Abstract
Regulation of mRNA translation offers the opportunity to diversify the expression and abundance of proteins made from individual gene products in cells, tissues and organisms. Emerging evidence has highlighted variation in the composition and activity of several large, highly conserved translation complexes as a means to differentially control gene expression. Heterogeneity and specialized functions of individual components of the ribosome and of the translation initiation factor complexes eIF3 and eIF4F, which are required for recruitment of the ribosome to the mRNA 5′ untranslated region, have been identified. In this Review, we summarize the evidence for selective mRNA translation by components of these macromolecular complexes as a means to dynamically control the translation of the proteome in time and space. We further discuss the implications of this form of gene expression regulation for a growing number of human genetic disorders associated with mutations in the translation machinery.
The central dogma of information flow from DNA to RNA to protein contains many regulatory stages. The relative contribution of mRNA translation, which is facilitated by several large multisubunit complexes (BOX 1), towards the ultimate expression of gene products is still a matter of substantial debate1–6. However, revolutions in genomics and proteomics technologies over the past decade now enable systematic comparison of mRNA levels (via RNA sequencing (RNA-seq)), ribosome occupancy (via polysome profiling7 and ribosome profiling8) and protein levels (via quantitative mass spectrometry) to build comprehensive systems-level maps of gene regulation. Analysis of these data sets has revealed that transcript levels by themselves are not sufficient to predict protein levels and that translational control is likely to have an important role in the regulation of gene expression, particularly during dynamic transitions such as embryonic development, cellular differentiation, responses to stimuli and stress5. Comparison of mRNA expression to ribosome occupancy and quantitative mass spectrometry data in cell lines has found that protein abundance is better predicted by ribosome occupancy than by transcript expression levels9. A similar study examining the gene expression profiles of stimulated and unstimulated dendritic cells attributed most changes in protein abundance to alterations at the RNA level, although a substantial portion of the proteome — including the translational machinery itself — was regulated at the level of translation as well10. Also, mRNA and protein levels are often uncoupled for genes that are differentially expressed across humans, chimpanzees and rhesus macaques11, suggesting an important evolutionary role for post-transcriptional control. In fact, hundreds of mRNAs underlying tissue-specific developmental processes are regulated at the translation level12. These include several key evolutionarily conserved developmental regulators and signalling pathways, such as HOX genes and MAPK, PI3K, SHH, WNT and Hippo pathway components, which have been identified as translationally regulated across key tissues within the developing mouse embryo12,13. It is interesting to speculate that the translational regulation of such fundamental regulators of tissue patterning, which are highly conserved across metazoans, may in fact vary to fit the developmental needs of each species. Indeed, the discoveries of pervasive translational regulation of the core developmental cell signalling circuitry and regulated rates of protein synthesis within stem cells and during cellular differentiation14–17 suggest that translational regulation is central to proper cellular function and organismal development.
Box 1 |. Cap-dependent translation initiation.
The majority of the eukaryotic genome is translated via cap-dependent translation initiation, which begins with the formation of the 43S preinitiation complex (PIC), composed of the 40S subunit of the ribosome, the eIF3 initiation factor complex, several smaller initiation factors (eIF1 and eIF1a), and the eIF2 GTPase bound to GTP and the initiator tRNA. The human 40S subunit, made up of 33 ribosomal proteins (RPs) and 1 ribosomal RNA (rRNA), contains the mRNA channel of the ribosome and three sites for tRNA: an aminoacyl (A) site, where new tRNAs enter; a peptidyl (P) site, where the growing polypeptide is attached; and an exit (e) site, where deacetylated tRNAs are transferred before leaving the ribosome. The initiator tRNA, containing the methionine that begins the peptide sequence, is positioned in the P site of the 40S subunit in the PIC. eIF3 wraps around the solvent-exposed side of the 40S subunit, with many of the subunits making direct contact and eIF3j even projecting towards the decoding centre of the ribosome180,181.
The 43S PIC is loaded onto the mRNA transcript via the eIF4F complex, composed of the major cap-binding protein eIF4e, the scaffolding protein eIF4G and the RNA helicase eIF4A. eIF4G also associates with the poly(A) binding protein (PABP), which circularizes the mRNA, and with eIF3 via the eIF3c, eIF3d and eIF3e subunits182, thereby recruiting the 43S PIC to the cap. once at the 5′ end of the mRNA, the 43S PIC travels towards the 3′ end in a process called ribosome scanning until the start codon is found. The codon then base pairs with the anticodon on the tRNA in the P site of the ribosome, and the resulting complex is called the 48S PIC.
Once the start codon is selected, the phosphate from the GTP hydrolysed by eIF2 is released, reducing the affinity of eIF2 for the tRNA and allowing binding of a new initiation factor, eIF5B-GTP. upon eIF5B-GTP binding, the other initiation factors dissociate from the complex and the large 60S ribosomal subunit is recruited. The 60S subunit, made up of 47 RPs and 3 rRNAs and containing the peptide exit tunnel, stimulates eIF5B GTP hydrolysis, resulting in the dissociation of eIF5B-GDP and any other remaining initiation factors. The 60S subunit joins the 40S subunit to create the translationally competent 80S ribosome, which can then proceed with translation elongation.
While this process is used to translate the bulk of eukaryotic mRNAs, there are several frequent variations. First, the start codon selection process has some flexibility: while it is usually an AUG in the optimal Kozak sequence context, the start codon can be other AUGs flanked by seemingly less favoured sequences. In fact, the start codon does not have to be AUG; CUG, GUG, UUG and other near-cognate codons can be used instead183. Whether the mechanism of translation initiation differs for these alternative start codons has not been fully elucidated. Additionally, rather than translating from a single open reading frame (ORF) to make a single protein, ribosomes can initiate at multiple places along a transcript. When a complete ORF occurs 5′ to the main protein-coding ORF, it is called an upstream ORF (uORF). In some cases, translation of uORFs siphons away PICs from the main ORF, resulting in decreased translation of the primary protein-coding sequence; in other cases, ribosomes can reinitiate onto the main ORF after uORF translation has terminated. This process is thought to be facilitated by lingering interactions between the elongating ribosome, eIF3 and eIF4F components. After uORF translation termination and subunit separation, the 40S subunit, if it is still associated with eIF3, eIF4A and eI4G, can resume scanning and participate in the translation of the main ORF103.
How translational regulation of gene expression is mechanistically controlled is an area of intense research. Recent work examining the ribosome and several translation initiation factor complexes across cell populations has revealed heterogeneity and specialized functions of individual components. This includes heterogeneity in the composition of the ribosome, with specialized subunits present at substoichiometric levels even within one cell type. Unlike the classic biochemical definition of specialization reflecting only ‘one enzyme, one substrate’, we consider the translation machinery specialized by the ability of certain components to preferentially translate particular groups of substrates even if the complex as a whole can bind and translate a greater fraction of transcripts across the genome. For example, within mouse embryonic stem (mES) cells, a ribosome subtype distinguished by the presence of the 40S core ribosomal protein (RP) S25 (also known as eS25) preferentially translates almost all genes in the vitamin B12 metabolism pathway18, although it does not exclusively translate only this subset of mRNAs. In this Review, we discuss with a particular focus on vertebrates how functional specialization and heterogeneity in the composition of the ribosome, the eIF3 complex and the eIF4F complex are critical for the regulated translation of the genome, and how mutations in these components underlie many human genetic disorders.
Ribosome heterogeneity and specialization
In 1958, following the discovery of the structure of DNA, Francis Crick proposed a bold hypothesis to explain the flow of genetic information from the nucleus to the cytoplasm. Armed with the knowledge that ribosomes accounted for the majority of RNA in the cell, he posited that ribosomes are the intermediate genetic information carriers, and therefore each of the millions of ribosomes in the cell is customized for only one gene product19. This ‘one gene, one ribosome, one protein’ hypothesis reflected the most extreme version of ribosome heterogeneity that was debated by the RNA Tie club (the exclusive group of 20 scientists — one per amino acid — that met and corresponded to discuss the genetic code). As more careful characterization of the ribosome did not reveal large differences in ribosomal RNA (rRNA) size to match the differences in the size of cellular proteins, Crick first revised his theory to “only part of the ribosomal RNA acts as a template” (REF.20) and eventually assigned all specificity to a theoretical non-ribosomal “genetic RNA” (REF.21) species. Concurrently, Francois Jacob and Jacques Monod postulated that ribosomes of the same composition indiscriminately translated all mRNAs they encounter22. This notion prevailed when the original hypothesis of gene-specific ‘bespoken’ ribosomes was impugned by the seminal 1961 Brenner, Jacob and Meselson manuscript that identified a phage RNA ‘messenger’ that was efficiently translated by bacterial ribosomes, leading to the conclusion that “ribosomes are non-specialized structures which synthesize, at a given time, the protein dictated by the messenger they happen to contain” (REF.23). This landmark paper — as well as other heterologous interspecies translation assays performed in mammalian reticulocyte lysates, which showed that ribosomes of one species could translate the mRNAs of another24 — reduced the ‘one gene, one ribosome, one protein’ hypothesis to an outdated and misguided model of the genetic code.
Of note, correlative evidence — including differences in RP transcript expression or abundance, tissue-specific functions attributable to one RP but not another, as well as RP paralogues exerting unique functions — suggests that ribosomes have far greater regulatory functions than simple “automatons” (REFS13,25–29). Our discussion will be predominantly focused on vertebrates; for examples from such diverse organisms as bacteria, yeast, plants and invertebrates, we direct readers to other reviews30,31. As discussed in these perspectives on the specialization of ribosomes30,31, direct evidence for ribosome heterogeneity and the functional roles for distinct ribosomes in controlling gene expression, however, were until very recently lacking. To date, most modern molecular biology textbooks still discuss the ribosome as a singular, uniform molecular machine.
Intracellular heterogeneity in ribosome composition.
Although the ribosome is an ancient macromolecular machine, a progressive step-wise increase in ribosome size is evident from prokaryotes to unicellular eukaryotes to mammals. The human ribosome contains 2,650 more nucleotides in rRNA and 2,452 more amino acids, including 26 additional RPs, compared with prokaryotic ribosomes. Human ribosomes are a full MDa larger than Saccharomyces cerevisiae ribosomes, with 1,706 additional rRNA nucleotides and one more RP32. While sharing a catalytic rRNA core with the prokaryotic ribosome33, the eukaryotic ribosome contains vast, tentacle-like expansion segments of rRNA that create a larger solvent-exposed outer RNA shell34. Expansion segments have vastly increased in size during eukaryotic evolution and may serve as docking points for a multitude of additional ribosome-associated proteins (RAPs). This tremendous increase in ribosome size and complexity may have provided increased regulation and specialization to the process of translation. Indeed, the range of 5′ untranslated region (UTR) lengths has expanded dramatically in metazoans relative to yeast, with individual human 5′ UTRs containing as many as several thousands of bases35, suggesting a concurrent acquisition of cis-regulatory mRNA motifs (discussed below) as the translation machinery increased in complexity. Although these evolutionary relationships are speculative, evidence is increasing that the ribosome can accommodate multiple compositions, both with respect to core RPs and RAPs18,36.
Direct evidence for ribosome heterogeneity has been enabled by new quantitative techniques to measure ribosome component stoichiometry, as well as by genome-wide profiling tools that assess the contributions of heterogeneous ribosomes to the control of gene expression (FIG. 1). Absolute measurement of macromolecular composition requires meticulous and precise quantitative methods, which have only recently been brought to bear on this important biological question. A recent study from our laboratory employed selected reaction monitoring (SRM) to measure the stoichiometry of 15 RPs in the polysomes of primary mES cells and identified several as being substoichiometric18. This finding indicates that actively translating ribosomes in mES cells can lack one or more core RPs (FIG. 2a). The biological functions of such heterogeneous ribosomes have been revealed by endogenously tagging substoichiometric RPs and profiling the mRNAs that they are translating genome-wide (discussed below).
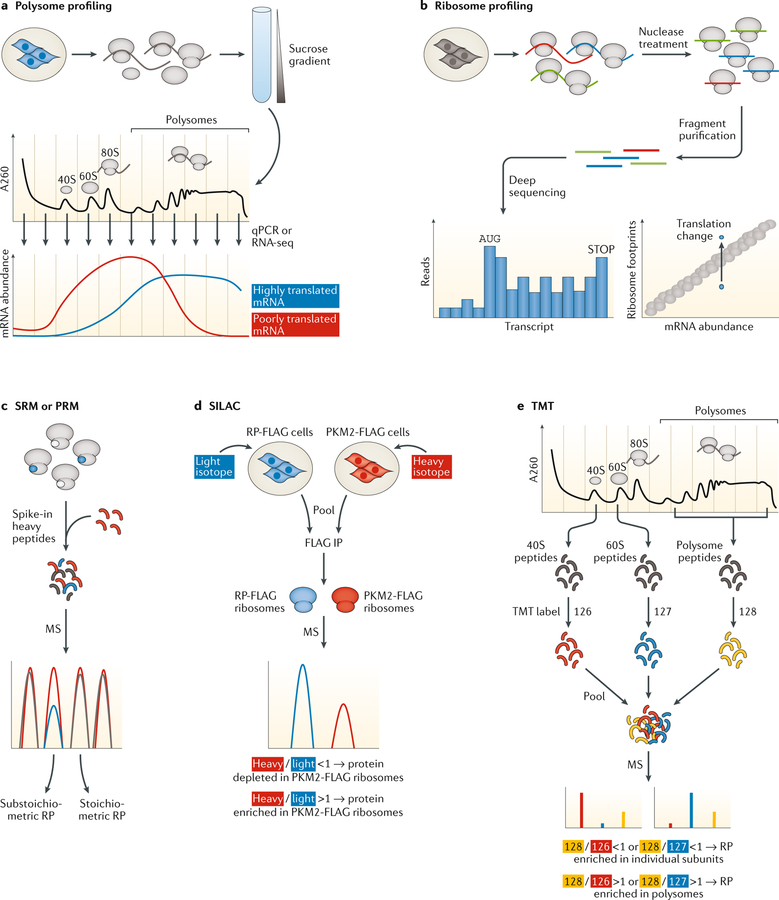
Fig. 1 |. Technologies for the quantification and characterization of translation machinery heterogeneity and functional specialization.
a | Translation efficiency measured by polysome profiling: lysates from cells treated with the drugs that immobilize elongating ribosomes on mRNAs are loaded onto sucrose gradients and spun in an ultracentrifuge. The free subunits, 80S ribosome and polysomes are separated into defined fractions in the gradient. RNA is isolated from each individual fraction, and the abundance of genes of interest is determined by quantitative PCR (qPCR) or RNA sequencing (RNA-seq). Highly translated mRNAs are enriched in the heavier polysome fractions (blue line), while poorly translated mRNAs are enriched in lighter fractions (red line). b | Translation efficiency measured by ribosome profiling: ribosomes are immobilized on mRNA, isolated by density centrifugation and treated with nucleases to digest unprotected mRNA, and then the ~30-nucleotide ribosome-protected mRNA fragments are sequenced. Mapping of these fragments to the transcriptome calculates the number of ribosomes at each position on mRNAs genome-wide. Translation efficiency is determined by comparing ribosome occupancy to mRNA abundance. Genes with the same transcript levels but different ribosome footprints are under different forms of translational regulation. c | Absolute protein quantification by selected or parallel reaction monitoring (SRM or PRM, respectively): complexes of interest, such as the ribosome, are isolated, digested into peptides, spiked with a known quantity of heavy-labelled peptides from the ribosomal proteins (RPs) of interest and analysed by mass spectrometry (MS). The absolute quantity of each RP is calculated by comparing the intensity of its peptides relative to the heavy-labelled standards. RPs present at one copy on every ribosome would have the same abundance; a substoichiometric RP found only on half of ribosomes would have a 50% lower abundance. d | Relative protein quantification by stable isotope labelling with amino acids in cell culture (SILAC): two cell populations, such as those containing a tagged RP or a tagged PKM2 allele, are treated with light (blue) or heavy (red) isotopes, respectively, and then pooled. The resulting heavy or light ribosomes are isolated by immunoprecipitation (IP) and digested into peptides, and the component proteins are identified by MS. The heavy/light ratio for each protein equals the relative abundance of the protein in the PKM2-containing ribosomes compared with total ribosomes. e | Relative protein quantification by tandem mass tags (TMTs): protein samples of interest, such as the individual ribosomal subunits and polysomes, are digested into peptides, are labelled with unique TMT reagents, are pooled and undergo MS. For each protein of interest, the ratios of the TMT labels determine the relative abundance of that protein in the free subunits compared with the polysomes.
