Abstract
Pain frequently co-occurs with elevated post-traumatic stress symptoms (PTSS); women are at elevated risk for their co-occurrence. PTSS and pain are associated with poor sleep quality; yet, little research has examined how sleep impacts their co-occurrence. The current study examines the indirect role of sleep on the relationship between PTSS and pain. A community sample of 182 women completed psychometrically-sound questionnaires assessing PTSS, sleep quality, pain characteristics, depression and anxiety symptoms, and anxiety sensitivity. We examined how sleep quality impacted associations among PTSS and pain intensity and pain interference, while controlling for key psychological factors. Greater PTSS was associated with worse pain interference, and poor sleep quality had a significant indirect effect on this relationship. Sleep may represent a modifiable behavioral mechanism that contributes to the mutual maintenance of PTSS and pain in women. Future research is needed to further clarify the role of sleep quality in their co-occurrence.
Keywords: Chronic pain, Posttraumatic stress disorder, Sleep quality
Introduction
Chronic pain, defined as pain persisting longer than three months, is one of the most common (Johannes et al., 2010), costly (Gaskin & Richard, 2012), and debilitating (Burke et al., 2015; Nahin, 2015) health problems. Individuals with chronic pain are at risk for developing internalizing mental health problems such as posttraumatic stress disorder (PTSD), or sub-clinical PTSD symptoms (PTSS). PTSS include trauma-related symptoms of re-experiencing, avoidance, negative thoughts or feelings, and arousal and reactivity, which persist for longer than one month (APA, 2013). Whereas the 12-month prevalence of PTSD in the United States is around 4% (Kessler et al., 2012), the rate of PTSD among community samples with chronic pain is around 10% (Siqveland et al., 2017). Compared to men, women are at elevated risk for developing both PTSD (8.5% vs. 3.4%; McLean et al., 2011) and chronic pain (34% vs. 27%; Johannes et al., 2010), and thus may be at particular risk for developing comorbid chronic pain and PTSD. However, limited research has investigated co-occurring PTSD and pain in a community sample of women.
A number of theories explain the co-occurrence of PTSS and pain symptoms. These theories emphasize overlapping psychological factors between PTSS and chronic pain symptomology, the mutually reinforcing and bidirectional nature of PTSS and chronic pain symptoms (Sharp & Harvey, 2001), as well as their shared physiological and neurobiological factors (e.g., hyperarousal; Holley et al., 2016; Sharp & Harvey, 2001). Sharp and Harvey (2001) posited that psychological (i.e., depression symptoms, anxiety symptoms, anxiety sensitivity) and cognitive (i.e., attentional biases, reminders of the trauma, cognitive demand) factors are common to both disorders, and may mutually maintain their co-occurrence. Asmundson et al. (2002) further posited that shared vulnerability factors, such as anxiety sensitivity, may predispose individuals to develop co-occurring PTSS and chronic pain. Indeed, it is established that psychological factors including depression symptoms (Morasco et al., 2013), anxiety symptoms and anxiety sensitivity (Asmundson & Katz, 2009) are implicated in the co-occurrence of chronic pain and PTSS.
Although less studied in the context of this co-occurrence, poor sleep quality is another factor shared by chronic pain and PTSS. A great deal of literature demonstrates direct associations between poor sleep and PTSS. For example, a recent review concluded that poor sleep quality is ubiquitous in both veteran and civilian populations with PTSD (Cox & Olatunji, 2016). A review of longitudinal studies in this area concluded that sleep may be a mechanism in the maintenance of PTSD (Babson & Feldner, 2010) and research has demonstrated direct links between poor sleep quality and chronic pain (Smith & Haythornthwaite, 2004). Chronic pain and sleep deficiency share a number of underlying neurobiological (e.g., increased activation of the limbic system) and neuro-chemical (e.g., dysregulation of the hypothalamic pituitary adrenal axis) correlates (Boakye et al., 2016). Around half of adults with chronic pain also have insomnia, and around half of adults with insomnia have chronic pain (Taylor et al. 2007a, b). Indeed, poor sleep quality in individuals with chronic pain has been demonstrated in cross-sectional (Kelly et al., 2011) and longitudinal (Axén, 2016; Ødegård et al., 2013) studies.
Given the presence of poor sleep in both PTSS and chronic pain, poor sleep may represent a mutually reinforcing factor underlying co-occurring PTSS and chronic pain that relates to symptoms in both domains. For example, poor sleep may relate to alterations in mood and exacerbate cognitive biases and hypervigilance towards pain (O’brien et al., 2010; Pavlova et al., 2017). Despite compelling evidence drawing direct links between poor sleep and both chronic pain and PTSS, little research has empirically examined the indirect effect of poor sleep quality on the relationship between PTSS and pain. The few published studies indicate that poor sleep quality helps explain the relationship between total PTSS and pain intensity and interference in adult cancer survivors (Lillis et al., 2017) and in youth with chronic pain (Noel et al., 2018). It is important to build on this existing research and examine the indirect role of sleep in elevated PTSS and pain in adult women, who are at elevated risk for both. Pediatric pain is distinct from pain in adulthood, with neurological, development and social differences distinguishing the two. Pain in adults with cancer is also distinct, with consideration to the psychological distress that often accompanies cancer diagnosis, treatment and survivorship (Deimling et al. 2006). Finally, it is critical that future research account for those psychological factors that have been identified as critical considerations in co-occurring PTSS and pain, specifically: depression symptoms, anxiety symptoms and anxiety sensitivity (Sharp & Harvey, 2001). Ultimately, clarifying these relationships has important treatment implications for individuals with elevated PTSS and pain, as targeted sleep interventions have been shown to result in improvements in both domains. For example, a recent meta-analysis concluded that cognitive behavioral therapy based interventions targeting sleep disturbances in adults led to reduced PTSS and improved sleep quality, effects that were moderate in magnitude (Ho et al., 2016).
