This preplanned secondary analysis of a randomized clinical trial assesses the 5- and 10-year incremental cost-effectiveness ratios of ranibizumab therapy compared with panretinal photocoagulation for treating patients diagnosed with proliferative diabetic retinopathy.
Key Points
Question
What is the incremental cost-effectiveness ratio of therapy with ranibizumab, 0.5 mg, compared with panretinal photocoagulation at 5- and 10-year horizons for treating patients diagnosed with proliferative diabetic retinopathy?
Findings
This preplanned secondary analysis of a randomized clinical trial found that the estimated 10-year incremental cost-effectiveness ratio of the ranibizumab group compared with panretinal photocoagulation for those without center-involved diabetic macular edema at baseline is $742 202 per quality-adjusted life-year, and for those with baseline center-involved diabetic macular edema, $63 930 per quality-adjusted life-year.
Meaning
This study’s findings suggest that, at the 5- and 10-year horizons, therapy with ranibizumab, 0.5 mg, may be within the frequently cited range considered cost-effective in the United States for treating patients with proliferative diabetic retinopathy and with baseline center-involved diabetic macular edema and vision loss but not for those without.
Abstract
Importance
The DRCR Retina Network Protocol S randomized clinical trial suggested that the mean visual acuity of eyes with proliferative diabetic retinopathy (PDR) treated with ranibizumab is not worse at 5 years than that of eyes treated with panretinal photocoagulation (PRP). Moreover, the ranibizumab group had fewer new cases of diabetic macular edema (DME) with vision loss or vitrectomy but had 4 times the number of injections and 3 times the number of visits. Although 2-year cost-effectiveness results of Protocol S were previously identified, incorporating 5-year data from Protocol S could alter the longer-term cost-effectiveness of the treatment strategies from the perspective of the health care system.
Objective
To evaluate 5- and 10-year cost-effectiveness of therapy with ranibizumab, 0.5 mg, compared with PRP for treating PDR.
Design, Setting, and Participants
A preplanned secondary analysis of the Protocol S randomized clinical trial using efficacy, safety, and resource utilization data through 5 years of follow-up for 213 adults diagnosed with PDR and simulating results through 10 years.
Interventions
Intravitreous ranibizumab, 0.5 mg, at baseline and as frequently as every 4 weeks based on a structured retreatment protocol vs PRP at baseline for PDR; eyes in both groups could receive ranibizumab for concomitant DME with vision loss.
Main Outcomes and Measures
Incremental cost-effectiveness ratios (ICERs) of ranibizumab therapy compared with PRP were evaluated for those with and without center-involved DME (CI-DME) and vision loss (Snellen equivalent, 20/32 or worse) at baseline.
Results
The study included 213 adults with a mean (SD) age of 53 (12) years, of whom 92 (43%) were women and 155 (73%) were white. The ICER of the ranibizumab group compared with PRP for patients without CI-DME at baseline was $582 268 per quality-adjusted life-year (QALY) at 5 years and $742 202/QALY at 10 years. For patients with baseline CI-DME, ICERs were $65 576/QALY at 5 years and $63 930/QALY at 10 years.
Conclusions and Relevance
This study suggests that during 5 to 10 years of treatment, ranibizumab, 0.5 mg, as given in the studied trial compared with PRP may be within the frequently cited range considered cost-effective in the United States for eyes presenting with PDR and vision-impairing CI-DME, but not for those with PDR but without vision-impairing CI-DME. Substantial reductions in anti–vascular endothelial growth factor cost may make the ranibizumab therapy cost-effective within this range even for patients without baseline CI-DME.
Trial Registration
ClinicalTrials.gov identifier: NCT01489189
Introduction
The DRCR Retina Network Protocol S 5-year outcomes showed that anti–vascular endothelial growth factor (anti-VEGF) therapy with ranibizumab, 0.5 mg, vs panretinal photocoagulation (PRP) for eyes with proliferative diabetic retinopathy (PDR) results in good mean visual acuity for eyes in both groups.1 Many of the outcomes at 2 years were sustained through 5 years, supporting the 2-year conclusion that ranibizumab therapy or PRP are both viable treatments for patients with PDR.2 The 5-year study had a high loss to follow-up in both groups, bringing into question whether the groups remained comparable at 5 years. Nevertheless, outcomes favoring the ranibizumab group included a decreased development of center-involved diabetic macular edema (CI-DME) associated with visual acuity loss (Snellen equivalent of 20/32 or worse) and a trend toward decreased development of retinal detachments. In contrast, outcomes favoring PRP included one-third the number of visits and one-quarter the number of injections. Only approximately 6% of eyes in each group had substantial visual acuity loss, and both groups experienced progressive visual field loss in years 3 through 5, diminishing the difference in visual field loss between the groups at 5 years compared with 2-year results. A smaller percentage of eyes in the ranibizumab group had a vitreous hemorrhage at 2 years, although 50% of the eyes in each group experienced at least 1 vitreous hemorrhage by 5 years.
