Key Points
Question
Is plasma lipidome alteration associated with progression of carotid artery atherosclerosis in the context of HIV infection?
Findings
In this cohort study using a plasma lipidomic profiling of 211 lipid species among 737 participants from 2 HIV cohorts, 12 lipid species were identified, representing independent signals for 10 lipid classes associated with risk of carotid artery plaque. Most of these plaque-associated lipid species were elevated in HIV-infected individuals, particularly among those using antiretroviral therapy.
Meaning
HIV/antiretroviral therapy–associated lipidome alteration may be linked with carotid artery atherosclerosis in HIV infection.
Abstract
Importance
Lipid metabolism disruption and excess risk of cardiovascular disease (CVD) have been observed in HIV-infected individuals, but the associations among HIV infection, plasma lipidome, and CVD risk have not been well understood.
Objective
To evaluate plasma lipidomic profiles and their associations with carotid artery atherosclerosis in individuals with HIV and individuals without HIV.
Design, Setting, and Participants
Prospective analysis in the Women’s Interagency HIV Study and Multicenter AIDS Cohort Study during a 7-year follow-up (from 2004-2006 to 2011-2013) at multicenter HIV cohorts in the United States. The study included 737 participants aged 35 to 55 years (520 with HIV and 217 without HIV) without CVD or carotid artery plaque at baseline. Data were analyzed between April 2017 and July 2019.
Exposures
Two hundred eleven plasma lipid species.
Main Outcomes and Measures
Poisson regression was used to examine the associations of baseline lipid species with risk of plaque measured by repeated B-mode carotid artery ultrasonography imaging.
Results
Of the 737 included participants, 398 (54%) were women, 351 (48%) were African American (non-Hispanic), 156 of 737 (21%) were nonwhite Hispanic, and the mean (SD) age was 45 (6) years. After adjusting for demographic and behavioral factors, we identified 12 lipid species, representing independent signals for 10 lipid classes, associated with risk of plaque. Nine lipid species remained significant after further adjusting for conventional CVD risk factors, although many of them showed moderate to high association with conventional blood lipids (eg, total and low-density lipoprotein cholesterols and triglycerides). Cholesteryl ester (16:1) (risk ratio [RR] per standard deviation, 1.28; 95% CI, 1.08-1.52), ceramide (16:0) (RR, 1.29; 95% CI, 1.02-1.63), lysophosphatidylcholine (20:4) (RR, 1.28; 95% CI, 1.05-1.58), lysophosphatidylethanolamine (16:0) (RR, 1.28; 95% CI, 1.05-1.57), phosphatidylethanolamine (38:6) (RR, 1.33; 95% CI, 1.08-1.64), phosphatidylethanolamine-plasmalogen (36:2) (RR, 1.25; 95% CI, 1.04-1.52), phosphatidylserine-plasmalogen (36:3) (RR, 1.19; 95% CI, 1.00-1.43), and triacylglycerol (54:6) (RR, 1.26; 95% CI, 1.04-1.54) were associated with increased risk of plaque, while phosphatidylcholine (36:4) (RR, 0.65; 95% CI, 0.54-0.77) was associated with decreased risk of plaque. Most of these plaque-increased lipid species showed higher levels in individuals with HIV, particularly among individuals with HIV using antiretroviral therapy compared with individuals without HIV. Network analysis identified 9 lipid modules, and 2 modules composed of triacylglycerols and phosphatidylcholines with long and unsaturated acyl chains, respectively, showed the strongest associations with increased risk of plaque.
Conclusions and Relevance
This study identified multiple plasma lipid species associated with carotid artery atherosclerosis, and alterations in these lipid species might be associated with HIV infection and antiretroviral therapy. Our data suggest unfavorable associations of long-chain and unsaturated triacylglycerols and phosphatidylcholines with carotid artery plaque formation.
This cohort study evaluates plasma lipidomic profiles and their associations with carotid artery atherosclerosis in individuals with HIV and individuals without HIV.
Introduction
The success of antiretroviral therapies (ART) has led to longer survival in people with HIV infection, and their risk of cardiovascular disease (CVD) has become a major concern.1,2,3 Excess CVD risk among people with HIV might be owing to complicated interactions among the effects of chronic HIV infection through immune activation and inflammation, metabolic toxicity of ART use, and HIV-unrelated risk factors. However, an understanding of the pathophysiology remains incomplete.1,2,4 The combination of HIV infection and ART use is associated with disrupted lipid metabolism, which may contribute to the excess CVD risk in people with HIV.2,5,6 HIV infection–associated and ART-associated dyslipidemia have been well documented, but most studies have focused on conventional blood lipids (eg, triglycerides, total cholesterol, low-density lipoprotein cholesterols, and high-density lipoprotein cholesterols).5,6,7,8 Conventional lipid biomarkers may not reflect the complex alteration of lipid metabolism in HIV infection, and studies are warranted to explore distinct lipid species that may contribute to the development of CVD.
Advances in lipidomics, an approach allowing the assessment of hundreds of lipid species across multiple pathways, provide a powerful platform for the discovery of new lipid biomarkers associated with CVD.9,10 In lipidomic studies of non-HIV populations, circulating lipid species and classes, such as sphingolipids, phospholipids, cholesteryl esters, and triacylglycerols, have been associated with atherosclerosis and CVD events.11,12,13,14,15,16 It has also been shown that adding newly identified lipid species in combination with traditional risk factors improved prediction of CVD events.11,12,13 However, lipidomics and CVD risk in HIV-infected populations have been explored only to a limited extent.17
Our prior work has demonstrated that HIV infection is associated with greater progression of carotid artery atherosclerosis,18 a validated surrogate for CVD outcomes.19 In this study, through a plasma lipidomic profiling, we examined the associations of 211 lipid species with progression of carotid artery atherosclerosis over approximately 7 years among 737 participants (520 infected with HIV and 217 not infected with HIV) from 2 prospective cohorts: the Women’s Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS).
Methods
Study Participants
Participants were from 2 prospective multicenter cohorts of women and men with or at risk for HIV infection, the WIHS and the MACS.20,21 Every 6 months, WIHS and MACS participants undergo a core visit with a comprehensive examination, providing biological specimens, and completing interviewer-administered questionnaires. Since 2004, the WIHS and MACS have collaborated on a uniform carotid artery imaging protocol19 to ascertain progression of subclinical carotid artery atherosclerosis.22 The primary exclusion criterion was a history of CVD. This study included participants who underwent carotid artery imaging for plaque assessment at a baseline visit (2004-2006) and at a follow-up visit (2011-2013).22,23 Exclusion criteria for the lipidomic study were age younger than 35 years, prevalent carotid artery plaque, or prevalent diabetes. A total of 398 women (291 with HIV and 107 without HIV) and 339 men (229 with HIV and 110 without HIV) were included in this study. All participants provided written informed consent, and protocols of this study were approved by the institutional review board at Albert Einstein College of Medicine.
