Abstract
Recent advances in techniques to differentiate human induced pluripotent stem cells (hiPSCs) hold the promise of an unlimited supply of human derived cardiac cells from both healthy and disease populations. That promise has been tempered by the observation that hiPSC-derived cardiomyocytes (hiPSC-CMs) typically retain a fetal-like phenotype, raising concern about the translatability of the in vitro data obtained to drug safety, discovery, and development studies. The Biowire II platform was used to generate 3D engineered cardiac tissues (ECTs) from hiPSC-CMs and cardiac fibroblasts. Long term electrical stimulation was employed to obtain ECTs that possess a phenotype like that of adult human myocardium including a lack of spontaneous beating, the presence of a positive force-frequency response from 1 to 4 Hz and prominent postrest potentiation. Pharmacology studies were performed in the ECTs to confirm the presence and functionality of pathways that modulate cardiac contractility in humans. Canonical responses were observed for compounds that act via the β-adrenergic/cAMP-mediated pathway, eg, isoproterenol and milrinone; the L-type calcium channel, eg, FPL64176 and nifedipine; and indirectly effect intracellular Ca2+ concentrations, eg, digoxin. Expected positive inotropic responses were observed for compounds that modulate proteins of the cardiac sarcomere, eg, omecamtiv mecarbil and levosimendan. ECTs generated in the Biowire II platform display adult-like properties and have canonical responses to cardiotherapeutic and cardiotoxic agents that affect contractility in humans via a variety of mechanisms. These data demonstrate that this human-based model can be used to assess the effects of novel compounds on contractility early in the drug discovery and development process.
Keywords: cardiomyocytes, contractility, engineered cardiac tissue, drug discovery, drug safety, in vitro models
Cardiovascular disease (CVD) remains a leading cause of morbidity and mortality worldwide (Benjamin et al., 2018). Patients with heart failure (HF) comprise a growing percentage of the CVD population (Benjamin et al., 2018). HF is instigated by both genetic, eg, inherited cardiomyopathy and acquired risk factors eg, coronary heart disease or hypertension (Stienen, 2015). Cardiotoxicity and HF can also be an unwanted consequence of treatment with a range of drugs in clinical usage (McNaughton et al., 2014; Onakpoya et al., 2016; Siramshetty et al., 2016). There remains the need for both novel therapies to prevent and treat HF as well as improved ways to assess the cardiac safety liabilities of candidate drug therapies.
Cardiac contractility is a consequence of a precise series of events known as excitation-contraction coupling (Eisner et al., 2017). This highly orchestrated process links electrical excitation of the surface membrane (the action potential) and changes in the cytoplasmic calcium concentration ([Ca2+]i) to muscle contraction and relaxation (Eisner et al., 2017). Aberrant contractility is a hallmark of HF and of numerous drugs with cardiotoxic effects and contributes to symptoms including dyspnea, fatigue, arrhythmia, and ischemia (Guth et al., 2015). Historically, contractility measurements have been performed using cardiac tissue isolated from patients or animals. The use of isolated tissues poses significant challenges, including limited availability and variability for the former, and species-to-species translation for the latter. These challenges have restricted the utility of these methods and highlight the need for novel models. For in vitro models to faithfully recapitulate this process they should demonstrate functional hallmarks of the electrical, calcium handling, and contractile machinery.
The recent development of robust cardiac differentiation protocols for human induced pluripotent stem cells (hiPSCs) has provided the potential for an unlimited supply of human derived cardiac cells from both healthy and diseased sources, but this potential has been limited by the observation that hiPSC-derived cardiomyocytes (hiPSC-CMs) typically retain a more fetal-like phenotype (Denning et al., 2016; Veerman et al., 2015). This raises concerns about the predictability and translatability of results obtained in vitro to the settings of drug safety, discovery, and development.
In this study, the Biowire II platform was used to generate 3D engineered cardiac tissues (ECTs) from hiPSC-CMs and cardiac fibroblasts and to make nondestructive contractility measurements of the ECTs. We present data demonstrating that ECTs produced using this platform and subjected to long term electrical stimulation have adult-like contractile properties. In addition, we show that contractility in the ECTs can be modulated by a variety of agents known to effect essential intracellular signaling pathways present in the human myocardium. These data demonstrate the utility of the platform as a tool for discovery of novel therapies and testing of drug safety in preclinical development, using a human model of myocardial tissue.
MATERIALS AND METHODS
Generation of ECTs
ECTs were generated in the Biowire II platform as described previously (Zhao et al., 2019). Briefly, 3D ECTs were formed in polystyrene microwells containing parallel poly(octamethylene maleate (anhydride) citrate) (POMaC) wires. Each ECT was comprised of 100 000 viable iCell Cardiomyocytes2 (Cellular Dynamics International, Madison, Wisconsin) and 10 000 human ventricular cardiac fibroblasts (Lonza, Allendale, New Jersey) in a collagen/Matrigel/fibrin gel. Following 7 days in culture, the hiPSC-CMs and cardiac fibroblasts self-organized into 3D ECTs and were suspended between the POMaC wires (Figure 1A). Custom chambers containing parallel carbon electrodes were used to provide electrical field stimulation using biphasic pulses of 2 ms duration, at twice the excitation threshold. Stimulation was started at 1 Hz and increased by 0.1 Hz increments daily to a maximum of 6 Hz. All ECTs used in this study were subjected to the same stimulation protocol for a minimum of 6 weeks. Compound effects on contractility were similar in tissues stimulated from 6 to 9 weeks.