To fill these gaps in the literature, the current study sought to investigate the indirect effect of poor sleep quality on the relationship between PTSS and pain in a community sample of women. Specifically, we aimed to determine the role of poor sleep quality in the relationship between total PTSS and pain intensity and pain interference, key pain characteristics related to PTSS (Asmundson et al., 2004; Stratton et al., 2014). We build on previous literature in this area by examining these relationships in a sample of adult women with pain while accounting for shared psychological factors of depression symptoms, anxiety symptoms and anxiety sensitivity. Leading theoretical accounts identify these three psychosocial factors as key potential covariates in the relationship between PTSD and sleep, and their consideration is essential in parsing the role of sleep in the relationship between PTSD and pain (Asmundson et al., 2002; Sharp & Harvey, 2001). We hypothesized significant positive relationships between PTSS and pain experiences, similar to prior research. Furthermore, we hypothesized that poor sleep quality would help explain these relationships, above and beyond other psychological symptoms. Importantly, we examined separate models examining the role of sleep on the relationship between PTSS and (1) pain intensity, and (2) pain interference. These distinct pain-related experiences carry different implications for understanding the impact of pain and its treatment, particularly in the context of sleep. For example, improving sleep in adults with chronic pain results in improved pain interference, but not pain intensity (Jungquist et al., 2010).
Methods
This is a secondary data analysis from a previously published study investigating the prevalence and impact of posttraumatic stress disorder in youth with and without chronic pain (Noel et al., 2016, 2018). This paper focuses on the caregivers of this sample, and the results have not previously been published. The original study included 195 caregivers (13 men, 182 women); however, given the predominance of women in this sample and because women are at heightened risk for both PTSD and pain (Johannes et al., 2010), we excluded the male caregivers from this analysis, resulting in a total of 182 participants. Detailed recruitment processes have been described in detail elsewhere (Noel et al., 2016), and thus are only briefly summarized below.
Participants and procedure
Participants were originally recruited, with their child aged 10–17 years, through their child’s pediatric chronic pain clinic, the community, or via a healthy participant research database, and were required to have fluency in English and access to the Internet to complete study measures through Research Electronic Data Capture (REDCap), a secure online server. Participants completed informed consent prior to completing study questionnaires online, and subsequently received gift cards for their participation. The institution’s Institutional Review Board approved all study procedures.
Measures
Demographic characteristics
Participants reported on their sex, race, marital status and educational attainment. Participants were not asked to report their age; however, because this sample was comprised of the mothers of youth aged 10–17, we expect that variability in age was restricted.
Pain characteristics
To identify the presence of chronic pain, participants responded to a yes/no question “Do you have a pain problem that has been present for at least 3 months and has your pain intensity been greater than 0 on a 0–10 scale at some time in the last month?” This assessment reflects chronic pain as defined by the International Association for the Study of Pain (Merskey & Bogduk, 1994) and aligns with chronic pain epidemiological research (Tunks et al., 2008). Women indicating the presence of chronic pain were then asked “how long have you had your pain problem” and responded in a free response field to report on pain duration.
PTSD checklist for DSM-5
Participants completed the PTSD Checklist for DSM-5 (PCL-5; Blevins et al., 2015), which is used to screen for PTSD and can be used in making a provisional PTSD diagnosis. Participants were provided with a list of 17 stressful events (e.g., ‘fire or explosion,’ ‘life threatening illness of injury’), and indicated whether each event had ‘happened to me,’ whether they ‘witnessed it,’ ‘learned about it,’ experienced it as ‘part of your job,’ if they were ‘not sure,’ or if the event ‘doesn’t apply.’
Next, participants were instructed to think of, and record, the event that bothered them the most and indicate the level to which they experienced 20 PTSD-related symptoms over the past month (e.g., “feeling very upset when something reminded you of the stressful experience,” “avoiding memories, thoughts, or feelings related to the stressful experience”). Each item is rated on a 5-point Likert scale, where 0 indicates “not at all” and 4 indicates “extremely.” A total score (range: 0–80) is calculated by summing responses to all 20 items, with higher scores indicating greater symptom severity. Four PTSD symptom subscale scores, which correspond to DSM-5 PTSD criteria, can also be calculated: re-experiencing, avoidance, negative cognitions and mood, and hyperarousal. A clinical psychologist (R.A.) coded participant’s description of the event that bothered them the most to determine exposure to a Criterion A trauma (exposure to actual or threatened death, serious injury, or sexual violence).
