Abstract
Background:
Children with Down Syndrome (DS) and obstructive sleep apnea (OSA) are difficult to treat, as first line therapies may not lead to significant improvement. Drug-induced sleep endoscopy (DISE) directed surgery may be particularly beneficial for these patients.
Objective:
To assess change in polysomnography (PSG) measures of patients with DS who underwent DISE-directed surgery.
Methods:
Retrospective chart review was performed on patients with DS who underwent DISE-directed surgery and had pre- and post-surgery PSG. Patients were analyzed in groups defined by previous adenotonsillectomy. Two-sided t-tests with equal variances were used to assess statistical significance.
Results:
Of 24 patients reviewed, 14 were surgically naïve and 10 had undergone prior adenotonsillectomy. The primary outcome was change in PSG parameters including apnea hypopnea index, obstructive apnea hypopnea index, oxygen nadir, oxygen desaturation index, and mean carbon dioxide level. While improvement was seen in all PSG parameters, only improvement in oxygen nadir in children who had undergone prior adenotonsillectomy was statistically significant (88.5% to 90.9%, p = 0.04).
Conclusions and Significance:
DISE-directed surgery may be beneficial for children with DS and OSA, with improvement in the means of main PSG measures observed. A larger, prospective study is warranted to further explore DISE utility.
Keywords: DISE, Drug-induced sleep endoscopy, Trisomy 21, Down Syndrome, Obstructive Sleep Apnea, Polysomnography
INTRODUCTION
Obstructive sleep apnea (OSA) is a common cause of childhood morbidity with a prevalence of approximately 1–4% in young children [1]. OSA has been shown to have cardiovascular complications, metabolic alterations, autonomic dysfunction and detrimental effects on behavior, quality of life, and cognitive function [1–3]. Certain populations have a markedly higher burden of disease, particularly children with Down Syndrome (DS) in which OSA is estimated to affect between 30%−70% [4–6]. Not only is OSA more common in children with DS, but it can also be more severe and have greater health impact, such as leading to cor pulmonale or adding to a high baseline cardiovascular risk [7,8].
The American Academy of Pediatrics recommends adenotonsillectomy (AT) as the first line of treatment for OSA [9]. However, up to 50% of children still have persistently abnormal post-operative polysomnograms (PSGs) after AT. Additionally, these guidelines are based on data that exclude complex populations and focus on otherwise healthy children with either adenotonsillar hypertrophy or obesity as underlying risk factors [9]. Children with complex comorbid conditions like DS are challenging to manage and often require additional treatment to appropriately impact their sleep apnea disease burden [10].
Drug-induced sleep endoscopy (DISE) is a novel adjunctive tool in the assessment of the upper airway in children with OSA [11–13]. General anesthesia is used to induce a sleep-like state after which a flexible fiberoptic endoscope is passed through the nares to examine the pharynx, larynx, and trachea [14]. Additional sites of airway obstruction are identified, and surgical interventions to alleviate the obstructions identified may be performed under the same anesthesia. Controversy remains as to how well DISE simulates physiologic sleep and by extension, its utility in improving sleep apnea.
This study aims first to compare obstruction levels within surgically naïve patients and patients who had OSA refractory to AT and second to examine whether PSG outcomes of DISE-directed surgery are improved in children with DS.
MATERIALS AND METHODS
Following IRB approval, pediatric patients with DS who underwent DISE-directed surgery from January 2013 to August 2016 were identified through retrospective chart review. Exclusion criteria included lack of pre- and post-DISE PSGs and prior airway surgery with the exception of previous AT. PSGs were performed within 12 months before and after DISE. Demographic data, PSG parameters, DISE scores, and DISE-directed interventions were collected and recorded in REDCap, an electronic data capture tool hosted at our institution [15]. Of note, not all PSG parameters were available on each PSG report as patients chose where to complete PSG testing, and not all centers reported each PSG parameter of interest to this study. Pediatric otolaryngologists performed DISE and graded the severity of obstruction using the previously validated Chan-Parikh (C-P) scoring system [16]. Five anatomic locations were assessed: adenoid, velum, lateral pharyngeal wall, tongue base, and supraglottis. Each site was graded on a 4-point ordinal scale with 0=no obstruction, 1 = <50% obstruction, 2 = >50% obstruction, and 3 = complete obstruction. Summation of the 5 sites yielded an aggregate obstructive C-P score (range 0–15).
