Abstract
Understanding how microbial communities develop is essential for predicting and directing their future states. Ecological theory suggests that community development is often influenced by priority effects, in which the order and timing of species arrival determine how species affect one another. Priority effects can have long-lasting consequences, particularly if species arrival history varies during the early stage of community development, but their importance to the human gut microbiota and host health remains largely unknown. Here, we explore how priority effects might influence microbial communities in the gastrointestinal tract during early childhood and how the strength of priority effects can be estimated from the composition of the microbial species pool. We also discuss factors that alter microbial transmission, such as delivery mode, diet and parenting behaviours such as breastfeeding, which can influence the likelihood of priority effects. An improved knowledge of priority effects has the potential to inform microorganism-based therapies, such as prebiotics and probiotics, which are aimed at guiding the microbiota towards a healthy state.
It is now widely recognized that the human body is colonized by many species of microorganisms that can influence a range of metabolic, developmental and physiological processes affecting host health. These microorganisms, especially those of the gut, help liberate and make available to their host otherwise inaccessible components of the diet1, stimulate development of the host immune system2 and protect against pathogen invasion3, among other functions beneficial to the host. The gut microbiota has also been implicated in several chronic gastrointestinal inflammatory disorders, including Crohn’s disease4,5, ulcerative colitis6,7, primary sclerosing cholangitis8, NAFLD9 and environmental enteropathy10,11 as well as other chronic disorders such as obesity12–14, chronic periodontitis15,16 and cardiovascular disease17.
Clinical studies correlating specific taxonomic groups with disease states have yielded valuable insight but have often assumed that host–microorganism interactions occur independently of the rest of the microbial community. Under this assumption, multispecies interactions that modulate the effect of specific taxa on health of the host would be overlooked. In community ecology, the field that focuses on multispecies interactions18, one phenomenon that is receiving increasing interest is priority effects, or the effects that the history of species arrival has on how species affect one another in communities19. Through this lens, human health can be viewed as the net result of dynamic interactions that involve both the host and its microbiota20. In this Review, we apply the concept of priority effects to the infant gut and explore how knowledge of the order and timing of microbial colonization of the infant gut might help predict the development of the early-life microbiota and guide it towards a healthy state. We focus on bacteria because more data are available for them than for other components of the microbiota, but the same concepts might apply to fungal, viral and other microbial components.
Gut microbiota assembly in early life
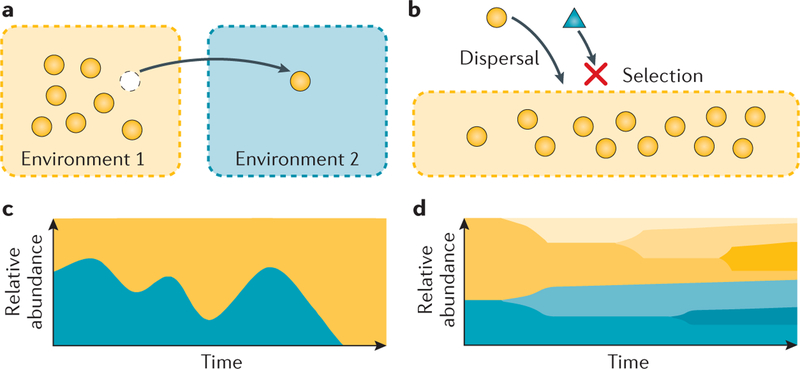
Community ecologists have proposed different concepts over the past century to explain observed patterns of species distribution and abundance. Mark Vellend synthesized these concepts by categorizing the processes that affect community assembly into four groups: dispersal, selection, drift and diversification21,22 (FIG. 1). Taxa are added to local sites through dispersal from the regional species pool and through in situ diversification, and the relative abundances of taxa are further shaped by selection and drift. In this section, we describe each process in reference to the infant gut to provide a context for discussing priority effects in the next section.
Figure 1 |. Four processes that affect ecological communities.
a | The arrow represents dispersal of an organism (orange circle) from Environment 1 (orange shading) to Environment 2 (blue shading). b | Deterministic fitness differences between two species (orange circle, blue triangle) cause the orange environment to select for one (orange circle) and against the other (blue triangle). c | Stochastic changes in the relative abundances of two species (orange area and blue area) result in changes in community structure within one environment through time. As a result, one population (blue) has gone locally extinct by the end of the time period. d | Mutation and/or recombination within a population (blue and orange areas) results in new genetic variation through time, leading to new strains (as denoted by different shades).
Dispersal.
The gastrointestinal tract of a newborn baby represents a large suite of physical and metabolic niches that microorganisms can colonize via dispersal23,24. Stool samples collected within the first 8 days of life suggest that initial colonizers largely originate from the maternal microbiota25. For example, the microbiota of vaginally delivered infants are dominated by taxa found in their mother’s vagina (Lactobacillus spp., Prevotella spp., Atopobium spp. or Sneathia spp.), whereas those of infants delivered by caesarian section are enriched for taxa found on human skin (Staphylococcus spp., Corynebacterium spp. and Propionibacterium spp.)25,26. The mother’s gut can also be a source of the initial microbial inoculum27–30, and the sharing of strains between mothers and newborn babies is commonly observed. Maternal strains of Helicobacter pylori 31,32, Escherichia coli33,34, Bacteroides vulgatus34 and Parabacteroides distasonis34 have been found to colonize the gastrointestinal tract of infants, as have Bifidobacterium longum subsp. longum and other Bifidobacterium spp.34–36. While species-level similarities between mothers and their children tend to increase over the first several years of life, strain-level sharing decreases over time. For example, one study found that 91% of strains were shared between the stool of mothers and their newborn babies 4 days after birth, yet that figure dropped to 55% 1 year later34. In addition, healthy, full-term infants can be influenced by their mother’s microbiota even before the rupture of amniotic membranes37. Although the existence of a persistent, metabolically active microbial community in the placenta remains controversial38,39, microbial DNA has been reported in the placenta40,41, amniotic fluid41,42 and meconium43–45. Microorganisms or microbial components can arrive in the prenatal intrauterine environment by ascending from the vagina46 or by spreading haematogenously from the oral cavity or gut47. It has also been postulated that dendritic cells or lymphoid tissues can translocate bacteria or bacterial DNA to the placenta48. However, we do not yet have enough information about the potential role of these events in healthy human pregnancies to assess how they might influence priority effects. Even if there were a microbial community in the placenta or amniotic sac, its contributions to the membership of the postnatal infant microbiota are likely overwhelmed by the vast numbers of microorganisms to which the infant is exposed at birth. Overall, specific taxa from the mother’s microbiota are commonly transmitted to the infant’s gut in early life. Variation in microbiota among mothers should therefore result in variation in dispersal among their infants. In addition to the mother, there are many other origins of microbial dispersal to the infant, which we will discuss later.
