Abstract
Background:
Respiratory disease is a leading cause of death and disability worldwide. These diseases frequently present with a sex-bias in occurrence and severity, yet the mechanisms responsible for these sex-biases is a critically understudied area of basic research.
Methods:
Male and female C57BL/6 mice were exposed to multi-walled carbon nanotubes (MWCNTs) or crystalline silica (cSiO2) via oropharyngeal aspiration. Acute assessments were conducted 24-hours and 7-days after a single exposure. In chronic experiments mice were exposed to respective particles once per week for 4 weeks and sacrificed 8 weeks after the last exposure. Lung lavage fluid (LLF) was assessed for markers of injury and inflammation. Immune cell populations were analyzed by flow cytometry and histopathology assessment was performed on lung tissue from chronically exposed mice.
Results:
Female mice exposed to a single dose of MWCNTs generated a greater eosinophilic response than males 24-hours and 7-days post-exposure. Eosinophilia was accompanied by elevated type 2 cytokine production in LLF. The exaggerated acute response in females was consistent with lung pathology observed in the chronic model: females had greater alveolitis and epithelial cell hyperplasia compared to males. There were no sex-differences 24-hours after cSiO2 exposure, but by 7-days post-exposure female mice had greater airspace neutrophilia and inflammatory cytokine levels compared to males. However, following repeated exposure to cSiO2, male mice had worse alveolitis and greater dendritic cell presence within the lungs.
Conclusions:
Female mice are more susceptible to acute and chronic MWCNT-induced inflammation, but male mice are more susceptible to chronic cSiO2-induced lung pathology.
Keywords: Multi-walled carbon nanotubes, crystalline silica, sex differences, eosinophils, lung inflammation
Introduction
Worldwide, 1 billion people suffer from acute or chronic respiratory conditions. Second only to cardiovascular disease, respiratory diseases account for more than 10% of all disability-adjusted life-years and 4 million people die prematurely every year due to chronic disease (Forum of International Respiratory Societies & European Respiratory Society 2017). The study of the role of sex-based biological variables in disease etiology and how this impacts sex-biases in pathology has long been overlooked in basic and clinical research. However, epidemiological and clinical data have provided a solid foundation of evidence that there are sex-biases in the occurrence and severity of many lung diseases, including those resulting from the inhalation of particulates (Carey, Card, Voltz, Arbes Jr, et al. 2007; Pinkerton et al. 2015; Neigh & Mitzelfelt 2016; Yoshizaki et al. 2017; Vidaillac et al. 2018; Fuentes & Silveyra 2018).
Sex-biases in the prevalence/severity of particulate-mediated lung disease is likely influenced by gender-based factors such as exposure paradigms and healthcare. However, the use of animal models to investigate inherent sex-differences in respiratory inflammation eliminates gender-based confounding factors present in human studies. As such, there is a growing body of evidence that these biases are primarily a result of biological sex-based differences in the respiratory response (Carey, Card, Voltz, Germolec, et al. 2007). Lung anatomy and the immune response have been reported to differ between males and females (Sathish & Prakash 2016; Klein & Flanagan 2016; Molgat-Seon et al. 2018), both of which may contribute to sex-differences in particle-mediated disease etiology. The immune response is of particular importance because inappropriate immune cell recruitment and inflammation can cause decreased lung function, such as seen during asthma (Papi et al. 2018). The immune system will mount a specific response to inhaled xenobiotics in order to convey the appropriate protection. Briefly, classical inflammation, or the Th1-type response, promotes cytotoxicity and is associated with inflammatory tissue damage and granulomas, while Th2-type inflammation promotes antibody production and is associated with allergy and asthma. Importantly, the literature suggests that males are predisposed to mounting a more severe Th1-type immune response, while females are more likely to produce an exaggerated Th2-type response (Speyer et al. 2005; Card et al. 2006; Falagas et al. 2007; Keselman & Heller 2015; Roved et al. 2017). Therefore, dependent upon which type of immune response is produced following the inhalation of a given particle, males or females may be predisposed to a more severe inflammatory response and subsequent lung injury.
Two particles of occupational and environmental inhalation concern are multi-walled carbon nanotubes (MWCNTs) and crystalline silica (cSiO2), both of which cause lung inflammation and compromised respiratory function (Pollard 2016; Kobayashi et al. 2017). Human data on sex-differences in MWCNT- and cSiO2-mediated lung injury is insufficient, however, preliminary rodent studies suggest that there may be sex-biases in disease severity (Ma-Hock et al. 2009; Brass et al. 2010; Latoche et al. 2016; Cartwright et al. 2016; Kasai et al. 2016; Mayeux et al. 2018). Furthermore, sex-differences in the acute inflammatory response have yet to be connected to long-term outcomes.
cSiO2 is a well-known respiratory toxicant causing both local and systemic inflammation and disease (Pollard 2016). Murine studies have reported that male animals are more susceptible to adverse outcomes following respiratory exposure to cSiO2 (Brass et al. 2010; Latoche et al. 2016; Mayeux et al. 2018). However, human data remains unclear due to the fact that most exposures occur in male-dominated trades such as construction and mining (Bang et al. 2015). Previous mechanistic studies have investigated sex-differences in acute outcomes following cSiO2 exposure (Brass et al. 2010; Latoche et al. 2016) though further research is required to elucidate whether or not these differences persist chronically and ultimately impact disease outcomes.
MWCNTs are versatile engineered nanomaterials that are utilized in a wide variety of consumer products such as electronics, sporting goods, and dental implants. During production and disposal, MWCNTs are easily aerosolized and capable of reaching the functional gas exchange region of the lungs when inhaled (Qiao et al. 2015). Human data on MWCNT inhalation is primarily focused on monitoring exposure levels, but rodent studies, albeit limited, suggest that there are sex-differences in carcinogenic and inflammatory outcomes following MWCNT exposure (Ma-Hock et al. 2009; Cartwright et al. 2016; Kasai et al. 2016). Ever-increasing production volumes coupled with the high pathogenic potential of MWCNTs necessitates further identification of potentially sensitive populations. The MWCNTs chosen for this study are flexible with a high aspect ratio and 5% by weight nickel contamination (Hamilton et al. 2012). The properties of the present MWCNTs are fairly representative of the MWCNTs typically found in consumer products. However, due to the high tunability of physical and chemical properties, CNTs are a heterogeneous family of nanomaterials. Many physiochemical properties may impact the biological activity of a given CNT, further necessitating thorough investigation of potential sex-based outcomes following CNT inhalation.
The purpose of this study was to investigate and define sex-differences in lung inflammation and injury following respiratory exposure to cSiO2 and MWCNTs. The acute respiratory inflammatory response was characterized in male and female mice, and for the first time, sex-differences in lung pathology resulting from chronic exposure to cSiO2 and MWCNTs was assessed in order to connect sex-differences in the acute response to long-term outcomes.
Methods
Mice:
Male and female C57BL/6 mice were housed in a specific pathogen-free vivarium at an ambient temperature of 22 ± 2°C with alternating 12 hour light/dark cycles. Mice were provided standard soy protein-free rodent chow (Envigo, Madison, WI) and deionized water ad libitum. Age-matched (9–12 weeks) male and female mice were used in all studies. All animal procedures were approved by, and in accordance with, the Institutional Animal Care and Use Committee at the University of Montana.
Experimental Instillations:
Preparation and characteristics of crystalline silica (cSiO2) is as previously described by our laboratory (Thakur et al. 2009). To remove metal contaminants, cSiO2 (Min-U-Sil-5, Pennsylvania Glass Sand Corporation) was washed in 1 M HCl at 100°C for 1 hour, followed by three washes with sterile water. The cSiO2 was dried in an oven at 200°C for several hours to remove all water. cSiO2 was determined endotoxin-free by Limulus Amebocyte Lysate assay (Cambrex, Walkersville, MD), data not shown. The zeta potential (surface charge) is −16.2 mV, however, particle size is heterogeneous with larger particles ranging from 1–2.5 μm in diameter and smaller particles 200 nm to 1 μm in diameter.