Means and standard deviations were calculated for age, body mass index (BMI), and DISE scores were; proportions were calculated for categorical variables including sex, presence of comorbidities, race and interventions performed. Fisher exact test was used to assess for statistically significant differences between surgically naïve and prior AT cohorts in categorical variables, while 2-sample t test was used for continuous variables. Mean PSG parameters including apnea-hypopnea index (AHI), obstructive apnea-hypopnea index (oAHI), oxygen (O2) nadir, oxygen desaturation index (ODI), and mean end-tidal carbon dioxide (CO2) level were calculated and two-sided t-tests with equal variances were used to assess for statistically significant improvements in these parameters following DISE-directed surgeries. Pediatric OSA severity based on AHI was defined as: <1 normal, 1–5 mild, 5–10 moderate, >10 severe. Abnormal values were defined as oAHI ≥1.5, O2 nadir <92%, ODI > 5, and mean CO2 > 45 mmHg [16]. Statistical analysis was performed using STATA/MP 13 software (StataCorp LP, College Station, Texas). For all tests, p-values≤0.05 were considered statistically significant and sufficient to reject the null hypothesis.
RESULTS
Twenty-four children with DS were identified who underwent DISE directed surgery and had pre- and post-operative PSG results available. Of these, 10 had and 14 had not undergone prior AT. Table 1 lists patient characteristics grouped by prior AT status. While age, sex, race, and comorbidity status were comparable between these groups, more children in the prior AT group were overweight or obese (p < 0.01). Accordingly, BMI percentile was also significantly higher in children who had undergone prior AT (p = 0.01).
Table 1:
Demographics and clinical characteristics of patients with Down syndrome who underwent DISE-directed surgery (N=24)
|
Sex Male |
Surgically naive (N=14) |
Previous AT (N=10) |
p - value* |
| No. (%) | |||
| 7 (50) | 8 (80) | 0.21 | |
| Normal weight (5th–84th percentile) | 13 (93) | 2 (20) | <0.01 |
| Overweight (85th-94th BMI percentile ) | 1 (7) | 6 (60) | |
| Obese (≥95th BMI percentile) | 0 | 2 (20) | |
| Race | |||
| White | 8 (57) | 8 (80) | 0.24 |
| Non White | 3 (36) | 0 (10) | |
| Declined to answert | 3 (7) | 2 (10) | |
| Comorbidities** | |||
| Hypotonia | 3 (21) | 0 | 0.24 |
| Pulmonary disorder | 3 (21) | 1 (10) | 0.62 |
| Developmental delay | 2 (14) | 1 (10) | 1.00 |
| Age (years) at DISE | Mean (SD) | ‡0.07 | |
| 3.9 (3.4) | 6.5 (3.2) | ||
| BMI percentile (%) | 55 (27) | 85 (16) | ‡<0.01 |
p-value from Fisher’s exact test
No subjects had a coexisting craniofacial condition, skeletal abnormality, or neurologic condition
p-value from 2 sample t-test with equal variances
Abbreviations: DISE, drug-induced sleep endoscopy; AT, adenoidectomy and tonsillectomy; BMI, Body Mass Index; SD, Standard Deviation
Patients who declined to answer were not included in the statistical comparison of white and non-white patients
DISE score and DISE-directed interventions received are presented in Table 2. The overall score was comparable between the two groups. However, obstruction was more common at the lateral pharyngeal wall in the group without prior AT, consistent with post-surgical changes expected at this level in the group who had undergone AT. The most common level of obstruction in the surgically naïve cohort was the lateral pharyngeal wall, followed by the supraglottis. The most common level of obstruction in the prior AT cohort was at the tongue base, followed by the supraglottis. Of the surgically naïve cohort, 17% had multilevel obstruction while 25% of the prior AT group had multilevel obstruction. All patients who had multilevel obstructions identified underwent multilevel DISE-directed surgery according to the obstruction level noted. In the surgically naïve cohort, the majority of multilevel surgery performed was AT with supraglottoplasty, while in the prior AT group the majority of multilevel surgery performed was lingual tonsillectomy with supraglottoplasty.
