Abstract
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death worldwide. Risk factors for HCC include chronic hepatitis B and hepatitis C, alcohol addiction, metabolic liver disease (particularly nonalcoholic fatty liver disease) and exposure to dietary toxins such as aflatoxins and aristolochic acid. All these risk factors are potentially preventable, highlighting the considerable potential of risk prevention for decreasing the global burden of HCC. HCC surveillance and early detection increase the chance of potentially curative treatment; however, HCC surveillance is substantially underutilized, even in countries with sufficient medical resources. Early-stage HCC can be treated curatively by local ablation, surgical resection or liver transplantation. Treatment selection depends on tumour characteristics, the severity of underlying liver dysfunction, age, other medical comorbidities, and available medical resources and local expertise. Catheter-based locoregional treatment is used in patients with intermediate-stage cancer. Kinase and immune checkpoint inhibitors have been shown to be effective treatment options in patients with advanced-stage HCC. Together, rational deployment of prevention, attainment of global goals for viral hepatitis eradication, and improvements in HCC surveillance and therapy hold promise for achieving a substantial reduction in the worldwide HCC burden within the next few decades.
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death in many parts of the world. Over the past few decades, considerable progress has been made in understanding the epidemiology, risk factors and molecular profiles of HCC. In addition, rational approaches to prevention, surveillance, early detection, diagnosis and treatment have been developed. Where these approaches have been applied in comprehensive programmes in high-incidence populations, they have shown their efficacy in preventing HCC and in curbing overall mortality from the disease. However, incidence and cancer-specific mortality still continue to increase in many countries, and the majority of HCC patients still present at an advanced stage in many parts of the world1. In this Review, we discuss these recent advances and propose an overall perspective on how the systematic deployment of realistic approaches could achieve a substantial reduction in the overall burden of HCC within the next few decades.
Epidemiology
HCC accounts for >80% of primary liver cancers worldwide2. HCC exacts a heavy disease burden and is a leading cause of cancer-related death in many parts of the world, being estimated to be the fourth most common cause of cancer-related death overall worldwide1.
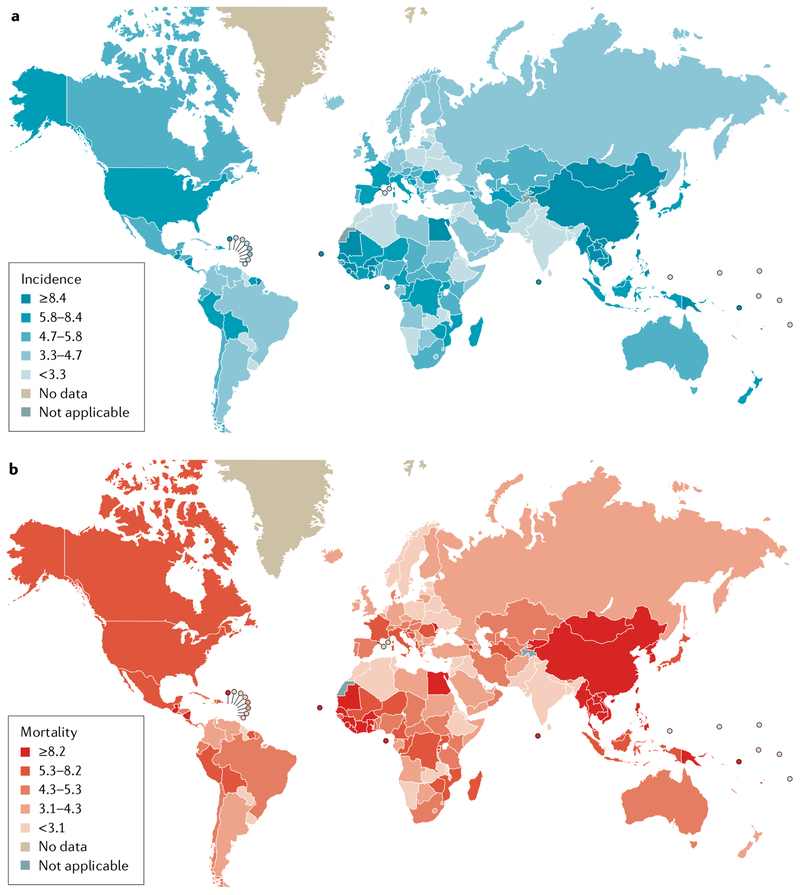
Substantial global variations in the incidence and mortality from HCC exist owing to differences in the timing and level of exposure to environmental and infectious risk factors, healthcare resource availability, and the ability to detect earlier stage HCC and provide potentially curative treatment (FIG. 1; Supplementary Tables 1,2). Almost 85% of HCC cases are estimated to occur in low-resource or middle-resource countries, particularly in Eastern Asia and sub-Saharan Africa3,4.
