Abstract
Bisphenol A (BPA) is a high production volume chemical widely used in plastics, food packaging, and many other products. It is well known that endocrine-disrupting chemicals might be harmful to human health due to interference with normal hormone actions. Recent studies report widespread usage and exposure to many BPA-like chemicals (BPs) that are structurally or functionally similar to BPA. However, the biological actions and toxicity of those BPs are still relatively unknown. To address this data gap, we used in vitro cell models to evaluate the ability of 22 BPs to induce or inhibit estrogenic and androgenic activity. BPA, Bisphenol AF (BPAF), bisphenol Z (BPZ), bisphenol C (BPC), tetramethyl bisphenol A (TMBPA), bisphenol S (BPS), bisphenol E (BPE), 4,4-bisphenol F (4,4-BPF), bisphenol AP (BPAP), bisphenol B (BPB), tetrachlorobisphenol A (TCBPA), and benzylparaben (PHBB) induced estrogen receptor (ER)α and/or ERβ-mediated activity. With the exception of BPS, TCBPA, and PHBB, these same BPs were also androgen receptor (AR) antagonists. Only 3 BPs were found to be ER antagonists. Bisphenol P (BPP) selectively inhibited ERβ-mediated activity and 4-(4-phenylmethoxyphenyl)sulfonylphenol (BPS-MPE) and 2,4-bisphenol S (2,4-BPS) selectively inhibited ERα-mediated activity. None of the BPs induced AR-mediated activity. In addition, we identify that the BPs can bind to ER or AR with varying degrees by a molecular modeling analysis. Taken together, these findings help us to understand the molecular mechanism of BPs and further consideration of their usage in consumer products.
Keywords: bisphenol analogs, estrogen receptor, androgen receptor, transcriptional activation, gene regulation, binding
Bisphenol A (BPA) is a high production volume chemical used in the manufacture of polycarbonate plastics, epoxy resins, and as a dye developer in thermal paper (US EPA, 2016). As such, it is found in a wide variety of consumer products including plastic dinnerware, eyeglass lenses, toys, the lining of food, and beverage cans, and in thermal papers such as cash register receipts and certain types of medical technical paper (eg, ultrasound and electrocardiogram printouts) (American Chemistry Council, 2013; Mendum et al., 2011). BPA was detected in 95.7% of Americans surveyed in the 2013–2014 National Health and Nutrition Examination Survey (NHANES), indicating widespread exposure (Lehmler et al., 2018). A growing number of reports, both epidemiological and from laboratory animal studies, indicates the potential for adverse effects following exposure to BPA at levels to which people are currently exposed (Rochester, 2013; Rochester et al., 2018; Vandenberg et al., 2013). For example, a recent systematic review and meta-analysis based on epidemiological and experimental rodent studies concluded that BPA is a “presumed” hazard to human health with regard to hyperactivity (Rochester et al., 2018).
Many of the health effects associated with BPA exposure are thought to be mediated by its activation of nuclear hormone receptors (Acconcia et al., 2015). Nuclear hormone receptors such as the estrogen receptors (ER) and androgen receptor (AR) are ligand inducible transcription factors that alter hormone responsive genes (Deroo and Korach, 2006). As such, ER and AR mediate several biological effects by modulating gene expression, and are important in directing growth, reproduction, and development (Burns and Korach, 2012; Deroo and Korach, 2006). For example, developmental exposure to BPA has been shown to disrupt the development of reproductive tissues such as the prostate and mammary glands (Prins et al., 2014; Vandenberg et al., 2007).
Concerns for human health effects due to BPA exposure has led to its gradual replacement in some consumer products. In turn, new products containing BPA-like chemicals (BPs) that are structurally or functionally similar to BPA have entered the marketplace. For example, some BPs have been detected in foodstuff (bisphenol AF: BPAF; bisphenol AP: BPAP; bisphenol B: BPB; 4,4-bisphenol F: 4,4-BPF; bisphenol P: BPP; bisphenol S: BPS; bisphenol Z: BPZ; Cacho et al., 2012; Liao and Kannan, 2013), household dust (BPAF, BPAP, BPB, 4,4-BPF, BPP, BPS; Liao et al., 2012b), personal care products (BPAF, BPAP, BPB, 4,4-BPF, BPP, BPS, BPZ; Liao and Kannan, 2014), or in thermal paper (BPS, D-8, Pergafast 201, D90, 3-Allyl-4-hydroyphenyl sulfone [TGSA], 4-(4-prop-2-enoxyphenyl)sulfonylphenol (BPS-MAE), 2,4-BPS, urea urethane compound; Becerra and Odermatt, 2013; Bjornsdotter et al., 2017; Eckardt and Simat, 2017; Liao et al., 2012c). BPs have also been detected in urine, blood, breast milk, or adipose tissue in human biomonitoring studies (eg, BPAF, BPAP, BPB, BPC, BPE, 4,4-BPF, bisphenol PH: BPPH, BPS, BPZ, D-8) (Cobellis et al., 2009, 2010; Cunha and Fernandes, 2010; Liao et al., 2012a; Zhou et al., 2013, 2014). The most recent NHANES data (2013–2014) suggest that exposure to BPs is ubiquitous, as BPS and 4,4-BPF were detected in 89.4% and 66.5% of Americans, respectively (Lehmler et al., 2018). There is some evidence that whereas BPA exposure is declining, BPS exposure is increasing (Ye et al., 2015).
Recent studies suggest that some BPs may be associated with health outcomes similar to those associated with BPA exposure (Charisiadis et al., 2018; Ferguson et al., 2018; Kataria et al., 2017; Liu et al., 2017; Mustieles et al., 2018; Wan et al., 2018; Zhang et al., 2019) and reviewed by Pelch et al. (2019). Because many BPA health effects are mediated through steroid hormone receptors (Acconcia et al., 2015), it would be beneficial to understand if BPs elicit the same cellular responses. Though the evidence suggesting that BPs have similar biological activity to BPA is growing (Pelch et al., 2017, 2019), it remains difficult to directly compare the effects of different BPs. This is because, to date, study protocols investigating ER and AR activity of BPs vary widely between published papers and laboratories (Pelch et al., 2017). For example, the type of cell, receptor (full, partial, or mutant receptor), and the specific response element used varies widely across the previously published studies (Pelch et al., 2017).
In contrast to a wealth of data available for BPA, there is a general lack of toxicological data on BPs. In fact, there are limited data on the potential of BPs to induce biological effects (eg, ER-mediated transcriptional activity) despite the fact that some are already in use. Available data on BPs suggest that the ER agonist activity indicated by transactivation of ERα/estrogen response element (ERE)-mediated reporter activity is the most sensitive assay for many BPs (Pelch et al., 2017; Tice et al., 2013). However, the available data for BPs are difficult to compare across studies given how widely the experimental variables differ across studies and between laboratories. In order to address these data gap, and to be able to directly make comparisons among the BPs, we tested 22 BPs in in vitro cell models to evaluate the effects of the BPs on ER and AR-mediated activities. We examined the estrogenic or androgenic activity of the BPs using a luciferase reporter gene assay and tested the effects on well-known ER target genes. In addition, we analyzed the binding ability to ER or AR using a molecular modeling analysis.
MATERIALS AND METHODS
Test chemicals
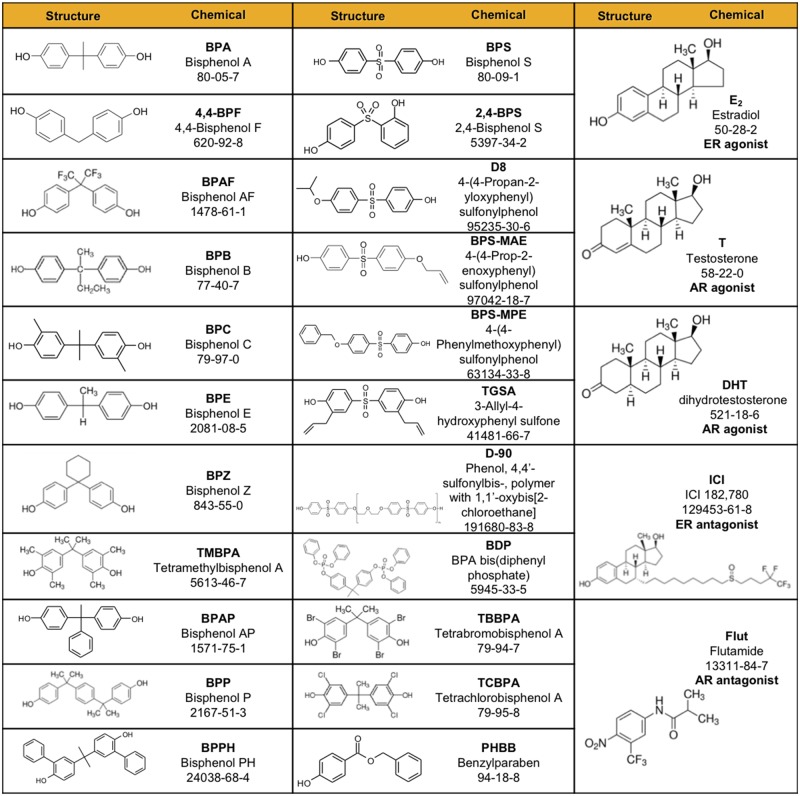
BPs were chosen for inclusion in this paper because they have been detected in the environment or human biological samples, or were identified by the U.S. EPA Design for the Environment as potential alternatives to BPA in thermal paper (US EPA, 2014). Chemicals for this experiment were supplied by MRI-Global under contract to NTP. The BPs were coded by MRI and laboratory staff remained blinded throughout the experiment until data analysis was complete. The original sources and manufacturers’ stated purity of the BPs evaluated are listed in Supplementary Table 1. Chemical Abstracts Services Registry Numbers (CASRN) and structures are in Figure 1 . Chemicals were dissolved in DMSO and stocks were maintained frozen at 10 mM.
Figure 1.
Structure and CASRN for tested bisphenol A-like chemicals (BPs). Chemical structures and Chemical Abstracts Service Registry Number (CASRN) are shown for 22 BPs, estradiol (E2), testosterone (T), dihydrotestosterone (DHT), ER antagonist ICI 182, 780 (ICI), and AR antagonist flutamide (Flut). Additional details can be found in Supplementary Table 1.
Plasmids
The pcDNA3 expression vector plasmid and the internal control plasmid for transfection efficiency, pRL-TK renilla luciferase (pRT-TK Luc) were purchased from Invitrogen (Carlsbad, California). The luciferase reporter plasmid, pGL/3xERE Luc, and human ERα and ERβ expression plasmids, pcDNA3/hERα and pcDNA3/hERβ have been described previously (Mueller et al., 2003).
Cell culture
The HepG2 human hepatocellular cancer and MCF7 human breast cancer cell lines were purchased from American Type Culture Collection (HB-8065 and HTB-22, respectively). The MDA-kb2 stably transfected human breast cancer cell line and the culture conditions for the cell lines were described previously (Li et al., 2012, 2013; Wilson et al., 2002). Briefly, HepG2 and MCF7 cells were maintained in phenol red-free minimum essential medium (MEM; Invitrogen) or Dulbecco’s Modified Eagle’s Medium (Invitrogen), respectively, supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gemini Bio Products, West Sacramento, California), 4 mM l-glutamine (Invitrogen), 100 U/ml penicillin (Invitrogen), and 100 µg/ml streptomycin (Invitrogen) at 37°C in 5% CO2. MDA-kb2 cells were maintained in L-15 medium (Invitrogen) supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C, without CO2. For serum-stripped conditions, 10% or 5% charcoal-dextran stripped heat-inactivated FBS (sFBS, Gemini Bio Products) was substituted for FBS for HepG2 or MCF7 cells, respectively. No antibiotics were added into the serum-stripped medium.