Table 2:
DISE scores and DISE-directed interventions performed
| Surgically naive (n=14) |
Previous AT (n=10) |
p- value* |
|
|---|---|---|---|
| Mean (SD) | |||
| DISE scores | |||
| Chan-Parikh score | 8.1 (2.9) | 7.0 (2) | 0.35 |
| Adenoid | 1.4 (1.1) | 0.6 (0.9) | 0.07 |
| Velum | 1.2 (1.3) | 1.4 (1.1) | 0.61 |
| Lateral pharyngeal wall | 2.1 (0.8) | 0.2 (0.7) | <0.01 |
| Tongue base | 1.5 (1.4) | 2.4 (0.7) | 0.09 |
| Supraglottis | 1.9 (1.4) | 2.3 (1.3) | 0.51 |
| No. (%) | |||
| Interventions performed | |||
| None | 1 (4) | 1 (4) | <0.01** |
| Adenoidectomy | 1 (4) | 0 | |
| Adenotonsillectomy | 7 (29) | 0 | |
| Lingual Tonsillectomy | 0 | 1 (4) | |
| Supraglottoplasty | 1 (4) | 2 (8) | |
| Multilevel | 4 (17) | 6 (25) | |
| Adenoidectomy + Lingual Tonsillectomy | 0 | 1 (4) | |
| Adenotonsillectomy + Supraglottoplasty | 3 (12) | 0 | |
| Lingual Tonsillectomy + Supraglottoplasty | 1 (4) | 4 (17) | 0.11** |
| Adenoidectomy + lingual tonsillectomy | 0 | 1 (4) | |
p-value from 2 sample t-test with equal variances
p-value from Fisher’s exact test
Severity of obstruction from DISE score: 0 = no obstruction, 1 = < 50% obstruction, 2 = >50% obstruction, and 3 = complete obstruction. Abbreviations: DISE, drug-induced sleep endoscopy; AT, adenoidectomy and tonsillectomy; SD, Standard Deviation
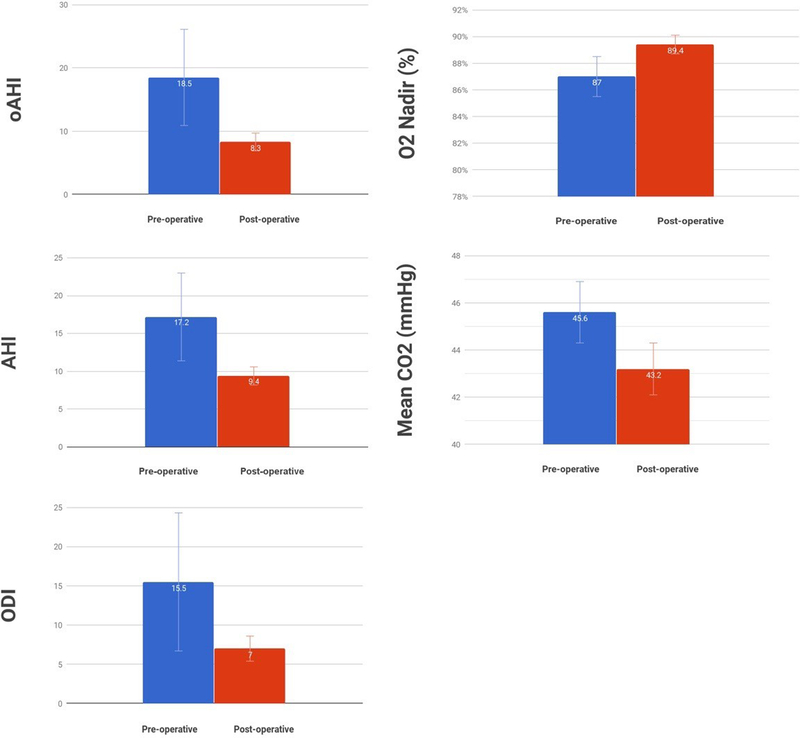
The primary outcome assessed was change in AHI, followed by change in four additional PSG parameters (presented in Table 3 and Figure 1). Improvements were noted in mean PSG parameters after DISE-directed intervention, both within those who had and those who had not had prior AT. Statistical trends were observed for improvement in AHI and ODI in children who had undergone prior AT; there was a statically significant improvement in O2 nadir in that group (p = 0.04).