Fig. 1 |. Global disease burden of primary liver cancer.
Global variation exists in the incidence (part a) and mortality (part b) of primary liver cancer, with the highest burden seen in East Asia and sub-Saharan Africa where medical resources are often limited. Hepatocellular carcinoma accounts for 80–90% of primary liver cancer. Numbers are per 100,000 person-years.
Data from Globocan 2018 (https://gco.iarc.fr/today/home).
In contrast to the decreasing disease burden and impact of many other major cancers, the overall burden of liver cancer worldwide is increasing over time. After lung cancer, liver cancer was the second leading cause of years of life lost from cancer worldwide between 2005 and 2015 with a 4.6% increase in absolute years of life lost (95% CI - 1.6% to 15.4%)3,4.
Despite a slowly decreasing trend in global age-standardized incidence rates (ASIRs) of liver cancer since the late 1990s, the total number of liver cancer cases has been increasing owing to ageing and population growth1. If the population age structure and size were the same in 2015 as they were in 2005, 8% fewer cases of liver cancer would have been diagnosed in 2015 compared with 2005. While ASIRs have been minimally decreasing globally since 2000, ASIRs have been increasing for high sociodemographic index countries since 1990 (REF.1). Indeed, the incidence rates of HCC in the USA have increased twofold to threefold over the past three decades5. The increase in the incidence rates of HCC in the USA is due in part to the high prevalence of HCV infection in the birth cohort born between 1945 and 1965, as well to the progressive increase in HCC due to obesity-related fatty liver disease over the past two decades6.
The age of onset of HCC varies in different parts of the world. HCC tends to occur later in life in Japan, North America and European countries, where the median age of onset is above 60 years. In contrast, in parts of Asia and most African countries, HCC is commonly diagnosed in the age range 30–60 years7. The HCC BRIDGE study of 18,031 patients with HCC from 42 sites in 14 countries showed that the mean age at HCC diagnosis was 69,65 and 62 years in Japan, Europe and North America, respectively, whereas it was 59 and 52 in South Korea and China, respectively7. In Africa, high-quality data from population-based studies are lacking, but a tertiary-referral-centre-based cohort study published in 2015 showed that the age of onset of HCC is low in sub-Saharan Africa. A study of 1,552 patients with HCC from 14 centres in seven African countries showed a median age at HCC diagnosis of 45 years8. For HBV-induced HCC, the age range having the most frequent HCC diagnoses was 32.5–37.5 years8. The early onset of HCC in individuals born in sub-Saharan Africa was also seen in an analysis of 59,907 patients with HCC diagnosed in the USA between 2000 and 2012 in the Surveillance, Epidemiology, and End Results (SEER) programme. Very early-onset HCC at <40 years of age was most strongly associated with being born in West Africa (adjusted odds ratio (AOR) 16.3, 95% CI 9.2–27.9; P<0.01), Central/South/other Africa (AOR 11.0, 95% CI 4.5–23.7; P<0.01), Oceania (AOR 4.9, 95% CI 2.9–8.0; P<0.01) and East Africa (AOR 3.5, 95% CI 1.5–6.8; P<0.01)9. The number of African-born Americans in the SEER studies is small, leading to wide confidence intervals in the AOR estimates. Importantly, the countries of West Africa, where the earliest onset of HCC is seen, include 384 million people, approximately 30% of the population of Africa. High-quality, population-based studies are needed to improve our understanding of HCC epidemiology in Africa.