A provisional PTSD diagnosis can be made by treating each item rated as a 2 or higher as a symptom endorsed, then following the DSM-5 diagnostic rule which requires at least one re-experiencing symptom, one avoidance symptom, two negative cognitions and mood symptoms, and two hyperarousal symptoms for a diagnosis of PTSD. The PCL5 is psychometrically sound, with excellent reliability and strong convergent and discriminant validity (Blevins et al., 2015). Psychometric studies also show good diagnostic utility for identifying provisional PTSD diagnoses, based on comparison to clinician-administered diagnostic assessment (Bovin et al., 2016). The internal consistency for this sample was excellent, α = .95 for the total score, and ranged from α = .86 to a = .91 for the subscale scores. Because the mechanisms proposed to underlie the co-occurrence of chronic pain and PTSS are proposed to function at the symptom (versus diagnostic) level (Asmundson et al., 2002; Sharp & Harvey, 2001), we consider total PTSS symptom score in our analyses of indirect effects, and provide rates of provisional PTSD diagnoses to help characterize our sample.
Pittsburgh sleep quality index
Participants completed the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) to assess their perceived sleep quality over the past 4 weeks. The PSQI contains 19 items (e.g., “cannot get to sleep within 30 min,” “taken medicine to help you sleep”) that generate seven component scores (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, daytime dysfunction), which have a range of 0–3. Total scores are computed by summing all component scores, with higher scores indicating poorer sleep quality. The PSQI has shown strong test–retest reliability and discriminant validity (Buysse et al., 1989). For the current study, the internal consistency for the total PSQI score was good, α = .86.
PROMIS-profile 29 version 2.1
The Patient-Reported Outcomes Measurement Information System (PROMIS) Profile instrument was used to assess physical, mental, and social health. The 29-item profile is a collection of short forms from eight domains (physical function, anxiety, depression, fatigue, sleep disturbance, ability to participate in social roles and activities, pain interference, and pain intensity) with the response frame over the past 7-days. For use in the present study, we included pain interference, anxiety, depression, and pain intensity domains. Participants completed 4-items which are used to assess pain interference (e.g., ‘how much did pain interfere with your daily activities?’). Items are scored on a 5-point Likert scale where 1 indicated “not at all” and 5 indicated “very much.” Participants completed 4-items about negative cognitions and affect to assess depressive symptoms (e.g., “I felt worthless,” “I felt depressed”), and 4-items about anxious cognitions, affect and behaviors to assess for anxiety symptoms (e.g., “I felt fearful,” “I found it hard to focus on anything other than my anxiety’’). Items for the depression and anxiety domains are scored on a 5-point Likert scale where 1 indicated “never” and 5 indicated “always.” The pain intensity domain includes a single question, “In the past 7 days, how would you rate your pain on average?” Participants responded using a 0–10 scale, where 0 indicated “no pain’ and 10 indicated “worst pain imaginable.’’
For the pain interference, depression, and anxiety domains, total raw scores were transformed into standardized T-scores for analyses. The T-score distribution has a mean of 50 (SD ± 10), with higher T-scores indicating greater interference or symptomatology. The PROMIS measures are normed against the United States general population as well as multiple disease populations, including chronic pain (Cella et al., 2010). Internal consistency for the pain interference, anxiety and depression domains ranged from good, α = .84 to excellent, α = .97. The pain intensity rating is one question, thereby preluding internal consistency analysis.
Anxiety sensitivity index
Participants completed the Anxiety Sensitivity Index—version 3 (ASI-3; S. Taylor et al. 2007a, b) to assess anxiety sensitivity, or fear of arousal-related sensations. The ASI contains 18 items that map onto three domains: physical concerns (e.g., “It scares me when my heart beats rapidly”), cognitive concerns (e.g., “When I feel ‘spacey’ or spaced out I worry that I may be mentally ill”), and social concerns (e.g., “I worry that other people will notice my anxiety”). Items are scored on a 5-point Likert scale where 0 indicates “very little” and 4 indicates “very much”. Domain scores are calculated by summing the corresponding items and a total score can be calculated by summing domain scores, with higher scores indicating greater anxiety sensitivity. The ASI has shown strong reliability, and convergent, discriminant and criterion validity (Taylor et al. 2007a, b). For the current study, internal consistency of the ASI was good, α = .89.
Data analysis
We conducted descriptive statistics to characterize our sample. Specifically, we characterize the number of women in our sample who reported the presence of a chronic pain problem, and the number of women meeting criteria for a provisional PTSD diagnosis. We described category of traumatic events, as well as rate of exposure to Criterion A trauma. We conducted a series of one way analysis of variance (ANOVAs) to determine whether demographic characteristics (race, education, employment status) were significantly related to key study variables (total PTSS, sleep quality, pain intensity, and pain interference scores). Because women in this study were initially recruited because they had a child with or without a chronic pain condition, we also conducted a series of ANOVAs to determine whether child pain status was significantly related key study variables.
