Abstract
Blunted activation in the reward circuitry has been associated with anhedonia, the inability to experience pleasure in previously rewarding activities. In healthy individuals, reward-related activation has been found to be modulated by acute contextual factors such as induced positive mood. Accordingly, blunted reward response in anhedonia might involve a failure to appropriately modulate reward-related activation as a function of context. To test this hypothesis, 29 participants (19 females, mean age of 24.14 ± 4.61, age range 18-34), with a wide range of anhedonic symptoms, underwent functional MRI while anticipating and receiving monetary rewards, before and after a positive mood induction. Change in neural activation from before to after mood induction was quantified, and effects of anhedonia were investigated through whole-brain, ROI, and functional connectivity analyses. Contrary to hypotheses, results indicated that during reward anticipation (but not receipt), nucleus accumbens activation decreased while its connectivity with the dorsolateral prefrontal cortex increased, following positive mood induction. Critically, anhedonia modulated both effects. The unexpected finding of decreased activation to reward cues following positive mood induction is compelling as it aligns with a prominent behavioral model of the effect of positive mood on exploration of rewarding and neutral stimuli. Furthermore, the modulation of this effect by anhedonia suggests that it may be a key process altered in anhedonia.
Keywords: Reward, Anhedonia, Positive Mood Induction, Nucleus Accumbens, dorsolateral prefrontal cortex, Monetary Incentive Delay
1. INTRODUCTION
Anhedonia, the inability to experience pleasure from previously rewarding activities, is a core clinical symptom of major depressive disorder (MDD), as well as of other neuropsychiatric disorders (Whitton et al., 2015). The presence of anhedonia is associated with greater illness severity and longer episode duration in depressed adolescents (Gabbay et al., 2015), and poorer treatment outcome in depressed adults (McIntyre et al., 2016; Spijker et al., 2001). Furthermore, anhedonia shows considerable heritability: 46% of between-individual variance in hedonic capacity is accounted for by genetic factors, and reduced hedonic capacity predicts depression onset (Berenbaum & Connelly, 1993; Bogdan & Pizzagalli, 2009; Rawal, Collishaw, Thapar, & Rice, 2013). As such, anhedonia has been proposed as an “endophenotype” of depression, and is thought to have a distinct neurobiological and behavioral profile (Pizzagalli, 2014). Therefore, investigating the neural signature of anhedonia may enhance our understanding of MDD and elucidate pathways toward development of therapeutic interventions.
Prior studies have described behavioral deficits in reward processing in individuals with MDD (Arrondo et al., 2015; Pizzagalli et al., 2009; Pizzagalli et al., 2008; Smoski et al., 2009; Steele et al., 2007). More recent evidence suggests that anhedonia might account for the failure to learn appropriately from rewarding stimuli (Fletcher et al., 2015; Harlé et al., 2017; Pizzagalli, 2014; Vrieze et al., 2013). Reward processing is mediated by the corticostriatal circuit, with the nucleus accumbens (NAc) recognized as a core hub, and anhedonia has been found to influence reward-related activation in this circuitry. Specifically, anhedonia correlated negatively with NAc activation to positive stimuli (Keedwell et al., 2005) and reward learning signals in the NAc (Gradin et al., 2011). Anhedonia was also associated with increased activation to positive stimuli in the prefrontal cortex, which may modulate (and potentially inhibit) activation in the NAc (Keedwell et al., 2005). The NAc is thought to represent the relative rewarding value of cues (reward anticipation) and stimuli (reward consumption), information that may guide adaptive behavioral responses (Knutson, Adams, Fong, & Hommer, 2001; Segarra et al., 2016). Decreased activation in this region in individuals with anhedonia may indicate aberrations in neural encoding of rewarding stimuli (Segarra et al., 2016).
Notably, although the major input to the NAc is the midbrain ventral tegmental area, the NAc is also extensively connected to areas involved in adapting behavior to a changing internal and external environment, such as the prefrontal cortex (PFC), amygdala, and hippocampus (Heshmati & Russo, 2015). This suggests that the reward-value NAc signal is modifiable based on inputs from these other brain regions, due to changes in internal state (e.g., mood, hunger status) or external state (e.g., noise, stimulation). Altered NAc activation in anhedonia, therefore, could indicate not only a change in response to rewarding stimuli, but also a change in state-based modulation of the NAc activity. Consistent with these notions, recent evidence demonstrated that while participants engaged in a task designed to put them in positive mood state, functional connectivity between the NAc and prefrontal cortex changed significantly in healthy individuals, but individuals with anhedonia failed to show such a change (Young et al., 2016). Another study similarly found that while healthy controls showed a change in corticostriatal connectivity following positive mood induction, individuals with remitted depression, who may experience anhedonia, showed no such change in connectivity (Admon & Pizzagalli, 2015). Interestingly, in the latter study, both healthy individuals and those with remitted depression showed acute increases in mood, but only healthy controls sustained this increase when tested after a delay. Therefore, individuals with past MDD might be impaired in sustaining a positive mood state, and it is possible that this failure to sustain mood is related to the observed failure to modulate brain connectivity based on positive mood state.
One possible mechanism through which changes in corticostriatal connectivity due to positive mood state could occur is a bottom-up shift in how the brain attends to rewarding stimuli. This possibility is supported by behavioral studies, which have demonstrated that positive mood induction changes how individuals respond to stimuli associated with rewards. The direction of this change remains equivocal: one study found increased orientation towards reward-related words following positive mood induction (Tamir & Robinson, 2007), while other studies found that positive mood induction decreased selective orientation towards rewarding stimuli, causing healthy individuals to “explore” other stimuli more by engaging equally with neutral (putatively non-rewarding) and positively-valenced (putatively rewarding) images (Wadlinger & Isaacowitz, 2006), and attending more to the environment surrounding positively-valenced images (Grol & De Raedt, 2014). Interestingly, the latter effect was not seen in individuals with high levels of depressive symptoms (Grol & De Raedt, 2014). Recently, Young & Nusslock (2016) provided initial evidence that such modulation of reward processing by positive mood induction occurs at a neural level too. They found that, among healthy controls, anticipation of reward elicited greater activation in reward-related regions such as the striatum and orbitofrontal cortex after positive mood vs. neutral mood induction.
Although Young & Nusslock investigated the effects of positive mood induction on reward-related brain activation acutely, and in healthy controls, no study has explored whether these effects sustain past the acute post-induction period. Furthermore, no study has investigated whether anhedonia affects the brain’s modulation of reward-related activation following positive mood induction. This latter investigation could be important to the study and treatment of anhedonia: if positive mood induction increases neural response to reward in individuals with anhedonia, as it has been reported in healthy individuals, it could potentially have therapeutic implications in individuals with anhedonia. Alternatively, positive mood induction could have differential effects on the brain’s response to reward in individuals with high anhedonia, compared to those with low anhedonia, suggesting an important neural substrate of the blunting of positive experiences in individuals with high anhedonia. It is further possible that the acute effects of positive mood induction on the brain’s response to reward are equivalent in individuals with high and low anhedonia, but the sustained effect is only observed in individuals with low anhedonia, dissipating quickly in individuals with high anhedonia.