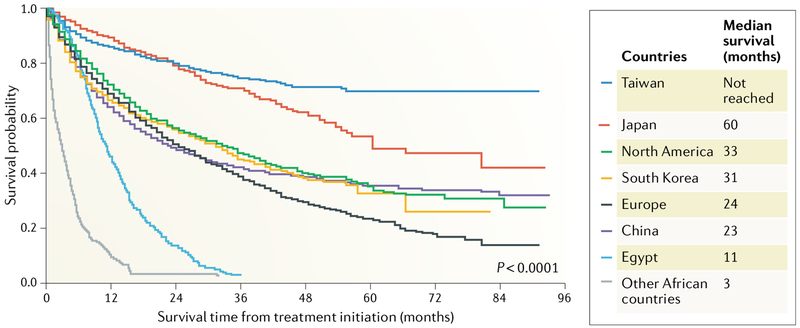
Overall survival of patients with HCC varies substantially across the world7,10 (FIG. 2). Median survival in Taiwan and Japan is significantly higher than in sub-Saharan Africa, where the median survival is only 2.5 months, highlighting the devastating effects of the combined lack of surveillance programmes and availability of effective treatments. Taiwan and Japan have the best clinical outcomes of patients with HCC. This is likely because both countries have comprehensive programmes for identifying all adults at risk of HCC and enrolling them in regular, intensive liver cancer surveillance programmes that use multiple tumour biomarkers (alpha-fetoprotein (AFP), the Lens culinaris agglutinin-reactive glycoform (AFP-L3), and des-gamma-carboxy prothrombin (DCP)) and liver ultrasonography reflexing to cross-sectional imaging in high-risk individuals developing new or suspicious liver nodules11. Consequently, >70% of HCCs diagnosed at major medical centres in these countries are detected at very early or early stages and are eligible for curative therapies. HCC outcomes in Korea and China or in North America and Europe are not as good as in Taiwan or Japan as ≥60% of patients in these countries present with intermediate-stage or advanced-stage HCC7. In Africa, the overall survival of patients with HCC in Egypt is longer than in the other sub-Saharan and East African countries studied. This finding is likely due to the lower proportion of patients presenting with advanced-stage or terminal-stage disease, representing 69% of HCC cases in Egypt compared with 95% in all the other African countries in the study (P<0.01), and the higher proportion of patients receiving any HCC treatment (76% in Egypt versus 3% in the other African countries; P<0.01).
Fig. 2 |. Global variation in the overall survival of patients with HCC.
Taiwan and Japan have the best clinical outcomes for patients with hepatocellular carcinoma (HCC), probably owing to the high proportion of HCCs that are detected at an early stage as a result of nationwide intensive surveillance programmes in both countries7. By contrast, outcomes in other East Asian countries are not as good as in Japan or Taiwan, as more patients present at an advanced stage. Overall survival of patients with HCC in Egypt was longer than in the other African countries, probably because more patients with HCC are diagnosed whilst under surveillance for HCC, so that a lower proportion of patients present with advanced- or terminal-stage disease and a higher proportion of patients receive HCC treatment9. Data from Park et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 35, 2155–2166 (2015)7 and Yang et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol. Hepatol. 2, 103–111 (2017)10.
Risk factors
Viral hepatitis B and C and cirrhosis.
Chronic HBV and HCV infection are the most important causes of HCC and account for 80% of HCC cases globally3,12. Chronic HBV infection is the leading cause of HCC in Eastern Asian countries and most African countries, except for northern Africa where the prevalence of HCV is highest7,8. It is estimated that 257 million individuals worldwide have chronic HBV infection and that 20 million deaths between 2015 and 2030 will be attributable to acute hepatitis, chronic hepatitis, cirrhosis and HCC caused by HBV, with 5 million deaths from HCC alone13. In addition, it is estimated that 57 million people have chronic HCV infection, of whom 10–20% will develop liver complications including decompensated cirrhosis and HCC6,14. HCV is the leading virus-related cause of HCC in North America, Europe, Japan, parts of central Asia including Mongolia, and northern Africa and the Middle East, particularly Egypt7,10.
In most instances, and particularly in high-resource countries, HCC develops as a sequel to protracted chronic hepatitis, occurring after patients develop liver cirrhosis from HBV or HCV infection. Overall, the annual incidence of HCC is 2–5% in patients with cirrhosis from chronic HBV or HCV infection15. However, HBV-associated HCC frequently occurs in the absence of cirrhotic liver disease, accounting for 30–50% of HCC in HBV endemic areas such as Eastern Asia and most African countries10. By contrast, in cohort studies from the USA where HBV is not endemic, >90% of patients with HBV-associated HCC have cirrhotic liver disease16. Differences in the mode of HBV transmission, onset and duration of infection, and environmental exposures may explain the higher frequency of HCC in patients with HBV without cirrhosis in HBV endemic areas.