To address our primary aim, we first conducted descriptive analyses of study variables (Means, SDs) as well as zero-order correlations between key study variables and psychological covariates. We then assessed the indirect effect of sleep quality on the relationships between PTSS total on pain intensity and pain interference. In both models, we entered PROMIS depression and anxiety symptom scores and total ASI score as covariates, to better isolate the unique influence of PTSS above and beyond that of other relevant psychological factors. We also included level of education attainment and employment status, as these demographic factors were significantly related to key variables in our model (i.e., PTSS, sleep quality, pain interference). We also included child pain status, as this was related to significantly higher PTSS, worse sleep quality, and greater pain interference. We then replicated these models with consideration to a potential confound: one PCL item assesses “trouble falling or staying asleep.” To account for the possibility that this sleep-related item inflated significant results, we calculated a total PCL score without this item. This new total was then entered as the independent variable in our models. This is similar to previous research in this area (Lillis et al., 2017; Noel et al., 2018). All indirect effect analyses were conducted in SPSS using Preacher and Hayes’ (Preacher & Hayes, 2008) PROCESS macro, using model 4. PROCESS generates, and we report, multiple regression models demonstrating the relationships between the independent variable and “mediator” (a path), “mediator” and dependent variable (b path), independent variable and dependent variable (c path), and the independent and dependent variable after accounting for the “mediator” (c’ path). In this case, the independent variable was always PCL total, the “mediator” was always PSQI global sleep quality, and the dependent variable was either pain intensity or pain interference (both scores generated from PROMIS). PROCESS also generates a bootstrapped confidence interval (CI); in this case, a 95% CI is generated from bootstrap measures of standard errors from 5000 resamples. When this interval is significant (i.e., does not include the value zero), the “mediator” has a significant indirect effect on the relationship between the independent and dependent variable. For each indirect effect analysis, we also reported this value.
Results
Participants
The mean pain intensity of the total sample was 2.52 (SD = 1.40) and mean pain interference was 49.14 (SD = 8.72). Of the total sample, 35.7% of participants reported having chronic pain, with an average duration of 11.69 years (SD = 12.63). Participant demographics are reported in Table 1. Based on PCL-5 scoring criteria (endorsement of DSM-5 PTSD Criteria B-E), 8.8% (n = 16) of women met criteria for a provisional PTSD diagnosis. Of the entire sample, 68.1% of women endorsed exposure to a Criterion A stressor. The most common traumatic event exposures were death (n = 57, 31.3%), illness (n = 27, 14.8%), accident (n = 25, 13.7%), and abuse (n = 22, 12.1%). More women reporting chronic pain had PTSD as compared to women not reporting chronic pain (n = 10, 15% vs. n = 6, 5%; X2(1) = 5.48, p = .019).
Table 1.
Participant demographics
| Demographic Variable | Percent of sample (%) |
|---|---|
| Race and ethnicity | |
| White, non-hispanic | 87.5 |
| White, hispanic | 2.2 |
| Asian or Asian American | 3.3 |
| Black or African American | 2.7 |
| Other | 4.3 |
| Marital status | |
| Married | 76.4 |
| Not married | 22.5 |
| Missing | 1.1 |
| Education | |
| High school or less | 4.4 |
| Vocational school/some college | 22.0 |
| College | 41.2 |
| Graduate/professional school | 32.4 |
| Employment status | |
| Full time | 53.9 |
| Part time | 28.0 |
| Not working | 17.0 |
| Missing | 1.1 |
Indirect effect of sleep on the relationship between PTSS and pain characteristics
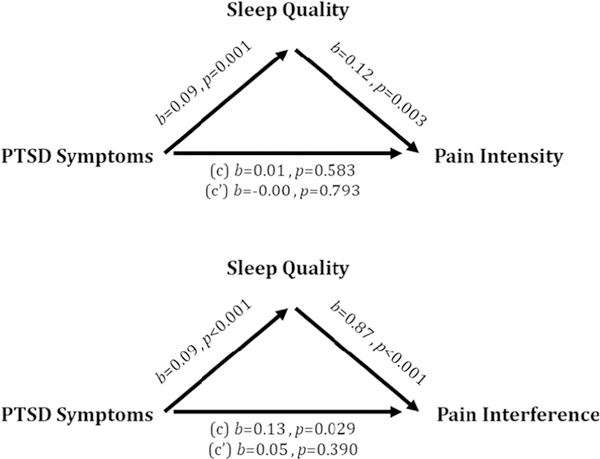
Means and standard deviations of key variables are presented in Table 2, as are zero-order Pearson correlations between key study variables and psychological covariates. We conducted indirect effect analysis examining whether sleep quality accounts for the relationship between PTSS and pain characteristics (intensity and interference). In Fig. 1, we report coefficients for the a path (i.e., relationship between PCL total and sleep quality), b path (i.e., relationship between sleep quality and pain), c path (i.e., relationship between PCL total and pain), and c’ path (i.e., relationship between PCL total and pain while accounting for sleep). We also report the significance of an indirect effect analysis using bootstrapped confidence intervals. In these visually depicted models, depression symptoms, anxiety symptoms, anxiety sensitivity, education attainment, employment status, and child pain status are entered as covariates. In Supplementary Material, we report indirect effect models with and without clinical and demographic covariates.
