INTRODUCTION
Emerging data suggest that the left atrium (LA) is much more than simply a conduit for left ventricular (LV) filling, and its size and remodeling are recognized as a predictor of poor outcomes in multiple disease states. LA dilation has been associated with increased risk of atrial fibrillation (AF), ischemic stroke, mortality after acute myocardial infarction, and heart failure with both reduced and preserved LV systolic function.1–4 In patients with heart failure, LA size provides incremental prognostic information over LV systolic and diastolic function.5
The causal pathway linking LA size with adverse outcomes is not entirely clear, which highlights the fact that LA dilation can be multifactorial, resulting from valvular disease, systemic hypertension, and any condition causing elevated LV filling pressures (Box 1). Furthermore, dilation itself predisposes to adverse outcomes, such as the development of AF and ischemic stroke.1,6 Hence, LA size may be considered a barometer for the combined effect of these conditions longitudinally.
Box 1. Prognostic value of left atrial size.
Atrial fibrillation
Heart failure with preserved ejection fraction
Heart failure with reduced ejection fraction
Coronary artery disease/myocardial infarction
Mitral regurgitation
Systemic hypertension
Ischemic stroke
Hypertrophic cardiomyopathy
Cardiovascular mortality
All-cause mortality
LEFT ATRIAL ANATOMY
Embryologically, most of the LA is derived from the primitive pulmonary vein and is characterized by smooth endocardium, whereas the left atrial appendage (LAA) is the only part from the primitive LA and has pectinate muscle and trabeculations. The LA is the most posterior of all the cardiac chambers, resting adjacent to the esophagus. In addition to the LAA, the LA is composed of a body, which receives passive pulmonary venous flow, a vestibule surrounding the mitral orifice, and a shared interatrial septum with the right atrium. The pulmonary veins drain into the posterior LA body, with the ostia of the left pulmonary veins higher than the right. The left circumflex artery and great cardiac vein run together in the left atrioventricular groove. The coronary sinus travels along the epicardial aspect of the posteroinferior wall.7
In utero, the interatrial septum is formed by the septum primum growing from the roof of the atria toward the endocardial cushion. Fenestrations then form within the septum primum. The septum secundum then forms by an in-folding of the atrial walls and through the overlap of the septum primum to the left acts as a conduit for right-to-left shunting of oxygenated blood in fetal circulation. After birth, if the primum and secundum septum fail to fuse, this results in a patent foramen ovale at the anterosuperior edge of the fossa ovalis.8
When viewed from the right atrium on transesophageal echocardiography (TEE), the atrial septum can appear falsely larger. The true septum is the fossa ovalis, and the muscular rim that surrounds it. The aortic mound is the anterior portion of the septum, which lies immediately behind the aortic root. Understanding LA anatomy and its relationship to other adjacent structures is very important, especially with the increase in percutaneous therapies requiring transseptal puncture.7
LEFT ATRIAL APPENDAGE ANATOMY
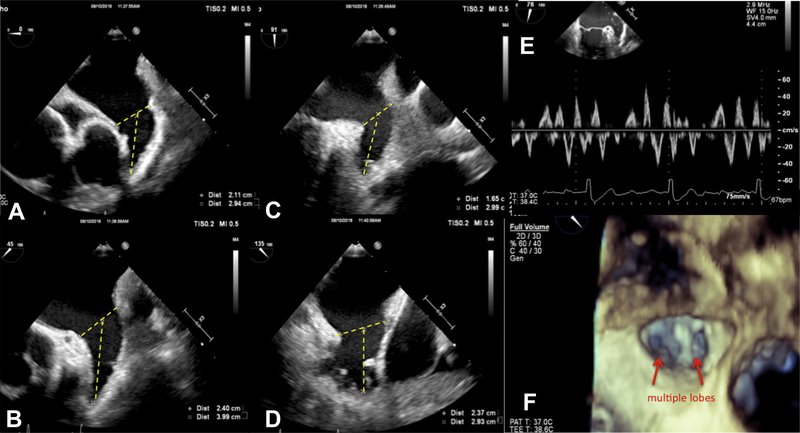
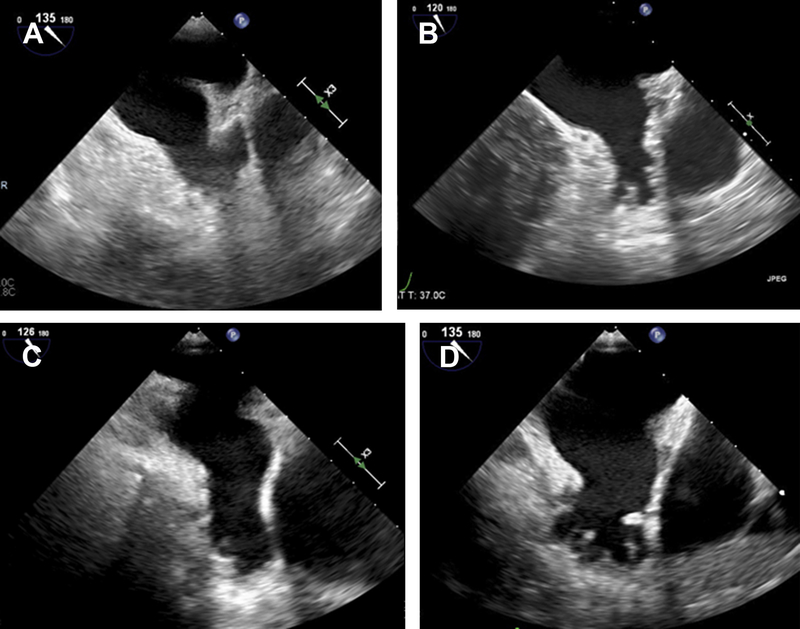
TEE been extensively used to characterize LAA structure and function and is the gold standard for assessing for the presence of LAA thrombus with a sensitivity of 92% to 100% and specificity of 98% to 99%.9 Although the LAA is sometimes visualized on transthoracic echocardiographic (TTE) imaging in the parasternal short axis through the cardiac base and the apical 2-chamber view, TEE is far superior to TTE in LAA assessment. In most patients, the LAA lies on the anterolateral aspect of the LA with an oval-shaped orifice separated from the left upper pulmonary vein by the left lateral ridge.7,10 There are significant variations in the size, shape, and relation to adjacent structures, and with the expanding use of the Watchman LAA occluder device for stroke prevention, it is important to clearly define the LAA anatomy. Fig. 1 shows a comprehensive assessment of the LAA before WATCHMAN implantation. The LAA can be single- or multilobed and morphologically has been classified having 4 broad categories. In one multimodality imaging study, the most common morphologies of the LAA were chicken wing (48%), cactus (30%), windsock (19%), and cauliflower (3%), as seen in Fig. 2 A–D. The chicken wing morphology, after adjusting for other comorbidities, has been associated with higher thromboembolic risk.11 The inner surface of the LAA has pectinate muscle bundles that can mimic thrombi or other intracardiac masses.