To investigate these important questions, the present study had two aims. The first aim was to quantify acute and sustained effects of positive mood induction on neural correlates of reward processing, expanding upon the work of Young & Nusslock (2016). The second aim was to investigate whether anhedonia influenced neural activation post mood induction. To accomplish these aims, neural responses to rewarding vs. neutral stimuli were measured using the Monetary Incentive Delay (MID) task, a well-validated probe of incentive motivation. Positive mood induction was accomplished using a positive performance feedback paradigm, adapted from Admon & Pizzagalli (2015; Figure 1). We were interested in capturing both acute and sustained effects of mood induction, as individuals with high anhedonia could differ either in the magnitude of their change in neural reward response following positive mood induction, or in how long the change lasts. Therefore, the present study probed neural responses to rewarding vs. neutral stimuli before (MID 1), immediately following (MID 2 – acute), and after a delay (MID 3 – sustained) following positive mood induction (Figure 2). To evaluate a sample with a wide range of depressive (including anhedonic) symptoms, we initially recruited equal numbers of individuals with low and high levels of depressive symptoms. We hypothesized that positive mood induction would increase NAc activation to reward relative to neutral stimuli, and that this change would be dampened by anhedonic symptoms. Specifically, we hypothesized that higher anhedonic symptoms would be associated with a smaller increase in reward-related neural activation in the NAc following positive mood induction, both immediately (acute effect) and after a delay (sustained effect). Additionally, we analyzed whole-brain functional connectivity with the nucleus accumbens at the same three time-points to explore potential alterations in connectivity with the prefrontal cortex.
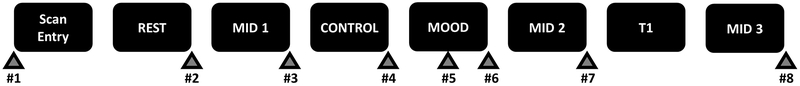
Figure 1: Full imaging sequence.
The study commenced with a resting-state scan, followed by the first run of the MID task. Next participants completed the control and humor runs of the positive mood induction, followed by the second run of the MID task, structural (T1-weighted) and third run of the MID task scans. Triangles indicate mood measurements (VAMS).
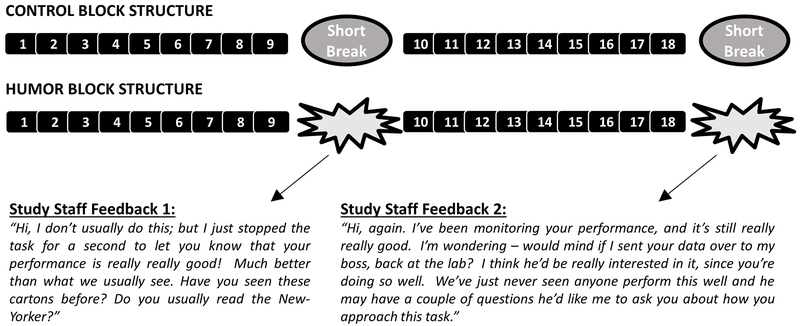
Figure 2: Positive Mood Induction procedure.
Participants experience two sequential runs: one “control” run in which they become accustomed to task design and receive no feedback, and one “humor” run in which they receive highly positive on-screen performance feedback on majority of trials, and were also given highly positive performance feedback from study staff twice (Study Staff Feedbacks 1 & 2). See Supplementary Methods for sample stimuli. See Admon & Pizzagalli (2015) for additional task details.
Findings revealed that positive mood induction indeed changed the brain’s response to rewarding stimuli, and the magnitude of this response decreased with increasing anhedonic symptoms. However, the direction of the change was opposite that anticipated, a surprising outcome that aligns with a prominent theory of positive mood, the “Broaden and Build” model (to be addressed in the Discussion). Changes in corticostriatal connectivity were also seen following positive mood induction and were also modulated by anhedonic symptoms.
2. METHODS
2.1. Participants
To ensure enrollment of participants with varying degrees of depressive (including anhedonic) symptoms, we recruited 23 individuals with low/no depressive symptoms (BDI-II score < 10) and no current or past psychiatric illness, and 17 individuals with elevated depressive symptoms. Participants were recruited from the Harvard College Psychology Department study pool and the community via the Craigslist website. Participants gave informed written consent to a protocol approved by Harvard University’s Committee on the Use of Human Subjects (CUHS) and were compensated with a $5 Amazon gift card for questionnaire completion. Participants deemed to be eligible were then contacted via phone call to confirm eligibility and set up a scanning session, which took place at Harvard University’s Center for Brain Science. To be eligible, participants had to be fluent in English, free of serious or unstable medical illnesses, right-handed, report no contraindications to MRI, report no current or history of substance abuse and dependence, and have normal or corrected to normal vision. Exclusion criteria for the group with low/no depressive symptoms included current or past psychiatric illnesses (assessed by phone screening). Exclusion criteria for the group with elevated depressive symptoms included use of any psychotropic medications in the past 2 weeks (6 months for dopaminergic drugs or antipsychotics; 6 weeks for fluoxetine; 2 weeks for benzodiazepines). Participants could not have smoked cannabis before the age of 14, or be regular users of nicotine or cannabis. All participants were non-cigarette smokers, except one individual who reported smoking no more than three cigarettes on any given day, but not smoking every day. Participants’ psychiatric status was assessed through an initial phone screening, which confirmed no present or past psychiatric illness in the individuals recruited to the “low/no depressive symptoms” group, and no present or past psychiatric illness other than depression in the individuals recruited to the “high depressive symptoms” group. However, as structured clinical interviews were not conducted, psychiatric diagnosis or lack thereof was not clinically confirmed.
Data from 29 participants were available for analyses of MID 1 and MID 2 (15 individuals with low/no depressive symptoms (11 females) and 14 individuals with high depressive symptoms (8 females). Data from 28 participants were available for analyses of MID 3. Rationale for participant exclusions are detailed in the results section. The age range for the 29 participants was 18 – 34, with a mean age of 24.14 ± 4.61. There was a broad range of anhedonia scores in the sample, from 14 (the lowest score possible) to 38 on the Snaith Hamilton Pleasure Scale, with a mean score of 25.24 ± 7.46. Importantly, although we collected data for two groups based on BDI, we found that anhedonia, our main measure of interest, was widely distributed within both the high depressive symptoms and the low/no depressive symptoms group – anhedonia ranged from 14 to 27 in the low/no depressive symptoms group (mean 20.47 ± 4.91), and from 14 to 38 in the high depressive symptoms group (mean 30.36 ± 6.28). Given the wide range of anhedonia scores present in the sample and the heterogeneity across both groups, all further analyses were conducted across the entire sample using a dimensional approach.
2.2. Study Design
Participants completed a single imaging session (Figure 1) during which they performed a monetary incentive delay task (Knutson et al., 2001; Supplementary Figure S1) before (MID 1) and immediately after (MID 2) a positive mood induction task (“humor intelligence test”; see below), as well as following a delay (~10 min; MID 3; Figure 1). Participants also completed a Visual Analog Mood Scale (VAMS) to assess their mood at baseline, and pre- and post-mood induction to gauge mood changes over time. In addition, they completed the Beck Depression Inventory-II (BDI-II; Beck et al., 1988), the Snaith Hamilton Pleasure Scale (SHPS; Snaith et al., 1995), Positive and Negative Affect Schedule (PANAS; Watson et al., 1988), Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995), and Perceived Stress Scale (PSS; Cohen et al., 1983) to assess depressive, anhedonic, afffect and perceived stress symptoms. They were compensated $50 for participation in the study, and $30 in “bonus” earnings from the MID task.
2.2.1. Visual Analog Mood Scale (VAMS)
Affective states were repeatedly assessed throughout the session using a visual analogue scale that asked participants to rate how they felt in the moment ranging from “Very Negative” (0) to “Very Positive” (100), by moving a slider bar to the appropriate distance along this scale.