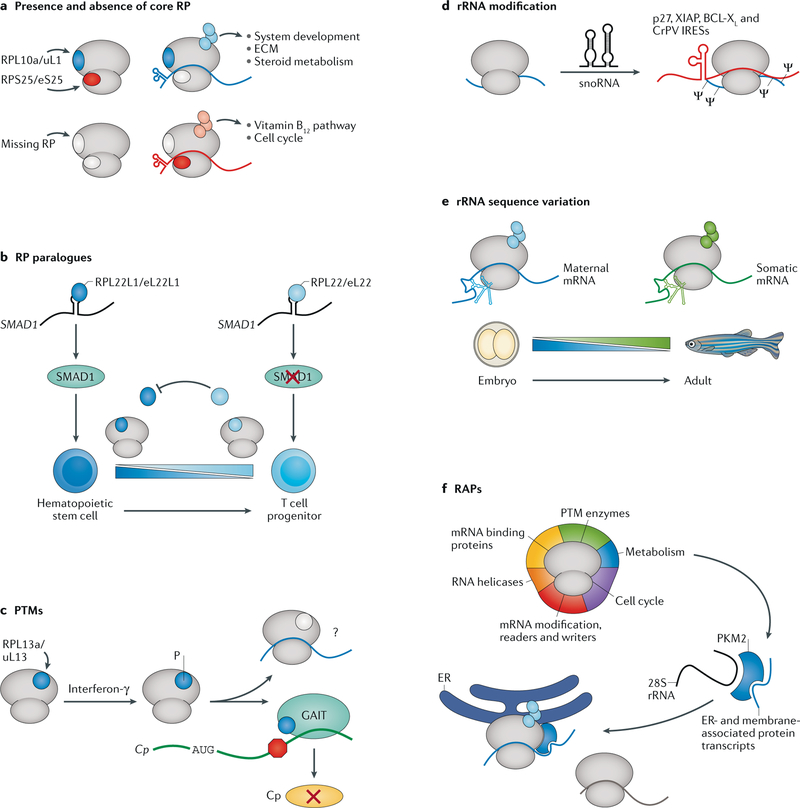
Fig. 2 |. Ribosome heterogeneity and specialization tune genetic networks.
Ribosome heterogeneity exists at multiple levels. a | Ribosomes containing or lacking specific core RPs, such as RPL10a/uL1 or RPS25/eS25, are specialized for the translation of mRNAs from specific cellular pathways. b | Ribosomes can contain paralogues of core ribosomal proteins (RPs). RPL22/eL22 represses expression of its paralogue, RPL22L1/eL22L1, as well as the important developmental regulator SMAD1. A switch from RPL22L1/eL22L1 to RPL22/eL22 expression is required for haematopoietic stem cell differentiation into T cell progenitors. c | Ribosomes are extensively post-translationally modified. Phosphorylation (P) of RPL13a/uL13 upon interferon-γ signalling causes its removal from the ribosome and incorporation into the γ-interferon inhibitor of translation (GAIT) complex, which binds the 3′ UTR of ceruloplasmin (Cp) mRNA and inhibits its translation. d | Ribosomal RNA (rRNA) is extensively modified. Small nucleolar RNAs (snoRNAs) can direct pseudouridylation (Ψ) of rRNA, and preventing this modification inhibits translation of several cellular and viral internal ribosome entry sites (IRESs). e | RNA can vary in sequence. During zebrafish development, ribosomes switch from an oocyte-specific rRNA sequence variant to a somatic sequence variant. These different rRNA sequences may promote translation of maternal or somatic mRNAs, respectively. f | Ribosome-associated proteins (RAPs) have many functions. One RAP, PKM2, is a metabolism enzyme that binds to the 28S rRNA and is enriched on ribosomes found at the endoplasmic reticulum (ER). PKM2 additionally binds to transcripts of ER-associated and membrane-associated proteins to upregulate their translation. CrPV, cricket paralysis virus; ECM, extracellular matrix; PTM, post-translational modification.
As there are estimated to be up to 10 million ribosomes in a mammalian cell, even small changes in RP abundance could affect the composition of hundreds of thousands of ribosomes. While SRM has many strengths, including its attomolar sensitivity, high precision and excellent reproducibility across multiple research groups (coefficient of variation typically ≤15%)37–39, it may be difficult to use for all RPs. In particular, the small size of many RPs poses a challenge for this analysis owing to the very small pool of candidate proteotypic peptides required for accurate quantification by SRM, although selection may be aided by a recently compiled SRM atlas40. This method also requires complete tryptic digestion of the proteins of interest and a specialized mass spectrometer to quantify the abundance of one protein of interest at a time. A higher-throughput approach that may be useful to apply to the question of translation complex stoichiometry is parallel reaction monitoring (PRM): operating under the same absolute quantification strategy as SRM, PRM also compares the abundance of each peptide to a spike-in standard but uses less advanced instrumentation and can assess the abundance of multiple peptides simultaneously41 (FIG. 1c).
An exciting alternative to analyse ribosome composition is a recently published native mass spectrometry protocol, which is capable of defining the mass of intact protein complexes as large as 9 MDa with high resolution42. Unlike the typical bottom-up mass spectrometry workflow, where proteins are digested into smaller peptides, this top-down approach can examine entire, fully assembled complexes, allowing missing proteins to be readily identified and calculated as substoichiometric. Recent advances in instrumentation have provided sufficient resolution to identify differences in the copy number of the uL7 and uL12 RPs on intact Escherichia coli ribosomes42. The advantage of this method, in addition to the fact that it does not require specific selection and synthesis of peptides, is that it can theoretically identify specific ribosomes lacking more than one RP simultaneously, which can reveal whether the incorporation of specific RPs on the ribosome is co-regulated. The potential drawback is that it may be difficult to obtain sufficient fragmentation efficiencies and coverage to reliably distinguish between RPs with relatively similar masses and charges, particularly if the level of heterogeneity is substantial (for example, many different combinations of proteins and post-translational modifications (PTMs) are present). False negatives could also emerge if other proteins take the place of a missing RP on the ribosome.
More high-throughput quantitative strategies, such as stable isotope labelling with amino acids in cell culture (SILAC) and tandem mass tags (TMTs), have been used to study ribosome composition, but these require relative quantification of ribosome components between two states (such as between free subunits and translationally active ribosomes18, monosomes and polysomes43 or different subcellular locales36) (FIG. 1d,e). A recent study from our laboratory employed these techniques to define the ribosome interactome in mES cells, revealing that it comprises hundreds of RAPs36, thus extending the potential for ribosome regulation and heterogeneity to the level of interacting proteins. Combining these methods with a proximity labelling technique, we were able to quantitatively identify RAPs enriched on ribosomes located at the endoplasmic reticulum (ER) versus the rest of the cytoplasm, which may exert specialized functions at this subcellular location, for example, the translation of ER-destined mRNAs36. Further work is required to expand the atlas of intracellular ribosome heterogeneity to encompass other cellular contexts, such as additional subcellular regions or during responses to cellular stimuli or stress.
Intercellular ribosome heterogeneity.
If ribosome composition can vary within a single cell type, can it also differ across tissues? This question has yet to be investigated with the necessary resolution at the protein level, but intriguing results have nevertheless emerged by examining RP transcript abundance. Multiple studies have shown that the transcript expression levels of the core RPs differ between vertebrate tissues13,44–49. In particular, the expression of several RP paralogues seems to be restricted to certain tissues, including the specific expression of RPL3L/uL3L in striated muscle50 and of RPS4Y1/eS4Y1 and RPS4Y2/eS4Y2 in the testis28. RNA-seq data sets have also revealed differences in the expression of certain RPs across individual cell types. For example, different cancer cell lines and haematopoietic cell types are marked by changes in expression levels of several core RPs26.
It would be valuable to use single-cell RNA-seq to assess RP expression differences, at least at the transcript level, between individual cells. However, both bulk and single-cell RNA-seq results are difficult to analyse, owing to the expression of several closely related RP paralogues and over 2,000 RP pseudogenes in the human genome. Indeed, data sets that previously reported 100-fold differences in RP transcript levels across human tissues, when reanalysed to properly account for pseudogenes, showed only threefold changes in RP expression44. Further difficulties in comparing results from multiple studies arise from differences in the developmental stage of the tissues and cell types examined. It is also important to note that whether such transcript expression differences are reflected by actual changes in RP incorporation into ribosomes has yet to be investigated.
How the changes in RP expression levels across cell or tissue types are generated remains an area of active research. The presence of binding sites for specific transcription factors in the promoters of RP genes26,45,51 suggests a point of regulation. Notably, the promoters of RPs specific to certain blood cell lineages contain the binding sites of corresponding lineage-specific transcription factors26. Although the actual promoter binding events have yet to be validated in these specific haematopoietic cell types, it suggests that RP expression is regulated at the level of transcription. Certain RPs can also negatively regulate the splicing of their own transcripts, creating negative feedback loops that may help stabilize RP expression52–54. mRNA stability may contribute as well, as in the case of RPL22L1/eL22L1, whose mRNA is destabilized by the binding of its paralogue, RPL22/eL22, to a stem-loop structure in its mRNA55 (FIG. 2b). RPs have been suggested to have evolved particular molecular features, such as their small size, to facilitate their coordinated and fast translation56, but evidence of RP-specific translational regulation has also emerged. For instance, RPL10a/uL1 is preferentially translated by RPL10a/uL1-containing ribosomes, creating a positive feedback loop18. At the protein level, it is possible for RPs to be regulated by protein degradation; in fact, excess RPs that are not incorporated into the ribosome are degraded by the proteasome57. Ribosome heterogeneity may additionally be achieved by the regulated incorporation and extraction of RPs from the ribosome. For example, given their high degree of similarity, RP paralogues likely bind to the same site on the ribosome and would therefore compete with each other for incorporation. By contrast, RPL13A/uL13 is extracted from the ribosome upon phosphorylation during interferon-γ signalling58 (FIG. 2c). Although the function of RPL13A/uL13 upon removal from the ribosome as part of an extra-ribosomal complex has been studied, it is not known whether ribosomes lacking RPL13A/uL13 have altered mRNA translational specificity. The regulatory mechanisms that generate ribosome heterogeneity are not yet known for most RPs; however, multiple strategies are likely to be employed to fine-tune ribosome composition for different cell types and environments.
An additional source of heterogeneity may be chemical modifications on RPs (BOX 2) or rRNA. Over 200 residues of human rRNA are modified by methylation, pseudouridylation, ribosylation or acetylation, and several sites of modification have been suggested to be variable. Pseudouridine modifications are guided by small nucleolar RNAs (snoRNAs), but whether there are cell-specific or stimulus-specific changes in snoRNA expression or activity that could create distinct patterns of rRNA modification remains to be determined (for more information on rRNA modifications, we direct readers to other recent reviews of the subject30,59–61). Pseudouridylation of rRNA does seem to have functional importance, as mutations in dyskerin, the enzyme that catalyses pseudouridylation, perturb rRNA modification and inhibit translation initiation at several viral and cellular internal ribosome entry sites (IRESs)62,63 (FIG. 2d). rRNA, which is encoded by hundreds of copies of ribosomal DNA (rDNA) in the vertebrate genome, is itself heterogeneous in sequence. In fact, during zebrafish embryogenesis, ribosomes initially contain oocyte-specific sequences for all four rRNAs and then later switch to somatic rRNA variants64,65 (FIG. 2e). Multiple rRNA sequence variants have also been identified in mice66,67, several of which have been shown to have tissue-specific expression patterns67. rRNA is similarly heterogeneous in humans66–68, as a recent study based on the 1000 Genomes Project estimated that 23% of Rrna nucleotides have sequence variants present in the general population, with each individual expressing on average 32 distinct alleles67.
Box 2 |. Post-translational modifications on the ribosome.
Numerous post-translational modifications (PTms), including phosphorylations, methylations, acetylations and hydroxylations, have been identified on the ribosome in unbiased proteomics experiments, although the majority of these have yet to be functionally characterized (reviewed in REF.187). Several modifications are highly abundant, including prolyl hydroxylation of RPS23/uS12, a ribosomal protein (RP) required for proper tRNA selection188, which increases translation fidelity in yeast and human cell lines189–191. Interestingly, missense mutations that decrease RPS23/uS12 hydroxylation result in a variety of tissue-specific phenotypes in human patients190 (TABLE 1), suggesting that some cell types rely on RPS23/uS12-mediated translation fidelity more than others. other modifications are induced upon specific stimuli, such as the phosphorylation of RACK1 in human cells infected with poxvirus192. Indeed, RACK1 is modified by a poxvirus kinase to co-opt the host ribosome for the translation of viral genes containing a poly(A)-leader cis element in their 5′ untranslated region (uTR)192, suggesting that these dynamic modifications expand ribosome heterogeneity and influence ribosome activity.
One of the more extensively studied PTms on the ribosome is ubiquitin, a small 8.5 kDa peptide that is covalently bonded to substrate proteins singularly (monoubiquitylation) or in long chains (polyubiquitylation) to either mark substrates for disposal by the proteasome (degradative ubiquitylation) or alter the function of the protein (regulatory ubiquitylation). The ribosome is both a source and substrate for the ubiquitin pool: in the human genome, two of the four genes encoding ubiquitin are ubiquitin–RP fusions (RPS27a/eS31 and RPL40/eL40)193. Although the ubiquitin moiety is post-translationally cleaved from the RPs, it is not completely equivalent to the other non-ribosomal ubiquitin sources. Depletion of the RPL40/eL40 ubiquitin fusion gene does not alter global ubiquitin levels but does decrease ubiquitylation of the ribosome. This ribosome modification is required for efficient translation, and thus the RPL40/eL40 deletion in mice is embryonic lethal194. Which ribosomal components are ubiquitylated by this particular ubiquitin pool is not known, but several RPs have been shown to be dynamically ubiquitylated in response to cellular conditions. RPS2/uS5, RPS3/uS3 and RPS20/uS10, for instance, undergo regulatory ubiquitylation during the unfolded protein response in yeast, fruit fly and human cell lines195. Prevention of this ubiquitylation sensitizes cells to endoplasmic reticulum stressors, revealing that ribosomal modification is required for the stress response, possibly owing to alteration of global translation rate or to selective translation of stress response genes.
Degradative ubiquitylation is also important for proper ribosomal function. excess RPs not incorporated into ribosomes are polyubiquitylated and degraded by the proteasome; inhibition of this process in yeast causes protein synthesis defects and decreases cell growth57,196. Intriguingly, RP degradation is also required for erythropoiesis. During the final stage of erythrocyte maturation, precursor cells eliminate their translation machinery. Concurrent to the removal of ribosomes, expression of several e2 and e3 ligases is induced, including UBE2O. UBE2O seems to act as both an e2 and e3 ligase to add multiple monoubiquitin modifications to several RPs, leading to their degradation by the proteasome197,198. UBE2O is necessary and sufficient for ribosome depletion, and the knockout of UBE2O in mice results in anaemia197. While the result of UBE2O activity is a global decrease in ribosome number, the rate of degradation of individual RPs varies, suggesting that ribosomes alter composition as the cells proceed to terminal differentiation. Whether these ribosomes have specific functions, such as being optimized for the translation of haemoglobin, has yet to be determined.
In addition to ubiquitin, several other related proteins have been found to modify the ribosome. NEDD8 and Sumo modifications on RPs seem to regulate protein stability and ribosome biogenesis, respectively, although mechanistic details are still lacking199–201. Another recently identified ubiquitin-related modification is ufm1, which was found conjugated to three RPs (RPS3/uS3, RPS20/uS10 and RPL10/uL16) in mouse embryonic stem cells36. Although the molecular function of this modification is not yet understood, mice lacking Ufl1, the E3 ligase for ufm1 and a ribosome-associated protein36, exhibit erythropoiesis defects reminiscent of other ribosomal mutation phenotypes92. The overlap of ufmylation and stress-responsive regulatory ubiquitylation on RPS3/uS3 and RPS20/uS10 is also intriguing, suggesting that certain RPs have combinatorial modifications.
The ribosome: a machine with selective cellular functions.