Assessment of estrogen agonist and antagonist activity in transiently transfected cells
HepG2 cells (1.4 × 107) were cultured in T75 flasks in 10% sFBS medium. The next day, cells were transfected using the Effectene Transfection Reagent (125 µl; Qiagen, Valencia, California) according to the manufacturer’s instructions with 5 µg DNA, including 2 µg of expression plasmid, 2 µg of reporter plasmid, and 1 µg of pRL-TK plasmid. Fresh 10% sFBS medium was applied after 5–7 h. The following day, transfected cells were lifted by incubation with 2X trypsin (Invitrogen) for 5 min at 37°C. Cells were then seeded from a homogenous cell suspension into 96-well plates (Costar, Corning, New York) at 7.5 × 104 cells/well in 200 µl fresh 10% sFBS medium and allowed to recover for 6 h. Chemicals were serially diluted in a deep-well block in medium containing 1% DMSO and 22.2 µl of diluted chemical was then added to the 96-well plate in triplicate. The final DMSO concentration was 0.1% DMSO in all wells. The final concentration of BPs was from 3 nM to 10 µM. The final concentration of the positive control for the ER/ERE-mediated agonism assays, E2, was from 0.001 to 100 nM. To assess ER/ERE-mediated antagonism, cells were treated with concentrations of BPs ranging from 3 nM to 10 µM in the presence of 1.0 nM E2. In our system, 1.0 nM E2 elicits about 90% maximum efficacy for ERα and 50% maximum efficacy for ERβ. The final concentration of the positive control for ER antagonism, ICI 182, 780 (ICI), was from 10 nM to 10 µM. The specificity for the ERα/ERE mechanism was confirmed for BPs considered active by treating transfected HepG2 cells with 1 µM of each BP in the presence or absence of 10 µM of the ER specific antagonist ICI.
Luciferase assay
After 18 h chemical treatment medium was aspirated from HepG2 or MDA-kb2 cells, and cells were lysed for at least 1 h in 40 µl passive lysis buffer (Promega, Madison, Wisconsin). Lysate (20 µl) was transferred to a white 96-well plate (Fisher Scientific, Hampton, New Hampshire) and luciferase activity was evaluated with the Dual Luciferase Reporter Activity System (Promega) according to the manufacturer’s recommendations on a CLARIOstar (BMG Labtech; Cary, North Carolina) plate reader equipped with reagent injectors.
Assessment of endogenous gene expression
MCF7 cells (3.5 × 105 cells/well) were cultured overnight in 6-well plates (Costar). Cells were maintained in 5% sFBS medium for 2 days then treated with vehicle control (0.01% DMSO), 10 nM E2 or 1 µM BPs as previously described (Li et al., 2012). The concentration of DMSO (0.01%) was constant for all treatments. After 18 h, cells were lysed by addition of RTL buffer (RNEasy, Qiagen). Lysates were passed through QIAshredder (Qiagen) columns and RNA was subsequently isolated with an on-column DNase treatment as per the manufacturer’s recommendations. RNA quality and quantity was determined using the UV-vis plate and CLARIOstar plate reader. First strand cDNA synthesis was performed on 2 µg RNA using Superscript Reverse Transcriptase (Invitrogen). Real-time PCR using SYBR Green technology was performed on an ABI PRISM 7900 Sequence Detection System and analysis software (Applied Biosystems, Foster City, California). Real-time RT-PCR reactions were performed in duplicate in 20 µl with primers at 100 nM each. The GenBank accession numbers (http://www.ncbi.nlm.nih.gov/genbank/. Accessed accessed 12, 2019) and sequences of primers used for real-time PCR were as follows: human progesterone receptor (PGR) (NM_000926.4): forward 5′-GACGTGGA GGGC GCAT AT-3′, reverse 5′-GCAG TCCGCTGTCCTTTTCT-3′; and human gene regulation by estrogen in breast cancer 1 (GREB1) (NM_014668): forward 5′-CAAAGAATAACCTGTTGGCCC-3′, reverse 5′-GACA TGCC TGCG CTCT CATAC-3. These primers have been previously described and were determined to be specific upon examination of the melting curves (Li et al., 2013). Human beta-actin (ACTB) (forward 5′-GACA GGATGCAGAAGGAGATCAC-3′, reverse 5′-GCTTCATACTCCAGCAGG-3′) was determined to be constitutively expressed in all samples and was used to normalize the expression of the other mRNAs using the method described by Livak and Schmittgen (2001). Fold changes (2ΔΔCT) relative to vehicle control are plotted as the mean ± standard error of the mean (SEM) in Graphpad Prism 6.00 for Mac (La Jolla, California). The assessment of endogenous gene expression in MCF7 cells was repeated 3 times. The differences between the CT value for GREB1 or PGR and ACTB (ΔCT) were compared by one-way analysis of variance (ANOVA) and Dunnett’s multiple comparison post hoc test to compare treated cells to vehicle treated cells.
Assessment of androgen agonist and antagonist activity in stably transfected cells
MDA-kb2 cells expressing endogenous AR and the stably transfected plasmid driven by the mouse mammary tumor virus promoter (pMMTV.luc.neo) were seeded from a homogenous cell suspension in fresh medium in 96-well plates (1 × 104 cells/well) (Wilson et al., 2002). Cells were treated with chemicals 6 h later, as per above (see “Assessment of estrogen agonist and antagonist activity in transiently transfected cells” section). The final concentrations of the positive control for the androgen receptor/mouse mammary tumor virus (AR/MMTV) mediated agonism assay, testosterone (T), were from 0.0001 to 100 nM, respectively. To assess AR antagonism, cells were treated with BPs (3 nM to 10 µM) in the presence of 1.0 nM T. The final concentration of the positive control for the AR/MMTV-mediated antagonism assay, flutamide (Flut) was from 1 nM to 10 µM. The luciferase reporter gene assay was performed as above.
Cytotoxicity assay
Cytotoxicity was determined for HepG2 and MDA-kb2 cells used in luciferase reporter gene assays. HepG2 and MDA-kb2 cells were seeded in white 96-well plates and treated with chemicals as per above. After 18–20 h of treatment, CellTox Green reagent (Promega) was diluted 1:50 in assay buffer and 2.2 µl/well was added to the cells (1:5000 final dilution). Fluorescence (excitation 485–500 nm and emission 520–530) was detected on a CLARIOstar plate reader after 15 min of incubation and 1 min of orbital shaking at room temperature.
Data analysis and statistics
All experimental data are available in CEBS at https://doi.org/10.22427/NTP-DATA-002-00060-0001-0000-9. All data analyses were conducted in Graphpad Prism. A positive, negative, and vehicle control were included in every luciferase reporter gene assay. For each luciferase reporter gene assay, technical replicates were normalized to the concurrent vehicle control and either the top of the E2 curve (for assays run in agonist mode) or 1.0 nM E2 or T (for assays run in antagonist mode) and averaged. Means from independent experiments were pooled and then log transformed and fit to a sigmoidal dose response with variable slope where the upper and lower limits were constrained to 0 (minimum) and 100 (maximum). The dose response curves were examined and only those in which the models converged or were ambiguous were considered further. All luciferase assays were repeated 3 times, with the exception of the AR agonism assay, which was only performed once. Individual data points were only removed from the analysis when there was a documented reason (eg, cells were missing from the well due to pipetting error). The mean ± SEM of the pooled independent experiments is presented. ANOVA followed by Dunnett’s post hoc test was used to determine if each data point was significantly different than control (vehicle control for agonist assays, 1.0 nM E2 or T for antagonist assays). For each BP, the maximum efficacy and inhibition is reported as the observed activity of the tested concentration that had the greatest response relative to the maximum activity of E2 or T (for agonist assays), or the greatest reduction in response relative to 1.0 nM E2 or T (for antagonist assays). The relative EC50 or IC50 (Rel EC50 or Rel IC50) was determined in Prism as the concentration at which the BP’s dose response curve crossed the line marking the 50% maximum response of E2 or T. To be considered “active,” BPs had to (1) induce (or reduce for antagonist assays) activity by ≥ 20% (Hsieh et al., 2015), (2) be statistically significant, and (3) be concentration dependent.
Cytotoxicity data were analyzed similarly. Fluorescence was normalized by determining the percentage difference from the concurrent vehicle control. One-way ANOVA followed by Dunnett’s post hoc test was used to determine if each data point was significantly different than vehicle control. BPs with a statistically significant increase in fluorescence signal ≥ 15% compared to vehicle were considered cytotoxic.
The specificity of the ERα/ERE mechanism was evaluated in HepG2 cells in the absence or presence of ICI. The fold induction of luciferase activity relative to vehicle treated cells in the absence of ICI was determined and averaged across 3 independent experiments. Unpaired t tests were run to compare the fold induction of luciferase activity in the presence or absence of ICI to determine statistical significance.
Molecular modeling of ER and AR binding
Two ERα structures were used as receptor configurations for molecular docking: (1) the receptor in the E2 and coactivator peptide src-1 bound agonist conformation (Protein Data Bank [PDB] ID: 3UUD), and (2) the receptor in the 4-hydroxytamoxifen bound antagonist conformation (PDB ID: 3ERT). For AR, dihydrotestosterone (DHT) bound agonist conformation was used for the receptor conformation (PDB ID: 3L3X). A potential antagonist form for AR was created by placing the C-terminal tail containing H12 helix of AR in a conformation similar to that of the 4-hydroxytamoxifen bound ERα. The ligand binding site of proteins was prepared for docking using the Receptor Setup module (Release 3.2.0.2) with the help of bound ligands (E2, 4-hydroxytamoxifen, or DHT) whereas docking studies were carried out using the FRED module (Release 3.2.0.2) (Kelley et al., 2015) of the OpenEye software package (OpenEye Scientific Software, Inc, Santa Fe, New Mexico; www.eyesopen.com. Accessed accessed 12, 2019). The scoring was done using the default ChemGauss4 scoring function (of the FRED module) which was based on the shape of the ligand, hydrogen bonding (H-bonding) between ligand and receptor, H-bonding interactions with implicit solvent, and metal-chelator interactions. Therefore, the binding scores are largely a representation of how well ligands fit into the receptor ligand binding pocket without being penalized for having unsuitable bonding environments (h-binding, hydrophobic, salt-bridging, etc.). BPs with larger negative values represent chemicals that bind better. Also, in the case of smaller and flexible ligands, comparable binding scores for agonist and antagonist conformations of the receptor can be found due to their adaptability to the area of the binding pocket without compromising other interaction parameters. Of note, the binding site of agonist and antagonist conformations of ER differ only by having variable orientations for the H12 helix. For AR, in the present study, we assume a similar scenario to estimate binding scores, even though no crystal conformation is available with a different H12 orientation for AR.