Carotid Artery Plaque Ascertainment
High-resolution B-mode carotid artery ultrasonography was used to image 6 locations in the right carotid artery of participants: the near and far walls of the common carotid artery, carotid bifurcation, and internal carotid artery. A standardized protocol was used at each of 2 carotid artery imaging visits for all sites.18,19 Focal plaque measures were obtained at a centralized reading center (University of Southern California). Coefficients of variation of repeated measurements of carotid artery intimamedia thickness (cIMT) at the common carotid artery have been published,24 and replicate image acquisition and interpretation studies were repeated to ensure consistency over time. We defined a focal plaque as an area with localized IMT greater than 1.5 mm in any of the 6 imaged carotid artery locations.25
Plasma Lipidomic Profiling
Plasma lipid species were profiled on stored frozen plasma specimens that had been collected at the core study visits closest to the baseline carotid artery imaging study visit. Lipidomics were accomplished using liquid chromatography-tandem mass spectrometry at the Broad Institute. Details on sample extraction, separation, and mass spectrometry settings are described in the eMethods in the Supplement. A total of 218 lipid species were quantified, and the median coefficient of variation (CV) was 4.7% (range, 2.8%-31.4%). We first excluded 4 lipid species with CV of at least 20%, and further excluded 3 lipid species with percentage of missing values at least 10%. The analysis included 211 lipid species from 11 classes (16 classes/subclasses). Detailed information on lipid classes/subclasses is shown in eTable 1 in the Supplement. Coefficients of variation and percentage of missing values for 211 lipid species are shown in eFigure 1 in the Supplement. No participants were excluded because there were no participants with missing data on 8 or more lipid species. Missing values were imputed with one-half minimum values for a given lipid species.
Assessments of HIV Infection and Covariates
Demographic, behavioral, clinical, and laboratory variables were collected using standardized protocols at semiannual core study visits.18 HIV infection was ascertained by enzyme-linked immunosorbent assay and confirmed by Western blot. HIV-specific parameters included CD4+ T-cell counts, HIV-1 viral load, and detailed information on classes of ART drugs (protease inhibitors, nonnucleoside reverse transcriptase inhibitors [NNRTI], and nucleoside reverse transcriptase inhibitors [NRTI] have been previously described).24 Cardiovascular disease–related risk factors included body mass index (BMI), systolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and current use of antihypertensive and lipid-lowering medications.23
Statistical Analysis
Baseline characteristics between patients with incident carotid artery plaque and patients without incident carotid artery plaque were compared using the Mann-Whitney U test (continuous variables) and χ2 test (categorical variables) in all participants as well as in men and women separately. Sex-specific inverse normal transformations were performed on each lipid species before analyses. Spearman correlation analyses were used to estimate correlation coefficients among all 211 lipid species. Poisson regression models with robust variance estimates were used to examine the association between each of 211 individual lipid species and risk of plaque in all participants, with adjustment for age, sex, race/ethnicity, education, study site, and current smoking status. False discovery rate was applied to control for multiple testing.26 Among significant lipid species (P < .05 after false discovery rate correction), we further conducted conditional analyses, conditioning on the top lipid species in a given lipid class, to identify potential secondary signals within each lipid class.
Subsequent analyses were focused on the associations of the identified top and secondary lipid species with risk of plaque by Poisson regression models, further adjusting for HIV infection and related parameters, crack cocaine use, injected drug use, hepatitis C virus infection, and traditional CVD risk factors. Potential lipid-sex interactions and lipid-HIV interactions on risk of plaque were tested with generalized score statistics. Risk discrimination was assessed by the C statistic27 and integrated discrimination improvement,28 and risk classification was estimated by net reclassification improvement (NRI).28,29
Multivariate general linear models were used to examine the associations of HIV infection and ART use with lipid species. Spearman correlation analyses were used to estimate correlation coefficients of lipid species with CVD risk factors and HIV-related factors.
Network analysis was conducted using a weighted correlation network analysis implemented in the WGCNA R package (the R Foundation).30 Hierarchical clustering was used to detect lipid modules, and the first principal component was estimated to represent the intramodular hub for each module (module score).30 Multivariate Poisson regression models were applied to examine the associations of lipid module scores and risk of plaque. All analyses were conducted using R, version 3.4.0 (the R Foundation) and the correlation networks were plotted by Cytoscape, version 3.6.0 (Institute for Systems Biology).31 A 2-sided P value less than .05 was considered statistically significant.
Results
Participant Characteristics
