Key Points
Questions
Are protein levels altered within synapses from the primary auditory cortex of individuals with schizophrenia and, if so, are these differences restricted to the synapse or present throughout the gray matter?
Findings
In this case-control study of 48 pairs of individuals with schizophrenia and matched control individuals, robust alterations were found in synaptic levels of mitochondrial and postsynaptic proteins, including glutamate and γ-aminobutyric acid receptors, that were highly coregulated and not associated with underlying differences in total gray matter levels.
Meaning
These findings suggest a broad and highly coordinated rearrangement of the synaptic proteome in schizophrenia that is best explained by alterations in local synaptic, but not tissuewide, protein homeostasis.
This case-control study examines whether protein levels are altered within synapses from the primary auditory cortex of individuals with schizophrenia and, if so, whether these differences are restricted to the synapse or occur throughout the gray matter.
Abstract
Importance
Findings from unbiased genetic studies have consistently implicated synaptic protein networks in schizophrenia, but the molecular pathologic features within these networks and their contribution to the synaptic and circuit deficits thought to underlie disease symptoms remain unknown.
Objective
To determine whether protein levels are altered within synapses from the primary auditory cortex (A1) of individuals with schizophrenia and, if so, whether these differences are restricted to the synapse or occur throughout the gray matter.
Design, Setting, and Participants
This paired case-control study included tissue samples from individuals with schizophrenia obtained from the Allegheny County Office of the Medical Examiner. An independent panel of health care professionals made consensus DSM-IV diagnoses. Each tissue sample from an individual with schizophrenia was matched by sex, age, and postmortem interval with 1 sample from an unaffected control individual. Targeted mass spectrometry was used to measure protein levels in A1 gray matter homogenate and synaptosome preparations. All experimenters were blinded to diagnosis. Mass spectrometry data were collected from September 26 through November 4, 2016, and analyzed from November 3, 2016, to July 15, 2019.
Main Outcomes and Measures
Primary measures were homogenate and synaptosome protein levels and their coregulation network features. Hypotheses generated before data collection were (1) that levels of canonical postsynaptic proteins in A1 synaptosome preparations would differ between individuals with schizophrenia and controls and (2) that these differences would not be explained by changes in total A1 homogenate protein levels.
Results
Synaptosome and homogenate protein levels were investigated in 48 individuals with a schizophrenia diagnosis and 48 controls (mean age in both groups, 48 years [range, 17-83 years]); each group included 35 males (73%) and 13 females (27%). Robust alterations (statistical cutoff set at an adjusted Limma P < .05) were observed in synaptosome levels of canonical mitochondrial and postsynaptic proteins that were highly coregulated and not readily explained by postmortem interval, antipsychotic drug treatment, synaptosome yield, or underlying alterations in homogenate protein levels.
Conclusions and Relevance
These findings suggest a robust and highly coordinated rearrangement of the synaptic proteome. In line with unbiased genetic findings, alterations in synaptic levels of postsynaptic proteins were identified, providing a road map to identify the specific cells and circuits that are impaired in individuals with schizophrenia A1.
Introduction
Patients with schizophrenia display impairments in the processing of auditory sensory information,1,2,3,4 including auditory event–related potentials (eg, mismatch negativity)1,2,5,6 that are generated in layer 3 of the primary auditory cortex (A1).7,8,9 Many individuals with schizophrenia also have deficits in auditory learning,10 limiting their functional recovery during targeted sensory and cognitive training.11 In A1, as in other cortical regions, learning requires the formation and stabilization of new dendritic spines,12,13,14 and decreased density of layer 3 dendritic spines has been reproducibly observed in multiple brain regions in patients with schizophrenia, including A1.15,16,17,18,19,20
Unbiased genetic studies of schizophrenia have implicated synaptic protein networks as having involvement in the disease pathology.21 This convergence of genetic risk factors is believed to underlie the dendritic spine deficits and information-processing alterations observed in the A1 and other cortical regions. Numerous studies22 have documented the essential role of local synaptic protein trafficking, translation, and degradation in spine formation and plasticity. This local protein homeostasis (proteostasis) is believed to be required for the rapid synaptogenesis and plasticity essential to cortical information processing and new learning.23,24 Indeed, blocking synaptic proteostatic processes impairs new synapse formation and learning.25,26 Thus, we hypothesized that protein levels at the synapse are altered in the A1 of patients with schizophrenia and that these synaptic alterations are distinct from protein alterations in total gray matter.
To investigate protein levels at the synapse, we used a high-precision targeted mass spectrometry (MS) approach with a [13C6]lysine–labeled brain proteome internal standard ([13C6]brain ISTD)27 to quantify more than 350 selected proteins in A1 synaptosome enrichments (to measure protein levels within synapses) from 48 pairs of individuals with schizophrenia and unaffected comparison individuals (controls) matched for age, sex, and postmortem interval (PMI). Although targeted MS is limited in the number of proteins assayable, it has exceptional precision, accuracy, and throughput. To determine whether the predicted alterations are synaptosome specific, identical studies were conducted in total A1 homogenate preparations to measure tissuewide protein levels. Technical controls were used to monitor reproducibility of synaptosome enrichment, sample preparation, and instrument performance. The effects of antipsychotic drug (APD) treatment and PMI were assessed in monkey and mouse models, respectively.
Methods
Human Tissue Sample Collection
For this case-control study, brain specimens were obtained from the Allegheny County Office of the Medical Examiner, Allegheny County, Pennsylvania, from September 16, 1996, through November 8, 2015 (Table and eTable 1 in Supplement 1). Oral informed consent for brain specimen donation was obtained from the next of kin, and all procedures were approved by the Committee for the Oversight of Research and Clinical Training Involving Decedents and the Institutional Review Board for Biomedical Research, University of Pittsburgh, Pittsburgh, Pennsylvania. Gray matter was harvested from the auditory cortex as previously described28,29 (details are given in Figure 1 and eMethods in Supplement 1). Data analysis was performed from November 3, 2016, to July 15, 2019.