RESULTS
Estrogenic Activity of BPs
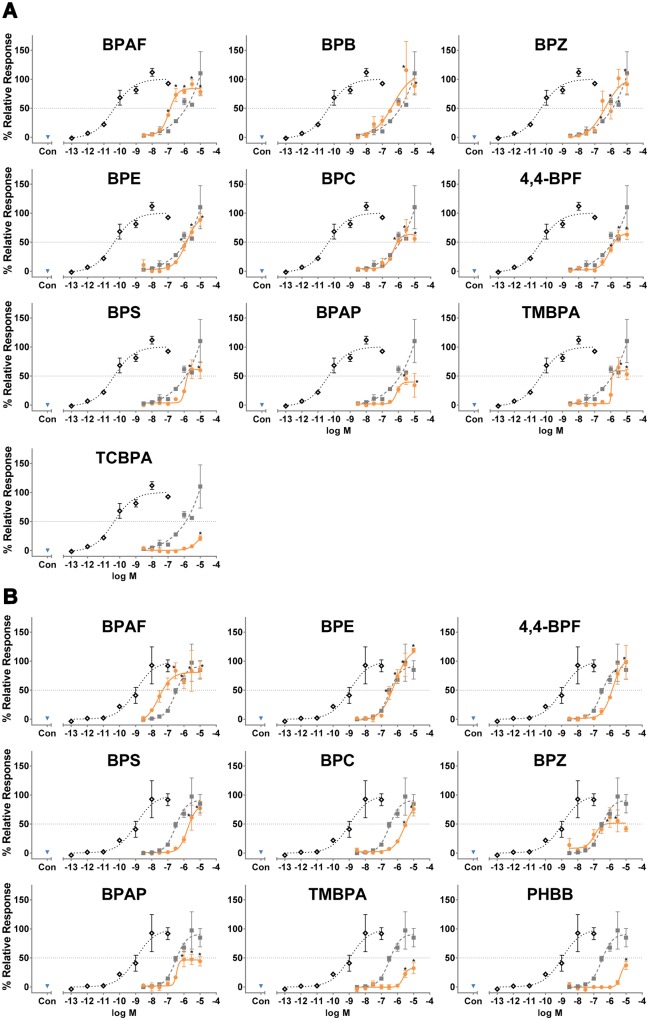
To evaluate the estrogenic activity of the 22 BPs, we performed luciferase reporter gene assays with HepG2 cells transiently transfected with ERα or ERβ (Table 1, Figure 2, Supplementary Figs. 1–3). For ERα, both E2 and BPA induced ERE-mediated transcriptional activity (Supplementary Figure 1), but BPA was over 28 000-fold less potent than E2 (Table 1). Induction of ERα/ERE-mediated activity by BPAF, BPB, and BPZ was 9.2, 3.8, and 3.0-fold more potent than by BPA, respectively (Table 1, Figure 2A). BPE had a similar potency and efficacy as BPA. Six BPs (BPC, 4,4-BPF, BPS, BPAP, TMBPA, TCBPA) were less efficacious than BPA (Figure 2A). Three BPs (BPP, BPPH, 2,4-BPS) had weak activity > 20% of the maximum activity induced by E2, but the findings were nonsignificant and therefore these BPs were considered inactive in this assay (Supplementary Figure 2). Of note, limited cytotoxicity between the concentrations of 3 × 10−8 and 1 × 10−6 M was observed in the CellTox Green assay for BPPH in HepG2 cells transfected with ERα. Four BPs (BPS-MAE, PHBB, D8, BPS-MPE) had significant activity at 1 × 10−5 M, but the activity was less than 20% of the maximum activity induced by E2, and therefore these BPs were considered inactive (Supplementary Figure 2). The remaining 4 BPs (D90, BPA bis(diphenyl phosphate) [BDP], Tetrabromobisphenol A [TBBPA], TGSA) were not statistically different from vehicle control and did not exceed 20% of the maximum response for E2, and were therefore considered inactive (Supplementary Figure 2). Other than that which was just mentioned for BPPH, cytotoxicity was not observed in HepG2 cells transfected with ERα and treated with BPs (data available in CEBS at https://doi.org/10.22427/NTP-DATA-002-00060-0001-0000-9. Accessed accessed 13, 2019). In addition, the ERα/ERE-mediated activity of BPAF, BPB, BPZ, BPE, BPC, 4,4-BPF, BPA, BPS, and BPAP was blocked by the ER antagonist ICI indicating the specificity of the ERα/ERE-mediated mechanism (Supplementary Figure 4).
Table 1.
Summary of Efficacy and Potency of BPs in ER and AR Luciferase Reporter Gene Assays
| ERα Agonism |
ERβ Agonism |
ERα Antagonism |
ERβ Antagonism |
AR Antagonism |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Max Efficacy (%) | Rel EC50 (M) | Max Efficacy (%) | Rel EC50 (M) | Max Inhibition (%) | Rel IC50 (M) | Max Inhibition (%) | Rel IC50 (M) | Max Inhibition (%) | Rel IC50 (M) | |
| E2 | 111.9 | 4.2E-11 | 92.8 | 1.1E-09 | 0 | na | 0 | — | na | na |
| ICI | na | na | na | na | 115.8 | — | 107.7 | — | na | na |
| T | na | na | na | na | na | na | na | na | 0 | na |
| Flut | na | na | na | na | na | na | na | na | 99.8 | 6.2E-07 |
| BPAF | 91.5 | 1.3E-07 | 85.3 | 4.6E-08 | 24.3 | — | −7.3 | — | 95.3 | 8.5E-07 |
| BPB | 115.7 | 3.2E-07 | 56.0 | — | 5.3 | — | −5.4 | — | 90.1 | 1.4E-06 |
| BPZ | 101.7 | 4.0E-07 | 61.3 | 5.0E-07 | 9.5 | — | 16.3 | — | 80.7 | 1.3E-06 |
| BPC | 71.0 | 7.8E-07 | 75.7 | 3.2E-06 | 9.4 | — | 29.9 | — | 96.2 | 1.0E-06 |
| TMBPA | 64.8 | 1.1E-06 | 32.3 | — | 32.2 | — | 34.4 | — | 99.1 | 7.4E-07 |
| BPA | 110.3 | 1.2E-06 | 97.4 | 3.5E-07 | 11.9 | — | 35.2 | — | 98.8 | 5.1E-07 |
| BPS | 61.6 | 1.3E-06 | 77.4 | 2.1E-06 | −4.5 | — | 6.1 | — | 30.7 | — |
| BPE | 88.4 | 1.4E-06 | 118.7 | 4.6E-07 | 2.1 | — | 19.9 | — | 89.2 | 1.0E-06 |
| 4,4-BPF | 63.1 | 1.6E-06 | 97.9 | 1.3E-06 | 37.6 | — | 12.5 | — | 66.2 | 5.9E-06 |
| BPP | 51.6 | 5.6E-06a | 3.4 | — | 65.0 | 4.3E-06a | 84.1 | 9.3E-07 | 62.5 | 6.5E-06 |
| BPS-MPE | 9.4 | — | 4.1 | — | 107.0 | 1.0E-06 | 36.7 | — | 17.5 | — |
| 2,4-BPS | 24.3 | — | 3.1 | — | 87.1 | 1.9E-06 | 57.4 | — | 22.1 | — |
| BPPH | 27.0 | — | 12.9 | — | 26.4 | — | 30.4 | — | 70.6 | 5.8E-06 |
| BPAP | 45.4 | — | 47.6 | — | −6.9 | — | 17.0 | — | 36.8 | — |
| TCBPA | 21.3 | — | 15.2 | — | 5.7 | — | 40.5 | — | 32.7 | — |
| PHBB | 16.4 | — | 37.2 | — | 18.1 | — | 38.4 | — | 29.4 | — |
| BPS-MAE | 19.7 | — | 6.0 | — | 39.2 | — | 38.7 | — | 38.0 | — |
| D8 | 11.2 | — | 6.1 | — | 16.7 | — | 98.0 | — | 54.0 | — |
| BDP | 10.3 | — | 10.5 | — | 26.4 | — | 18.1 | — | 18.1 | — |
| D90 | 6.6 | — | 3.1 | — | 45.4 | — | 30.2 | — | 36.3 | — |
| TBBPA | 3.6 | — | 7.6 | — | −0.2 | — | 22.0 | — | 26.2 | — |
| TGSA | 3.0 | — | 2.0 | — | 70.6 | — | 47.2 | — | 35.6 | — |
Maximum efficacy is reported as greatest % relative response of E2. Maximum inhibition is reported as the greatest % relative inhibition of 1.0 nM E2 or T. The reported maximum efficacy and inhibition are observed values. Relative (Rel) EC50 and Rel IC50 are the concentrations at which the modeled curve cross the line marking the 50% maximum response of E2 or T. Cells with “na” indicate that this was not tested in the given assay system. A dash (—) indicates that a value could not be calculated. Abbreviations: BPs, bisphenol A-like chemicals; ER, estrogen receptor; AR, androgen receptor; BPAF, Bisphenol AF; BPB, bisphenol B; BPZ, bisphenol Z; BPC, bisphenol C; TMBPA, tetramethyl bisphenol A; BPA, Bisphenol A; BPS, bisphenol S; BPE, bisphenol E; 4,4-BPF, 4,4-bisphenol F; BPP, Bisphenol P; BPS-MPE, 4-(4-phenylmethoxyphenyl)sulfonylphenol; 2,4-BPS, 2,4-bisphenol; BPPH, bisphenol PH; BPAP, bisphenol AP; TCBPA, tetrachlorobisphenol A; PHBB, benzylparaben; na, not applicable.
Rel EC50 or Rel IC50 could be calculated for these BPs though they are not considered active in the given assay because they were not statistically significant.
Figure 2.
Bisphenol A-like chemicals (BPs) induce estrogen receptor/estrogen response element (ER/ERE)-mediated transcriptional activity. Agonism of (A) ERα/ERE or (B) ERβ/ERE-mediated transcriptional activity in transiently transfected HepG2 cells. Each plot shows the vehicle control (blue down triangle), and the dose response curves for E2 (black open diamond), bisphenol A (BPA) (gray closed square) and the indicated BP (orange closed circle). A horizontal gray dotted line marks the 50% maximum response of E2. Only BPs considered active are shown in this figure; inactive BPs are shown in Supplementary Figure 2 (for ERα) and Supplementary Figure 4 (for ERβ). Data are presented as mean response normalized to vehicle control and the maximum E2 response ± SEM from n = 3 independent experiments. *p < .05 versus vehicle control by ANOVA and Dunnett’s multiple comparison post hoc test. For improved clarity, only statistics for the indicated BP are shown in this figure. See Supplementary Figure 1 for statistics for E2 and BPA.