A total of 737 participants (520 with HIV and 217 without HIV) without carotid artery plaque at baseline were included, and baseline demographic and socioeconomic characteristics among participants with HIV and without HIV were generally similar and comparable.32,33 Over a median 7-year follow-up, 112 incident plaque cases (67 men and 45 women) were identified. Table 1 shows baseline characteristics of participants by incident plaque cases and those without incident plaque. Although men and women were different on several characteristics, differences in characteristics between incident plaque cases and those without incident plaque were generally consistent for men and women. As expected, compared with participants without incident plaque, participants with incident plaque were more likely to have HIV infection, use antihypertensive and lipid-lowering medications, and have higher systolic blood pressure, triglyceride levels, and total cholesterol levels. Among participants without lipid-lowering medication use, higher levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol at baseline were significantly associated with increased risk of plaque (eTable 2 in the Supplement).
Table 1. Baseline Characteristics of Participants.
| Characteristics | No. (%) | |||||
|---|---|---|---|---|---|---|
| All | Men (MACS) | Women (WIHS) | ||||
| Without Incident Plaque | With Incident Plaque | Without Incident Plaque | With Incident Plaque | Without Incident Plaque | With Incident Plaque | |
| Participants, No. | 625 | 112 | 272 | 67 | 353 | 45 |
| Age, median (IQR), y | 44 (40-49) | 47 (43-52)a | 47 (43-51) | 47 (44-52) | 41 (38-46) | 45 (42-52)a |
| Race/ethnicity | ||||||
| White/other | 186 (29.8) | 44 (39.3) | 160 (58.8) | 39 (58.2) | 26 (7.4) | 5 (11.1) |
| Hispanic | 141 (22.6) | 15 (13.4) | 31 (11.4) | 6 (9.0) | 110 (31.2) | 9 (20.0) |
| African American | 298 (47.7) | 53 (47.3) | 81 (29.8) | 22 (32.8) | 217 (61.5) | 31 (68.9) |
| Education | ||||||
| Less than high school | 156 (25.0) | 23 (20.5) | 21 (7.7) | 4 (6.0) | 135 (38.2) | 19 (42.2) |
| High school | 147 (23.5) | 20 (17.9) | 37 (13.6) | 11 (16.4) | 110 (31.2) | 9 (20.0) |
| College and higher | 322 (51.5) | 69 (61.6) | 214 (78.7) | 52 (77.6) | 108 (30.6) | 17 (37.8) |
| Current smoking | 254 (40.6) | 55 (49.1) | 80 (29.4) | 27 (40.3) | 174 (49.3) | 28 (62.2) |
| Crack cocaine use | 65 (10.4) | 20 (17.9)a | 30 (11.0) | 14 (20.9)a | 35 (9.9) | 6 (13.3) |
| Injected drug use | 120 (19.2) | 27 (24.1) | 18 (6.6) | 11 (16.4)a | 102 (28.9) | 16 (35.6) |
| HCV infection | 136 (21.8) | 28 (25.0) | 32 (11.8) | 10 (14.9) | 104 (29.5) | 18 (40.0) |
| Medication use | ||||||
| Antihypertensive | 86 (13.8) | 30 (26.8)a | 38 (14.0) | 17 (25.4)a | 48 (13.6) | 13 (28.9)a |
| Lipid lowering | 60 (9.6) | 19 (17.0)a | 49 (18.0) | 18 (26.9) | 11 (3.1) | 1 (2.2) |
| BMI, median (IQR) | 26.8 (23.6-30.8) | 25.8 (23.2-28.2)a | 25.6 (23.0-28.3) | 25.4 (23.3-27.5) | 28.2 (24.2-32.3) | 27.1 (23.2-29.1)a |
| Systolic blood pressure, median (IQR), mm Hg | 118 (110-128) | 125 (115-132)a | 122 (115-130) | 126 (121-132)a | 115 (106-123) | 120 (111-129) |
| Triglycerides, median (IQR), mg/dL | 114.5 (75.0-172.0) | 138.0 (96.5-210.5)a | 129.0 (80.0-198.0) | 160.0 (108.5-227.0)a | 102.0 (73.0-147.0) | 121.0 (83.0-174.0) |
| Cholesterol, median (IQR), mg/dL | ||||||
| LDL | 106 (83-131) | 111 (93-132) | 114 (88-140) | 111 (88-132) | 101 (78-124) | 108 (99-132)a |
| HDL | 48 (39-58) | 45 (38-53) | 44 (37-54) | 45 (36-53) | 49 (40-61) | 45 (38-53) |
| Total | 182 (157-209) | 193 (168-221)a | 189 (163-214.5) | 196 (167-224) | 175 (152-202) | 187 (168-220)a |
| HIV infection | 430 (68.8) | 90 (80.4)a | 177 (65.1) | 52 (77.6)a | 253 (71.7) | 38 (84.4) |
| HIV-specific characteristicsb | ||||||
| CD4+ T-cell count, median (IQR), cells/mm3 | 495 (326-668) | 410 (273-579)a | 524 (365-728) | 504 (323-650) | 464 (309-638) | 314 (219-474)a |
| Undetectable viral load (≤80 copies/mL) | 236 (54.9) | 50 (55.6) | 116 (65.5) | 36 (69.2) | 120 (47.4) | 14 (36.8) |
| HIV-1 viral load, median (IQR), copies/mLc | 4557 (1100-20000) | 2647 (458-40500) | 5850 (1330-28172) | 2906 (558-81144) | 4000 (790-15000) | 1450 (410-36000) |
| ART use | ||||||
| Past 6 mo | 335 (77.9) | 71 (78.9) | 147 (83.1) | 44 (84.6) | 188 (74.3) | 27 (71.1) |
| PI | 177 (41.2) | 49 (54.4)a | 64 (36.2) | 32 (61.5)a | 113 (44.7) | 17 (44.7) |
| NNRTI | 160 (37.2) | 30 (33.3) | 87 (49.2) | 20 (38.5) | 73 (28.9) | 10 (26.3) |
| NRTI | 332 (77.2) | 69 (76.7) | 147 (83.1) | 42 (80.8) | 185 (73.1) | 27 (71.1) |
| Cumulative exposure, median (IQR), y | 4.1 (2.0-6.7) | 4.5 (2.8-6.7) | 4.5 (1.9-7.2) | 6.0 (4.0-7.6)a | 3.5 (2.5-6.5) | 3.5 (1.6-5.4) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HCV, hepatitis C virus; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; MACS, Multicenter AIDS Cohort Study; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, phosphatidylinositol; WIHS, Women’s Interagency HIV Study.
SI conversion factors: To convert cholesterol levels to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
P less than .05 between patients with and without incident plaque cases.
HIV-specific characteristics were based on the HIV-infected participants only.
Data from participants with detectable HIV-1 viral load.
Lipidomic Profiles and Risk of Carotid Artery Plaque
We first examined intercorrelations among all 211 lipid species. Most lipid species showed moderate to high associations with each other, especially those within the same lipid classes (eFigure 2 in the Supplement).
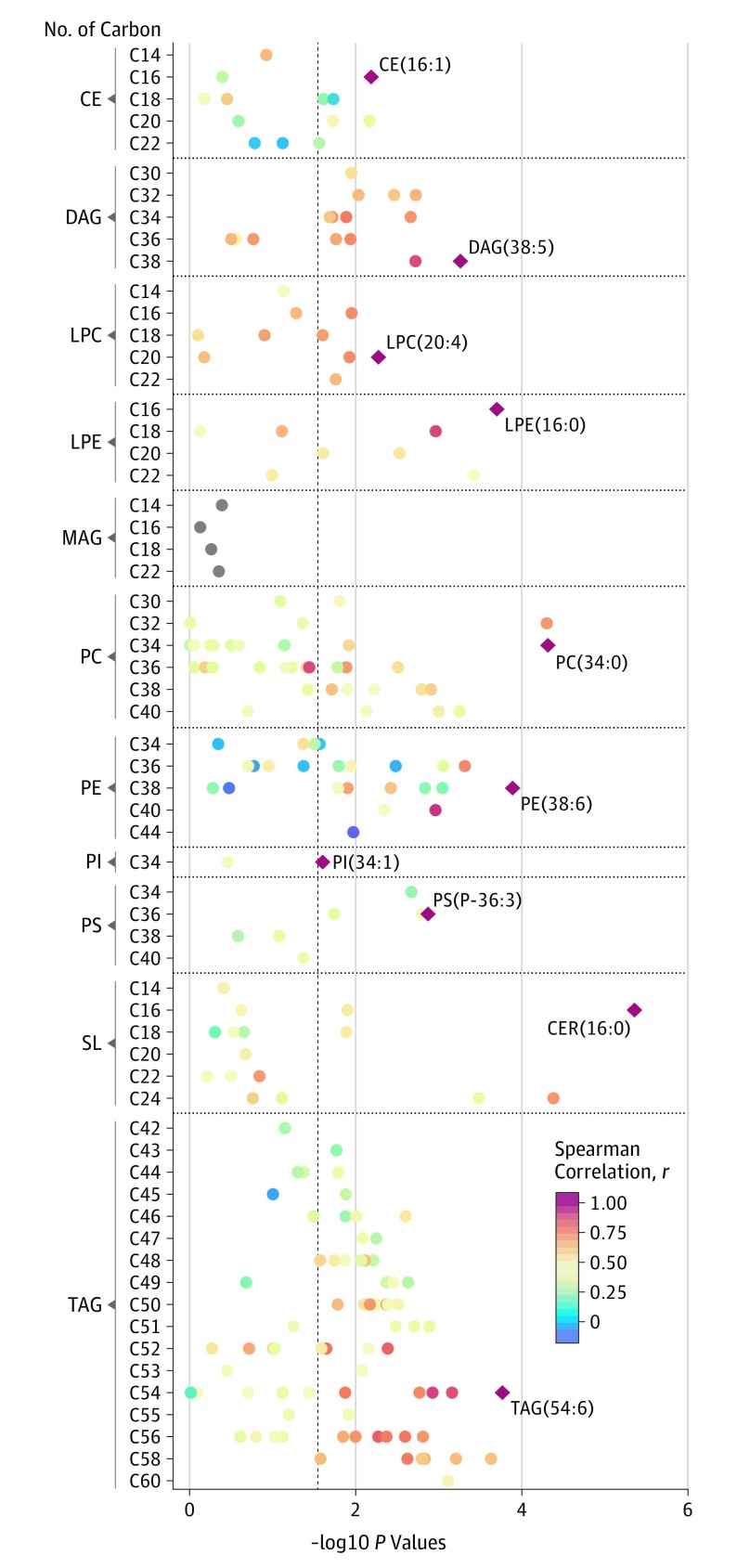
Among these 211 individual lipid species from 11 lipid classes, 120 lipid species from 10 lipid classes showed significant associations with risk of plaque (Figure 1). Within each of these 10 lipid classes, the top lipid species were ceramide (16:0), cholesteryl ester (16:1), diacylglycerol (DAG) (38:5), lysophosphatidylcholine (20:4), lysophosphatidylethanolamine (16:0), phosphatidylcholine (34:0), phosphatidylethanolamine (38:6), phosphatidylinositol (34:1), phosphatidylserine (P-36:3), and triacylglycerol (54:6). After conditioning on the top lipid species, most lipid species in the same lipid classes were not significantly associated with risk of plaque except for phosphatidylcholine (36:4) and phosphatidylethanolamine (P-36:2) (eTable 3 in the Supplement).