Multi-walled carbon nanotubes (MWCNT) were generously provided by Dr. Nigel Walker and Brad Collins at the National Toxicology Program (NTP) at the National Institute of Environmental Health Sciences (NIEHS). The MWCNTs used in this study (“FA21”; Sun Innovations, Inc., Fremont, CA) were 27 nm in diameter, 5–15 μm in length and contain 5.54% nickel and < .01% iron and molybdenum contamination by weight, as previously described (Hamilton et al. 2012).
Immediately prior to each experiment, 20 mg/ml cSiO2 and 1 mg/ml MWCNT suspensions were prepared in dispersion media (DM) vehicle (mouse serum albumin and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine). Uniform suspensions were generated by sonication for 5 minutes at 25% amplitude in a Qsonica Q500 Sonicator with VWR cooled circulating water bath. Mice were anesthetized and exposed to DM (50 μl/mouse), cSiO2 (1 mg/mouse), or MWCNT (50 μg/mouse) via oropharyngeal aspiration. Mice were euthanized 1 or 7 days after a single exposure, or exposed once per week for 4 weeks and euthanized 8 weeks after the last exposure. Euthanasia was performed by an overdose of sodium pentobarbital (Euthasol™).
Whole Lung Lavage:
A concentrated first-pull was collected for local cytokine analysis: lungs were removed from the chest cavity and 1 ml of PBS was used to wash the lungs three times with the same fluid; centrifugation was used to pellet any cells and the concentrated first-pull supernatant was collected for cytokine measurement. Following the concentrated first pull, four additional, separate washes (1 mL PBS each) were performed. The supernatant from these washes was discarded and the collected cells pooled with those from the concentrated first-pull for airspace immune cell analysis. Lung lavage total cell counts were measured by Z2 Coulter Particle Count and Size Analyzer.
Flow Cytometry:
Airspace immune cell populations were collected by lung lavage. Following lavage, lung tissue was digested with Collagenase IA (Sigma-Aldrich, Saint Louis, MO) in complete RPMI with 10% FBS (VWR International, Radnor, PA) at 37°C for 1.5 hours. Cells were then used for flow cytometry.
Flow cytometry was performed on Life Technologies Attune® NxT Acoustic Focusing Cytometer in the Flow Cytometry Core at the University of Montana. DAPI was used as a live/dead marker and anti-mouse antibodies were purchased from verified vendors: CD45-BV510 (clone 30-F11, Biolegend), CD11c-PerCP/Cy5.5 (clone N418, Biolegend), CD11b-APC (clone M1/70, Biolegend), Ly6G-AF700 (clone 30-F11, Biolegend), and Siglec F-APC/Cy7 (clone E50–2440 (RUO), BD Biosciences). The following populations were defined: alveolar macrophages (CD45+ Ly6G− Siglec F+ CD11c+ CD11b−), neutrophils (CD45+ Ly6G+ CD11b+ Siglec F−), eosinophils (CD45+ Ly6G− Siglec F+ CD11c− CD11b+), dendritic cells (CD45+ Ly6G− Siglec F− F4/80− CD11c+ MHCII+), and interstitial macrophages/monocytes (CD45+ Ly6G− F4/80− CD11b+ CD11c−). All data analysis was performed in FlowJo 10.4.
Eosinophil peroxidase assay:
Cells were collected by lung lavage and incubated with eosinophil peroxidase (EPX) substrate solution (0.1mM orthophenylene diamine, 50mM Tris-HCl, 0.1% Triton X-100, and 1mM hydrogen peroxide) for 10 minutes. Sulfuric acid was used to stop the reaction and optical density was read at 495nm. Horseradish peroxidase (Sigma-Aldrich, St. Louis, MO, United States) was used to create a standard curve.
Lung lavage fluid cytokines:
Cytokine levels in lung lavage fluid (IFNγ, IL-0, IL-13, IL-1β, IL-33, IL-6, TNFα, IL-4, IL-5) and plasma (TNFα) were measured by Meso Scale Discovery Multiplex Assay, according to manufacturer’s instruction. This high-sensitivity assay accurately measures cytokines present at levels well below the limit of detection in a standard ELISA (< 1 pg/ml).
Lung lavage fluid total protein content:
Extracellular protein content in concentrated first-pull LLF was determined by the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL) according to manufacturer’s instruction.
Histology:
The lungs from each mouse were inflation-fixed through the trachea with 3% paraformaldehyde-PBS and submerged in the same fixative overnight at 4°C. The lungs were washed with PBS, dehydrated with 70% ethanol, and embedded in paraffin (Leica ASP 300 Tissue Processor). Tissue sections (5 μm) were stained with hematoxylin-eosin (RAS Harris Hematoxylin and Shandon Alcohol Eosin) for histological analysis using a Leica ST 5010 automated stainer (Thermo Shandon, Thermo Fisher Scientific, Houston, TX).
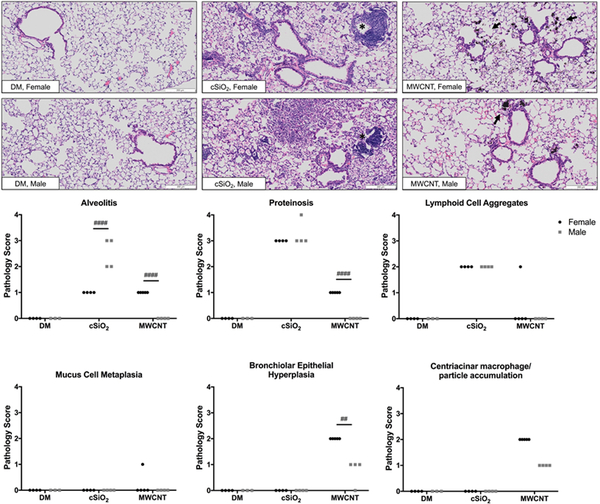
Tissues were scored semi-quantitatively in a blinded fashion by a board-certified veterinary pathologist (Dr. Jack Harkema, Michigan State University) for the following lung lesions: (a) presence of lymphoid aggregates within perivascular and peribronchiolar regions; (b) presence of alveolar proteinosis; (c) alveolitis defined as the increased accumulation of neutrophils, lymphocytes, and mononuclear/macrophages in the alveolar parenchyma; and (d) alveolar type II epithelial cell hyperplasia. Individual lungs were graded for these lesions using the following criteria (% of total pulmonary tissue examined): (0) no changes compared to control mice; (1) minimal (<10%); (2) mild (10–25%); (3) moderate (26–50%); (4) marked (51–75%) of total area affected, as previously described (Bates et al. 2018).
Statistics:
Statistical analyses involved comparison of means using two-way ANOVA followed by Dunnett’s test or Sidak’s post hoc testing. Because subjective histopathology scoring is ordinal level data, raw scores were rank-transformed prior to two-way ANOVA and post hoc testing. All probabilities were two-tailed unless otherwise stated. Statistical power was greater than 0.8. Statistical significance was defined as a probability of type I error occurring at less than 5% (p < 0.05). Graphics and analyses were performed on PRISM v.7.0 (GraphPad, San Diego, CA).
Results
Female mice have greater airspace eosinophilia 24-hours after a single exposure to MWCNTs.