Table. Summary of Subject Characteristicsa.
| Characteristic | Controls (n = 48) | Individuals With Schizophrenia (n = 48) |
|---|---|---|
| Age, mean (SD) [range], y | 48.6 (13.5) [17-82] | 48.1 (13.6) [17-83] |
| Sex, No. (%) | ||
| Male | 35 (73) | 35 (73) |
| Female | 13 (27) | 13 (27) |
| Handedness, No. (%) | ||
| Right | 30 (63) | 42 (88) |
| Left | 8 (17) | 4 (8) |
| Ambidextrous | 1 (2) | 0 |
| Unknown | 9 (19) | 2 (4) |
| PMI, mean (SD) | 17.7 (5.7) | 17.9 (7.1) |
| Brain tissue pH, mean (SD) | 6.70 (0.27) | 6.55 (0.31) |
| Storage time, mean (SD), mo | 155.4 (50.0) | 148.7 (54.0) |
| Suicide, No. (%) | 0 | 12 (25) |
| Schizoaffective disorder, No. (%) | NA | 14 (29) |
| ATOD use, No. (%) | ||
| Alcohol or substance abuse | 18 (38) | 29 (60) |
| Antipsychotic | 0 | 42 (88) |
Abbreviations: ATOD, alcohol, tobacco, or other drugs; NA, not applicable; PMI, postmortem interval.
There were no diagnostic group differences in age, sex, PMI, storage time, or the distribution of handedness between the diagnostic groups. There was a small but significant decrease in brain tissue pH in individuals with schizophrenia (P = .01).
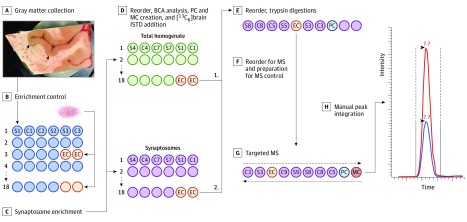
Figure 1. Study Methods for Human Samples.
A, Gray matter was collected from Heschl gyrus in 40-μm sections and frozen at −80 °C. B, The ultracentrifuge rotor used for synaptosome enrichment has limited capacity at 6 tubes. To monitor reproducibility across synaptosome enrichment, 1 g of superior temporal gyrus gray matter (pink powder in image) from a low–postmortem interval control was pulverized in liquid nitrogen and divided into 50-mg aliquots. C, Two aliquots of these enrichment controls (EC) were included in every third rotor run. D, The resulting homogenate and synaptosome fractions were then reordered into separate blocks and processed independently for the remainder of the protocol. Micro bicinchoninic acid (BCA) assay was used to determine total protein amounts. A pooled control (PC) was then created from all samples within each fraction (homogenate or synaptosome) and divided into 8 aliquots to monitor variability in addition of [13C6]lysine–labeled brain proteome internal standard ([13C6]brain ISTD) and trypsin digestion. E, The samples were again reordered and digested with trypsin. F, The samples were reordered a final time, and a single MS control (MC) to monitor instrument stability was created by mixing a homogenate PC and a synaptosome PC. G, All samples were analyzed in duplicate, with the sample order run first forward and then backward. The ECs were distributed randomly throughout the run order, while PCs and the MC were analyzed every 10 to 12 injections. H, Integration of all light and heavy peak pairs were manually checked and reintegrated, if needed, by trained personnel blinded to sample. C indicates control; MS, mass spectrometry; and S, schizophrenia.
Animal Tissue Samples
We obtained APD-treated monkey tissue specimens from previously completed experiments that have been described elsewhere30 (eMethods in Supplement 1). Twelve C57BL/6 adult mice, 2 each at 0, 6, 12, 18, 24, and 30 hours, were killed by carbon dioxide asphyxiation, followed by cervical dislocation. The carcasses were incubated at room temperature for two-thirds of the PMI duration and then placed at 4°C for the final one-third. The brain was then removed from the skull, the cerebellum discarded, and the hemispheres separated (giving 4 hemispheres per time point), flash frozen in isopentane on dry ice, and stored at −80°C.
Sample Preparation
Total gray matter homogenate and synaptosome preparations were obtained using a variation on our sucrose density gradient centrifugation method, validated for use in human postmortem brain tissue.27,31 The homogenate preparation was composed of all material present in the A1 gray matter, an aliquot of which was set aside before synaptosome enrichment. A detailed protocol is given in the eMethods in Supplement 1. To monitor variability in synaptosome preparation in the human cohorts, two 50-mg aliquots of pulverized superior temporal gyrus gray matter from a human control with a low PMI were included in every third run and assayed alongside patient samples (Figure 1). To monitor variability in sample preparation after synaptosome enrichment, a pooled control sample was prepared and assayed alongside patient samples (Figure 1).
Sample Preparation of Targeted MS
We mixed 10 μg of homogenate, synaptosome, or pooled controls with 10 μg of [13C6]brain ISTD27 prepared from stable isotope labeling in mammals (SILAM) mouse cortex tissue (Cambridge Isotopes). The human-[13C6] brain ISTD mixtures were subject to trypsin digestion by filter-aided sample preparation.32
Mass Spectrometry
Mass spectrometry data were collected from September 26 through November 4, 2016. Peptide-selected reaction monitoring was generated using data from discovery MS analyses of human gray matter homogenates as well as synaptosome and postsynaptic density enrichments and validated as previously described.27 Mass spectrometry analyses were conducted on a triple-stage quadrupole mass spectrometer (TSQ Quantiva; ThermoFisher Scientific) with high-performance liquid chromatography (UltiMate 3000; Dionex). Two microliters (approximately 1 μg of protein) were loaded on a 3-μm 120A with 105 mm Reprosil-PUR C18 PicoChip (New Objective) at 1 μL/min for 12 minutes and eluted at 400 nL/min over a 25-minute gradient from 3% to 35% mobile phase B (Acetonitrile, 0.1% formic acid). Transitions were monitored, allowing for a cycle time of 1.1-second, resulting in a dynamic dwell time never less than 10 milliseconds. The MS instrument variables were as follows: capillary temperature of 275 °C, spray voltage of 1350 V, and a collision gas of 1.4 mTorr (argon), resolving power at 0.7 Da for both quadrupoles.
Data Processing
Peak areas and area ratios were calculated within Skyline.33 All individual selected reaction monitoring transitions and integration areas were manually inspected and reintegrated as needed by trained experimenters.
Statistical Analysis
Proteins Enriched in Synaptosomes
Reproducibility of peptide quantification and calculation of protein level measures and effects of PMI are described in the eMethods and reported in eTables 3 and 4 and eFigures 2-4 in Supplement 1. Of the 348 proteins quantified in the synaptosome fractions, 159 had synaptosome levels that were significantly enriched compared with their homogenate levels, as defined by a Bonferroni-corrected P < .05, and a synaptosome-homogenate change greater than 1.25-fold (eTable 2 in Supplement 1). Statistical analysis of synaptosome differences between individuals with schizophrenia and controls was limited to these proteins. Effects of PMI are described in eMethods and eFigure 5 in Supplement 1.