Figure 1. Manhattan Plot for the Associations of 211 Lipid Species With Risk of Carotid Artery Plaque.
Individual lipid species are depicted by filled circles and arranged by lipid classes in 11 panels according to the number of total carbon atoms (x-axes) and –log10 P values (y-axes). Dash line represents a cutoff of false discovery rate less than .05. Diamond shapes with lipid names are the top lipid species associated with risk of carotid artery plaque in each of the lipid classes. Circle color indicates Spearman correlation coefficients between the top lipid species and other lipid species within each lipid class. CE indicates cholesteryl ester; CER, ceramide; DAG, diacylglycerol; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; MAG, monoacylglycerol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; SL, sphingolipid; TAG, triacylglycerol.
The multivariate-adjusted associations of these 10 top and 2 secondary lipid species with risk of plaque are shown in Table 2. After adjusting for demographic, socioeconomic, behavioral variables, and HIV-associated factors, most of these lipid species remained associated with increased risk of plaque (Table 2), while phosphatidylcholine (36:4) was associated with decreased risk of plaque (RR, 0.71; 95% CI, 0.60-0.84). After further adjustment for traditional CVD risk factors, all the associations were attenuated and 9 lipid species remained significantly associated with risk of plaque.
Table 2. Associations of 12 Top and Secondary Lipid Species With Risk of Incident Carotid Artery Plaquea.
| Lipid Species | All (N = 737) | Men (n = 339) | Women (n = 398) | HIV-Positive (n = 520) | HIV-Negative (n = 217) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| CE (16:1) | ||||||||||
| Model 1 | 1.23 (1.05-1.44) | .01 | 1.43 (1.16-1.76) | .001 | 0.99 (0.77-1.28) | .94 | 1.25 (1.05-1.49) | .01 | 1.30 (0.88-1.92) | .19 |
| Model 2 | 1.28 (1.08-1.52) | .004 | 1.45 (1.15-1.84) | .002 | 0.98 (0.71-1.35) | .88 | 1.34 (1.10-1.63) | .004 | 1.26 (0.85-1.85) | .25 |
| CER (16:0) | ||||||||||
| Model 1 | 1.39 (1.17-1.64) | <.001 | 1.28 (1.02-1.61) | .04 | 1.59 (1.20-2.12) | .001 | 1.36 (1.10-1.67) | .004 | 1.39 (1.00-1.92) | .05 |
| Model 2 | 1.29 (1.02-1.63) | .03 | 1.23 (0.93-1.61) | .15 | 1.46 (0.95-2.22) | .08 | 1.33 (1.00-1.77) | .05 | 1.16 (0.72-1.88) | .53 |
| DAG (38:5) | ||||||||||
| Model 1 | 1.29 (1.08-1.53) | .004 | 1.22 (0.98-1.53) | .08 | 1.49 (1.13-1.97) | .005 | 1.25 (1.04-1.51) | .02 | 1.46 (0.94-2.26) | .09 |
| Model 2 | 1.26 (0.98-1.61) | .07 | 1.07 (0.79-1.46) | .64 | 2.15 (1.40-3.30) | <.001 | 1.23 (0.93-1.63) | .14 | 1.30 (0.69-2.43) | .42 |
| LPC (20:4) | ||||||||||
| Model 1 | 1.32 (1.10-1.60) | .003 | 1.26 (1.00-1.59) | .05 | 1.56 (1.08-2.23) | .02 | 1.31 (1.06-1.61) | .01 | 1.44 (0.92-2.25) | .11 |
| Model 2 | 1.28 (1.05-1.58) | .02 | 1.21 (0.92-1.59) | .17 | 1.56 (1.08-2.28) | .02 | 1.30 (1.04-1.63) | .02 | 1.37 (0.83-2.24) | .22 |
| LPE (16:0) | ||||||||||
| Model 1 | 1.36 (1.13-1.64) | .001 | 1.35 (1.05-1.73) | .02 | 1.46 (1.10-1.94) | .008 | 1.35 (1.10-1.65) | .004 | 1.41 (1.00-1.99) | .05 |
| Model 2 | 1.28 (1.05-1.57) | .01 | 1.23 (0.95-1.60) | .11 | 1.47 (1.06-2.03) | .02 | 1.29 (1.03-1.62) | .03 | 1.19 (0.83-1.70) | .35 |
| PC (34:0) | ||||||||||
| Model 1 | 1.37 (1.15-1.64) | <.001 | 1.24 (0.98-1.56) | .07 | 1.60 (1.22-2.10) | .001 | 1.34 (1.09-1.63) | .005 | 1.67 (1.14-2.45) | .008 |
| Model 2 | 1.26 (0.99-1.60) | .06 | 1.12 (0.85-1.48) | .41 | 1.69 (1.07-2.67) | .02 | 1.32 (0.98-1.78) | .07 | 1.23 (0.84-1.82) | .29 |
| PE (38:6) | ||||||||||
| Model 1 | 1.33 (1.12-1.58) | .001 | 1.50 (1.19-1.91) | .001 | 1.16 (0.88-1.53) | .28 | 1.25 (1.05-1.50) | .01 | 1.69 (1.21-2.37) | .002 |
| Model 2 | 1.33 (1.08-1.64) | .008 | 1.44 (1.11-1.86) | .005 | 1.25 (0.79-1.97) | .34 | 1.26 (1.00-1.58) | .05 | 1.78 (1.03-3.09) | .04 |
| PI (34:1) | ||||||||||
| Model 1 | 1.15 (0.95-1.38) | .15 | 1.21 (0.95-1.54) | .13 | 0.99 (0.73-1.36) | .96 | 1.11 (0.90-1.38) | .33 | 1.23 (0.83-1.82) | .29 |
| Model 2 | 1.01 (0.81-1.26) | .95 | 1.12 (0.84-1.49) | .44 | 0.77 (0.49-1.22) | .26 | 1.03 (0.79-1.34) | .83 | 0.91 (0.54-1.52) | .71 |
| PS (P-36:3) | ||||||||||
| Model 1 | 1.26 (1.06-1.49) | .007 | 1.41 (1.13-1.77) | .002 | 1.14 (0.85-1.52) | .38 | 1.24 (1.04-1.48) | .02 | 1.41 (0.88-2.26) | .15 |
| Model 2 | 1.19 (1.00-1.43) | .05 | 1.28 (1.03-1.60) | .03 | 1.19 (0.81-1.77) | .38 | 1.19 (0.98-1.45) | .08 | 1.21 (0.68-2.13) | .52 |
| TAG (54:6) | ||||||||||
| Model 1 | 1.32 (1.13-1.55) | .001 | 1.36 (1.10-1.68) | .005 | 1.34 (1.03-1.73) | .03 | 1.29 (1.09-1.53) | .003 | 1.48 (1.01-2.15) | .04 |
| Model 2 | 1.26 (1.04-1.54) | .02 | 1.24 (0.97-1.57) | .08 | 1.53 (1.10-2.12) | .01 | 1.24 (1.00-1.54) | .05 | 1.52 (0.94-2.44) | .09 |
| PC (36:4)b | ||||||||||
| Model 1 | 0.71 (0.60-0.84) | <.001 | 0.68 (0.55-0.85) | .001 | 0.69 (0.48-1.00) | .05 | 0.70 (0.58-0.85) | <.001 | 0.66 (0.44-0.99) | .04 |
| Model 2 | 0.65 (0.54-0.77) | <.001 | 0.61 (0.49-0.76) | <.001 | 0.65 (0.43-0.97) | .03 | 0.66 (0.54-0.81) | <.001 | 0.49 (0.33-0.74) | .001 |
| PE (P-36:2)b | ||||||||||
| Model 1 | 1.25 (1.05-1.48) | .01 | 1.06 (0.87-1.31) | .56 | 1.58 (1.15-2.17) | .005 | 1.21 (1.00-1.47) | .05 | 1.42 (1.00-2.02) | .05 |
| Model 2 | 1.25 (1.04-1.52) | .02 | 1.04 (0.84-1.30) | .72 | 1.70 (1.13-2.57) | .01 | 1.27 (1.01-1.59) | .04 | 1.24 (0.79-1.96) | .35 |
Abbreviations: CE, cholesteryl ester; CER, ceramide; DAG, diacylglycerol; HIV, human immunodeficiency virus; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; RR, risk ratio; TAG, triacylglycerol.