2.2.2. Monetary Incentive Delay Task (MID)
The MID task was designed to probe reward anticipation and reward consumption, and has been extensively utilized and validated (Supplementary Figure S1; Knutson et al., 2008; Kumar et al., 2015). Briefly, each trial began with a visual cue (0.5s) indicating the potential outcome (reward: +$; no-incentive: 0$), associated with performance. After a variable inter-stimulus interval (2.4-3.9s), a red target square was briefly presented (0.16s) to which participants responded by pressing a button as quickly as possible. After a second variable delay (2.25-3.75s), visual feedback indicated the trial outcome (reward or no change). A variable inter-trial interval (1.5-4.5s) separated the trials. Participants were told that the outcome depended on their speed with which they pressed the button after the presentation of the target. In the reward condition, wins were associated with monetary gains, which were randomly selected values within the range of $1.96-$2.33. There was no gain associated with reward trials in which participants’ reaction times fell out of the 70th percentile window (determined after the practice block) or with no-incentive trials. The task included 16 reward and 16 no-incentive trials, divided into two blocks. To obtain individual RT cutoffs, participants initially completed a practice task identical to the design described above except no feedback was displayed.
2.2.3. Humor Intelligence Task (Positive Mood Induction)
The humor task used in this study was a modified version of a mood manipulation recently developed (Figure 2; Admon & Pizzagalli, 2015). The task was divided into one control and one humor block, each with 18 trials. During both blocks, participants were shown a cartoon and three captions underneath, with one of the captions randomly marked with a cursor. All cartoons were taken from the New Yorker magazine Cartoon Caption Contest, a contest in which readers are asked to submit their funniest caption and the public then votes on which of three finalist captions should win.
Control block:
During the control block, participants were shown one cartoon and three descriptive sentences; they were instructed to choose the most accurate sentence that described the cartoon. They were further informed that they would not receive any feedback on their performance and the goal of this block was to become familiarized with the task. Each cartoon and three sentences were presented until the participant made a selection (or up to 18s in case no selection was made), and were then followed by an empty screen (null feedback; 6s). The block (~15 min) was separated into two sections of 9 trials with a short break in between. Participants completed a VAMS rating after this block, which was used as their pre-induction mood rating.
Humor block:
Participants then completed the humor block, in which they were again shown sequential cartoons, but this time with the three finalist captions. Participants were instructed to choose, for each cartoon, which one of the three captions they thought won the New Yorker Caption Contest. The overall design of the humor block was identical to the control block, only this time participants were told that they could receive feedback on their performance. Regardless of their accuracy, on 14 of the 18 trials, participants were presented with a screen indicating that their selection was correct (positive feedback). Positivity and believability of the feedback was enforced by including participants’ actual response time in the feedback screen, and indicating that they were faster than prior participants by a random number of seconds (1-3s). No feedback was given if no selection was made, and no feedback on response time was given if slower than the participant’s mean+2SD until that trial. Intensity of positive mood induction was further increased by explicit feedback in two stages. Halfway through the humor block (at the end of the first 9 trials), the scan was “paused” and study staff delivered positive feedback on their performance (Study Staff Feedback 1; exact text shown in Figure 2). Participants then completed another VAMS rating. At the end of the second 9 trials, study staff delivered further positive feedback on their performance (Study Staff Feedback 2; exact text shown in Figure 2). Participants completed another VAMS rating at the end of the humor block. More information about reliability of task stimuli and design can be found in Admon & Pizzagalli (2015). The final design and believability of the mood induction was optimized by running three pilot versions involving 62 independent participants (see Supplement).
2.3. fMRI Data Acquisition
Participants were scanned on a Siemens Magnetom Prisma 3T scanner at the Center for Brain Science, Harvard University using a 64-channel phase arrayed coil. Structural images were acquired using a T1 magnetized-prepared rapid acquisition with gradient echo (MPRAGE) imaging sequence with the following acquisition parameters: 176 sagittal slices, repetition time = 2530ms, echo time = 1.69ms, 7° flip angle, 1 × 1 × 1 mm voxels, field of view = 256mm. Functional images were acquired using a T2*-weighted multiband EPI sequence developed at the University of Minnesota Center for Magnetic Resonance Research (Feinberg et al., 2010; Moeller et al., 2010; Xu et al., 2013). The sequence included the following acquisition parameters: repetition time = 2000ms, echo time = 30ms, 80° flip angle, 1.5 x 1.5 x 1.5 mm voxels, field of view = 204mm, multiband factor = 3.
2.4. fMRI Data Preprocessing
Functional MRI data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm). Raw functional and structural images were inspected for artifacts. Functional images were then realigned to the mean image of the series, corrected for motion and slice timing related artifacts, and co-registered to an anatomical image. Functional images were then normalized to the 2x2x2 MNI template, and smoothed with a 4mm Gaussian kernel. Manual checks were performed following motion correction, co-registration and segmentation. Following motion correction, motion parameters were examined for each participant and the data of any participant showing > 3mm movement in any of the three assessed directions were discarded. Structural images were segmented into white matter, gray matter, and CSF using SPM.
2.5. Data Analyses
Analyses for affective ratings, reaction times, and neural data focused on investigating acute and sustained effects of positive mood induction on reward processing. Acute phase refers to change from Time 1 (baseline) to Time 2 (immediate post-mood induction) and sustained phase refers to change from Time 2 (immediate post-mood induction) to Time 3 (delayed post-mood induction). To this end, two repeated measures ANOVA were run to investigate acute and sustained effects separately.
2.5.1. Behavioral Analyses
Change in mood from pre- to post-mood induction (measured by the Visual Analog Mood Scale) was assessed using a paired t-test across the whole cohort. Acute mood effects on reaction time during the MID task was analyzed with a 2 × 2 repeated measures ANOVA with Incentive (Reward, Neutral) and Time (MID 1, MID 2) as within-subject factors. Similarly, sustained effects were analyzed with a repeated measures Incentive (Reward, Neutral) and Time (MID2, MID 3) ANOVA. Relationships with Anhedonia were investigated for each of these measures.
2.5.2. fMRI Analyses
Statistical analyses of single-subject fMRI data were implemented using a general linear model (GLM) with regressors corresponding to reward cue, no-incentive (neutral) cue, successful reward feedback, unsuccessful reward feedback, and no-change feedback (no-incentive condition). Each event was constructed with a hemodynamic response function, modeled using a gamma function, convolved with onset times of events and stimulus duration. The six rigid-body motion time courses from the motion correction, target, errors (i.e., when the button was pressed before the target presentation) and inter-stimulus intervals (ISIs) were included as covariates of no interest, with a total of 15 regressors in each single-subject design matrix. Contrast maps were constructed for reward anticipation (reward vs. neutral cue) and consumption (gain vs. no-change feedback) for each MID session: MID 1, MID 2 and MID 3. These contrast maps were used in ROI-based statistical analyses to test a priori hypotheses as well as for whole-brain main effects analysis evaluating brain regions affected by the task. To investigate the neural correlates of reward anticipation and consumption at baseline (MID 1) and to validate the task, a one-sample t-test was conducted across all subjects. Cluster correction at p < 0.05 family-wise error (FWE) with an initial voxel forming threshold of p < 0.001 was utilized.
2.5.3. ROI Analyses
To test a priori hypotheses that positive mood induction would affect reward-related activation in the nucleus accumbens, we created left and right anatomical NAc ROIs from the FSL Harvard-Oxford Subcortical Atlas using a 40% probability threshold. For each MID run, parameter estimates from each of the two ROIs (left and right NAc) were extracted from reward vs. no-incentive cue (hereby referred to as “Anticipation”) and gain vs. no-change feedback (hereby referred to as “Consumption”) contrast maps of each subject and were entered into SPSS (version 22). Throughout the analyses, data were inspected for the presence of outliers. Values that exceeded three times the inter-quartile range (the difference between the third and first quartile) of mean parameter estimates were deemed to be outliers and were further investigated to identify if they were due to motion, registration error, or other sources of artifacts. If no problems could be identified and corrected, outlier data points were removed from the analyses.