The 2-year cost-effectiveness analysis of Protocol S showed that ranibizumab therapy compared with PRP was unlikely to be cost-effective for patients with PDR without CI-DME and vision loss but was possibly cost-effective for patients with PDR and CI-DME with vision loss who were receiving anti-VEGF therapy for DME.3 It was not appropriate to expand these findings across 5 to 10 years without knowing the visual acuity, number of visits and injections, incidence of CI-DME with vision loss, and safety outcomes beyond 2 years. The 5-year relative cost-effectiveness, from the health care system perspective, and an estimate of the 10-year cost-effectiveness using simulations based on 5-year outcomes may be calculated from the Protocol S 5-year results.
Methods
Overview
The DRCR Retina Network Protocol S randomized clinical trial was undertaken from February 2012 through February 2018 at 55 clinical sites in the United States.1 All participants had PDR in the study eye. In the efficacy analysis, both eyes were eligible; however, because it is difficult to allocate costs and improved quality-of-life by eye, this analysis included only 213 individuals with 1 study eye (70% of the study participants).
The ranibizumab group was treated as often as monthly, and the PRP group received PRP at baseline and supplemental PRP during follow-up if neovascularization worsened. All eyes could receive ranibizumab, 0.5 mg, for vision-impairing CI-DME. Analyses were conducted separately for individuals with and without vision-impairing CI-DME at baseline. Multiple institutional review boards approved the study, and all patients provided written informed consent to participate. The study protocol and the statistical analysis plan were previously published.1
Analysis
Eyes had best-corrected visual acuity measured at baseline and every 16 weeks. The protocol specified collection of resource utilization related to ocular conditions and systemic adverse events for cost-utility analysis. Because the association of ocular use of anti-VEGF agents with systemic adverse events was not strong, costs of systemic adverse events were not included in the base analyses but were included in a sensitivity analysis. Data were collected through 5 years, and 95% CIs were calculated. Outcomes beyond the 5-year period were simulated up to a 10-year horizon. Assuming that vision remained constant, injections continued at the same frequency observed in year 5, and (in a sensitivity analysis) long-term serious adverse events that occurred in years 1 through 5 (cerebrovascular accident and myocardial infarction) continued to have long-term costs of care. These assumptions were varied in sensitivity analyses.
Costs
Costs were applied to the resources used that were captured in the trial. The unit costs, including physician and facility fees, were based on the 2018 Medicare fee schedule of allowable charges. A dose of ranibizumab, 0.5 mg, cost $1866.82; the injection procedure, $104.40; and PRP, $351.00.4,5 Costs were grouped into clinic visits or diagnostic procedures, study procedures, intraocular therapies, and (in the sensitivity analysis) systemic adverse events. Short- and long-term costs for cerebrovascular accidents and myocardial infarction were based on the literature.6 Costs were considered from the health system perspective and were measured in 2018 US dollars, and future costs were discounted by 3% annually (eTables 1 and 2 in the Supplement). Baseline characteristics of the study population are provided in eTable 3 in the Supplement.
Health Utility
Health-related quality of life was measured using 2 methods. For the main analysis, visual acuities from the better-seeing eye at the 16-, 32-, 52-, 68-, 84-, 104-, 156-, 208-, and 260-week visits were converted into quality-adjusted life-years (QALYs) using the methods of Brown et al7 and were compared with the baseline utility associated with the participant’s best-corrected visual acuity in the better-seeing eye at randomization (eTable 1 in the Supplement). This approach is commonly used but is limited by the top of the scale being anchored at perfect vision instead of perfect health.8 The second approach used the best-corrected visual acuities from the study eye and was anchored at perfect health.9 In both methods, if an observation was missing, the last observation was carried forward. The QALYs gained were discounted by 3% annually.