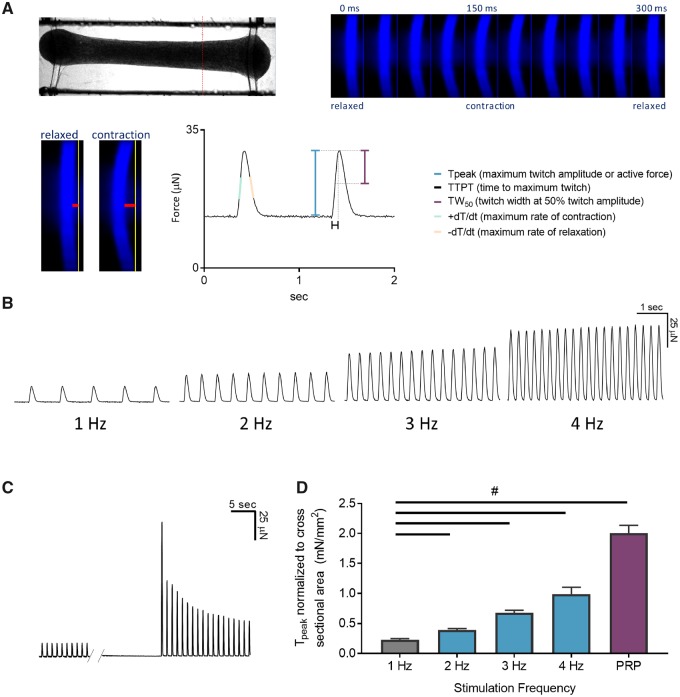
Figure 1.
Engineered cardiac tissues (ECTs) generated in the Biowire II platform have adult-like contractile properties. A, A representative image of an ECT suspended in the Biowire II platform (top left). Twitch force is measured by acquiring a video of the contracting tissues under field stimulation and converting the pixel displacement of the poly(octamethylene maleate (anhydride) citrate) (POMaC) wire (bottom left, red horizontal bars) into a force measurement (Zhao et al., 2019). A time lapse of the POMaC displacement is shown on the top right panel. Once pixel movements are converted to force, parameters of contractility including maximal twitch amplitude or active force (Tpeak), time constants and rates of contraction and relaxation are derived (bottom right). B, ECTs stimulated in the Biowire II platform showed positive force-frequency relationship and (C) prominent postrest potentiation after 6 weeks of electrical stimulation. D, Summary of force-frequency relationship and postrest potentiation normalized to ECT cross-sectional area. (n = 6) ECTs. Data presented as mean ± SEM. #p < .05 using one-way ANOVA.
Image-based contractility measurements
ECT contractility was measured by tracking the deflection of the POMaC wires as a function of time as previously described (Zhao et al., 2019). Stimulated ECTs were placed in a custom chamber containing parallel carbon electrodes to provide external field stimulation in an environmental chamber at 37°C and 5% CO2. ECTs were stimulated at twice the excitation threshold during acquisition. The POMaC wires were illuminated using 350 nm excitation. Videos were acquired at a rate of 100 frames per second using a Zyla 4.2 sCMOS camera (Andor, South Windsor, Connecticut) with a 470 nm emission filter using NIS-Elements Advanced Research software (Nikon Instruments Inc, Edgewood, New York). To measure contractile force, the videos were analyzed using custom software to track the location of the POMaC wire (Figure 1A). The pixel movements were converted to force as previously described (Zhao et al., 2019).
The force-frequency relationship (FFR) was determined by pacing ECTs at 1 Hz and increasing the stimulation frequency by 1 Hz every 30 s to a final frequency of 4 Hz. Following 4 Hz stimulation, the postrest potentiation was determined by turning off the external field stimulation for 10 s and recording the twitch transients after resuming field stimulation at 1 Hz. To determine the cross-sectional area of the tissue, ECTs were fixed in 10% neutral buffered formalin for 2 h followed by 3 washes in phosphate buffered saline. Tissue was cut halfway between the midsection of the tissue and the POMaC wire (red dotted line in Figure 1A). A photo of the transverse section was acquired, and the cross-sectional area was determined using ImageJ. Average tissue cross-sectional area was 0.066 ± 0.001 mm2 (n = 6 ECTs).
Compound testing
At the end of the electrical stimulation protocol, ECTs were assessed for automaticity (ie, spontaneous beat rate), the FFR (active force at 1 Hz through 4 Hz), and postrest potentiation. ECTs without spontaneous activity that exhibited a positive FFR and postrest potentiation were used for compound testing.
Tests were conducted in a 37°C, 5% CO2 environmental chamber under field stimulation at 1 Hz. Tissues were incubated for 30 min in the environmental chamber after which a baseline contractility video was acquired for 30 s. One-third of the tissue culture medium volume (6 ml total) was pipetted from the well containing the ECTs twice to equilibrate the ECTs to the shear stress induced by the procedure. A video of 30 s duration was acquired 10 min after the pipetting (baseline). The test article was added to the well to provide the desired final concentration and one-third of the media volume was pipetted twice to gently mix. The test article stock solutions were prepared in either dimethyl sulfoxide or water as appropriate, and serially diluted in medium. Following 10–30 min of incubation a video of 30 s duration was acquired. The same procedure was followed for all subsequent doses (lowest to highest) such that 1 ECT was incubated with all doses. Contractility videos were analyzed using a custom analysis software. The force at each dose was normalized by dividing the force values in the presence of compound by the baseline force values of the same tissue.
Statistics
Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc, La Jolla, California). Data are presented as mean ± SEM. Results were considered statistically significant (#) if p < .05 using one-way ANOVA followed by a Tukey's post hoc multiple comparison test or where appropriate repeat measure one-way ANOVA followed by Dunnett’s post hoc multiple comparison test. The replicate numbers are indicated in the figure captions. For EC50/IC50 calculation, the nonlinear fit function of GraphPad Prism (Sigmoidal with 3 parameters) was used to find the best fit for the data.
RESULTS
Cardiac ECTs Generated via the Biowire II Platform Have Adult-Like Contractile Properties
The functionality of the contractile machinery was assessed by measuring the frequency-dependent regulation of contractility, ie, the FFR. FFR is a property of the adult heart where a stepwise increase in force is observed when the frequency of stimulation is increased within a physiologically relevant range (Buckley et al., 1972). Prior to electrical stimulation ie, 1-week postseeding, the ECTs were spontaneously beating at approximately 1.5 Hz and had limited capture at 3 and 4 Hz impairing the ability to assess the FFR. After 7 weeks of culture (6 weeks of electrical stimulation), a positive FFR was observed ie, increased force of contraction with increasing stimulation frequencies (Figs. 1B and 1D).
We further investigated the contractile machinery of the ECTs by assessing postrest potentiation of force, an indicator of the capacity of the sarcoplasmic reticulum (SR) to store and release Ca2+. In large mammals and humans, short periods of cardiac rest give rise to an increased force of contraction of the first beats following restimulation. This results from to an increased uptake and subsequent release of Ca2+ from the SR (Pieske et al., 1996). Due to spontaneous beating, we could not assess postrest potentiation at 1-week postseeding. After 6 weeks of electrical stimulation, we observed a significant postrest potentiation of force following 10 s of rest (Figs. 1C and 1D).