In order to investigate potential sex-differences in the respiratory response to inhaled particles, two relevant particles of interest were selected for the study: micron-sized cSiO2 and nano-sized MWCNTs. Male and female C57BL/6 mice were exposed to a single dose of cSiO2 (1 mg), MWCNTs (50 μg), or dispersion media (DM) vehicle control (50 μl), by oropharyngeal aspiration and harvested 24-hours post-exposure. Whole lung lavages were performed to collect immune cells located within the airspace and assess the local inflammatory response. The term airspace will henceforth be used to describe lavageable areas of the lower respiratory system including the trachea, bronchioles, and acinus.
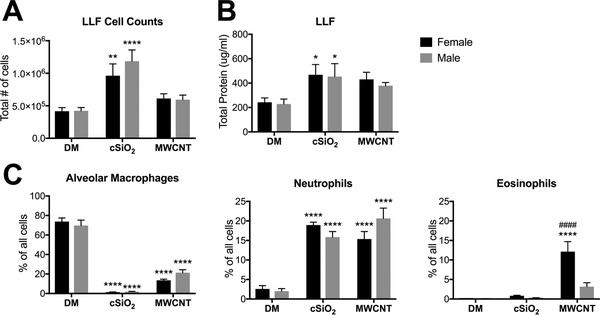
Total cell counts showed a significant increase in cell numbers in the lung lavage fluid (LLF) following exposure to cSiO2, but not MWCNTs, for both sexes (Fig. 1A). Results were similar for the two particles for airspace protein content (Fig. 1B). Cytometric analysis of airspace immune cell populations only revealed sex-differences in eosinophil recruitment following MWCNT exposure; female animals had significantly greater airspace eosinophilia compared to males (Fig. 1C). In contrast, very little eosinophilia was observed following cSiO2 exposure, however a dramatic reduction in alveolar macrophages (AMs) and influx of neutrophils was observed, regardless of sex (Fig. 1C). The reduction in AM frequency (% of total LLF cells) following particle exposure was due to increases in other immune cell populations, but also particle-mediated toxicity. The observed immune cell profiles were consistent with previous reports on the characteristic responses to cSiO2 (Rabolli et al. 2014) and MWCNTs (Beamer et al. 2013; Carvalho et al. 2018).
Figure 1. Sex-differences in immune cell populations in LLF 24-hours post-exposure to a single dose of cSiO2 or MWCNTs.
Male and female C57BL/6 mice were exposed to a single dose of cSiO2 (1 mg), MWCNTs (50μg), or dispersion media (DM) vehicle control (50 μl), and euthanized 24-hours post-exposure. Whole lung lavage fluid (LLF) was collected for total cell counts (A), extracellular protein assessment (B), and cytometric analysis of immune cell populations within the airspace (C). Data represented as mean ± SEM, n = 4–12. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared to same-sex DM control, # compared to males of the same treatment.
Female mice have greater type 2 cytokine production than males 24-hours after a single exposure to MWCNTs.
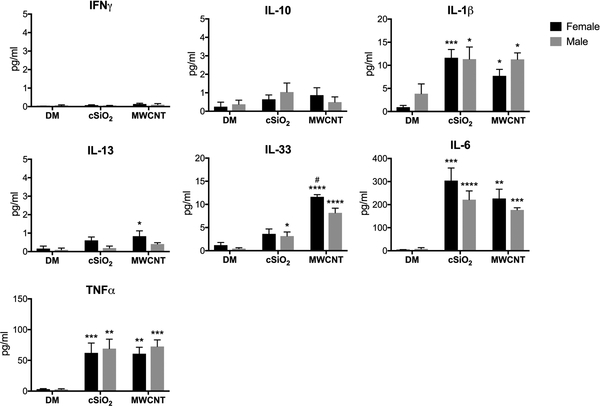
In an effort to help explain the MWCNT sex-differences, inflammatory cytokine signaling was analyzed in male and female animals 24-hours following a single particle exposure. LLF was collected from exposed mice and local cytokine levels were measured. Classical acute-phase inflammatory cytokines IL-6, IL-1β, and TNFα were significantly upregulated in both treatment groups, regardless of sex (Fig. 2). While cSiO2 exposure elicited a more classical M1/Th1-type inflammatory response in male and female animals, MWCNT exposure was most notably characterized by an induction of the M2/Th2-type cytokine IL-33. Consistent with the observed eosinophilia, female animals produced greater levels of IL-33 and IL-13 in response to MWCNTs than males (Fig. 2). Our laboratory has previously demonstrated that IL-33 is an essential mediator of MWCNT-induced inflammation (Beamer et al. 2013); this signaling pathway is associated with initiating allergic-type inflammation and eosinophil recruitment to the airways (Ding et al. 2018).
Figure 2. Cytokine analysis in the LLF of mice 24-hours post-exposure to a single dose of cSiO2 or MWCNTs.
Male and female C57BL/6 mice were exposed to a single dose of cSiO2 (1 mg), MWCNTs (50μg), or dispersion media (DM) vehicle control (50 μl), and euthanized 24-hours post-exposure. Concentrated first-pull lung lavage fluid was collected for cytokine expression analysis. Data represented as mean ± SEM, n = 4. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared to same-sex DM control, # compared to males of the same treatment.
7-days post-exposure, there is greater MWCNT-induced eosinophil peroxidase activity and cSiO2-mediated neutrophil recruitment in the airspace of female mice.
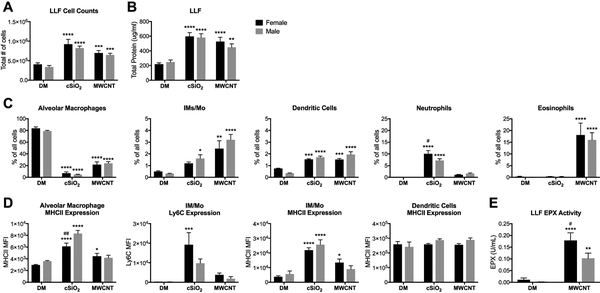
To further characterize sex-differences in the progression of the acute inflammatory response to cSiO2 and MWCNTs, mice were harvested 7-days following a single exposure to respective particles. By 7-days post-exposure to a single dose of cSiO2 or MWCNTs, there was significant infiltration of inflammatory cells into the airspace. Compared to vehicle controls, all treatment groups had increased total LLF cell counts and extracellular protein content, consistent with impaired AM function and surfactant clearance (Fig. 3A, B).
Figure 3. Sex-differences in immune cell populations in LLF 7-days post-exposure to a single dose of cSiO2 or MWCNTs.
Male and female C57BL/6 mice were exposed to a single dose of cSiO2 (1 mg), MWCNTs (50μg), or dispersion media (DM) vehicle control (50 μl), and euthanized 7-days post-exposure. Whole lung lavage fluid (LLF) was collected for total cell counts (A), extracellular protein assessment (B), cytometric analysis of immune cell populations within the airspace (C, D), and eosinophil peroxidase (EPX) measurement (E). The frequency of specific immune populations expressed as a percentage of all non- and immune-related cell types; data represented as mean ± SEM, n = 4–15. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared to same-sex DM control, # compared to males of the same treatment. IM/Mo, interstitial macrophage/monocytes; MFI, median fluorescence intensity.
This buildup of surfactant within the airspace could be explained by the dramatic reduction in AM frequency (Fig. 3C) and number (data not shown), due to particle toxicity. There were no apparent sex-differences in the frequency (Fig. 3C) or number (data not shown) of MWCNT-induced antigen presenting cells (APCs) recruited to the airspace; there was significant infiltration of interstitial macrophages/monocytes (IM/Mo) and dendritic cells (DCs) in both sexes. As expected, female mice continued to maintain a significant eosinophilic response to MWCNTs at this time-point. However, male mice have also begun to recruit a significant number of eosinophils to the airspace (Fig. 3C). AM and IM/Mo expression of MHCII was only increased over controls in MWCNT-treated females, but there were no statistically significant sex-differences in MHCII or Ly6C expression levels in MWCNT-treated males and females (Fig. 3D). While there was no significant difference between the total LLF cell counts and frequency of eosinophils in MWCNT-treated males and females due to inter-animal variability, females trended higher in both groups. Furthermore, eosinophil peroxidase (EPX) measurements, which reflect the overall number of eosinophils present in the airspace, exhibited significantly greater enzyme activity in female mice compared to males (Fig. 3E).