An open question in the field is: what is the purpose of ribosome heterogeneity? One possibility is that the presence or absence of specific ribosome components may create pools of ribosomes with defined functions in translation, enabling careful regulation of gene expression through changes in the composition of ribosomes at different times and places. In support of this hypothesis, ribosomes containing RPL10a/uL1 or RPS25/eS25, which are present at substoichiometric levels in mES cells, were found to preferentially translate several hundred genes18 (FIG. 2a). By contrast, ribosomes containing RPL22/eL22, an RP that is likely to be present on all ribosomes in mES cells, showed little transcript specificity, indicating that the selective translation patterns of ribosomes containing RPL10a/uL1 or RPS25/eS25 are distinct. RPL10a/uL1 was shown to direct the translation of genes in several gene ontology categories, including extracellular matrix organization and steroid metabolism, as well as important mRNAs that promote growth and metastasis; conversely, RPL10a/uL1-containing ribosomes were depleted in mRNAs involved in vitamin metabolism and several genes required for the stress response and cell death18 (FIG. 2a). Importantly, transcripts that are preferentially bound to RPL10a/uL1-containing ribosomes also require this RP for their efficient translation. Upon knockdown of RPL10a/uL1, the translational efficiency of these mRNAs is diminished, revealing the functional importance of ribosome heterogeneity. Several mRNAs that rely on RPL10a/uL1 for their translation contain IRES elements in their 5′ UTRs that require RPL10a/uL1 for their activation, although the exact mechanism is unknown. RPL10a/uL1 also regulates translation from the hepatitis C virus (HCV) and cricket paralysis virus (CrPV) IRESs but not the encephalomyocarditis virus (EMCV) IRES, revealing a specific role for this RP in promoting translation of particular classes of viral IRESs18. This finding reveals that ribosomes of specific compositions can coordinately regulate distinct classes of genes: changing the abundance of RPL10a/uL1 on the ribosome results in a systems-level remodelling of specific networks of mRNAs for unified cellular responses.
Another example of this regulation is the selective translation of genes by RPS25/eS25-containing ribosomes. RPS25/eS25 has been well studied as a regulator of viral IRES translation initiation, as it is required for multiple classes of viral IRESs (such as CrPV, EMCV, HCV and poliovirus) but is dispensable for cap-dependent translation initiation69,70. Crosslinking and cryo-electron microscopy studies have shown that RPS25/eS25 contacts the HCV and CrPV IRESs directly71,72, suggesting that it is RPS25 itself and not an intermediary protein that controls IRES-mediated translation initiation. RPS25/eS25 has also been shown to regulate the translation of eukaryotic mRNAs, some of which are known to contain IRESs, possibly also via direct contact with the mRNA18,70. Similar to RPL10a/uL1-containing ribosomes in mES cells, those containing RPS25/eS25, another substoichiometric RP, also preferentially translate select mRNAs belonging to distinct functional categories, including those composing whole metabolic pathways. A noteworthy example is the requirement of RPS25/eS25-containing ribosomes for the translation of all components of the vitamin B12 pathway18 (FIG. 2a). This finding suggests a level of regulation analogous to the bacterial operon system, where genes in the same pathway are simultaneously regulated, in this instance by their ability to be translated by specific classes of ribosomes.
RP functions in development and disease.
Intriguing genetic evidence for more selective functions of individual RPs in specific cell and tissue types comes from RP mutations in human diseases, known as ribosomopathies (TABLE 1). Mutations in several RPs underlie Diamond–Blackfan anaemia (DBA), which is characterized primarily by bone marrow failure resulting in anaemia as well as an increased predisposition to tumour development. Depending on the nature of the mutated RPs, other tissue-specific congenital birth defects, such as heart, craniofacial and thumb abnormalities, are observed in patients with DBA73. Other striking examples of disease states associated with RP mutations include mutations in RPSA/uS2, which cause asplaenia74; RPL10/uL16, which may underlie autism spectrum disorders75; and RPL21/eL21, which cause hereditary hypotrichosis simplex, resulting in the loss of body hair76.
Table 1 |.
Ribosomal proteins with specialized translation functions and human and mouse phenotypes
| RP | Human phenotype | Mouse phenotype | Effects of RP downregulation on translation | Specialized translation functions |
|---|---|---|---|---|
| RPS6/eS6 | Unknown | • Embryonic lethal203 • Keratinocyte-specific heterozygosity: hyperpigmentation of ears, tail and footpads204 • Bone marrow-specific heterozygosity: decreased haematopoietic stem cell number, decreased erythropoiesis, macrocytic anaemia and haematopoietic defects205 • Thymus-specific heterozygosity: decrease in numbers of peripheral T cells206 |
Ribosome biogenesis defects in haploinsufficient embryos203 and in activated heterozygous T cells206 | NR |
| RPS7/eS7 | Diamond–Blackfan anaemia207 | Missense mutations: microphthalmia and uveal coloboma, fused and disorganized vertebrae, cortical thinning, eye, brain and skeletal abnormalities, and white belly spot208 | Ribosome biogenesis defects in heterozygous mice208 and in patient cells207 | NR |
| RPS10/eS10 | Diamond–Blackfan anaemia209 | Unknown | Ribosome biogenesis defect in patient cells209 | NR |
| RPS14/uS11 | 5q-Myelodysplastic syndrome210 | Macrocytic anaemia211 | Decreased protein synthesis upon knockdown in TF-1 cells and ribosome biogenesis defects in 5q-myelodysplastic syndrome patient cells210 | NR |
| RPS15a/uS8 | Diamond–Blackfan anaemia212 | Unknown | Ribosome biogenesis defect in K562 cells heterozygous for the mutation observed in patients with Diamond–Blackfan anaemia212 | NR |
| RPS17/eS17 | Missense mutations in start codon: Diamond–Blackfan anaemia213,214 | Unknown | Unknown | NR |
| RPS19/eS19 | Diamond–Blackfan anaemia215 | Missense mutation: hyperpigmentation of ears, tail and footpads; white belly spot; mild reduction in red blood cell counts; and erythroid defects204 | Decreased protein synthesis (knockdown in K562 cells or mouse erythroblasts)84,90 | Promotes cellular IRES activity in mouse erythroblasts84 |
| RPS20/uS10 | Unknown | Missense mutation: hyperpigmentation of ears, tail and footpads204 | Unknown | NR |
| RPS23/uS12 | Missense mutations: microcephaly , hearing loss, autism spectrum disorders, craniofacial abnormalities, atypical palm creases and finger pads and short fingers190 | Unknown | No global protein synthesis changes in patient cells190 | Promotes translation fidelity188 |
| RPS24/eS24 | Diamond–Blackfan anaemia216 | Unknown | Unknown | NR |
| RPS25/eS25 | Unknown | Unknown | – | • Promotes viral and cellular IRES
activity69,70 • Promotes translation of several hundred mRNAs in mES cells18 |
| RPS26/eS26 | Diamond–Blackfan anaemia209,217 | Unknown | Ribosome biogenesis defects in patient cells209 | Promotes translation from start codons in Kozak sequence contexts in yeast218 |
| RPS27/eS27 | Diamond–Blackfan anaemia and abnormal skin pigmentation219 | Unknown | Ribosome biogenesis defects (knockdown in K562 cells)219 | NR |
| RPS28/eS28 | Missense mutation in start codon: Diamond–Blackfan anaemia220 | Unknown | Unknown | NR |
| RPS29/uS14 | Diamond–Blackfan anaemia221 | Unknown | Ribosome biogenesis defect in patient mononuclear cells221 | NR |
| RPS30/eS30 | Unknown Heterozygous deletion of entire FAU/MNSFβ gene: decreased female fertility222 | Unknown | NR | RPS30/eS30 |
| RPSA/uS2 | Isolated congenital asplenia74 | • No phenotype observed for
heterozygote • Homozygote embryonic lethal74 |
No ribosome biogenesis defects in patient cells74 | NR |
| RACK1 | Unknown | White belly spot and hypopigmented paws and tail87 | • Decreased protein synthesis in livers
from heterozygous mice87 • No change in protein synthesis upon knockdown in primary normal human dermal fibroblasts192 or S2 cells85 |
• CrPV and HCV IRES
translation85 • Translation of poxvirus genes192 |
| RPLP1 | Unknown | Brain atrophy , male infertility and kinked tail223 | No global protein synthesis changes in mouse embryonic fibroblasts lacking RPLP1 (Ref.223) | NR |
| RPL5/uL18 | Diamond–Blackfan anaemia, heart abnormalities, craniofacial defects (including cleft lip and palate), thumb malformations (including triphalangeal thumbs) and tumour predisposition207 | Unknown | Decreased protein synthesis (knockdown in HeLa cells)207 | NR |
| RPL10/uL16 | Missense mutations: autism spectrum disorders, intellectual disability , microcephaly and seizures in males75,224 | Unknown | Decreased protein synthesis upon knockdown in zebrafish224 and upon introduction of the mutant human RPL10/uL16 alleles into yeast75 | NR |
| RPL10a/uL1 | Unknown | Unknown | – | Promotes translation of several hundred genes, including several with known RPL10a/uL1-dependent IRES elements, in mES cells; promotes HCV and CrPV IRES activity18 |
| RPL11/uL5 | Diamond–Blackfan anaemia, thumb malformations (including triphalangeal thumbs) and tumour predisposition207 | • Embryonic lethal • Heterozygosity induced in adult mice: anaemia and tumour predisposition225 |
Decreased protein synthesis (knockdown in HeLa cells207) and ribosome biogenesis defects in RPL11-deficient mouse embryonic fibroblasts and spleens225 | Promotes cellular IRES activity in mouse erythroblasts84 |
| RPL13a/uL13 | Unknown | Macrophage-specific knockout: increased inflammation upon endotoxin stimulation226 | Increased expression of GAIT complex targets but no effect on global protein synthesis in macrophages lacking RPL13a/uL13 or upon knockdown in U937 cells226,227 | Inhibition of translation of target mRNAs as part of GAIT complex58 |
| RPL15/eL15 | Diamond–Blackfan anaemia228 | Unknown | Decreased protein synthesis (knockdown in HeLa cells)228 | NR |
| RPL21/eL21 | Missense mutation: hereditary hypotrichosis simplex76 | Unknown | Unknown | NR |
| RPL22/eL22 | T cell acute lymphoblastic leukaemia229 | Knockout: αβ T cell ablation78 | No change in global protein synthesis in heterozygous mice229; mild decrease in protein synthesis in knockout mice78 | Represses smad1 translation in zebrafish29 |
| RPL22L/eL22L | Unknown | Knockout: embryonic lethal79 | No change in global protein synthesis observed upon morpholino knockdown in zebrafish embryos29 | NR |
| RPL24/eL24 | Unknown | Abnormal retinal cell differentiation, incomplete or absent optic nerve, white belly spot, kinked tail and polydactyly77 | Decreased protein synthesis in primary mouse embryonic fibroblasts from haploinsufficient mice77 | NR |
| RPL26/uL24 | Diamond–Blackfan anaemia, cleft palate, missing or narrowed external auditory meatus, left kidney agenesis, bicuspid aortic valve, missing or fused radius and ulna, and oligodactyly230 | Unknown | Decreased protein synthesis (knockdown in HeLa cells)230 | NR |
| RPL27/eL27 | Diamond–Blackfan anaemia and atrial septal defect219 | Unknown | Ribosome biogenesis defects (knockdown in K562 cells)219 | NR |
| RPL27A/uL15 | Unknown | Cerebellar ataxia; hyperpigmentation of ears, tail, footpads and genital areas; and pancytopenia231 | Unknown | NR |
| RPL29/eL29 | Unknown | Homozygous deletion: small size and increased bone fragility232,233 | Decreased protein synthesis in primary mouse embryonic fibroblasts from null mice233 | NR |
| RPL31/eL31 | Diamond–Blackfan anaemia, triphalangeal thumb and thenar hypoplasia and fused radius and ulna234 | Unknown | Ribosome biogenesis defects in Diamond–Blackfan anaemia patient primary cells and upon knockdown in K562 cells234 | NR |
| RPL35A/eL33 | Diamond–Blackfan anaemia, ventricular septal defect, hypertelorism and low-set ears235 | Unknown | Decreased protein synthesis (knockdown in UT-7/Epo and HEK293 cell lines)235 | NR |
| RPL38/eL38 | Unknown | Midline facial cleft, microphthalmia and incomplete optic fissure closure, exencephaly , kinked tail and homeotic axial skeleton transformations13 | Decreased translation of several Hox genes but no effects on global translation in neural tubes and somites of haploinsufficient mice13 | Promotes translation of several Hox genes via IRES elements in their 5′ UTRs13,83 |
| RPL40/eL40 | Unknown | Homozygous deletion of entire UBA52 gene: embryonic lethal194 | Decreased protein synthesis (knockdown in DLD-1 cells)194 | Promotes translation of VSV genes and several stress response transcripts in yeast88 |
Published human diseases and mouse models resulting from mutations in core ribosomal proteins (RPs) are listed with their phenotypes. All phenotypes are the result of haploinsufficient RP expression unless noted otherwise. Where known, the resulting impact on translation is also listed, as are any additional specialized translation functions, which may come from studies in a variety of model systems. CrPV, cricket paralysis virus; GAIT, γ-interferon inhibitor of translation; HCV, hepatitis C virus; IRES, internal ribosome entry site; mES, mouse embryonic stem; NR, not reported; UTR, untranslated region; VSV, vesicular stomatitis virus.
In addition to human diseases, RP haploinsufficiency in mouse models also produces tissue-specific phenotypes (TABLE 1). The specificity of the phenotypes can be striking; for instance, mice hypomorphic for RPL38/eL38 have unique homeotic transformations, the replacement of one skeletal element with that of another, along the vertebral column not seen in any other RP mouse model or ribosomopathy13, whereas mice deficient in RPL24/eL24 exhibit polydactyly (extra digits)77. Even between highly similar RP paralogues, there are differences in organismal phenotypes; for instance, Rpl22/eL22 knockout mice are phenotypically normal except for abnormal T cell development78, whereas knockout of its paralogue Rpl22l1/eL22l1 is embryonic lethal79. Together, these findings suggest that different RPs are required at different times and places during development.
Connecting the organismal genetics of RP haploinsufficiency and ribosomopathies to a deeper molecular understanding has been challenging. It is important to first properly classify the phenotypes resulting from mutations in RPs. While it is tempting to group many RP phenotypes under the category of ‘skeletal defects’, this grouping does not accurately reflect the different aetiologies80. For example, the changes in vertebral identity seen in RPL38/eL38 haploinsufficient mice arise from a particular homeotic transformation patterning defect that is distinct from the RPL24/eL24-deficient mouse phenotype of polydactyly. The vertebrae and digits in fact derive from separate embryonic tissues: the axial skeleton arises from the paraxial mesoderm and the limbs from the lateral plate mesoderm. Neither of these phenotypes is in any way equivalent to the bone marrow defects that cause anaemia in many ribosomopathies, and anaemia does not always accompany RP mutations. For example, RPL38/eL38 haploinsufficient mice do not show bone marrow or haematopoietic defects81. Of note, many of the RP mouse models were made on the same C57BL6 genetic background; therefore, their distinct phenotypes are due to genuine differences in RP functions. Clarifying these issues in the field is necessary to move forward with the molecular mechanisms under-pinning these phenotypes. Further work is also required to properly ascribe the RP loss-of-function phenotypes to translational control versus possible extraribosomal functions or nucleolar stress, the p53-mediated stress response to defects in ribosome biogenesis82, and to identify the biochemical mechanisms of these activities.
Cis elements that interface with ribosomes for RP-regulated gene expression.
Insights into cis-regulatory elements within the genome that may interface with selective RPs have emerged by characterizing the homeotic transformations and defects in body plan formation associated with haploinsufficiency of RPL38/eL38 in mouse models. RPL38/eL38 haploinsufficiency decreases the translation of a small subset of Hox genes, which are master regulators of body plan formation, without affecting global protein synthesis13. Notably, the homeotic transformations in RPL38/eL38 haploinsufficient mice mirror the loss-of-function phenotypes of these Hox genes. The transcript-specific regulation of these Hox genes by RPL38/eL38 can be achieved by a combination of two cis-regulatory elements in their 5′ UTRs: a potent translation inhibitory element (TIE) near the 5′ cap that suppresses general cap-dependent initiation and a structured IRES-like element closer to the main AUG that relies on RPL38/eL38 for its activity83 (BOX 3). The detailed biochemical mechanisms by which RPL38/eL38 can influence the preferential translation of Hox IRES elements remain to be determined, as does their applicability to the specificity of other RPs for their target mRNAs.
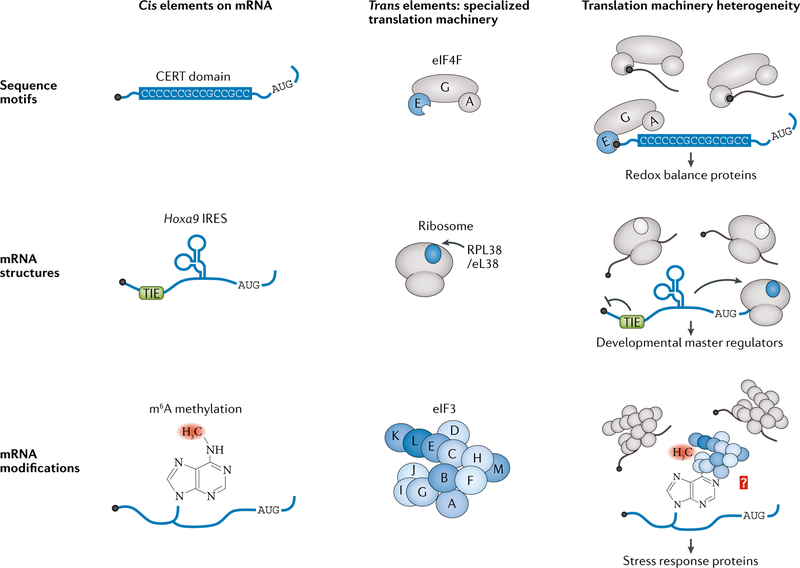
Box 3 |. Cis-regulatory mRNA elements recognized by specialized translation machinery.