Extrapolation of Longer-term Results
Resource use, costs, and QALYs were aggregated across the 5 years of the trial based on observed data. Additional outcomes were simulated from years 5 through 10, making assumptions about continuing cost and vision. Rates of injections in year 5 in both groups were assumed to remain constant throughout years 6 through 10. Specifically, eyes in the ranibizumab group would continue to receive 3 ranibizumab injections per year and those in the PRP group would continue to receive a mean (SD) of 0.09 (0.283) ranibizumab injections per year for eyes without CI-DME and vision loss at baseline and a mean (SD) of 0.9 (0.276) injections per year for eyes with CI-DME and vision loss at baseline. In a sensitivity analysis, individuals who experienced serious systemic events in the first 5 years were assumed to have continuing care for those conditions through year 10. The last observed visual acuity (and utility) was assumed to remain the same through year 10. These assumptions were varied in sensitivity analyses.
The main outcome was the incremental cost-effectiveness ratio (ICER), which was calculated by taking the incremental total cost of the ranibizumab group vs the PRP group and dividing by incremental overall QALYs gained of ranibizumab vs PRP. The ICERs were computed for subgroups with and without concomitant baseline DME and vision loss. A lower ICER indicated better cost-effectiveness. Although there is no strict threshold for cost-effectiveness in the United States, values of $50 000, $100 000, and $150 000/QALY were focused on because they are frequently cited as being cost-effective.10
Sensitivity Analysis
Sensitivity analyses were undertaken to show how results were sensitive to input assumptions. One-way sensitivity analyses identified the variables that were most influential on the results. Probabilistic sensitivity analysis, in which all parameters were varied simultaneously using Monte Carlo simulation to better understand the overall uncertainty and confidence in the results.11 In the probabilistic sensitivity analysis, synthetic trial results were created by bootstrapping individuals from the trial,12 and randomly selecting parameter values (unit costs and vision-to-utility conversion values). The Monte Carlo simulation used 10 000 iterations to create cost-effectiveness acceptability curves.
Results
The study included 213 adults with a mean (SD) age of 53 (12) years, of whom 92 (43%) were women, 121 (57%) were men, and 155 (73%) were white.
Costs
For participants without baseline CI-DME and vision loss over a 5-year period, the mean (SD) total costs were $8887 ($10 375) for the PRP group and $32 300 ($19 903) for the ranibizumab group (difference, $23 413 [95% CI, $18 567-$28 258]). For participants with baseline CI-DME and vision loss, the mean (SD) costs were $22 355 ($18 730) for the PRP group and $40 825 ($23 457) for the ranibizumab group (difference, $18 470 [95% CI, $6320-$30 619]).
Costs increased during a 10-year period. For participants without baseline CI-DME and vision loss, the simulated mean (SD) cost for the PRP group was $9509 ($10 394) and $53 183 ($19 789) for the ranibizumab group (difference, $43 675 [95% CI, $38 849-$48 500]). For participants with baseline CI-DME and vision loss, the simulated mean total costs for participants in the PRP group were $28 889 ($18 928) and for the ranibizumab group, $60 979 ($21 667) (difference, $32 090 [95% CI, $20 486-$43 693]). Most costs were for the ranibizumab therapy or treatment of systemic adverse events (Table 1).
Table 1. Mean per-Patient Costs of Treatment With Ranibizumab, 0.5 mg, and PRP.
| Variable | With Vision-Impairing CI-DME at Baselinea | Without Vision-Impairing CI-DME at Baseline | ||
|---|---|---|---|---|
| PRP for PDR, Ranibizumab for CI-DME (n = 25) | Ranibizumab for PDR and CI-DME (n = 21) | PRP (n = 87) | Ranibizumab (n = 80) | |
| At 5 y of follow-up, $ | ||||
| Clinic visits/diagnostic procedures | 1282 | 1954 | 1051 | 1971 |
| PRP | 351 | 16 | 351 | 21 |
| Anti-VEGF injection procedureb | 947 | 1916 | 255 | 1542 |
| Ranibizumab, 0.5 mg, therapy | 16 925 | 34 264 | 4562 | 27 572 |
| Vitrectomies | 1096 | 1336 | 1404 | 387 |
| Other intraocular therapiesc | 1755 | 1338 | 1264 | 807 |
| Total (range) | 22 355 (15 013-29 697) | 40 825 (30 793-50 858) | 8887 (6707-11 067) | 32 300 (27 938-36 661) |
| Simulated 10 y, $ | ||||
| Additional costs of ranibizumab injection procedures and drug | 6534 | 20 154 | 621 | 20 883 |
| Total (range) | 28 889 (21 470-36 309) | 60 979 (51 712-70 246) | 9509 (7324-11 693) | 53 183 (48 847-57 520) |
Abbreviations: anti-VEGF, anti–vascular endothelial growth factor; CI-DME, center-involved diabetic macular edema; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation.