Selection.
Selection occurs when fitness and niche differences among taxa cause them to reproduce or die at different rates. In the infant gut, two primary sources of selection are the immune system and the diet. For instance, commensal E. coli strains colonizing the gastrointestinal tract of Rag2−/− mice, which lack B cells and T cells, adapted more slowly than strains colonizing mice with an intact adaptive immune system49. In gnotobiotic zebrafish, a statistical model that assumed that species are identical to one another in their birth and death rates predicted microbiota composition well in early life, but selection became more important as the adaptive immune system of the fish became active50. Similarly, as an infant’s immune system matures, it might exert increasing selection on the microbiota, causing largely homogeneous communities to become increasingly body-site-specific23,51.
Drift.
After a microorganism colonizes the infant gut, its growth rate and abundance can be shaped not just through deterministic forces such as selection but also via stochastic processes such as ecological drift. Drift is the random changes in population size that occur regardless of species identity52. The effect of drift is stronger on low-abundance species because they are more likely to be stochastically pushed to local extinction. Some species are at low abundance in the gut because they arrive infrequently as a small population or because they experience large reductions in number by a major perturbation such as diarrhoea53 or antibiotic treatment54. These species can be affected by drift more strongly than by selection. However, the effect of drift on gut microbiota assembly has not been well characterized, in part because factors that cause drift often alter selection as well, making it difficult to tease apart the two processes.
Diversification.
Microorganisms, with their large population sizes, high growth rates and high mutation and recombination rates, are able to rapidly diversify and adapt when faced with the strong selective regimes found in the human body. One example is the diversification of Pseudomonas aeruginosa in the airways of patients with cystic fibrosis. Several adaptations were observed over a decade of mostly constant selective pressures inside the cystic fibrosis lung55,56. By comparison, communities that assemble in the infant gut experience frequently shifting selective regimes related to immune system development, the addition of complementary foods, the cessation of breastfeeding and increasing competition resulting from increased taxonomic diversity. Because diversification often requires persistent selective pressure, the extent of diversification in the infant gut during assembly remains uncertain.
Some factors affect more than one of the four processes simultaneously. For example, breast milk affects both dispersal and selection because it is both a source of microorganisms dispersing to the gut and the primary nutrient source for the infant and their microbiota57. Breast milk commonly harbours Bifidobacterium spp.57–60, Lactobacillus spp.57,59–61, Staphylococcus spp.57,62 and Streptococcus spp.59,60,62 and is composed of a rich mix of proteins, fats and human milk oligosaccharides (HMOs), which are a diverse set of unconjugated glycans that cannot be digested by the host and can be digested by only a subset of the microbiota63. The complex composition of breast milk selects for both HMO specialists and mucus-adapted species with a wide range of glycoside hydrolases capable of metabolizing diverse carbon sources that become abundant after complementary foods are introduced into the infant’s diet64. Breast milk also contains many antimicrobial factors such as lysozyme, lactoferrin and secretory immunoglobulin A (IgA)65,66, which impose additional selection on the gut microbial community. Formula milk, by contrast, lacks many of these bioactive compounds as well as the microorganisms that are adapted to the milk environment, which might result in altered dispersal and selection compared with those related to breast milk, although the effects of these foods remain unclear.
What makes the four processes interesting and challenging to understand is that they do not always have simple additive effects but can instead exert complex interacting effects on community assembly67. Priority effects are an example of such interactive effects in which dispersal history modulates how selection, drift and diversification influence community structure.
Priority effects in the infant gut
Each local microbial community can be viewed as a subsample of the regional pool of species that passed through a set of biotic and abiotic filters. From this perspective, it might seem that species composition at equilibrium is predictable from the local environmental conditions and the list of species that are available to colonize the local habitat. However, the order and timing of dispersal can have large effects on final species composition, even if environmental conditions and regional species pools are identical19. It is these effects of the order and timing of past species immigrations on interspecies interactions that are known as priority effects (FIG. 2).
Figure 2 |. Contrasting hypothetical patterns of community assembly in the infant gut.
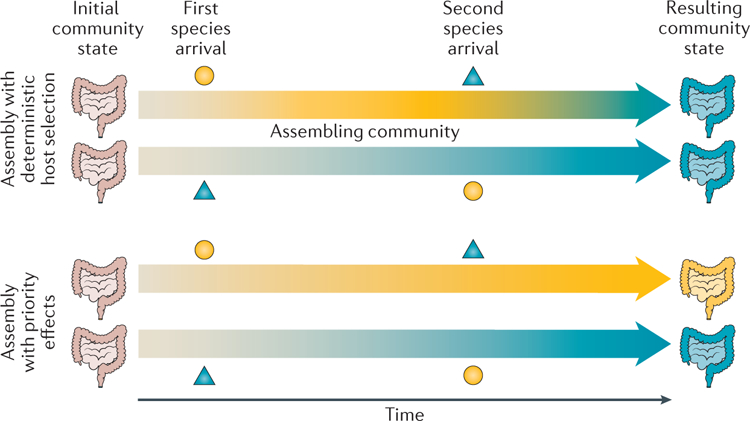
An illustration of how infant microbial communities assemble with deterministic host selection (top) or priority effects (bottom). The shapes represent different taxa, while the colours represent the community state. Under deterministic host selection, the state of the assembling community is determined by host features that select for the blue microorganisms regardless of colonization order. With priority effects, colonization order can matter more than species identity.