At the 7-day time-point, female animals also produced a greater neutrophilic response to cSiO2 compared to males, reflected both in frequency (Fig. 3C) and cell number (data not shown). Female mice also recruited a greater amount of Ly6C-expressing IM/Mo (Fig. 3C, D), indicative of increased inflammatory signaling. Yet male AMs upregulated MHCII expression to a greater extent than females (Fig. 3D), suggesting increased cSiO2-mediated antigen presentation capacity in male animals.
7-days post-exposure to cSiO2 and MWCNTs female mice have increased levels of inflammatory cytokines in the LLF compared to males.
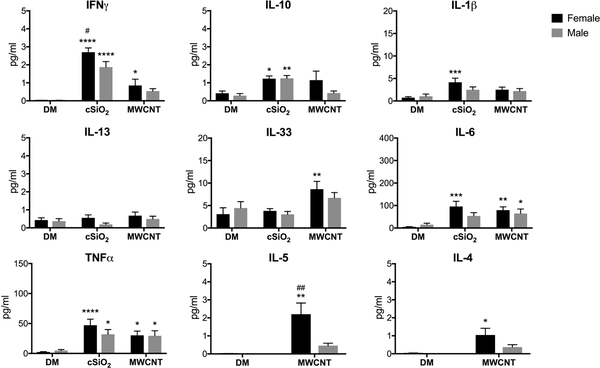
By 7-days post-exposure, expression of the inflammatory cytokines IL-1β, IL-6, and TNFα began to decline in all treatment groups (Fig. 4). IL-1β and IL-6 expression remained elevated over controls in cSiO2-treated females but not males, which supports the notion that there was greater respiratory inflammation in female mice 7-days following cSiO2 exposure. Despite the down-regulation of other acute-phase inflammatory cytokines, IFNγ levels were significantly increased over controls in the lungs of both cSiO2- and MWCNT-treated female mice, but only in cSiO2-exposed males (Fig. 4). Consistent with other inflammatory cytokines and immune cell recruitment, particle-induced upregulation of IFNγ was greater in female mice compared to males.
Figure 4. Cytokine analysis in the LLF of mice 7-days post-exposure to a single dose of cSiO2 or MWCNTs.
Male and female C57BL/6 mice were exposed to a single dose of cSiO2 (1 mg), MWCNTs (50μg), or dispersion media (DM) vehicle control (50 μl), and euthanized 7-days post-exposure. Concentrated first-pull lung lavage fluid was collected for cytokine expression analysis. Data represented as mean ± SEM, n = 5–12. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared to same-sex DM control, # compared to males of the same treatment.
IL-33 expression continued to remain elevated in MWCNT-exposed females; the continued activation of the ST2/IL-33 axis was consistent with elevated levels of MWCNT-induced IL-4 and IL-5 in female mice (Fig. 4). IL-5 is a potent eosinophil chemoattractant that is produced by innate immune cells in response to IL-33 signaling (Ding et al. 2018); IL-4 is also a type 2 inflammatory cytokine associated with eosinophilia (Chen et al. 2004). Despite the significant eosinophilia in male mice (Fig. 3C), IL-4 and IL-5 levels were not significantly increased above control animals.
In a chronic exposure model, male mice have worse cSiO2-mediated lung pathology and female mice have worse MWCNT-mediated pathology.
To address the question of whether or not sex-differences in the acute immune response to cSiO2 and MWCNTs translated to long-term lung pathology outcomes, chronic experiments were conducted by repeated exposures to both particles. Mice were exposed to cSiO2 or MWCNTs, respectively, once per week for four weeks and euthanized eight weeks after the last exposure. Blinded pathology scoring of H&E lung sections was then performed by a certified veterinary pathologist. Following repeated exposure to cSiO2 both male and female mice developed mild aggregation of lymphocytes (T cells, B cells) and DCs (suggestive of ectopic lymphoid structures) in the perivascular and peribronchiolar regions and moderate alveolar proteinosis (Fig. 5). However, males had significantly greater accumulation of immune cells in the alveolar parenchyma (alveolitis) compared to females (Fig. 5).
Figure 5. Histopathology of lung tissue in a chronic exposure model.
Male and female C57BL/6 mice were exposed to cSiO2 (1 mg), MWCNTs (50μg), or dispersion media (DM) vehicle control (50 μl), once per week for 4 weeks and harvested 8 weeks after the last exposure. Representative images (10X) of Hematoxalin & Eosin stained lung sections (A), which were graded individually for severity of lung inflammation (B): no findings (0), minimal (1), mild (2), moderate (3), or severe (4). Asterisks indicate lymphoid cell aggregates; arrows denote centriacinar macrophage/MWCNT accumulation. Data points represent individual mice of respective treatments, n = 3–5. # p < .05, ## p < .01, ### p < .001, #### p < .0001 compared to mice of the opposite sex from the same exposure group.
In contrast to the cSiO2 pathology results, but as predicted by acute inflammatory measures, female mice developed worse MWCNT-induced lung pathology than males, as measured by alveolitis, proteinosis, epithelial cell hyperplasia, and the accumulation of MWCNT-carrying macrophages in the centriacinar regions of the lungs (the beginning of the lung parenchyma where the bronchioles meet the alveolar ducts) (Fig. 5). Despite the previously observed type 2 inflammatory response in acute studies and epithelial hyperplasia, no significant mucus cell metaplasia was observed in response to MWCNTs at this time-point (Fig. 5).
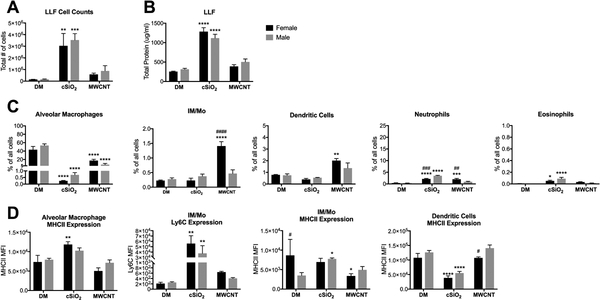
In the LLF 8-weeks post-exposure, MWCNT-treated females have increased phagocytic cells and cSiO2-treated males have greater neutrophilia.
In a separate repeated, chronic exposure experiment, cells present within the airspace were collected by lung lavage and analyzed by flow cytometry. Total LLF cell counts were not different between male and female mice within respective treatment groups, and only cSiO2-exposed animals had increased cell counts over controls (Fig. 6A). Consistent with histopathology proteinosis assessment, airspace protein content was significantly elevated in cSiO2-exposed mice, but not different between sexes (Fig. 6B). However, protein content in MWCNT-exposed mice was not significantly increased over vehicle controls (Fig. 6B), suggesting a resolution of inflammation and recovery of lung macrophage function by this time post-exposure.
Figure 6. Sex-differences in immune cell populations in LLF 8-weeks post-exposure to cSiO2 or MWCNTs.