Data are RRs and 95% confidence intervals of incident carotid artery plaque per SD increment of lipid species, adjusted for age, sex, race/ethnicity, education, study site, current smoking, HIV infection, and treatment status (without HIV, antiretroviral therapy user with HIV, antiretroviral therapy nonuser with HIV), CD4+ T-cell count, HIV-1 viral load, crack cocaine use, injected drug use, and HCV infection (model 1); and further adjusted for systolic blood pressure, high-density lipoprotein cholesterol, total cholesterol, triglycerides, body mass index, antihypertensive medication use and lipid lowering medication use (model 2).
Secondary lipid species.
Results were generally consistent between MACS men and WIHS women. No effect modifications by HIV infection were observed. Among HIV-infected participants, results were also consistent between those with and without ART use (eTable 4 in the Supplement). We performed several sensitivity analyses by excluding participants with lipid-lowering medication use, crack cocaine use, injected drug use, or hepatitis C virus infection, and results did not change materially (eTable 5 in the Supplement).
Plaque-Associated Lipid Species, HIV Infection, and CVD Risk Factors
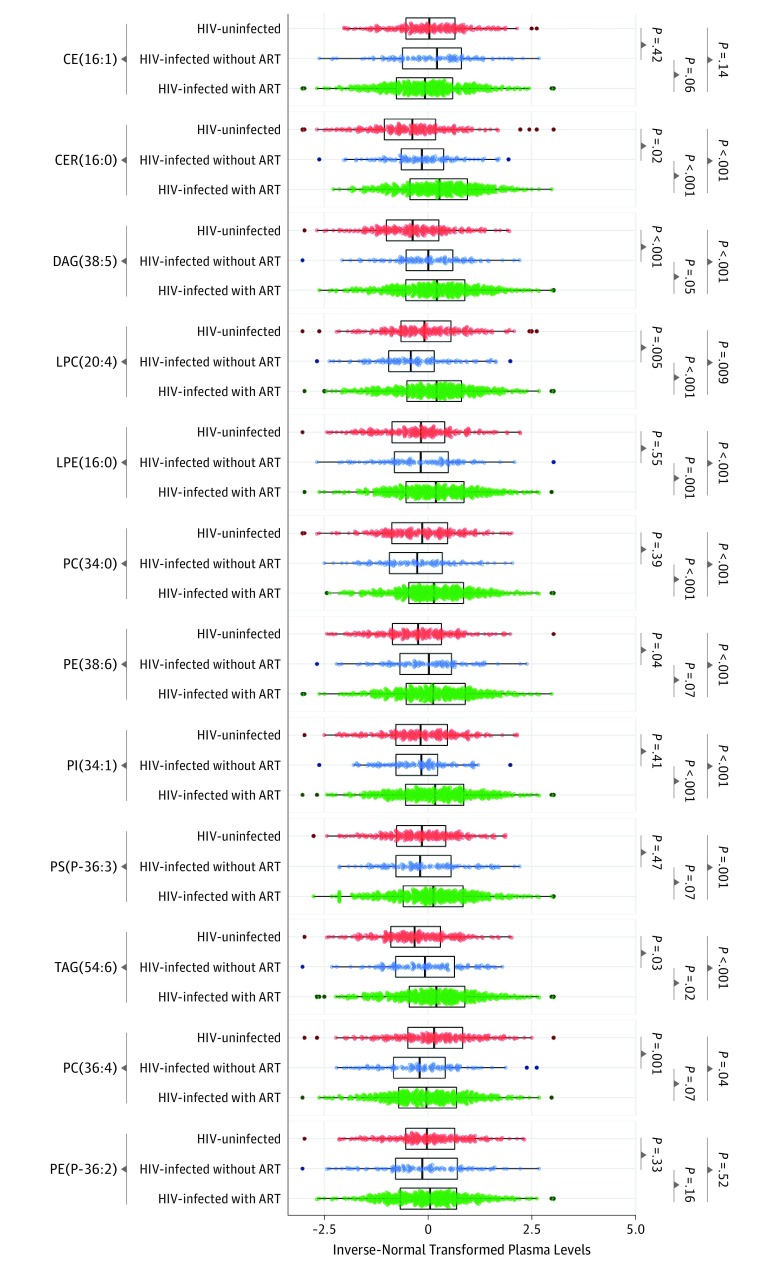
Eight of these 12 plaque-associated lipid species, including ceramide (16:0), diacylglycerol (38:5), lysophosphatidylethanolamine (16:0), phosphatidylcholine (34:0), phosphatidylethanolamine (38:6), phosphatidylinositol (34:1), phosphatidylserine (P-36:3), and triacylglycerol (54:6), showed significantly higher levels in individuals with HIV compared with those without HIV infection (eTable 6 in the Supplement). Further analyses showed that levels of these 8 lipid species were highest in individuals with HIV receiving ART (Figure 2), and 6 of them showed significant positive associations with ART use after multivariate adjustment (eTable 6 in the Supplement). We also found that protease inhibitor use was associated with higher levels of most of these 12 plaque-associated lipid species, while there were no significant differences in these lipid species between those with and without NNRTI use (eFigure 3 in the Supplement).
Figure 2. Plasma Levels of Carotid Artery Plaque–Associated Lipid Species According to HIV Infection and Antiretroviral Therapies (ART) Use.
Data are inverse normal transformed levels of 12 carotid artery plaque–associated lipid species in participants without HIV (red), participants with HIV without ART use (blue), and participants with HIV with ART use (green). P values for comparisons between groups were calculated using Wilcoxon test. CE indicates cholesteryl ester; CER, ceramide; DAG, diacylglycerol; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; TAG, triacylglycerol.