In aggregate the data demonstrate that the ECTs manifest contractile parameters that approach that seen in adult human myocardium.
ECTs Generated via Biowire II Display Positive Inotropic Responses to Drugs That Modulate Messenger 3′-5′-Cyclic Adenosine Monophosphate Production and Degradation
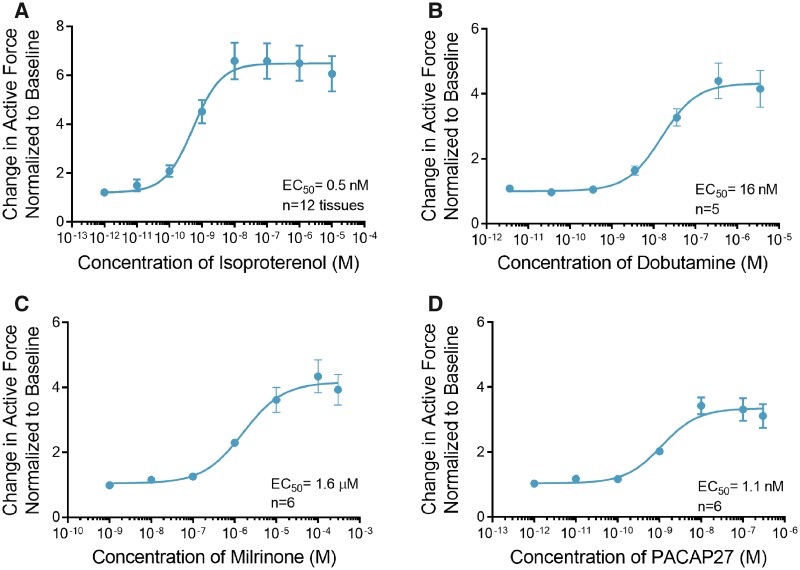
β-adrenergic signaling is an important physiological regulator of cardiac contractility (de Lucia et al., 2018). It results in an increase in the production of the intracellular second messenger cyclic adenosine monophosphate (cAMP) and a positive inotropic response (Bers, 2008). ECTs treated with the β-agonist isoproterenol showed a maximal 6.6 ± 0.8-fold increase in contractile force at a concentration of 10 nM with a half maximal effective concentration (EC50) of 0.5 nM (Figure 2A, time constants and rates of contraction and relaxation are summarized in Supplemental Table 1). Similarly, addition of dobutamine, a predominantly β1 agonist increased the maximal contractile force by 4.4 ± 0.6-fold at 360 nM with EC50 of 16 nM (Figure 2B).
Figure 2.
Engineered cardiac tissues (ECTs) respond to effectors of the cyclic adenosine monophosphate (cAMP) signaling pathway. A, Treatment with isoproterenol induced an increase in contractile force with EC50 of 0.5 nM (n = 12). B, Like isoproterenol, dobutamine induced an increase in contractile force with EC50 of 16 nM (n = 5). C, Milrinone, a phosphodiesterase-3 (PDE3) inhibitor also increased contractile force with EC50 of 1.6 µM (n = 6). D, Treatment with pituitary adenylate cyclase-activating peptide (PACAP27) induced an increase in contractile force with EC50 of 1.1 nM (n = 6). Data are presented as mean ± SEM.
Further, we investigated the intracellular pathways involved in adrenergic signaling, using small molecule modulators that effect distinct signaling pathways. Degradation of cAMP is mediated by a cAMP-dependent enzyme, phosphodiesterase-3 (PDE3). We observed a maximal 4.3 ± 1.2-fold increase in the force of contraction when ECTs were treated with the PDE3 inhibitor milrinone at a concentration of 100 µM with an EC50 of 1.6 μM (Figure 2C, Supplemental Table 1).
Pituitary adenylate cyclase-activating polypeptide (PACAP27) is a 27-amino acid peptide that activates adenylyl cyclase via binding to its cognate G-protein-coupled receptor. We observed a maximal 3.4 ± 1.1-fold increase in force of contraction when ECTs were treated with 10 nM PACAP27 with an EC50 of 1.1 nM (Figure 2D, Supplemental Table 1).
ECTs Have Functional L-Type Ca2+ Channels, SR, and Na+-Ca2+ Exchangers
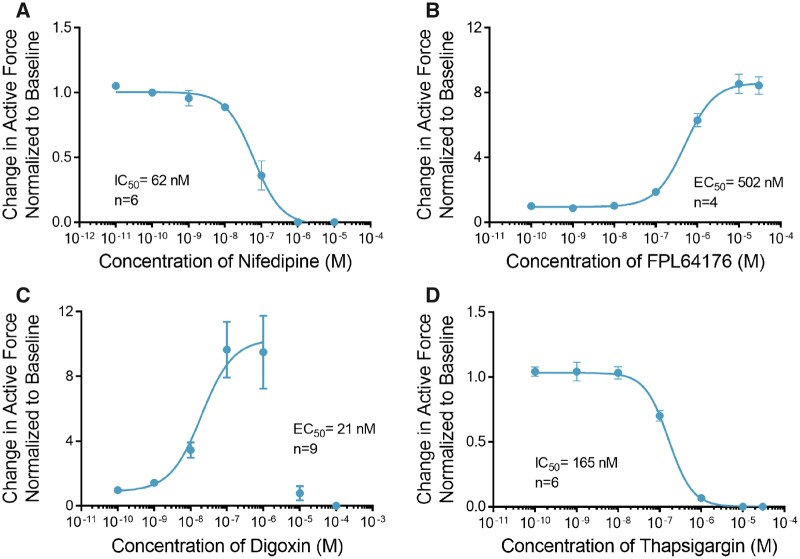
The voltage-gated L-type Ca2+ Channels (LTCCs) are the primary mediators of Ca2+ influx into the cardiomyocyte, which is essential for initiation of cardiac excitation-contraction coupling (Benitah et al., 2010; Bers, 2002). Treatment of ECTs with the LTCC blocker nifedipine completely inhibited contraction at a concentration of 1 μM with a half maximal inhibitory concentration (IC50) of 62 nM (Figure 3A, Supplemental Table 2), whereas treatment with the LTCC activator FPL 64176 induced a maximal 8.5 ± 0.6-fold increase in the force of contraction at a concentration of 10 µM with an EC50 of 502 nM (Figure 3B, Supplemental Table 2).