Table 3:
Changes in polysomnography parameters among all Down syndrome participants
| PSG Parameter | Mean Pre- operative score (SD) |
Mean Post- operative score (SD) |
Mean Difference Score |
95% CI | p-value* |
|---|---|---|---|---|---|
| All subjects (N) | |||||
| AHI (23) | 17.6 (30.0) | 10.5 (9.1) | 7.1 | −46 – 18.8 | 0.22 |
| oAHI (23) | 16.1 (30.3) | 8.9 (8.6) | 7.2 | −4.5 – 18.9 | 0.21 |
| O2 Nadir (%) (23) | 86.4 (7.3) | 88.3 (3.2) | −1.9 | −4.9 – 1.2 | 0.22 |
| ODI (18) | 15.8 (41.2) | 8.4 (7.5) | 7.4 | −10.5 – 25.3 | 0.39 |
| MeanCO2 (mmHg) (21) | 45.6 (6.8) | 43.2 (5.6) | 2.4 | −1.5 – 6.3 | 0.21 |
| Surgically naïve (N) | |||||
| AHI (13) | 22.6 (39.6) | 13.2 (11.2) | 9.4 | −12.3 – 31.2 | 0.36 |
| oAHI (13) | 21.2 (40.0) | 11.2 (10.7) | 10.0 | −11.6 – 31.6 | 0.33 |
| O2 Nadir (%) (13) | 84.8 (8.9) | 86.3 (6.7) | −1.4 | 7.0 – 4.1 | 0.58 |
| ODI (10) | 25.0 (54.7) | 12.7 (12.6) | 12.3 | −22.7 – 47.2 | 0.45 |
| MeanCO2(mmHg)(11) | 45.2 (8.0) | 43.1 (6.6) | 2.1 | −4.5 – 8.7 | 0.50 |
| Previous AT (N) | |||||
| AHI (10) | 11.0 (4.2) | 6.9 (3.1) | 4.1 | −0.2 – 8.4 | 0.06 |
| oAHI (8) | 10.0 (4.9) | 5.8 (3.5) | 4.3 | −1.6 – 10.1 | 0.13 |
| O2 Nadir (%) (10) | 88.5 (4.1) | 90.9 (2.3) | −2.5 | −4.7 – −0.2 | 0.04 |
| ODI (8) | 4.3 (2.0) | 2.9 (1.9) | 1.4 | −0.3 – 3.0 | 0.09 |
| MeanCO2 (mmHg)(10) | 46.0 (5.5) | 43.3 (4.6) | 2.8 | −2.6 – 8.1 | 0.27 |
p-value from paired t-test with equal variances
Abbreviations: PSG, polysomnography; SD, Standard Deviation; CI, Confidence Interval; AHI, Apnea Hypopnea Index; oAHI, obstructive Apnea Hypopnea Index; O2, oxygen saturation; ODI, Oxygen Desaturation Index; MeanCO2, mean end tidal carbone dioxide; AT, adenoidectomy and tonsillectomy
Figure 1.
Changes in mean polysomnography parameters in Trisomy 21 patients following DISE-directed surgery. Error bars represent standard error. DISE: drug-induced sleep endoscopy; AHI: apnea-hypopnea index; oAHI: obstructive apnea hypopnea index; ODI: oxygen desaturation index; O2: oxygen saturation.