Fig. 1.
TEE examination of the LAA before Watchman occluder device implantation, including 2DE images of the LAA at 0°, 45°, 90°, and 135° (A–D), pulsed Doppler LAA velocities (E), and 3DE enface of the LAA ostium (F).
Fig. 2.
TTE examinations of LAA morphologies: chicken wing (A), cactus (B), windsock (C), and cauliflower (D).
If the images of the LAA are suboptimal, microbubble contrast agents can be used to help eliminate artifacts, enhance visualization of the cavity, and assess for filling defects that can represent thrombus. Three-dimensional echocardiography (3DE) is a helpful adjunct in assessing the LAA by differentiating thrombus from pectinate muscle and defining the LAA’s relationship to surrounding structures. In a TEE and computed tomography (CT) study, LAA orifice area on CT correlated well with area on 3D TEE (r = 0.98) but not with area on 2-dimensional (2D) TEE calculated assuming an ellipsoid shape using diameters obtained from the orthogonal plane (r = 0.13). Bland-Altman analysis demonstrated that 2D TEE systematically underestimated LAA orifice area compared with 3D TEE.12
3DE is also superior in planning for and real-time imaging guidance during percutaneous device therapy for LAA occlusion. Several catheter-based LAA closure devices have been developed over the years, and currently, the Watchman Left Atrial Appendage Closure Implant (Boston Scientific, Massachusetts, USA) is commercially available in the United States. The Amplatzer Cardiac Plug (Abbott, Illinois, USA) is available in select international markets. In the PROTECT Atrial Fibrillation, a prospective, randomized trial, LAA closure with the Watchman was noninferior to warfarin therapy in preventing cardiovascular death, stroke, or systemic embolization in patients with nonvalvular AF after 3.8 years of follow-up and was superior for cardiovascular and all-cause mortality.13 Five-year follow-up data show that LAA closure with the Watchman device provides stroke prevention in nonvalvular AF comparable to warfarin, with additional reductions in major bleeding and mortality.14
In sinus rhythm, the LAA is highly contractile with cavity obliteration at its apex, which prevents thrombus formation. LAA contraction can be assessed by pulsed-wave Doppler in the proximal third of the LAA and in normal subjects is biphasic with velocities ranging from 50 ± 6 cm/s to 83 ± 25 cm/s with filling velocities ranging from 46 ± 12 cm/s to 60 ± 19 cm/s. AF causes LAA remodeling with sac dilation and reduction in pectinate muscles. Doppler examination in AF shows loss of the normal pattern and lower velocities (see Fig. 1E). Velocities less than 40 cm/s are associated with higher risk of stroke and spontaneous echo contrast and less than 20 cm/s with identification of LAA thrombus.9,15,16 After cardioversion, albeit spontaneous, chemical, or electrical, there is temporary stunning with a paradoxic worsening of LA and LAA mechanical function and reduction in LAA flow velocities that typically resolve after a few days, underscoring the importance of adequate anticoagulation.17
LEFT ATRIAL PHYSIOLOGY
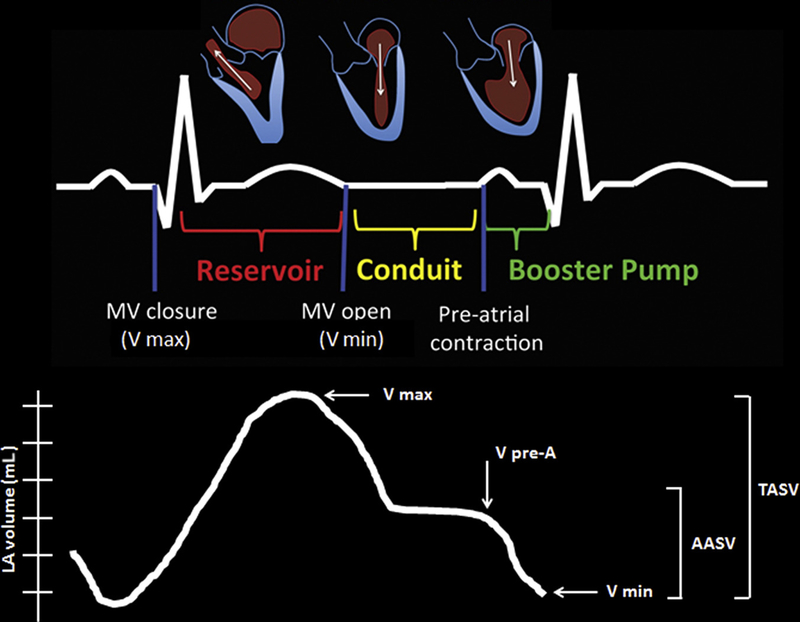
The LA is a complex chamber with multiple functions, and it is important to recognize the dynamic relationship between LA and LV performance. The principal role of the LA is to modulate LV filling via its reservoir, conduit, and booster functions. During the reservoir phase, which is governed by LA compliance, the LA stores pulmonary venous return during LV contraction and isovolumic relaxation. In the conduit phase, the LA passively transfers blood to the LV. Last, LA contraction during the booster phase in late diastole contributes about a quarter of LV stroke volume18,19 (Fig. 3, top row).
Fig. 3.
Left atrial reservoir, conduit, and booster LA function in relation to the cardiac cycle (top) with the corresponding LA volume curves during these phases (bottom). The maximal volume (Vmax) at end-systole just before the opening of the mitral valve, minimal volume (Vmin) at end-diastole before mitral valve closure, and the volume before atrial contraction (VpreA) before mitral valve reopening at the time of the P wave on ECG. TASV is Vmax − Vmin and the AASV is the VpreA − Vmin, which represents the LA booster phase.
The volumes of the LA during these phases can be defined as maximal volume (Vmax) at end-systole just before the opening of the mitral valve, minimal volume (Vmin) at end-diastole before mitral valve closure, and the volume before atrial contraction (VpreA) before mitral valve reopening at the time of the P wave on electrocardiogram (ECG) (see Fig. 3, bottom row). From these 3 volumes, the following parameters can be obtained:
Total atrial stroke volume (TASV): Vmax − Vmin.
Total atrial emptying fraction: TASV/Vmax × 100.
Active atrial stroke volume (AASV): VpreA − Vmin.
Active atrial emptying fraction: AASV/VpreA × 100.
Atrial expansion index: TASV/Vmin × 100.