Figure 3.
Engineered cardiac tissues (ECTs) respond to calcium handling effectors. A, Treatment with nifedipine decreased contractile force with IC50 of 62 nM (n = 6). B, Treatment with FPL64176 induced a robust increase in contractile force with EC50 of 502 nM (n = 4). C, Treatment with digoxin induced a robust increase in contractile force at concentrations below 1 µM (n = 9). Higher concentrations of digoxin showed toxic effects with no contractility observed at doses higher than 10 µM. To calculate the EC50 for the positive inotropic effects of digoxin, concentration values above 1 µM where not included in the curve fit. D, Thapsigargin completely abolished contractile force at concentrations above 1 µM (IC50 = 165 nM, n = 6). Data are presented as mean ± SEM.
The Na+-Ca2+ exchanger (NCX) is the primary means by which cardiomyocytes regulate intracellular Ca2+ and hence a critical modulator of excitation-contraction coupling. To assess the functionality of the NCX in this model we treated ECTs with digoxin. Digoxin is a cardiac glycoside, a class of compounds that directly inhibits the Na+/K+ ATPase and requires a functional NCX for its inotropic effect (Altamirano et al., 2006; Ozdemir et al., 2008). The inhibition of the Na+/K+ ATPase results in increased intracellular Na+ accumulation, which reduces the electrochemical drive for Ca2+ efflux through NCX. In this setting excess intracellular Ca2+ is primarily removed from the cytosol by the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), which loads the SR with Ca2+ leading to an increase in cardiac contractility (Ottolia et al., 2013). Treatment of ECTs with digoxin induced a maximal 9.7 ± 1.7-fold increase in contractile force at a concentration of 100 nM with an EC50 of 21 nM (Figure 3C, Supplemental Table 2). Digoxin at concentration of 1 µM induced ectopic activity and significantly prolonged the duration of contraction (Supplemental Figure 1). At concentrations above 10 μM, ECT contractility was significantly diminished and contraction could not be elicited in the majority of the ECTs using external field stimulation.
Thapsigargin, a noncompetitive inhibitor of SERCA activity reduces the SR calcium load and leads to increased cytosolic calcium and reduced contractility. Treatment of ECTs with thapsigargin completely inhibited contraction at concentrations above 1 μM with IC50 of 165 nM (Figure 3D, Supplemental Table 2).
ECTs Respond to Sarcomere Modulators
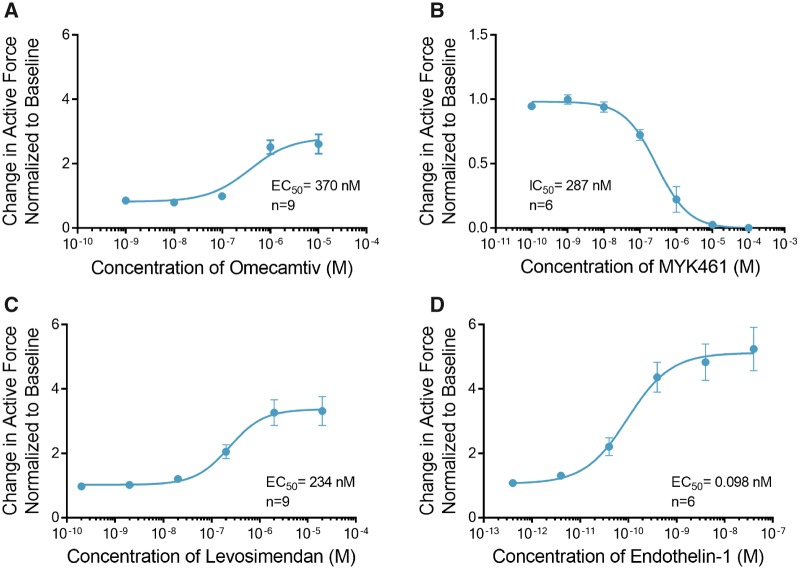
In the cardiac sarcomere, energy derived from myosin-mediated ATP hydrolysis is used to drive contraction (Malik et al., 2011). Omecamtiv mecarbil (OM) is a novel positive inotrope that binds to and stimulates the ATPase activity of myosin resulting in an increased force of contraction in humans (Planelles-Herrero et al., 2017). Treatment of ECTs with OM elicited a maximal 2.6 ± 0.3-fold increase in contractile force at a concentration of 10 µM with an EC50 of 370 nM (Figure 4A). OM at 10 μM significantly increased the time of contraction and relaxation and had no effect on the rates of contraction and relaxation (Supplemental Table 3). In contrast, treatment of ECTs with MYK-461 (mavacamten), an inhibitor of cardiac myosin ATPase, completely abolished contraction at concentrations above 10 μM with IC50 of 287 nM (Figure 4B, Supplemental Table 3).
Figure 4.
Engineered cardiac tissues (ECTs) display canonical inotropic responses to sarcomere modulators and activation of phospholipase C. A, Omecamtiv increased the contractile force with EC50 of 370 nM (n = 9). B, Conversely, increasing doses of MYK461 decreased the contractile force with IC50 of 287 nM (n = 6). C, Treatment with levosimendan increased the contractile force (n = 9) with EC50 of 234 nM. D, Treatment with endothelin-1 which stimulates the activity of phospholipase C (PLC), increased the contractile force with EC50 of 98 pM (n = 6). Data are presented as mean ± SEM.
Cardiac troponin is a trimeric protein complex in the sarcomere (Sorsa et al., 2004). It plays a critical role in regulating sensitivity to Ca2+. Levosimendan is a positive inotrope that modulates troponin-C, 1 protein of the trimer and increases its sensitivity to regulation by Ca2+ (Pollesello et al., 1994; Robertson et al., 2016). Treatment of ECTs with levosimendan induced a maximal 3.3 ± 0.4-fold increase in contractile force at a concentration of 2 μM with an EC50 of 234 nM (Figure 4C).