With visual acuity letter score less than 78 (approximate Snellen equivalent, 20/32 or worse) at baseline.
Participants in all groups received ranibizumab, 0.5 mg, if they developed DME during the trial.
Includes treatment for ocular adverse events, such as endophthalmitis.
Health Utilities
For participants without CI-DME causing vision loss at baseline, the improvement from ranibizumab over PRP was 0.04 QALYs (95% CI, −0.019 to 0.099) at 5 years and 0.059 QALYs (95% CI, −0.059 to 0.177) at 10 years. For those with CI-DME causing vision loss at baseline, the improvement was 0.282 QALYs (95% CI, 0.11-0.454) at 5 years and 0.502 QALYs (95% CI, 0.169-0.835) at 10 years (Table 2). Differences using the QALY calculation method based on the treated eye showed similar or smaller differences (eTables 4 and 5 in the Supplement).
Table 2. Change in QALYs and Cost-effectiveness Results (Utilities Converted From Visual Acuity in the Better-Seeing Eye)a.
| Cost-effectiveness Parameter | With Vision-Impairing DME at Baselineb | Without Vision-Impairing CI-DME at Baseline | ||||
|---|---|---|---|---|---|---|
| PRP for PDR and Ranibizumab for CI-DME (n = 25) | Ranibizumab, 0.5 mg, for PDR and CI-DME (n = 21) | Difference | PRP (n = 87) | Ranibizumab, 0.5 mg (n = 80) | Difference | |
| At 5 yb | ||||||
| Mean costs (95% CI), $ | 22 355 | 40 825 | 18 470 (6320 to 30 619) |
8887 | 32 300 | 23 413 (18 567 to 28 258) |
| QALYs (95% CI) | 0.02 | 0.30 | 0.282 (0.11 to 0.454) |
0.015 | 0.055 | 0.04 (−0.019 to 0.099) |
| ICER, $ | NA | NA | 65 576 | NA | NA | 582 268 |
| At 10 yb | ||||||
| Mean costs (95% CI), $ | 28 889 | 60 979 | 32 090 (20 486 to 43 693) |
9509 | 53 183 | 43 675 (38 849 to 48 500) |
| QALYs (95% CI) | 0.04 | 0.54 | 0.502 (0.169 to 0.835) |
0.040 | 0.098 | 0.059 (−0.059 to 0.177) |
| ICER, $ | NA | NA | 63 930 | NA | NA | 742 202 |
Abbreviations: CI-DME, center-involved diabetic macular edema; DME, diabetic macular edema; ICER, incremental cost-effectiveness ratio; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation; QALYs, quality-adjusted life-years.
Individuals who died had a utility of 0 after the date of death. In converting from best-corrected visual acuity, letter scores were converted to Snellen visual acuities and then to utility levels using the mapping from Brown et al.7
Participants received ranibizumab, 0.5 mg, or PRP for PDR with and without concomitant baseline DME (utilities converted from visual acuity in the better-seeing eye).
Cost-effectiveness
Treatment with ranibizumab vs PRP in participants without CI-DME at baseline causing vision loss had an ICER of $582 268/QALY at 5 years and $742 202/QALY at 10 years. For participants with CI-DME at baseline causing vision loss, the ICER of ranibizumab vs PRP was $65 576/QALY at 5 years and $63 930/QALY at 10 years. Estimates of the uncertainty in these values are presented in the next section with the probabilistic sensitivity analysis.
Sensitivity Analyses
A sensitivity analysis that included costs related to systemic adverse events was performed because rates of systemic adverse events were slightly higher in the ranibizumab group. The ICERs increased slightly once systemic adverse events were included (eTable 6 in the Supplement). For participants with CI-DME and vision loss at baseline, incremental costs increased by $10 000 at 5 years and $7000 at 10 years, leading to ICERs of $106 289/QALY at 5 years and $92 114/QALY at 10 years. The ICERs for participants without CI-DME and vision loss at baseline also had slight increases to $637 613/QALY at 5 years and $812 185/QALY at 10 years (eTable 6 in the Supplement).