Table 2.
Descriptive statistics and correlation matrix
| Range | Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. PTSD symptoms (total) | 0–80 | 12.30 (13.98) | |||||||
| 2. PTSD symptoms (excluding sleep item) | 0–76 | 11.53 (13.23) | 1.00 (<.001) | ||||||
| 3. Pain intensity | 0–10 | 2.52 (1.40) | .14 (.097) | .12 (.142) | |||||
| 4. Pain interference | 10–90 | 49.14 (8.72) | .41 (<.001) | .40 (<.001) | .67 (<.001) | ||||
| 5. Sleep quality | 0–57 | 6.70 (3.93) | .55 (<.001) | .54 (<.001) | .33 (<.001) | .55 (<.001) | |||
| 6. Depression | 10–90 | 49.05 (7.90) | .48 (<.001) | .49 (<.001) | .21 (.012) | .35 (<.001) | .46 (<.001) | ||
| 7. Anxiety | 10–90 | 51.53 (7.98) | .57 (<.001) | .55 (<.001) | .16 (.048) | .36 (<.001) | .51 (<.001) | .64 (<.001) | |
| 8. Anxiety sensitivity | 0–90 | 10.86 (9.47) | .38 (<.001) | .38 (<.001) | .30 (<.001) | .41 (<.001) | .39 (<.001) | .51 (<.001) | .56 (<.001) |
Depicts zero-order correlations between key study variables and psychological covariates. PTSD was measured using the PCL; Sleep Quality was measured using the PSQI; Anxiety Sensitivity was measured using the ASI; All other variables (pain intensity, pain interference, depression,anxiety) were assessed using PROMIS
Fig. 1.
Models depicting direct paths between key study variables. Indirect effect analysis using bootstrapping revealed a significant indirect effect of sleep on the relationship between PTSD Symptoms and Pain Intensity (b = .01, CI [.003, .03]) and pain interference (b = .08, CI [.04, .14])
For the model examining the indirect effect of sleep quality on the relationship between total PTSS and pain intensity, poor sleep quality was associated with greater total PTSS and higher pain intensity. There was not a significant relationship between PTSS and pain intensity; however, examination of bootstrapped confidence intervals revealed a small, significant indirect effect of sleep on this relationship [.003, .03].
For the model examining the indirect effect of sleep quality on the relationship between total PTSS and pain interference, there were statistically significant relationships between all three variables. Greater total PTSS was associated with poorer sleep quality and greater pain interference. The significant relationship between PTSS and pain interference became nonsignificant once accounting for the effect of sleep quality. Further supporting the significance of poor sleep quality for explaining the significant positive relationship between total PTSS and pain interference, bootstrapped confidence intervals also suggested a significant indirect effect of poor sleep quality [.04, .14].
Finally, we then re-ran the two indirect effect models described previously, with an alternate PTSD total score variable that excluded the PCL-5 sleep item as the independent variable. Both models, examining the indirect effect of sleep quality on the relationships between PTSD and pain intensity and pain interference had the same pattern of findings (see Supplementary Material), reflecting significant indirect effects of sleep quality on the relationship between PTSS and pain interference.
Discussion
This study assessed PTSS in women with varying pain experiences to examine the role of poor sleep quality on relationships between PTSS and pain intensity and interference. To our knowledge, this is the first study to examine these relationships in a non-clinical sample of adult women, who are at heightened risk for both PTSS and pain. Compared to large prevalence studies, rates of reported chronic pain (35.7%) and provisional PTSD diagnoses (8.8%) in the current community sample were similar (Johannes et al., 2010; McLean et al., 2011). As expected, poor sleep quality was related to greater PTSS and worse pain symptoms, and had an indirect effect on relationships between PTSS and pain interference and intensity. We extend previous literature in adult women by showing these findings remain consistent even when accounting for theoretically-derived psychological covariates. Predominant theory about the co-occurrence between PTSD and chronic pain posits that shared symptoms mutually maintain both conditions (Sharp & Harvey, 2001). A wealth of literature draws direct links between poor sleep quality and PTSD as well as between poor sleep quality and chronic pain, pointing to poor sleep quality as a shared symptom that may contribute to this mutual maintenance. The current study examined the indirect effect of poor sleep on the relationship between PTSS and pain characteristics, suggesting that indeed, poor sleep quality partially accounts for the positive relationship between PTSS and pain interference. The role of poor sleep quality on the positive relationship between PTSS and pain intensity is less clear. Future iterations of existing conceptual models of PTSS and pain-related physical interference may consider including poor sleep quality as a key underlying behavioral mechanism.