Passive atrial emptying fraction: (Vmax − VpreA)/Vmax× 100.20
There is some controversy regarding the impact of aging on total LA volume, by both 2-dimensional echocardiography (2DE) and 3DE, but there are data to suggest that phasic -contributions to LA volume change with age. As patients age, there is a decrease in conduit phase (passive emptying) and an increase in booster (active emptying) contribution representing impaired LV diastolic relaxation.21–23 In a study of 276 healthy volunteers, 3DE LA volumes indexed to body surface area increased with age,24 whereas another multicenter of the Normal Reference Ranges for Echocardiography in 371 healthy individuals found a trend toward increased LA volume with age that did not reach statistical significance.23 LA volume is greater in men compared with women, but this difference is attributable to differences in body surface area; therefore, indexed volumes are similar.24
CLINICAL IMPLICATIONS OF LEFT ATRIAL ABNORMALITIES
LA preload is largely volume dependent. The LA manifests adaptive changes in its structure and mechanics in response to changes in compliance of the LV, the primary determinant of LA afterload. These changes are well described in the setting of abnormal patterns of LV filling. Previous studies have shown that increasing LA volume and pressure leads to LA dilation with an initial increase in contractile function followed by worsening LA function with further dilation, similar to the Frank-Starling pressure volume relationship in the LV.18 In the absence of mitral valve disease and AF, an increase in LA size most commonly reflects increased wall tension as a result of chronic elevation of LA pressure.25–27 This increase in LA size also results in impaired LA function due to atrial myopathy.28 LA size has been found to be an important marker for the chronicity of elevated LV filling pressures and a powerful predictor of adverse cardiovascular outcomes, including stroke, development of AF, congestive heart failure, and death.1,2,5,6,29–32 There are also data to suggest using LA size is a therapeutic target. Medical therapy with angiotensin-converting enzyme inhibitor and angiotensin-receptor blockers resulted in reverse remodeling and decrease in LA size.33,34 Therefore, accurate and reproducible measurement of atrial volumes is important in clinical practice.
MEASUREMENT OF LEFT ATRIAL SIZE
The LA can be imaged with cardiac CT, cardiac MRI, and with the echocardiography being best suited in most situations with the ability to image in real time with good spatial and temporal resolution to assess not only size but also function with Doppler and strain imaging.35–42 TTE is the most frequently used imaging modality for measuring atrial size. TTE is superior to TEE in evaluating LA size. Because of the close proximity of the LA to the esophagus, it is difficult to view the entire endocardial boundary in the TEE sector. In addition, because patients referred for TEE usually have underlying cardiac pathologic condition, reference values for LA size by TEE have not been established.
Among the different echocardiographic parameters available for assessing LA size, including diameter, area, and the LA volumes has been identified as the most accurate and robust predictor of cardiovascular outcomes.43 Attention has turned toward establishing the most accurate echocardiographic method to measure LA volumes and identifying normal values and partitions for severity of atrial enlargement, given its clinical implications.
Historically, LA size was assessed using M-mode echocardiography acquired from the parasternal long-axis view. In this transducer position, the anteroposterior dimension of the LA was recorded. Because this measurement is highly reproducible, it was widely adopted by echocardiography laboratories worldwide. It soon became obvious, however, that the LA does not dilate symmetrically in all directions when it enlarges. In fact, there are data to suggest that left atrial enlargement in the anteroposterior direction is constricted by the presence of the spine and sternum, and accordingly, most LA enlargement tends to occur in the superior-inferior direction.38 Because of this, the use of M-mode echocardiography (which measures LA size in the anteroposterior direction) was strongly discouraged by the American Society of Echocardiography (ASE) 2005 chamber quantification guidelines, and measurement of LA volumes was recommended for clinical practice.44
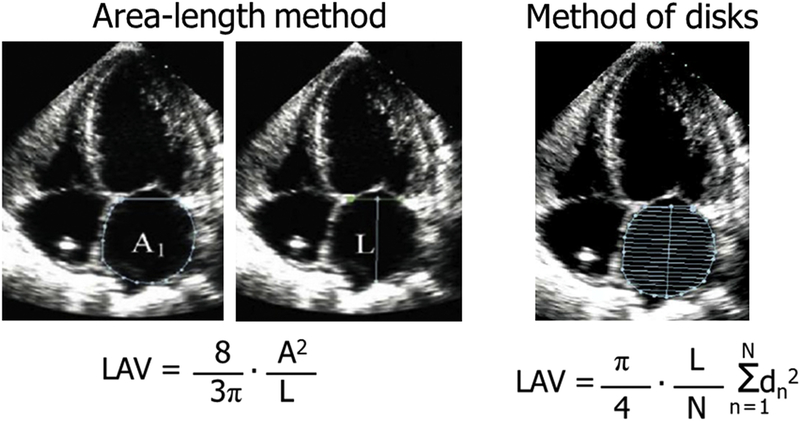
2DE measurements of atrial volumes assume a certain atrial shape and as a result depend on the specific imaging plane used. For standardization purposes, current 2015 ASE guidelines recommend that the atria be measured in apical 4- and 2-chamber views while excluding the LAA and pulmonary ostia at ventricular end systole.45 However, these views maximize often the long axis of the left ventricle, rather than the dimensions of the atria, resulting in foreshortening of the atria. Both the 2005 and the current 2015 chamber quantification guidelines recommend the use of the biplane method of disks or alternatively the area-length method for the measurement of LA volumes. The LA endocardial border is traced and volume computed by adding the volume of a stack of 20 cylinders of length (L) and area calculated by orthogonal minor and major transverse axes (ai and bi) assuming an oval shape.
The LA endocardial borders should be traced in both the apical 4- and the 2-chamber views. Alternatively, a biplane calculation could also be performed using the LA areas and length measured from both the apical 4- (A1) and 2-chamber (B1) views. The LA volume is calculated as using the area-length method:
where L is the shortest distance between the midline of the plane of mitral annulus to the opposite superior side (roof) of the LA measured in either the 4- or 2-chamber views. As well, it is assumed that the difference between L measured in the 2- and 4-chambers views is no more than 5 mm (Fig. 4). Although the area-length method still assumes an ellipsoidal LA shape, it has the advantage of reducing linear dimensions to a single measurement. The area-length method has been shown to result in atrial volumes that are slightly larger than those obtained using the biplane method of disks.41
Fig. 4.
Apical 4-chamber images of the LA depicting both the area-length and biplane method of disks equations for calculation of LA volumes.