Specialized translation regulation requires the translation machinery to recognize selected mRNAs, demarcated by particular cis regulons. Such regulons, found in the untranslated regions (UTRS) of mRNA transcripts, can be sequence motifs, mRNA structures, mRNA modifications or a combination of all three (see the figure).
One of the best-characterized RNA regulatory elements recognized by specialized translation machinery is the internal ribosome entry site (IRES). First described in RNA viruses, whose uncapped transcripts cannot follow the canonical eukaryotic translation initiation pathway, these elements are capable of recruiting the translation machinery directly within a transcript (reviewed in REF.184). IRESs are also found in cellular mRNAs, with genomic screens suggesting that there are as many as hundreds within the human genome185, although the importance of these IRESs for in vivo gene expression is unknown. To address this, the minimal IRES elements would need to be identified and removed from the 5′ UTR and the translation efficiency of the truncated transcript compared with that of the wild-type mRNA. Such characterization has been done for cellular IRESs found in mouse Hox gene 5′ UTRS. These IRESs, the activity of which is dependent on RPL38/eL38 (REF.83), are not only at least as strong as the hepatitis C virus (HCV) viral IRES in bicistronic reporters but also required for the majority of protein expression in vivo, as shown for HOXA9.
Other cis elements that promote translation are sequence based. Several 5′ UTR sequence motifs are required for target gene expression activation upon mTOR signalling, including the 5′ terminal oligopyrimidine tract (5′ TOP) and the pyrimidine-rich translational element (PRTe). The 5′ TOP, consisting of a polypyrimidine sequence at the very 5′ end of the transcript, is widespread across the genes encoding translational machinery, providing a mechanism for the global upregulation of translation occurring during mTOR signalling149. The PRTe, while similar to the 5′ TOP motif, is found further within the 5′ UTR and is additionally regulated by eIF4e activity148. eIF4e also mediates translation activation via another 5′ UTR sequence element, the cytosine-enriched regulator of translation (CERT) domain, spanning 15 nucleotides and comprising mostly cytosine147. Chemical modifications can also occur on RNA nucleotides within sequence motifs, adding another layer of regulation. A prominent example is the N6-methyladenosine (m6A) modification, which occurs across transcript UTRS and coding sequences on adenines within the GAC sequence motif. When in the 5′ UTR, m6A can stimulate cap-independent translation initiation via binding of eIF3 (REF.97). While the mechanism of eIF3 recognition of m6A is unclear, it is possible that it relies not just on the sequence motif but also on neighbouring RNA structures.
Finally, several cis motifs impede translation. one well-known element is the upstream open reading frame (uORF), where translation of a reading frame upstream of the main protein-coding sequence inhibits translation of the downstream ORF (see BOX 1). Another example is the translation inhibitory element (TIE), identified in several Hox genes83. The TIE, present at the 5′ end of the IRES elements in the Hox 5′ UTRS, is a potent repressor of cap-dependent translation initiation. No sequence conservation is evident across the Hox TIEs, and structural information is lacking. The pairing of the TIE and IRES elements suggests that the TIE has evolved to promote dependence of Hox mRNAs on the IRES, thereby creating a reliance on specialized translation machinery for their expression.
The mechanisms underlying many of these cis regulons remain uncharacterized. Further work is required to assess the contributions of RNA sequence versus structure, to identify unknown trans-acting factors and to determine how these translational regulatory elements may be dynamically regulated within the cell or across the developing organism. Recent technological advances may aid in this endeavour, such as using ribosome profiling to measure start codon usage or translation complex profile sequencing (TCP-seq186), a related technique, to examine the dynamics of ribosomal scanning in the 5′ UTR.
While RPL38/eL38 and the Hox gene IRESs provide an intriguing paradigm for ribosomal regulation of translation, an outstanding question in the field is what features of the transcribed genome create this rich regulatory landscape for fine-tuned control of gene expression. One possibility is that IRESs represent a widespread cis element for translation regulation by specialized ribosomes. In fact, multiple RPs have been shown to regulate IRES elements, including RPL38/eL38, RPS25/eS25 and RPL10a/uL1, as discussed above. Other RPs that also regulate IRES elements are RPL11/uL5 and RPS19/eS19, whose knockdown in mouse erythroblasts reduces expression of Bag1 and Csde1 by decreasing their cellular IRES activity84. RACK1 has also been shown to mediate translation of the CrPV IRES in Drosophila melanogaster S2 cells and the HCV IRES in a human cell line85. Interestingly, while RACK1 depletion does not affect survival, proliferation or global cap-dependent initiation in cell culture, homozygous deletion of RACK1 in D. melanogaster and mice is embryonic lethal86,87. The heterozygous mouse is viable but has pigmentation defects, suggesting that RACK1 has additional specialized roles in embryonic development, although whether this is mediated by IRES elements in cellular mRNAs is not yet known.
Other cis-regulatory RNA motifs in addition to IRESs seem to interface with trans factors on the ribosome. For instance, RPL40/eL40 is required in human cell lines for the translation of vesicular stomatitis virus (VSV) mRNAs which, unlike IRES-reliant RNA viruses, are capped and adenylated identically to cellular mRNAs88. RPL40/eL40 activates translation of VSV mRNAs in a cap-dependent manner; which cis-regulatory RNA motifs enable this recognition is unknown. Interestingly, RPL40/eL40 regulates translation of specific stress-responsive transcripts in yeast, and if this activity is conserved in mammals, it may represent an endogenous specialized translation function for RPL40/eL40. Additionally, there are examples of RPs with negative functions on target mRNA expression, such as RPL22/eL22. Despite being 73% identical in amino acid sequence, RPL22/eL22 and its paralogue RPL22L1/eL22L1 have opposing and sequential roles in T cell differentiation in zebrafish and mice. RPL22L1/eL22L1 is required for the formation of haematopoietic stem cells and the seeding of the thymus; RPL22/eL22 is needed subsequently for the development of αβ T cells29,78. This switch is mediated by competition between RPL22/eL22 and RPL22L1/eL22L1 for binding to an RNA motif89 in the key haematopoiesis regulator smad1 5′ UTR: Rpl22l1/eL22l1 binding permits smad1 expression, allowing haematopoiesis to occur, whereas Rpl22/eL22 represses smad1 translation to promote further development29. As mentioned previously, this is compounded by a direct antagonistic relationship between the paralogues, whereby RPL22/eL22 can repress expression of RPL22L1/eL22L1 (REF.55) (FIG. 2b). Further research will be required to determine whether these activities take place on the ribosome, but this phenomenon represents an interesting paradigm for regulation of gene expression by RP paralogues.
Finally, global protein synthesis defects have been proposed to have more selective effects on specific mRNA transcripts80. Mutations in several RPs that cause DBA decrease protein production, which might suggest that their common phenotype — anaemia — is an effect of global downregulation of synthesis, whereas their divergent symptoms — such as specific congenital birth defects — are due to the loss of more specific RP functions. Indeed, the haematopoietic system seems particularly sensitive to changes in translation efficiency, as genetic manipulations to increase or decrease global protein synthesis rates in haematopoietic stem cells impair cell survival and differentiation17. One explanation for this phenomenon is the principle that certain mRNAs may be tuned to particular translation rates. A proposed example of this is the gene GATA1, an important regulator of erythropoiesis with decreased expression in patients with DBA, whose 5′ UTR is highly structured. While there are no known specific regulatory motifs in this 5′ UTR, the strong secondary structure in general may impede translation initiation, thereby causing the gene to rely on the cell having highly efficient translation machinery for this protein to be successfully translated90. In this way, a general mutation effect — global protein synthesis changes — may have specific effects on particular mRNAs in certain cell types, but more examples are required to determine whether this is a common phenomenon.
Specialized functions of ribosome-associated proteins.
In addition to the core RPs, many other proteins can be part of the ribosome. A recent study from our lab developed an affinity purification methodology to isolate ribosomes and performed mass spectrometry to identify hundreds of RAPs in mES cells36. The list of identified RAPs is likely not exhaustive, as additional RAPs might be specific to certain cell types or cell states, and some RAPs might be only loosely associated with the ribosome and thus not identified by these assays. Nonetheless, the identified RAPs already include a wide array of protein-modifying and RNA-modifying enzymes, metabolism enzymes and cell redox homeostasis pathway members. These proteins not only connect the ribosome to other important cell signalling pathways but also may enable the ribosome to translate specific mRNAs. For instance, the protein-modifying RAPs include kinases, ubiquitin ligases and methyltransferases, which may modify RPs on the ribosome to alter their activity. Interestingly, one of the identified RAPs is UFL1, a protein required for the recently identified PTM ufmylation (UFM1)91,92, which was shown to modify specific RPs36 (BOX 2). It is interesting to speculate that RP modifications could alter ribosome interactions with specific mRNAs, as one of the ubiquitylated and ufmylated RPs, RPS3/uS3, has a role in start codon selection in yeast93.
In addition, several of the identified RAPs are known to directly activate or repress translation of specific transcripts. For instance, fragile X mental retardation protein (FMRP), the loss of function of which causes fragile X syndrome, associates with polysomes in the mammalian brain. FMRP directly binds to the coding sequences of several hundred mRNAs associated with synaptic signalling pathways and autism spectrum disorders and stalls translation of these transcripts94,95. How FMRP recognizes its target mRNAs and stalls the 80S ribosome is not yet fully understood; a number of mRNA sequence and structural motifs have been suggested, including G-quadruplexes, which FMRP is capable of binding in vitro96, but convincing validation has been lacking. It is possible that the binding sites contain more subtle sequence, structure or RNA modification patterns that have yet to be identified, or that additional protein cofactors are involved in the mRNA selectivity of FMRP. On the activation side, the RAPs include several ‘readers’ of N6-methyladenosine (m6A) modifications on mRNA, which can allow the mRNA to be translated by a cap-independent mechanism97. These proteins may specialize their associated ribosomes for this specific type of translation initiation, although direct evidence for this regulation on the ribosome remains to be established. In addition, several metabolism enzymes were identified as RAPs. Such enzymes have been suggested to be capable of binding mRNA98, and one of these RAPs, PKM2, was shown to bind ER-associated mRNA transcripts to promote their translation36 (FIG. 2f). PKM2 itself is enriched on ER-associated ribosomes relative to cytoplasmic ribosomes, raising the intriguing question of whether more RAPs could be localized on ribosomes in particular cellular compartments, providing specialized spatial regulation to their translation functions.
With each identification of a new RAP, more questions arise. Analysis of each RAP individually is required to assess its in vivo function, but the evidence already present from FMRP, PKM2 and others suggests that these proteins are major regulators of translation. Even for RAPs that have been previously investigated, more work remains to determine whether their association with the ribosome is differentially regulated across cell types and subcellular localizations and how this could be accomplished mechanistically. Even at a basic biochemical level, much remains to be understood: for instance, what is the binding affinity for each of these RAPs to the ribosome? Could any of these high-affinity RAPs be considered core components of the ribosome? For more loosely bound RAPs, is there frequent exchange between ribosome-associated and cytoplasmic pools, and what is its importance for cellular function? Elucidation of these questions could revolutionize our understanding of the ribosome, revealing its role not only as an active regulator of translation but also as a key nexus of regulation within the cell.
Specialization in translation initiation factors
In addition to the ribosome, several other large protein complexes are required for efficient translation initiation (BOX 1). These initiation factors, similarly to the ribosome, are composed of multiple modules that work together to perform the initiation function of the complex as a whole. We propose in this Review that the subunits of these large complexes may have evolved individual functions to suit the intricate gene expression regulatory needs of metazoans. We discuss the emerging evidence of specialization in detail for the eIF3 and eIF4F initiation complexes below, with a focus on vertebrates.
eIF3: a modular and extensively modified translation initiation factor.
The mammalian eIF3 complex (FIG. 3a) is required for efficient cap-dependent translation initiation. eIF3 binds to the 40S ribosomal subunit and the scaffolding protein eIF4G, thereby bringing the 40S subunit to the 5′ cap, as well as to several other initiation factors that aid in proper subunit joining at the start codon99,100 (BOX 1). Additionally, eIF3 has been proposed to function in translation reinitiation after termination in yeast and in mammalian in vitro translation systems101–103, suggesting that it remains on elongating ribosomes during translation of the coding sequence.
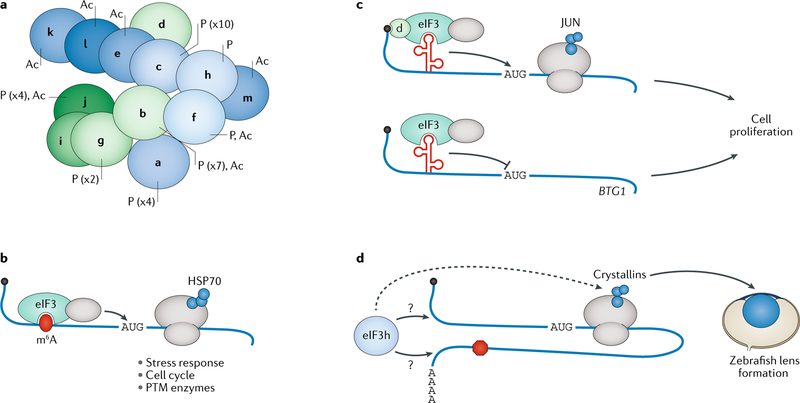
Fig. 3 |. eIF3 is required for translation of specific cellular mRNAs.
a | Schematic of the mammalian eIF3 complex, which consists of 13 subunits, 8 of which form a core octamer (blue), while the remaining 5 peripheral subunits (green) seem to be more conformationally flexible202. Post-translational modifications (PTMs) identified on individual subunits108 are labelled (P, phosphorylation; Ac, acetylation). b | eIF3 binds to N6-methyladenosine (m6A) modifications (red) in the 5′ untranslated regions (UTRs) of mRNAs involved in cell stress, including HSP70 (blue circles), and in additional pathways to promote recruitment of the 40S ribosomal subunit to the start codon (AUG). This process is cap-independent, but the precise mechanism is unknown. c | eIF3 regulates cellular proliferation by repressing expression of BTG1 and activating expression of JUN in a cap-dependent manner. Regulation of both of these genes requires eIF3 binding to a hairpin structure (in red) in their 5′ UTRs. In the case of JUN, the 5′ cap is additionally bound by eIF3 (via the eIF3d subunit) instead of the canonical eIF4F cap-binding complex. d | In zebrafish, eIF3h regulates translation of crystallin proteins (blue circles), which are required for proper development of the eye lens. Whether eIF3h makes direct contact with these mRNAs is unknown, but both the 5′ UTR and the 3′ UTR are required for eIF3h-mediated translational activation.
The eIF3 complex is conserved across eukaryotes but has dramatically increased in size in metazoans. Of the 13 mammalian eIF3 subunits, only 6 are conserved in S. cerevisiae104, suggesting that more specialized functions have arisen during evolution. Interestingly, several eIF3 subunits seem to be dispensable for global cap-dependent initiation105,106, suggesting that they have evolved more specialized roles in translation and in fact may not need to be present on all eIF3 complexes. Indeed, the modular makeup of eIF3 is suggestive of a regulated ability of subunits to dissociate from the larger complex. One subunit in particular, eIF3j, is only loosely associated with the eIF3 complex and has been suggested to regulate start codon selection107. Future studies will be required to measure the exact stoichiometry of the other eIF3 subunits on the 43S preinitiation complex (PIC) in specific cell types and environments.
The eIF3 subunits are extensively modified, with 29 phosphorylation sites, several acetylations and multiple amino-terminal methionine cleavages identified by mass spectrometry on eIF3 extracted from HeLa cells108 (FIG. 3a). Importantly, several phosphorylation sites are substoichiometric (18–36% abundance), which may produce functional as well as physical heterogeneity of eIF3 complexes109,110. Which enzymes modify the eIF3 subunits, whether these PTMs are dynamically regulated within a cell or across tissues and whether these PTMs specialize eIF3 for the translation of specific mRNAs are crucial open questions in the field.
eIF3 specialization from cells to organisms.