For ERβ, both E2 and BPA induced ERE-mediated activity (Supplementary Figure 1), but BPA was nearly 300-fold less potent than E2 (Table 1). Induction of ERβ/ERE-mediated activity by BPAF was 7.6-fold more potent than by BPA (Table 1, Figure 2B). BPE once again had a similar potency compared to BPA (Table 1, Figure 2B). Induction by 4,4-BPF reached the same maximum efficacy as BPA, but was less potent than BPA (Table 1, Figure 2B). In contrast, BPS, BPC, and BPZ were less efficacious and less potent than BPA (Table 1, Figure 2B). BPAP, TMBPA, and PHBB were active but did not reach 50% of the maximum response of E2 (Table 1, Figure 2B). BPB tended to be active, with a similar potency to BPA, but the data from one of the replicate runs was very variable. Ultimately, because none of the BPB concentrations tested were significantly different from vehicle, BPB was considered inactive (Supplementary Figure 3). TCBPA had significant activity at 1 × 10−5 M, but the activity was less than 20% of the maximum value for E2, and therefore TCBPA was considered inactive (Supplementary Figure 3). The remaining 10 BPs (BPPH, BDP, TBBPA, D8, BPS-MAE, BPS-MPE, BPP, 2,4-BPS, D90, TGSA) were inactive for ERβ receptor activity (Supplementary Figure 3). In HepG2 cells transfected with ERβ and treated with BPs, cytotoxicity was only observed in the CellTox Green assay for BPS-MPE at 1 × 10−7 M (data available in CEBS at https://doi.org/10.22427/NTP-DATA-002-00060-0001-0000-9. Accessed accessed 13, 2019).
To further investigate the ER-dependent response of the BPs that showed activation in the luciferase reporter gene assays, we examined the effects of those BPs on 2 well-characterized ER target genes, GREB1 and PGR, using real-time RT-PCR analysis in ERα positive MCF7 cells (Figure 3). All the selected BPs significantly induced expression of GREB1 and PGR, excepting TCBPA, which did not induce the expression of either gene. These results indicate that BPs can alter transcriptional regulation directly on endogenous ER target genes.
Figure 3.
Bisphenol A-like chemicals (BPs) induce endogenous estrogen receptor (ER) target gene expression in MCF7 cells. Cells were treated with 10 nM E2 or 1 µM of an individual BP. Total RNA was extracted and used as a template for cDNA synthesis. Gene expression was measured by qPCR. Results are average fold change relative to vehicle control + SEM from n = 3 independent experiments. *p < .05 compared to vehicle control.
The BPs exhibited a wide range of responses in the assays for estrogenic activity. All of the estrogenic BPs were less potent than E2 at inducing ERα/ERE- or ERβ/ERE-mediated activity. BPAF was more potent than BPA in both ERα/ERE and ERβ/ERE-mediated assays. Some of the BPs were active on both receptors (BPAF, BPZ, BPE, BPC, 4,4-BPF, BPS, BPAP, TMBPA), though the potency and efficacy relative to BPA and E2 differed in some instances for the 2 receptors (eg, 4,4-BPF, BPC). In contrast, BPB and TCBPA were only active in the assays for ERα/ERE-mediated activity, whereas PHBB was only active in the assay for ERβ/ERE-mediated activity. BPP, tended to induce ERα/ERE-mediated activity, but ultimately this was not statistically significant, though BPP did induce expression of GREB1 in MCF7 cells (data not shown). BPPH, 2,4-BPS, BPS-MAE, D8, BDP, BPS-MPE, D90, TBBPA, and TGSA were not estrogenic in any of the assays.
Antiestrogenic Activity by BPs
The antagonistic potential of the BPs was tested using the luciferase reporter gene assays with transiently transfected HepG2 cells exposed to each BP in the presence of 1.0 nM E2 (Table 1, Figure 4, Supplementary Figs. 5 and 6). ICI fully blocked both E2-induced ERα/ERE- and ERβ/ERE-mediated activity between 1 × 10−5 and 1 × 10−8 M, whereas BPA did not antagonize E2 in cells transfected with either ERα or ERβ (Supplementary Figure 1). Only BPS-MPE and 2,4-BPS were considered ERα antagonists (Figure 4A). BPP, BPS-MAE, 4,4-BPF, TMBPA, and BDP reduced E2-induced ERα/ERE-mediated activity by ≥ 20%, but their activities were not significantly different from that of 1.0 nM E2 (Supplementary Figure 5), and were therefore considered inactive. The remaining BPs (TGSA, D90, BPPH, BPAF, PHBB, D8, BPZ, BPC, TCBPA, BPB, BPE, TBBPA, BPS, BPAP) did not antagonize E2-induced ERα/ERE-mediated activity (Supplementary Figure 5). Likewise, only BPP was considered an ERβ antagonist (Figure 4B). 2,4-BPS reduced E2-induced ERβ/ERE-mediated activity by ≥ 20%, but its activity was not significantly different from that of 1.0 nM E2 (Supplementary Figure 6). The remaining BPs (D8, D90, BPS-MAE, TGSA, TCBPA, PHBB, BPS-MPE, TMBPA, BPPH, BPC, TBBPA, BPE, BDP, BPAP, BPZ, 4,4-BPF, BPS, BPB, BPAF) did not antagonize E2-induced ERβ/ERE-mediated activity (Supplementary Figure 6).
Figure 4.
Bisphenol A-like chemicals (BPs) antagonize E2 induction of estrogen receptor/estrogen response element (ER/ERE)-mediated transcriptional activity. Antagonism of (A) ERα/ERE or (B) ERβ/ERE-mediated transcriptional activity in transiently transfected HepG2 cells concurrently treated with 1.0 nM E2. Each plot shows hormone free vehicle control (blue down triangle), 1.0 nM E2 (black open diamond), and the dose response curves for ICI (green X), bisphenol A (BPA) (gray closed square) and the indicated BP (orange closed circle). A horizontal gray dotted line marks 50% of the maximum response of 1.0 nM E2. Only BPs considered active are shown in this figure; inactive BPs are shown in Supplementary Figure 5 (for ERα) and Supplementary Figure 6 (for ERβ). Data are presented as mean response normalized to vehicle control and the 1.0 nM E2 response ± SEM from n = 3 independent experiments. *p < .05 versus 1.0 nM E2 by ANOVA and Dunnett’s multiple comparison post hoc test. For improved clarity, only statistics for the indicated BP are shown in this figure. See Supplementary Figure 1 for statistics for ICI and BPA.
Cytotoxicity was measured using the CellTox Green reagent in order to ensure that a decrease in signal was due to receptor-mediated antagonism rather than an increase in cell death. No cytotoxicity was observed in the CellTox Green assay for the BPs considered active ERα or ERβ antagonists. Thus, the observed decrease in E2-induced ER/ERE-mediated activity observed for BPS-MPE, 2,4-BPS, and BPP is specific antagonistic activity not due to cytotoxicity. In HepG2 cells transfected with ERα and treated with BPs in the presence of E2, cytotoxicity was observed only for D8 between the concentrations of 1 × 10−8 and 1 × 10−6 M and for TGSA between the concentrations of 3 × 10−9 and 3 × 10−6 M. No cytotoxicity was observed in the CellTox Green assay in HepG2 cells transfected with ERβ and treated with BPs in the presence of E2 (data available in CEBS at https://doi.org/10.22427/NTP-DATA-002-00060-0001-0000-9. Accessed accessed 13, 2019).
Androgenic and Antiandrogenic Activity by BPs
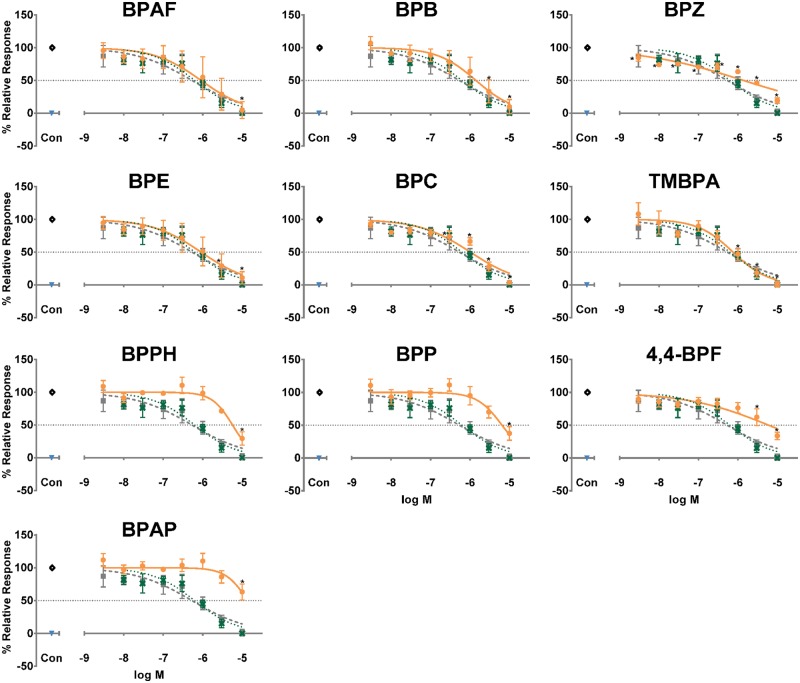
To investigate the androgenic activity of the BPs, we performed the luciferase reporter gene assay in MDA-kb2 cells stably transfected with the MMTV luciferase reporter (Wilson et al., 2002). None of the BPs induced AR/MMTV-mediated activity (Supplementary Figure 7). For AR antagonism, the positive control antiandrogen, Flut, antagonized T-induced AR/MMTV-mediated activity with an IC50 of 6.2 × 10−7 M and BPA antagonized T with similar potency (5.1 × 10−7 M) (Table 1, Supplementary Figure 1). Six BPs (BPAF, BPB, BPZ, BPE, BPC, TMBPA) had similar potencies and fully antagonized T-induced AR-mediated activity (Table 1, Figure 5). Three BPs (BPPH, BPP, 4,4-BPF) reduced activity > 50% but were less potent than BPA and Flut (IC50 of 5.8 × 10−6, 6.5 × 10−6, and 5.9 × 10−6) (Table 1, Figure 5). BPAP reduced activity by 37% (Table 1, Figure 5). Four BPs (BPS-MAE, TGSA, D90, TCBPA) significantly reduced activity ≥ 20%, but lacked a clear dose response curve and were therefore considered inactive (Supplementary Figure 8). The remaining BPs (TBBPA, D8, 2,4-BPS, PHBB, BPS, BDP, BPS-MPE) did not significantly reduce activity by ≥ 20%, so were considered inactive (Supplementary Figure 8). In addition, cytotoxicity was measured in tandem using the CellTox Green reagent in order to ensure that a decrease in signal was due to receptor-mediated antagonism rather than an increase in cell death. No cytotoxicity was observed in the CellTox Green assay in MDA-kb2 cells treated with BPs in the presence of T supporting the determinations of specific and true receptor antagonism (data available in CEBS at https://doi.org/10.22427/NTP-DATA-002-00060-0001-0000-9. Accessed accessed 13, 2019).
Figure 5.
Bisphenol A-like chemicals (BPs) antagonize T-induced AR/MMTV-mediated transcriptional activity. Antagonism of AR/MMTV-mediated activity in stably transfected MDA-kb2 cells treated concurrently with 1.0 nM T. Each plot shows hormone free vehicle control (blue down triangle), 1.0 nM T (black open diamond), and the dose response curves for Flut (green X), bisphenol A (BPA) (gray closed square) and the indicated BP (orange closed circle). A horizontal gray dotted line marks 50% of the maximum response of 1.0 nM T. Only BPs considered active are shown in this figure; inactive BPs are shown in Supplementary Figure 8. Data are presented as mean response normalized to vehicle control and the 1.0 nM T response ± SEM from n = 3 independent experiments. *p < .05 versus 1.0 nM T by ANOVA and Dunnett’s multiple comparison post hoc test. For improved clarity, only statistics for the indicated BP are shown in this figure. See Supplementary Figure 1 for statistics for Flut and BPA.