After inspection for outliers, a 2 x 2 repeated measures ANOVA with Time (MID 1, MID 2) and Hemisphere (Right NAc, Left NAc) as within-subject factors was run to investigate acute mood induction effects on both reward anticipation and consumption. A second 2 x 2 repeated measures ANOVA with Time (MID 2, MID 3) and Hemisphere (Right NAc, Left NAc) as within-subject factors was run to investigate sustained mood induction effects on both reward anticipation and consumption. As our primary aim was to investigate the influence of anhedonia on the neural changes associated with positive mood induction, anhedonia was entered as a covariate in these ANOVAs. Post-hoc analyses proceeded based on the results of the ANOVAs, using paired t-tests and Pearson correlations. Comparison of correlations were conducted through Meng’s test, which was used to test for a significant difference between two dependent correlation coefficients (Meng, Rosenthal, & Rubin, 1992).
2.5.4. Whole-Brain Analyses
An exploratory whole-brain analysis was also performed to investigate brain regions beyond the NAc that were affected by positive mood induction. To investigate the neural correlates of reward anticipation and consumption at baseline (MID 1), as well as acute (MID 1 – MID 2) and sustained effects (MID 2 – MID 3) of positive mood induction, one-sample and paired t-tests, respectively, were conducted across all subjects. Cluster correction at p < 0.05 family-wise error (FWE) with an initial voxel forming threshold of p < 0.001 was utilized.
2.5.5. PPI Connectivity Analyses
To assess potential alterations in connectivity between the NAc and other reward-related brain regions following positive mood induction, functional connectivity analyses were implemented via the gPPI toolbox (http://www.nitrc.org/projects/gppi) using the left NAc and right NAc as seeds, separately. Anhedonia score was entered as a covariate at the whole-brain level to assess the effects of anhedonia on NAc connectivity change post-mood induction. Parameter estimates (i.e., mean connectivity values) were extracted from clusters that survived p < 0.05 family-wise error (FWE) correction (initial voxel forming threshold of p < 0.001) and follow-up analyses were conducted in SPSS.
3. RESULTS
3.1. Participant Exclusions
Of the 34 participants who completed the study, the full datasets from five participants were excluded from the final analyses (two due to computer error, one due to excessive movement > 3mm, and two due to task non-compliance). For one additional participant, data for MID 1 and MID 2 were included, but data were from MID 3 were excluded as they failed to complete the task during this block. No participants were removed as outliers from the statistical analyses, as no parameter values exceeded three times the interquartile range for any participant.
3.2. Behavioral Results
3.2.1. Effectiveness of the Positive Mood Induction
The mood induction was extensively piloted to ensure validity prior to the present study (see Supplementary Methods for information regarding pilot studies). Full mood data from two participants were lost due to technical errors, and these participants were not included in mood analyses. Four additional participants were missing one mood score each and were included in mood analyses. Consistent with prior work, baseline mood (collected just before the scan), as measured by visual analog mood scale, correlated with anhedonia (r = −0.47, p = 0.014). To test the effects of the mood induction, mood data collected just after the control block (VAMS timepoint #4) were subtracted from mood data collected just after the second positive feedback (VAMS timepoint #6; see Figure 3).
Figure 3: Average mood change across study.
Each point represents an averaged measurement of mood ratings on a 0-100 scale. * denotes p < .05.
Acute:
A paired t-test confirmed a significant increase in mood [t(26) = 2.96, p = 0.006, dz = 0.71] immediately following mood induction [from before positive mood induction (VAMS timepoint #4) to after second positive feedback (VAMS timepoint #6); Figure 3]. Change in mood, however, was not correlated with anhedonia score (r = −0.07, p = 0.73).
Sustained:
A paired t-test demonstrated a significant decrease in mood [t(26) = 2.9, p = 0.006, dz = 0.50] from immediately after mood induction [after second positive feedback (VAMS timepoint #6) to the “sustained mood” time-point (before MID 3; VAMS timepoint #7]. There was no correlation between change in mood from the acute to the sustained time-point and anhedonia (r = 0.32, p = 0.10).
3.2.2. Reaction Time during MID tasks
Consistent with prior studies utilizing the MID task (Supplementary Figure S1), faster reaction times in response to reward vs. neutral cues were observed across all participants during MID 1 [t(29) = 9.91, p < 0.001, dz = 1.75], confirming that the task elicited the intended behavioral effects.
Acute:
A 2 × 2 Incentive (Reward, Neutral) x Time (MID 1, MID 2) repeated measures ANOVA of reaction times during the MID task revealed a significant main effect of Incentive [F(1,28) = 101.20, p < 0.01, η2p = 0.78]. No main effect of Time or Incentive x Time interaction emerged (all ps > 0.31). Paired t-tests confirmed that, similar to MID 1, participants were faster to reward vs. neutral cues during MID 2 [t(29) = 6.89, p < 0.001, dz = 1.60]. There was no correlation between anhedonia and reaction time to reward or neutral cues at MID 1 or MID 2 (all ps > 0.45).
Sustained:
A 2 × 2 Incentive (Reward, Neutral) x Time (MID 2, MID 3) repeated measures ANOVA on reaction times during the MID task revealed a significant effect of Incentive [F(1,27) = 65.62, p < 0.01, η2p = 0.71]. No main effect of Time or Incentive x Time interaction emerged (all ps > 0.25). Paired t-tests confirmed significant differences between reaction time to reward vs. neutral cues at MID 3 [t(27) = 6.83, p < 0.001, dz = 1.40], in line with results during MID 1 and MID 2. Similarly, there was no correlation between anhedonia and reaction time to reward or neutral cues at MID 3 (all ps > 0.32).
Collectively, these findings indicate that the current version of the MID elicited the intended behavioral effects, which were similar across blocks, and that such effects were not further modulated by anhedonia.
3.3. Imaging Results
3.3.1. Whole Brain Analyses: MID Task Validation (MID 1)
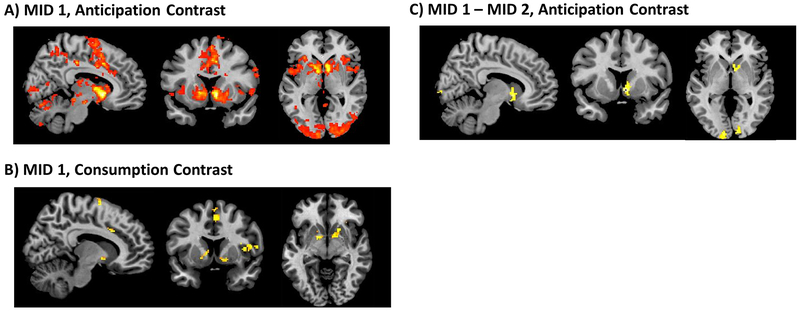
Replicating prior findings (Knutson et al., 2001), a whole-brain analysis across all subjects during MID 1 revealed significant clusters in the NAc, caudate and midcingulate during reward anticipation (Figure 4A; full cluster list in Supplementary Table 1). During reward consumption, significant clusters emerged in an array of regions including the bilateral insula, dorsolateral prefrontal and orbitofrontal cortices, in line with prior studies using the MID task (Kumar et al., 2014; Oldham et al., 2018) (Figure 4B; full cluster list in Supplementary Table 2).
Figure 4: Whole-brain main effects for whole group (n = 29).