Similarly to RPs, eIF3 may serve to regulate the translation of specific networks of mRNAs via cis-regulatory elements in their 5′ UTRs. In support of this model, several genes have been shown to rely on different subunits of eIF3 for their expression. These include several viral IRESs111–113 and a distinct, recently described mechanism of translation initiation on mRNAs containing the m6A modification in their 5′ UTRs. m6A is found in GAC motifs across the 5′ UTR, 3′ UTR and coding sequence of hundreds of genes. When in the 5′ UTR, even one single m6A base is sufficient to stimulate cap-independent translation of a transcript, and this activity requires binding of eIF3 to the m6A residue97 (FIG. 3b). How eIF3 recognizes m6A is not fully understood; it does require the full GAC motif, but whether there is a broader sequence or structural context or whether specific eIF3 subunits are needed is not clear. m6A is a dynamic RNA modification, and changing the expression levels of its methyltransferase or demethylase is sufficient to alter levels of m6A on 5′ UTRs and, consequently, the translation efficiency of the transcripts97. Intriguingly, in human cell lines under specific cellular stresses, such as ultraviolet treatment or heat shock, m6A 5′ UTR levels increase on genes belonging to specific cellular pathways, such as cell cycle regulation97. This suggests that m6A is a means of retaining active translation on particular transcripts during cellular stress, when canonical cap-dependent mechanisms are downregulated.
In addition to its cap-independent roles, eIF3 is required for the translation of specific cellular mRNAs, such as JUN, via a cap-dependent mechanism (FIG. 3c). In photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP) experiments performed in 293T cells, eIF3a, eIF3b, eIF3d and eIF3g bind to a region of the JUN 5′ UTR that contains a stem-loop structure, which, when deleted or mutated, abrogates gene expression114. Whether this regulation is dependent on the 5′ UTR sequence or the hairpin structure is not well established, as the researchers did not make complementary mutations to alter sequence but restore secondary structure. Similarly, the sufficiency of this motif was not investigated by placing the sequence in a 5′ UTR not known to be regulated by eIF3. Nonetheless, this represents a novel form of translational regulation. Indeed, recognition of the JUN 5′ cap seems to rely not on eIF4F, the canonical cap-binding complex, but instead on eIF3d115. eIF3 had been previously uncovered in crosslinking experiments to bind to the 5′ cap116,117, albeit with no previously known function or specificity. The mechanism allowing eIF3d to specifically recognize the 5′ cap of JUN and not that of other mRNAs is unclear.
Another open question is whether this regulation is unique to JUN or is more widespread throughout the genome. Cap-dependent translation of BTG1 mRNA, which was also bound by four eIF3 (a, b, d and g) subunits in PAR-CLIP experiments, in fact seems to be negatively regulated by eIF3114, suggesting that eIF3 has opposing effects on gene expression, possibly mediated by additional cofactors (FIG. 3c). An additional several hundred mRNAs were identified as being bound by one or a combination of the eIF3 subunits, predominantly via the 5′ UTR, but further functional characterization is required. As many of these mRNAs are associated with the regulation of cell growth, it is possible that changes in eIF3 activity control cancer development. Indeed, overexpression of eIF3a, eIF3b, eIF3c, eIF3h or eIF3i promotes transformation of NIH3T3 cells118, and overexpression or underexpression of eight eIF3 subunits is associated with a variety of human cancers (reviewed in REF.119).
eIF3 may also have important regulatory roles in vertebrate development. eIF3h regulates the translation of a specific subset of genes in zebrafish embryos. Knockdown of eIF3ha in zebrafish embryos using two distinct morpholinos resulted in largely normal development, except for severe brain and eye defects120 (FIG. 3d). This is in contrast to both control morpholinos, which exhibited no phenotype, and to morpholinos targeting eIF3c, which caused general degeneration and early lethality120. eIF3ha depletion did not cause global translation inhibition, unlike eIF3c morphants, but did cause decreased translation of specific mRNAs121. Compellingly, these genes included a subfamily of crystallin proteins (key components of the lens), although the mechanism behind this regulation has not yet been fully elucidated. eIF3h has been implicated in reinitiation on transcripts containing uORFs in Arabidopsis thaliana122,123, but whether reinitiation is compromised in eIF3ha-depleted zebrafish or in the more recently published but phenotypically uncharacterized eIF3h knockout mouse124 has yet to be shown.
The diversity of mRNAs dependent on eIF3 for their translation, and the variety of mechanisms this specialization entails, suggests a complex network of protein–mRNA interactions to recruit and activate eIF3 for translation initiation. Further work is required to define these interactions at the structural level and to expand this regulation to a genomic context. It will also be important to define the dynamics of this novel form of translational regulation across standard and stressed growth conditions, as well as across development and evolution.
eIF4F heterogeneity during evolution.
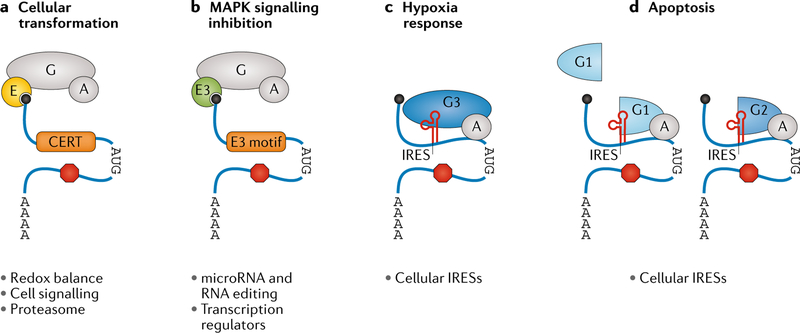
The eIF4F complex, which brings the 43S preinitiation complex to the 5′ cap (BOX 1), may also allow for specialized translation initiation in different cell types and cell states. eIF4F is a highly conserved heterotrimeric complex containing eIF4E, eIF4A and eIF4G. eIF4E directly binds the 5′ cap, while eIF4A is a helicase that may help unwind the 5′ UTR. eIF4G is a scaffolding protein that binds to eIF4E and eIF4A, as well as to other initiation factors. Each of these components is found across eukaryotes, but multiple homologous genes can be expressed within a species. For instance, in trypanosomatids, a family of pathogenic protozoa that cause diseases such as sleeping sickness, there are four eIF4E homologues, two eIF4A homologues and five eIF4G homologues, several of which have verified roles in translation125–127. Mammals contain three known variants for each eIF4F component: eIF4E1–eIF4E13, eIF4A1–eIF4A13, and eIF4G1, eIF4G2 (also called p97 or DAP5) and eIF4G3. The eIF4A homologues are not far diverged (85% similarity in humans), whereas the eIF4E and eIF4G variants have more substantial differences (approximately 40% and 30% similarity, respectively). Of the eIF4E homologues, eIF4E1 is conserved across eukaryotes, eIF4E2 (also known as 4E-HP) is metazoan-specific, and eIF4E3 is vertebrate-specific, suggesting that these homologues have developed specialized roles during evolution128. The canonical mammalian eIF4F complex contains eIF4G1, eIF4E1 and eIF4A1, but other homologues can be incorporated in distinct combinations. For instance, eIF4G1 is capable of binding both eIF4E1 and eIF4E3, whereas eIF4G3 complexes with eIF4E2 (REFS128,129). eIF4G2, on the other hand, does not bind eIF4E and thus likely acts in a cap-independent manner130. Within a single human cell line, multiple homologues can be co-expressed and incorporated into eIF4F complexes129,131. Additionally, several subunit homologues have tissue-specific expression patterns at the mRNA level in mammals128,132, suggesting that different cell types contain distinct sets of eIF4F complexes. The regulation of eIF4F homologues may also differ: for instance, eIF4E activity is canonically regulated by the binding of eIF4E binding proteins (4E-BPs), which prevent eIF4E from associating with eIF4G. 4E-BP activity is in turn inhibited by mTOR signalling, allowing translation to be regulated by growth factors and nutrient abundance. However, the 4E-BPs bind to the eIF4E1 homologues with varying affinities128,133. Finally, eIF4F subunit composition seems to change in cancerous cells and under specific cellular stimuli, such as hypoxia and mitogenic signalling, creating specialized initiation programmes for each cell state (see below)134. Although these findings suggest that both intracellular and intercellular eIF4F heterogeneity exist, more proteomic work is required to determine the stoichiometry of each component and to establish a protein-level map of eIF4F composition across cell types and cell states.
To add further complexity to this system, the eIF4F complex is extensively post-translationally modified. eIF4E is phosphorylated by the kinases MNK1 and MNK2 during MAPK pathway activation (see below)135, and eIF4G contains up to 20 known phosphorylation sites in HeLa cells, of which 14 have calculated stoichiometries (all within the range of less than 5% to 75%)110. Although the function and dynamic regulation of most of these phosphorylation sites are unknown, one eIF4G phosphorylation site regulates the binding of MNK kinases to the complex136, and thereby also the phosphorylation of eIF4E. eIF4G also has a number of phosphorylation sites of unknown functional importance that respond dynamically to serum treatment in culture137 and to the cell cycle138. eIF4E and eIF4G are also sumoylated, though the exact roles of these modifications are not yet fully understood139,140. These findings suggest that PTMs form a complex array of interactions that further modulate eIF4F composition as well as function.
eIF4F complexes in translation control and cellular responses to stimuli.
At the organismal level, different eIF4F subunits seem to be required for the development of specific tissues, particularly gametogenesis. In mice, a homozygous missense mutation in eIF4G3 causes meiotic arrest during spermatogenesis, likely owing to decreased translation of a critical chaperone141. Spermatogenesis in D. melanogaster also seems to rely on particular eIF4G isoforms142, and loss of two eIF4G isoforms in Caenorhabditis elegans impairs oogenesis but via distinct mechanisms for each isoform143. Similarly, specific eIF4E isoforms are required for spermatogenesis and oogenesis in D. melanogaster142,144. However, Eif4e2 knockout in mice results in smaller body size, unexpanded lungs and perinatal lethality145. In somatic tissues, eIF4G has also been proposed to regulate differentiation: as human haematopoietic stem cells differentiate into megakaryocytes, eIF4G3 rapidly increases in abundance in the eIF4F complex131. Even in the unicellular trypanosomatids, different eIF4E homologues seem to be required at different stages of the parasites’ life cycle. The effects of this change in eIF4F composition on gene expression are not known, nor the generality of this model to cell differentiation overall.
Canonical eIF4E has historically been considered one of the rate-limiting components of cap-dependent translation initiation146; however, Eif4e heterozygous mice with a 50% reduction in eIF4E protein have no apparent phenotype and normal translation of both cap-dependent and cap-independent transcripts147, suggesting that eIF4E is expressed in abundance of the actual cellular need. As the Eif4e heterozygote mouse embryonic fibroblasts are resistant to cellular transformation, eIF4E level reduction may have an impact on select transcripts required for cancer development. In fact, a small group of genes critical for cellular transformation or tumour cell survival are sensitive to reduction in eIF4E levels via a sequence motif in their 5′ UTRs, termed the cytosine-enriched regulator of translation (CERT) domain147 (BOX 3; FIG. 4a). More work is needed to determine the sufficiency of the CERT domain for this novel type of translational regulation and the exact mechanism connecting this 5′ UTR sequence element with eIF4E; nonetheless, this is an exciting example of a translation initiation factor specialized for the expression of particular genes in certain cellular contexts.
Fig. 4 |. eIF4F specialization is regulated by cellular stimuli.
a | eIF4E promotes translation of mRNAs containing the cytosine-enriched regulator of translation (CERT) element in their 5′ untranslated regions (UTRs). These mRNAs encode genes involved in multiple genetic networks that promote tumour development. b | Upon MAPK signalling inhibition, the eIF4E3 homologue is incorporated into eIF4F and promotes translation of mRNAs encoding RNA editing and transcription regulators with particular sequence motifs in their 5′ UTRs. c | During hypoxia, when cap-dependent translation initiation is downregulated, eIF4F complexes containing eIF4G3 initiate translation of mRNAs containing internal ribosome entry site (IRESs) in their 5′ UTRs. d | During apoptosis, eIF4G1 is cleaved by caspases into several fragments, each containing different initiation factor binding domains. While this effectively eliminates formation of the preinitiation complexes required for cap-dependent translation initiation, the eIF4G1 fragment that retains the binding domains for eIF4A and eIF3 can initiate translation at cellular IRESs. eIF4G2, which is homologous to this eIF4G1 fragment, can similarly activate cellular IRES translation initiation during apoptosis after cleavage by caspases removes an inhibitory domain.
Intriguingly, the CERT domain is not the only 5′ UTR cis-regulatory element associated with eIF4E activity. In a human prostate cancer cell line with constitutively active mTOR signalling, over 100 target genes were found to be translationally activated as a consequence of eIF4E hyperactivation, including many known regulators of cell proliferation and metastasis. Approximately two-thirds of these genes contained a pyrimidine-rich translational element (PRTE) in their 5′ UTRs (BOX 3), and this PRTE domain was necessary for eIF4E-mediated translational regulation148. Another frequent 5′ UTR element was the 5′ terminal oligopyrimidine tract (5′ TOP), which is in fact present on most of the mRNAs encoding most of the translational machinery and is viewed as a mechanism to coordinately regulate their expression in response to nutrient availability149. While the 5′ TOP is known to be regulated by mTOR signalling, the connection specifically to eIF4E is yet to be determined.
In addition to canonical eIF4E1, the other eIF4E homologues have stimulus-specific translation functions. Upon MAPK signalling, which coordinates the cellular response to growth factors and other stimuli, the kinase MNK regulates the balance between eIF4F complexes containing eIF4E1 or eIF4E3 in several cancer cell lines129. Phosphorylation of eIF4E1 at serine 209 by MNK results in downregulation of eIF4E3 levels, leading to predominantly eIF4E1-containing eIF4F initiation complexes. Upon inhibition of MNK kinase activity, eIF4E3 increases in abundance. While eIF4E3 recognizes the 5′ cap using a distinct mechanism from eIF4E1 (REF.150), eIF4E3 still interacts with eIF4G and eIF4A129, suggesting that it forms functional eIF4F complexes. In fact, overexpression of eIF4E1 or eIF4E3 increased the translation of distinct subsets of mRNAs, suggesting that they programme the eIF4F complexes to activate translation of specific transcripts, though more validation at physiological levels of eIF4E1 and eIF4E3 is needed (FIG. 4b). These eIF4E1-specific and eIF4E3-specific mRNAs seem to have distinct sequence motifs in their 5′ UTRs, but the necessity and sufficiency of these cis RNA features, as well as how the mechanism underlying their specificity for a particular eIF4F complex, are unknown. The functional role of the eIF4E3-specific gene expression programme requires further investigation as well: eIF4E3 can act as a tumour suppressor in vitro150, but its in vivo role has not yet been elucidated. Interestingly, mice lacking MNK kinases151 or harbouring a non-phosphorylatable eIF4E1 knock-in allele seem to develop normally but do show decreased tumorigenesis152 and altered pain sensitivity153. These findings suggest that eIF4E1-specific and eIF4E3-specific regulation of translation contribute to cellular function and cancer progression.
Another stimulus-specific eIF4F complex important for cancer progression is induced under hypoxic conditions. Hypoxic tumour cells are able to survive by adapting their metabolism and producing pro-angiogenic factors, both of which require remodelling of the gene expression programmes of the cells. However, cap-dependent translation is downregulated during hypoxia via increased activity of 4E-BPs, which prevent the association of eIF4E with eIF4G154. While eIF4E1 is potently repressed by 4E-BPs, eIF4E2 escapes their inhibition, likely owing to their lower binding affinity133 and is the predominant cap-binding eIF4E homologue in human cell lines grown in low oxygen conditions155. Interestingly, the hypoxic eIF4F complex contains not only eIF4E2 but also a noncanonical eIF4G homologue, eIF4G3. eIF4G3 but not eIF4G1 is required for hypoxic translation156. eIF4G3 aids in cap-independent translation initiation, which is upregulated in hypoxic conditions (FIG. 4c). In fact, in hypoxic breast cancer models, eIF4G expression increases, resulting in more translation initiation at the cellular IRESs of HIF1α, a master regulator of hypoxic gene expression, and VEGFA, which promotes angiogenesis157. Taken together, eIF4E2, eIF4G3 and eIF4A may create a hypoxia-specific eIF4F complex, enabling reprogramming of translation to promote expression of genes, demarcated by cis-regulatory mRNA elements that are required for the cellular response to hypoxic stimuli.