Many studies have demonstrated elevated rates of PTSS among individuals with chronic pain (Siqveland et al., 2017), and the current findings extend this finding to a sample of adult, civilian women. Using bootstrapping, we found a significant indirect effect of sleep quality on the relationship between PTSS and pain characteristics, consistent with other research in this area (Lillis et al., 2017; Noel et al., 2018). Critically, this indirect effect was present even after accounting for key shared psychological symptomology in the mutual maintenance of PTSD and chronic pain, namely depression symptoms, anxiety symptoms and anxiety sensitivity, supporting the robust role of poor sleep quality. Examination of the direct paths between these variables suggests this effect may be stronger for the relationship between PTSS and pain interference. Even after accounting for key psychosocial and demographic covariates, there was a statistically significant relationship between PTSS and pain interference. Further supporting the indirect effect of sleep on this relationship, the relationship became nonsignificant after accounting for sleep quality. These findings are similar to those in adults with cancer-related pain (Lillis et al., 2017). Departing from Lillis’ et al. (2017) findings, there was no statistically significant relationship between PTSS and pain intensity, after accounting for psychological covariates. This highlights the importance of considering psychological and demographic covariates in the relationship between PTSS and pain, as well as possible divergences in an adult cancer populations versus a non-clinical sample of women with pain. Whereas the relationship between PTSS and pain intensity could largely be accounted for by these covariates, the relationship between PTSS and pain interference was more robust. The posited alterations in cognition and mood related to elevated PTSS might not increase pain perception, but more broadly drive behavioral avoidance which more directly affects the experience of pain interference rather than pain intensity. This has important clinical implications given that changes in pain interference and disability, versus reductions in pain intensity, are often the primary focus for intervention and indicator of chronic pain treatment success (e.g., McCracken & Vowles, 2014).
The results provide further rationale for targeting poor sleep in psychosocial interventions for individuals with co-occurring PTSS and pain. Cognitive behavioral therapy for insomnia (CBT-I) is a short-term and targeted intervention that results in meaningful improvement to sleep quality in individuals with insomnia (Trauer et al., 2015). Specifically in adults with PTSS, CBT-I has been shown to improve sleep quality and reduce PTSS (Ho et al., 2016). There have also been applications of CBT-I to adults with chronic pain, finding improvements in sleep quality and improved pain interference but not pain intensity (Jungquist et al., 2010). This is consistent with the present findings showing differential relationships between PTSS and pain interference and pain intensity. CBT-I also results in small to medium improvements in depression and anxiety symptoms in adults with insomnia (Taylor & Pruiksma, 2014), other factors that contribute to the PTSD and chronic pain comorbidity. Targeting an underlying modifiable mechanism like sleep, particularly in individuals who are least likely to respond to conventional pain treatments, could improve physical functioning in this vulnerable population.
An important limitation of the current study is its cross-sectional design, which precludes examination of temporal pathways between poor sleep quality, PTSD and pain experiences. Growing research suggests that poor sleep quality may precede the onset of chronic pain (Finan et al., 2013) and PTSD (Babson & Feldner, 2010). For example, greater insomnia symptoms in the year following hospitalization for motor vehicle crashes predicts PTSD diagnosis at one-year follow-up (Koren et al., 2002). Researchers have applied advanced longitudinal analysis to characterize the relationship between sleep and pain over time, concluding that sleep reliably and robustly predicts subsequent pain intensity and frequency, with stronger relationships than the reverse direction of pain predicting subsequent sleep quality (Bigatti et al., 2008; Edwards et al., 2008; Quartana et al., 2010). Moreover, adverse childhood events have been linked to the onset of chronic pain (Felitti et al., 1998; Nelson et al., 2017), which suggests that trauma symptoms might precede the development of chronic pain. Future longitudinal research is needed to delineate temporal pathways between poor sleep quality, PTSD and pain: if it is the case that poor sleep drives co-occurring pain and insomnia, sleep intervention would be particularly important to implement.
A second limitation of the current study is its exclusive reliance on self-report measures of core constructs. While self-report assessment is arguably the ideal way to assess internalizing mental health symptoms and pain, future research should utilize objective measures of sleep to capture additional parameters beyond perceived sleep quality. Use of ambulatory EEG and actigraphy would enable a more objective assessment of sleep duration, sleep efficiency, wake after sleep onset, and slow wave sleep (de Souza et al., 2003; Finan et al., 2016). In addition, subjective assessment of pre-sleep arousal and insomnia might be particularly relevant given their close connection with anxiety and trauma. Parasomnias, such as nightmares, should also be assessed given the presence of re-experiencing symptoms in PTSD that can manifest during sleep (APA, 2013). This study did not utilize clinical interviews to assess PTSD at the diagnostic level. Rather, participants completed a measure that assessed exposure to traumatic events as well as PTSD symptoms that they attributed to their most distressing event. While this measure provides calculation of provisional PTSD diagnoses, future research should incorporate clinical interviews to more fully capture the nature and presence of PTSD. A number of other covariates may be considered in future studies. Most notably, we were unable to examine age as a moderator in the models in this study; while we expect limited variability in age given participants were all mothers to children aged 10–17 years, age may have been a meaningful factor in the observed relationships. Other psychological (e.g., catastrophizing), cognitive (e.g., attention bias), physiological (e.g., BMI) and neurobiological (e.g., hyperarousal) factors may be considered in future research to shed additional light on the relationship between pain and PTSD symptoms.