A) MID 1 Anticipation (Cue Reward – Cue Neutral), B) MID 1 Consumption (Feedback Reward – Feedback Neutral), and C) Change in reward anticipation (Cue Reward – Cue Neutral) from MID 1 to MID 2. Clusters significant at FWE p < 0.05 with initial cluster forming threshold p < 0.001. Significant clusters reported in Supplementary Methods.
3.3.2. ROI Analyses: NAc Activation to Reward Anticipation Following Mood Induction
Acute:
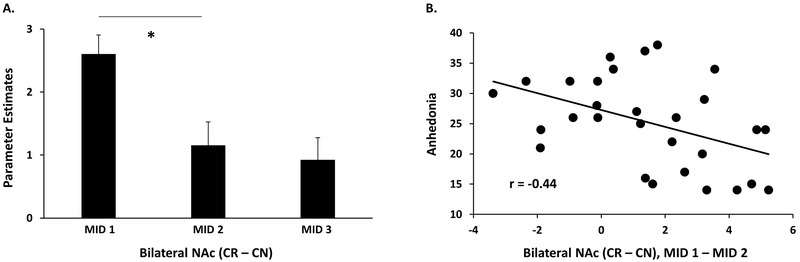
A repeated measures Time (MID 1, MID 2) x Hemisphere (Left NAc, Right NAc) ANOVA for reward anticipation with Anhedonia as covariate revealed a significant main effect of Time [F(1,27) = 11.94, p = 0.002, η2p = 0.31] and a significant Time x Anhedonia interaction [F(1,27) = 6.49, p = 0.017, η2p = 0.19]. Given lack of an interaction with Hemisphere, the left and right NAc were merged into a bilateral NAc ROI for further analyses. Contrary to our hypothesis, a paired t-test demonstrated a significant decrease, from before to after mood induction, in bilateral NAc activation during anticipation [t(28) = 3.30, p = 0.003, ds = 0.80; Figure 5A]. Moreover, a Pearson correlation showed that this change in activation was negatively associated with anhedonia (r = −0.44, p = 0.017), in line with the observed Time x Anhedonia interaction (Figure 5B). Follow-up analyses to further interrogate the data revealed two important findings. First, even though the individual correlations at MID 1 and MID 2 did not survive statistical significance, the correlation between anhedonia and reward anticipation was significantly different between MID 1 (r = −0.28, p = 0.15) and MID 2 (r = 0.29, p = 0.12); Meng’s test: z = −2.27, p = 0.002 (Meng et al., 1992; Supplementary Figures S2A & S2B). This was driven by the fact that anhedonia was associated with lower NAc activation to reward anticipation pre-mood induction, but with higher NAc activation to reward anticipation post-mood induction. Specifically, and unexpectedly, higher anhedonia was associated with a relative increase in NAc activation during reward anticipation after the positive mood induction. Median split analyses quantifying this difference in activation change in individuals with high vs. low anhedonia are included in the Supplementary Methods for visualization of these results (Supplementary Figure S3A). Second, follow-up analyses of valence-specific effects revealed that anhedonia interacted differently in response to reward vs. neutral cues, from MID 1 to MID 2. Even though the valence-specific individual correlations with anhedonia were not significant, the correlation between anhedonia and NAc activation was significantly different during reward (r = −0.17, p = 0.39), and neutral cues (r = 0.10, p = 0.62) as shown by Meng’s test (z = −2.25, p = 0.02; Supplementary Figures S2C & S2D). Therefore, the NAc activation to reward and neutral cues approached equalization following positive mood induction in individuals with low anhedonia, but not in those with high anhedonia.
Figure 5: Bilateral NAc activation during reward anticipation and its relationship with anhedonia.
A). Mean activation of bilateral NAc during reward anticipation (Cue Reward – Cue Neutral) from MID 1, MID 2 and MID 3. B) Correlation between anhedonia and change in NAc activity during reward anticipation (Cue Reward – Cue Neutral) from MID 1 (before mood induction) to MID 2 (after mood induction). CR = Cue Reward, CN = Cue Neutral.
Sustained:
A 2 x 2 Time (MID 2, MID 3) x Hemisphere (Left, Right) ANOVA for reward Anticipation with Anhedonia as a covariate of interest demonstrated no significant main effects or interactions (Figure 5A, all ps > 0.12), suggesting that the effects of the positive mood induction persisted from acute to the sustained time-point, independent of anhedonia.
3.3.3. ROI Analyses: NAc Activation to Reward Consumption Following Mood Induction
Acute:
A repeated measures Time (MID 1, MID 2) x Hemisphere (Left NAc, Right NAc) ANOVA for reward consumption with Anhedonia as a covariate of interest revealed no significant main effects or interactions (all ps > 0.20).
Sustained:
A repeated measures Time (MID 2, MID 3) x Hemisphere (Left NAc, Right NAc) ANOVA for reward consumption with Anhedonia as a covariate of interest revealed no significant main effects or interactions (all ps> 0.13).
3.3.4. Exploratory Whole Brain analyses: Change in Reward Anticipation Following Mood Induction
Acute:
A whole-brain paired t-test between reward anticipation during MID 1 (pre-mood) vs. MID 2 (acute post-mood) confirmed a significant cluster in the right NAc (Figure 4C; full cluster list in Supplementary Table 1), characterized by greater activity in the right NAc for the reward anticipation contrast (reward cue vs. neutral cue) during MID 1 compared to MID 2, consistent with results from the ROI analysis.
Sustained:
A whole brain paired t-test between reward anticipation during MID 2 vs. MID 3 revealed no significant clusters.
3.3.5. Exploratory Whole Brain analyses: Change in Reward Consumption following Mood Induction
Acute:
A whole brain paired t-test between reward consumption during MID 1 vs. MID 2 revealed no significant clusters.
Sustained:
A whole brain paired t-test between MID 2 vs MID 3 also revealed no significant clusters.
3.3.6. Functional Connectivity (PPI) Analyses: Changes in NAc Connectivity Following Mood Induction during Reward Anticipation
Acute (MID 1 – MID 2):
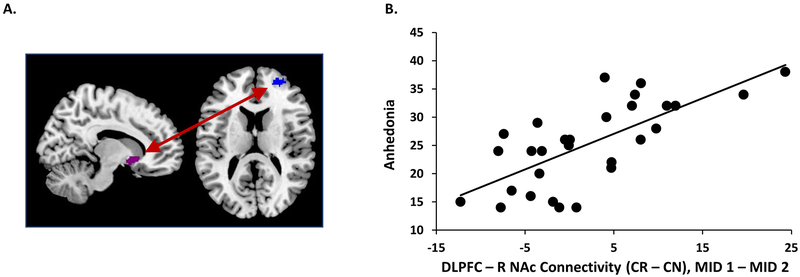
As significant changes in NAc activation to reward anticipation, but not reward consumption, were apparent immediately following mood induction, functional connectivity analyses focused only on the effects of acute mood induction on reward anticipation. The right and left NAc ROIs were used as seed regions and Anhedonia as covariate of interest. A significant dorsolateral prefrontal (DLPFC) cluster emerged with the right NAc seed (Figure 6A), whereas no significant clusters emerged with the left NAc (initial voxel forming threshold of p < 0.001 and p < 0.05 FEW cluster correction). Beta weights were extracted from the DLPFC cluster to further evaluate these effects. As shown in Figure 6B, there was a robust correlation between anhedonia and change in DLPFC-right NAc connectivity during reward anticipation, from MID 1 to MID 2: specifically, higher anhedonia predicted a smaller increase in the DLPFC-right NAc connectivity. Further, a bivariate correlation demonstrated that the change in the DLPFC-NAc connectivity during anticipation from MID 1 to MID 2 was negatively correlated with the change in anticipatory right NAc activity from MID 1 to MID 2 (r = −0.47, p = 0.001, n = 29).