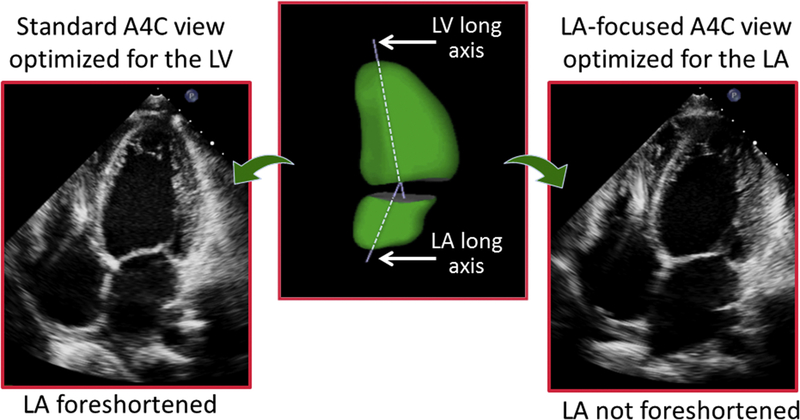
There was a major increase in the published values for normal LA volumes between the 2005 and 2015 chamber quantification guidelines. The upper normal reference value increased from 28 mL/m2 for both men and women in 2005 to 34 mL/m2 in 201544,45 (Table 1). The main reason for this change is that the 2015 document had access to normative LA volume data obtained from a large number of studies conducted after the 2005 guidelines had been published. Just as it is important not to foreshorten the left ventricle when obtaining measurements of LV volumes and ejection fraction, it is just as crucial to not foreshorten the LA. The need for such “atrial-focused views” has been recognized for over a decade. The long axes of the left ventricle and LA almost always lie in different planes, which explains why dedicated acquisitions of the LA must be obtained to optimize volume measurements (Fig. 5). In these LA-focused views, care must be taken to maximize the long-axis length and the base of the LA in both the apical 4- and the apical 2-chamber views in order to avoid foreshortening. If acquired adequately, the length of the LA in the 2 apical views should be nearly identical. As outlined in Table 2, 6 of the 13 studies with a total of 3066 subjects out of the total 4701 normal subjects (65%) used to define normative values specifically stated that non-foreshortened atrial-focused views were used.2,4,19,29–31,35,37,40,42,46–48 This large percentage of the data can probably explain the increase in the recommended normal values in the revised 2015 guidelines.49
Table 1.
Normative left atrial values
| Left Atrial Parameter | Reference | Value |
|---|---|---|
| LA volume (2DE) | Lang et al,45 2015 | LAVi upper limit 34 mL/m2 |
| LA volume (3DE) | Wu et al,51 2013, n = 124 | LAVi upper limit 33 mL/m2 |
| Badano et al,24 2016, n = 276 | LAVi upper limit 43 mL/m2 | |
| Sugimoto et al,23 2018, n = 371 | LAVi upper limit 41 mL/m2 | |
| LA volume (CMR) | Maceira et al,39 2010, n = 120 | LAVi mean 40 ± 7 mL/m2 |
| Hudsmith et al,65 2005, n = 108 | LAV mean 103 ± 30 mL (men), 89 ± 21 mL (women) | |
| LA strain | Miglioranza et al,56 2016, P-wave reference, n = 171 | LA LS pos 19.7% ± 5.6%, LA LS neg −14.5% ± 2.4%, and LA LS total 33.3% ± 5.7% |
| Saraiva et al,55 2010, P-wave reference, n = 64 | LA LS pos 23.2% ± 6.7%, LA LS neg −14.6% ± 3.5%, and LA LS total 37.6% ± 7.6% | |
| Sun et al,61 2013, R-wave reference, n = 121 | LA LS pos 46.8% ± 7.7% | |
Abbreviations: LAVi, left atrial volume index; LA LS, left atrial longitudinal strain positive, negative, total.
Fig. 5.
Example of an apical 4-chamber view, optimized to depict maximal length of the LV (left). In this view, the LA is foreshortened, in contrast with an LA-focused view specifically optimized to visualize the atrium at its maximal length (right). Atrial foreshortening occurs because the long axes of the ventricle are not the same, as depicted in this 3D reconstruction of both left heart chambers (center).
Table 2.
Studies cited by the recent chamber quantification guidelines update as a basis for an increase in normal values for left atrial volumes
| Source | Total Patients | Normal Subjects | Method | View | Indexed Mean LA Volume (mL/m2) |
|---|---|---|---|---|---|
| Tsang et al,29 2002 | 140 | 44 | Biplane A-L | Standard apical | 22 ± 5 (no diastolic dysfunction) |
| Barnes et al,4 2004 | 1554 | 1462 | Biplane A-L | Standard apical | 34 ± 14 (no history of stroke) |
| Takemoto et al,31 2005 | 1375 | 1237 | Biplane A-L | Standard apical | 32 ± 12 (non-HF) |
| Orban et al,46 2008 | 24 | 24 | Biplane A-L | Standard apical | 17 ± 3 |
| Yoshida et al,2 2009 | 111 | 20 | Biplane MOD | Standard apical | 14 ± 4 |
| Whitlock et al,35 2010 | 103 | 18 | Biplane A-L | Standard apical | 23 ± 6 |
| Cacciapuoti et al,47 2012 | 77 | 15 | Biplane MOD | Standard apical | 23 ± 4 |
| Thomas et al,19 2002 | 92 | 92 | Biplane MOD | Zoomed apical | 23 ± 7 |
| Tsang et al,33 2006 | 423 | 255 | Biplane A-L | Non-forshortened atrial apical | 36 ± 10 (sinus rhythm, no CV events) |
| Yamaguchi et al,48 2006 | 105 | 105 | Biplane MOD | Non-forshortened atrial apical | 22 ± 4 |
| Nistri et al,37 2011 | 418 | 418 | Biplane A-L | Dedicated LA | 32.2 ± 9 |
| Iwataki et al,42 2012 | 200 | 77 | Biplane MOD | Non-forshortened atrial apical | 27 ± 5 |
| Kou et al,40 2014 | 734 | 734 | Both | Atrial focused | 29 ± 7 (A-L) and 26 ± 6 (MOD) |
Abbreviations: A-L, area-length; CV, cardiovascular; HF, heart failure; MOD, method of disks.
3-DIMENSIONAL ASSESSMENT OF THE LEFT ATRIUM
Previous studies have shown that 3DE minimizes the inaccuracies associated with geometric assumptions and mostly eliminates the errors associated with foreshortening by allowing the operator to manually select orthogonal planes that maximize the long axis of the chamber being quantified.50 In order to obtain good-quality 3D images, first, the 2DE image should be optimized in an apical LA focused view as described above by modifying the gain, compress, and time gain compensation controls. For best temporal resolution, a multibeat, wide-angle full-volume acquisition, including the entire LA cavity in the pyramidal scan, should be obtained. This acquisition should be done during a breath-hold to minimize stitch artifact from respiratory motion. Simultaneous real-time multiplanar mode should be used to minimize any dropout, especially of the posterior LA wall.