Differential Expression and Network Analyses
The Limma-voom method34 was performed to detect the difference between patient and control samples in homogenate and synaptosome proteins. Unadjusted and adjusted P values were obtained (eTables 1 and 2 in Supplement 2). Weighted gene coexpression network analysis35 was used to investigate the pattern of coregulated protein expression (eMethods in the Supplement).
A comparison with PsychENCODE transcriptome findings is given in the eMethods and eTables 12 and 13 in Supplement 1. eTables 1 and 2 in Supplement 2 report the module memberships for homogenate and synaptosome proteins, respectively. eTables 7 and 8 in Supplement 1 report the protein eigenvalues for each individual from the homogenate and synaptosome modules. eTables 9 and 10 in Supplement 1 report the full list of enriched terms for the homogenate and synaptosome modules, respectively.
Results
Synaptosome and homogenate protein levels were investigated in brain tissue samples from 48 individuals with schizophrenia and 48 controls, for a total of 96 study individuals. Mean (SD) age was 48 years (range, 17-83 years) for both groups; each group included 35 male (73%) and 13 female (27%) individuals.
Synaptosome Protein Yield in A1 in Individuals With Schizophrenia
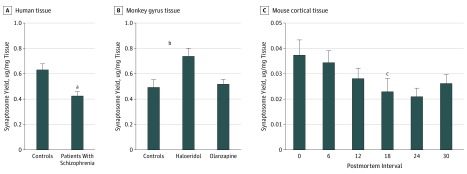
Total synaptosome yield was decreased 33% in individuals with schizophrenia (mean yield, −0.21 μg protein/mg gray matter; 95% CI, −0.31 to −0.10 μg protein/mg gray matter; paired t test, P < .001) (Figure 2A). Haloperidol-treated monkeys had 1.5-fold greater synaptosome yield compared with vehicle (mean [SD] yield, 0.25 [0.80] μg protein/mg gray matter; 95% CI, 0.05-0.44 μg protein/mg gray matter; unpaired t test, P = .02) (Figure 2B). Postmortem interval was associated with decreased synaptosome yield in the mouse PMI model (r = −0.84; 95% CI = −0.98 to −0.10; P = .04) (Figure 2C), but not in the human cohort (eFigure 1A in Supplement 1). Tissue pH correlated with synaptosome yield in the human cohort (r = 0.37; 95% CI, 0.1746-0.5418; P < .001) (eFigure 1B in Supplement 1); however, yield was still significantly decreased in individuals with schizophrenia when controlling for tissue pH (estimate (SD), −0.4715 [0.1817]; t = −2.595; linear regression, P = .01). To control for the lower synaptosome yield in individuals with schizophrenia, the same amount of total synaptosome protein from all individuals and animals was used for MS analyses.
Figure 2. Synaptosome Yield.
Total micrograms of protein in each synaptosome preparation were measured by micro bicinchoninic acid assay. This value was then normalized to wet tissue weight to calculate synaptosome yield. Error bars indicate standard error of the mean. P values were calculated for comparison of all postmortem intervals.
aP ≤ .001.
bP = .02.
cP < .001.
Homogenate and Synaptosome Proteome Alterations in Individuals With Schizophrenia
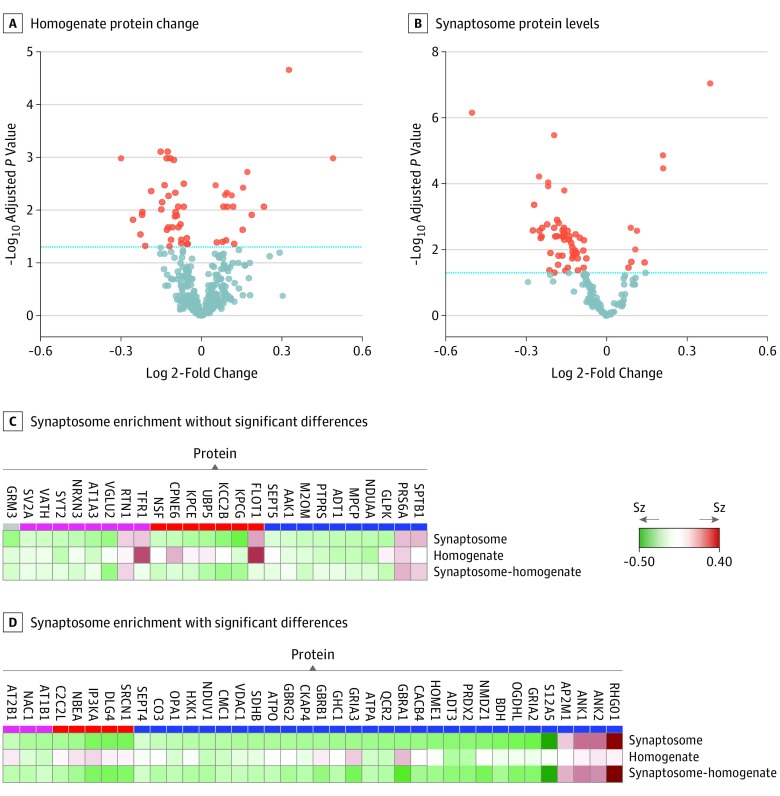
Fifty-five of 402 proteins in homogenates (13.7%) and 64 of 155 in synaptosomes (41.3%) differed significantly between individuals with schizophrenia and controls (adjusted Limma cut point, P < .05) (Figure 3A and B). Of the 64 proteins altered in synaptosomes, only 26 alterations could be explained by concurrent changes in homogenate levels of these proteins (Figure 3C and D).
Figure 3. Volcano Plots and Heat Maps of Schizophrenia (Sz) Case-Control Differences.
A and B, Volcano plots chart the − log10 P value and the log 2-fold change (individuals with Sz vs controls) for protein levels of homogenate (of 402 proteins measured, 55 were significant) and synaptosome (of 155 proteins measured, 64 were significant). The blue dashed lines are set at an adjusted Limma P = .05. Heat maps report the fold change (individuals with Sz vs controls) for the 63 significantly altered synaptosome proteins in the synaptosome fraction and the fold-change of those proteins in the homogenate fraction, as well as the synaptosome enrichment values (the ratio of a protein’s synaptosome level to its homogenate level) for each individual. Synaptosome-homogenate rows report the fold change (individuals with Sz vs controls) for this metric. C, Proteins for which the synaptosome enrichment values did not differ significantly between individuals with Sz and controls. D, Proteins for which the synaptosome enrichment values significantly differed between individuals with Sz and controls in the same direction as the synaptosome level changes.