Little is known about how priority effects shape microbial community assembly in early childhood because few studies report the timing and order of colonization, but indirect evidence suggests that priority effects are plausible. For example, in a 2016 study, Yassour et al.68 classified infant gut microbiota into two groups based on the abundance of Bacteroides spp. present in the first 6 months of life. Of the 35 infants in their cohort, 11 were characterized as having low levels of Bacteroides spp. and were instead dominated by either Proteobacteria or Actinobacteria (especially Bifidobacterium spp.)68. These ‘low-Bacteroides’ microbiota remained less diverse than the ‘high-Bacteroides’ group for at least the first 36 months of life, well after Bacteroides spp. membership expanded in relative abundance68. In other work, facultative anaerobes such as Enterobacteriaceae (for example, Escherichia spp.) have been found in high abundance in meconium or early stools but gradually yield to strict anaerobes such as Bifidobacterium spp., Bacteroides spp. and Clostridium spp. over the first few months of life28,69. Collectively, these findings suggest a degree of mutual exclusion between Bacteroides spp., Escherichia spp. and lactic acid producers such as Bifidobacterium spp. and Lactobacillus spp. that might be partially mediated by the infant’s exposure history and the patterns of dispersal from various sites in or on their mother.
Priority effects occur when microorganisms either pre-empt or modify a given ecological niche and thereby alter the ability of subsequent microbial immigrants to colonize. For example, Bifidobacterium spp. consume a wide range of HMOs found in breast milk60,70. Their arrival soon after birth likely depletes the intestinal lumen of these carbon sources, thereby limiting the ability of later species to colonize60,64,70–72. Niche pre-emption necessarily results in the inhibition of later immigrants, but taxa that modify niches can either inhibit or facilitate later immigrants. For example, the gut commensal Bacteroides thetaiotaomicron liberates mucus-derived sugars such as fucose and sialic acid, which provide efficient carbon sources for late-arriving pathogens such as Clostridium difficile and Salmonella enterica subsp. enterica serovar Typhimurium73. Similarly, some early colonizers such as E. coli deplete oxygen in the infant gut, facilitating subsequent colonization by obligate anaerobes such as Bacteroides spp.69 while making the environment less hospitable to facultative anaerobes.
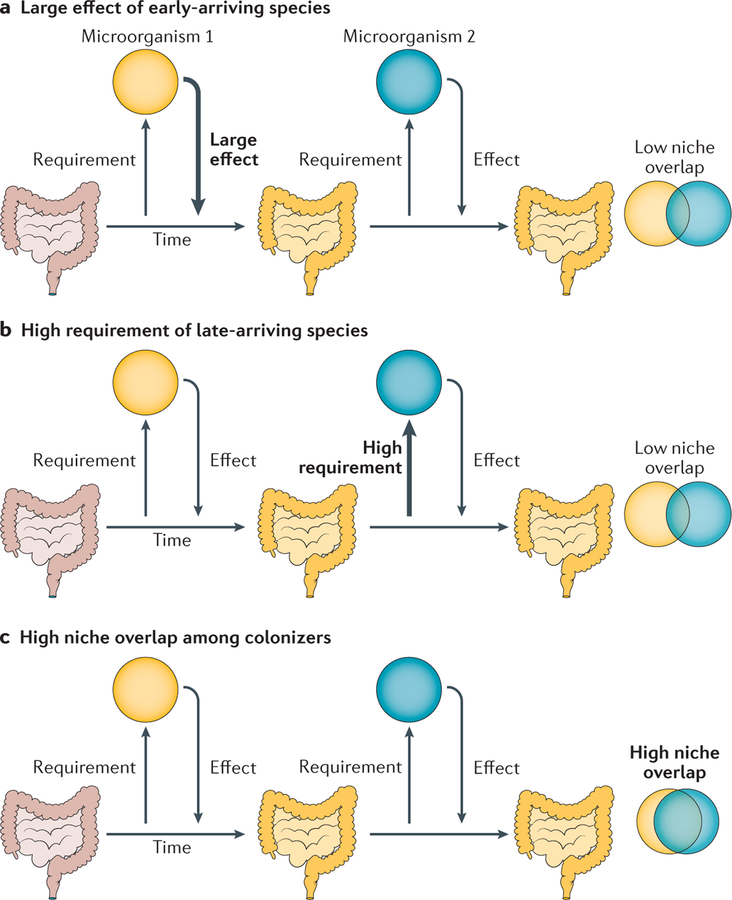
Newly arriving microbial taxa vary in both the effect they have on the local environment and the resources that they must acquire from it for survival and reproduction. Strong priority effects can occur when early-arriving species have a large effect on the local environment or when late-arriving species have high environmental requirements19 (FIG. 3a,b). Furthermore, for an early-arriving species to pre-empt a niche from a late-arriving species, the two must have a high degree of niche overlap19,74 (FIG. 3c). This condition has been demonstrated in mouse colonization models in which isogenic strains with complete niche overlap exhibit strong priority effects over one another75,76. Although the mechanisms of priority effects are usually unknown, Lee et al. identified a bacterial genetic locus, commensal colonization factor (ccf), that mediates priority effects in host-associated Bacteroides spp.77. Consistent with the niche overlap expectation (FIG. 3c), gnotobiotic mice that are colonized with a single Bacteroides sp. are resistant to colonization by the same, but not different, species77. The ccf locus enables Bacteroides spp. to associate with colonic crypts, thereby excluding later immigrants77. In fact, non-toxin-producing Bacteroides fragilis can limit the colonization of enterotoxigenic B. fragilis in specific pathogen-free (SPF) mice, demonstrating that priority effects through niche pre-emption could be a powerful tool in the design of probiotic-based prophylaxis78.
Figure 3 |. Hypotheses on species features causing strong priority effects.