In 1-way sensitivity analyses, most parameter changes resulted in an ICER for those with CI-DME and vision loss remaining between $50 000 and $85 000/QALY. However, if only 1.5 annual ranibizumab injections were required after the fifth year, the ICER for participants with CI-DME and vision loss would decrease to $43 854/QALY. If the utility associated with good vision (20/20 to 20/25) were better or utility with moderate vision loss (20/50 to 20/200) were worse, then the ICER for participants with CI-DME and vision loss would decrease to approximately $50 000/QALY (eTable 7a and eFigure 1 in the Supplement). The 1-way sensitivity analyses showed ranibizumab was not likely to be cost-effective for those without baseline CI-DME and vision loss. If only 1.5 annual ranibizumab injections were required after the fifth year, the ICER for participants without CI-DME and vision loss would decrease to $375 100/QALY (eTables 7a and 7b in the Supplement).
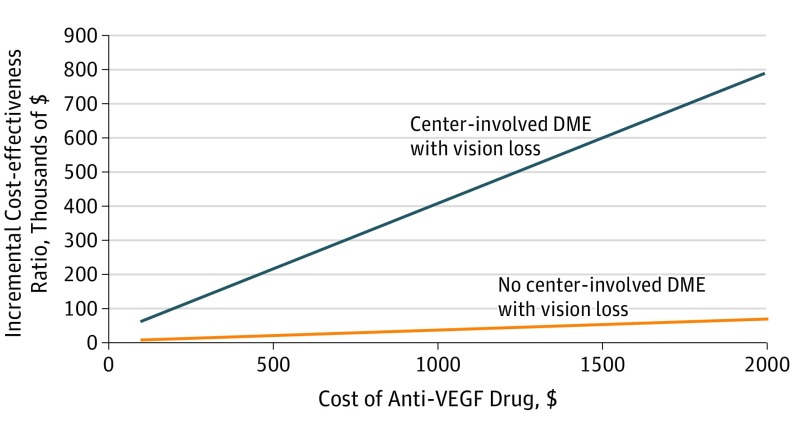
Figure 1 shows how the cost of ranibizumab injections altered the ICER. To have an ICER below $100 000/QALY gained, injections of ranibizumab, 0.5 mg, must cost less than $198 per dose for patients without baseline CI-DME and vision loss and less than $2988 for those with CI-DME and vision loss. eFigure 2 in the Supplement shows similar results using utility conversion based on the treated eye.
Figure 1. One-Way Sensitivity Analysis of Varying Cost of Anti–Vascular Endothelial Growth Factor (Anti-VEGF) Assuming Effectiveness of Ranibizumab, 0.5 mg.
The incremental cost-effectiveness ratios of anti-VEGF therapy vs panretinal photocoagulation. Although there are no official thresholds of cost-effectiveness in the United States, interventions costing less than $50 000 to $150 000 may be considered cost-effective.7 The orange line represents results for patients with center-involved diabetic macular edema (DME) and vision loss; the blue line, patients without center-involved DME and vision loss.
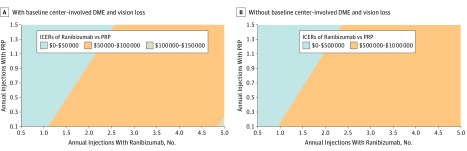
Figure 2A shows how the number of annual injections after 5 years affected cost-effectiveness. If fewer than 2 injections of ranibizumab are required per year, then ranibizumab would have an ICER less than $50 000/QALY for participants with baseline CI-DME and vision loss. The ICERs remained high for participants without baseline CI-DME and vision loss (Figure 2B). eFigures 3a and 3b in the Supplement showed similar results using utility conversion based on the treated eye.
Figure 2. Two-Way Sensitivity Analysis of Number of Annual Injections With and Without Baseline Center-Involved Diabetic Macular Edema (DME) and Vision Loss.
The colors in the figures represent the incremental cost-effectiveness ratios (ICERs) of therapy with ranibizumab, 0.5 mg, vs panretinal photocoagulation (PRP). Although there are no official thresholds of cost-effectiveness in the United States, interventions costing less than $50 000 to $150 000 may be considered cost-effective.7 The color scales are an order of magnitude different.
Two-way sensitivity analyses on price vs utility (eFigure 4 in the Supplement), utility vs effectiveness (eFigure 5 in the Supplement), and price vs effectiveness (eFigure 6 in the Supplement), showed conditions under which ranibizumab may be cost-effective for those with baseline CI-DME and vision loss. Ranibizumab may be more cost-effective for combinations of patients with worse utility associated with moderate vision loss, lower ranibizumab prices, or better long-term vision outcomes with ranibizumab therapy.