Fatty liver disease and diabetes.
Nonalcoholic fatty liver disease (NAFLD) is now the most common liver disease and a major risk factor for HCC in most developed countries17,18. Between 10% and 20% of HCC cases in the USA are now attributed to NAFLD19,20. High-quality, population-based studies investigating the association between NAFLD and HCC are lacking. Several studies have investigated the strength of the association between NAFLD and HCC and the population-attributable fraction of NAFLD for HCC using the US SEER-Medicare database, which is representative of the general population with Medicare benefits, most of whom are aged over 65 years19,21. One study reported that NAFLD was associated with a 2.6-fold increased risk of HCC19. Diabetes mellitus and/or obesity, major clinical risk factors for NAFLD, had the highest population-attributable fraction of 37% for HCC in the USA21. As the patient age at onset of HCC is associated with the aetiology of the underlying liver disease, NAFLD-associated HCC as compared with virus-associated HCC (as an example) is more common in elderly patients than young patients, and thus the findings of studies with a preponderance of persons aged over 65 years should be interpreted with caution as these studies might over-represent NAFLD-associated HCC. NAFLD-associated HCC also occurs frequently in the absence of cirrhosis20,22. A population-based study of 93 patients with HCC in Olmsted County, Minnesota, showed that 27% of patients with NAFLD-HCC did not have cirrhosis20. Similarly, a Veterans Health Administration study of 1,500 patients with HCC in the USA showed that patients with NAFLD-associated HCC had a more than fivefold risk of having HCC in the absence of cirrhosis, compared with patients with HCV-associated HCC22.
Diabetes mellitus is associated with a twofold to threefold increased risk of HCC15,23,24. Insulin resistance and consequent production of reactive oxygen species that trigger hepatic inflammation are thought to have a role in hepatocarcinogenesis25–27. One study suggests that diabetes increases the risk of HCC even in patients with liver cirrhosis15. Whether interactions between diabetes and the aetiology of liver cirrhosis influence the risk of HCC remains controversial15,24.
Alcohol.
Alcoholic cirrhosis is the second most common risk factor for HCC in the USA and Europe7. A meta-analysis of 19 studies [n = 5,650) by the World Cancer Research Fund found a statistically significant increased risk of 4% per 10 g alcohol intake per day (relative risk 1.04, 95% CI 1.02–1.06)28. However, the absolute risk of developing HCC in patients with alcohol-related cirrhosis seems to be lower than that in patients with cirrhosis from chronic viral hepatitis29,30. A Danish nationwide population-based study showed that the 5-year cumulative HCC risk was 1.0% (95% CI 0.8–1.3%) among all Danish citizens with a first-time hospital diagnosis of alcoholic cirrhosis from 1993 to 2005 [n = 8.482)30. Similarly, a population-based study of 3,107 cirrhosis patients using the UK General Practice Research Database (1987–2006) found that patients with alcohol-related cirrhosis had a twofold to threefold lower risk of HCC than patients with cirrhosis due to viral hepatitis29. A single-centre retrospective cohort study of 450 patients with alcoholic cirrhosis showed that older age (≥55 years) and thrombocytopenia (platelet count <125,000 per mm3) were independent risk factors for the development of HCC31.
Aflatoxin and aristolochic acid.
Aflatoxins are mycotoxins with strong hepatocarcinogenic effects that contaminate many staple cereals and oilseeds32. Aflatoxin contamination is widespread in areas with a high incidence of HCC. For example, >90% of the general population of several West African countries are exposed to aflatoxins due to inappropriate postharvest processing, whereas exposure is minimal in Western countries33. The main form of aflatoxin involved in liver carcinogenesis is aflatoxin B1 (AFB1) produced by Aspergillus sp. Aflatoxin exposure is thought to at least partially account for the early onset of HCC in many sub-Saharan African countries12,32,34,35. AFB1 predominantly causes mutations at codon 249 in the TP53 tumour suppressor gene (AGG to AGT), resulting in substitution of arginine for serine (R249S), which is rarely observed in cancers other than HCC33. The R249S mutation accounts for 50–90% of TP53 mutations found in HCCs from regions with high aflatoxin exposure levels; this percentage drops to <6% of TP53 mutations in HCCs from patients in the USA33,36.