Both early-colonizing (Microorganism 1) and late-colonizing (Microorganism 2) microorganisms have their own set of requirements for colonizing a given environment as well as a distinct effect on that environment74. The width of the arrow denotes the strength of each microorganism’s effect niche and requirement niche. a | Microorganism 1 has a large effect on its environment, resulting in a modified niche that inhibits colonization by Microorganism 2. b | Microorganism 2 has a high niche requirement and is therefore more sensitive to smaller modifications to the niche that can inhibit its colonization. c | Microorganisms 1 and 2 have high niche overlap, meaning that Microorganism 1 is able to pre-empt the niche and inhibit colonization by Microorganism 2. Niche overlap is not necessary if the priority effects occur by way of the environment, as in parts a and b.
Microorganisms can also modify niches found in the human body through interactions with the host immune system. For example, Bacteroides spp. colonization can affect innate immune signalling79, endotoxin tolerance79 and T helper 1 (TH1) cell immune responses27, and Bifidobacterium spp. can modulate vaccine response80 and increase cytokine production in vitro81. These immune-mediated effects can occur even as the result of prenatal microbial exposure. Colonization of pregnant mice with the HA107 strain of E. coli, which was genetically engineered to be unable to persist in the intestine, demonstrated that microbial metabolites, independent of the microorganisms themselves, can increase both intestinal group 3 innate lymphoid cells and F4/80+ CD11c+ mononuclear cells in neonate pups while also decreasing bacterial translocation to the mesenteric lymph nodes82. The transient gestational colonization affecting both immune development and microbiota structure in offspring suggests that priority effects can occur before microorganisms even have the opportunity to colonize82.
Species pools in early life
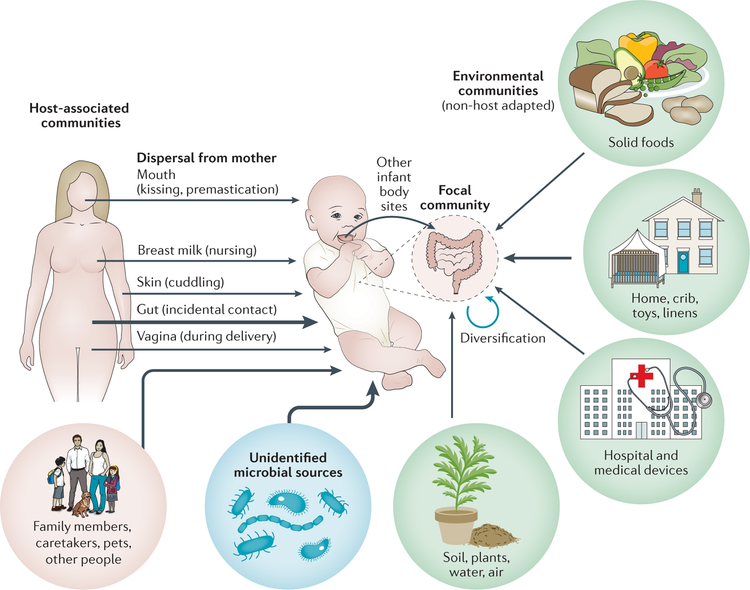
The role that priority effects play during community assembly is determined by the characteristics of the microbial taxa contained in the pool of potential colonizers19. For example, a species pool that is taxonomically and functionally more diverse might be more likely to contain taxa that yield priority effects19. Therefore, to understand whether priority effects influence community assembly, it is helpful to characterize the set of microorganisms that have the potential to colonize the infant gut in early life, including those originating from host-associated, environmental and yet unknown sources (FIG. 4). However, defining a species pool is often challenging, and few investigations of early-life colonization have attempted to characterize all sources of microorganisms that are capable of colonizing an infant.
Figure 4 |. Local species pools that contribute to the regional pool of microorganisms available for colonization of the infant gut.
Infants are colonized by microorganisms from host-associated communities, environmental communities that are not host adapted, and unknown microbial sources. The thickness of the arrows denotes the hypothesized relative contributions of microorganisms from different sources that disperse to and stably colonize the local community (infant gastrointestinal tract).
The microbiota of family members, medical personnel, birth attendants and other caretakers can all contribute to the species pool of an infant’s gut (FIG. 4). The first site with which many infants come into contact is the maternal birth canal. Vaginal communities have been classified into five distinct community state types (CSTs), with four of the five exhibiting somewhat low diversity and domination by a distinct Lactobacillus spp.83. If delivered via caesarian section, infants can instead first come into contact with the mother’s skin26, which harbours more diverse communities than the vagina and therefore might contain more species capable of causing priority effects. Although delivery mode is correlated with differences in early postnatal microbiota structure, mothers who deliver via caesarian section (both planned and emergency) are commonly prescribed antibiotics84 and are often not able to breastfeed as early as those who deliver vaginally85, confounding the effect of delivery mode on microbiome assembly. Nonetheless, some mothers intentionally wipe their caesarian-delivered infants with their vaginal secretions in an attempt to simulate the priority effects that occur following vaginal delivery, although the health benefits remain unproven86.
The skin microbiota has ample opportunity to disperse while the infant is in contact with their mother during sleep or feeding23,87,88. Kangaroo mother care, or immediate and continual skin-to-skin contact between mothers and newborn babies immediately following birth, is commonly recommended for pre-term infants because it decreases the risk of sepsis and increases breastfeeding rates89, effects that could be partially mediated by increased transmission of commensal bacteria90. As discussed earlier, breast milk contains bacteria, although its composition varies with lactational stage, delivery mode and the mother’s health59,91. Microbial diversity and abundance are several orders of magnitude higher in the gastrointestinal tract, and transmission of the mother’s gut microbiota to the newborn baby has been observed in many studies28,35,36,68. A mother’s diet affects both the structure of her gut microbiota and the nutritional and microbial composition of her breastmilk92,93. In addition to mothers, studies have reported an effect of fathers25,94, older siblings95,96, furry pets97 and day care attendance92 on microbiota assembly.