Figure 6: DLPFC-Right NAc connectivity during reward anticipation and its relationship with anhedonia.
A). The connectivity between the seed - right NAc (pink) and DLPFC (blue) from the whole brain interaction with anhedonia. Peak voxel [32 56 14], 50 voxels, cluster significant at p = 0.05 (FWE), with p < 0.001 initial cluster forming threshold. B.) Scatterplot showing the correlations between anhedonia and right NAc-DLPFC connectivity change during Anticipation (Cue Reward – Cue Neutral) from MID 1 (before mood induction) and MID 2 (after mood induction).
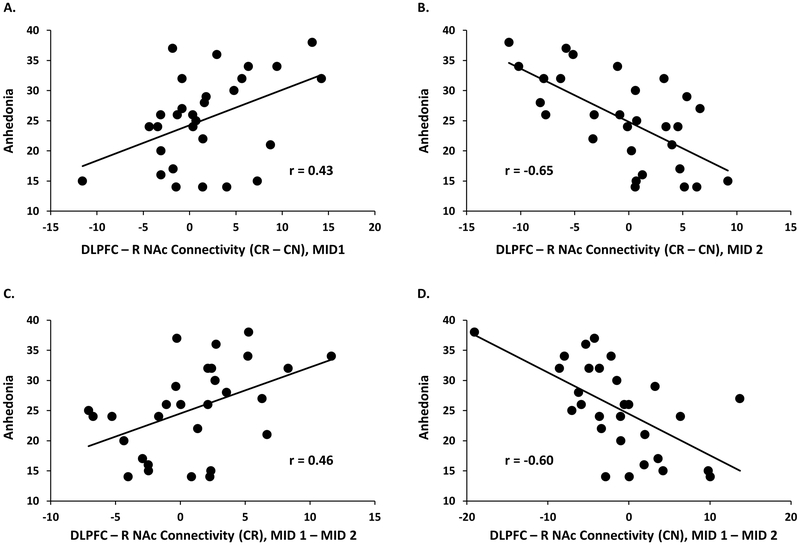
This correlation was due to a reversal of the relationship between anhedonia and DLPFC-right NAc connectivity, from MID 1 to MID 2 (Figure 7). At MID 1, anhedonia scores were positively correlated with DLPFC-right NAc connectivity during anticipation (r = 0.43, p = 0.02; Figure 7A) while at MID 2, they were negatively correlated with DLPFC-right NAc connectivity during anticipation (r = −0.65, p < 0.001; Figure 7B) and these correlations were significantly different (Meng’s test z = 3.64, p < 0.001). To determine the valence-specific effects on the relationship between anhedonia and connectivity change, we extracted connectivity values during each condition separately, calculating a connectivity change value as [connectivity MID 1 – connectivity MID 2]. From before to after positive mood induction, anhedonia was positively correlated with DLPFC-right NAc connectivity change from MID 1 to MID 2 during reward cues, indicating that lower anhedonia was associated with an increase in connectivity strength during reward cues, while higher anhedonia was associated with a decrease in connectivity strength during reward cues with positive mood induction (r = 0.46, p = 0.012; Figure 7C). In contrast, anhedonia was negatively correlated with DLPFC-right NAc connectivity change from MID 1 to MID 2 during neutral cues, indicating that lower anhedonia was associated with a decrease in connectivity strength during neutral cues, while higher anhedonia was associated with an increase in connectivity strength during neutral cues (r = −0.60, p = 0.001; Figure 7D). A Meng’s test confirmed that these correlations were significantly different (z = 3.86, p < 0.001). Median split analyses quantifying these differences in connectivity change in individuals with high vs. low anhedonia are included in the Supplementary Methods (Supplementary Figure S3B)
Figure 7: DLPFC-Right NAc connectivity and its relationship with anhedonia.
A). Correlations between anhedonia and DLPFC-right NAc connectivity during reward anticipation (Cue Reward – Cue Neutral) of MID 1. B) Correlations between anhedonia and DLPFC-right NAc connectivity during reward anticipation (Cue Reward – Cue Neutral) of MID 2. C). Correlations between anhedonia and DLPFC-right NAc connectivity from MID 1 to MID 2 during reward cues (CR). D) Correlations between anhedonia and DLPFC-right NAc connectivity from MID 1 to MID 2 during neutral cues (CN).
Sustained (MID 2 – MID 3):
The whole brain connectivity analyses revealed no significant clusters with both right and left NAc as seeds and Anhedonia as a covariate of interest, suggesting that the effects of the positive mood induction on the connectivity between NAc and DLPFC, persisted from acute to the sustained time-point, independent of anhedonia.
4. DISCUSSION
Contrary to our hypothesis, this study demonstrated that, across all subjects, positive mood induction led to a decrease in bilateral NAc activation during anticipation of rewarding vs. neutral outcomes, a decrease which persisted at the sustained time-point. As hypothesized, anhedonia modulated this effect, although in the opposite direction than envisioned. Specifically, following a positive mood induction, lower anhedonia was associated with a greater reduction in bilateral NAc activation during reward anticipation. Furthermore, after positive mood induction, lower anhedonia was also associated with an increase in functional connectivity between the DLPFC and right NAc during reward anticipation. Further analyses revealed that this change was valence-specific: lower anhedonia was associated with an increase in DLPFC-right NAc connectivity during reward cues and a decrease in DLPFC-right NAc connectivity during neutral cues, pre- to post-mood induction. Critically, change in mood did not correlate with anhedonia; individuals with high and low anhedonia alike experienced an increase in mood due to the paradigm. Therefore, the neural differences observed are unlikely to be simply due to decreased efficacy of the positive mood induction paradigm in individuals with high anhedonia.
Although our findings contradict our initial hypotheses (informed by a recent study in healthy individuals), they do align with prior accounts of the effects of positive mood in healthy individuals. One of the most prominent models of the effect of positive mood is Frederickson’s broaden-and-build model (Fredrickson, 2004), which proposes that when an individual is in a positive emotional state, she/he will react to environmental cues in a “broader” fashion, trying out ambiguous options rather than simply pursuing those guaranteed to be positive. This model is consistent with a rich animal literature demonstrating that in more positive (enriched) environmental contexts, animals engage in greater exploratory vs. exploitative reward-related behavior (Charnov, 1976; Widman & Rosellini, 1990). Of note, recent research demonstrated this pattern of increased exploration due to environmental richness during a foraging paradigm in humans (Constantino & Daw, 2015). Further, positive mood induction itself causes healthy individuals to visually explore their environment more, sampling both neutral and positively-valenced images rather than being biased towards positive images, and increasing visual attention to the environment surrounding positive stimuli (Wadlinger & Isaacowitz, 2006; Grol & de Raedt, 2014). Compellingly, recent evidence suggests that the choice between exploratory and exploitative behavior may be mediated within dopaminergic corticostriatal circuits, in both animals and humans. In human imaging studies, activation in the frontopolar cortex is maximized during exploratory decisions, while activation in the striatum and ventromedial prefrontal cortex are maximized during exploitative decisions (Daw, O’Doherty, Dayan, Seymour, & Dolan, 2006). Furthermore, tonic dopamine levels and genes encoding corticostriatal dopamine, have been shown to influence an animal’s predilection for exploitative vs. exploratory behavior (Beeler, Daw, Frazier, & Zhuang, 2010; Frank, Doll, Oas-Terpstra, & Moreno, 2009).