There is a strong interaction between HBV and aflatoxin exposure in liver cancer risk37. Chronic HBV infection may induce the cytochrome P450s that metabolize inactive AFB1 to the mutagenic AFB1–8,9-epoxide. Hepatocyte necrosis and regeneration from chronic HBV infection also increase the probability of the AFB1-induced TP53 mutations. In addition, nuclear excision repair, which is normally responsible for removing AFB1-DNA adducts, is inhibited by HBV oncogenic protein38.
Aristolochic acid (AA) is a highly mutagenic compound found in plants known as Aristolichia or Asarum (Chinese wild ginger) which grow worldwide39. Plants containing AA have been used in traditional Chinese herbal medicines for centuries. Next-generation sequencing studies have shown that a proportion of HCCs in patients from Asia, particularly China, Taiwan, Vietnam and Southeast Asia, show high rates of mutations matching a mutational signature characteristic of AA exposure40,41. The trinucleotide contexts characteristic of AA exposure included a prominent peak at 5′-CTG-3′ (5′-CAG-3′ on the complementary strand)41. A large study of 1,400 HCCs from diverse geographical regions showed that 78% of HCCs from Taiwan, 47% of HCCs from China, 29% of HCCs from Southeast Asia, 13% of HCCs from Korea, 2.7% of HCCs from Japan, 4.8% of HCCs from North America and 1.7% of HCCs from Europe showed the AA signature41. One study of a randomly sampled cohort of 200,000 National Health Insurance patients in Taiwan between 1997 and 2003 showed that about one-third of the Taiwanese population had been exposed to AA42. A dose-response relationship has also been shown in Taiwan between AA exposure and risk of HCC43.
Other causes of cirrhosis.
Other chronic liver diseases, such as chronic biliary disease and genetic or metabolic liver diseases, can lead to cirrhosis and promote the development of HCC, but the proportion of HCCs caused by these other aetiologies is <5% to 10% worldwide12.
Protective factors.
Coffee, statins, metformin and aspirin have been shown to be protective against the development of HCC in multiple observational studies44–47. Although none of these protective associations have been confirmed in randomized controlled trials (RCTs), coffee use is now recommended by the European Association for the Study of the Liver (EASL) 2018 clinical practice guidelines for HCC48.
Pharmacological suppression of HBV or clearance of HCV by highly potent antiviral treatment seems to decrease the risk of HCC in infected individuals by 50–80%49–52.The effect of direct-acting antiviral (DAA) treatment for HCV infection on hepatocarcinogenesis, tumour recurrence and progression has become a matter of intense discussion, owing to findings of an association between DAA treatment and an increased risk of HCC recurrence after curative cancer treatment in a retrospective study in four Spanish referral hospitals53. An Italian retrospective cohort study including 59 patients with previous HCC showed similar results54. Another retrospective study confirmed a nonsignificant trend towards an increased risk of HCC recurrence after liver transplantation in 5 (28%) of 18 DAA-treated patients compared with 6 (10%) of 63 nontreated patients55. A small single-centre study from the USA showed high incidence rates of de novo HCC after DAA treatment in patients with HCV-related cirrhosis56. However, several subsequent studies have failed to show an association between DAA treatment and increased risk of HCC recurrence57–59. A retrospective multicentre study published in 2019 of 793 patients with HCV-associated HCC of whom 38% received DAA therapy showed that, after adjusting for covariates, DAA therapy was not associated with HCC recurrence (HR 0.90, 95% CI 0.70–1.16)59. Overall, the initial potential warning signals that DAAs might increase the risk of HCC recurrence after curative treatment do not seem to be confirmed in larger studies; however, well-designed prospective multicentre studies are needed to fully characterize the clinical effect of DAA therapy on the risk of HCC recurrence.
Although the association between DAA treatment and the risk of HCC recurrence after curative treatment remains controversial, successful DAA treatment does seem to decrease the risk of de novo HCC development. A nationwide Veterans Health Administration study in the USA showed that DAA treatment decreased the risk of HCC in patients with HCV-related cirrhosis, particularly after successful achievement of a sustained virologic response (SVR)52. An SVR with DAA treatment was associated with a 72% reduction in risk of HCC (HR 0.28, 95% CI 0.22–0.36). Among patients with an SVR, the presence of cirrhosis and a history of alcohol abuse were independent risk factors for HCC52. A large French multicentre study of 1,270 patients with compensated biopsy-proven HCV-associated cirrhosis showed that the crude 3-year cumulative incidences of HCC were 5.9% in the DAA group versus 3.1% in the group of patients who achieved an SVR following an interferon-based regimen (HR 2.03, 95% CI 1.07–3.84; P = 0.030). However, after adjusting for other known risk factors, there was no statistically significant increase in risk for HCC associated with DAA use, suggesting that the apparent increased risk of HCC in the DAA group was possibly due to patient characteristics, such as age, diabetes and/or impaired liver function60.