In addition to microbiota found on the mother’s body or other family members’ bodies, a newborn infant is exposed to a myriad of other microorganisms in their environment, each with their own habitat-specific features (FIG. 4). Experiments with gnotobiotic mice demonstrate that microorganisms from many diverse environmental and host-associated habitats can colonize the mouse gut98. Competitive invasion assays showed that a soil-derived Ruminococcus sp. was able to invade gut-adapted microbial communities98. Furthermore, bacteria from the human gut colonize co-housed germ-free mice via coprophagy even faster than microorganisms from conventionally raised mice98. This counterintuitive result can be explained in part by priority effects because well-adapted species are limited in their ability to diversify. Specifically, it is possible that well-adapted species can outcompete less-adapted mutants and dominate regardless of their colonization order, while nonadapted strains are able to diversify rapidly and exert priority effects, but this occurs only if they arrive early enough to pre-emptively exploit the resources in that niche19.
The role of microorganisms from the built environment might be underappreciated during host-associated assembly. Infants born at home are less frequently colonized by E. coli and C. difficile than those born in a hospital95, although these environmentally acquired microorganisms can also vary between hospitals99. This effect is especially apparent in premature infants that lack normal immune development and might therefore be more susceptible to priority effects owing to reduced host selection100.
Infant-care-associated behaviours (ICABs) that affect microbial dispersal have evolved owing to changes in societal and family structures, diets, medical practices, travel, migration patterns, urbanization and housing environments. Parents can transmit oral microorganisms to their infants by kissing101, premasticating solid foods102, cleaning pacifiers with their mouths103 or other ICABs that place newborn babies in contact with an adult’s microbiota (FIG. 4). ICABs are variable across cultures. For example, anointing newborn babies with oil or other emollients104 is a common practice across southeast Asia. In many parts of India, it is customary to not breastfeed for the first several days of an infant’s life and instead administer prelacteal foods that include honey, ghee (clarified butter), water, tea, jaggery (brown sugar) and ghutti (a herbal paste)105–107. Infants themselves instinctively explore their local environment with their hands and put their hands and other nonfood items in their mouth, which when persistent is characterized as a psychological disorder known as pica108. A large comparative study found that the gut microbiota of Guahibo Amerindian mothers living in Venezuela were more similar to those of their own child than to those of unrelated children, while mother–child dyads from Malawi were not more similar than unrelated pairs109. Differences in the occurrence and timing of ICABs among cultures could explain some of the observed differences in microorganisms that are shared between mothers and their infants. If priority effects are a major driver of gut microbiota assembly, it might be possible to steer the trajectory of microbiome assembly towards a healthy adult-like state by modifying ICABs related to parturition and early life.
Consequences of priority effects
Priority effects could explain some puzzling observations of microbiota assembly and consequences for the host. For example, one study based on the use of an SPF porcine model of microbiota assembly discovered strong batch effects during efforts to replicate the findings110. Two groups of identical animals housed in the same SPF animal facility ended up with divergent communities. The investigators found that stochastic variation in Clostridia colonization in the first day of life caused sustained, broad colonization differences at day 35 (REF. 110). Priority effects driving the communities towards alternative states might have been responsible for this finding. Priority effects can also cause a community to enter an oscillating compositional cycle or cause more complex patterns, such as those arising from nontransitive or ‘rock-paper-scissors’ types of interactions111. Examples of compositional cycles include predator–prey dynamics, such as those that have been observed during infant gut assembly between strains of Staphylococcus epidermidis and their bacteriophages112. The population dynamics of these communities are historically contingent because their composition is dependent on the specific sequences of species arrival.
Broad-spectrum antibiotics are commonly used in early life in humans, often with little regard for potential long-term consequences of priority effects for microbiota assembly113. Broad-spectrum antibiotics cause a strong perturbation of the microbial communities in the infant gut, possibly altering its maturational trajectory68,69,95,114–117. Antibiotic use by mothers can also alter the regional species pool of the infant118. These perturbations can have lasting effects on host metabolism, immune development and health, especially if they occur during critical immune developmental windows early in life117,119. Realizing the benefits of probiotics in mitigating these adverse effects requires an understanding of possible priority effects and the consequences of alternative assembly patterns. Priority effects can be particularly important to consider when they involve catalytic species, which, given the right timing, invade a community, change its composition and then go locally extinct120. These species create ‘Humpty-Dumpty’ communities; that is, communities that cannot be reassembled just from the set of species that they contain120. Such circumstances underscore the need for detailed records of colonization history.
Future research needs
Perhaps the greatest challenge for investigating factors that influence gut microbiota assembly is the limited set of opportunities for experiments with humans. Experimental manipulation of bacterial colonization history, which is necessary to rigorously evaluate priority effects, might pose health risks to the developing infant and therefore should not be implemented without careful review. Nevertheless, some clinical situations might be amenable to interventional studies in which bacterial exposure is intentionally altered through the use of antibiotics, probiotics or techniques such as vaginal microbiota transfer86. For example, when populations of comparable infants vary in the timing of probiotic supplementation relative to antibiotic use, this variation could be used to test for priority effects and clinical consequences for the host. In fact, the results of probiotic interventions might depend on the specific organism and the timing and dosage of its administration, which might be in part caused by priority effects in the microbiota. One study found that Bifidobacterium breve BBG-001 administered within the first 48 hours of life had no effect on necrotizing enterocolitis or late-onset sepsis121, while another found that use of Lactobacillus plantarum ATCC-202195 in conjunction with a prebiotic fructooligosaccharide in the first week of life reduced neonatal sepsis by 40%122. Broad conclusions, such as the suggestion in a 2014 Cochrane review that probiotics can help prevent necrotizing enterocolitis in preterm infants123, seem premature at this stage. This meta-analysis pooled results from 24 randomized trials using a range of organisms, including Saccharomyces boulardii, Lactobacillus spp., Bifidobacterium spp. or a mixture of several bacteria and/or fungal taxa, administered at different time points and for different durations. The effects of specific strains and the timing of their administration on priority effects and subsequent microbiota beneficial services should be examined carefully before this practice is widely endorsed.