Male and female C57BL/6 mice were exposed to cSiO2 (1 mg), MWCNTs (50μg), or dispersion media (DM) vehicle control (50 μl), once per week for 4 weeks and harvested 8 weeks after the last exposure. Whole lung lavage fluid (LLF) was collected for total cell counts (A), extracellular protein assessment (B), and cytometric analysis of immune cell populations within the airspace (C, D). The frequency of specific immune populations expressed as a percentage of all non- and immune-related cell types; data represented as mean ± SEM, n = 4–5. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared to same-sex DM control, # compared to males of the same treatment. IM/Mo, interstitial macrophage/monocytes; MFI, median fluorescence intensity.
Consistent with the observed increase in immune cells within the alveolar space of male animals (Fig. 5), the frequency of neutrophils present within the airspace of cSiO2-exposed males was greater than cSiO2-exposed females (Fig. 6C). At this time-point, eosinophil presence was modest, but significantly increased over controls in both cSiO2 groups. Lung macrophage levels were comparable between sexes (Fig. 6C), but have opposing regulation of MHCII expression in response to cSiO2. AMs from females, but not males, had increased expression of MHCII compared to controls. However, MHCII expression on IM/Mo’s was only significantly elevated in males due to greater homeostatic expression in control females (Fig. 6D). In both sexes, DC MHCII expression was downregulated compared to controls. Ly6C, an inflammatory marker, was significantly upregulated in IM/Mo of both sexes. These results are in accordance with previous literature reporting that inflammatory macrophages are the primary mediators of cSiO2-induced lung inflammation and injury (Iyer et al. 1996).
MWCNT-mediated IM/Mo, DC, and neutrophil recruitment was greater in females compared to males (Fig. 6C). Despite the continued influx of phagocytes in female mice (Fig. 6C) and the persistence of MWCNTs in the lungs (Fig. 5), MWCNT-induced eosinophil recruitment (Fig. 6C) and upregulation of APC inflammatory markers (Fig. 6D) has begun to resolve in both sexes. Compared to control animals, infiltrating IM/Mo and DCs did not upregulate the inflammatory marker Ly6C nor antigen-presenting MHCII in MWCNT-treated males or females (Fig. 6D).
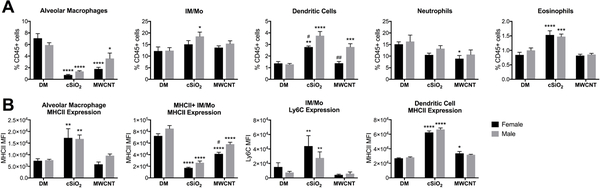
Male mice have a greater frequency of phagocytic cells in the lung tissue 8-weeks post-exposure to MWCNTs and cSiO2.
To further address the question of changes in immune cell populations within the lungs, whole lung tissue was digested for immune cell analysis by flow cytometry. Using whole lung tissue allows for the detection of any sex-differences within the lung interstitium that may not have been apparent in the LLF, which only reflects immune cell populations located within the airspace. Several sex-differences that were not observed in LLF analysis, were detected in the lung tissue. Compared to controls, the frequency of IM/Mo in the lung interstitium increased in cSiO2-treated males, but not females (Fig. 7A); similar to the LLF, inflammatory Ly6C expression was upregulated on IM/Mo of both sexes (Fig. 7B). Interestingly, of those IM/Mo that expressed MHCII, expression levels of this complex was downregulated (Fig. 7B), but the percentage of IM/Mo that expressed MHCII increased with cSiO2 exposure (data not shown). In both male and female cSiO2-treated mice, the frequency of neutrophils did not change, but eosinophils increased modestly over controls (Fig. 7A). Notably, the frequency of DCs within the lung tissue increased in both sexes, but was significantly greater in males compared to females. Additionally, MHCII expression was significantly upregulated in DCs of both sexes (Fig. 7B). These results contrast with LLF data, which reported a downregulation of MHCII expression in both sexes (Fig. 6D).
Figure 7. Sex-differences in immune cell populations in the lung tissue 8-weeks post-exposure to cSiO2 or MWCNTs.
Male and female C57BL/6 mice were exposed to cSiO2 (1 mg), MWCNTs (50μg), or dispersion media (DM) vehicle control (50 μl), once per week for 4 weeks and harvested 8 weeks after the last exposure. Whole lung tissue was digested and used for flow cytometry to identify immune cell populations (A) and cell surface marker expression as determined by median fluorescence intensity (MFI) (B). Cell population frequencies expressed as a percentage of CD45+ cells within the lung tissue; data represented as mean ± SEM, n = 4–5. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared to same-sex DM control, # compared to males of the same treatment. IM/Mo, interstitial macrophage/monocytes.
In response to MWCNTs, a significantly greater frequency of DCs and upregulation of MHCII on lung tissue IM/Mo was observed in males compared to females (Fig. 7A, B). However, only female mice had a significant decrease in neutrophil frequency within the lung tissue (Fig. 7A). Interestingly, these results conflict with the LLF (Fig. 6C, D). No other changes or sex-differences of note were observed in the lung tissue of MWCNT-treated mice.
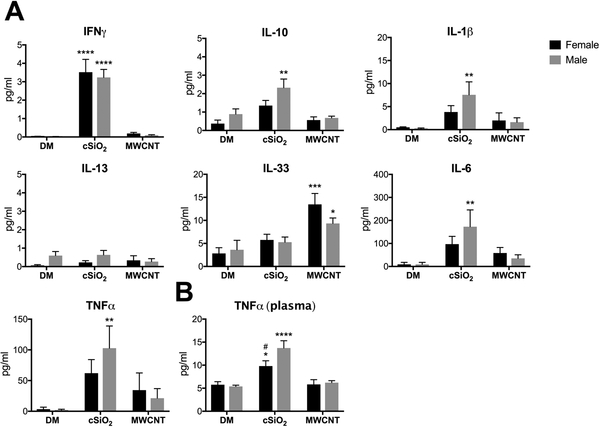
Male mice have greater local and systemic inflammatory cytokine levels than females after chronic cSiO2 exposure.
Cytokine levels were measured in LLF and plasma from male and female mice following chronic particle exposure. cSiO2-induced pro-inflammatory signaling was generally higher in males compared to females. IFNγ and IL-13 expression was comparable between males and females; however, IL-1β, IL-6, and TNFα LLF levels were all significantly increased over controls in cSiO2-exposed males but not females (Fig. 8A). This increased inflammatory signaling was paralleled by higher levels of anti-inflammatory IL-10 in the LLF of cSiO2-exposed males but not females. Furthermore, systemic inflammatory signaling, as measured by plasma levels of TNFα, was significantly greater in cSiO2-exposed males in comparison to controls and MWCNT-treated females (Fig. 8B). These results support histopathology (Fig. 5) and flow cytometry data (Fig. 6 C, D; Fig. 7) showing that 8-weeks after repeated exposure to cSiO2 male mice have increased lung inflammation and injury when compared to female mice also exposed to cSiO2. Consistent with the previously observed resolution of inflammation in MWCNT-treated animals, no cytokines, with the exception of IL-33, were elevated above control animals at this time-point (Fig. 8).
Figure 8. LLF and plasma cytokine expression in mice chronically exposed to cSiO2 and MWCNTs.
Male and female C57BL/6 mice were exposed to cSiO2 (1 mg), MWCNTs (50μg), or dispersion media (DM) vehicle control (50 μl), once per week for 4 weeks and harvested 8 weeks after the last exposure. Concentrated first-pull lung lavage fluid (A) and plasma (B) was collected for cytokine expression analysis. Data represented as mean ± SEM, n = 4–5. * p < .05, ** p < .01, *** p < .001, **** p < .0001 compared to same-sex DM control, # compared to males of the same treatment.