APD and Protein Levels
Of the 55 homogenate alterations, 10 showed alterations (uncorrected P < .05) in the same direction in APD monkey tissue (eFigure 7A and eTable 5 in Supplement 1). Of the 64 synaptosome alterations, 1 (ANK2) was similarly altered by APDs (uncorrected cut point, P < .05) (eFigure 7B and eTable 6 in Supplement 1). Two proteins (GRIA2 and GBRB1) with significantly decreased levels in synaptosomes in individuals with schizophrenia had increased levels (uncorrected cut point, P < .05) in haloperidol- and/or olanzapine-treated monkeys compared with vehicle (eFigure 7B in Supplement 1). Additional potential confounds are discussed in the eMethods in Supplement 1 and reported eTables 3 and 4 in Supplement 2.
Protein Coregulation Networks
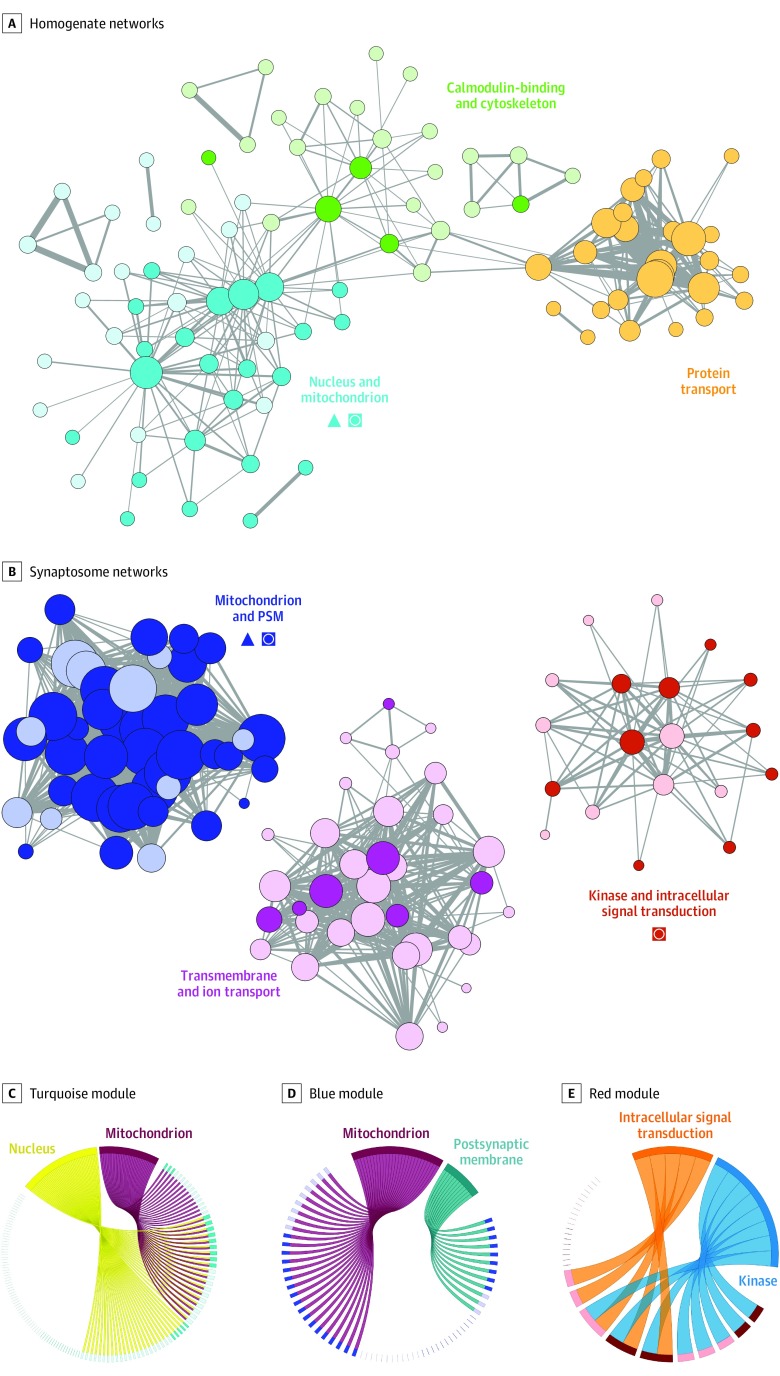
The homogenate proteome organized into 3 interlinked modules (Figure 4A and eFigure 6A in Supplement 1) enriched for terms relating to nucleus and mitochondrion (P = 7.5 × 10−6), protein transport (P = 1.3 × 10−2), and calmodulin-binding and cytoskeleton (P = 2.1 × 10−4 and P = 3 × 10−3, respectively). The nucleus and mitochondrion module was enriched for proteins that differed between individuals with schizophrenia and controls (P = 2.6 × 10−6), and its protein eigenvalues differed significantly between individuals with schizophrenia and controls (P = 2.6 × 10−4) (eTable 11 in Supplement 1). Figure 4C depicts the number of proteins in the turquoise module that contributed to the selected gene ontologies and the proportion of those that were altered individuals with schizophrenia in a chord diagram.
Figure 4. Homogenate and Synaptosome Protein Network Alterations.
Protein coregulation networks were constructed for the homogenate and synaptosome proteomes (eFigure 6 in the Supplement). These networks were then visualized for homogenate (A) and synaptosome (B) samples. Each node (circle) represents a protein. Color denotes module membership. Darker shading denotes proteins that were significantly altered in individuals with schizophrenia. Node sizes are proportional to each protein’s degree of connectivity. Line weights represent the strength of between-protein correlations. Only proteins with a weighted node connectivity of greater than 0.15 were visualized. Triangles indicate modules significantly enriched for proteins that differed significantly between individuals with schizophrenia and controls; squares with circle, modules whose protein eigenvalues differed significantly between individuals with schizophrenia and controls. The chord figures depict the number of proteins in the turquoise (C), blue (D), and red (E) modules that contributed to the selected gene ontologies. Proteins contributing to ontologic findings are depicted as rectangles, with darker colors denoting those significantly altered in individuals with schizophrenia. Proteins not contributing to the ontologies are depicted as lines, with darker colors denoting significant alterations in individuals with schizophrenia.