In conclusion, the present study shows that poor sleep quality helps explain significant positive relationships between PTSS and pain experiences, particularly physical interference related to pain, and may be a modifiable behavioral mechanism that contributes to the mutual maintenance of PTSS and chronic pain in women, who are at greater risk for both disorders. Future research is needed to clarify the role of sleep quality in this common comorbidity and to delineate temporal pathways between these factors.
Supplementary Material
Acknowledgements:
We thank Caitlin Murrary for her assistance in the revision of this manuscript.
Funding: Rachel Aaron is supported by T32GM086270 awarded to Tonya Palermo; Tonya Palermo is supported by K24HD060068; Melanie Noel is supported by the Vi Riddell Pain Initiative of the Alberta Children’s Hospital Research Institute. This research was supported by a Hearst Grant awarded to Melanie Noel by the Center for Child Health, Behavior and Development at the Seattle Children’s Hospital Research Institute.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Human and animal rights and Informed consent All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Board at Seattle Children’s Research Institute approved all study procedures and informed consent was obtained from all individual participants included in the study.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10865-019-00016-5) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rachel Aaron, University of Washington & Seattle Children’s Research Institute.
Melanie Noel, University of Calgary & Alberta Children’s Hospital and Research Institute.
Joanne Dudeney, Seattle Children’s Research Institute.
Anna Wilson, Oregon Health & Science University.
Amy Holley, Oregon Health & Science University.
Tonya Palermo, University of Washington & Seattle Children’s Research Institute.
References
- APA. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Coons MJ, Taylor S, & Katz J. (2002). PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. The Canadian Journal of Psychiatry, 47, 930–937. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, & Katz J. (2009). Understanding the co-occurrence of anxiety disorders and chronic pain: State-of-the-art. Depression and Anxiety, 26, 888–901. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Wright KD, & Stein MB (2004). Pain and PTSD symptoms in female veterans. European Journal of Pain, 8, 345–350. [DOI] [PubMed] [Google Scholar]
- Axén I. (2016). Pain-related sleep disturbance: A prospective study with repeated measures. The Clinical journal of pain, 32, 254–259. [DOI] [PubMed] [Google Scholar]
- Babson KA, & Feldner MT (2010). Temporal relations between sleep problems and both traumatic event exposure and PTSD: A critical review of the empirical literature. Journal of Anxiety Disorders, 24, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigatti SM, Hernandez AM, Cronan TA, & Rand KL (2008). Sleep disturbances in fibromyalgia syndrome: Relationship to pain and depression. Arthritis Care & Research, 59, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, & Domino JL (2015). The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28, 489–498. [DOI] [PubMed] [Google Scholar]
- Boakye PA, Olechowski C, Rashiq S, Verrier MJ, Kerr B, Witmans M, et al. (2016). A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption. The Clinical Journal of Pain, 32, 327–336. [DOI] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, et al. (2016). Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders—fifth edition (PCL-5) in veterans. Psychological Assessment, 28, 1379. [DOI] [PubMed] [Google Scholar]
- Burke AL, Mathias JL, & Denson LA (2015). Psychological functioning of people living with chronic pain: A meta-analytic review. British Journal of Clinical Psychology, 54, 345–360. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. (2010). Initial adult health item banks and first wave testing of the patient-reported outcomes measurement information system (PROMISTM) network: 2005–2008. Journal of Clinical Epidemiology, 63, 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RC, & Olatunji BO (2016). A systematic review of sleep disturbance in anxiety and related disorders. Journal of Anxiety Disorders, 37, 104–129. [DOI] [PubMed] [Google Scholar]
- de Souza L, Benedito-Silva AA, Pires MLN, Poyares D, Tufik S, & Calil HM (2003). Further validation of actigraphy for sleep studies. Sleep, 26, 81–85. [DOI] [PubMed] [Google Scholar]
- Deimling GT, Bowman KF, Sterns S, Wagner LJ, & Kahana B. (2006). Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer, 15, 306–320. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, & Smith MT (2008). Duration of sleep contributes to next-day pain report in the general population. PAIN®, 137, 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, & Smith MT (2013). The association of sleep and pain: an update and a path forward. The Journal of Pain, 14, 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Richards JM, Gamaldo CE, Han D, Leoutsakos JM, Salas R, et al. (2016). Validation of a wireless, self-application, ambulatory electroencephalographic sleep monitoring device in healthy volunteers. Journal of Clinical Sleep Medicine, 12, 1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin DJ, & Richard P. (2012). The economic costs of pain in the United States. The Journal of Pain, 13, 715–724. [DOI] [PubMed] [Google Scholar]
- Ho FY-Y, Chan CS, & Tang KN-S (2016). Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clinical Psychology Review, 43, 90–102. [DOI] [PubMed] [Google Scholar]