Discussion
The purpose of this study was to investigate potential sex-differences in the acute and chronic inflammatory responses to particles of occupational and environmental inhalation concern. Nano-sized MWCNTs and micron-sized cSiO2 were chosen for this study based on their ability to elicit robust inflammatory responses (Hamilton et al. 2008; Hamilton et al. 2012). In this study, we showed that the acute airspace inflammatory response to MWCNTs, but not cSiO2, was predictive of sex-based chronic pathology outcomes. Female mice generated a rapid eosinophilic response to MWCNTs at 24-hours post-exposure (Fig. 1C) which was maintained until 7-days post-exposure, but male mice did not develop significant airspace eosinophilia until day 7 (Fig. 3C). Eosinophilia was accompanied by increased IL-33 production in both males and females, but at 7-days post-exposure only MWCNT-treated females had elevated IL-5 and IL-4 in the lungs compared to controls (Fig. 4). IL-5 is a potent eosinophil chemokine, but eosinophil recruitment may be mediated by alternative chemotactic signaling in males, such as Eotaxins 1 and 2. IL-4 is produced by a variety of cell types (Paul 2015) but the cell type responsible for elevated IL-4 levels following MWCNT exposure is unknown. Future identification of the source of IL-4 may explain the lack of IL-4 in male LLF. The exaggerated acute eosinophilic/type 2 inflammatory response in females was consistent with long-term pathology outcomes: female mice had worse alveolitis, proteinosis, and epithelial cell hyperplasia after repeated exposure to MWCNTs (Fig. 5). The increased susceptibility of female mice to MWCNT-mediated type 2 inflammation is consistent with previously published animal models of allergic-type inflammation (Carey, Card, Voltz, Germolec, et al. 2007; Blacquière et al. 2010), as well as human data (Carey, Card, Voltz, Arbes Jr, et al. 2007; Keselman & Heller 2015).
Furthermore, clearance of MWCNTs from the lungs appears to be more efficient in male animals. The greater persistence of MWCNT-laden cells in the centriacinar regions of female animals (Fig. 5) may contribute to worsened lung pathology. There is a growing body of evidence that females may be at a disadvantage when it comes to mucociliary clearance due to lung anatomy and/or sex hormone-mediated regulation of epithelial cell function (Townsend et al. 2012; Jain et al. 2012; Raghavan & Jain 2016). Both of which may negatively impact the ability of MWCNT-containing AMs to traffic out of the lungs. Immune cell analysis suggests that there is an upregulation of phagocyte (IM/Mo, DCs, neutrophils) trafficking from the interstitium (Fig. 7A) into the airspace (Fig. 6C) of female animals in an effort to clear accumulated MWCNTs from the lungs.
The sustained production of the alarmin IL-33 in female animals is likely due to the continued presence of MWCNTs in the lungs, which are damaging epithelial cells and prompting them to release IL-33. However, the specific cell type responsible for propagating the IL-33 mediated (Beamer et al. 2013) type 2 inflammatory signaling following MWCNT exposure is unknown. AMs and ILC2s are both resident innate immune cells within the lungs that express the IL-33 receptor ST2 (Li et al. 2014) and therefore logical candidates as mediators of MWCNT-induced inflammation. ILC2s are emerging as critical initiators of allergic lung inflammation and IL-33 is a potent activator of ILC2-derived cytokine production. IL-33 is required, but not sufficient for, ILC2 activation; activation also requires the presence of TSLP, IL-2, or IL-7 (Martinez-Gonzalez et al. 2015). Future studies measuring the levels of these cytokines in the LLF following MWCNT exposure would help indicate whether or not ILC2s are activated and playing a role in sex-differences in eosinophil recruitment following MWCNT exposure.
AMs, however, are the primary resident immune cells within the lungs, which regularly phagocytose and degrade foreign materials and have been reported as key mediators of MWCNT-induced inflammation (Gustafson et al. 2015). AMs integrate cues from both endogenous and exogenous sources in order to adopt a spectrum of phenotypes which initiate specific immune responses (Martinez & Gordon 2014). Alternatively activated M2a macrophages promote the Th2-type immune response and are therefore associated with allergic inflammation. As the eosinophil recruitment reported in this study indicates, inhalation of MWCNTs has been reported to induce a type 2 immune response in human and animal models (Fatkhutdinova et al. 2016; Dong & Ma 2018a, p. 2), and even more specifically, induction of the M2a phenotype in AMs (Dong & Ma 2018b). IL-33 has been reported to amplify the M2a phenotype in AMs and subsequent eosinophil recruitment (Kurowska-Stolarska et al. 2009; Bunting et al. 2013). Furthermore, IL-33 production can be induced in AMs during inflammatory challenge (Chang et al. 2011; Li et al. 2014), which has the potential to act in an autocrine manner to propagate M2a polarization and cytokine production. Moreover, M2a polarization in allergic inflammation has been reported to be enhanced in female mice compared to males, as mediated by estrogen receptor alpha (ERα) signaling (Keselman et al. 2017). Therefore, it is reasonable to hypothesize that a combination of IL-33- and ERα-mediated signaling amplifies M2a AM signaling in female mice following MWCNT exposure, which leads to an increased type 2 immune response and subsequently worse lung pathology compared to males. However, further investigation will be required to elucidate the exact mechanism involved.
In this study there were no sex-differences in the respiratory immune response 24-hours post-exposure to cSiO2 (Fig. 1), but at 7-days post-exposure female mice had greater airspace neutrophilia (Fig. 3C) and local inflammatory cytokine levels (Fig. 4) than males. However, when examining lung pathology following repeated exposure, males had increased alveolitis (Fig. 5) and DC infiltration (Fig. 7A) than females; this pathology was accompanied by elevated local and systemic inflammatory signaling (Fig. 8) in males. These results may suggest that the increased acute airspace immune response in females serves a partially-protective function against chronic cSiO2-mediated disease. However, a limitation of this study includes only analyzing the LLF at acute time-points, which may not reflect population changes in the lung interstitium that may correlate better with long-term pathology outcomes.
Previous work on sex-differences in response to instilled cSiO2 report similar findings: C57BL/6 female mice recruit more inflammatory cells to the airspace at 3- and 14-days post-exposure but there is more hydroxyproline in the lung tissue of male mice, suggesting males develop worse fibrosis compared to females (Brass et al. 2010). Mechanistic investigation suggested that estrogen-mediated repression of secreted phosphoprotein 1 (SPP1) production contributes to the relative resistance of female mice to cSiO2-induced fibrosis (Latoche et al. 2016), with AMs or epithelial cells being the most likely cell types to be producing SPP1. However, these studies were limited to acute time-points following a single exposure. Interestingly, 12-weeks after a single exposure to cSiO2 in the diversity outbred (DO) murine model, male mice have worse lung inflammation and greater systemic autoantibody production (Mayeux et al. 2018). Taken together, these results corroborate the findings in the present study: female mice develop exaggerated acute inflammation to cSiO2, however, chronic disease is worse in males.
The observed development of lymphoid aggregates in the lungs (Fig. 5), known as ectopic lymphoid structures (ELS) or tertiary lymphoid structures, is a previously described cSiO2-associated lung pathology (Bates et al. 2018). ELS serve as secondary lymphoid organs within the lungs and possess germinal centers for antigen presentation and antibody production (Bates et al. 2018). The presence of these structures is often associated with autoimmune diseases, such as systemic lupus erythematosus (SLE), which is also associated with cSiO2 exposure in humans (Pollard 2012). Histopathology data did not show a sex-bias in lung ELS development (Fig. 5), however, the higher frequency of DCs in the lung tissue of male animals following cSiO2 exposure suggests that there should be a greater capacity for antigen presentation in male mice compared to females. It is of interest to note that in humans SLE occurs dramatically more often in women than men (9:1) (Pollard 2012). However, previous literature on autoantibody production (Mayeux et al. 2018) and the current study’s data on APC presence in the lung tissue (Fig. 7A) indicates that male mice are more susceptible to cSiO2-induced autoimmunity. These data suggest that cSiO2-induced autoimmunity may possess some unique attributes which may well depend on the model under examination.
