease: new horizons. Proc Am Thorac Soc. 2005;2:334–339. doi: 10.1513/pats.200504-024SR. [DOI] [PubMed] [Google Scholar]
- 16.MacIntyre I., Zaidi M., Alam A.S., Datta H.K., Moonga B.S., Lidbury P.S. Osteoclastic inhibition: an action of nitric oxide not mediated by cyclic GMP. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2936–2940. doi: 10.1073/pnas.88.7.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasten T.P., Collin-Osdoby P., Patel N., Osdoby P., Krukowski M., Misko T.P. Potentiation of osteoclast bone-resorption activity by inhibition of nitric oxide synthase. Proc. Natl. Acad. Sci. U. S. A. 1994;91:3569–3573. doi: 10.1073/pnas.91.9.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwaki F., Amano H., Ohura K. Nicorandil inhibits osteoclast differentiation in vitro. Eur J Pharmacol. 2016;793:14–20. doi: 10.1016/j.ejphar.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Yaroslavskiy B.B., Zhang Y., Kalla S.E., Palacios V.G., Sharrow A.C., Li Y. NO-dependent osteoclast motility: reliance on cGMP-dependent protein kinase I and VASP. J Cell Sci. 2005;118:5479–5487. doi: 10.1242/jcs.02655. [DOI] [PubMed] [Google Scholar]
- 20.Davison N.L., ten Harkel B., Schoenmaker T., Luo X., Yuan H., Everts V. Osteoclast resorption of beta-tricalcium phosphate controlled by surface architecture. Biomaterials. 2014;35:7441–7451. doi: 10.1016/j.biomaterials.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 21.Costa D.O., Prowse P.D., Chrones T., Sims S.M., Hamilton D.W., Rizkalla A.S. The differential regulation of osteoblast and osteoclast activity by surface topography of hydroxyapatite coatings. Biomaterials. 2013;34:7215–7226. doi: 10.1016/j.biomaterials.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Semmler J., Gebert U., Eisenhut T., Moeller J., Schonharting M.M., Allera A. Xanthine derivatives: comparison between suppression of tumour necrosis factor-alpha production and inhibition of cAMP phosphodiesterase activity. Immunology. 1993;78:520–525. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.