The synaptosome proteome organized into 3 highly distinct modules (Figure 4B and eFigure 6B in Supplement 1) enriched for terms relating to mitochondrion and postsynaptic membrane (P = 6.1 × 10−10 and P = 2.9 × 10−3, respectively), kinase and intracellular signal transduction (P = 1.2 × 10−2 and P = 4.6 × 10−2, respectively), and transmembrane and ion transport (P = 1.4 × 10−8 and P = 8.5 × 10−6, respectively). The mitochondrion and postsynaptic membrane module was enriched for proteins with levels that differed between individuals with schizophrenia and controls (P = 1.5 × 10−5), and its protein eigenvalues differed significantly between individuals with schizophrenia and controls (P = 2 × 10−4) (eTable 11 in Supplement 1). Figure 4D depicts the numbers of proteins in the blue module that contribute to the selected gene ontologies, as well as the proportion of those that were altered in individuals with schizophrenia. In line with the analysis above, the 38 proteins with synaptosome alterations not explained by homogenate alterations (Figure 3D) were enriched for proteins from the mitochondrion and postsynaptic membrane module (P = 1.5 × 10−3). Finally, protein eigenvalues for the kinase and intracellular signal transduction (red) module differed between individuals with schizophrenia and controls (P = .01) (Figure 4E and eTable 11 in Supplement 1). Expanded chord diagrams for all homogenate and synaptosome modules, with protein identifiers and additional ontologies, are presented in eFigures 8 to 13 in Supplement 1.
The protein eigenvalues from the calmodulin-binding and cytoskeleton (green) module were significantly inversely correlated with the protein transport (orange) and mitochondrion (turquoise) modules (eFigure 14A in Supplement 1). We observed a significant correlation between the homogenate mitochondrion (turquoise) and synaptosome mitochondrion and postsynaptic membrane (blue) modules in the controls but not the individuals with schizophrenia (eFigure 14B in Supplement 1).
Similarities With Reported Transcriptome Alterations
We did not observe a significant overlap between messenger RNA (mRNA) and homogenate protein alterations or mRNA and synaptosome protein alterations (eFigure 15A and B in Supplement 1). However, a significant correlation (r = 0.15; 95% CI, 0.05-0.24; P = 3 × 10−3) was found between mRNA and homogenate protein fold changes in individuals with schizophrenia and controls (eFigure 15C in Supplement 1). No correlation was observed between mRNA and synaptosome protein fold changes in individuals with schizophrenia and controls (eFigure 15D in Supplement 1).
Discussion
We observed robust differences in A1 homogenate and synaptosome protein levels in individuals with schizophrenia. These differences organized into distinct coregulation network alterations. Synaptosome levels of mitochondrion and postsynaptic membrane proteins were generally lower in individuals with schizophrenia, highly coregulated, and did not appear to be associated with lower protein levels in the total homogenate. Homogenate levels of nuclear and mitochondrion proteins were lower in individuals with schizophrenia but were not correlated with synaptosome mitochondrion and postsynaptic membrane proteins in disease.
We observed robust and highly coregulated differences in the synaptic levels of canonical mitochondrial and postsynaptic proteins between groups, including glutamate and γ-aminobutyric acid (GABA) receptors. Tissuewide levels of most of these proteins did not differ significantly in individuals with schizophrenia. These findings suggest a broad and coordinated rearrangement of the synaptic proteome likely associated with synapse and circuit pathologic features. That these synaptic proteome alterations were not associated with tissuewide protein level differences suggests that they were associated with impairments in synaptic, not cellwide, proteostasis.
Synaptosome Mitochondrion and Postsynaptic Membrane Proteins
Synaptosome alterations in the mitochondrion and postsynaptic membrane module included a diverse array of proteins that were highly coregulated, were mostly (but not uniformly) decreased in individuals with schizophrenia, and are believed to localize to different synaptic microdomains. Specifically, this module included mitochondrial proteins (eg, ADT3 and ATPA), ionotropic glutamate receptor subunits (NMDZ1, GRIA2, and GRIA3), GABA receptor subunits (GBRA1, GBRB1, and GBRG2), ion transporters (S12A5), adaptor proteins (ANK1 and ANK2), second messengers (RHG01), and a complement component (CO3). In the cortex, mitochondria are present in presynaptic boutons but not dendritic spines36,37; and ionotropic glutamate and GABA receptors are largely localized to different postsynaptic compartments. Because decreases in presynaptic boutons or inhibitory synapses in the A1 have not been observed in individuals with schizophrenia,38,39 decreased synaptosome levels of mitochondrial proteins and GABA receptors are likely driven by alterations in their respective synaptic microdomains, not the loss of synaptic compartments. Although we observed decreased density of dendritic spines in the A1 region of individuals with schizophrenia, we believe that it is unlikely that the observed decreases in synaptosome levels of postsynaptic glutamate receptors are driven by loss of the postsynaptic compartment; levels of other canonical postsynaptic proteins (ANK1, ANK2, and RGH01) were increased and levels of AMPA1 did not differ significantly. Placed in the context of what is known about schizophrenia cytoarchitectonics, our findings suggest a robust, broad, and highly coordinated rearrangement of the synaptic proteome affecting glutamate and GABA synapses as well as presynaptic bioenergetics. However, a subset of our findings may be driven by the loss of specific cellular or synaptic compartments in concert with altered protein levels in others. This question will ultimately have to be addressed by multiple-label confocal microscopy, RNA-scope, or electron microscopy studies guided by our findings.
Most of these synaptosome alterations cannot be explained by underlying changes in total homogenate protein levels, further suggesting that they are driven by alterations in local mitochondrial trafficking or synaptic proteostasis. Indeed, decreased mitochondrial numbers at axon terminals have been observed in individuals with schizophrenia,40 and cellular mechanisms that regulate synaptic proteostasis are essential for synapse formation and plasticity and have previously been implicated in schizophrenia. For example, the ubiquitin-proteasome system, a key regulator of synaptic protein stability, is essential for long-term potentiation and learning41,42 and has been implicated in schizophrenia pathology by unbiased genetic analyses.43,44 Thus, it is not surprising that ubiquitin-proteasome system alterations have been directly observed in tissue from patients with schizophrenia.45,46,47 Similarly, phosphorylation is a well-documented regulator of glutamate receptor trafficking,48 and altered kinase activity has recently been observed in tissue from deceased individuals with schizophrenia.49,50 In addition to posttranslational modification, alternative isoform expression and nonsynonymous mutations can produce proteins with drastically different synaptic trafficking or anchoring properties51,52 and have also been recently implicated in schizophrenia.53 In line with the results of these previous studies, our findings support a role for altered mitochondrial trafficking and synaptic proteostasis in schizophrenia.