DISCUSSION
DISE-directed surgery is a promising technique for identifying sites of obstruction in order to guide treatment decisions, especially in patients with refractory OSA. Studies of DISE-directed surgery on adults with OSA have shown improved outcomes, but few studies have focused on the pediatric population and fewer still focus on DS patients with OSA [17]. No studies have compared characteritics between Down syndrome patients who are surgically naïve and those who have undergone prior AT, making this study unique. Compared with non-syndromic children, those with DS are more likely to have OSA refractory to AT [4–6], likely secondary to their higher rates of multilevel upper airway collapse [18]. There are several features of DS that may increase risk for sleep-disordered breathing including decreased pharyngeal muscle tone, retroglossia, macroglossia, midfacial hypoplasia, lymphoid hyperplasia, and high BMI [6]. Commonly identified sites of obstruction include the lingual tonsils, tongue base and the supraglottis, none of which can be seen on direct physical exam, but all of which can be corrected surgically [19–20].
We found multisite airway obstruction to be common in this series. Scores for both surgically naïve children and those who had undergone prior AT were over 7 out of 15 (Table 2). The most common sites of obstruction in children with DS who had undergone prior AT were the tongue base and supraglottis, suggesting that these sites may be responsible for the high prevalence of residual OSA seen in DS patients after AT. Our findings also revealed that DS patients with OSA who are surgically naïve are more likely to be of normal weight, but still have lateral pharyngeal wall collapse (Table 1 and 2). The implication that lateral pharyngeal wall collapse can be obstructive in DS patients that are of normal weight is clinically important to management considerations.
AT remains a first line therapy for children with OSA and DS. However, the success rate of AT in children with DS is lower than in the general population, compounding the clinical challenge of managing these patients. This study sought to test whether DISE-directed surgery may be of benefit for children with DS. We found that PSG parameters improved for both surgically naïve patients and those who underwent prior AT, although the majority of improvements did not reach statistical significance. DISE can be especially useful in OSA due to multilevel upper airway collapse as seen in children with DS. For these patients, the paradigm of DISE-directed surgery in which site(s) of anatomic airway obstruction are identified by DISE and then addressed surgically may be an improvement from the existing paradigm of initial empiric AT. A recent review of 5 case series (total n=234) of both surgically naïve and non-naïve children recommended that DISE be reserved for surgically naïve children without hypertrophic tonsils and for children with persistent OSA after AT [21]. The case series included both healthy children and children with sleep-related comorbidities.
LIMITATIONS
The main limitation of this analysis of 24 patients was a lack of power to detect statistically significant changes in PSG parameters. An additional limitation was that this study was retrospective and occurred at a single tertiary care institution, where there may be systematic biases and differences in patient selection, clinical management, and surgical technique. DISE-directed surgery may have inherent limitations as well, as it uses anesthesia to mimic natural sleep and may not accurately reveal locations of obstruction.
CONCLUSION
This study confirms a high proportion of multisite airway obstruction in DS patients with OSA. Although we observed an improvement across PSG measures, this study lacked power to detect statistically significant changes. DISE-directed surgery holds promise as a beneficial tool for children with DS but a larger prospective study is needed before specific recommendations may be made on incorporating DISE into the OSA diagnostic and treatment algorithm for children with DS.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health [5T32DC000018–33].
The time provided by Dr. Akkina was supported by the United States National Institute on Deafness and Other Communication Disorders under Grant T32 DC000018.
Footnotes
DECLARATION OF INTERESTS
There are no financial disclosures or conflicts of interest.