In addition to hypoxia, eIF4G homologues are required for IRES-mediated translation during apoptosis. In apoptosis, global cap-dependent translation is inhibited, while pro-apoptotic proteins remain expressed via IRES elements. To accomplish this, caspases cleave eIF4G to separate the regions that bind to the other initiation factors, thereby preventing formation of the 48S preinitiation complex158–160 (FIG. 4d). Cellular IRESs, however, can successfully initiate translation with the truncated eIF4G161. These cleavage events can additionally occur in eIF4G2, which also leads to translation initiation on cellular IRES elements161,162 (FIG. 4d). In fact, eIF4G2 is a more potent activator of the MYC, XIAP and IAP1 IRESs in HeLa cell extracts than eIF4G1163 and can activate the IRES within its own 5′ UTR to create a positive feedback loop162,163. Despite these similarities, eIF4G1 and eIF4G2 are not fully interchangeable. While knockdown of either eIF4G1 or eIF4G2 with small interfering RNA (siRNA) in cell culture moderately decreased protein synthesis and cell proliferation rates, eIF4G2 overexpression rescued only the eIF4G2 knockdown phenotype and had no effect on eIF4G1-depleted cells164.
Finally, while we hypothesize that specific eIF4F complexes are required for the regulation of gene expression in particular cell states, these eIF4F homologues might also ‘moonlight’ as members of other protein complexes in the cell. One such example is a complex formed by eIF4E2, GIGYF2 and ZNF598 that can repress translation145. Both mouse embryos lacking eIF4E2 and HeLa cells with eIF4E2 knocked down have increased protein synthesis, suggesting that eIF4E2 regulates the translation of many genes or a small number of abundant mRNAs that are highly translated in the absence eIF4E2. Specificity for particular mRNAs could be facilitated by ZNF598, an E3 ubiquitin ligase and zinc-finger protein that can bind to RNA and add regulatory ubiquitin moieties to several ribosomal proteins165. Intriguingly, eIF4E2 can interact with GIGYF2 while binding the 5′ cap, suggesting that eIF4E2–GIGYF2–ZNF598 serves as an alternative, repressive cap-binding complex.
Taken together, these findings suggest a role for eIF4F as a signalling hub, linking mRNA translation to such crucial cellular cues as oxygen availability and mitogen activity, and reveal the power of the translational machinery to tune genetic output to fit the needs of the cell. The fact that this complex is critical for tumour development is consistent with the observations that increased mitogen signalling and the ability to tolerate hypoxia are hallmarks of many cancers (reviewed in REFS166–168). Further work into the mechanisms of the regulation of eIF4F composition, its specificity for particular mRNAs and its importance for normal organismal development will hopefully clarify how this translational regulation has evolved and reveal therapeutic strategies to selectively perturb eIF4F oncogenic activity in cancer cells (reviewed in REF.169).
Perspectives
The concept of gene expression regulation by specialized functions of heterogeneous translational machinery is growing in importance. The ribosome, eIF3 and eIF4F not only serve general translation functions but also have components required for the translation of specific transcripts. This model of large multisubunit complexes with both general and specialized activities is not unique to translation: it is analogous to how the Mediator complex, a global regulator of transcription, can activate expression of particular genes through changes in its composition and activity170. It is interesting to speculate that the large size of these complexes, particularly when compared with their considerably smaller prokaryotic or yeast counterparts, may have evolved to allow orchestrated expression of the metazoan genome.
While great advancements have been made, future work in the field will need to broaden the map of heterogeneity and specialization of the translation machinery to encompass more cell types and stimuli, as well as to more carefully examine protein stoichiometry and PTMs. Relative and absolute quantification mass spectrometry are excellent approaches to answer these questions, coupled with existing mass spectrometry strategies to identify specific PTMs (FIG. 1). As omics technologies improve, it may also be possible to examine ribosome and initiation factor heterogeneity within a single cell. Single-cell RNA-seq is already well established and has been used to great effect171,172, but no analogous proteomics method has been developed; until it is, techniques such as single-cell western blotting173, mass cytometry174 and high-resolution imaging may be able to begin examining expression level differences in translational machinery components (though not necessarily incorporation into functional translation complexes) across individual cells. It is possible to measure global protein synthesis rates at a single-cell level by using O-propargylpuromycin (OP-puro), a small molecule that covalently attaches to synthesizing peptides and can be conjugated to fluorophores, combined with flow cytometry. This has been successfully employed to compare translation rates across haematopoietic lineages and has revealed clear differences in protein synthesis between haematopoietic stem cells and terminally differentiated cells17. However, methods to measure the translation efficiency of individual mRNAs, such as polysome profiling or ribosome profiling, have yet to be adapted for single cells.
Another frontier for the study of translation machinery composition is the question of subcellular heterogeneity. Localized translation of specific transcripts is critical for many cells, from neurons to the intestinal epithelium175, and it is possible that translation machinery of specific compositions may reside in distinct subcellular regions to facilitate this process. Laser microdissection is sufficient to isolate large cellular regions in highly polarized cells175, but more finesse is required for smaller or less rigidly bounded areas. Proximity labelling, which has been successfully used to characterize ER-bound ribosomes36, can be readily applied to many cellular structures, as can recently published techniques for live imaging of translation176–178. Solving the structures of heterogeneous translation complexes using, for example, cryo-electron microscopy techniques coupled with biotin-streptavidin pull-downs179, will also be invaluable to the field. The application of these techniques will hopefully produce a multidimensional atlas of translation machinery composition spanning subcellular space, tissue types, growth conditions and developmental time.
Importantly, heterogeneity must be connected to function. The field is at an exciting point where important examples of specialized regulation of gene expression are mounting, but the mechanisms underlying many of them remain a mystery. Functional characterization will require the identification of not just cis-regulatory elements in the mRNAs translated by specialized translation machinery but also the mechanisms by which trans-acting factors bind these regulons (if not the translation machinery directly), the prevalence of the motifs across the genome, and the sequence and structural features required for function. It is also likely that mRNAs contain multiple cis regulons that work in tandem (as in the case of Hox IRESs and TIEs) or combinatorially or are active in distinct environments or cell types; accordingly, studies performed on elements only in isolation from the rest of the mRNA or in one cell type may miss dynamic aspects of this activity. Similarly, genetic evidence from mouse models or human disease needs to carefully ascribe phenotypes to specific molecular causes. This includes determining whether a developmental defect is due to a specialized function of the mutated gene or is a product of nucleolar stress82, as well as improved methods of perturbing ribosome and initiation factor composition. An example would be mice with conditional deletions induced at particular times or tissue types for genes whose organism-wide heterozygous deletion is embryonic lethal. In this way, we will be able not only to map the intricate relationships between the ribosome, initiation factors and mRNA but also to understand their importance from cells to developing organisms.
Finally, we lack a comprehensive understanding of how heterogeneity and specialization of the translational machinery are regulated. Heterogeneity may originate via changes in the expression or incorporation of a given component at multiple steps during the assembly of these multisubunit complexes or via PTMs — these possibilities must be evaluated to identify what upstream pathways control these processes and how these are in turn regulated in each individual cellular environment. Once these regulatory pathways are identified, they can be perturbed to alter ribosome and initiation complex functionality. This approach would provide a useful framework not only to investigate the roles of specialized translation but also to develop synthetic biology tools and therapeutic applications. For example, customized ribosomes and eIF3 and eIF4 complexes could be tailored to optimize the production of particular proteins. Small molecules targeting specialized translation machinery components may also be effective treatments for ribosomopathies and cancers associated with translation misregulation169. These directions will likely bring heterogeneity and specialization of translational machinery to the forefront of science and medicine, revealing novel forms of gene regulation crucial for organismal development and human disease.
Ribosome
The macromolecular machine that synthesizes proteins, consisting of a large (60S) and a small (40S) subunit that come together to form the translationally active eukaryotic 80S ribosome.
Start codon
The codon where translation is initiated, encoding the first amino acid of the peptide; typically an AUG encoding methionine.
Kozak sequence
The optimal sequence context for a start codon.
Open reading frame
(ORF). A region of mRNA, beginning with a start codon and ending with a stop codon, that has the capability of being translated into a peptide.
Core ribosomal protein
(RP). one of the 80 canonical RPs, which were originally identified by their high-affinity association with the ribosome.
Ribosomal RNA
(rRNA). The RNA components of the ribosome. Three eukaryotic rRNAs (5S, 5.8S and 28S) are in the large ribosomal subunit, while one (18S) is in the small subunit.
Expansion segments
Sections of ribosomal RNA (rRNA) that are longer in eukaryotes relative to prokaryotes. Many of these expansion segments also show growth between unicellular and multicellular organisms.
Untranslated region
(UTR). The portions of the mature mRNA that do not encode a protein. The sequence before the start codon is the 5′ UTR, while the section after the stop codon is the 3′ UTR.
Paralogues
Genes, typically created by a duplication event, with high homology to another gene within the same species.
Stoichiometry
The quantity of each constituent of a complex. if a subunit is not present on every complex, it is considered substoichiometric.
Polysomes
Multiple ribosomes that are translating the same mRNA transcript.
Proximity labelling
A technique to identify proteins in a subcellular region of interest. A promiscuous biotinylating enzyme is localized to a particular organelle or fused to a protein of interest and labels any proteins in its vicinity with biotin. Streptavidin pull-down and mass spectrometry can be used to identify these protein interactors.
Pseudogenes
A paralogue that can no longer be functionally expressed.
Internal ribosome entry sites
(iReSs). Regions in mRNA with the capability of initiating translation by recruiting translation machinery independently of a 5′ cap.
Ribosomopathies
Human diseases caused by ribosomal defects.
Diamond–Blackfan anaemia
(DBA). A common ribosomopathy caused by mutations in ribosomal proteins that results in anaemia and a variety of congenital birth defects.
Haploinsufficiency
When the expression of a gene from a single functional allele is not sufficient for normal cellular function.
5′ cap
A methylated guanine that is present at every 5′ end of all eukaryotic transcripts.
Translation efficiency
A measure of the rate of translation for a particular transcript of interest; typically calculated by normalizing ribosome occupancy to mRNA abundance.
Preinitiation complex
(PiC). The ribosome and associated initiation factors that come together at specific stages of translation initiation. The eukaryotic 43S PiC contains the ribosome, eif3, eif2, eif1, eif1A and the initiator tRNA; the 48S PiC has the addition of eIF4F.
Acknowledgements
The authors thank members of the Barna laboratory for thoughtful comments on this work. This work was supported by the New York Stem Cell Foundation NYSCF-R-I36 (M.B.), National Institutes of Health grant 1R01HD086634 (M.B.) and Pew Scholars Award (M.B.). N.R.G. is supported by National Science Foundation Graduate Research Fellowship DGE-114747. M.B. is a New York Stem Cell Foundation Robertson Investigator.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kong J & Lasko P Translational control in cellular and developmental processes. Nat. Rev. Genet 13, 383–394 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Sonenberg N & Hinnebusch AG Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136, 731–745 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis D, Lehmann R & Zamore P Translational regulation in development. Cell 81, 171–178 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Schwanhausser B et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Beyer A & Aebersold R On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535–550 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Fortelny N, Overall CM, Pavlidis P & Freue GVC Can we predict protein from mRNA levels? Nature 547, E19–E20 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Floor SN & Doudna JA Tunable protein synthesis by transcript isoforms in human cells. eLife 5, e10921 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingolia NT Ribosome footprint profiling of translation throughout the genome. Cell 165, 22–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu TY et al. Time-resolved proteomics extends ribosome profiling-based measurements of protein synthesis dynamics. Cell Syst 4, 636–644 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jovanovic M et al. Dynamic profiling of the protein life cycle in response to pathogens. Science 347, 1259038 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan Z et al. Primate transcript and protein expression levels evolve under compensatory selection pressures. Science 342, 1100–1104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii K, Shi Z, Zhulyn O, Denans N & Barna M Pervasive translational regulation of the cell signalling circuitry underlies mammalian development. Nat. Commun 8, 14443 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondrashov N et al. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell 145, 383–397 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slaidina M & Lehmann R Translational control in germline stem cell development. J. Cell Biol 207, 13–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampath P et al. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell 2, 448–460 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Shalaby NA & Buszczak M Changes in rRNA transcription influence proliferation and cell fate within a stem cell lineage. Science 343, 298–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Signer RAJ, Magee JA, Salic A & Morrison SJ Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 509, 49–54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Z et al. Heterogeneous ribosomes preferentially translate distinct subpools of mRNAs genome-wide. Mol. Cell 67, 71–83 (2017).This study uses absolute quantification mass spectrometry techniques to calculate the stoichiometry of several RPs on the ribosome, revealing heterogeneity in ribosome composition within mES cells.
- 19.Crick FHC On protein synthesis. Symp. Soc. Exp. Biol 12, 138–163 (1958). [PubMed] [Google Scholar]
- 20.Crick FHC & Brenner S Some footnotes on protein synthesis: a note for the RNA Tie Club. Profiles in Medicine https://profiles.nlm.nih.gov/ps/access/SCBBFV.pdf (1959).
- 21.Brenner S & Crick FHC What are the properties of genetic RNA? A note for the RNA Tie Club. Profiles in Medicine https://profiles.nlm.nih.gov/ps/access/SCBBFZ.pdf (1960).
- 22.Jacob F & Monod J Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol 3, 318–356 (1961). [DOI] [PubMed] [Google Scholar]
- 23.Brenner S, Jacob F & Meselson M An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature 190, 576–581 (1961). [DOI] [PubMed] [Google Scholar]
- 24.Lamfrom H Factors determining the specificity of hemoglobin synthesized in a cell-free system. J. Mol. Biol 3, 241–252 (1961). [DOI] [PubMed] [Google Scholar]
- 25.Marygold SJ, Coelho CMA & Leevers SJ Genetic analysis of RpL38 and RpL5, two minute genes located in the centric heterochromatin of chromosome 2 of Drosophila melanogaster. Genetics 169, 683–695 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guimaraes JC & Zavolan M Patterns of ribosomal protein expression specify normal and malignant human cells. Genome Biol 17, 236 (2016).This study shows distinct RP expression signatures at the RNA level in particular cell types, most notably in the haematopoietic lineage. The observed patterns in RP expression may be regulated by cell type-specific transcription factors.
- 27.Ramagopal S Induction of cell-specific ribosomal proteins in aggregation-competent nonmorphogenetic Dictyostelium discoideum. Biochem. Cell Biol 68, 1281–1287 (1990). [DOI] [PubMed] [Google Scholar]
- 28.Lopes AM et al. The human RPS4 paralogue on Yq11.223 encodes a structurally conserved ribosomal protein and is preferentially expressed during spermatogenesis. BMC Mol. Biol 11, 33 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y et al. Control of hematopoietic stem cell emergence by antagonistic functions of ribosomal protein paralogs. Dev. Cell 24, 411–425 (2013).This study reveals distinct and antagonistic developmental functions for two RP paralogues, RPL22/eL22 and RPL22L1/eL22L1.
- 30.Xue S & Barna M Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat. Rev. Mol. Cell Biol 13, 355–369 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrgazov K, Vesper O & Moll I Ribosome heterogeneity: another level of complexity in bacterial translation regulation. Curr. Opin. Microbiol 16, 133–139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melnikov S et al. One core, two shells: bacterial and eukaryotic ribosomes. Nat. Struct. Mol. Biol 19, 560–567 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Noller HF, Hoffarth V & Zimniak L Unusual resistance of peptidyl transferase to protein extraction procedures. Science 256, 1416–1419 (1992). [DOI] [PubMed] [Google Scholar]
- 34.Anger AM et al. Structures of the human and Drosophila 80S ribosome. Nature 497, 80–85 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Leppek K, Das R & Barna M Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol 19, 158–174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simsek D et al. The mammalian ribo-interactome reveals ribosome functional diversity and heterogeneity. Cell 169, 1051–1065 (2017).This study uses quantitative mass spectrometry techniques to identify hundreds of RAPs.
- 37.Anderson L & Hunter CL Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteom 5, 573–588 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Picotti P & Aebersold R Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat. Methods 9, 555–566 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Addona TA et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat. Biotechnol 27, 633–641 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kusebauch U et al. Human SRMAtlas: a resource of targeted assays to quantify the complete human proteome. Cell 166, 766–778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson AC, Russell JD, Bailey DJ, Westphall MS & Coon JJ Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol. Cell. Proteom 11, 1475–1488 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van de Waterbeemd M et al. High-fidelity mass analysis unveils heterogeneity in intact ribosomal particles. Nat. Methods 14, 283–286 (2017).This study improves native mass spectrometry techniques to provide sufficient resolution to identify heterogeneity in prokaryotic ribosomes.