ECTs Respond to Activation of Phospholipase C
Endothelin-1 (ET-1) is a 21-amino acid peptide that binds to the ET receptor, a Gq-protein-coupled receptor, and activates phospholipase C (Sugden, 2003). Second messenger effects are proposed to mediate positive inotropic events that involve modulation of the intracellular Ca2+ transients and myofilament Ca2+ sensitivity. Treatment of ECTs with ET-1 induced a maximal 5.2 ± 0.7-fold increase in contractile force at 40 nM with an EC50 of 98pM (Figure 4D, Supplemental Table 3).
DISCUSSION
Here we evaluated the contractile function of human 3D ECTs generated from hiPSC-CMs in the Biowire II platform. We demonstrate that the platform enables the generation of 3D ECTs that remain viable and retain their functionality for weeks. These ECTs develop properties of the adult myocardium and exhibit robust responses to a variety of inotropic compounds with distinct mechanisms of action selected to confirm the presence, and interrogate the function of, pathways known to regulate cardiac function.
G-protein coupled receptor signaling pathways play a critical role in functional adaptation in the heart by regulating inotropic and chronotropic responses. Endothelin-1, a potent vasoactive peptide signaling via the phospholipase-C/protein kinase-C pathway, has been shown to regulate cardiac contractility both in vivo and in isolated human myocardium (MacCarthy et al., 2000; Pieske et al., 1999). ECTs generated a positive inotropic response, in a concentration-dependent manner, upon exposure to ET-1; confirming the presence of a functional ET-1 receptor signaling cascade. In patients suffering from HF, dysregulation of β-adrenergic signaling is a common feature of cardiac pathophysiologies and a target of therapy eg, β-adrenergic antagonists are a cornerstone of therapy (Bristow et al., 1990). We demonstrated canonical responses to the well-studied β-agonists isoproterenol and dobutamine. Inotropic responses are observed for PACAP27, which activates adenylyl cyclase via receptors distinct from isoproterenol and milrinone (an inhibitor of cAMP degradation) indicating that a range of cAMP-mediated signaling cascades are also present in the ECTs. Although observation of the stimulatory effects of β-adrenergic agonism in engineered human heart tissues is not novel, previous reports have suggested little to no significant effect on contractility (Hirt et al., 2014), but rather an increase in chronotropy. Additionally, others have observed positive inotropic responses at concentrations far right-shifted and therefore, less physiologically relevant to effects observed in the in this study (Zhang et al., 2013). Interestingly, milrinone has been shown to produce a positive inotropic response in fibroblast containing ECTs (Ravenscroft et al., 2016), whereas when tissues were prepared without fibroblasts, there was no inotropic response to milrinone as compared to human heart tissue (Mannhardt et al., 2017); suggesting maturity in the adrenergic pathway and/or enhanced PDE3A expression in fibroblast containing ECTs. We observed a significant increase in the magnitude of force generated in response to milrinone in ECTs electrically stimulated for 6 weeks as compared to 3 weeks, demonstrating that long term electrical stimulation is also an important factor that contributes to the increased inotropic response to milrinone (Supplemental Figure 2). Further studies are required to determine the mechanism by which the inotropic response to milrinone was increased in ECTs generated in the Biowire II platform.
Calcium channels are both the direct and indirect target of various cardiac therapies. LTCC antagonists are a mainstay in the treatment of hypertension, cardiac ischemia, and arrhythmias, but can elicit cardio depressant effect. Conversely, compounds such as Bay K-8644 and the more potent FPL 64176 were designed to stimulate LTCC activity and have the consequent effect of increasing the contractile force. As such, treatment of the ECTs with the LTCC blocker, nifedipine and with FPL 64176 yielded the expected result of decreased and increased contractility respectively, indicating the LTCC of the ECT was functional and could be regulated by external stimuli. One of the oldest HF therapies is digoxin, a cardiac glycoside isolated from the foxglove plant. Digoxin induces a positive inotropic effect via its ability to promote Ca2+ loading in the SR. A biphasic increase in force was observed when ECTs were treated with digoxin at or below 1 μM demonstrating that contractility can be regulated at the level of NCX and that the SR modulates Ca2+ levels in a physiologically relevant manner. Treatment of tissues with digoxin at concentrations above 1 μM showed toxic effects commonly observed with this compound (Supplemental Figure 1) (Mannhardt et al., 2017; Ruch et al., 2003).
The recent discovery of agents that modulate sarcomere proteins holds great promise for the development of novel treatments for HF. OM is an agent that directly regulates cardiac myosin and levosimendan is an agent that regulates the function of troponin-C, the Ca2+ sensor of the sarcomere. Data from studies in HF patients show OM and levosimendan can promote a positive inotropic effect and hemodynamic improvements (Follath et al., 2002; Teerlink et al., 2016b) without a significant increase in oxygen consumption (Lilleberg et al., 1998; Shen et al., 2010; Ukkonen et al., 2000). Consistent with data from clinical and animal studies, both compounds were able to modulate contractility in ECTs eliciting significant increases in contractile force with concentrations typical for these compounds (Nagy et al., 2015). MYK461, a small molecule inhibitor of myosin also shows potential in the treatment of HF. Tissue treated with MYK461 showed significant decrease in contractility in the range consistent with published results (Green et al., 2016).