Molecular Modeling of BPs Binding to ERα and AR
A series of molecular dockings of the compounds used in this experiment were performed with both ERα and AR receptors (Tables 2 and 3). Using the docking protocol, E2, the endogenous ligand for ERα, was found to be docked into a conformation resembling the X-ray crystal structure found in PDB ID: 3UUD with the strongest binding score of −16.95 (Table 2). BPAP and BPZ exhibited weaker but similar binding scores for ERα among the scores for compounds listed in Table 2. Several BPs (BPC, TMBPA, BPAF, BPA, BPB, BPE, TCBPA, BPPH, 4,4-BPF, BPP, PHBB, TBBPA, TGSA, BPS) fall into the next category in which binding of the ligand to the ER receptor in the agonist confirmation was slightly stronger than in the antagonist confirmation. In contrast, compounds such as 2,4-BPS, BPS-MAE, D8, BDP, BPS-MPE, and D90 had a preference for antagonist conformations when their binding’s scores were compared (Table 2).
Table 2.
BPs Binding Scores for ERα, Residues Making H-bonding, and the Contact Area by the Molecular Modeling Analysis
| Ligands/BPs | Binding Score: Agonist | Binding Score: Antagonist | H-bonding | Contacts |
|---|---|---|---|---|
| E2 | −16.95 | −14.81 | R394, E353, H524 | A350, L346, L387, L525, G521, L391, M388, I424, L384, L428, M421, F404 |
| BPAP | −15.36 | −13.14 | L387, R394 | A350, L346, L525, L391, M388, I424, L384, L428, M421, F404 |
| BPZ | −15.33 | −14.64 | R394, E353 | A350, L346, L387, L525, L391, L540, M388, L384, M421, F404 |
| BPC | −13.83 | −12.76 | E353, G521, H524 | A350, L346, L387, L525, L349, L391, M388, I424, L384, L428, M421, F404 |
| TMBPA | −13.68 | −11.94 | L346, G521, H524 | A350, L387, L525, E353, L349, M388, I424, L384, L428, M421, F404 |
| BPAF | −13.54 | −12.68 | L346, L387, L525, M522, E353, G521, L391, L384, M421, F404 | |
| BPA | −13.54 | −12.85 | E353, G521, H524 | A350, L346, L525, L349, L391, M388, L384, L428, M421, F404 |
| BPB | −13.47 | −13.07 | R394, E353 | A350, L346, L387, L525, L391, L540, M388, L384, M421, F404 |
| BPE | −13.32 | −12.83 | E353, G521, H524 | A350, L346, L525, L349, L391, M388, L384, L428, M421, F404 |
| TCBPA | −13.32 | −11.5 | E353, G521, H524 | A350, L346, L387, L525, L349, M388, L384, L428, M421, F404 |
| BPPH | −13.15 | −12.5 | G521, H524, L384 | A350, L387, L525, F425, R394, E353, L391, L540, M388, I424, L428, M421, F404 |
| 4,4-BPF | −13.08 | −11.97 | E353, G521, H524 | A350, L346, L525, L349, L384, M421, F404 |
| BPP | −13.07 | −11.3 | A350, D351, W384, L387, L525, L354, L384, L391, L525, F404, T347 | |
| PHBB | −12.78 | −12.5 | G521, H524 | A350, L346, L387, L525, M343, F425, L391, I424, L428, M421, F404 |
| TBBPA | −12.25 | −11.15 | L346, G521, H524 | A350, L346, L387, L525, L349, L391, M388, I424, L384, L428, M421, F404 |
| TGSA | −12.2 | −11.29 | L384, G521, H524 | A350, L346, L387, L525, M343, F425, L391, M388, I424, L428, M421, F404 |
| 2,4-BPS | −11.6 | −11.92 | L387, R394 | A350, L346, L525, E353, G521, L391, M388, L384, F404 |
| BPS | −11.23 | −11.04 | R394, G521 | A350, L346, L387, L525, E353, L391, L384, F404 |
| BPS-MAE | −10.31 | −11.56 | L387, R394 | A350, E353, W383, D351, L346, L354, L384, L391, L525, M388, F404, T347 |
| D8 | −9.95 | −11.24 | E353 | A350, L346, L387, L349, L525, W383, R394, D351, L354, L384, L525, F404, T347 |
| BDP | −9.28 | −11.71 | A350, E353, I358, L379, L387, M421, W383, D351, E380, H524, L346, L354, L384, L391, L525, M388, F404, T347 | |
| BPS-MPE | −6.73 | −10.93 | E353 | A350, L387, W383, R394, D351, L346, L384, L391, L525, F404, T347 |
| D90 | −5.2 | −11.24 | R394, E353 | A350, I358, L354, L384, L391, F404, W383, E380, L346, L379, L387, L525, M388, T347 |
The larger negative values indicate better binding ligands. Ligands are listed in decreasing predicted binding strength in the agonist confirmation. Small ligands that can easily adapt to the shape of the ligand binding site have similar agonist and antagonist binding scores.
Abbreviations: BPs, bisphenol A-like chemicals; ER, estrogen receptor; BPAP, bisphenol AP; BPZ, bisphenol Z; BPC, bisphenol C; TMBPA, tetramethyl bisphenol A; BPAF, Bisphenol AF; BPA, Bisphenol A; BPB, bisphenol B; BPE, bisphenol E; TCBPA, tetrachlorobisphenol A; BPPH, bisphenol PH; 4,4-BPF, 4,4-bisphenol F; BPP, Bisphenol P; PHBB, benzylparaben; 2,4-BPS, 2,4-bisphenol; BPS, bisphenol S; BPS-MPE, 4-(4-phenylmethoxyphenyl)sulfonylphenol.
Table 3.
BPs Binding Scores for AR, Residues Making H-bonding, and the Contact Area by the Molecular Modeling Analysis
| Ligands/BPs | Binding Score: Agonist | Binding Score: Antagonist | H-bonding | Contacts |
|---|---|---|---|---|
| DHT | −16.26 | −14.53 | N705, T877 | G708, L704, L880, M745, M780, M895, F876, Q711, M742, M749, F764, V746 |
| T | −16.19 | −14.38 | N705, T877 | G708, L704, L880, M745, M895, F876, Q711, M742, M749, F764, V746 |
| BPP | −15.31 | −14.61 | R752, T877, F764 | N705, L704, L880, M745, M895, F876, T877, R752, L707, M742, M749, W741, V746 |
| BPC | −13.93 | −12.91 | N705, T877 | G708, L704, M745, M780, M895, Q711, L707, M742, M749, F764, W741, V746 |
| TGSA | −13.77 | −12.22 | N705, T877 | G708, L704, M745, M780, M895, R752, Q711, L701, L707, L873, M742, M749, F764, W741, V746 |
| BPAP | −13.48 | −13.19 | N705, T877 | G708, I899, L704, M745, M780, M895, Q711, L707, L873, M742, M787, F764, F891, W741, V746 |
| BPB | −13.28 | −13.16 | N705, F764 | G708, L704, M745, M895, Q711, L707, L873, M742, M749, W741, V746 |
| BPA | −13.19 | −12.49 | N705, F764 | L704, M745, M895, Q711, L707, M742, M749, W741, V746 |
| BPAF | −13.11 | −12.67 | F764 | G708, L704, M745, M780, R752, Q711, L707, L873, M742, M787, W741, V746 |
| BPS-MPE | −13.02 | −12.3 | R752, Q711, F764 | N705, L704, L880, M745, M895, F976, T877, L701, M742, M749, W741, V746 |
| BPZ | −12.97 | −12.25 | R752, F764 | G708, L704, M745, M780, M895, L707, L873, M742, M749, M787, W741, V746 |
| TMBPA | −12.87 | −11.9 | F764 | N705, L704, M745, M780, R752, Q711, L707, L873, M742, M749, W741, V746 |
| PHBB | −12.8 | −12.31 | N705, T877 | G708, L704, L880, M745, Q711, L707, M742, M749, F764 |
| TCBPA | −12.67 | −11.97 | F764 | N705, G708, L704, M745, M780, T877, R752, Q711, L707, L873, M742, M749, F764, W741 |
| TBBPA | −12.59 | −11.64 | F764 | N705, G708, L704, M745, M780, T877, R752, Q711, L707, L873, M742, M749, M787, W741 |
| BPE | −12.56 | −11.79 | N705, F764 | G708, L704, M745, M895, Q711, M742, M749, W741 |
| BPS-MAE | −12.45 | −11.66 | F764 | G708, L704, M745, M780, F876, T877, Q711, L701, L707, L873, M742, M749 |
| D8 | −12.09 | −11 | F764 | G708, L704, L880, M745, M780, F876, T877, Q711, L707, M742, M749, W741 |
| BPPH | −11.98 | −13.48 | T877, N705, M742 | G708, L704, M745, M780, F764, T877, R752, Q711, L873, M749, W741, V746 |
| 4,4-BPF | −11.91 | −11.12 | N705, F764 | G708, L704, M745, M895, Q711, L707, M749, W741 |
| BPS | −11.88 | −10.83 | N705, F764 | G708, L704, M745, M895, Q711, M742, M749, W741 |
| 2,4-BPS | −11.38 | −10.93 | N705, M742 | G708, L704, M745, M895, Q711, M749, F764, W741, V746 |
| BDP | −7.9 | −10.92 | N705, G708, L704, L712, M745, M780, F876, T877, Q711, I737, Q738, L707, L873, M742, F764, W741 | |
| D90 | 1.68 | −10.82 | R752, Q711, L712, F764 | N705, G708, Q738, I737, M745, L704, M742, M749, W741, V715, V716, V746 |
The larger negative values indicate better binding ligands. Ligands are listed in decreasing predicted binding strength in the agonist confirmation. Small ligands that can easily adapt to the shape of the ligand binding site have similar agonist and antagonist binding scores.
Abbreviations: BPs, bisphenol A-like chemicals; AR, androgen receptor; DHT, dihydrotestosterone; BPP, Bisphenol P; BPC, bisphenol C; BPAP, bisphenol AP; BPB, bisphenol B; BPA, Bisphenol A; BPAF, Bisphenol AF; BPS-MPE, 4-(4-phenylmethoxyphenyl)sulfonylphenol; BPZ, bisphenol Z; TMBPA, tetramethyl bisphenol A; PHBB, benzylparaben; TCBPA, tetrachlorobisphenol A; BPE, bisphenol E; BPPH, bisphenol PH; 4, 4-BPF, 4, 4-bisphenol F; BPS, bisphenol S; 2, 4-BPS, 2, 4-bisphenol.
Most compounds exhibit H-bonding in their receptor bound form, except in the cases of BPAF, BPP, and BDP (Table 2). Both hydroxyl groups of E2 present at each end of the molecule had H-bonding; one with the salt bridge forming amino acid residues R394 and E353 and the other with H524 (see Supplementary Figure 8 for labelling). Most compounds showed strong H-bond forming capability particularly with only 1 of the hydroxyl groups (or an analog). Twelve ER residues were involved in E2 contacts, and most compounds maintain similar contacts with the same residues involved in ER contacts. 4,4-BPF, maintained the least number of contacts with ERα, with only 7 of the 12 contacts maintained. Likewise, BPS and its derivative 2,4-BPS also have a smaller number of contacting residues (8 and 9, respectively).