Among all participants, these 12 plaque-associated lipid species were more highly associated with blood cholesterol and triglyceride levels vs other CVD risk factors (eFigure 4 in the Supplement). In particular, phosphatidylinositol (34:1), phosphatidylcholine (34:0), and ceramide (16:0) were more highly associated with total cholesterol (r = 0.42, r = 0.55, and r = 0.41, respectively; all P < .001) and triglyceride levels (r = 0.40, r = 0.36, and r = 0.44, respectively; all P < .001) compared with other lipid species. The correlation patterns between these lipid species and CVD risk factors were similar between participants with HIV and participants without HIV.
Carotid Artery Plaque Risk Discrimination and Classification
The addition of 5 previously reported metabolites (tryptophan,33 kynurenic acid,33 Trimethylamine-N-Oxide,32 short-chain acylcarnitine score,34 and ceramide [16:00]35) to the conventional risk factor model increased the C index from 0.708 to 0.737 (P = .04) and resulted in an integrated discrimination improvement of 0.032 (P = .001), and further addition of 11 currently reported lipid species (ceramide [16:00] was previously reported35 and thus it was not included) increased the C index to 0.778 (P < .001) and had an integrated discrimination improvement of 0.094 (P < .001). The addition of previously reported metabolites on top of conventional risk factors showed a significant improvement in risk classification (categorical NRI = 0.165; P = .009), and further addition of currently reported lipid species further improved risk classification (categorical NRI = 0.250; P < .001). The improvement was driven mainly by correct reclassification of participants without incident plaque (categorical NRI = 0.203; P < .001). The significant improvements in discrimination and classification by further addition of 11 currently reported lipid species were observed among individuals with HIV. We repeated these analyses in 2 HIV cohorts (MACS and WIHS) separately, and results were generally consistent. Details of these estimates are summarized in eTable 7 in the Supplement.
Lipid Correlation Networks, Carotid Artery Plaque, and HIV Infection
Lipid network analysis of the 211 lipid species identified 9 lipid subnetwork modules (eFigure 5 in the Supplement). Lipid species with similar structures of the acyl chains in the same lipid classes were generally constructed into the same lipid subnetwork modules (Table 3). Two modules composed of TAGs and PCs with relatively higher carbon numbers and more double bonds, respectively, showed the strongest associations with increased risk of plaque (RR, 1.36; 95% CI, 1.16-1.58 and 1.35; 95% CI, 1.13-1.62 per SD of the lipid module score; P = .001 and .006, respectively). Another 2 modules, which contained mostly TAGs and PCs, respectively, with relatively lower carbon numbers and fewer double bonds, were also associated with increased risk of plaque. We then plotted the association results of all 211 lipid species by lipid class/subclass and carbon/double-bond number (eFigure 6 in the Supplement). This plot illustrated the similar associations of lipid species, corresponding to lipid modules, by chain length and number of double bonds, with risk of plaque.
Table 3. Associations Between Lipid Subnetwork Modules and Risk of Incident Carotid Artery Plaque.
| Lipid Modules | Lipid Species | Median (Range) | RR (95% CI)a | P Value | No. of Lipid Species Showed Positive Associations | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Major | No. of Carbon Atoms | No. of Double Bonds | With Carotid Artery Plaque Only | With HIV Infection Only | Both | |||
| Blue | 23 | TAGs | 56 (52-60) | 8 (5-12) | 1.36 (1.16-1.58) | .001 | 4 | 0 | 19 |
| Pink | 13 | PCs | 39 (36-40) | 6 (5-10) | 1.35 (1.13-1.62) | .006 | 9 | 0 | 2 |
| Turquoise | 72 | TAGs | 49 (42-56) | 2 (0-6) | 1.27 (1.08-1.51) | .02 | 4 | 14 | 45 |
| Green | 16 | PCs | 32 (30-36) | 1 (0-4) | 1.27 (1.06-1.52) | .03 | 5 | 0 | 1 |
| Red | 15 | LPCs | 18 (16-20) | 1 (0-5) | 1.29 (1.04-1.58) | .04 | 1 | 2 | 7 |
| LPEs | 18 (16-22) | 0 (0-4) | |||||||
| Yellow | 17 | PCs | 36 (34-38) | 2 (0-4) | 1.19 (1.01-1.41) | .06 | 0 | 3 | 6 |
| Brown | 22 | PCs | 36 (34-40) | 3 (1-7) | 1.19 (1.01-1.40) | .06 | 7 | 0 | 0 |
| PEs | 36 (34-38) | 3 (2-6) | |||||||
| Black | 15 | SMs | 18 (14-24) | 0 (0-2) | 1.17 (1.00-1.38) | .07 | 6 | 0 | 0 |
| Magenta | 11 | TAGs | 54 (52-56) | 3 (3-5) | 1.12 (0.95-1.31) | .19 | 0 | 11 | 0 |
Abbreviations: FDR, false discovery rate; LPCs, lysophosphatidylcholines; LPEs, lysophosphatidylethanolamines; PCs, phosphatidylcholines; PEs, phosphatidylethanolamines; RR, risk ratio; SMs, sphingomyelins; TAGs, triacylglycerols.
Data are RRs and 95% confidence intervals of carotid artery plaque per SD increment of lipid module scores, adjusted for age, sex, race/ethnicity, education, study site and current smoking. P values were corrected by FDR.
We found that most lipid species in the long and unsaturated TAG lipid module were positively associated with both carotid artery plaque and HIV infection, while most lipid species in the long and unsaturated PC lipid module were associated with carotid artery plaque only but not with HIV infection (Table 3). However, the associations of these 2 lipid modules with risk of plaque were similar between participants with HIV and participants without HIV (eTable 8 in the Supplement).
Discussion
In 2 combined prospective HIV cohorts, our plasma lipidomic profiling identified multiple lipid species associated with risk of carotid artery plaque. The top lipid species associated with risk of plaque in the cholesteryl ester, lysophosphatidylethanolamine, and phosphatidylcholine classes (ie, ceramide [16:1], lysophosphatidylethanolamine [16:0], and phosphatidylcholine [34:0]) had relatively lower carbon numbers and double-bond contents, which are consistent with previous results in non-HIV studies.11,12,13,14 Ceramides, especially ceramide (16:0), have been reported to be implicated in the development of atherosclerosis and CVD in our and others’ previous analyses.11,35,36,37,38 The top-ranked phosphatidylethanolamines in our study were those with medium acyl chains and double bonds (ie, phosphatidylethanolamine [38:6] and phosphatidylethanolamine [P-36:2]), and similar medium-chain phosphatidylethanolamines have been associated with increased risk of CVD in non-HIV cohorts.13,14 The associations between lysophosphatidylcholines and CVD are controversial.11,13,14 Nevertheless, our finding on lysophosphatidylcholines is supported by a study that showed elevated lysophosphatidylcholine content in human carotid artery plaque tissues.39
Consistent with previous lipidomic/metabolomic studies in non-HIV cohorts,11,12,13 our study also found that plasma lipidomic profiling may improve cardiovascular risk discrimination and classification beyond traditional risk factors. The results were consistent between our 2 HIV cohorts (MACS and WIHS), but external validations in other independent HIV studies are needed. The potential clinical use of these lipid species in the prediction of CVD among HIV-infected people remains unknown because the end point of this study is incident carotid artery plaque, a subclinical CVD measure. Future HIV studies with incident CVD data are warranted to evaluate the clinical utility of these biomarkers.