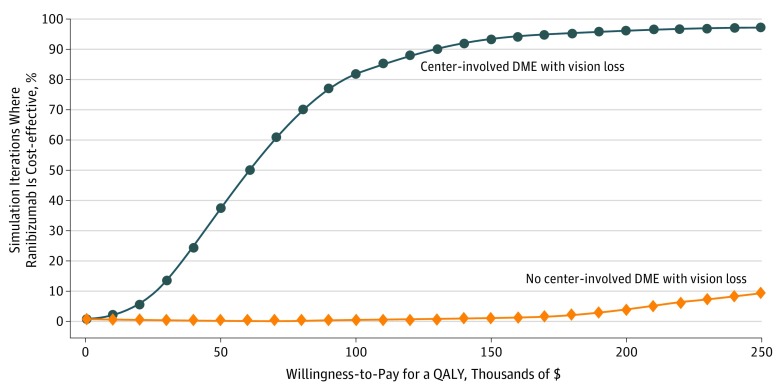
The cost-effectiveness acceptability curves generated as part of the probabilistic sensitivity analysis showed the probability that ranibizumab injections may be cost-effective for various levels of willingness to pay for QALYs while taking into account the overall parameter uncertainty. Figure 3 shows that ranibizumab injections have a 37% chance of being cost-effective at a threshold of $50 000/QALY, 82% at a threshold of $100 000/QALY, and 93% at $150 000/QALY for participants with CI-DME and vision loss. There was only a 9% chance that ranibizumab injections may be cost-effective at a high threshold of $250 000/QALY for those without CI-DME and vision loss. Similar results using utility conversion based on the treated eye are shown in eFigure 7 in the Supplement.
Figure 3. Cost-effectiveness Acceptability Curves.
Results of the Monte Carlo simulation, which simultaneously varied all parameter estimates and created synthetic populations of patients from the trial using bootstrapping. The lines represent the fraction of simulation iterations in which ranibizumab was cost-effective compared with panretinal photocoagulation (y-axis) at various levels of willingness to pay for quality-adjusted life-year (QALY) gains (x-axis). The orange line represents results for patients with center-involved diabetic macular edema (DME) and vision; the blue line, patients without center-involved DME and vision loss.
Discussion
Based on this analysis of Protocol S across a 5- or 10-year horizon, treatment with ranibizumab injections is likely to be cost-effective for patients with PDR and CI-DME causing vision loss at baseline for whom ranibizumab therapy would be administered to treat the DME. In contrast, it is unlikely that ranibizumab injections compared with PRP would be cost-effective for patients with PDR but without CI-DME causing vision loss at the time of initiating treatment for PDR across a 5- to 10-year horizon. Results are consistent with our prior analysis, which used study results through 2 years.3
The cost of ranibizumab is substantially associated with the cost-effectiveness of ranibizumab therapy for treating PDR. Although ranibizumab, 0.3 mg, is available and less expensive, Protocol S used ranibizumab, 0.5 mg; therefore, the costs of the 0.5-mg dose were used for this analysis. While use of the 0.3-mg dose may reduce costs, it is unknown how this dosage would alter the effectiveness of the drug; therefore, we cannot calculate with certainty how the use of ranibizumab, 0.3 mg, would have altered the ICERs reported in this study. It is also difficult to extrapolate this cost-effectiveness analysis to other readily available anti-VEGF agents, including aflibercept or bevacizumab because the 2- to 5-year outcomes of those agents with regard to visual acuity, number of visits or injections, incidence of DME with vision loss, vitrectomy for vitreous hemorrhage or traction retinal detachment, or peripheral visual field loss are unknown at this time. However, if other anti-VEGF agents were equally as effective as ranibizumab, 0.5 mg, but cost much less (eg, $50 per dose, which is close to the cost of intravitreous bevacizumab in the United States and in many other countries), then anti-VEGF therapy may be cost-effective even for those without DME causing vision loss at baseline (Figure 1 and eFigure 1 in the Supplement). In addition, ranibizumab may be more cost-effective for certain subgroups of patients with CI-DME. For example, for patients with greater fear concerning vision loss (lower utility with moderate vision loss), ranibizumab may be more cost-effective. For patients without CI-DME, we did not find subgroups for whom ranibizumab would be cost-effective.
The probabilistic sensitivity analysis (Figure 3 and eFigure 7 in the Supplement) showed that there is substantial uncertainty in the precise ICER of ranibizumab therapy vs PRP for patients with PDR and CI-DME causing vision loss at baseline. This is likely because there were only 46 patients enrolled whose 1 eye also had baseline CI-DME and vision loss.