Our findings are thus in line with a rich animal and behavioral literature demonstrating a tendency towards more exploratory cue sampling in a highly positive state, and provide initial evidence for a neural correlate of this behavioral change, within the corticostriatal circuit shown to be a central mediator of the explore/exploit trade-off.
Critically, this neural effect was modulated by anhedonia – as anhedonia increased, change in neural response to reward vs. neutral cues became less evident. This is in line with the fact that the broadening effect of positive mood attention to positive stimuli is not seen in individuals with high levels of depressive symptoms (Grol & de Raedt, 2014). These results suggest that the ability to adapt the brain’s reward response following positive mood induction may be a key deficit in individuals with anhedonia. Importantly, this modulation by anhedonia was seen immediately following positive mood induction, and was also present at the sustained time-point. We initially had predicted that individuals with high anhedonia might have intact change in reward response at the acute time-point, but show a dissipation of the change in reward response at the sustained time point, indicating an inability to sustain the effects of positive mood induction. However, the present results suggest that while individuals with low anhedonia show an initial robust modulation of reward response to positive mood induction, and sustain this modulation following a delay, individuals with high anhedonia are incapable of modulation of reward response based on positive mood induction even at the acute phase.
Interestingly, the decrease in selective activation to reward vs. neutral cues from before to after the positive mood induction was accompanied by an increase in connectivity between the DLPFC and right NAc. Like the change in activation to reward vs. neutral cues, the change in functional connectivity scaled inversely with anhedonia; a greater change in functional connectivity was seen in individuals with lower anhedonia. Functional connectivity provides no information about directionality, so a causal role cannot be inferred for the DLPFC in the present study. However, a modulatory role of the DLPFC over the striatum has been suggested repeatedly in reward paradigms. Specifically, a study modeling prefrontal-striatal activity during reward anticipation utilizing effective connectivity methods found that initial DLPFC responses to reward cues are followed by an increase in DLPFC-NAc connectivity (Ballard et al., 2011). Further, an increase in DLPFC activation and a decrease in striatal activation, as well as an increase in DLPLC-NAc connectivity, were found during intentional reappraisal of reward-wanting (Delgado et al., 2008; Kober et al., 2010; Staudinger et al., 2011) and neural response to food cues after satiation (Thomas et al., 2015). Finally, disrupting activation in the DLPFC shifts behavior away from a “model-based” strategy to a “model free” strategy of reinforcement learning (Smittenaar et al., 2013). Model-based strategies of reinforcement learning attempt to build a model of future reward probability, taking into account past and present environmental circumstances and physical/mental state, whereas model-free strategies focus only on the history of rewards delivered vs. missed without considering broader situational factors (Dayan & Berridge, 2014). A potential interpretation for our findings is, therefore, that positive mood induction, which represents a key past occurrence, influences top-down regulation of the NAc by the DLPFC. This alteration could result in a decreased NAc response to reward cues and an increased NAc response to neutral cues, in line with the effects of positive context on exploratory behavior and the broaden-and-build model of positive mood.
The observed differences in connectivity change between individuals with high vs. low anhedonia are particularly interesting given an extant report of failure to update NAc-prefrontal connectivity in a pleasant context, in individuals with anhedonia (Young et al., 2016). Further, a recent study of the effects of accelerated intermittent theta-burst stimulation over the left DLPFC of depressed individuals found that the treatment decreased neural response to reward in individuals with low levels of anhedonia, but increased it in individuals with high levels of anhedonia, suggesting different modulation of the striatal circuitry by the DLPFC in high anhedonia (Duprat et al., 2017). Importantly, studies of depression implicate striatal-DLPFC connectivity in predicting treatment response to transcranial magnetic stimulation (TMS) to the DLPFC (Avissar et al., 2017), suggesting that the aberrant connectivity seen in individuals with higher anhedonia may be clinically relevant. The present study adds to the literature elucidating the brain basis of anhedonia by demonstrating a context-specific alteration in brain activity and connectivity that scales with severity of anhedonia.
4.1. Limitations
Several limitations of our study must be considered. First, although our data suggest the importance of the clinical symptom of anhedonia, this symptom was assessed only through self-report; an ideal assessment method would have involved a structured clinical interview. Second, the mood induction may not have held the same meaning (or potency) for each individual. It is possible that individuals with higher anhedonia experienced the positive mood induction as less confidence-boosting and more stressful, although analysis of self-report following debriefing did not indicate such a trend. Third, since causal analyses were not performed on the data, direct modulation of the NAc by the DLPFC following positive mood induction cannot be inferred from the present findings.
4.2. Conclusion
Findings from the present study indicate that, positive mood induction significantly alters the human brain’s response to reward; we found a significant decrease in the difference between NAc activation to reward vs. neutral cues, from before to immediately after, and persistent following a delay after, positive mood induction. This decrease in NAc activation to reward cues was accompanied by an increase in DLPFC-NAc connectivity during reward cues, and a decrease in DLPFC-NAc connectivity during neutral cues. These changes might indicate that, while in a positive mood, individuals might react to environmental cues in a “broader” fashion, consistent with Frederickson’s broaden-and-build model (Fredrickson, 2004). Furthermore, these effects are relatively persistent, lasting into the “sustained” phase of our experiment. Critically, anhedonia modulated these changes: higher anhedonia was associated with a smaller magnitude of change in response to reward vs. neutral cues in the NAc, following mood induction, as well as an opposite pattern of connectivity change. The present study thus suggests that in addition to baseline differences in reward processing, anhedonia is associated with key differences in how the brain adapts its response to reward following induction of a positive mood. This, we believe, is a critical finding: while most studies have investigated differences in the reward circuit in anhedonia in a single, static affective environment, individuals with high anhedonia may also differ in how the reward circuit responds to changing environmental conditions, with inadequate or inappropriate adaptation contributing to pathology. Future studies should continue to probe the flexible adaptation of the reward circuit based on contextual factors such as mood, in individuals with varying levels of anhedonia. It may be that dynamic changes in connectivity and reward-circuit activation are critical to appropriate experience of a given context or mood state, and that the alterations demonstrated in high anhedonia in the present study hold significant relevance to the lack of pleasure, and lack of motivation to pursue pleasurable activities, reported in clinical populations.
Supplementary Material
Acknowledgments
This study was partially supported by the Harvard College Research Program, the Herchel Smith Undergraduate Science Research Program and the Harvard College Program for Research in Science and Engineering (awarded to IWG). DAP was partially supported by R37 MH068376 and R01 MH101521. PK was partially supported by R21 MH105775 and a Brain and Behavior Foundation (formerly NARSAD) Young Investigator award. We thank Mr. Robert T. Mankoff, chief editor, the New Yorker Caption Contest, for providing all the captions and cartoons that were used in the task, as well as for his fruitful thoughts throughout the construction of the task. Data for this study were collected at the Harvard Center for Brain Science. This work involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program; specifically, grant number S10OD020039. The SMS-BOLD sequence was developed at the Center for Magnetic Resonance Research (CMRR) at the University of Minnesota. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Caroline West, Dr. Stephanie McMains, Dr. Ross Mair, and Tammy Moran from the Center for Brain Science for their expertise and support throughout the project; Dr. Leah Somerville for critical commentary and review; and the members of the Pizzagalli lab for their scientific insight and personal encouragement.
Footnotes
Financial Disclosures
Over the past three years, Dr. Pizzagalli received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehreinger Ingelheim, Compass, and Takeda for activities unrelated to the present study.