Other opportunities to observe and investigate priority effects with limited additional risk to the infant include cases in which there is natural variation in microbial colonization or in the species pools, such as during cross-cultural comparisons of ICABs, although confounding variables make inference complicated. Moreover, stool provides only a limited view of the microbial interactions that occur throughout the lumen of the gut and poorly reflects interactions among mucosa-associated microorganisms, especially those that take place in more proximal regions of the gastrointestinal tract4,124. Endoscopic biopsies, mucosal brushings and other sampling approaches are likely necessary to observe these fine-scale interactions. In addition, studying how communities assemble at other body sites that are more amenable to experimental intervention, such as transplant experiments among skin or oral communities125, might yield insight into the factors that shape community assembly in the gut. In concert with experimental and clinical data collection, statistical techniques for analysing the data should be improved, and methods developed in the ecological literature126 should be helpful.
We have focused primarily on bacteria, but priority effects are also possible across domains of life (that is, between bacteria and archaea and/or eukaryotic microorganisms)127–130. In particular, diverse fungal communities are present in infants131. Fungi are transmitted from mother to infant in early life, their dispersal history can be highly variable among infants, and once immigrated, they can interact strongly with bacteria87. Yet we have little understanding of how they affect microbial community assembly via priority effects. Studies on the infant gut should consider the broadly defined microbial community.
Conclusions
We have discussed the mechanisms, conditions and consequences of priority effects that might affect microorganisms in the gastrointestinal tract. Ecological theory and circumstantial evidence strongly suggest that priority effects are important to infant health, but definitive direct evidence is largely lacking. Given that we now have the foundational concepts from community ecology and many of the molecular and computational tools needed to study the microbiome, we believe the time is ripe for studying priority effects by use of clinically relevant data to improve microbiome management.
Community assembly
The construction and maintenance of local communities through sequential, repeated immigration of species from a regional species pool.
Regional species pool
The set of species that could potentially colonize and establish within a community.
Niche pre-emption
Occurs when the first species to arrive in a given habitat uses or otherwise sequesters resources and, as a consequence, inhibits the colonization of later species.
Community state types
(CSTs). Categories of stereotypical microbial communities that are typically defined by their dominant taxa and found at a given body site.
Key points.
Infant gut microbiota assembly is driven by four ecological processes — dispersal, diversification, drift and selection — and can be understood by resolving their relative contributions, mechanisms and interactive effects
Priority effects, whereby the order and timing of dispersal alters how diversification, drift and selection affect infant gut microbiota assembly, could have long-lasting consequences for host health
Priority effects in the infant gut are influenced by the regional species pool, which is made up of numerous local communities, some of which are host-associated, while others are not
To understand the role of priority effects in the infant gut, future studies in model systems should intentionally vary dispersal order and timing
In future studies, when intentional variation in dispersal order is not feasible, dispersal order should be carefully recorded along with relevant environmental variables
An understanding of the processes that govern priority effects can be used to inform microorganism-based therapies and manage strategies aimed at guiding the microbiota towards a healthy state
Acknowledgements
The authors’ work is supported by the US National Science Foundation (NSF) Graduate Research Fellowship award number DGE-114747 (D.S.), the National Institute of General Medical Sciences of the NIH under award number T32GM007276 (D.S.), the Thomas C. and Joan M. Merigan Endowment at Stanford University (D.A.R.), The Leona and Harry B. Helmsley Foundation grant number 2014PG-IBD014 (D.A.R.), US NSF award numbers DEB-1555786 and DEB-1737758 (T.F.) and the Terman Fellowship of Stanford University (T.F.). Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the US NSF or the NIH. The authors especially thank E. Costello for her helpful feedback.
Footnotes
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koropatkin NM, Cameron EA & Martens EC How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol 10, 323–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gensollen T, Iyer SS, Kasper DL & Blumberg RS How colonization by microbiota in early life shapes the immune system. Science 352, 539–544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buffie CG & Pamer EG Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol 13, 790–801 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gevers D et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15, 382–392 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson AR et al. Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of Crohn’s disease. PLoS ONE 7, e49138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez C et al. Unstable composition of the fecal microbiota in ulcerative colitis during clinical remission. Am. J. Gastroenterol 103, 643–648 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Lavelle A et al. Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut 64, 1553–1561 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabibian JH, O’Hara SP & Lindor KD Primary sclerosing cholangitis and the microbiota: current knowledge and perspectives on etiopathogenesis and emerging therapies. Scand. J. Gastroenterol 49, 901–908 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang W et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep 5, 8096 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donowitz JR et al. Small intestine bacterial overgrowth and environmental enteropathy in Bangladeshi children. mBio 7, e02102–02115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown EM et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat. Commun 6, 7806 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridaura VK et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1131 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Kirst ME et al. Dysbiosis and alterations in predicted functions of the subgingival microbiome in chronic periodontitis. Appl. Environ. Microbiol 81, 783–793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abusleme L et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J 7, 1016–1025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeth RA et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med 19, 576–585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morin PJ Community Ecology (Wiley-Blackwell, 2011). [Google Scholar]
- 19.Fukami T Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst 46, 1–23 (2015).This review lays out a conceptual framework for understanding and studying the role of historical contingency in community assembly.
- 20.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM & Relman DA The application of ecological theory toward an understanding of the human microbiome. Science 336, 1255–1262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vellend M Conceptual synthesis in community ecology. Q. Rev. Biol 85, 183–206 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Vellend M The Theory of Ecological Communities (Princeton Univ. Press, 2016).This book provides a theoretical foundation for understanding how ecological communities arise and change though time.
- 23.Costello EK, Carlisle EM, Bik EM, Morowitz MJ & Relman DA Microbiome assembly across multiple body sites in low-birthweight infants. mBio 4, e00782–e00713 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu DM et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med 23, 314–326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer C, Bik EM, DiGiulio DB, Relman DA & Brown PO Development of the human infant intestinal microbiota. PLoS Biol 5, e177 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominguez-Bello MG et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).This paper provides early evidence that birth mode affects early infant colonization.