Bibiolography
- 1.Marcus CL. Childhood obstructive sleep apnea syndrome: unanswered questions. Chest 2008; 134:1114–1115. [DOI] [PubMed] [Google Scholar]
- 2.Marrone O, Bonsignore MR. The puzzle of metabolic effects of obstructive sleep apnoea in children. The European respiratory journal 2016; 47:1050–1053. [DOI] [PubMed] [Google Scholar]
- 3.Muzumdar H, Arens R. Physiological effects of obstructive sleep apnea syndrome in childhood. Respiratory physiology & neurobiology 2013; 188:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DelRosso LM. Epidemiology and Diagnosis of Pediatric Obstructive Sleep Apnea. Current problems in pediatric and adolescent health care 2016; 46:2–6. [DOI] [PubMed] [Google Scholar]
- 5.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Sleep problems and obstructive sleep apnea in children with down syndrome, an overwiew. International journal of pediatric otorhinolaryngology 2016; 82:12–15. [DOI] [PubMed] [Google Scholar]
- 6.Churchill SS, Kieckhefer GM, Landis CA, Ward TM. Sleep measurement and monitoring in children with Down syndrome: a review of the literature, 1960–2010. Sleep medicine reviews 2012; 16:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies CR, Harrington JJ. Impact of Obstructive Sleep Apnea on Neurocognitive Function and Impact of Continuous Positive Air Pressure. Sleep medicine clinics 2016; 11:287–298. [DOI] [PubMed] [Google Scholar]
- 8.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics 1991; 88:132–139. [PubMed] [Google Scholar]
- 9.Marcus CL, Brooks LJ, Draper KAet al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012; 130:e714–755. [DOI] [PubMed] [Google Scholar]
- 10.Lee CH, Hsu WC, Chang WH, Lin MT, Kang KT. Polysomnographic findings after adenotonsillectomy for obstructive sleep apnoea in obese and non-obese children: a systematic review and meta-analysis. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 2016; 41:498–510. [DOI] [PubMed] [Google Scholar]
- 11.Lin AC, Koltai PJ. Sleep endoscopy in the evaluation of pediatric obstructive sleep apnea. International journal of pediatrics 2012; 2012:576719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteller E, Mulas D, Haspert R, Matino E, Lopez R, Girabent-Farres M. Drug-induced sleep-endoscopy in children’s sleep related breathing disorders. Acta otorrinolaringologica espanola 2016; 67:212–219. [DOI] [PubMed] [Google Scholar]
- 13.Durr ML, Meyer AK, Kezirian EJ, Rosbe KW. Drug-induced sleep endoscopy in persistent pediatric sleep-disordered breathing after adenotonsillectomy. Archives of otolaryngology--head & neck surgery 2012; 138:638–643. [DOI] [PubMed] [Google Scholar]
- 14.Charakorn N, Kezirian EJ. Drug-Induced Sleep Endoscopy. Otolaryngologic clinics of North America 2016; 49:1359–1372. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan DK, Liming BJ, Horn DL, Parikh SR. A new scoring system for upper airway pediatric sleep endoscopy. JAMA Otolaryngol Head Neck Surg. 2014;140:595–602. [DOI] [PubMed] [Google Scholar]
- 17.Quante M, Merkenschlager A, Kiess W et al. [The impact of sleep endoscopy for obstructive sleep-disordered breathing in children and adolescents]. Laryngo- rhino- otologie 2014; 93:831–839. [DOI] [PubMed] [Google Scholar]
- 18.Maris M, Verhulst S, Saldien V, Van de Heyning P, Wojciechowski M, Boudewyns A. Drug-induced sedation endoscopy in surgically naive children with Down syndrome and obstructive sleep apnea. Sleep medicine 2016; 24:63–70. [DOI] [PubMed] [Google Scholar]
- 19.Digoy GP, Shukry M, Stoner JA. Sleep apnea in children with laryngomalacia: diagnosis via sedated endoscopy and objective outcomes after supraglottoplasty. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2012; 147:544–550. [DOI] [PubMed] [Google Scholar]
- 20.Mase CA, Chen ML, Horn DL, Parikh SR. Supraglottoplasty for sleep endoscopy diagnosed sleep dependent laryngomalacia. International journal of pediatric otorhinolaryngology 2015; 79:511–515. [DOI] [PubMed] [Google Scholar]
- 21.Galluzi F, Pignataro L, Gaini RM, Garavello W. Drug induced sleep endoscopy in the decision-making process of children with obstructive sleep apnea. Sleep Med 2015; 16:331–5. [DOI] [PubMed] [Google Scholar]