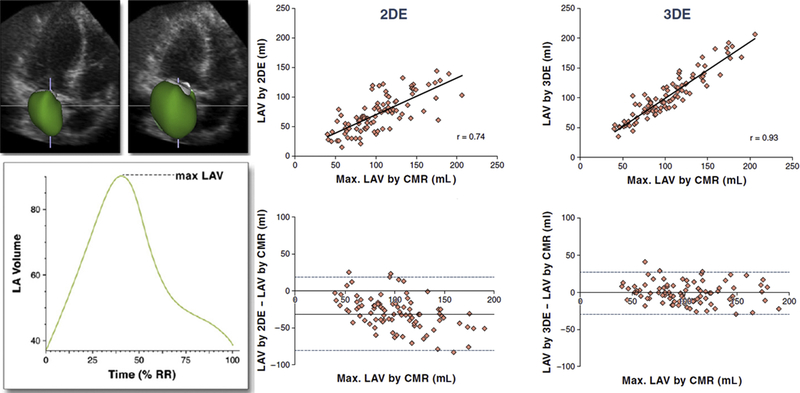
To perform 3DE analysis, depending on the software used, a combination of 2-, 3-, and 4-chamber views is selected from 3DE pyramidal data set. In these views, the LA boundaries can be manually initialized on 2 frames depicting minimal and maximal left atrial volumes (LAVs). These initialized LA boundaries are then used to reconstruct the LA endocardial surface throughout the cardiac cycle. This reconstruction can be repeated for each frame of the cardiac cycle, resulting in a dynamic cast of LA cavity, and for each consecutive frame, the voxel count inside the 3D surface is used to measure the LA volume. This analysis results in a smooth interpolated LA volume time curve with effective temporal resolutions of 150 to 200 samples per second (Fig. 6, left).50 It has been suggested that because the LA wall lacks the trabeculations found in the LV wall, 3D LA volumes more closely approximate those obtained with cardiac magnetic resonance imaging (CMR).36 Fig. 6 (right) highlights the results of the comparisons between the 2DE and 3DE measurements of the maximal LA volumes, respectively, against the corresponding CMR values in a study of 92 patients.50 2DE-derived values of the LA volume correlated well with CMR reference values (r = 0.74). However, Bland-Altman analysis revealed negative biases of 31 mL (P<.001), reflecting a systematic underestimation of the LA volume by the 2DE technique. There was a trend toward increased bias in patients with enlarged atria compared with those with normal atrial sizes. The corresponding 3DE measurements resulted in even better correlations with CMR (r = 0.93) with only minimal bias of 1 mL ( not significant) for maximal LA volume. The limits of agreement for the 3DE measurements were considerably tighter than those of the 2DE data.
Fig. 6.
Example of the LA cavity cast shown at 2 different phases of the cardiac cycle depicting the minimal and maximal LAV and the corresponding time curve depicting the LAV throughout the cardiac cycle from 0% to 100% of the R-R interval (left). Linear regression and Bland-Altman analyses of 2DE and 3DE measurements of maximal LAV. Correlation coefficients (r values) are shown; solid horizontal lines depict the bias of each technique (mean difference from the CMR reference, whereas dashed lines indicate the limits of agreement; 2 standard deviations around the mean difference) (right).
There are conflicting data on the relationship between 3DE- and 2DE-derived LA volumes with some studies finding significantly larger normal reference values for maximum LA volumes obtained by 3D echocardiography (20%–30% larger) versus those obtained using the 2D biplane Simpson method performed on atrial-focused views, where others have found similar values between the 2 methods.24,49,51 In those with differences in 2DE and 3DE LA volume, the 3DE-derived LA volume had a stronger and additive prognostic value with higher risk ratios compared with 2DE-derived volume.51 2DE, 3DE, and CMR normative LA volumes are summarized in Table 1.
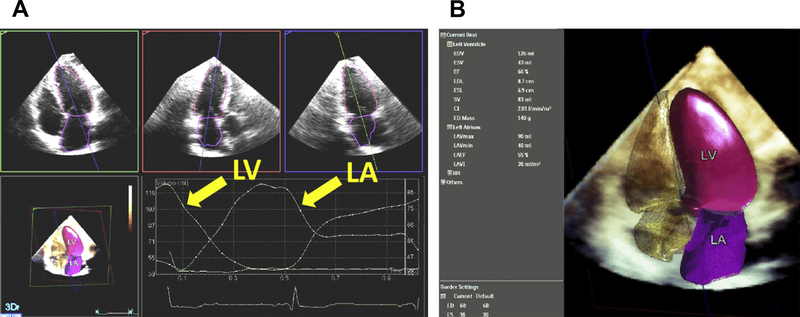
Despite the well-known advantages of 3DE, this modality is not routinely used in clinical practice for a variety of reasons, including the need for 3DE-specific expertise and the additional time needed for 3DE imaging. Philips HeartModel A.I. is a fully automated program, validated and found to be reasonably accurate when compared with CMR measurements in a group of more than 150 patients, which simultaneously detects LA and LV endocardial surfaces using an adaptive analytics algorithm that consists of knowledge-based identification of initial global shape and orientation followed by patient-specific adaptation. In a study of 30 patients, the average acquisition time for a 3DE full-volume data set of the LA and LV was 20 seconds, and analysis time was 17 seconds, providing 3D volumes throughout the cardiac cycle (Fig. 7). By automating some of the manual steps required for 3D analysis, integration of 3D analysis into the workflow of a busy echocardiography laboratory52 may become possible in the future.
Fig. 7.
Dynamic HeartModel A.I. application display showing the dynamic contours on the automatically aligned AP4, AP3, and AP2 views along with the volume waveform and the 3D shell of the left atrial and ventricular cavity.
3DE also allows for the assessment of LA shape, which may help to risk-stratify increases in LA volume. Study of LA shape has provided insight into the potential mechanism that determines blood stasis, which predisposes to embolic events in patients with mitral stenosis. It has been reported that patients in whom the LA remodels from an ellipsoidal to a more spherical shape are at greater risk of embolic events.53 Spherical remodeling is thought to result in an increase in atrial wall tension that predisposes patients to AF and is less effective for atrial contraction. Although these findings are physiologically important, at this time the clinical utility of LA shape remains uncertain.
DIASTOLIC FUNCTION AND THE LEFT ATRIUM
In addition to volumetric data throughout the cardiac cycle as described above, LA and diastolic function can be assessed with spectral Doppler of transmitral, pulmonary venous and LAA flow, tissue Doppler, and LA strain.18,54–57 Dysfunction in LA phases leads to impaired LV filling and the development of heart failure with preserved ejection fraction (HFpEF). In the absence of atrial arrhythmias and significant mitral valve disease, LA size and function can act as a surrogate for LV diastolic disease, thus assessing that the LA is vital in diagnosing these patients.2,32 According to the 2016 ASE Diastolic Function guidelines, LA volume index greater than 34 mL/m2 along with abnormal mitral annular tissue velocities (septal <7 cm/s, lateral <10 m/s), average E/eʹ ratio >14, and peak tricuspid regurgitation velocity greater than 2.8 m/s are the 4 parameters used to assess for diastolic dysfunction (Table 3). LV diastolic function is normal if more than half of the available variables do not meet the cutoff values for identifying abnormal function. LV diastolic dysfunction is present if more than half of the available parameters meet these cutoff values. The study is inconclusive if half of the parameters do not meet the cutoff values.58 The mitral E velocity reflects the LA-LV pressure gradient in early diastole and is affected by LV relaxation and LA pressure. The mitral A velocity is the LA-LV pressure gradient in late diastole affected by LV compliance and LA contractile function. The mitral inflow velocities are used to identify LV filling patterns. Along with tissue Doppler, these can be used to estimate filling pressures. Mean LA pressure can also be assessed with pulmonary venous S/D ratio, isovolumic relation time, Ar-A duration, and in the absence of pulmonary disease, diastolic PA pressure from a pulmonic regurgitation jet. These updated and simplified guidelines for the estimation of filling pressures are more user-friendly and efficient than the 2009 guidelines and provide accurate estimates of LV filling pressure in most patients when compared with invasive measurements.59 The simplicity of the new algorithm did not compromise its accuracy and is likely to encourage its incorporation into clinical decision making.