For the AR receptor, the endogenous ligands, DHT and T, showed the highest binding scores followed by BPP (Table 3). With the exception of BPPH, BDP, and D90, the BPs showed a slight preference in binding score toward the agonist form (Table 3). Only 1 compound, BDP, did not show any H-bonding in the docked conformation. The endogenous ligands T and DHT maintained 11 and 12 contacts, respectively with AR residues, whereas BPE, BPS, and 4,4-BPF maintained only 8 contacts (Table 3).
DISCUSSION
The aim of the current study was to concurrently test a large set of BPs that are structurally or functionally similar to BPA and may be used as replacements for BPA in consumer products. BPs, like other endocrine active chemicals, display cell type specific effects (Li et al., 2012). To date, studies that evaluate BPs ability to induce ER-mediated activity have been conducted in many mammalian cell types, including: human cervical cancer (HeLa), breast cancer (MCF7, T47D, BG1), endometrial cancer (Ishikawa), and liver cancer (HepG2) cell lines, as well as monkey kidney cells (CV1) (see Supplementary Figure 8 of Pelch et al., 2017 for references to primary literature). As an example of cell type specific responses, BPA and E2 are nearly equipotent in inducing activation of ERα/ERE-mediated reporter activity in HeLa cells, though BPA is less potent than E2 in Ishikawa and HepG2 cells (Li et al., 2012). These cell type specific effects are likely due to the presence or absences of different coregulators that may be involved in the ER-mediated activation in various cell types (Li et al., 2018). Therefore, testing the BPs under identical cell culture conditions is an important consideration if one has the goal of making comparisons across BPs in terms of relative efficacy and potency.
Here we used a luciferase reporter gene assay and find that 9 BPs (BPAF, BPZ, BPE, BPC, 4,4-BPF, BPS, BPAP, TMBPA, BPA) have agonistic activity for both ERα and ERβ, 2 BPs (BPB, TCBPA) have ERα specific agonistic activity, and 1 (PHBB) has ERβ specific agonistic activity. Eleven of these BPs also induce the expression of at least 1 endogenous estrogen responsive gene, GREB1 and/or PGR, suggesting that the actions are ER dependent. With that said, many of the BPs identified here as ER agonists have been previously reported as such in a variety of in vitro assays, including reporter gene assays like those conducted in this study and in MCF7 cell proliferation assays. For example, BPAF was the most potent ER agonist in this study, which is consistent with previous reports (Bermudez et al., 2010; Deliperi et al., 2003; Fic et al., 2014; Kitamura et al., 2005; Li et al., 2012; Matsushima et al., 2010; Song et al., 2014; Zhang et al., 2009) and reviewed by Pelch et al. (2017). Further, in previously published literature, BPS and TMBPA tend to be some of the least potent ER agonists (Delfosse et al., 2012; Grignard et al., 2012; Kang et al., 2014; Kitamura et al., 2005; Kuruto-Niwa et al., 2005; Molina-Molina et al., 2013; Rosenmai et al., 2014; Ruan et al., 2015; Teng et al., 2013). In the current study they were less efficacious than E2 and BPA, but their relative potency was very similar to that of BPA. These differences may be related to the cell types used in the different reports, as previous reports have all used cell types other than HepG2 (see Supplementary Figure 8 of Pelch et al., 2017). In addition, the relative estrogenic activity of BPs reported here is similar to that recently reported by Grimaldi et al., who investigated 13 of the same BPs in stably transfected HeLa cervical carcinoma cells (Grimaldi et al., 2019).
ER/ERE-mediated antagonist activity has not been well characterized for many BPs (see Supplementary Figure 8 in Pelch et al., 2017). In the current study only 3 BPs showed ERα or ERβ antagonistic activity. BPP antagonized E2-induced ERβ/ERE-mediated activity and tended to antagonize E2-induced ERα/ERE-mediated activity. Grimaldi et al., also recently identified BPP as an ERα and ERβ antagonist (Grimaldi et al., 2019). In contrast, BPS-MPE and 2,4-BPS specifically antagonized E2-induced ERα/ERE-mediated activity. To our knowledge, this is the first report of ER antagonistic activity for these 2 BPs (Pelch et al., 2019). The results we observed agree with previous reports of a lack of ER antagonistic activity for 4,4-BPF (Fic et al., 2014; Gaido et al., 2000; Kitamura et al., 2005), BPB (Kitamura et al., 2005; Wang et al., 2014), BPC (Wang et al., 2014), BPE (Gaido et al., 2000; Kitamura et al., 2005), and BPS (Kitamura et al., 2005). Further, in the current study we did not observe ER antagonism by BPAF. This is consistent with previous reports that find no ER antagonism by BPAF in HepG2 cells (Li et al., 2012), which is the cell type used in this study, though BPAF does appear to be a potent ERβ antagonist in HeLa cells (Delfosse et al., 2012; Li et al., 2012; Matsushima et al., 2010). Importantly, ERα and ERβ antagonism was observed in the absence of any significant cytotoxicity, suggesting specific receptor inhibition rather than confounding variables.
Generally speaking, the BPs that were identified as ER agonists were also AR antagonists (BPAF, BPB, BPZ, BPC, TMBPA, BPA, BPE, 4,4-BPF, BPAP). A notable exception is BPS, which induced both ERα/ERE and ERβ/ERE-mediated activity, but did not antagonize T-induced AR/MMTV-mediated activity. Additionally, BPP, which antagonized T-induced AR/MMTV-mediated activity, was not considered active in either of the assays of ER/ERE-mediated activity. Twelve BPs have previously been evaluated for their ability to inhibit androgen-mediated AR transcriptional activity in a variety of cell types (see Supplementary Figure 15 in Grimaldi et al., 2019; Pelch et al., 2017). BPAF, BPB, BPZ, BPE, BPC, TMBPA, 4,4-BPF, BPS, BPAP, TCBPA, and BPP have all been reported as AR antagonists (eg, Grimaldi et al., 2019; Kitamura et al., 2007; Kolsek et al., 2015; Rosenmai et al., 2014; and others). In prior studies, TMBPA and BPAF were the most potent antiandrogens, with TMBPA reportedly 10 times more potent that the positive control antiandrogen flutamide in NIH3T3 mouse fibroblast cells (Kitamura et al., 2005). In the current study using MDA-kb2 cells, TMBPA and BPAF had similar potency to BPA, which may signal cell type specific effects. Of the antiandrogenic BPs, BPS appeared to be the weakest, acting as an antiandrogen in some previously published reports (Roelofs et al., 2015) but not others (Molina-Molina et al., 2013). In the current report, we did not find BPS to be an AR antagonist. Only 1 prior study of AR antagonism used the same cell line we present here, MDA-kb2 cells, but the 5 BPs tested by Kolsek et al. (BPF, BPAF, BPC, BPS, BPZ) were tested only at a single dose, so their relative potency cannot be calculated or compared to our results (Kolsek et al., 2015). Our results, are however, comparable to those recently reported by Grimaldi et al., with the exceptions that we identified BPPH as an AR antagonist and Grimaldi et al., did not, and that Grimaldi et al., identified TCBPA as a weak AR antagonist, whereas we did not (Grimaldi et al., 2019). Importantly, AR antagonism was observed in the absence of any significant cytotoxicity, suggesting specific receptor inhibition rather than confounding variables.
Though we were able to accomplish our goal of providing a comprehensive data set of BPs tested under identical laboratory conditions, it is important to note that many data gaps still remain. To our knowledge, this is the first study to report on biological activity of BPS-MPE (Pelch et al., 2019). Further, it is only the second to report on the in vitro biological activity of BPS-MAE, TGSA, and 2,4-BPS, the third to report on the in vitro biological activity of BPPH, and the fifth to report on the in vitro biological activity of D8, despite that these chemicals are known to be in use in thermal receipt paper (Bjornsdotter et al., 2017; Goldinger et al., 2015; Kobayashi et al., 2006; NICCA USA Inc, 1996; Nishigori et al., 2012). We report here for the first time that BPS-MPE is a selective ERα antagonist. Our results also support previous studies that have found a lack of estrogenic activity for BPS-MAE, TGSA, and D8 (Bjornsdotter et al., 2017; Kuruto-Niwa et al., 2005; Terasaki et al., 2007). To our knowledge, however, there still remains no peer-reviewed studies evaluating the biological activity of other BPs that may be used as replacements to BPA in consumer and industrial products, including: BTUM (151882-81-4), DD-70 (93589-69-6), MBHA (5129-00-0), and Urea Urethane Compound (321860-75-7) (Pelch et al., 2019). Unfortunately, these BPs were not commercially available and as such we could not obtain them, or 2 additional poorly characterized BPs: 2,2-BPF (2467-02-9), and Pergafast 201 (232938-43-1) for testing in our in vitro assays.
In the current study, most of the BPs that induced ERα/ERE and ERβ/ERE-mediated activity in vitro were derivatives of BPA. The molecular modeling of these BPs predicted ERα binding favoring the agonist confirmation. The one BPA derivative predicted to favor the antagonist confirmation, BDP, did not induce or antagonize ERα/ERE or ERβ/ERE-mediated activity. In contrast, the BPS-derivatives (ie, those that contain a sulfonyl group) were active in fewer in vitro assays and tended to have predicted ERα binding favoring the antagonist confirmation. The one notable exception to this statement is BPS, which induced both ERα/ERE and ERβ/ERE-mediated activity and had predicted ERα binding favoring the agonist confirmation. The only other BPS-derivatives that were active in in vitro assays, BPS-MPE and 2,4-BPS, were found to be ERα specific antagonists, which supported the molecular modeling findings that these 2 BPs favored the antagonist confirmation. The remaining BPS-derivatives (BPS-MAE, D8, D90, TGSA) were considered inactive in the in vitro assays. It could be noted though, that the induction of ERα/ERE-mediated activity by BPS-MAE was just under the 20% cut off to be considered active in the agonist assay (19.8% activation relative to the maximum E2 response), and that the inhibition of E2-induced ERα/ERE-mediated activity was > 20% (39.2% inhibition of 1.0 nM E2 response), but the findings were not statistically significant. This may suggest that this chemical should be carefully considered in future studies.
Because, in the present study, molecular modeling depends only on the docking, it is rather difficult to directly evaluate the possible refolding events of the receptors (ER and AR) that result in agonist or antagonist forms without actually performing lengthy molecular dynamics simulations. However, for ERα we took advantage of using crystal structures of the receptor available in agonist or antagonist conformations depending on the bound ligand. In contrast, for AR, there is no such distinct difference observed in the available crystal structures when various agonist and antagonist ligands bind to AR. Binding scores are more representative of the binding strength of the ligand, and serve only partially in the characterization of the agonist or antagonist behavior of receptors resulting from ligand binding. This may explain why most of the BPs had a slight preference in binding score toward the agonist form of AR, though none of the BPs were found to induce AR/MMTV-mediated agonist activity.