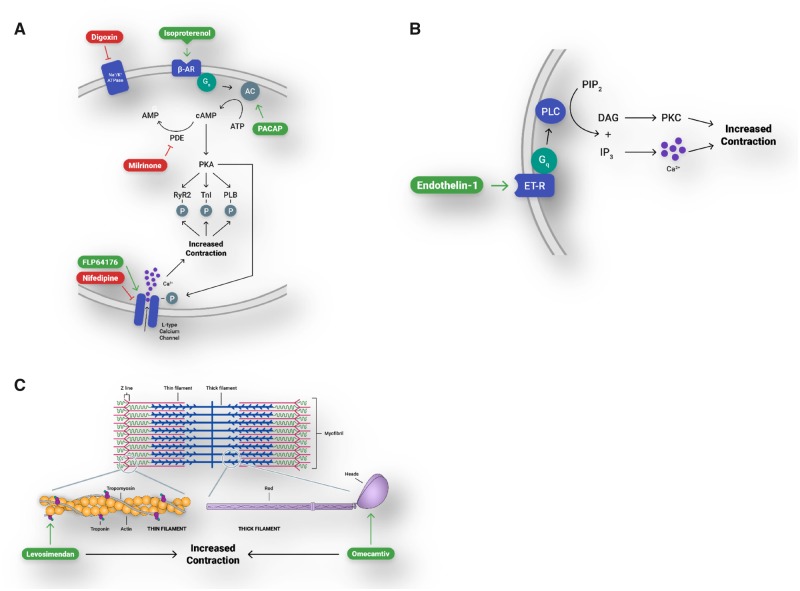
In conclusion, human ECTs created in the Biowire II platform have adult-like canonical responses to compounds known to affect contractility via an array of physiologically relevant pathways (Figure 5, Summary data presented in Table 1). These studies can be conducted under external stimulation, providing investigators with a high degree of experimental control, and with the ability to assess compounds using a nondestructive measurement of contractility. This model can be used to assess the effects of novel compounds in a human-based model of contractility early in the drug discovery and development process. These results suggest the utility of the Biowire II platform to create disease models via the use of patient derived iPSC-CMs.
Figure 5.
Engineered cardiac tissues (ECTs) generated in Biowire II platform have canonical responses to compounds that affect contractility via physiologically relevant pathways. A, β-adrenergic/cAMP-mediated pathway and the L-type calcium channel. β-AR, β-adrenergic receptor; AC, adenylyl cyclase; PACAP, pituitary adenylate cyclase-activating peptide; cAMP, cyclic adenosine monophosphate; ATP, adenosine triphosphate; PDE, phosphodiesterase; PKA, protein kinase A; RYR2, ryanodine receptor; TnI, troponin-I; PLB, phospholamban. B, Gq-protein-coupled receptor signaling. ET-R, endothelin receptor; PLC, phospholipase C; PIP2, phosphatidylinositol 4, 5-bisphosphate; IP3, inositol 1, 4, 5-trisphosphate; DAG, diacylglycerol; PKC, protein kinase C. C, Modulation of proteins of the cardiac sarcomere.
Table 1.
Summary of Engineered Cardiac Tissues (ECT) Inotropic Responses
| Compound | ECT EC50/IC50 | Effective Plasma Concentration or EC50/IC50 | Reference |
|---|---|---|---|
| Isoproterenol | 0.5 nM | 27 nMa | EC50 in isolated human ventricular muscle strip (Flesch et al., 1999) |
| Dobutamine | 16 nM | 133–632 nM | Plasma levels of 133 nM were associated with significant increase in cardiac index with linear increase in index up to 632 nM (Leier et al., 1979) |
| Milrinone | 1.6 µM | 0.791 µM | Plasma concentration associated with 50% increase in cardiac index (Bailey et al., 1994) |
| PACAP27 | 1.1 nM | 0.5 nMa | Recalculated EC50 in dog isolated left ventricle (Hirose et al., 1998) |
| Nifedipine | 62 nM | 10–81 nM | Steady state of 10 nM (Raemsch and Sommer, 1983), plasma levels above 81 nM were associated with decreased dp/dtmax (Clifton et al., 1990) |
| FPL64176 | 502 nM | 600 nMa | Recalculated EC50 in guinea pig papillary muscle (Rampe et al., 1993) |
| Digoxin | 21 nM | 1.5–2.6 nM | Plasma level range in patients with improved fractional shortening (Guyatt et al., 1988) |
| Thapsigargin | 165 nM | 300 nMa | Complete inhibition of contraction in isolated adult rat cardiomyocytes (Wrzosek et al., 1992) |
| Omecamtiv | 370 nM | 792 nM | Maximum plasma level associated with increased stroke volume (Teerlink et al., 2016a) |
| MYK461 | 287 nM | 180 nMa | IC50 in adult rat ventricular cardiomyocytes (Green et al., 2016) |
| Levosimendan | 234 nM | 351 nM | Plasma level associated with increased stroke volume (Kivikko et al., 2003) |
| Endothelin-1 | 0.1 nM | 9.1 nMa | EC50 in isolated human ventricular trabeculae (Saetrum Opgaard et al., 2000) |
The EC50/IC50 for each compound was compared to the effective therapeutic dose of the compound where data were available. For compounds where effective therapeutic dose was not available, comparison was made with published results from in vitro or animal models and is indicated by superscript letter (a).
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
The authors received no financial support for the research, authorship and for the publication of this article.
DECLARATION OF CONFLICTING INTERESTS
N.T.F., I.P., R.S., D.R.B., M.G., M.P.G., and R.A.-S. are employees and shareholders of TARA Biosystems Inc.