Taken together, the literature and results of the present study provide strong evidence that sex-steroid hormones play a role in regulating the immune response to both MWCNTs and cSiO2. Additionally, the opposing sex-biases in particle-induced lung injury observed in this study may be attributed to the different immune responses produced. Instillation of MWCNTs elicited a Th2/M2a-type response, which has been reported to be enhanced by estrogen signaling (Campbell et al. 2014; Keselman et al. 2017). However, testosterone has been shown to promote a more severe Th1/M1-type response (Speyer et al. 2005; Card et al. 2006), similar to what was observed following chronic exposure to cSiO2. Further investigation of these mechanisms is required to elucidate the role of sex-steroid hormones in the immune response to inhaled particles.
One of the limitations of this study was the use of a single dose for each particle, which was not adjusted based on the body weight of the mice. Sex-specific outcomes may differ based on administered dose and a dose-response study would improve the characterization of sex-differences in inflammatory outcomes. However, due to the contrasting sex-specific pathologies observed in cSiO2- and MWCNT-exposed animals, we would not anticipate that the increased sensitivity of female animals to MWCNTs was simply due to smaller lung size/increased particle burden. But rather that these differences are due to inherent differences in the inflammatory responses of male and female mice to inhaled particles. An additional limitation of this study was the focused immune cell analysis to only those of myeloid-lineage. The development of a type 2 response to MWCNTs and the formation of ELS in cSiO2-treated mice indicate that lymphocytes are also contributing to sex-differences in lung inflammation.
Upon respiratory exposure, cSiO2 and MWCNTs induce distinctive immune response profiles (Th1 vs. Th2) in mice. Overall, these immune profiles are comparable between male and female mice, however, there is a sex-based susceptibility to the severity of particle-induced lung inflammation and injury. Taken together, the results in the present study provide compelling evidence for further sex-based characterization of acute and chronic inflammatory responses to inhaled particles. As well as the need to investigate how sex-based biological variables, such as sex-steroid hormones, contribute to the pathology of particle-mediated lung disease. Furthermore, there are sex-biases in respiratory diseases not caused by particulates. For example, autoimmune lung disease and pulmonary hypertension are more common in women; however, idiopathic pulmonary fibrosis and lower respiratory tract infections are more common in men (Falagas et al. 2007; Fuentes & Silveyra 2018). Understanding how sex-based biological variables contribute to respiratory inflammation will advance our scientific understanding of both non- and particle-mediated disease etiology. This will improve the quality of life of millions of people worldwide through the identification of potential therapeutic targets and the prevention of deleterious exposures in sensitive populations.
Acknowledgements
We would like to thank Dr. Nigel Walker and the National Toxicology Program of NIEHS for providing the MWCNTs and the Research Triangle Institute for the characterization of the MWCNTs. We would also like to thank Pam Shaw at the University of Montana’s Fluorescence Cytometry Core (Center for Environmental Health Sciences), and Mary Buford and Britten Postma in the Inhalation and Pulmonary Physiology Core (Center for Environmental Health Sciences) for technical assistance. The authors are indebted to Jack Harkema, DVM, PhD, DACVP of Michigan State University (Department of Pathobiology and Diagnostic Investigation) for performing the histopathology scoring assessments.
Source(s) of support and Disclaimers: Research reported in this publication was supported by the National Institute of Environmental Health Sciences under Award Number R01 ES023209 and the National Institute of General Medical Sciences under Award Number P30 GM103338. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of Interest: This work was supported by the National Institutes of Health [P30 GM103338 and R01 ES023209]. The authors report no conflict of interest.
References Cited
- Bang KM, Mazurek JM, Wood JM, White GE, Hendricks SA, Weston A, Centers for Disease Control and Prevention (CDC). 2015. Silicosis mortality trends and new exposures to respirable crystalline silica - United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 64:117–120. [PMC free article] [PubMed] [Google Scholar]
- Bates MA, Akbari P, Gilley KN, Wagner JG, Li N, Kopec AK, Wierenga KA, Jackson-Humbles D, Brandenberger C, Holian A, et al. 2018. Dietary Docosahexaenoic Acid Prevents Silica-Induced Development of Pulmonary Ectopic Germinal Centers and Glomerulonephritis in the Lupus-Prone NZBWF1 Mouse. Front Immunol [Internet]. [cited 2019 Jun 20]; 9 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6143671/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer CA, Girtsman TA, Seaver BP, Finsaas KJ, Migliaccio CT, Perry VK, Rottman JB, Smith DE, Holian A. 2013. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 7:1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacquière MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, Melgert BN. 2010. Airway inflammation and remodeling in two mouse models of asthma: comparison of males and females. Int Arch Allergy Immunol. 153:173–181. [DOI] [PubMed] [Google Scholar]
- Brass DM, McGee SP, Dunkel MK, Reilly SM, Tobolewski JM, Sabo-Attwood T, Fattman CL. 2010. Gender influences the response to experimental silica-induced lung fibrosis in mice. Am J Physiol - Lung Cell Mol Physiol. 299:L664–L671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting MM, Shadie AM, Flesher RP, Nikiforova V, Garthwaite L, Tedla N, Herbert C, Kumar RK. 2013. Interleukin-33 Drives Activation of Alveolar Macrophages and Airway Inflammation in a Mouse Model of Acute Exacerbation of Chronic Asthma. BioMed Res Int [Internet]. [cited 2019 Jul 1]. Available from: https://www.hindawi.com/journals/bmri/2013/250938/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Emmerson E, Williams H, Saville CR, Krust A, Chambon P, Mace KA, Hardman MJ. 2014. Estrogen Receptor-Alpha Promotes Alternative Macrophage Activation during Cutaneous Repair. J Invest Dermatol. 134:2447–2457. [DOI] [PubMed] [Google Scholar]
- Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. 2006. Gender Differences in Murine Airway Responsiveness and Lipopolysaccharide-Induced Inflammation. J Immunol Baltim Md 1950 177:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Arbes SJ Jr, Germolec DR, Korach KS, Zeldin DC. 2007. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 18:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. 2007. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol - Lung Cell Mol Physiol. 293:L272–L278. [DOI] [PubMed] [Google Scholar]
- Cartwright MM, Schmuck SC, Corredor C, Wang B, Scoville DK, Chisholm CR, Wilkerson H-W, Afsharinejad Z, Bammler TK, Posner JD, et al. 2016. The pulmonary inflammatory response to multiwalled carbon nanotubes is influenced by gender and glutathione synthesis. Redox Biol. 9:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho S, Ferrini M, Herritt L, Holian A, Jaffar Z, Roberts K. 2018. Multi-Walled Carbon Nanotubes Augment Allergic Airway Eosinophilic Inflammation by Promoting Cysteinyl Leukotriene Production. Front Pharmacol [Internet]. [cited 2018 Jul 15]; 9 Available from: https://www.frontiersin.org/articles/10.3389/fphar.2018.00585/full#h10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-J, Kim HY, Albacker LA, Baumgarth N, McKenzie ANJ, Smith DE, DeKruyff RH, Umetsu DT. 2011. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 12:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Grabowski KA, Xin J, Coleman J, Huang Z, Espiritu B, Alkan S, Xie HB, Zhu Y, White FA, et al. 2004. IL-4 Induces Differentiation and Expansion of Th2 Cytokine-Producing Eosinophils. J Immunol. 172:2059–2066. [DOI] [PubMed] [Google Scholar]
- Ding W, Zou G-L, Zhang W, Lai X-N, Chen H-W, Xiong L-X. 2018. Interleukin-33: Its Emerging Role in Allergic Diseases. Mol J Synth Chem Nat Prod Chem [Internet]. [cited 2019 Jul 2]; 23 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6099536/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Ma Q. 2018a. Type 2 Immune Mechanisms in Carbon Nanotube-Induced Lung Fibrosis. Front Immunol [Internet]. [cited 2019 Apr 24]; 9 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5972321/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Ma Q. 2018b. Macrophage polarization and activation at the interface of multi-walled carbon nanotube-induced pulmonary inflammation and fibrosis. Nanotoxicology. 12:153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Mourtzoukou EG, Vardakas KZ. 2007. Sex differences in the incidence and severity of respiratory tract infections. Respir Med. 101:1845–1863. [DOI] [PubMed] [Google Scholar]
- Fatkhutdinova LM, Khaliullin TO, Vasil’yeva OL, Zalyalov RR, Mustafin IG, Kisin ER, Birch ME, Yanamala N, Shvedova AA. 2016. Fibrosis biomarkers in workers exposed to MWCNTs. Toxicol Appl Pharmacol. 299:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forum of International Respiratory Societies, European Respiratory Society. 2017. The global impact of respiratory disease. [place unknown].