- 27.Jakobsson HE et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 63, 559–566 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Bäckhed F et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Biasucci G et al. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev 86 (Suppl. 1), 13–15 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Gosalbes MJ et al. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 43, 198–211 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Didelot X et al. Genomic evolution and transmission of Helicobacter pylori in two South African families. Proc. Natl Acad. Sci. USA 110, 13880–13885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz S, Morelli G, Kusecek B & Manica A Horizontal versus familial transmission of Helicobacter pylori. PLoS Pathog 4, e1000180 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Muinck EJ et al. Diversity, transmission and persistence of Escherichia coli in a cohort of mothers and their infants. Environ. Microbiol. Rep 3, 352–359 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Nayfach S, Rodriguez-Mueller B, Garud N & Pollard KS An integrated metagenomics pipeline for strain profiling reveals novel patterns of bacterial transmission and biogeography. Genome Res 26, 1612–1625 (2016).This paper shows that strain-level sharing between mothers and children changes over time.
- 35.Makino H et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS ONE 8, e78331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milani C et al. Exploring vertical transmission of bifidobacteria from mother to child. Appl. Environ. Microbiol 81, 7078–7087 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wassenaar TM & Panigrahi P Is a foetus developing in a sterile environment? Lett. Appl. Microbiol 59, 572–579 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Hornef M & Penders J Does a prenatal bacterial microbiota exist? Mucosal Immunol 10, 598–601 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Lauder AP et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 4, 29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aagaard K et al. The placenta harbors a unique microbiome. Sci. Transl Med 6, 237ra65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collado MC, Rautava S, Aakko J, Isolauri E & Salminen S Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep 6, 23129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiGiulio DB Diversity of microbes in amniotic fluid. Semin. Fetal Neonatal Med 17, 2–11 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Ardissone AN et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS ONE 9, e90784 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiménez E et al. Is meconium from healthy newborns actually sterile? Res. Microbiol 159, 187–193 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Moles L et al. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS ONE 8, e66986 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witkin SS The vaginal microbiome, vaginal anti-microbial defence mechanisms and the clinical challenge of reducing infection-related preterm birth. BJOG 122, 213–218 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Fardini Y, Chung P, Dumm R, Joshi N & Han YW Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infect. Immun 78, 1789–1796 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Funkhouser LJ & Bordenstein SR Mom knows best: the universality of maternal microbial transmission. PLoS Biol 11, e1001631 (2013).This review gives a comparative view of maternal microbial transmission across the animal kingdom.
- 49.Barroso-Batista J, Demengeot J & Gordo I Adaptive immunity increases the pace and predictability of evolutionary change in commensal gut bacteria. Nat. Commun 6, 8945 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burns AR et al. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J 10, 655–664 (2016).This paper assesses the role of neutral processes in community assembly by fitting observations in a powerful experimental model to a mathematical model.
- 51.Olm MR et al. Identical bacterial populations colonize premature infant gut, skin, and oral microbiomes and exhibit different in situ growth rates. Genome Res 27, 601–612 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hubbell SP The Unified Neutral Theory of Biodiversity and Biogeography. (Princeton Univ. Press, 2001). [DOI] [PubMed] [Google Scholar]
- 53.Fukuyama J et al. Multidomain analyses of a longitudinal human microbiome intestinal cleanout perturbation experiment. PLoS Comput. Biol 13, e1005706 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dethlefsen L & Relman DA Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4554–4561 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marvig RL, Sommer LM, Molin S & Johansen HK Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet 47, 57–64 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Folkesson A et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat. Rev. Microbiol 10, 841–851 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Martín V et al. Sharing of bacterial strains between breast milk and infant feces. J. Hum. Lact 28, 36–44 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Grönlund MM et al. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin. Exp. Allergy 37, 1764–1772 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Khodayar-Pardo P, Mira-Pascual L, Collado MC & Martínez-Costa C Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol 34, 599–605 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Solís G, de Los Reyes-Gavilan CG, Fernández N, Margolles A & Gueimonde M Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe 16, 307–310 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Martín R, Heilig GHJ, Zoetendal EG, Smidt H & Rodríguez JM Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J. Appl. Microbiol 103, 2638–2644 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Hunt KM et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 6, e21313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bode L Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22, 1147–1162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marcobal A et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 10, 507–514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rogier EW et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc. Natl Acad. Sci. USA 111, 3074–3079 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Planer JD et al. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature 534, 263–266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vellend M, Srivastava DS, Anderson KM & Brown CD Assessing the relative importance of neutral stochasticity in ecological communities. Oikos 123, 1420–1430 (2014). [Google Scholar]
- 68.Yassour M et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl Med 8, 343ra81 (2016).This longitudinal study examines the role of environmental factors in early-life colonization patterns.
- 69.Bokulich NA et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl Med 8, 343ra82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sela DA et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl Acad. Sci. USA 105, 18964–18969 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koenig JE et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4578–4585 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcobal A & Sonnenburg JL Human milk oligosaccharide consumption by intestinal microbiota. Clin. Microbiol. Infect 18 (Suppl. 4), 12–15 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ng KM et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502, 96–99 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vannette RL & Fukami T Historical contingency in species interactions: towards niche-based predictions. Ecol. Lett 17, 115–124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lam LH & Monack DM Intraspecies competition for niches in the distal gut dictate transmission during persistent Salmonella infection. PLoS Pathog 10, e1004527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Devevey G, Dang T & Graves CJ First arrived takes all: inhibitory priority effects dominate competition between co-infecting Borrelia burgdorferi strains. BMC Microbiol 15, 61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee SM et al. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature 501, 426–429 (2013).This paper identifies the ccf locus as a possible basis of priority effects for B. fragilis.