Molecular patterns of carcinogenesis
Integrative studies combining exome sequencing, transcriptome analysis and genomic characterization of HCCs have shown that HCC is heterogeneous at the histomolecular level, with variable molecular features and clinical outcomes61–63. One validated analysis has identified six robust subgroups of HCC, designated G1-G6, that are associated with specific genetic and clinical characteristics64,65. Mutations in the TERT promoter (occurring in 44–65% of patients with HCC and regulating transcription of the catalytic subunit of telomerase), CTNNB1 (27–40%, encoding β-catenin, a proto-oncogene in the WNT signalling pathway) and TP53 (21–31%, the master cell cycle regulator) are the most common61,66. Specific aetiologies of HCC are associated with particular genetic alterations66. For example, TERT promoter and TP53 mutations are the most frequent genetic events in HBV-associated HCC, whereas CTNNB1 mutations are strongly associated with alcohol-related HCC. Furthermore, TP53 mutations are associated with reduced survival61,64. IL-6-Janus kinase-signal transducer and activator of transcription pathway activation without TERT, CTNNB1 or P53 pathway alterations is frequently seen in the steatohepatitic subtype of HCC66. These integrated analyses emphasize the molecular diversity of HCC and the associations of different aetiologies with distinct mechanisms of hepatocarcinogenesis.
Overall, around one quarter of HCCs seem to contain molecular or genetic alterations potentially targetable by currently approved drugs, fuelling an interest in the use of molecular signatures for designing targeted therapeutic trials62,67. Studies prospectively characterizing HCC by next-generation sequencing in patients treated with systemic therapies are beginning to provide insights into the interactions between alterations in different cell signalling pathways and rates of disease control in response to specific classes of systemic therapies. For example, for patients with HCC treated with immune checkpoint inhibitors, activating mutations in the Wnt/β-catenin signalling pathway have been associated with a lower disease control rate and survival67.
Prevention and surveillance
Preventing chronic HBV and HCV carriage.
From a global perspective multiple approaches exist to prevent HCC. Primary prevention is essential — and perhaps the only realistic and sustainable approach — for decreasing the burden of HCC in low-resource countries where viral hepatitis is endemic and resources for the management of viral hepatitis and HCC are limited. In endemic areas, HBV is mainly transmitted through contact with infected blood, frequently by vertical transmission from mother to baby in utero or during delivery, as well as by horizontal transmission from family members to infants and children12. A population-based study in Taiwan showed that HCC incidence was fourfold higher in the HBV unvaccinated cohort than in the vaccinated birth cohorts68. The study used data from two Taiwan HCC registry systems on patients 6–26 years old diagnosed with HCC from 1983 to 2011. Among the 1,509 patients, 1,343 were born before and 166 were born after the start of the HBV vaccination programme68. The relative risks for HCC in patients 6–9 years old, 10–14 years old, 15–19 years old and 20–26 years old who were vaccinated versus those who were unvaccinated were 0.26 (95% CI 0.17–0.40), 0.34 (95% CI 0.25–0.48), 0.37 (95% CI 0.25–0.51) and 0.42 (95% CI 0.32–0.56), respectively68. Although neonatal HBV vaccination is available and recommended in most countries, vaccine coverage is only 40–70% for the largest and most populated African countries with high incidences of HCC69, providing a clear window of opportunity for improvement in prevention. In addition, evidence exists that antiviral treatment of pregnant mothers who have high HBV loads in the third trimester of pregnancy reduces the risk of mother-to-child transmission70.Whether administration of hepatitis B immunoglobulin to pregnant mothers is useful for the prevention of mother-to-child transmission is under debate70,71.
Prevention of HCV by vaccination is not currently possible, but minimizing transmission of viral hepatitis through the screening of blood products before transfusion, the use of single-use disposable needles and other incidental supplies, and sterilization of surgical and dental instruments are cornerstone strategies to decrease iatrogenic transmission of HBV and HCV12. Screening for HCV infection is currently recommended for the high-risk birth cohort born in the USA between 1945 and 1965 (REF.72). While population-based HBV and HCV screening and provision of antiviral treatment to infected individuals in highly endemic areas are currently lacking, the implementation of a strategy for worldwide elimination of chronic viral hepatitis by 2030 has the potential to substantially alleviate the HCC disease burden50,51,73,74.