Studies in populations without HIV infection have suggested that structural features of the acyl chains, such as the length of chains and the number of double bonds, may play a critical role in the associations between lipid species and CVD risk.13,14 In this study, although we confirmed the positive associations of carotid artery plaque with triacylglycerols and phosphatidylcholines with shorter acyl chains and fewer of double bonds, we did not observe inverse associations for long and unsaturated triacylglycerols or phosphatidylcholines. In contrast, our network analysis identified that 2 lipid clusters of triacylglycerols and phosphatidylcholines with long and unsaturated acyl chains, respectively, showed the strongest associations with increased risk of plaque. It should be noted that this study examined carotid artery plaque, while previous studies focused on the clinical CVD events.13,14 Our results were partially supported by a previous study14 that showed enrichments of polyunsaturated cholesteryl esters with long-chain fatty acids in human carotid artery plaque tissue, suggesting a potential role of long-chain polyunsaturated lipid species in the formation of carotid artery plaque.
Another interesting finding of our study is that most top-ranked lipids species associated with higher plaque risk were elevated in individuals with HIV, particularly among those using ART. Our further analyses indicated that protease inhibitor use, but not NNRTI use, was associated with higher levels of lipid species. This is in line with observations that lipid metabolism disorders are more evident among individuals using PI-based ART compared with those using NNRTI-based ART.7,8 A 2018 study40 of 155 ART-naive individuals with HIV also reported that initiation of ART may lead to significant changes in the plasma lipidome at 48 weeks, but it was found that NNRTI-based ART showed greater influences on the plasma lipidome compared with PI-based or NRTI-based ART. Thus, further studies are needed to examine the long-term effects of different ART regimens on the lipidome and the underlying mechanisms associated with CVD risk. Findings from future studies may help guide the choice of ART regimens for people with HIV in the prevention of cardiometabolic diseases and thus may have very important clinical implications.
The consistent association patterns of lipid species with HIV infection and progression of carotid artery atherosclerosis (ie, lipid species elevated in HIV infection and/or ART use and associated with increased risk of plaque) suggest that these lipid species might be involved in the links of HIV/ART with CVD risk. However, we also found that some lipid species (eg, long and unsaturated phosphatidylcholines) were associated with risk of plaque but not with HIV infection, suggesting the underlying mechanisms for these lipid species in association with CVD risk might be different.
Limitations
To our knowledge, this is the first prospective lipidomic study of atherosclerosis among HIV-infected and HIV-uninfected individuals with similar demographic characteristics and risk behaviors. The main strengths of this study include 2 well-characterized HIV cohorts, longitudinal measurement of carotid artery atherosclerosis, high-throughput and high-quality lipidomics, and careful consideration of multiple covariates including HIV parameters and CVD risk factors. Our study has several limitations. Although our untargeted lipidomics approach had broad coverage of lipid species, levels of lipid species were semiquantified without absolute values; and thus targeted methods on lipid species of particular interests are needed to better understand the underlying biological mechanisms. While our study did not examine incident CVD events, we evaluated a subclinical measure of carotid artery atherosclerosis that has been validated as a surrogate preclinical marker of clinical CVD events.19,41,42 Regarded as an intermediate phenotype, this subclinical measure may provide important biological information before the onset of CVD, and it can be assessed noninvasively and quickly through ultrasonography.41,42 However, the difficulty to adequately image all carotid artery segments has been noted.43 Ultrasonography-derived carotid artery metrics (eg, plaque presence, common cIMT, and internal cIMT) have shown various predictive abilities for CVD among different studies.41,42,43 In this study, we focused on incident plaque as a measure of carotid artery atherosclerosis progression because our previous studies have shown that HIV infection was associated with risk of carotid plaque but not with changes in cIMT,18 and carotid artery plaque but not cIMT was associated with mortality in our HIV cohorts.44 Potential sex differences could not be adequately examined owing to differences in sociodemographic characteristics between men and women. This study was underpowered to detect effect modification by HIV infection because of a relatively small number of plaque cases in participants without HIV.
Conclusions
In summary, this lipidomic study in 2 HIV cohorts identified multiple lipid species associated with progression of carotid artery atherosclerosis, independent of traditional CVD risk factors. Most of these lipid species were elevated in HIV-infected individuals receiving ART. Our network analysis identified 2 lipid subnetwork modules containing lipid species with long and unsaturated acyl chains that showed the strongest associations with increased risk of carotid artery plaque. These intriguing data implicate structure features of the acyl chains of lipid species in the formation of carotid artery plaques.
eMethods
eTable 1. Number of lipid species in each of lipid classes/sub-classes
eTable 2. Associations of traditional blood lipids with risk of carotid artery plaque
eTable 3. Conditional analysis results in the PC and PE lipid classes.
eTable 4. Associations between 12 lipid species and risk of carotid artery plaque among HIV-infected individuals stratified by ART use
eTable 5. Sensitivity analyses by excluding participants with lipid medication use, with illicit drug use, or with HCV infection
eTable 6. Associations of 12 carotid artery plaque-associated lipid species with HIV infection and ART use
eTable 7. Risk of carotid artery plaque discrimination and classification
eTable 8. Associations between lipid sub-network modules and risk of carotid artery plaque by the HIV-infection status
eFigure 1. Coefficient of variations (CVs) and percentage of missing sample size count for 211 lipid species from 16 lipid classes/sub-classes
eFigure 2. Pairwise correlation heatmap of 211 individual lipid species in 11 lipid classes
eFigure 3. Plasma levels of carotid artery associated lipid species according to HIV infection and classes of ART use
eFigure 4. Partial Spearman correlation of carotid artery plaque associated lipid species with CVD and HIV related factors
eFigure 5. Lipid network with blue and pink sub-network modules
eFigure 6. Associations of 211 individual lipid species with risk of carotid artery plaque according to numbers of carbon atoms and double bond contents
References
- 1.Kaplan RC, Hanna DB, Kizer JR. Recent insights into cardiovascular disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep. 2016;13(1):44-52. doi: 10.1007/s11904-016-0301-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thienemann F, Sliwa K, Rockstroh JK. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J. 2013;34(46):3538-3546. doi: 10.1093/eurheartj/eht388 [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525-1533. doi: 10.1016/S0140-6736(13)61809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown TT, Glesby MJ. Management of the metabolic effects of HIV and HIV drugs. Nat Rev Endocrinol. 2011;8(1):11-21. doi: 10.1038/nrendo.2011.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feeney ER, Mallon PW. HIV and HAART-associated dyslipidemia. Open Cardiovasc Med J. 2011;5:49-63. doi: 10.2174/1874192401105010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggi P, Di Biagio A, Rusconi S, et al. Cardiovascular risk and dyslipidemia among persons living with HIV: a review. BMC Infect Dis. 2017;17(1):551. doi: 10.1186/s12879-017-2626-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nduka C, Sarki A, Uthman O, Stranges S. Impact of antiretroviral therapy on serum lipoprotein levels and dyslipidemias: a systematic review and meta-analysis. Int J Cardiol. 2015;199:307-318. doi: 10.1016/j.ijcard.2015.07.052 [DOI] [PubMed] [Google Scholar]