- 43.Slavov N, Semrau S, Airoldi E, Budnik B & van Oudenaarden A Differential stoichiometry among core ribosomal proteins. Cell Rep 13, 865–873 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta V & Warner JR Ribosome-omics of the human ribosome. RNA 20, 1004–1013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishii K et al. Characteristics and clustering of human ribosomal protein genes. BMC Genomics 7, 37 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bortoluzzi S, D’Alessi F, Romualdi C & Danieli GA Differential expression of genes coding for ribosomal proteins in different human tissues. Bioinformatics 17, 1152–1157 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Wong QW-L et al. RPL39L is an example of a recently evolved ribosomal protein paralog that shows highly specific tissue expression patterns and is upregulated in ESCs and HCC tumors. RNA Biol 11, 33–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Klerk E et al. Assessing the translational landscape of myogenic differentiation by ribosome profiling. Nucleic Acids Res 43, 4408–4428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugihara Y et al. Proteomic analysis of rodent ribosomes revealed heterogeneity including ribosomal proteins L10-like, L22-like 1, and L39-like. J. Proteome Res 9, 1351–1366 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Chaillou T, Zhang X & Mccarthy JJ Expression of muscle-specific ribosomal protein L3-like impairs myotube growth. J. Cell. Physiol 231, 1894–1902 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry RP The architecture of mammalian ribosomal protein promoters. BMC Evol. Biol 5, 15 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macias S, Bragulat M, Tardiff DF & Vilardell J L30 binds the nascent RPL30 transcript to repress U2 snRNP recruitment. Mol. Cell 30, 732–742 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Malygin AA, Parakhnevitch NM, Ivanov AV, Eperon IC & Karpova GG Human ribosomal protein S13 regulates expression of its own gene at the splicing step by a feedback mechanism. Nucleic Acids Res 35, 6414–6423 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivanov AV, Malygin A. a. & Karpova GG Human ribosomal protein S26 suppresses the splicing of its pre-mRNA. Biochim. Biophys. Acta 1727, 134–140 (2005). [DOI] [PubMed] [Google Scholar]
- 55.O’Leary MN et al. The ribosomal protein Rpl22 controls ribosome composition by directly repressing expression of its own paralog, Rpl22l1. PLoS Genet 9, e1003708 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reuveni S, Ehrenberg M & Paulsson J Ribosomes are optimized for autocatalytic production. Nature 547, 293–297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lam YW, Lamond AI, Mann M & Andersen JS Analysis of nucleolar protein dynamics reveals the nuclear degradation of ribosomal proteins. Curr. Biol 17, 749–760 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazumder B et al. Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell 115, 187–198 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Roundtree IA, Evans ME, Pan T & He C Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma S & Lafontaine DLJ ‘View from a bridge’: a new perspective on eukaryotic rRNA base modification. Trends Biochem. Sci 40, 560–575 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Mcmahon M, Contreras A & Ruggero D Small RNAs with big implications: new insights into H/ACA snoRNA function and their role in human disease. Wiley Interdiscip. Rev. RNA 6, 173–189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellodi C et al. H/ACA small RNA dysfunctions in disease reveal key roles for noncoding RNA modifications in hematopoietic stem cell differentiation. Cell Rep 3, 1493–1502 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon A et al. Impaired control of IRES-mediated translation in X-linked Dyskeratosis Congenita. Science 312, 902–907 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Locati MD et al. Expression of distinct maternal and somatic 5.8S, 18S, and 28S rRNA types during zebrafish development. RNA 23, 1188–1199 (2017).This paper reveals a switch in ribosome composition, from an embryonic-specific rRNA allele to a somatic rRNA with a different sequence, during zebrafish development.
- 65.Locati MD et al. Linking maternal and somatic 5S rRNA types with different sequence-specific non-LTR retrotransposons. RNA 23, 446–456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnheim N & Southern EM Heterogeneity of the ribosomal genes in mice and men. Cell 11, 363–370 (1977). [DOI] [PubMed] [Google Scholar]
- 67.Parks MM et al. Variant ribosomal RNA alleles are conserved and exhibit tissue-specific expression. Sci. Adv 4, eaao0665 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuo BA, Gonzalez IL, Gillespie DA & Sylvester JE Human ribosomal RNA variants from a single individual and their expression in different tissues. Nucleic Acids Res 24, 4817–4824 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landry DM, Hertz MI & Thompson SR RPS25 is essential for translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes Dev 23, 2753–2764 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hertz MI, Landry DM, Willis AE, Luo G & Thompson SR Ribosomal protein S25 dependency reveals a common mechanism for diverse internal ribosome entry sites and ribosome shunting. Mol. Cell. Biol 33, 1016–1026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quade N, Boehringer D, Leibundgut M, van den Heuvel J & Ban N Cryo-EM structure of Hepatitis C virus IRES bound to the human ribosome at 3.9-Å resolution. Nat. Commun 6, 7646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishiyama T, Yamamoto H, Uchiumi T & Nakashima N Eukaryotic ribosomal protein RPS25 interacts with the conserved loop region in a dicistroviral intergenic internal ribosome entry site. Nucleic Acids Res 35, 1514–1521 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boria I et al. The ribosomal basis of diamond-blackfan anemia: mutation and database update. Hum. Mutat 31, 1269–1279 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bolze A et al. Haploinsufficiency in humans with isolated congenital asplenia. Science 340, 976–978 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klauck SM et al. Mutations in the ribosomal protein gene RPL10 suggest a novel modulating disease mechanism for autism. Mol. Psychiatry 11, 1073–1084 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Zhou C et al. Mutation in ribosomal protein L21 underlies hereditary hypotrichosis simplex. Hum. Mutat 32, 710–714 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Oliver ER, Saunders TL, Tarle SA & Glaser T Ribosomal protein L24 defect in Belly spot and tail (Bst), a mouse Minute. Development 131, 3907–3920 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anderson SJ et al. Ablation of ribosomal protein L22 selectively impairs αβ T cell development by activation of a p53-dependent checkpoint. Immunity 26, 759–772 (2007). [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y et al. Ribosomal proteins Rpl22 and Rpl22l1 control morphogenesis by regulating pre-mRNA splicing. Cell Rep 18, 545–556 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mills EW & Green R Ribosomopathies: there’s strength in numbers. Science 358, eaan2755 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Brotherton TW, Chui DHK, McFarland EC & Russell ES Fetal erythropoiesis and hemoglobin ontogeny in tail-short (Ts/+) mutant mice. Blood 54, 673–683 (1979). [PubMed] [Google Scholar]
- 82.Zhang Y & Lu H Signaling to p53: ribosomal proteins find their way. Cancer Cell 16, 369–377 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xue S et al. RNA regulons in Hox 5′ UTRs confer ribosome specificity to gene regulation. Nature 517, 33–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horos R et al. Ribosomal deficiencies in Diamond-Blackfan anemia impair translation of transcripts essential for differentiation of murine and human erythroblasts. Blood 119, 262–272 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Majzoub K et al. RACK1 controls IRES-mediated translation of viruses. Cell 159, 1086–1095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kadrmas JL, Smith MA, Pronovost SM & Beckerle MC Characterization of RACK1 function in Drosophila development. Dev. Dyn 236, 2207–2215 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Volta V et al. RACK1 depletion in a mouse model causes lethality, pigmentation deficits and reduction in protein synthesis efficiency. Cell. Mol. Life Sci 70, 1439–1450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee AS, Burdeinick-Kerr R & Whelan SPJ A ribosome-specialized translation initiation pathway is required for cap-dependent translation of vesicular stomatitis virus mRNAs. Proc. Natl Acad. Sci. USA 110, 324–329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dobbelstein M & Shenk T In vitro selection of RNA ligands for the ribosomal L22 protein associated with Epstein-Barr virus-expressed RNA by using randomized and cDNA-derived RNA libraries. J. Virol 69, 8027–8034 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ludwig LS et al. Altered translation of GATA1 in Diamond-Blackfan anemia. Nat. Med 20, 748–753 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Komatsu M et al. A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J 23, 1977–1986 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang M et al. RCAD/Ufl1, a Ufm1 E3 ligase, is essential for hematopoietic stem cell function and murine hematopoiesis. Cell Death Differ 22, 1922–1934 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dong J et al. Rps3/uS3 promotes mRNA binding at the 40S ribosome entry channel and stabilizes preinitiation complexes at start codons. Proc. Natl Acad. Sci. USA 114, e2126–e2135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Darnell JC et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen E, Sharma MR, Shi X, Agrawal RK & Joseph S Fragile X mental retardation protein regulates translation by binding directly to the ribosome. Mol. Cell 54, 407–417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vasilyev N et al. Crystal structure reveals specific recognition of a G-quadruplex RNA by a β-turn in the RGG motif of FMRP. Proc. Natl Acad. Sci. USA 112, E5391–E5400 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meyer KD et al. 5′ UTR m6A promotes cap-independent translation. Cell 163, 999–1010 (2015).This study reveals that m6 A methylation in the 5′ UTR promotes cap-independent translation initiation via the eIF3 complex.
- 98.Castello A et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Jackson RJ, Hellen CUT & Pestova TV The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol 10, 113–127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hinnebusch AG The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem 83, 779–812 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Mohammad MP, Munzarová Pondelícková V, Zeman J, Gunišová S & Valášek LS In vivo evidence that eIF3 stays bound to ribosomes elongating and terminating on short upstream ORFs to promote reinitiation. Nucleic Acids Res 45, 2658–2674 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cuchalová L et al. The RNA recognition motif of eukaryotic translation initiation factor 3 g (eIF3g) is required for resumption of scanning of posttermination ribosomes for reinitiation on GCN4 and together with eIF3i stimulates linear scanning. Mol. Cell. Biol 30, 4671–4686 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pöyry TAA, Kaminski A & Jackson RJ What determines whether mammalian ribosomes resume scanning after translation of a short upstream open reading frame? Genes Dev 18, 62–75 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hinnebusch AG eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem. Sci 31, 553–562 (2006). [DOI] [PubMed] [Google Scholar]
- 105.Masutani M, Sonenberg N, Yokoyama S & Imataka H Reconstitution reveals the functional core of mammalian eIF3. EMBO J 26, 3373–3383 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagner S, Herrmannová A, Malík R, Peclinovská L & Valášek LS Functional and biochemical characterization of human eukaryotic translation initiation factor 3 in living cells. Mol. Cell. Biol 34, 3041–3052 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.ElAntak L et al. The indispensable N-terminal half of eIF3j/HCR1 cooperates with its structurally conserved binding partner eIF3b/PRT1-RRM and with eIF1A in stringent AUG selection. J. Mol. Biol 396, 1097–1116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Damoc E et al. Structural characterization of the human eukaryotic initiation factor 3 protein complex by mass spectrometry. Mol. Cell. Proteom 6, 1135–1146 (2007).This study performs both bottom-up and top-down proteomics methods to identify PTMs on the eIF3 complex.
- 109.Jia W, Andaya A & Leary JA Novel mass spectrometric method for phosphorylation quantification using cerium oxide nanoparticles and tandem mass tags. Anal. Chem 84, 2466–2473 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andaya A, Villa N, Jia W, Fraser CS & Leary JA Phosphorylation stoichiometries of human Eukaryotic initiation factors. Int. J. Mol. Sci 15, 11523–11538 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cai Q et al. Distinct regions of human eIF3 are sufficient for binding to the HCV IRES and the 40S ribosomal subunit. J. Mol. Biol 403, 185–196 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hashem Y et al. Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit. Nature 503, 539–543 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siridechadilok B, Fraser CS, Hall RJ, Doudna JA & Nogales E Structural roles for human translation factor eIF3 in initiation of protein synthesis. Science 310, 1513–1515 (2005). [DOI] [PubMed] [Google Scholar]
- 114.Lee ASY, Kranzusch PJ & Cate JHD eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 522, 111–114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee AS, Kranzusch PJ, Doudna JA & Cate JHD eIF3d is an mRNA cap-binding protein that is required for specialized translation initiation. Nature 536, 96–99 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sonenberg N, Morgan MA, Testa D, Colonno RJ & Shatkin AJ Interaction of a limited set of proteins with different mRNAs and protection of 5′ caps against pyrophosphatase digestion in initiation complexes. Nucleic Acids Res 7, 15–29 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar P, Hellen CUT & Pestova TV Toward the mechanism of eIF4F-mediated ribosomal attachment to mammalian capped mRNAs. Genes Dev 30, 1573–1588 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang L, Pan X & Hershey JWB Individual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cells. J. Biol. Chem 282, 5790–5800 (2007). [DOI] [PubMed] [Google Scholar]
- 119.Hershey JWB The role of eIF3 and its individual subunits in cancer. Biochim. Biophys. Acta - Gene Regul. Mech 1849, 792–800 (2015). [DOI] [PubMed] [Google Scholar]
- 120.Choudhuri A, Evans T & Maitra U Non-core subunit eIF3h of translation initiation factor eIF3 regulates zebrafish embryonic development. Dev. Dyn 239, 1632–1644 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choudhuri A, Maitra U & Evans T Translation initiation factor eIF3h targets specific transcripts to polysomes during embryogenesis. Proc. Natl Acad. Sci. USA 110, 9818–9823 (2013).This paper identifies a developmental function for a subunit of eIF3 in the zebrafish eye. Knockdown of the subunit decreased translation of multiple mRNAs, including those encoding proteins required for proper eye formation.
- 122.Zhou F, Roy B & von Arnim AG Translation reinitiation and development are compromised in similar ways by mutations in translation initiation factor eIF3h and the ribosomal protein RPL24. BMC Plant Biol 10, 193 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim T, Kim B, Yahalom A, Chamovitz DA & von Arnim AG Translational regulation via 5′ mRNA leader sequences revealed by mutational analysis of the Arabidopsis translation initiation factor subunit eIF3h. Plant Cell 16, 3341–3356 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Daxinger L et al. A forward genetic screen identifies eukaryotic translation initiation factor 3, subunit H (eIF3h), as an enhancer of variegation in the mouse. G3 2, 1393–1396 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dhalia R et al. Translation initiation in Leishmania major: characterisation of multiple eIF4F subunit homologues. Mol. Biochem. Parasitol 140, 23–41 (2005). [DOI] [PubMed] [Google Scholar]
- 126.Freire ER et al. The four trypanosomatid eIF4E homologues fall into two separate groups, with distinct features in primary sequence and biological properties. Mol. Biochem. Parasitol 176, 25–36 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moura DMN et al. Two related trypanosomatid eIF4G homologues have functional differences compatible with distinct roles during translation initiation. RNA Biol 12, 305–319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Joshi B, Cameron A & Jagus R Characterization of mammalian eIF4E-family members. Eur. J. Biochem 271, 2189–2203 (2004). [DOI] [PubMed] [Google Scholar]
- 129.Landon AL et al. MNKs act as a regulatory switch for eIF4E1 and eIF4E3 driven mRNA translation in DLBCL. Nat. Commun 5, 5413 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sugiyama H et al. Nat1 promotes translation of specific proteins that induce differentiation of mouse embryonic stem cells. Proc. Natl Acad. Sci. USA 114, 340–345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caron S, Charon M, Cramer E, Sonenberg N & Dusanter-Fourt I Selective modification of eukaryotic initiation factor 4F (eIF4F) at the onset of cell differentiation: recruitment of eIF4GII and long-lasting phosphorylation of eIF4E. Mol. Cell. Biol 24, 4920–4928 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hernández G & Vazquez-Pianzola P Functional diversity of the eukaryotic translation initiation factors belonging to eIF4 families. Mech. Dev 122, 865–876 (2005). [DOI] [PubMed] [Google Scholar]
- 133.Tee AR, Tee JA & Blenis J Characterizing the interaction of the mammalian eIF4E-related protein 4EHP with 4E-BP1. FEBS Lett 564, 58–62 (2004). [DOI] [PubMed] [Google Scholar]
- 134.Ho JJD & Lee SA Cap for every occasion: alternative eIF4F complexes. Trends Biochem. Sci 41, 821–823 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Waskiewicz AJ, Flynn A, Proud CG & Cooper JA Mitogen-activated protein kinases activate the serine/threonine kinaseses Mnk1 and Mnk2. EMBO J 16, 1909–1920 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dobrikov M, Dobrikova E, Shveygert M & Gromeier M Phosphorylation of eukaryotic translation initiation factor 4G1 (eIF4G1) by protein kinase Cα regulates eIF4G1 binding to Mnk1. Mol. Cell. Biol 31, 2947–2959 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raught B et al. Serum-stimulated, rapamycin-sensitive phosphorylation sites in the eukaryotic translation initiation factor 4GI. EMBO J 19, 434–444 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dobrikov MI, Shveygert M, Brown MC & Gromeier M Mitotic phosphorylation of eukaryotic initiation factor 4G1 (eIF4G1) at Ser1232 by Cdk1:Cyclin B inhibits eIF4A helicase complex binding with RNA. Mol. Cell. Biol 34, 439–451 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu X, Vatsyayan J, Gao C, Bakkenist CJ & Hu J Sumoylation of eIF4E activates mRNA translation. EMBO Rep 11, 299–304 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jongjitwimol J et al. The S. pombe translation initiation factor eIF4G is sumoylated and associates with the SUMO protease Ulp2. PLoS ONE 9, e94182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun F, Palmer K & Handel MA Mutation of Eif4g3, encoding a eukaryotic translation initiation factor, causes male infertility and meiotic arrest of mouse spermatocytes. Development 137, 1699–1707 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ghosh S & Lasko P Loss-of-function analysis reveals distinct requirements of the translation initiation factors eIF4E, eIF4E-3, eIF4G and eIF4G2 in Drosophila spermatogenesis. PLoS ONE 10, e0122519 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Contreras V, Richardson M.a, Hao E & Keiper BD Depletion of the cap-associated isoform of translation factor eIF4G induces germline apoptosis in C. elegans. Cell Death Differ 15, 1232–1242 (2008). [DOI] [PubMed] [Google Scholar]
- 144.Friday AJ & Keiper BD Positive mRNA translational control in germ cells by initiation factor selectivity. Biomed. Res. Int 2015, 327963 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morita M et al. A novel 4EHP-GIGYF2 translational repressor complex is essential for mammalian development. Mol. Cell. Biol 32, 3585–3593 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Duncan R, Milburn SC & Hershey JWB Regulated phosphorylation and low abundance of HeLa cell initiation factor 4F suggest a role in translational control. Heat shock effect on eIF4F. J. Biol. Chem 262, 380–388 (1987). [PubMed] [Google Scholar]
- 147.Truitt ML et al. Differential requirements for eIF4E dose in normal development and cancer. Cell 162, 59–71 (2015).This study presents an eIF4E haploinsufficiency mouse model that reveals that eIF4E expression is in excess for normal development but not for cancer formation and that eIF4E is required for translation of specific mRNAs via a novel 5′ UTR element.