REFERENCES
- Admon R, & Pizzagalli DA (2015). Corticostriatal pathways contribute to the natural time course of positive mood. Nature Communications, 6(May), 10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, … Murray GK (2015). Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Frontiers in Psychology, 6, 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar M, Powell F, Ilieva I, Respino M, Gunning FM, Liston C, & Dubin MJ (2017). Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimulation, 10(5), 919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, & Adcock RA (2011). Dorsolateral Prefrontal Cortex Drives Mesolimbic Dopaminergic Regions to Initiate Motivated Behavior. Journal of Neuroscience, 31(28):10340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Garbin MG (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–100. [Google Scholar]
- Beeler JA, Daw N, Frazier CRM, & Zhuang X (2010). Tonic dopamine modulates exploitation of reward learning. Frontiers in Behavioral Neuroscience, 4, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum H, & Connelly J (1993). The effect of stress on hedonic capacity. Journal of Abnormal Psychology, 102(3), 474–481. [DOI] [PubMed] [Google Scholar]
- Bogdan R, & Pizzagalli DA (2009). The heritability of hedonic capacity and perceived stress: a twin study evaluation of candidate depressive phenotypes. Psychological Medicine, 39(2), 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov EL (1976). Optimal foraging, the marginal value theorem. Theoretical Population Biology, 9(2), 129–136. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. [PubMed] [Google Scholar]
- Constantino SM, & Daw ND (2015). Learning the opportunity cost of time in a patch-foraging task. Cognitive, Affective and Behavioral Neuroscience, 15(4), 837–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, O’Doherty JP, Dayan P, Seymour B, & Dolan RJ (2006). Cortical substrates for exploratory decisions in humans. Nature, 441(7095), 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, & Berridge KC (2014). Model-based and model-free Pavlovian reward learning: revaluation, revision, and revelation. Cognitive, Affective & Behavioral Neuroscience, 14(2), 473–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, & Phelps EA (2008). Regulating the expectation of reward via cognitive strategies. Nature Neuroscience, 11(8), 880–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat R, Wu GR, De Raedt R, & Baeken C (2017). Accelerated iTBS treatment in depressed patients differentially modulates reward system activity based on anhedonia. World Journal of Biological Psychiatry, pp. 1–12. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, … Yacoub E (2010). Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PloS One, 5(12), e15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher K, Parker G, Paterson A, Fava M, Iosifescu D, & Pizzagalli DA (2015). Anhedonia in melancholic and non-melancholic depressive disorders. Journal of Affective Disorders, 184, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, & Moreno F (2009). Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nature Neuroscience, 12(8), 1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (2004). The broaden-and-build theory of positive emotions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 359(1449), 1367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Johnson AR, Alonso CM, Evans LK, Babb JS, & Klein RG (2015). Anhedonia, but not Irritability, Is Associated with Illness Severity Outcomes in Adolescent Major Depression. Journal of Child and Adolescent Psychopharmacology, 25(3), 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, … Steele JD (2011). Expected value and prediction error abnormalities in depression and schizophrenia. Brain, 134(6), 1751–1764. [DOI] [PubMed] [Google Scholar]
- Harlé KM, Guo D, Zhang S, Paulus MP, & Yu AJ (2017). Anhedonia and anxiety underlying depressive symptomatology have distinct effects on reward-based decision-making. Plos One, 12(10), e0186473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati M, & Russo SJ (2015). Anhedonia and the brain reward circuitry in depression. Current Behavioral Neuroscience Reports, 2(3), 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, & Phillips ML (2005). The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry, 58(11), 843–853. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, & Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 21(16),RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, & Gotlib IH (2008). Neural responses to monetary incentives in major depression. Biological Psychiatry, 63(7), 686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, & Hommer D (2001). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport, 12(17), 3683–7. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, & Ochsner KN (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America, 107(33), 14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, & Pizzagalli DA (2014). Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience, 266, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Slavich GM, Berghorst LH, Treadway MT, Brooks NH, Dutra SJ, … Pizzagalli DA (2015). Perceived life stress exposure modulates reward-related medial prefrontal cortex responses to acute stress in depression. Journal of Affective Disorders, 180, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Woldeyohannes HO, Soczynska JK, Maruschak NA, Wium-Andersen IK, Vinberg M, … Kennedy SH (2016). Anhedonia and cognitive function in adults with MDD: results from the International Mood Disorders Collaborative Project. CNS Spectrums, 21(5), 362–366. [DOI] [PubMed] [Google Scholar]
- Meng X, Rosenthal R, & Rubin DB (1992). Comparing correlated correlation coefficients. Psychological Bulletin, 111(1), 172–175. [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, & Uğurbil K (2010). Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magnetic Resonance in Medicine, 63(5), 1144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, Youssef G, Yücel M, & Lorenzetti V (2018). The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Human Brain Mapping, 39(8), 3398–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, … Fava M (2009a). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry, 166(6), 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, … Fava M (2009b). Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals With Major Depressive Disorder. American Journal of Psychiatry, 166(6), 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, & Fava M (2008). Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research, 43(1), 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal A, Collishaw S, Thapar A, & Rice F (2013). ‘The risks of playing it safe’: a prospective longitudinal study of response to reward in the adolescent offspring of depressed parents. Psychological Medicine, 43(1), 27–38. [DOI] [PubMed] [Google Scholar]
- Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Dudas RB, … Murray GK (2016). Abnormal Frontostriatal Activity During Unexpected Reward Receipt in Depression and Schizophrenia: Relationship to Anhedonia. Neuropsychopharmacology, 41(8):2001–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smittenaar P, FitzGerald THB, Romei V, Wright ND, & Dolan RJ (2013). Disruption of Dorsolateral Prefrontal Cortex Decreases Model-Based in Favor of Model-free Control in Humans. Neuron, 80(4): 914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, & Dichter GS (2009). fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders, 118(1–3), 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P (1995). A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British Journal of Psychiatry, 167(1), 99–103. [DOI] [PubMed] [Google Scholar]
- Spijker J, Bijl RV, de Graaf R, & Nolen WA (2001). Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatrica Scandinavica, 103(2), 122–30. [DOI] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, & Walter H (2011). Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cerebral Cortex (New York, N.Y.: 1991), 21(11), 2578–2588. [DOI] [PubMed] [Google Scholar]
- Steele JD, Kumar P, & Ebmeier KP (2007). Blunted response to feedback information in depressive illness. Brain, 130(9), 2367–2374. [DOI] [PubMed] [Google Scholar]
- Tamir M, & Robinson MD (2007). The happy spotlight: Positive mood and selective attention to rewarding information. Personality and Social Psychology Bulletin, 33(8), 1124–1136. [DOI] [PubMed] [Google Scholar]
- Thomas JM, Higgs S, Dourish CT, Hansen PC, Harmer CJ, & McCabe C (2015). Satiation attenuates BOLD activity in brain regions involved in reward and increases activity in dorsolateral prefrontal cortex: An fMRI study in healthy volunteers. American Journal of Clinical Nutrition, 101(4), 697–704. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, De Boer P, … Claes S (2013). Reduced reward learning predicts outcome in major depressive disorder. Biological Psychiatry, 73(7), 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and Validation of Brief Measures of Positive and Negative Affect. The PANAS Scales. Journal of personality and social psychology, 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, & McCormick RA (1995). Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology, 104(1), 3–14. [DOI] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, & Pizzagalli DA (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 28(1), 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widman DR, & Rosellini RA (1990). Restricted daily exposure to environmental enrichment increases the diversity of exploration. Physiology and Behavior, 47(1), 57–62. [DOI] [PubMed] [Google Scholar]
- Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, … Uğurbil K (2013). Evaluation of slice accelerations using multiband echo planar imaging at 3T. NeuroImage, 83, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, & Menon V (2016). Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Translational Psychiatry, 6, e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CB, & Nusslock R (2016). Positive mood enhances reward-related neural activity. Social Cognitive and Affective Neuroscience, 11(6), 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.