Because neuronal excitation, inhibition, and bioenergetics are tightly balanced, it is not surprising that synaptic levels of these proteins are highly coregulated. Considering the large body of work implicating postsynaptic glutamate receptor signaling in schizophrenia and synaptic plasticity, it is a reasonable hypothesis that proteostatic alterations at excitatory postsynaptic compartments are the primary mechanism of action or insult. For example, synaptosome levels of GRIA2, GRIA3, and S12A5 were decreased in individuals schizophrenia. Trafficking of GRIA2/3-containing AMPA receptors to new spines is essential for their potentiation and stabilization,54 as is synaptic S12A5.55,56,57 Subsequent decreases in synaptic mitochondrial and GABA receptors would then reflect homeostatic plasticity downstream of this decreased excitatory drive. Alternatively, presynaptic mitochondria are essential for bouton development58 and activity.59 Thus, altered mitochondrial transport to the presynaptic compartment could also contribute to synaptic pathologic findings in schizophrenia.
Cellular Homogenate Mitochondrion Proteins
We found reduced mitochondrion protein levels in cellular homogenates in individuals with schizophrenia, consistent with numerous reports of lower tissuewide expression of mitochondrial transcripts,44,60,61,62 proteins,63 and enzymatic activity64,65 in the cortex of individuals with schizophrenia. Because alterations in cell numbers have not been observed in the A1 of individuals with schizophrenia, these alterations likely reflect differences in cellular protein levels. Of interest, cellular homogenate nuclear and mitochondrion protein eigenvalues and synaptosome mitochondrion and postsynaptic membrane protein eigenvalues were highly correlated in controls but not in individuals with schizophrenia (eFigure 14 in the Supplement), suggesting that cellular and synaptic mitochondria regulation are uncoupled in disease. This finding is consistent with the dynamic and responsive nature of the mitochondrial organelle; mitochondria localized to synaptic compartments would be specifically reactive to an altered synaptic microenvironment compared with mitochondria in other cellular domains.
Association With Transcriptome Alterations
The homogenate protein alterations were weakly but significantly correlated with previously observed mRNA alterations in individuals with schizophrenia,53 which is in line with multiple reports that mRNA and protein levels are only modestly correlated.66,67,68 Multiple processes beyond transcription regulate synaptic protein levels, such as RNA trafficking, translation, and protein trafficking. Thus, it is not surprising that synaptic protein alterations were not associated with previously reported mRNA alterations in schizophrenia. This absence of a link between the synaptic proteome and the transcriptome (and, by extension, the genome) highlights a critical gap in the current understanding of the cause of schizophrenia and the need to investigate additional molecular features that regulate the synaptic proteome in patient tissue.
Limitations
This study was conducted in human postmortem brain tissue. We have strived to control for confounds associated with this approach via individual matching, animal models, and statistics. In addition, although we observed significant alterations in synaptosome protein levels, future studies will be required to determine the cause of these alterations.
Conclusions
In this study, we identified robust evidence of synaptic proteome alterations in the A1 in individuals with schizophrenia. Some of these alterations appeared to be driven by tissuewide changes, whereas others appeared to be exclusive to the synaptic compartment. Our findings are in line with previous reports of postsynaptic glutamate receptor and mitochondrial impairments in schizophrenia by identifying synapse-specific alterations as well as providing a possible link between them. These findings may provide a map to guide future studies into the upstream drivers these synaptic impairments as well as their effects on specific cortical cells and circuits. Future proteomic studies appear to be required to determine how altered ubiquitination, phosphorylation, and isoform expression may each contribute to synaptic proteostasis impairments in schizophrenia, whereas microscopy studies appear to be required to identify the cortical cell types and circuits to which these postsynaptic and mitochondrial alterations localize.
eTable 1. Participant Demographics
eTable2. Synaptosome Enrichment
eTable 3. Mouse Homogenate PMI
eTable 4. Mouse Synaptosome PMI
eTable 5. Monkey APD Homogenate
eTable 6. Monkey APD Synaptosome
eTable 7. Eigenprotein Human Homogenate
eTable 8. Eigenprotein Human Synaptosome
eTable9. Homogenate Modules DAVID
eTable10. Synaptosome Modules DAVID
eTable11. Module Eigenprotein Differences
eTable12. Aligned Sz-Control Homogenate Proteome and Transcriptome Findings
eTable13. Aligned Sz-Control Syntaptosome Proteome and Transcriptome Findings
eTable14. List of Protein SRMs from Assay Stages
eMethods. Participants and Materials
eFigure 1. Synaptosome Yield, PMI, and pH in Human Tissue
eFigure 2. Distribution of Technical and Variance in Peptide Quantification
eFigure 3. Homogenate Protein Levels and PMI in Mice
eFigure 4. Synaptosome Protein Levels and PMI
eFigure 5. Exploratory Module Preservation Analysis
eFigure 6. Homogenate and Synaptosome Protein Networks
eFigure 7. Comparison of Antipsychotic Drug Effects and Sz Alterations
eFigures 8-13. Expanded Chord Diagrams for All Homogenate and Synaptosome Modules
eFigure 14. Associations Between Module Eigenprotein Values
eFigure 15. Comparison of Proteomic and Transcriptomic Analysis of Sz Tissue
eReferences.