Strengths and Limitations
We are unaware of other large randomized clinical trials with a follow-up of 5 years evaluating anti-VEGF vs PRP for PDR with which to compare these results. However, the strengths of this study include random assignment of a relatively large cohort, excellent adherence to treatment regimens with few protocol deviations that could likely alter the outcomes analyzed, and prospective collection of data for this preplanned secondary outcome on cost-effectiveness through 5 years. Considering the stability of visual acuity, number of injections, and number of PRP sessions in years 3 through 5, there is greater confidence to the assumptions made when simulating the 10-year cost-effectiveness results.
Limitations of this analysis include the large proportion of individuals in each group lost to follow-up, although post hoc analyses of these groups did not identify obvious differences among the 5-year completers in each group.1 Also, these 5-year results and simulations for 10-year horizons used best-corrected visual acuity in the better-seeing eye or the study eye as a surrogate for overall health-related quality of life. This surrogate outcome may not capture all aspects of quality of well-being associated with receiving these interventions and do not capture the effects of these therapies on measurements of peripheral visual fields. There are also concerns comparing utilities anchored at perfect vision with cost-effectiveness thresholds anchored at perfect health.8 When we used a method with utilities anchored at perfect health (eTable 5 in the Supplement), ranibizumab therapy became less cost-effective. Furthermore, only direct medical costs of select events were captured rather than other costs, such as caregiver burden, transportation costs to visits, and costs associated with time away from work. These latter variables may have an adverse effect on the cost-effectiveness in the ranibizumab group, which had a 3-fold greater number of injections and a 4-fold greater number of visits compared with the PRP group at 5 years. These additional burdens could make ranibizumab less desirable from the perspective of patients. In addition, when PRP was performed at the time of this study, there was a 90-day global period before payment for additional PRP could be given. In 2019, the reimbursement for PRP is much less, and the global period is 10 days. It is unclear how or whether these changes may affect costs associated with PRP in routine clinical care, but they do not affect the efficacy of PRP as performed in this study. An additional limitation, the number of eyes with CI-DME with vision loss at baseline was small in this study, although the sensitivity analyses, particularly the probabilistic sensitivity analysis, explore some of that uncertainty. Finally, as noted for the cost-effectiveness analysis at 2 years, the use of investigator discretion to assess CI-DME treatment after the baseline visit may affect these 5-year cost-effectiveness analyses.
Conclusions
Our analysis suggests that ranibizumab, 0.5 mg, vs PRP as given in this trial for treating PDR is within the $50 000/QALY to $150 000/QALY range, which is frequently cited as cost-effective in the United States for eyes presenting with, but not for those without, PDR and CI-DME causing vision loss at 5 years and a simulated 10-year horizon. A lower price for an equally effective or more efficacious drug could also make anti-VEGF cost-effective as a PDR treatment for individuals without baseline CI-DME and vision loss. Because the 2- and 5-year outcomes from Protocol S support ranibizumab therapy or PRP as viable treatments for PDR,1,2 when patient-specific factors, including anticipated visit compliance, cost, and frequency of visits, are taken into account, this cost-effectiveness analysis through 5 years and simulated for a 10-year horizon may be considered when choosing treatments for PDR.
eTable 1. Input Parameters
eTable 2. Details on Procedure Costing
eTable 3. Baseline Characteristics of the Study Population
eTable 4. Change in QALYs
eTable 5. Cost-effectiveness Results
eTable 6. Scenario Analysis: Including Costs of Systemic Adverse Events
eTable 7. Incremental Cost-effectiveness Ratio Values as Each Parameter Assumption is Changed From Low to High, One-at-a-Time
eFigure 1. Two-Way Sensitivity Analysis on Utility With Good Vision (20/20–20/25) Versus Moderate Vision Loss (20/50- 20/200) With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 2. One-Way Sensitivity Analysis Varying Cost of Anti–Vascular Endothelial Growth Factor With Utilities Converted From Visual Acuity in Treated Eye Assuming Effectiveness of 0.5-mg Ranibizumab
eFigure 3. Two-Way Sensitivity Analysis on Number of Annual Injections With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 4. Two-Way Sensitivity Analysis on Utility With Moderate Vision Loss (20/50–20/200) Versus Cost of Anti-VEGF Drug Injections With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 5. Two-Way Sensitivity Analysis on Utility With Moderate Vision Loss (20/50–20/200) Versus Effectiveness in Terms of Long-term Improvements in Vision With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 6. Two-Way Sensitivity Analysis on Cost of Anti-VEGF Drug Versus Effectiveness in Terms of Long-term Improvements in Vision With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 7. Cost-effectiveness Acceptability Curves With Utilities Converted From Visual Acuity in Treated Eye
eReferences.