- Fuentes N, Silveyra P. 2018. Endocrine regulation of lung disease and inflammation. Exp Biol Med. 243:1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. 2015. Nanoparticle Uptake: The Phagocyte Problem. Nano Today. 10:487–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RF, Buford M, Xiang C, Wu N, Holian A. 2012. NLRP3 inflammasome activation in murine alveolar macrophages and related lung pathology is associated with MWCNT nickel contamination. Inhal Toxicol. 24:995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RF, Thakur SA, Holian A. 2008. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med. 44:1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Hamilton RF, Li L, Holian A. 1996. Silica-induced apoptosis mediated via scavenger receptor in human alveolar macrophages. Toxicol Appl Pharmacol. 141:84–92. [DOI] [PubMed] [Google Scholar]
- Jain R, Ray JM, Pan J, Brody SL. 2012. Sex Hormone–Dependent Regulation of Cilia Beat Frequency in Airway Epithelium. Am J Respir Cell Mol Biol. 46:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, Matsumoto M, Fukushima S. 2016. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part Fibre Toxicol [Internet]. [cited 2019 Jan 24]; 13 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5064785/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keselman A, Fang X, White PB, Heller NM. 2017. Estrogen Signaling Contributes to Sex Differences in Macrophage Polarization during Asthma. J Immunol. 199:1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keselman A, Heller N. 2015. Estrogen Signaling Modulates Allergic Inflammation and Contributes to Sex Differences in Asthma. Front Immunol [Internet]. [cited 2017 Nov 19]; 6 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4644929/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol. 16:626–638. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Izumi H, Morimoto Y. 2017. Review of toxicity studies of carbon nanotubes. J Occup Health. 59:394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, Rooijen N van, et al. 2009. IL-33 Amplifies the Polarization of Alternatively Activated Macrophages That Contribute to Airway Inflammation. J Immunol. 183:6469–6477. [DOI] [PubMed] [Google Scholar]
- Latoche JD, Ufelle AC, Fazzi F, Ganguly K, Leikauf GD, Fattman CL. 2016. Secreted Phosphoprotein 1 and Sex-Specific Differences in Silica-Induced Pulmonary Fibrosis in Mice. Environ Health Perspect. 124:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Guabiraba R, Besnard A-G, Komai-Koma M, Jabir MS, Zhang L, Graham GJ, Kurowska-Stolarska M, Liew FY, McSharry C, Xu D. 2014. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. 134:1422–1432.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, Mertler M, Wiench K, Gamer AO, van Ravenzwaay B, Landsiedel R. 2009. Inhalation Toxicity of Multiwall Carbon Nanotubes in Rats Exposed for 3 Months. Toxicol Sci. 112:468–481. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep [Internet]. [cited 2017 May 23]; 6 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3944738/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez I, Steer CA, Takei F. 2015. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. 36:189–195. [DOI] [PubMed] [Google Scholar]
- Mayeux JM, Escalante GM, Christy JM, Pawar RD, Kono DH, Pollard KM. 2018. Silicosis and Silica-Induced Autoimmunity in the Diversity Outbred Mouse. Front Immunol [Internet]. [cited 2018 May 16]; 9 Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2018.00874/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgat-Seon Y, Peters CM, Sheel AW. 2018. Sex-differences in the human respiratory system and their impact on resting pulmonary function and the integrative response to exercise. Curr Opin Physiol. 6:21–27. [Google Scholar]
- Neigh GN, Mitzelfelt MM, editors. 2016. Sex Differences in Physiology. Amsterdam: Elsevier/Academic Press. [Google Scholar]
- Papi A, Brightling C, Pedersen SE, Reddel HK. 2018. Asthma. The Lancet. 391:783–800. [DOI] [PubMed] [Google Scholar]
- Paul WE. 2015. History of Interleukin-4. Cytokine. 75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton KE, Harbaugh M, Han MK, Jourdan Le Saux C, Van Winkle LS, Martin WJ, Kosgei RJ, Carter EJ, Sitkin N, Smiley-Jewell SM, George M. 2015. Women and Lung Disease. Sex Differences and Global Health Disparities. Am J Respir Crit Care Med. 192:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM. 2012. Gender differences in autoimmunity associated with exposure to environmental factors. J Autoimmun. 38:J177–J186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM. 2016. Silica, Silicosis, and Autoimmunity. Front Immunol [Internet]. [cited 2017 Oct 6]; 7 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4786551/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Liu W, Gu H, Wang D, Wang Y. 2015. The Transport and Deposition of Nanoparticles in Respiratory System by Inhalation. J Nanomater [Internet]. [cited 2019 May 4]. Available from: https://www.hindawi.com/journals/jnm/2015/394507/ [Google Scholar]
- Rabolli V, Badissi AA, Devosse R, Uwambayinema F, Yakoub Y, Palmai-Pallag M, Lebrun A, De Gussem V, Couillin I, Ryffel B, et al. 2014. The alarmin IL-1α is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part Fibre Toxicol. 11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan D, Jain R. 2016. Increasing awareness of sex differences in airway diseases. Respirology. 21:449–459. [DOI] [PubMed] [Google Scholar]
- Roved J, Westerdahl H, Hasselquist D. 2017. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav. 88:95–105. [DOI] [PubMed] [Google Scholar]
- Sathish V, Prakash YS. 2016. Sex Differences in Pulmonary Anatomy and Physiology: Implications for Health and Disease In: Neigh GN, Mitzelfelt MM, editors. Sex Differ Physiol. Amsterdam: Elsevier/Academic Press. [Google Scholar]
- Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, Ward PA. 2005. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol - Cell Physiol. 288:C881–C890. [DOI] [PubMed] [Google Scholar]
- Thakur SA, Hamilton R, Pikkarainen T, Holian A. 2009. Differential Binding of Inorganic Particles to MARCO. Toxicol Sci. 107:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Miller VM, Prakash YS. 2012. Sex Differences and Sex Steroids in Lung Health and Disease. Endocr Rev. 33:1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaillac C, Yong VFL, Jaggi TK, Soh Min-Min, Chotirmall SH. 2018. Gender differences in bronchiectasis: a real issue? Breathe. 14:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Brito JM, Silva LF, Lino-dos-Santos-Franco A, Frias DP, e Silva RCR, Amato-Lourenço LF, Saldiva PHN, de Fátima Lopes Calvo Tibério I, Mauad T, Macchione M. 2017. The effects of particulate matter on inflammation of respiratory system: Differences between male and female. Sci Total Environ. 586:284–295. [DOI] [PubMed] [Google Scholar]