eTable 1. Human Homogenate
eTable 2. Human Synaptosome
eTable 3. Hom Confounds
eTable 4. Syn Confounds
References
- 1.Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52(7):550-558. doi: 10.1001/archpsyc.1995.03950190032005 [DOI] [PubMed] [Google Scholar]
- 2.Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J Abnorm Psychol. 1997;106(2):315-324. doi: 10.1037/0021-843X.106.2.315 [DOI] [PubMed] [Google Scholar]
- 3.Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111(10):1733-1737. doi: 10.1016/S1388-2457(00)00377-1 [DOI] [PubMed] [Google Scholar]
- 4.Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch Gen Psychiatry. 2000;57(12):1149-1155. doi: 10.1001/archpsyc.57.12.1149 [DOI] [PubMed] [Google Scholar]
- 5.Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37(1):65-79. doi: 10.1016/S0920-9964(98)00138-8 [DOI] [PubMed] [Google Scholar]
- 6.Ahveninen J, Jääskeläinen IP, Osipova D, et al. . Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biol Psychiatry. 2006;60(6):612-620. doi: 10.1016/j.biopsych.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 7.Hill SK, Beers SR, Kmiec JA, Keshavan MS, Sweeney JA. Impairment of verbal memory and learning in antipsychotic-naïve patients with first-episode schizophrenia. Schizophr Res. 2004;68(2-3):127-136. doi: 10.1016/S0920-9964(03)00125-7 [DOI] [PubMed] [Google Scholar]
- 8.Mohn C, Torgalsbøen AK. Details of attention and learning change in first-episode schizophrenia. Psychiatry Res. 2018;260:324-330. doi: 10.1016/j.psychres.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 9.Kantrowitz JT, Epstein ML, Beggel O, et al. . Neurophysiological mechanisms of cortical plasticity impairments in schizophrenia and modulation by the NMDA receptor agonist D-serine. Brain. 2016;139(pt 12):3281-3295. doi: 10.1093/brain/aww262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biagianti B, Fisher M, Neilands TB, Loewy R, Vinogradov S. Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology. 2016;30(8):998-1008. doi: 10.1037/neu0000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdlow NR, Bhakta SG, Light GA. Room to move: plasticity in early auditory information processing and auditory learning in schizophrenia revealed by acute pharmacological challenge. Schizophr Res. 2018;199:285-291. doi: 10.1016/j.schres.2018.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moczulska KE, Tinter-Thiede J, Peter M, et al. . Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall. Proc Natl Acad Sci U S A. 2013;110(45):18315-18320. doi: 10.1073/pnas.1312508110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Liu DQ, Huang W, et al. . Selective synaptic remodeling of amygdalocortical connections associated with fear memory. Nat Neurosci. 2016;19(10):1348-1355. doi: 10.1038/nn.4370 [DOI] [PubMed] [Google Scholar]
- 14.Berry KP, Nedivi E. Spine dynamics: are they all the same? Neuron. 2017;96(1):43-55. doi: 10.1016/j.neuron.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosoklija G, Toomayan G, Ellis SP, et al. . Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57(4):349-356. doi: 10.1001/archpsyc.57.4.349 [DOI] [PubMed] [Google Scholar]
- 16.Garey LJ, Ong WY, Patel TS, et al. . Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65(4):446-453. doi: 10.1136/jnnp.65.4.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65-73. doi: 10.1001/archpsyc.57.1.65 [DOI] [PubMed] [Google Scholar]
- 18.Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162(6):1200-1202. doi: 10.1176/appi.ajp.162.6.1200 [DOI] [PubMed] [Google Scholar]
- 19.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34(2):374-389. doi: 10.1038/npp.2008.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71(12):1323-1331. doi: 10.1001/jamapsychiatry.2014.1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez-Castelao B, Schuman EM. The regulation of synaptic protein turnover. J Biol Chem. 2015;290(48):28623-28630. doi: 10.1074/jbc.R115.657130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127(1):49-58. doi: 10.1016/j.cell.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg T, Gal-Ben-Ari S, Dieterich DC, et al. . The roles of protein expression in synaptic plasticity and memory consolidation. Front Mol Neurosci. 2014;7:86. doi: 10.3389/fnmol.2014.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Salon M, Alonso M, Vianna MR, et al. . The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci. 2001;14(11):1820-1826. doi: 10.1046/j.0953-816x.2001.01806.x [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Choi JH, Lee N, et al. . Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319(5867):1253-1256. doi: 10.1126/science.1150541 [DOI] [PubMed] [Google Scholar]
- 27.MacDonald ML, Ciccimaro E, Prakash A, et al. . Biochemical fractionation and stable isotope dilution liquid chromatography-mass spectrometry for targeted and microdomain-specific protein quantification in human postmortem brain tissue. Mol Cell Proteomics. 2012;11(12):1670-1681. doi: 10.1074/mcp.M112.021766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deo AJ, Cahill ME, Li S, et al. . Increased expression of kalirin-9 in the auditory cortex of schizophrenia subjects: its role in dendritic pathology. Neurobiol Dis. 2012;45(2):796-803. doi: 10.1016/j.nbd.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deo AJ, Goldszer IM, Li S, et al. . PAK1 protein expression in the auditory cortex of schizophrenia subjects. PLoS One. 2013;8(4):e59458. doi: 10.1371/journal.pone.0059458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konopaske GT, Dorph-Petersen KA, Sweet RA, et al. . Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63(8):759-765. doi: 10.1016/j.biopsych.2007.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn CG, Banerjee A, Macdonald ML, et al. . The post-synaptic density of human postmortem brain tissues: an experimental study paradigm for neuropsychiatric illnesses. PLoS One. 2009;4(4):e5251. doi: 10.1371/journal.pone.0005251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6(5):359-362. doi: 10.1038/nmeth.1322 [DOI] [PubMed] [Google Scholar]
- 33.MacLean B, Tomazela DM, Shulman N, et al. . Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26(7):966-968. doi: 10.1093/bioinformatics/btq054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Law CW, Chen Y, Shi W, Smyth GK. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15(2):R29. doi: 10.1186/gb-2014-15-2-r29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santuy A, Turégano-López M, Rodríguez JR, Alonso-Nanclares L, DeFelipe J, Merchán-Pérez A. A quantitative study on the distribution of mitochondria in the neuropil of the juvenile rat somatosensory cortex. Cereb Cortex. 2018;28(10):3673-3684. doi: 10.1093/cercor/bhy159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afifi AK. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 38.Moyer CE, Delevich KM, Fish KN, et al. . Intracortical excitatory and thalamocortical boutons are intact in primary auditory cortex in schizophrenia. Schizophr Res. 2013;149(1-3):127-134. doi: 10.1016/j.schres.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moyer CE, Delevich KM, Fish KN, et al. . Reduced glutamate decarboxylase 65 protein within primary auditory cortex inhibitory boutons in schizophrenia. Biol Psychiatry. 2012;72(9):734-743. doi: 10.1016/j.biopsych.