Maintaining healthy lifestyles.
Maintaining a healthy lifestyle and avoiding HCC risk factors are additional strategies for the prevention of HCC. Avoiding heavy alcohol use and a hypercaloric diet leading to obesity and metabolic syndrome will decrease fatty liver-related liver injury and cirrhosis and the associated burden of HCC75. Smoking is an established risk factor for HCC and pooled data of 14 prospective US cohorts suggest that the risk of HCC among individuals who quit smoking >30 years ago was almost equivalent to that in never-smokers (HR 1.09, 95% CI 0.74–1.61), suggesting that smoking cessation reduces the risk of HCC76.
Minimizing dietary aflatoxin exposure is a crucial step for decreasing HCC burden in areas with a high endemic burden. A meta-analysis of 19 studies estimated the population attributable risk of aflatoxin-related HCC at 17%, being higher in HBV carriers (21–23%) than in noncarriers (8–9%)77. Studies included in the metaanalysis used AFB1-albumin adducts, urinary aflatoxin metabolites, AFB1-DNA adducts and dietary history (peanut butter and corn consumption) as surrogates for aflatoxin exposure. In 2014, the International Agency for Research on Cancer convened a working group that evaluated the effectiveness of various intervention strategies to reduce human exposure to aflatoxins32. These measures include the selection of genetically resistant seeds, improved postharvest processing, primary prevention with mycotoxin-trapping enterosorbents and opportunities for chemoprevention. Postharvest measures, in particular, can result in a marked decrease in the levels of biomarkers of aflatoxin contamination in individuals participating in these interventions78,79.
Surveillance.
HCC surveillance is a secondary prevention strategy to decrease the burden of HCC through early tumour detection and appropriate early management. HCC surveillance is indicated in patients with liver cirrhosis or chronic HBV infection with high risk features, in other words Asian male hepatitis B carriers >40 years of age, Asian female hepatitis B carriers >50 years of age, hepatitis B carriers with a family history of HCC and African or North American black individuals with chronic hepatitis B80. The EASL guidelines recommend HCC surveillance in high-risk, patients with chronic HBV infection according to the PAGE-B classification based on platelet count, age and gender, which was developed to predict the 5-year risk of HCC in white patients receiving entecavir or tenofovir for chronic hepatitis B48,81. No high-quality RCTs exist, but a metaanalysis of 47 studies including 15,158 patients found that HCC surveillance is associated with improved overall survival through detection of HCC at a very early or early stage, when patients are eligible to receive potentially curative treatments82. Although the benefits of surveillance are well acknowledged among the clinical community and guideline writing committees48,80, the US National Cancer Institute seems less convinced with their website stating that “Based on fair evidence, screening of persons at elevated risk does not result in a decrease in mortality from hepatocellular cancer”83. This discrepancy in recommendations is due to the lack of high-quality evidence that HCC surveillance decreases HCC mortality, despite numerous observational study results supporting the impact of surveillance in improving overall survival in patients with HCC82. However, it seems that a randomized study of HCC screening is not feasible when informed consent is provided as informed patients prefer surveillance84.
Liver ultrasonography is the standard HCC surveillance test endorsed by the American Association for the Study of Liver Diseases (AASLD), the EASL and the Asia Pacific Association for the Study of the Liver48,80,85. The optimum surveillance interval is 6 months, based on the median doubling time of HCC ranging from 3 months to 9 months and studies showing that the 6-month interval is equivalent in efficacy to 3-month to 4-month intervals and is better than a 12-month surveillance interval86,87.