Four different variants in the binding scores were observed for ER. Two compounds with high binding scores (BPAP and BPZ) show variable H-bonding and residue contact when compared with the high scoring E2 ligand binding. Both BPAP and BPZ lost H-bonding with H524 and it is possible that loss of this H-bond may be critical in localizing the ligands in the binding site. Our previous molecular dynamics studies (Li et al., 2018) of BPA, BPAF, and BPS binding to ER displayed molecular rotations in the bound ligands at the ligand binding cavity, displaying potential dynamic behavior in the coregulator binding surface. As observed from Table 2, the differences in H-bonding and residue contacts may easily be translated to cause significant differences in the coregulator binding surface that ultimately dictate whether the receptor acts like an agonist or antagonist. Interestingly, for ERα, 2 BPs (2,4-BPS, BPS-MPE) that lead to antagonist activity of the receptor displayed better scores when the antagonist conformation of ER was used for docking while showing the weakest calculated binding scores among the tested compounds.
For most molecular receptors, the location of the helix H12 in the ligand binding domain is important in deciding whether the ligand binding imposes an agonist or antagonist conformation. However, it is unclear whether this study is relevant for AR because no crystal structure of antagonist bound AR is available (Tan et al., 2015). The binding scores for the AR antagonists identified in our current experiments (BPZ, BPAF, BPE, BPC, TMBPA, BPB, BPA, 4,4-BPF, BPAP) are at least 2 units smaller than the endogenous androgens T and DHT. The 2 endogenous ligands make H-bonds with residues N705 and T877 while keeping contacts with 11 to 12 receptor residues. Interestingly, the AR antagonist BPZ makes 2 H-bonds with the same 2 residues as the endogenous ligands while keeping contacts with 12 residues. Both BPAF and TMBPA make only 1 H-bond with F764; BPB and BPE make an additional H-bond with N705; and BPZ makes an H-bond with R752. The rest of ligands make variable contacts with 8 to 12 residues. Once again, the variations of H-bonding and ligand-contacting residue composition found in the docking study may point to variabilities in the coregulator binding surface that dictates the agonistic or the antagonistic nature of the ligand. Importantly, which coregulators are present and available for binding depends on the cell type (Bhattacharjee et al., 2013).
Moving forward, it has been suggested that BPs be considered as a class in health hazard and risk assessments, and that attention should be paid to avoiding situations of regrettable substitution when moving away from BPA in consumer products (Thayer et al., 2016). To this end, information from well characterized BPs like BPA, BPS, BPAF, and 4,4-BPF could be used to make predictions about the biological activity of data poor BPs. This is particularly important given that some of the data poor BPs are already known to be used in consumer products (eg, BDP, Pergafast 201, TGSA; Bjornsdotter et al., 2017; Eckardt and Simat, 2017). In particular, the data set presented here should be useful in supporting future read across efforts. Importantly, however, read across efforts should work to incorporate the totality of the body of evidence, given the known cell type specific differences in potency and efficacy of BPs. Further there are known metabolism and toxicokinetic differences among the BPs, and uncertainty remains about the ability for in vitro assays of potency to predict in vivo potency (Conley et al., 2016; Gramec Skledar et al., 2015; Karrer et al., 2018). The speed and process by which BPs are metabolized in an organism and the cellular context in which a biological response is being evaluated are important considerations for future read across efforts that aim to assess BPs as a class.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
ACKNOWLEDGMENTS
We thank for Christina Teng, Raymond Tice, Andrew Rooney, Scott Auerbach, Michael DeVito, Yukitomo Arao for discussion. We thank Kembra Howdeshell and Sylvia Hewitt for helpful comments and review of the manuscript. We thank Brad Collins as coordinator of NTP for obtaining the compounds from MRI-Global.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences.
REFERENCES
- Acconcia F., Pallottini V., Marino M. (2015). Molecular mechanisms of action of BPA. Dose Response 13, 1559325815610582.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Chemistry Council (2013). About Bisphenol A: Information Sheets Available at: http://www.bisphenol-a.org/about/bpa-info/bpa-plastics.html. Accessed January 20, 2019.
- Becerra V., Odermatt J. (2013). Interferences in the direct quantification of bisphenol S in paper by means of thermochemolysis. J. Chromatogr. A 1275, 70–77. [DOI] [PubMed] [Google Scholar]
- Bermudez D. S., Gray L. E. Jr, Wilson V. S. (2010). Modeling the interaction of binary and ternary mixtures of estradiol with bisphenol A and bisphenol AF in an in vitro estrogen-mediated transcriptional activation assay (T47D-KBluc). Toxicol. Sci. 116, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S., Renganaath K., Mehrotra R., Mehrotra S. (2013). Combinatorial control of gene expression. Biomed Res. Int. 2013, 407263.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdotter M. K., Jonker W., Legradi J., Kool J., Ballesteros-Gomez A. (2017). Bisphenol A alternatives in thermal paper from the Netherlands, Spain, Sweden and Norway. Screening and potential toxicity. Sci. Total Environ. 601–602, 210–221. [DOI] [PubMed] [Google Scholar]
- Burns K. A., Korach K. S. (2012). Estrogen receptors and human disease: An update. Arch. Toxicol. 86, 1491–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacho J. I., Campillo N., Vinas P., Hernandez-Cordoba M. (2012). Stir bar sorptive extraction coupled to gas chromatography-mass spectrometry for the determination of bisphenols in canned beverages and filling liquids of canned vegetables. J. Chromatogr. A 1247, 146–153. [DOI] [PubMed] [Google Scholar]
- Charisiadis P., Andrianou X. D., van der Meer T. P., den Dunnen W. F. A., Swaab D. F., Wolffenbuttel B. H. R., Makris K. C., van Vliet-Ostaptchouk J. V. (2018). Possible obesogenic effects of bisphenols accumulation in the human brain. Sci. Rep. 8, 8186.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobellis L., Colacurci N., Trabucco E., Carpentiero C., Grumetto L. (2009). Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. 23, 1186–1190. [DOI] [PubMed] [Google Scholar]
- Cobellis L., Panariello A., Campitiello M. R., Nocerino A., Pacilio C., Salzillo M. E., Castaldi M. A., Boccia O., Borrelli A. (2010). Relationship between endometriosis and exposure to BPA and BPB. Giornale Italiano di Ostetricia e Ginecologia 32, 44–48. [Google Scholar]
- Conley J. M., Hannas B. R., Furr J. R., Wilson V. S., Gray L. E. Jr (2016). A demonstration of the uncertainty in predicting the estrogenic activity of individual chemicals and mixtures from an in vitro estrogen receptor transcriptional activation assay (T47D-KBluc) to the in vivo uterotrophic assay using oral exposure. Toxicol. Sci. 153, 382–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha S. C., Fernandes J. O. (2010). Quantification of free and total bisphenol A and bisphenol B in human urine by dispersive liquid-liquid microextraction (DLLME) and heart-cutting multidimensional gas chromatography-mass spectrometry (MD-GC/MS). Talanta 83, 117–125. [DOI] [PubMed] [Google Scholar]
- Delfosse V., Grimaldi M., Pons J. L., Boulahtouf A., Le Maire A., Cavailles V., Labesse G., Bourguet W., Balaguer P. (2012). Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. U.S.A. 109, 14930–14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliperi S., Bardwell D. N., Papathanasiou A. (2003). Effect of different polymerization methods on composite microleakage. Am. J. Dent. 16, 73A–76A. [PubMed] [Google Scholar]
- Deroo B. J., Korach K. S. (2006). Estrogen receptors and human disease. J. Clin. Invest. 116, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt M., Simat T. J. (2017). Bisphenol A and alternatives in thermal paper receipts—A German market analysis from 2015 to 2017. Chemosphere 186, 1016–1025. [DOI] [PubMed] [Google Scholar]
- Ferguson K. K., Meeker J. D., Cantonwine D. E., Mukherjee B., Pace G. G., Weller D., McElrath T. F. (2018). Environmental phenol associations with ultrasound and delivery measures of fetal growth. Environ. Int. 112, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fic A., Zegura B., Gramec D., Masic L. P. (2014). Estrogenic and androgenic activities of TBBA and TBMEPH, metabolites of novel brominated flame retardants, and selected bisphenols, using the XenoScreen XL YES/YAS assay. Chemosphere 112, 362–369. [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Maness S. C., McDonnell D. P., Dehal S. S., Kupfer D., Safe S. (2000). Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: Structure-activity studies. Mol. Pharmacol. 58, 852–858. [PubMed] [Google Scholar]
- Goldinger D. M., Demierre A. L., Zoller O., Rupp H., Reinhard H., Magnin R., Becker T. W., Bourqui-Pittet M. (2015). Endocrine activity of alternatives to BPA found in thermal paper in Switzerland. Regul. Toxicol. Pharmacol. 71, 453–462. [DOI] [PubMed] [Google Scholar]
- Gramec Skledar D., Troberg J., Lavdas J., Peterlin Masic L., Finel M. (2015). Differences in the glucuronidation of bisphenols F and S between two homologous human UGT enzymes, 1A9 and 1A10. Xenobiotica 45, 511–519. [DOI] [PubMed] [Google Scholar]
- Grignard E., Lapenna S., Bremer S. (2012). Weak estrogenic transcriptional activities of bisphenol A and bisphenol S. Toxicol. In Vitro 26, 727–731. [DOI] [PubMed] [Google Scholar]
- Grimaldi M., Boulahtouf A., Toporova L., Balaguer P. (2019). Functional profiling of bisphenols for nuclear receptors. Toxicology 420, 39–45. [DOI] [PubMed] [Google Scholar]
- Hsieh J. H., Sedykh A., Huang R., Xia M., Tice R. R. (2015). A data analysis pipeline accounting for artifacts in Tox21 quantitative high-throughput screening assays. J. Biomol. Screen. 20, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. S., Choi J. S., Kim W. K., Lee Y. J., Park J. W. (2014). Estrogenic potency of bisphenol S, polyethersulfone and their metabolites generated by the rat liver S9 fractions on a MVLN cell using a luciferase reporter gene assay. Reprod. Biol. Endocrinol. 12, 102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer C., Roiss T., von Goetz N., Gramec Skledar D., Peterlin Masic L., Hungerbuhler K. (2018). Physiologically based pharmacokinetic (PBPK) modeling of the bisphenols BPA, BPS, BPF, and BPAF with new experimental metabolic parameters: Comparing the pharmacokinetic behavior of BPA with its substitutes. Environ. Health Perspect. 126, 077002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria A., Levine D., Wertenteil S., Vento S., Xue J., Rajendiran K., Kannan K., Thurman J. M., Morrison D., Brody R., et al. (2017). Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr. Res. 81, 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley B. P., Brown S. P., Warren G. L., Muchmore S. W. (2015). POSIT: Flexible shape-guided docking for pose prediction. J. Chem. Inf. Model. 55, 1771–1780. [DOI] [PubMed] [Google Scholar]