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts RC, Barksdale KA, Roche JK, Lahti AC. Decreased synaptic and mitochondrial density in the postmortem anterior cingulate cortex in schizophrenia. Schizophr Res. 2015;168(1-2):543-553. doi: 10.1016/j.schres.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegde AN. Proteolysis, synaptic plasticity and memory. Neurobiol Learn Mem. 2017;138:98-110. doi: 10.1016/j.nlm.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mabb AM, Ehlers MD. Ubiquitination in postsynaptic function and plasticity. Annu Rev Cell Dev Biol. 2010;26:179-210. doi: 10.1146/annurev-cellbio-100109-104129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C, Bousman CA, Pantelis C, et al. . Pathway-wide association study identifies five shared pathways associated with schizophrenia in three ancestral distinct populations. Transl Psychiatry. 2017;7(2):e1037. doi: 10.1038/tp.2017.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arion D, Corradi JP, Tang S, et al. . Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry. 2015;20(11):1397-1405. doi: 10.1038/mp.2014.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott MR, Meador-Woodruff JH. Intracellular compartment-specific proteasome dysfunction in postmortem cortex in schizophrenia subjects [published online January 25, 2019]. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology. 2013;38(10):1910-1920. doi: 10.1038/npp.2013.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim P, Scott MR, Meador-Woodruff JH. Abnormal expression of ER quality control and ER associated degradation proteins in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res. 2018;197:484-491. doi: 10.1016/j.schres.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53(3):362-368. doi: 10.1016/j.neuropharm.2007.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGuire JL, Depasquale EA, Funk AJ, et al. . Abnormalities of signal transduction networks in chronic schizophrenia. NPJ Schizophr. 2017;3(1):30. doi: 10.1038/s41537-017-0032-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGuire JL, Hammond JH, Yates SD, et al. . Altered serine/threonine kinase activity in schizophrenia. Brain Res. 2014;1568:42-54. doi: 10.1016/j.brainres.2014.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X, Coulombe-Huntington J, Kang S, et al. . Widespread expansion of protein interaction capabilities by alternative splicing. Cell. 2016;164(4):805-817. doi: 10.1016/j.cell.2016.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson DS, Rodrigues DM, Hyndman BD, Crupi MJ, Nicolescu AC, Mulligan LM. Alternative splicing results in RET isoforms with distinct trafficking properties. Mol Biol Cell. 2012;23(19):3838-3850. doi: 10.1091/mbc.e12-02-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandal MJ, Zhang P, Hadjimichael E, et al. ; PsychENCODE Consortium . Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362(6420):eaat8127. doi: 10.1126/science.aat8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Man HY. GluA2-lacking, calcium-permeable AMPA receptors: inducers of plasticity? Curr Opin Neurobiol. 2011;21(2):291-298. doi: 10.1016/j.conb.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Llano O, Smirnov S, Soni S, et al. . KCC2 regulates actin dynamics in dendritic spines via interaction with β-PIX. J Cell Biol. 2015;209(5):671-686. doi: 10.1083/jcb.201411008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Khirug S, Cai C, et al. . KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56(6):1019-1033. doi: 10.1016/j.neuron.2007.10.039 [DOI] [PubMed] [Google Scholar]
- 57.Tornberg J, Voikar V, Savilahti H, Rauvala H, Airaksinen MS. Behavioural phenotypes of hypomorphic KCC2-deficient mice. Eur J Neurosci. 2005;21(5):1327-1337. doi: 10.1111/j.1460-9568.2005.03959.x [DOI] [PubMed] [Google Scholar]
- 58.Lee CW, Peng HB. The function of mitochondria in presynaptic development at the neuromuscular junction. Mol Biol Cell. 2008;19(1):150-158. doi: 10.1091/mbc.e07-05-0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flippo KH, Strack S. An emerging role for mitochondrial dynamics in schizophrenia. Schizophr Res. 2017;187:26-32. doi: 10.1016/j.schres.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet. 2005;14(2):241-253. doi: 10.1093/hmg/ddi022 [DOI] [PubMed] [Google Scholar]
- 61.Vawter MP, Tomita H, Meng F, et al. . Mitochondrial-related gene expression changes are sensitive to agonal-pH state: implications for brain disorders. Mol Psychiatry. 2006;11(7):615, 663-679. doi: 10.1038/sj.mp.4001850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 2002;22(7):2718-2729. doi: 10.1523/JNEUROSCI.22-07-02718.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karry R, Klein E, Ben Shachar D. Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol Psychiatry. 2004;55(7):676-684. doi: 10.1016/j.biopsych.2003.12.012 [DOI] [PubMed] [Google Scholar]
- 64.Cavelier L, Jazin EE, Eriksson I, et al. . Decreased cytochrome-c oxidase activity and lack of age-related accumulation of mitochondrial DNA deletions in the brains of schizophrenics. Genomics. 1995;29(1):217-224. doi: 10.1006/geno.1995.1234 [DOI] [PubMed] [Google Scholar]
- 65.Bergman O, Ben-Shachar D. Mitochondrial oxidative phosphorylation system (OXPHOS) deficits in schizophrenia: possible interactions with cellular processes. Can J Psychiatry. 2016;61(8):457-469. doi: 10.1177/0706743716648290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5(12):1512-1526. doi: 10.1039/b908315d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang B, Gaiteri C, Bodea LG, et al. . Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153(3):707-720. doi: 10.1016/j.cell.2013.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma K, Schmitt S, Bergner CG, et al. . Cell type- and brain region-resolved mouse brain proteome. Nat Neurosci. 2015;18(12):1819-1831. doi: 10.1038/nn.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Participant Demographics
eTable2. Synaptosome Enrichment
eTable 3. Mouse Homogenate PMI
eTable 4. Mouse Synaptosome PMI
eTable 5. Monkey APD Homogenate
eTable 6. Monkey APD Synaptosome
eTable 7. Eigenprotein Human Homogenate
eTable 8. Eigenprotein Human Synaptosome
eTable9. Homogenate Modules DAVID
eTable10. Synaptosome Modules DAVID
eTable11. Module Eigenprotein Differences
eTable12. Aligned Sz-Control Homogenate Proteome and Transcriptome Findings
eTable13. Aligned Sz-Control Syntaptosome Proteome and Transcriptome Findings
eTable14. List of Protein SRMs from Assay Stages
eMethods. Participants and Materials
eFigure 1. Synaptosome Yield, PMI, and pH in Human Tissue
eFigure 2. Distribution of Technical and Variance in Peptide Quantification
eFigure 3. Homogenate Protein Levels and PMI in Mice
eFigure 4. Synaptosome Protein Levels and PMI
eFigure 5. Exploratory Module Preservation Analysis
eFigure 6. Homogenate and Synaptosome Protein Networks
eFigure 7. Comparison of Antipsychotic Drug Effects and Sz Alterations
eFigures 8-13. Expanded Chord Diagrams for All Homogenate and Synaptosome Modules
eFigure 14. Associations Between Module Eigenprotein Values
eFigure 15. Comparison of Proteomic and Transcriptomic Analysis of Sz Tissue
eReferences.
eTable 1. Human Homogenate
eTable 2. Human Synaptosome
eTable 3. Hom Confounds
eTable 4. Syn Confounds