References
- 1.Gross JG, Glassman AR, Liu D, et al. ; Diabetic Retinopathy Clinical Research Network . Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-1148. doi: 10.1001/jamaophthalmol.2018.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross JG, Glassman AR, Jampol LM, et al. ; Writing Committee for the Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. doi: 10.1001/jama.2015.15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutton DW, Stein JD, Bressler NM, Jampol LM, Browning D, Glassman AR; Diabetic Retinopathy Clinical Research Network . Cost-effectiveness of intravitreous ranibizumab compared with panretinal photocoagulation for proliferative diabetic retinopathy: secondary analysis from a Diabetic Retinopathy Clinical Research Network Randomized Clinical Trial. JAMA Ophthalmol. 2017;135(6):576-584. doi: 10.1001/jamaophthalmol.2017.0837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Medicare and Medicaid Services Medicare ASP drug pricing files. 2018; https://www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/Downloads/2018-Oct-ASP-Pricing-File.zip. Accessed December 28, 2018.
- 5.Centers for Medicare and Medicaid Services Medicare fee for service payment: physician fee schedule. https://www.cms.gov/Medicare/Medicare-Fee-For-Service-Payment/PhysicianFeeSched/Index.html. Published 2018. Accessed November 1, 2018.
- 6.Bonafede MM, Johnson BH, Richhariya A, Gandra SR. Medical costs associated with cardiovascular events among high-risk patients with hyperlipidemia. Clinicoecon Outcomes Res. 2015;7:337-345. doi: 10.2147/CEOR.S76972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48(2):204-223. doi: 10.1016/S0039-6257(02)00457-5 [DOI] [PubMed] [Google Scholar]
- 8.Lee BS, Kymes SM. Re: Brown et al.: Cataract surgery cost utility revisited in 2012: a new economic paradigm (Ophthalmology. 2013;120:2367-76). Ophthalmology. 2015;122(3):e18. doi: 10.1016/j.ophtha.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 9.Mitchell P, Annemans L, Gallagher M, et al. . Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol. 2012;96(5):688-693. doi: 10.1136/bjophthalmol-2011-300726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 11.Taylor M. What Is Sensitivity Analysis. Heslington, England: York Health Economics Consortium, University of York; 2009:1-8. [Google Scholar]
- 12.Lord J, Asante MA. Estimating uncertainty ranges for costs by the bootstrap procedure combined with probabilistic sensitivity analysis. Health Econ. 1999;8(4):323-333. doi: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Input Parameters
eTable 2. Details on Procedure Costing
eTable 3. Baseline Characteristics of the Study Population
eTable 4. Change in QALYs
eTable 5. Cost-effectiveness Results
eTable 6. Scenario Analysis: Including Costs of Systemic Adverse Events
eTable 7. Incremental Cost-effectiveness Ratio Values as Each Parameter Assumption is Changed From Low to High, One-at-a-Time
eFigure 1. Two-Way Sensitivity Analysis on Utility With Good Vision (20/20–20/25) Versus Moderate Vision Loss (20/50- 20/200) With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 2. One-Way Sensitivity Analysis Varying Cost of Anti–Vascular Endothelial Growth Factor With Utilities Converted From Visual Acuity in Treated Eye Assuming Effectiveness of 0.5-mg Ranibizumab
eFigure 3. Two-Way Sensitivity Analysis on Number of Annual Injections With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 4. Two-Way Sensitivity Analysis on Utility With Moderate Vision Loss (20/50–20/200) Versus Cost of Anti-VEGF Drug Injections With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 5. Two-Way Sensitivity Analysis on Utility With Moderate Vision Loss (20/50–20/200) Versus Effectiveness in Terms of Long-term Improvements in Vision With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 6. Two-Way Sensitivity Analysis on Cost of Anti-VEGF Drug Versus Effectiveness in Terms of Long-term Improvements in Vision With and Without Baseline Center-Involved Diabetic Macular Edema and Vision Loss
eFigure 7. Cost-effectiveness Acceptability Curves With Utilities Converted From Visual Acuity in Treated Eye
eReferences.