- 78.Hecht AL et al. Strain competition restricts colonization of an enteric pathogen and prevents colitis. EMBO Rep 17, 1281–1291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vatanen T et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165, 842–853 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huda MN et al. Stool microbiota and vaccine responses of infants. Pediatrics 134, e362–372 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arboleya S et al. Production of immune response mediators by HT-29 intestinal cell-lines in the presence of Bifidobacterium-treated infant microbiota. Benef. Microbes 6, 543–552 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Gomez de Aguero M et al. The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302 (2016). [DOI] [PubMed] [Google Scholar]
- 83.DiGiulio DB et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl Acad. Sci. USA 112, 11060–11065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smaill FM & Grivell RM Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst. Rev 10, CD007482 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zanardo V et al. Elective cesarean delivery: does it have a negative effect on breastfeeding? Birth 37, 275–279 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Dominguez-Bello MG et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med 22, 250–253 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagata R et al. Transmission of the major skin microbiota, Malassezia, from mother to neonate. Pediatr. Int 54, 350–355 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Song SJ et al. Cohabiting family members share microbiota with one another and with their dogs. eLife 2, e00458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Conde-Agudelo A & Díaz-Rossello JL Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst. Rev 4, CD002771 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Hendricks-Muñoz KD et al. Skin-to-skin care and the development of the preterm infant oral microbiome. Am. J. Perinatol 32, 1205–1216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cabrera-Rubio R et al. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr 96, 544–551 (2012). [DOI] [PubMed] [Google Scholar]
- 92.Thompson AL, Monteagudo-Mera A, Cadenas MB, Lampl ML & Azcarate-Peril MA Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front. Cell. Infect. Microbiol 5, 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chu DM et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med 8, 77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subramanian S et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Penders J et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Laursen MF et al. Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol 15, 154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nermes M, Endo A, Aarnio J, Salminen S & Isolauri E Furry pets modulate gut microbiota composition in infants at risk for allergic disease. J. Allergy Clin. Immunol 136, 1688–1690.e1 (2015). [DOI] [PubMed] [Google Scholar]
- 98.Seedorf H et al. Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 159, 253–266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taft DH et al. Intestinal microbiota of preterm infants differ over time and between hospitals. Microbiome 2, 36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brooks B et al. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2, 1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kort R et al. Shaping the oral microbiota through intimate kissing. Microbiome 2, 41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Han CS et al. Salivary microbiomes of indigenous Tsimane mothers and infants are distinct despite frequent premastication. PeerJ 4, e2660 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thompson JC & Dolen WK Pacifier cleaning practices and risk of allergy development. Pediatrics 134, S136–S137 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Darmstadt GL et al. Effect of topical emollient treatment of preterm neonates in Bangladesh on invasion of pathogens into the bloodstream. Pediatr. Res 61, 588–593 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Choudhry UK Traditional practices of women from India: pregnancy, childbirth, and newborn care. J. Obstet. Gynecol. Neonatal Nurs 26, 533–539 (1997). [DOI] [PubMed] [Google Scholar]
- 106.McKenna KM & Shankar RT The practice of prelacteal feeding to newborns among Hindu and Muslim families. J. Midwifery Womens Health 54, 78–81 (2009). [DOI] [PubMed] [Google Scholar]
- 107.Singh S Can establishment of human microbiome be customized after birth with local traditions of first feed and intimate kissing? J. Lab. Physicians 7, 73–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams DE & McAdam D Assessment, behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res. Dev. Disabil 33, 2050–2057 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Yatsunenko T et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Merrifield CA et al. Neonatal environment exerts a sustained influence on the development of the intestinal microbiota and metabolic phenotype. ISME J 10, 145–157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steiner CF & Leibold MA Cyclic assembly trajectories and scale-dependent productivity-diversity relationships. Ecology 85, 107–113 (2004). [Google Scholar]
- 112.Sharon I et al. Time series community genomics analysis reveals rapid shifts in bacterial species, strains, and phage during infant gut colonization. Genome Res 23, 111–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blaser MJ Antibiotic use and its consequences for the normal microbiome. Science 352, 544–545 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeissig S & Blumberg RS Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat. Immunol 15, 307–310 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Deshmukh HS et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med 20, 524–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gray J et al. Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci. Transl Med 9, eaaf9412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cho I et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lemas DJ et al. Exploring the contribution of maternal antibiotics and breastfeeding to development of the infant microbiome and pediatric obesity. Semin. Fetal Neonatal Med 21, 406–409 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Cox LM et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Warren PH, Law R & Weatherby AJ Mapping the assembly of protist communities in microcosms. Ecology 84, 1001–1011 (2003). [Google Scholar]
- 121.Costeloe K, Hardy P, Juszczak E, Wilks M & Millar MR Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 387, 649–660 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Panigrahi P et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548, 407–412 (2017). [DOI] [PubMed] [Google Scholar]
- 123.AlFaleh K & Anabrees J Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev 4, CD005496 (2014). [DOI] [PubMed] [Google Scholar]
- 124.Budding AE et al. Rectal swabs for analysis of the intestinal microbiota. PLoS ONE 9, e101344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Costello EK et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ottosson E et al. Species associations during the succession of wood-inhabiting fungal communities. Fungal Ecol 11, 17–28 (2014). [Google Scholar]
- 127.Doublet V, Natsopoulou ME, Zschiesche L & Paxton RJ Within-host competition among the honey bees pathogens Nosema ceranae and deformed wing virus is asymmetric and to the disadvantage of the virus. J. Invertebr. Path 124, 31–34 (2015). [DOI] [PubMed] [Google Scholar]
- 128.Malakar R, Elkinton JS, Hajek AE, & Burand JP Within-host interactions of lymantria dispar (Lepidoptera: Lymantriidae) nucleopolyhedrosis virus and Entomophaga maimaiga (Zygomycetes: Entomophthorales). J. Invertebr. Path 73, 91–100 (1999). [DOI] [PubMed] [Google Scholar]
- 129.Tucker CM & Fukami T Environmental variability counteracts priority effects to facilitate species coexistence: evidence from nectar microbes. Proc. R. Soc. B. Biol. Sci 281, 20132637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martins FS et al. Inhibition of tissue inflammation and bacterial translocation as one of the protective mechanisms of Saccharomyces boulardii against Salmonella infection in mice. Microbes Infect 15, 270–279 (2013). [DOI] [PubMed] [Google Scholar]
- 131.Ward TL, Knights D & Gale CA Infant fungal communities: current knowledge and research opportunities. BMC Med 15, 30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]