- 8.da Cunha J, Maselli LM, Stern AC, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: old and new drugs. World J Virol. 2015;4(2):56-77. doi: 10.5501/wjv.v4.i2.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JM, Hazen SL. Seeking a unique lipid signature predicting cardiovascular disease risk. Circulation. 2014;129(18):1799-1803. doi: 10.1161/CIRCULATIONAHA.114.009224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ummarino D. Diabetes: lipidomics refines CVD risk prediction. Nat Rev Cardiol. 2016;13(12):697. doi: 10.1038/nrcardio.2016.180 [DOI] [PubMed] [Google Scholar]
- 11.Alshehry ZH, Mundra PA, Barlow CK, et al. Plasma lipidomic profiles improve on traditional risk factors for the prediction of cardiovascular events in type 2 diabetes mellitus. Circulation. 2016;134(21):1637-1650. doi: 10.1161/CIRCULATIONAHA.116.023233 [DOI] [PubMed] [Google Scholar]
- 12.Paynter NP, Balasubramanian R, Giulianini F, et al. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137(8):841-853. doi: 10.1161/CIRCULATIONAHA.117.029468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stegemann C, Pechlaner R, Willeit P, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129(18):1821-1831. doi: 10.1161/CIRCULATIONAHA.113.002500 [DOI] [PubMed] [Google Scholar]
- 14.Toledo E, Wang DD, Ruiz-Canela M, et al. Plasma lipidomic profiles and cardiovascular events in a randomized intervention trial with the Mediterranean diet. Am J Clin Nutr. 2017;106(4):973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Ke C, Liu H, et al. Large-scale metabolomic analysis reveals potential biomarkers for early stage coronary atherosclerosis. Sci Rep. 2017;7(1):11817. doi: 10.1038/s41598-017-12254-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng JM, Suoniemi M, Kardys I, et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis. 2015;243(2):560-566. doi: 10.1016/j.atherosclerosis.2015.10.022 [DOI] [PubMed] [Google Scholar]
- 17.Wong G, Trevillyan JM, Fatou B, et al. Plasma lipidomic profiling of treated HIV-positive individuals and the implications for cardiovascular risk prediction. PLoS One. 2014;9(4):e94810. doi: 10.1371/journal.pone.0094810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis. 2015;61(4):640-650. doi: 10.1093/cid/civ325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128(4):262-269. doi: 10.7326/0003-4819-128-4-199802150-00002 [DOI] [PubMed] [Google Scholar]
- 20.Bacon MC, von Wyl V, Alden C, et al. The women’s interagency HIV study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310-318. doi: 10.1093/aje/126.2.310 [DOI] [PubMed] [Google Scholar]
- 22.Hanna DB, Guo M, Bůžková P, et al. HIV infection and carotid artery intima-media thickness: pooled analyses across 5 cohorts of the NHLBI HIV-CVD collaborative. Clin Infect Dis. 2016;63(2):249-256. doi: 10.1093/cid/ciw261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanna DB, Lin J, Post WS, et al. Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J Infect Dis. 2017;215(9):1352-1361. doi: 10.1093/infdis/jix082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22(13):1615-1624. doi: 10.1097/QAD.0b013e328300581d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011): an update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290-296. doi: 10.1159/000343145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289-300. [Google Scholar]
- 27.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361-387. doi: [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11-21. doi: 10.1002/sim.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498-2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shan Z, Clish CB, Hua S, et al. Gut microbial-related choline metabolite trimethylamine-n-oxide is associated with progression of carotid artery atherosclerosis in HIV infection. J Infect Dis. 2018;218(9):1474-1479. doi: 10.1093/infdis/jiy356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi Q, Hua S, Clish CB, et al. Plasma tryptophan-kynurenine metabolites are altered in human immunodeficiency virus infection and associated with progression of carotid artery atherosclerosis. Clin Infect Dis. 2018;67(2):235-242. doi: 10.1093/cid/ciy053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua S, Scott JM, Hanna DB, et al. Plasma acylcarnitines and progression of carotid artery atherosclerosis in HIV infection. AIDS. 2019;33(6):1043-1052. doi: 10.1097/QAD.0000000000002142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao W, Wang X, Deik AA, et al. Elevated plasma ceramides are associated with antiretroviral therapy use and progression of carotid artery atherosclerosis in HIV infection. Circulation. 2019;139(17):2003-2011. doi: 10.1161/CIRCULATIONAHA.118.037487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havulinna AS, Sysi-Aho M, Hilvo M, et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol. 2016;36(12):2424-2430. doi: 10.1161/ATVBAHA.116.307497 [DOI] [PubMed] [Google Scholar]
- 37.Wang DD, Toledo E, Hruby A, et al. Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevención Con Dieta Mediterránea). Circulation. 2017;135(21):2028-2040. doi: 10.1161/CIRCULATIONAHA.116.024261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laaksonen R, Ekroos K, Sysi-Aho M, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37(25):1967-1976. doi: 10.1093/eurheartj/ehw148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stegemann C, Drozdov I, Shalhoub J, et al. Comparative lipidomics profiling of human atherosclerotic plaques. Circ Cardiovasc Genet. 2011;4(3):232-242. doi: 10.1161/CIRCGENETICS.110.959098 [DOI] [PubMed] [Google Scholar]
- 40.Trevillyan JM, Wong G, Puls R, et al. ; ALTAIR Study Group . Changes in plasma lipidome following initiation of antiretroviral therapy. PLoS One. 2018;13(8):e0202944. doi: 10.1371/journal.pone.0202944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365(3):213-221. doi: 10.1056/NEJMoa1012592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorenz MW, Schaefer C, Steinmetz H, Sitzer M. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? ten-year results from the Carotid Atherosclerosis Progression Study (CAPS). Eur Heart J. 2010;31(16):2041-2048. doi: 10.1093/eurheartj/ehq189 [DOI] [PubMed] [Google Scholar]
- 43.Nambi V, Chambless L, He M, et al. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J. 2012;33(2):183-190. doi: 10.1093/eurheartj/ehr192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanna DB, Moon JY, Haberlen SA, et al. Carotid artery atherosclerosis is associated with mortality in HIV-positive women and men. AIDS. 2018;32(16):2393-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Number of lipid species in each of lipid classes/sub-classes
eTable 2. Associations of traditional blood lipids with risk of carotid artery plaque
eTable 3. Conditional analysis results in the PC and PE lipid classes.
eTable 4. Associations between 12 lipid species and risk of carotid artery plaque among HIV-infected individuals stratified by ART use
eTable 5. Sensitivity analyses by excluding participants with lipid medication use, with illicit drug use, or with HCV infection
eTable 6. Associations of 12 carotid artery plaque-associated lipid species with HIV infection and ART use
eTable 7. Risk of carotid artery plaque discrimination and classification
eTable 8. Associations between lipid sub-network modules and risk of carotid artery plaque by the HIV-infection status
eFigure 1. Coefficient of variations (CVs) and percentage of missing sample size count for 211 lipid species from 16 lipid classes/sub-classes
eFigure 2. Pairwise correlation heatmap of 211 individual lipid species in 11 lipid classes
eFigure 3. Plasma levels of carotid artery associated lipid species according to HIV infection and classes of ART use
eFigure 4. Partial Spearman correlation of carotid artery plaque associated lipid species with CVD and HIV related factors
eFigure 5. Lipid network with blue and pink sub-network modules
eFigure 6. Associations of 211 individual lipid species with risk of carotid artery plaque according to numbers of carbon atoms and double bond contents