Table 3.
2016 diastolic function guidelines
| LA volume index >34 mL/m2 Abnormal mitral annular tissue velocities (septal <7 cm/s, lateral <10 m/s) Peak TR velocity >2.8 m/s Average E/e′ ratio >14 |
LV diastolic function is normal if more than half of the available variables do not meet the cutoff values for identifying abnormal function. LV diastolic dysfunction is present if more than half of the available parameters meet these cutoff values. |
LEFT ATRIAL STRAIN
Strain imaging using 2D speckle tracking of the LA has been used for the assessment of left atrial function. LA strain is angle independent, and thus less susceptible to the limitations of Doppler echocardiographic assessment of strain. Alterations in LA strain have been described in patients with hypertension, AF, and diastolic heart failure.56,57 Reduction in LA strain was found to be an important predictor in separating patients with clinical HFpEF and asymptomatic diastolic dysfunction.60 To obtain LA strain, using 2D speckle tracking software, the LA endocardial border is traced in the apical 4-chamber view, taking care to exclude the appendage and pulmonary veins from the LA cavity, generating an LA longitudinal strain curve throughout the cardiac cycle. The peak negative strain corresponds to the LA contractile function and the peak positive strain corresponds to the LA conduit function. The sum of the peak positive and negative strains is considered to be total LA strain, corresponding to LA reservoir function. Studies using either the R wave (Fig. 8A) or the P wave (see Fig. 8B) as the zero-reference point have generated completely different normative values.55,56,61 The single additional measurement of LA strain using 2D speckle tracking may be a valuable diagnostic tool in the evaluation of diastolic dysfunction (Fig. 9).62 In addition, changes in LA strain have been shown to be independent of LA volume in patients with HFpEF63 and correlated well with filling pressures in patients with systolic heart failure.64 However, peak LA strain is susceptible to the effects of age, obesity, valvular disease, such as mitral regurgitation, and AF.54
Fig. 8.
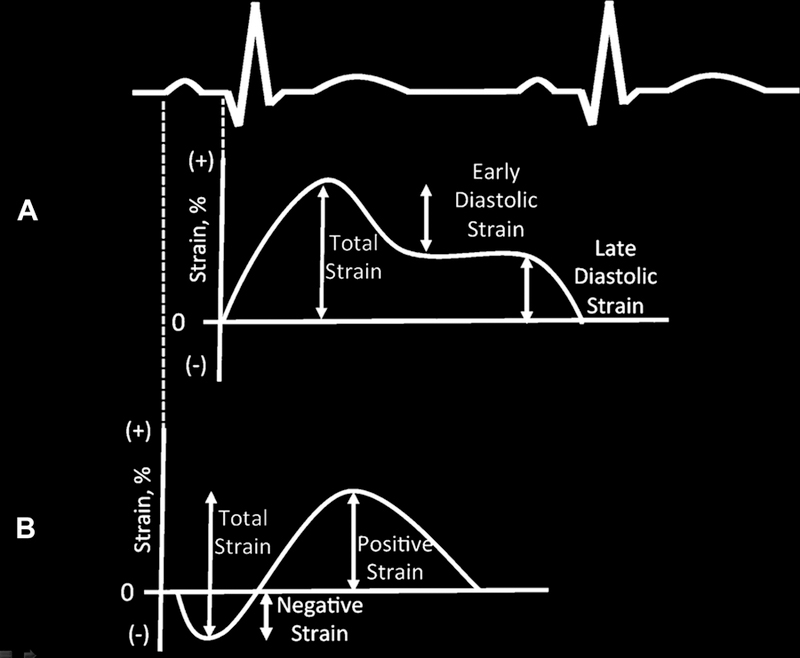
LA strain time curves and an electrocardiogram using an R-wave zero reference (A) and P-wave zero reference point (B). Using the R-wave reference point, the total LA strain is positive and the sum of the early and late diastolic strain. Using the P-wave reference point, the total LA strain is the sum of the negative and positive strain.
Fig. 9.
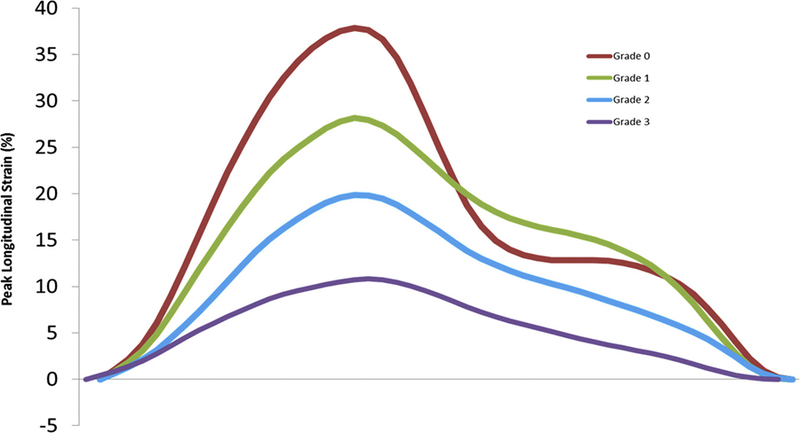
Peak longitudinal strain curves are depicted as the mean of each subgroup of diastolic dysfunction from grade 0 to grade 4. Diastolic dysfunction grade based on the 2009 ASE guidelines.
SUMMARY
Alterations in LA size and function have been associated with adverse cardiovascular outcomes. LA enlargement is both a marker of severity and chronicity of diastolic dysfunction and magnitude of LA pressure elevation. LA size assessment is important in routine clinical practice because it holds clinical and prognostic significance. LA volumes should be measured using dedicated, focused views and reported indexed to body surface area. Although 2DE methods for measuring LA volumes are recommended, 3DE methods are likely more accurate and are a stronger predictor of mortality. However, routine use of 3DE to obtain LA volumes is limited by the time required to analyze the data set to obtain this measurement and the lack of large population-based normal values. These issues are being addressed by the development of automated chamber quantification programs for 3DE data, and large 3DE studies on LA size in normal and abnormal patients. In addition, changes in LA strain are associated with clinical HFpEF and elevated filling pressures in patients with LV systolic dysfunction. Last, it has been demonstrated that medical therapy can result in reverse remodeling of the LA with improvement in size and function,33,34 suggesting the possibility of using LA as a future therapeutic target.