- Kitamura S., Suzuki T., Sanoh S., Kohta R., Jinno N., Sugihara K., Yoshihara S., Fujimoto N., Watanabe H., Ohta S. (2005). Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 84, 249–259. [DOI] [PubMed] [Google Scholar]
- Kitamura S., Suzuki T., Sugihara K., Uramaru N., Kuroki H., Fujimoto N., Ohta S. (2007). Thyroid hormonal and estrogenic activity of OH-PCB and OH-PBDE in cell culture. Organohalogen Compd. 69, 213/1–213/3. [Google Scholar]
- Kobayashi S., Shinohara H., Tabata K., Yamamoto N., Miyai A. (2006). Stereo structure-controlled and electronic structure-controlled estrogen-like chemicals to design and develop non-estrogenic bisphenol A analogs based on chemical hardness concept. Chem. Pharm. Bull. 54, 1633–1638. [DOI] [PubMed] [Google Scholar]
- Kolsek K., Gobec M., Mlinaric Rascan I., Sollner Dolenc M. (2015). Screening of bisphenol A, triclosan and paraben analogues as modulators of the glucocorticoid and androgen receptor activities. Toxicol. In Vitro 29, 8–15. [DOI] [PubMed] [Google Scholar]
- Kuruto-Niwa R., Nozawa R., Miyakoshi T., Shiozawa T., Terao Y. (2005). Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ. Toxicol. Pharmacol. 19, 121–130. [DOI] [PubMed] [Google Scholar]
- Lehmler H. J., Liu B., Gadogbe M., Bao W. (2018). Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 3, 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Burns K. A., Arao Y., Luh C. J., Korach K. S. (2012). Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor alpha and beta in vitro. Environ. Health Perspect. 120, 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luh C. J., Burns K. A., Arao Y., Jiang Z., Teng C. T., Tice R. R., Korach K. S. (2013). Endocrine-disrupting chemicals (EDCs): In vitro mechanism of estrogenic activation and differential effects on ER target genes. Environ. Health Perspect. 121, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Perera L., Coons L. A., Burns K. A., Tyler Ramsey J., Pelch K. E., Houtman R., van Beuningen R., Teng C. T., Korach K. S. (2018). Differential in vitro biological action, coregulator interactions, and molecular dynamic analysis of bisphenol A (BPA), BPAF, and BPS ligand-ERalpha complexes. Environ. Health Perspect. 126, 017012.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., Kannan K. (2013). Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric. Food Chem. 61, 4655–4662. [DOI] [PubMed] [Google Scholar]
- Liao C., Kannan K. (2014). A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch. Environ. Contam. Toxicol. 67, 50–59. [DOI] [PubMed] [Google Scholar]
- Liao C., Liu F., Alomirah H., Loi V. D., Mohd M. A., Moon H. B., Nakata H., Kannan K. (2012a). Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures. Environ. Sci. Technol. 46, 6860–6866. [DOI] [PubMed] [Google Scholar]
- Liao C., Liu F., Guo Y., Moon H. B., Nakata H., Wu Q., Kannan K. (2012b). Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: Implications for human exposure. Environ. Sci. Technol. 46, 9138–9145. [DOI] [PubMed] [Google Scholar]
- Liao C., Liu F., Kannan K. (2012c). Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ. Sci. Technol. 46, 6515–6522. [DOI] [PubMed] [Google Scholar]
- Liu B., Lehmler H. J., Sun Y., Xu G., Liu Y., Zong G., Sun Q., Hu F. B., Wallace R. B., Bao W. (2017). Bisphenol A substitutes and obesity in US adults: Analysis of a population-based, cross-sectional study. Lancet Planet. Health 1, e114–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Matsushima A., Liu X., Okada H., Shimohigashi M., Shimohigashi Y. (2010). Bisphenol AF is a full agonist for the estrogen receptor ERalpha but a highly specific antagonist for ERbeta. Environ. Health Perspect. 118, 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendum T., Stoler E., VanBenschoten H., Warner J. C. (2011). Concentration of bisphenol A in thermal paper. Green Chem. Lett. Rev. 4, 81–86. [Google Scholar]
- Molina-Molina J. M., Amaya E., Grimaldi M., Saenz J. M., Real M., Fernandez M. F., Balaguer P., Olea N. (2013). In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 272, 127–136. [DOI] [PubMed] [Google Scholar]
- Mueller S. O., Hall J. M., Swope D. L., Pedersen L. C., Korach K. S. (2003). Molecular determinants of the stereoselectivity of agonist activity of estrogen receptors (ER) alpha and beta. J. Biol. Chem. 278, 12255–12262. [DOI] [PubMed] [Google Scholar]
- Mustieles V., Williams P. L., Fernandez M. F., Minguez-Alarcon L., Ford J. B., Calafat A. M., Hauser R., Messerlian C., Environment., and Reproductive Health Study, T. (2018). Maternal and paternal preconception exposure to bisphenols and size at birth. Hum. Reprod. 33, 1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICCA USA Inc (1996). Initial submission: Letter from NICCA U.S.A. Inc to USEPA regarding genotoxicity testing of phenol, 2-[(4-hydroxyphenyl)sulfonyl], with attachments and dated 3/5/96. Govt Reports Announcements & Index (GRA&I), Issue 07, 2009. Available from Toxline Database..
- Nishigori M., Nose T., Shimohigashi Y. (2012). Highly potent binding and inverse agonist activity of bisphenol A derivatives for retinoid-related orphan nuclear receptor RORgamma. Toxicol. Lett. 212, 205–211. [DOI] [PubMed] [Google Scholar]
- Pelch K., Wignall J. A., Goldstone A. E., Ross P. K., Blain R. B., Shapiro A. J., Holmgren S. D., Hsieh J.-H., Svoboda D., Auerbach S. S., et al. (2019). A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 424, 152235. [DOI] [PubMed] [Google Scholar]
- Pelch K. E., Wignall J. A., Goldstone A. E., Ross P. K., Blain R. B., Shapiro A. J., Holmgren S. D., Hiseh J.-H., Svoboda D., Auerbach S. S., et al. (2017). NTP research report on biological activity of bisphenol A (BPA) structural analogues and functional alternatives Natl Toxicol Program Res Rep Ser. Research Triangle Park (NC): National Toxicology Program.(RR-04), 1–80. [PubMed] [Google Scholar]
- Prins G. S., Hu W. Y., Shi G. B., Hu D. P., Majumdar S., Li G., Huang K., Nelles J. L., Ho S. M., Walker C. L., et al. (2014). Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 155, 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester J. R. (2013). Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 42, 132–155. [DOI] [PubMed] [Google Scholar]
- Rochester J. R., Bolden A. L., Kwiatkowski C. F. (2018). Prenatal exposure to bisphenol A and hyperactivity in children: A systematic review and meta-analysis. Environ. Int. 114, 343–356. [DOI] [PubMed] [Google Scholar]
- Roelofs M. J., van den Berg M., Bovee T. F., Piersma A. H., van Duursen M. B. (2015). Structural bisphenol analogues differentially target steroidogenesis in murine MA-10 Leydig cells as well as the glucocorticoid receptor. Toxicology 329, 10–20. [DOI] [PubMed] [Google Scholar]
- Rosenmai A. K., Dybdahl M., Pedersen M., Alice van Vugt-Lussenburg B. M., Wedebye E. B., Taxvig C., Vinggaard A. M. (2014). Are structural analogues to bisphenol a safe alternatives? Toxicol. Sci. 139, 35–47. [DOI] [PubMed] [Google Scholar]
- Ruan T., Liang D., Song S., Song M., Wang H., Jiang G. (2015). Evaluation of the in vitro estrogenicity of emerging bisphenol analogs and their respective estrogenic contributions in municipal sewage sludge in China. Chemosphere 124, 150–155. [DOI] [PubMed] [Google Scholar]
- Song M., Liang D., Liang Y., Chen M., Wang F., Wang H., Jiang G. (2014). Assessing developmental toxicity and estrogenic activity of halogenated bisphenol A on zebrafish (Danio rerio). Chemosphere 112, 275–281. [DOI] [PubMed] [Google Scholar]
- Tan M. H., Li J., Xu H. E., Melcher K., Yong E. L. (2015). Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 36, 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C., Goodwin B., Shockley K., Xia M., Huang R., Norris J., Merrick B. A., Jetten A. M., Austin C. P., Tice R. R. (2013). Bisphenol A affects androgen receptor function via multiple mechanisms. Chem. Biol. Interact. 203, 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M., Shiraishi F., Fukazawa H., Makino M. (2007). Occurrence and estrogenicity of phenolics in paper-recycling process water: Pollutants originating from thermal paper in waste paper. Environ. Toxicol. Chem. 26, 2356–2366. [DOI] [PubMed] [Google Scholar]
- Thayer K. A., Pelch K. E., Birnbaum L. S., Bucher J. R. (2016). Bisphenols: More unnecessary surprises. Endocr. Disruptors 4, e1131032. [Google Scholar]
- Tice R. R., Austin C. P., Kavlock R. J., Bucher J. R. (2013). Improving the human hazard characterization of chemicals: A Tox21 update. Environ. Health Perspect. 121, 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA (2014). Bisphenol A Alternatives in Thermal Paper (Final Report January 2014) Available at: http://www.epa.gov/dfe/pubs/projects/bpa/about.htm. Accessed March 31, 2014.
- US EPA (2016). Bisphenol A (BPA) Action Plan Available at: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/bisphenol-bpa-action-plan. Accessed July 20, 2016.
- Vandenberg L. N., Ehrlich S., Belcher S. M., Ben-Jonathan N., Dolinoy D. C., Hugo E. R., Hunt P. A., Newbold R. R., Rubin B. S., Saili K. S., et al. (2013). Low dose effects of bisphenol A. Endocr. Disruptors 1, e26490. [Google Scholar]
- Vandenberg L. N., Maffini M. V., Wadia P. R., Sonnenschein C., Rubin B. S., Soto A. M. (2007). Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology 148, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Huo W., Xu S., Zheng T., Zhang B., Li Y., Zhou A., Zhang Y., Hu J., Zhu Y., et al. (2018). Relationship between maternal exposure to bisphenol S and pregnancy duration. Environ. Pollut. 238, 717–724. [DOI] [PubMed] [Google Scholar]
- Wang S., Rijk J. C., Besselink H. T., Houtman R., Peijnenburg A. A., Brouwer A., Rietjens I. M., Bovee T. F. (2014). Extending an in vitro panel for estrogenicity testing: The added value of bioassays for measuring antiandrogenic activities and effects on steroidogenesis. Toxicol. Sci. 141, 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. S., Bobseine K., Lambright C. R., Gray L. E. Jr (2002). A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol. Sci. 66, 69–81. [DOI] [PubMed] [Google Scholar]
- Ye X., Wong L. Y., Kramer J., Zhou X., Jia T., Calafat A. M. (2015). Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ. Sci. Technol. 49, 11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. C., Chen L. Y., Liu S. S., Yin D. Q. (2009). Jointed estrogenic activities of bisphenol A and three of its analogs. Huan Jing Ke Xue 30, 260–265. [PubMed] [Google Scholar]
- Zhang Y., Dong T., Hu W., Wang X., Xu B., Lin Z., Hofer T., Stefanoff P., Chen Y., Wang X., et al. (2019). Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 123, 325–336. [DOI] [PubMed] [Google Scholar]
- Zhou F., Zhang L., Liu A., Shen Y., Yuan J., Yu X., Feng X., Xu Q., Cheng C. (2013). Measurement of phenolic environmental estrogens in human urine samples by HPLC-MS/MS and primary discussion the possible linkage with uterine leiomyoma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 938, 80–85. [DOI] [PubMed] [Google Scholar]
- Zhou X., Kramer J. P., Calafat A. M., Ye X. (2014). Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 944, 152–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.