Supplementary Material
REFERENCES
- Altamirano J., Li Y., DeSantiago J., Piacentino V. 3rd, Houser S. R., Bers D. M. (2006). The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+- exchanger function. J. Physiol. 575, 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Levy J. H., Kikura M., Szlam F., Hug C. C. Jr. (1994). Pharmacokinetics of intravenous milrinone in patients undergoing cardiac surgery. Anesthesiology 81, 616–622. [DOI] [PubMed] [Google Scholar]
- Benitah J. P., Alvarez J. L., Gomez A. M. (2010). L-type Ca(2+) current in ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 48, 26–36. [DOI] [PubMed] [Google Scholar]
- Benjamin E. J., Virani S. S., Callaway C. W., Chamberlain A. M., Chang A. R., Cheng S., Chiuve S. E., Cushman M., Delling F. N., Deo R., et al. (2018). Heart disease and stroke statistics-2018 update: A report from the American heart association. Circulation 137, e67–e492. [DOI] [PubMed] [Google Scholar]
- Bers D. M. (2002). Cardiac excitation-contraction coupling. Nature 415, 198–205. [DOI] [PubMed] [Google Scholar]
- Bers D. M. (2008). Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 70, 23–49. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Hershberger R. E., Port J. D., Gilbert E. M., Sandoval A., Rasmussen R., Cates A. E., Feldman A. M. (1990). Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation 82, I12–I25. [PubMed] [Google Scholar]
- Buckley N. M., Penefsky Z. J., Litwak R. S. (1972). Comparative force-frequency relationships in human and other mammalian ventricular myocardium. Pflugers Arch. 332, 259–270. [DOI] [PubMed] [Google Scholar]
- Clifton G. D., Booth D. C., Hobbs S., Boucher B. A., Foster T. S., McAllister R. G. Jr, DeMaria A. N. (1990). Negative inotropic effect of intravenous nifedipine in coronary artery disease: Relation to plasma levels. Am. Heart J. 119, 283–290. [DOI] [PubMed] [Google Scholar]
- de Lucia C., Eguchi A., Koch W. J. (2018). New insights in cardiac beta-adrenergic signaling during heart failure and aging. Front. Pharmacol. 9, 904.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning C., Borgdorff V., Crutchley J., Firth K. S., George V., Kalra S., Kondrashov A., Hoang M. D., Mosqueira D., Patel A., et al. (2016). Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta 1863, 1728–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Caldwell J. L., Kistamas K., Trafford A. W. (2017). Calcium and excitation-contraction coupling in the heart. Circ. Res. 121, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch M., Kilter H., Cremers B., Laufs U., Sudkamp M., Ortmann M., Muller F. U., Bohm M. (1999). Effects of endotoxin on human myocardial contractility involvement of nitric oxide and peroxynitrite. J. Am. Coll. Cardiol. 33, 1062–1070. [DOI] [PubMed] [Google Scholar]
- Follath F., Cleland J. G., Just H., Papp J. G., Scholz H., Peuhkurinen K., Harjola V. P., Mitrovic V., Abdalla M., Sandell E. P., et al. (2002). Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the lido study): A randomised double-blind trial. Lancet 360, 196–202. [DOI] [PubMed] [Google Scholar]
- Green E. M., Wakimoto H., Anderson R. L., Evanchik M. J., Gorham J. M., Harrison B. C., Henze M., Kawas R., Oslob J. D., Rodriguez H. M., et al. (2016). A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 351, 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth B. D., Chiang A. Y., Doyle J., Engwall M. J., Guillon J. M., Hoffmann P., Koerner J., Mittelstadt S., Ottinger S., Pierson J. B., et al. (2015). The evaluation of drug-induced changes in cardiac inotropy in dogs: Results from a HESI-sponsored consortium. J. Pharmacol. Toxicol. Methods 75, 70–90. [DOI] [PubMed] [Google Scholar]
- Guyatt G. H., Sullivan M. J., Fallen E. L., Tihal H., Rideout E., Halcrow S., Nogradi S., Townsend M., Taylor D. W. (1988). A controlled trial of digoxin in congestive heart failure. Am. J. Cardiol. 61, 371–375. [DOI] [PubMed] [Google Scholar]
- Hirose M., Furukawa Y., Lakhe M., Chiba S. (1998). Regional differences in cardiac effects of pituitary adenylate cyclase-activating polypeptide-27 in the isolated dog heart. Eur. J. Pharmacol. 349, 269–276. [DOI] [PubMed] [Google Scholar]
- Hirt M. N., Boeddinghaus J., Mitchell A., Schaaf S., Bornchen C., Muller C., Schulz H., Hubner N., Stenzig J., Stoehr A., et al. (2014). Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol. 74, 151–161. [DOI] [PubMed] [Google Scholar]
- Kivikko M., Lehtonen L., Colucci W. S. (2003). Sustained hemodynamic effects of intravenous levosimendan. Circulation 107, 81–86. [DOI] [PubMed] [Google Scholar]
- Leier C. V., Unverferth D. V., Kates R. E. (1979). The relationship between plasma dobutamine concentrations and cardiovascular responses in cardiac failure. Am. J. Med. 66, 238–242. [DOI] [PubMed] [Google Scholar]
- Lilleberg J., Nieminen M. S., Akkila J., Heikkila L., Kuitunen A., Lehtonen L., Verkkala K., Mattila S., Salmenpera M. (1998). Effects of a new calcium sensitizer, levosimendan, on haemodynamics, coronary blood flow and myocardial substrate utilization early after coronary artery bypass grafting. Eur. Heart J. 19, 660–668. [DOI] [PubMed] [Google Scholar]
- MacCarthy P. A., Grocott-Mason R., Prendergast B. D., Shah A. M. (2000). Contrasting inotropic effects of endogenous endothelin in the normal and failing human heart: Studies with an intracoronary ET(A) receptor antagonist. Circulation 101, 142–147. [DOI] [PubMed] [Google Scholar]
- Malik F. I., Hartman J. J., Elias K. A., Morgan B. P., Rodriguez H., Brejc K., Anderson R. L., Sueoka S. H., Lee K. H., Finer J. T., et al. (2011). Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science 331, 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhardt I., Eder A., Dumotier B., Prondzynski M., Kramer E., Traebert M., Sohren K. D., Flenner F., Stathopoulou K., Lemoine M. D., et al. (2017). Blinded contractility analysis in hiPSC-cardiomyocytes in engineered heart tissue format: Comparison with human atrial trabeculae. Toxicol. Sci. 158, 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton R., Huet G., Shakir S. (2014). An investigation into drug products withdrawn from the EU market between 2002 and 2011 for safety reasons and the evidence used to support the decision-making. BMJ Open 4, e004221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L., Kovacs A., Bodi B., Pasztor E. T., Fulop G. A., Toth A., Edes I., Papp Z. (2015). The novel cardiac myosin activator omecamtiv mecarbil increases the calcium sensitivity of force production in isolated cardiomyocytes and skeletal muscle fibres of the rat. Br. J. Pharmacol. 172, 4506–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onakpoya I. J., Heneghan C. J., Aronson J. K. (2016). Worldwide withdrawal of medicinal products because of adverse drug reactions: A systematic review and analysis. Crit. Rev. Toxicol. 46, 477–489. [DOI] [PubMed] [Google Scholar]