KEY POINTS.
The cause of left atrial enlargement is multifactorial and associated with adverse outcomes in multiple disease states.
Growing evidence supports the clinical importance of left atrial size and function for risk-stratification of patients with heart failure.
The left atrium modulates left ventricular filling via its reservoir, conduit, and booster functions. These can be measured volumetrically and with speckle tracking strain, are altered in response to age and diastolic function, and are correlated with outcomes.
Increasing accessibility and automation of 3-dimensional echocardiography and longitudinal strain analyses allow the application of left atrial size and function in routine clinical practice.
Acknowledgments
RML received a research grant from Philips Healthcare.
Footnotes
Disclosure: K.Y. Kebed and K. Addetia have nothing to disclose.
REFERENCES
- 1.Tsang TS, Barnes ME, Bailey KR, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc 2001;76(5):467–75. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida C, Nakao S, Goda A, et al. Value of assessment of left atrial volume and diameter in patients with heart failure but with normal left ventricular ejection fraction and mitral flow velocity pattern. Eur J Echocardiogr 2009;10(2):278–81. [DOI] [PubMed] [Google Scholar]
- 3.Moller JE, Hillis GS, Oh JK, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation 2003;107(17):2207–12. [DOI] [PubMed] [Google Scholar]
- 4.Barnes ME, Miyasaka Y, Seward JB, et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc 2004;79(8):1008–14. [DOI] [PubMed] [Google Scholar]
- 5.Rossi A, Temporelli PL, Quintana M, et al. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail 2009;11(10):929–36. [DOI] [PubMed] [Google Scholar]
- 6.Tsang TS, Barnes ME, Gersh BJ, et al. Risks for atrial fibrillation and congestive heart failure in patients ≤65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol 2004;93(1):54–8. [DOI] [PubMed] [Google Scholar]
- 7.Ho SY, McCarthy KP, Faletra FF. Anatomy of the left atrium for interventional echocardiography. Eur J Echocardiogr 2011;12(10):i11–5. [DOI] [PubMed] [Google Scholar]
- 8.Calvert PA, Rana BS, Kydd AC, et al. Patent foramen ovale: anatomy, outcomes, and closure. Nat Rev Cardiol 2011;8(3):148–60. [DOI] [PubMed] [Google Scholar]
- 9.Beigel R, Wunderlich NC, Ho SY, et al. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging 2014;7(12): 1251–65. [DOI] [PubMed] [Google Scholar]
- 10.Agmon Y, Khandheria BK, Gentile F, et al. Echocardiographic assessment of the left atrial appendage. J Am Coll Cardiol 1999;34(7):1867–77. [DOI] [PubMed] [Google Scholar]
- 11.Di Biase L, Santangeli P, Anselmino M, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60(6):531–8. [DOI] [PubMed] [Google Scholar]
- 12.Shah SJ, Bardo DM, Sugeng L, et al. Real-time three-dimensional transesophageal echocardiography of the left atrial appendage: initial experience in the clinical setting. J Am Soc Echocardiogr 2008;21(12):1362–8. [DOI] [PubMed] [Google Scholar]
- 13.Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014; 312(19):1988–98. [DOI] [PubMed] [Google Scholar]
- 14.Reddy VY, Doshi SK, Kar S, et al. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol 2017;70(24):2964–75. [DOI] [PubMed] [Google Scholar]
- 15.Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Ann Intern Med 1998;128(8): 639–47. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Fernandez MA, Torrecilla EG, San Roman D, et al. Left atrial appendage Doppler flow patterns: implications on thrombus formation. Am Heart J 1992;124(4):955–61. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Suwa M, Otake Y, et al. Assessment of left atrial appendage function after cardioversion of atrial fibrillation: relation to left atrial mechanical function. Am Heart J 1998;135(6 Pt 1):1020–6. [DOI] [PubMed] [Google Scholar]
- 18.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63(6):493–505. [DOI] [PubMed] [Google Scholar]
- 19.Thomas L, Levett K, Boyd A, et al. Compensatory changes in atrial volumes with normal aging: is atrial enlargement inevitable? J Am Coll Cardiol 2002; 40(9):1630–5. [DOI] [PubMed] [Google Scholar]
- 20.Anwar AM, Soliman OI, Geleijnse ML, et al. Assessment of left atrial volume and function by real-time three-dimensional echocardiography. Int J Cardiol 2008;123(2):155–61. [DOI] [PubMed] [Google Scholar]
- 21.Boyd AC, Schiller NB, Leung D, et al. Atrial dilation and altered function are mediated by age and diastolic function but not before the eighth decade. JACC Cardiovasc Imaging 2011;4(3):234–42. [DOI] [PubMed] [Google Scholar]
- 22.Nikitin NP, Witte KK, Thackray SD, et al. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr 2003;4(1):36–42. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto T, Robinet S, Dulgheru R, et al. Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2018;19(6): 630–8. [DOI] [PubMed] [Google Scholar]
- 24.Badano LP, Miglioranza MH, Mihaila S, et al. Left atrial volumes and function by three-dimensional echocardiography: reference values, accuracy, reproducibility, and comparison with two-dimensional echocardiographic measurements. Circ Cardiovasc Imaging 2016;9(7) [pii:e004229]. [DOI] [PubMed] [Google Scholar]
- 25.Appleton CP, Galloway JM, Gonzalez MS, et al. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol 1993;22(7):1972–82. [DOI] [PubMed] [Google Scholar]
- 26.Geske JB, Sorajja P, Nishimura RA, et al. The relationship of left atrial volume and left atrial pressure in patients with hypertrophic cardiomyopathy: an echocardiographic and cardiac catheterization study. J Am Soc Echocardiogr 2009;22(8):961–6. [DOI] [PubMed] [Google Scholar]
- 27.Guron CW, Hartford M, Rosengren A, et al. Usefulness of atrial size inequality as an indicator of abnormal left ventricular filling. Am J Cardiol 2005; 95(12):1448–52. [DOI] [PubMed] [Google Scholar]
- 28.Ersboll M, Andersen MJ, Valeur N, et al. The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging 2013;6(1):26–33. [DOI] [PubMed] [Google Scholar]
- 29.Tsang TS, Barnes ME, Gersh BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002; 90(12):1284–9. [DOI] [PubMed] [Google Scholar]
- 30.Tsang TS, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 2006;47(5):1018–23. [DOI] [PubMed] [Google Scholar]
- 31.Takemoto Y, Barnes ME, Seward JB, et al. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or 5 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol 2005;96(6):832–6. [DOI] [PubMed] [Google Scholar]
- 32.Gottdiener JS, Kitzman DW, Aurigemma GP, et al. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or 565 years of age (the cardiovascular health study). Am J Cardiol 2006;97(1):83–9. [DOI] [PubMed] [Google Scholar]