- Holley A, Wilson A, Noel M, & Palermo T. (2016). Posttraumatic stress symptoms in children and adolescents with chronic pain: A topical review of the literature and a proposed framework for future research. European Journal of Pain, 20, 1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, & Dworkin RH (2010). The prevalence of chronic pain in United States adults: results of an Internet-based survey. The Journal of Pain, 11, 1230–1239. [DOI] [PubMed] [Google Scholar]
- Jungquist CR, O’Brien C, Matteson-Rusby S, Smith MT, Pigeon WR, Xia Y, et al. (2010). The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Medicine, 11, 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GA, Blake C, Power CK, O’keeffe D, & Fullen BM (2011). The association between chronic low back pain and sleep: A systematic review. The Clinical journal of pain, 27, 169–181. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, & Wittchen HU (2012). Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research, 21, 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren D, Arnon I, Lavie P, & Klein E. (2002). Sleep complaints as early predictors of posttraumatic stress disorder: A 1-year prospective study of injured survivors of motor vehicle accidents. American Journal of Psychiatry, 159, 855–857. [DOI] [PubMed] [Google Scholar]
- Lillis TA, Gerhart J, Bouchard LC, Cvengros J, O’Mahony S, Kopkash K,… Burns J. (2017). Sleep disturbance mediates the association of post-traumatic stress disorder symptoms and pain in patients with cancer. American Journal of Hospice and Palliative Medicine®, 1049909117739299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, & Vowles KE (2014). Acceptance and commitment therapy and mindfulness for chronic pain: Model, process, and progress. American Psychologist, 69, 178. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, & Hofmann SG (2011). Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research, 45, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey H, & Bogduk N. (1994). Classification of chronic pain (2nd ed.). Seattle: IASP. [Google Scholar]
- Morasco BJ, Lovejoy TI, Lu M, Turk DC, Lewis L, & Dobscha SK (2013). The relationship between PTSD and chronic pain: mediating role of coping strategies and depression. Pain, 154, 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahin RL (2015). Estimates of pain prevalence and severity in adults: United States, 2012. The Journal of Pain, 16, 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cunningham NR, & Kashikar-Zuck S. (2017). A conceptual framework for understanding the role of adverse childhood experiences in pediatric chronic pain. The Clinical Journal of Pain, 33, 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel M, Vinall J, Tomfohr-Madsen L, Holley A, Wilson AC, & Palermo TM (2018). Sleep mediates the association between PTSD symptoms and chronic pain in youth. The Journal of Pain, 19, 67–75. [DOI] [PubMed] [Google Scholar]
- Noel M, Wilson AC, Holley A, Durkin L, Patton M, & Palermo TM (2016). Posttraumatic stress disorder symptoms in youth with vs without chronic pain. Pain, 157, 2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, et al. (2010). Negative mood mediates the effect of poor sleep on pain among chronic pain patients. The Clinical Journal of Pain, 26, 310–319. [DOI] [PubMed] [Google Scholar]
- Ødegård SS, Sand T, Engstrøm M, Zwart J-A, & Hagen K. (2013). The impact of headache and chronic musculoskeletal complaints on the risk of insomnia: longitudinal data from the Nord-Trøndelag health study. The journal of headache and pain, 14, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova M, Ference J, Hancock M, & Noel M. (2017). Disentangling the sleep-pain relationship in pediatric chronic pain: The mediating role of internalizing mental health symptoms. Pain research and management, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. [DOI] [PubMed] [Google Scholar]
- Quartana PJ, Wickwire EM, Klick B, Grace E, & Smith MT (2010). Naturalistic changes in insomnia symptoms and pain in temporomandibular joint disorder: A cross-lagged panel analysis. PAIN®, 149, 325–331. [DOI] [PubMed] [Google Scholar]
- Sharp TJ, & Harvey AG (2001). Chronic pain and posttraumatic stress disorder: mutual maintenance? Clinical Psychology Review, 21, 857–877. [DOI] [PubMed] [Google Scholar]
- Siqveland J, Hussain A, Lindstrøm JC, Ruud T, & Hauff E. (2017). Prevalence of posttraumatic stress disorder in persons with chronic pain: A meta-analysis. Frontiers in Psychiatry, 8, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, & Haythornthwaite JA (2004). How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medicine Reviews, 8, 119–132. [DOI] [PubMed] [Google Scholar]
- Stratton KJ, Clark SL, Hawn SE, Amstadter AB, Cifu DX, & Walker WC (2014). Longitudinal interactions of pain and posttraumatic stress disorder symptoms in US Military service members following blast exposure. The Journal of Pain, 15, 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, & Bush AJ (2007a). Comorbidity of chronic insomnia with medical problems. Sleep, 30, 213–218. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, & Pruiksma KE (2014). Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: A systematic review. International Review of Psychiatry, 26, 205–213. [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, et al. (2007b). Robust dimensions of anxiety sensitivity: Development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment, 19, 176. [DOI] [PubMed] [Google Scholar]
- Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, & Cunnington D. (2015). Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Annals of Internal Medicine, 163, 191–204. [DOI] [PubMed] [Google Scholar]
- Tunks ER, Crook J, & Weir R. (2008). Epidemiology of chronic pain with psychological comorbidity: Prevalence, risk, course, and prognosis. The Canadian Journal of Psychiatry, 53, 224–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.