- Ottolia M., Torres N., Bridge J. H., Philipson K. D., Goldhaber J. I. (2013). Na/Ca exchange and contraction of the heart. J. Mol. Cell. Cardiol. 61, 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir S., Bito V., Holemans P., Vinet L., Mercadier J. J., Varro A., Sipido K. R. (2008). Pharmacological inhibition of Na/Ca exchange results in increased cellular Ca2+ load attributable to the predominance of forward mode block. Circ. Res. 102, 1398–1405. [DOI] [PubMed] [Google Scholar]
- Pieske B., Beyermann B., Breu V., Loffler B. M., Schlotthauer K., Maier L. S., Schmidt-Schweda S., Just H., Hasenfuss G. (1999). Functional effects of endothelin and regulation of endothelin receptors in isolated human nonfailing and failing myocardium. Circulation 99, 1802–1809. [DOI] [PubMed] [Google Scholar]
- Pieske B., Sutterlin M., Schmidt-Schweda S., Minami K., Meyer M., Olschewski M., Holubarsch C., Just H., Hasenfuss G. (1996). Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J. Clin. Invest. 98, 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planelles-Herrero V. J., Hartman J. J., Robert-Paganin J., Malik F. I., Houdusse A. (2017). Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat. Commun. 8, 190.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollesello P., Ovaska M., Kaivola J., Tilgmann C., Lundstrom K., Kalkkinen N., Ulmanen I., Nissinen E., Taskinen J. (1994). Binding of a new Ca2+ sensitizer, levosimendan, to recombinant human cardiac troponin C. A molecular modelling, fluorescence probe, and proton nuclear magnetic resonance study. J. Biol. Chem. 269, 28584–28590. [PubMed] [Google Scholar]
- Raemsch K. D., Sommer J. (1983). Pharmacokinetics and metabolism of nifedipine. Hypertension 5, II18–II24. [DOI] [PubMed] [Google Scholar]
- Rampe D., Anderson B., Rapien-Pryor V., Li T., Dage R. C. (1993). Comparison of the in vitro and in vivo cardiovascular effects of two structurally distinct Ca++ channel activators, BAY K 8644 and FPL 64176. J. Pharmacol. Exp. Ther. 265, 1125–1130. [PubMed] [Google Scholar]
- Ravenscroft S. M., Pointon A., Williams A. W., Cross M. J., Sidaway J. E. (2016). Cardiac non-myocyte cells show enhanced pharmacological function suggestive of contractile maturity in stem cell derived cardiomyocyte microtissues. Toxicol. Sci. 152, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I. M., Pineda-Sanabria S. E., Yan Z., Kampourakis T., Sun Y. B., Sykes B. D., Irving M. (2016). Reversible covalent binding to cardiac troponin C by the Ca2+-sensitizer levosimendan. Biochemistry 55, 6032–6045. [DOI] [PubMed] [Google Scholar]
- Ruch S. R., Nishio M., Wasserstrom J. A. (2003). Effect of cardiac glycosides on action potential characteristics and contractility in cat ventricular myocytes: Role of calcium overload. J. Pharmacol. Exp. Ther. 307, 419–428. [DOI] [PubMed] [Google Scholar]
- Saetrum Opgaard O., Moller S., de Vries R., Edvinsson L., Saxena P. R. (2000). Positive inotropic responses mediated by endothelin ET(A) and ET(B) receptors in human myocardial trabeculae. Clin. Sci. (Lond.) 99, 161–168. [DOI] [PubMed] [Google Scholar]
- Shen Y. T., Malik F. I., Zhao X., Depre C., Dhar S. K., Abarzua P., Morgans D. J., Vatner S. F. (2010). Improvement of cardiac function by a cardiac myosin activator in conscious dogs with systolic heart failure. Circ. Heart Fail. 3, 522–527. [DOI] [PubMed] [Google Scholar]
- Siramshetty V. B., Nickel J., Omieczynski C., Gohlke B. O., Drwal M. N., Preissner R. (2016). WITHDRAWN—a resource for withdrawn and discontinued drugs. Nucleic Acids Res. 44, D1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsa T., Pollesello P., Solaro R. J. (2004). The contractile apparatus as a target for drugs against heart failure: Interaction of levosimendan, a calcium sensitiser, with cardiac troponin C. Mol. Cell. Biochem. 266, 87–107. [DOI] [PubMed] [Google Scholar]
- Stienen G. J. (2015). Pathomechanisms in heart failure: The contractile connection. J. Muscle Res. Cell Motil. 36, 47–60. [DOI] [PubMed] [Google Scholar]
- Sugden P. H. (2003). An overview of endothelin signaling in the cardiac myocyte. J. Mol. Cell. Cardiol. 35, 871–886. [DOI] [PubMed] [Google Scholar]
- Teerlink J. R., Felker G. M., McMurray J. J., Solomon S. D., Adams K. F. Jr., Cleland J. G., Ezekowitz J. A., Goudev A., Macdonald P., Metra M., et al. (2016a). Chronic oral study of myosin activation to increase contractility in heart failure (COSMIC-HF): A phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet 388, 2895–2903. [DOI] [PubMed] [Google Scholar]
- Teerlink J. R., Felker G. M., McMurray J. J. V., Ponikowski P., Metra M., Filippatos G. S., Ezekowitz J. A., Dickstein K., Cleland J. G. F., Kim J. B., et al. (2016b). Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: The ATOMIC-AHF study. J. Am. Coll. Cardiol. 67, 1444–1455. [DOI] [PubMed] [Google Scholar]
- Ukkonen H., Saraste M., Akkila J., Knuuti J., Karanko M., Iida H., Lehikoinen P., Nagren K., Lehtonen L., Voipio-Pulkki L. M. (2000). Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin. Pharmacol. Ther. 68, 522–531. [DOI] [PubMed] [Google Scholar]
- Veerman C. C., Kosmidis G., Mummery C. L., Casini S., Verkerk A. O., Bellin M. (2015). Immaturity of human stem-cell-derived cardiomyocytes in culture: Fatal flaw or soluble problem? Stem Cells Dev. 24, 1035–1052. [DOI] [PubMed] [Google Scholar]
- Wrzosek A., Schneider H., Grueninger S., Chiesi M. (1992). Effect of thapsigargin on cardiac muscle cells. Cell Calcium 13, 281–292. [DOI] [PubMed] [Google Scholar]
- Zhang D., Shadrin I. Y., Lam J., Xian H. Q., Snodgrass H. R., Bursac N. (2013). Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34, 5813–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Rafatian N., Feric N. T., Cox B. J., Aschar-Sobbi R., Wang E. Y., Aggarwal P., Zhang B., Conant G., Ronaldson-Bouchard K., et al. (2019). A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 176, 913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.