- 33.Tsang TS, Barnes ME, Abhayaratna WP, et al. Effects of quinapril on left atrial structural remodeling and arterial stiffness. Am J Cardiol 2006;97(6):916–20. [DOI] [PubMed] [Google Scholar]
- 34.Gerdts E, Wachtell K, Omvik P, et al. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: losartan intervention for endpoint reduction in hypertension trial. Hypertension 2007;49(2):311–6. [DOI] [PubMed] [Google Scholar]
- 35.Whitlock M, Garg A, Gelow J, et al. Comparison of left and right atrial volume by echocardiography versus cardiac magnetic resonance imaging using the area-length method. Am J Cardiol 2010;106(9): 1345–50. [DOI] [PubMed] [Google Scholar]
- 36.Rodevan O, Bjornerheim R, Ljosland M, et al. Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. Int J Card Imaging 1999;15(5):397–410. [DOI] [PubMed] [Google Scholar]
- 37.Nistri S, Galderisi M, Ballo P, et al. Determinants of echocardiographic left atrial volume: implications for normalcy. Eur J Echocardiogr 2011;12(11): 826–33. [DOI] [PubMed] [Google Scholar]
- 38.Maddukuri PV, Vieira ML, DeCastro S, et al. What is the best approach for the assessment of left atrial size? Comparison of various unidimensional and two-dimensional parameters with three-dimensional echocardiographically determined left atrial volume. J Am Soc Echocardiogr 2006;19(8):1026–32. [DOI] [PubMed] [Google Scholar]
- 39.Maceira AM, Cosin-Sales J, Roughton M, et al. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010; 12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kou S, Caballero L, Dulgheru R, et al. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging 2014;15(6):680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiamsripong P, Honda T, Reuss CS, et al. Three methods for evaluation of left atrial volume. Eur J Echocardiogr 2008;9(3):351–5. [DOI] [PubMed] [Google Scholar]
- 42.Iwataki M, Takeuchi M, Otani K, et al. Measurement of left atrial volume from transthoracic three-dimensional echocardiographic datasets using the biplane Simpson’s technique. J Am Soc Echocardiogr 2012;25(12):1319–26. [DOI] [PubMed] [Google Scholar]
- 43.Pritchett AM, Jacobsen SJ, Mahoney DW, et al. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol 2003; 41(6):1036–43. [DOI] [PubMed] [Google Scholar]
- 44.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18(12):1440–63. [DOI] [PubMed] [Google Scholar]
- 45.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16(3):233–70. [DOI] [PubMed] [Google Scholar]
- 46.Orban M, Bruce CJ, Pressman GS, et al. Dynamic changes of left ventricular performance and left atrial volume induced by the mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am J Cardiol 2008;102(11):1557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cacciapuoti F, Scognamiglio A, Paoli VD, et al. Left atrial volume index as indicator of left ventricular diastolic dysfunction: comparation between left atrial volume index and tissue myocardial performance index. J Cardiovasc Ultrasound 2012; 20(1):25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi K, Tanabe K, Tani T, et al. Left atrial volume in normal Japanese adults. Circ J 2006;70(3): 285–8. [DOI] [PubMed] [Google Scholar]
- 49.Kebed K, Kruse E, Addetia K, et al. Atrial-focused views improve the accuracy of two-dimensional echocardiographic measurements of the left and right atrial volumes: a contribution to the increase in normal values in the guidelines update. Int J Cardiovasc Imaging 2017;33(2):209–18. [DOI] [PubMed] [Google Scholar]
- 50.Mor-Avi V, Yodwut C, Jenkins C, et al. Real-time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging 2012;5(8): 769–77. [DOI] [PubMed] [Google Scholar]
- 51.Wu VC, Takeuchi M, Kuwaki H, et al. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc Imaging 2013;6(10): 1025–35. [DOI] [PubMed] [Google Scholar]
- 52.Narang A VV, Tamborini G, et al. 3D echocardiographic automated quantification of left ventricular and left atrial time-volume curves: comparison with MRI. Nashville (TN): American Society of Echocardiography; 2018. [Google Scholar]
- 53.Nunes MC, Handschumacher MD, Levine RA, et al. Role of LA shape in predicting embolic cerebrovascular events in mitral stenosis: mechanistic insights from 3D echocardiography. JACC Cardiovasc Imaging 2014;7(5):453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh A, Medvedofsky D, Mediratta A, et al. Peak left atrial strain as a single measure for the noninvasive assessment of left ventricular filling pressures. Int J Cardiovasc Imaging 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 55.Saraiva RM, Demirkol S, Buakhamsri A, et al. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr 2010;23(2): 172–80. [DOI] [PubMed] [Google Scholar]
- 56.Miglioranza MH, Badano LP, Mihaila S, et al. Physiologic determinants of left atrial longitudinal strain: a two-dimensional speckle-tracking and three-dimensional echocardiographic study in healthy volunteers. J Am Soc Echocardiogr 2016;29(11): 1023–34.e3. [DOI] [PubMed] [Google Scholar]
- 57.Buggey J, Hoit BD. Left atrial strain: measurement and clinical application. Curr Opin Cardiol 2018; 33(5):479–85. [DOI] [PubMed] [Google Scholar]
- 58.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17(12): 1321–60. [DOI] [PubMed] [Google Scholar]
- 59.Balaney B, Medvedofsky D, Mediratta A, et al. Invasive validation of the echocardiographic assessment of left ventricular filling pressures using the 2016 diastolic guidelines: head-to-head comparison with the 2009 guidelines. J Am Soc Echocardiogr 2018;31(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurt M, Wang J, Torre-Amione G, et al. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2009;2(1):10–5. [DOI] [PubMed] [Google Scholar]
- 61.Sun JP, Yang Y, Guo R, et al. Left atrial regional phasic strain, strain rate and velocity by speckletracking echocardiography: normal values and effects of aging in a large group of normal subjects. Int J Cardiol 2013;168(4):3473–9. [DOI] [PubMed] [Google Scholar]
- 62.Singh A, Addetia K, Maffessanti F, et al. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 2017;10(7):735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santos AB, Kraigher-Krainer E, Gupta DK, et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 2014; 16(10):1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cameli M, Lisi M, Mondillo S, et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound 2010;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hudsmith LE, Petersen SE, Francis JM, et al. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson 2005;7(5):775–82. [DOI] [PubMed] [Google Scholar]