- 148.Hsieh AC et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485, 55–61 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Levy S, Avni D, Hariharan N, Perry RP & Meyuhas O Oligopyrimidine tract at the 5′ end of mammalian ribosomal protein mRNAs is required for their translational control. Proc. Natl Acad. Sci. USA 88, 3319–3323 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Osborne MJ et al. eIF4E3 acts as a tumor suppressor by utilizing an atypical mode of methyl-7-guanosine cap recognition. Proc. Natl Acad. Sci. USA 110, 3877–3882 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S & Fukunaga R Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol. Cell. Biol 24, 6539–6549 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Furic L et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc. Natl Acad. Sci. USA 107, 14134–14139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moy JK et al. The MNK-eIF4E signaling axis contributes to injury-induced nociceptive plasticity and the development of chronic pain. J. Neurosci 37, 7841–7499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Connolly E, Braunstein S, Formenti S & Schneider RJ Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol. Cell. Biol 26, 3955–3965 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Timpano S & Uniacke J Human cells cultured under physiological oxygen utilize two cap-binding proteins to recruit distinct mRNAs for translation. J. Biol. Chem 291, 10772–10782 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ho JJD et al. Systemic reprogramming of translation efficiencies on oxygen stimulus. Cell Rep 14, 1293–1300 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Braunstein S et al. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell 28, 501–512 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Clemens MJ, Bushell M & Morley SJ Degradation of eukaryotic polypeptide chain initiation factor (eIF) 4G in response to induction of apoptosis in human lymphoma cell lines. Oncogene 17, 2921–2931 (1998). [DOI] [PubMed] [Google Scholar]
- 159.Marissen WE & Lloyd RE Eukaryotic translation initiation factor 4G is targeted for proteolytic cleavage by caspase 3 during inhibition of translation in apoptotic cells. Mol. Cell. Biol 18, 7565–7574 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bushell M et al. Cleavage of polypeptide chain initiation factor eIF4GI during apoptosis in lymphoma cells: characterisation of an internal fragment generated by caspase-3-mediated cleavage. Cell Death Differ 7, 628–636 (2000). [DOI] [PubMed] [Google Scholar]
- 161.Nevins TA, Harder ZM, Korneluk RG & Holcík M Distinct regulation of internal ribosome entry site-mediated translation following cellular stress is mediated by apoptotic fragments of eIF4G translation initiation factor family members eIF4GI and p97/DAP5/NAT1. J. Biol. Chem 278, 3572–3579 (2003). [DOI] [PubMed] [Google Scholar]
- 162.Henis-Korenblit S et al. The caspase-cleaved DAP5 protein supports internal ribosome entry site-mediated translation of death proteins. Proc. Natl Acad. Sci. USA 99, 5400–5405 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hundsdoerfer P, Thoma C & Hentze MW Eukaryotic translation initiation factor 4GI and p97 promote cellular internal ribosome entry sequence-driven translation. Proc. Natl Acad. Sci. USA 102, 13421–13426 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ramirez-Valle F, Braunstein S, Zavadil J, Formenti SC & Schneider RJ eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J. Cell Biol 181, 293–307 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Garzia A et al. The E3 ubiquitin ligase and RNA-binding protein ZNF598 orchestrates ribosome quality control of premature polyadenylated mRNAs. Nat. Commun 8, 16056 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.De Benedetti A & Graff JR eIF-4E expression and its role in malignancies and metastases. Oncogene 23, 3189–3199 (2004). [DOI] [PubMed] [Google Scholar]
- 167.Pelletier J, Graff J, Ruggero D & Sonenberg N Targeting the eIF4F translation initiation complex: a critical nexus for cancer development. Cancer Res 75, 250–263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Howard A & Rogers AN Role of translation initiation factor 4G in lifespan regulation and age-related health. Ageing Res. Rev 13, 115–124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Blagden SP & Willis AE The biological and therapeutic relevance of mRNA translation in cancer. Nat. Rev. Clin. Oncol 8, 280–291 (2011). [DOI] [PubMed] [Google Scholar]
- 170.Poss ZC, Ebmeier CC & Taatjes DJ The Mediator complex and transcription regulation. Crit. Rev. Biochem. Mol. Biol 48, 575–608 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Villani A et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356, eaah4573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Trapnell C Defining cell types and states with single-cell genomics. Genome Res 25, 1491–1498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hughes AJ et al. Single-cell western blotting. Nat. Methods 11, 749–755 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Giesen C et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422 (2014). [DOI] [PubMed] [Google Scholar]
- 175.Moor AE et al. Global mRNA polarization regulates translation efficiency in the intestinal epithelium. Science 2399, 1299–1303 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Morisaki T et al. Real-time quantification of single RNA translation dynamics in living cells. Science 352, 1425–1429 (2016). [DOI] [PubMed] [Google Scholar]
- 177.Wu B, Eliscovich C, Yoon YJ & Singer RH Translation dynamics of single mRNAs in live cells and neurons. Science 352, 1430–1435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yan X, Hoek TA, Vale RD & Tanenbaum ME Dynamics of translation of single mRNA molecules in vivo. Cell 165, 976–989 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Han BG, Watson Z, Cate JHD & Glaeser RM Monolayer-crystal streptavidin support films provide an internal standard of cryo-EM image quality. J. Struct. Biol 200, 307–313 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.des Georges A et al. Structure of mammalian eIF3 in the context of the 43S preinitiation complex. Nature 525, 491–495 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fraser CS, Berry KE, Hershey JWB & Doudna JA eIF3j is located in the decoding center of the human 40S ribosomal subunit. Mol. Cell 26, 811–819 (2007). [DOI] [PubMed] [Google Scholar]
- 182.Villa N, Do A, Hershey JWB & Fraser CS Human eukaryotic initiation factor 4G (eIF4G) protein binds to eIF3c, -d, and -e to promote mRNA recruitment to the ribosome. J. Biol. Chem 288, 32932–32940 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ingolia NT, Lareau LF & Weissman JS Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 147, 789–802 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fraser CS & Doudna JA Structural and mechanistic insights into hepatitis C viral translation initiation. Nat. Rev. Microbiol 5, 29–38 (2007). [DOI] [PubMed] [Google Scholar]
- 185.Weingarten-Gabbay S et al. Systematic discovery of cap-independent translation sequences in human and viral genomes. Science 351, aad4939 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Archer SK, Shirokikh NE, Beilharz TH & Preiss T Dynamics of ribosome scanning and recycling revealed by translation complex profiling. Nature 535, 570–574 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Simsek D & Barna M An emerging role for the ribosome as a nexus for post-translational modifications. Curr. Opin. Cell Biol 45, 92–101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sharma D, Cukras AR, Rogers EJ, Southworth DR & Green R Mutational analysis of S12 protein and implications for the accuracy of decoding by the ribosome. J. Mol. Biol 374, 1065–1076 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Loenarz C et al. Hydroxylation of the eukaryotic ribosomal decoding center affects translational accuracy. Proc. Natl Acad. Sci. USA 111, 4019–4024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Paolini NA et al. A ribosomopathy reveals decoding defective ribosomes driving human dysmorphism. Am. J. Hum. Genet 100, 506–522 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Singleton RS et al. OGFOD1 catalyzes prolyl hydroxylation of RPS23 and is involved in translation control and stress granule formation. Proc. Natl Acad. Sci. USA 111, 4031–4036 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jha S et al. Trans-kingdom mimicry underlies ribosome customization by a poxvirus kinase. Nature 546, 651–655 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Finley D, Bartel B & Varshavsky A The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature 338, 394–401 (1989). [DOI] [PubMed] [Google Scholar]
- 194.Kobayashi M et al. The ubiquitin hybrid gene UBA52 regulates ubiquitination of ribosome and sustains embryonic development. Sci. Rep 6, 36780 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Higgins R et al. The unfolded protein response triggers site-specific regulatory ubiquitylation of 40S ribosomal proteins. Mol. Cell 59, 35–49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sung MK et al. A conserved quality-control pathway that mediates degradation of unassembled ribosomal proteins. eLife 5, e19105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nguyen AT et al. UBE2O remodels the proteome during terminal erythroid differentiation. Science 357, eaan0218 (2017).This paper reveals extensive ubiquitylation of several RPs by a ubiquitin ligase, UBE2O, which is required for erythropoiesis.
- 198.Yanagitani K, Juszkiewicz S & Hegde RS UBE2O is a quality control factor for orphans of multiprotein complexes. Science 357, 472–475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xirodimas DP et al. Ribosomal proteins are targets for the NEDD8 pathway. EMBO Rep 9, 280–286 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Matafora V, D’Amato A, Mori S, Blasi F & Bachi A Proteomics analysis of nucleolar SUMO-1 target proteins upon proteasome inhibition. Mol. Cell. Proteom 8, 2243–2255 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Panse VG et al. Formation and nuclear export of preribosomes are functionally linked to the small-ubiquitin-related modifier pathway. Traffic 7, 1311–1321 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sun C et al. Functional reconstitution of human eukaryotic translation initiation factor 3 (eIF3). Proc. Natl Acad. Sci. USA 108, 20473–20478 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Panic L et al. Ribosomal protein S6 gene haploinsufficiency is associated with activation of a p53-dependent checkpoint during gastrulation. Mol. Cell. Biol 26, 8880–8891 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.McGowan KA et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat. Genet 40, 963–970 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mcgowan KA et al. Reduced ribosomal protein gene dosage and p53 activation in low-risk myelodysplastic syndrome. Blood 118, 3622–3633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sulic S et al. Inactivation of S6 ribosomal protein gene in T lymphocytes activates a p53-dependent checkpoint response. Genes Dev 19, 3070–3082 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gazda HT et al. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan Anemia patients. Am. J. Hum. Genet 83, 769–780 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Watkins-Chow DE et al. Mutation of the Diamond-Blackfan anemia gene Rps7 in mouse results in morphological and neuroanatomical phenotypes. PLoS Genet 9, e1003094 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Doherty L et al. Ribosomal protein genes RPS10 and RPS26 are commonly mutated in Diamond-Blackfan anemia. Am. J. Hum. Genet 86, 222–228 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ebert BL et al. Identification of RPS14 as a 5q-syndrome gene by RNA interference screen. Nature 451, 335–339 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Barlow JL et al. A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q-syndrome. Nat. Med 16, 59–66 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ikeda F et al. Exome sequencing identified RPS15A as a novel causative gene for Diamond-Blackfan anemia. Haematologica 102, e93–e96 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cmejla R, Cmejlova J, Handrkova H, Petrak J & Pospisilova D Ribosomal protein S17 gene (RPS17) is mutated in Diamond-Blackfan anemia. Hum. Mutat 28, 1178–1182 (2007). [DOI] [PubMed] [Google Scholar]
- 214.Song M et al. A novel initiation codon mutation in the ribosomal protein S17 gene (RPS17) in a patient with Diamond-Blackfan anemia. Pediatr. Blood Cancer 54, 629–631 (2010). [DOI] [PubMed] [Google Scholar]
- 215.Draptchinskaia N et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat. Genet 21, 169–175 (1999). [DOI] [PubMed] [Google Scholar]
- 216.Gazda HT et al. Ribosomal protein S24 gene is mutated in Diamond-Blackfan anemia. Am. J. Hum. Genet 79, 1110–1118 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Farrar JE et al. Ribosomal protein gene deletions in Diamond-Blackfan anemia. Blood 118, 6943–6951 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ferretti MB, Ghalei H, Ward EA, Potts EL & Karbstein K Rps26 directs mRNA-specific translation by recognition of Kozak sequence elements. Nat. Struct. Mol. Biol 24, 700–707 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang R et al. Loss of function mutations in RPL27 and RPS27 identified by whole-exome sequencing in Diamond-Blackfan anaemia. Br. J. Haematol 168, 854–864 (2015). [DOI] [PubMed] [Google Scholar]
- 220.Gripp KW et al. Diamond-Blackfan anemia with mandibulofacial dystostosis is heterogeneous, including the novel DBA genes TSR2 and RPS28. Am. J. Med. Genet. Part A 164, 2240–2249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mirabello L et al. Whole-exome sequencing and functional studies identify RPS29 as a novel gene mutated in multicase Diamond-Blackfan anemia families. Blood 124, 24–32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gu YAN et al. Deficiency of monoclonal non-specific suppressor factor beta (MNSFB) promotes pregnancy loss in mice. Mol. Reprod. Dev 82, 475–488 (2015). [DOI] [PubMed] [Google Scholar]
- 223.Perucho L et al. RPLP1, a crucial ribosomal protein for embryonic development of the nervous system. PLoS ONE 9, e99956 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brooks SS et al. A novel ribosomopathy caused by dysfunction of RPL10 disrupts neurodevelopment and causes X-linked microcephaly in humans. Genetics 198, 723–733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Morgado-Palacin L et al. Partial loss of Rpl11 in adult mice recapitulates Diamond-Blackfan anemia and promotes lymphomagenesis. Cell Rep 13, 712–722 (2015). [DOI] [PubMed] [Google Scholar]
- 226.Poddar D et al. An extraribosomal function of ribosomal protein L13a in macrophages resolves inflammation. J. Immunol 190, 3600–3612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chaudhuri S et al. Human ribosomal protein L13a is dispensable for canonical ribosome function but indispensable for efficient rRNA methylation. RNA 13, 2224–2237 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Landowski M et al. Novel deletion of RPL15 identified by array-comparative genomic hybridization in Diamond-Blackfan anemia. Hum. Genet 132, 1265–1274 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rao S et al. Inactivation of ribosomal protein L22 promotes transformation by induction of the stemness factor, Lin28B. Blood 120, 3764–3773 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gazda HT et al. Frameshift mutation in p53 regulator RPL26 is associated with multiple physical abnormalities and a specific pre-ribosomal RNA processing defect in Diamond-Blackfan anemia. Hum. Mutat 33, 1037–1044 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Terzian T et al. Rpl27a mutation in the sooty foot ataxia mouse phenocopies high p53 mouse models. J. Pathol 224, 540–552 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Oristian DS et al. Ribosomal protein L29 / HIP deficiency delays osteogenesis and increases fragility of adult bone in mice. J. Orthop. Res 27, 28–35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kirn-Safran CB et al. Global growth deficiencies in mice lacking the ribosomal protein HIP/RPL29. Dev. Dyn 236, 447–460 (2007). [DOI] [PubMed] [Google Scholar]
- 234.Farrar JE et al. Exploiting pre-rRNA processing in Diamond Blackfan anemia gene discovery and diagnosis. Am. J. Hematol 89, 985–991 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Farrar JE et al. Abnormalities of the large ribosomal subunit protein, Rpl35a, in Diamond-Blackfan anemia. Blood 112, 1582–1592 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]