Among blood-based surveillance tests, detection of elevated levels of serum AFP is commonly used as an adjunct to liver ultrasonography, although its use as a surveillance test for HCC remains controversial owing to its low sensitivity of 40–60% with a specificity of 80–90% at a cut-off of 20 ng/ml (REF.88). A study of 1,597 patients with cirrhosis from Taiwan showed that measuring AFP levels in addition to standard ultrasonography substantially improves the sensitivity of surveillance compared with ultrasonography alone without a substantial loss of specificity. Ultrasonography alone achieved a sensitivity of 92.0% and a specificity of 74.2%, whereas the combination of ultrasonography and AFP achieved a sensitivity and specificity of 99.2% and 68.3%, respectively88. A large population-based study of 1,487 hepatitis B surface antigen-positive Alaskan native carriers showed that surveillance using AFP levels alone could detect most HCCs at a resectable stage and substantially prolonged survival, suggesting that AFP can be used as an HCC surveillance test when ultrasonography is not widely available owing to limited resources89. A multicentre retrospective study from the USA showed that the performance of AFP measurement improves substantially in patients with cirrhosis and viral hepatitis when they have normal alanine aminotransferase levels90. A nationwide Veterans Affairs registry study from the USA also showed that the use of AFP for detection of HCC had better performance in patients with low serum alanine aminotransferase levels, reflecting low levels of hepatic inflammation91. Assessment of the trend of change in AFP levels or the standard deviation of AFP measurements can improve the performance of single estimations of the AFP level, which are frequently assessed in studies but rarely used in isolation by experienced practitioners92.
Other serum-based biomarkers, such as AFP-L3 and DCP, have been used for surveillance for HCC, particularly in Japan93,94. In addition, the GALAD score, which is derived from gender, age, AFP-L3, AFP and DCP and has been shown to be a highly accurate model for the detection of HCC, has been proposed for early detection of HCC95,96. A 2018 multicentre phase II biomarker study showed that GALAD is superior to ultrasonography for the detection of HCC97. This study included a total of 111 patients with HCC and 180 controls with cirrhosis or chronic hepatitis B97. The area under the curve of the GALAD score for HCC detection was 0.95 (95% CI 0.93–97), which was higher than the area under the curve for ultrasonography (0.82, P<0.01). At a cut-off of −0.76, the GALAD score had a sensitivity of 91% and a specificity of 85% for HCC detection97. The performance of the GALAD score should be further investigated in prospective biomarker studies in comparison or in combination with imaging tests, and its cost-effectiveness should also be carefully assessed. This score might be a useful test, as it can be easily calculated based on patient demographics and measurement of blood-based tumour markers using a small volume of peripheral blood. If the assay costs can be reduced, the GALAD score might be a practical, effective surveillance test in low-resource countries where ultrasonography equipment and trained radiologists are scarce, which might improve compliance with surveillance and lead to improved effectiveness of cancer control programmes.
Following advances in molecular technologies and genomics, circulating cancer biomarkers, such as microRNAs, specific peptides identified by proteomics, DNA mutations and differentially methylated regions have been shown to have potential use for surveillance and early detection of HCC98–102. However, such candidate biomarkers have not yet been used in clinical practice.
Although the benefit of HCC surveillance has been studied extensively, selection of appropriate individuals for surveillance remains very important to avoid overdiagnosis of HCC. For example, patients with cirrhosis and severe hepatic dysfunction (Child-Pugh score class C) should undergo HCC surveillance only if they are eligible for liver transplantation, as their expected survival is very short owing to their high risk of death from liver failure in the absence of transplantation48. Attempting to detect early HCC in this group of patients is considered overdiagnosis of HCC, which can lead to overtreatment, increasing costs, adverse physical effects and psychological harms without improving overall survival or quality of life103,104.
Diagnosis and staging
Noninvasive radiological diagnosis.
Traditionally, the diagnosis of HCC has been established based on cytology or histology. Following advances in understanding HCC-specific radiological features during phasic vascular perfusion of contrast during cross-sectional imaging with CT and MRI, the diagnosis of HCC in patients with cirrhosis who are under surveillance can now be made reliably without biopsy. The AASLD and EASL guidelines state that the diagnosis of HCC can be made radiologically if a new mass measuring ≥1 cm is found that demonstrates arterial hyper-enhancement and venous washout in a cirrhotic liver using either multiphasic contrast CT or MRI48,80.
In 2011, the Liver Imaging Reporting and Data System (LI-RADS) was introduced to standardize the reporting and data collection of CT and MRI for HCC105. LI-RADS classifies new hepatic lesions into five classes based on their size, extent of interval growth and patterns of enhancement. Of note, lesions with a low LI-RADS class 1–2 should also be followed carefully as the system has low specificity for the prediction of benign lesions106. A large prospective validation of the LI-RADS system is still needed.
Staging.
Most staging systems were developed for prognostication of patients with HCC, but a few staging systems also propose an optimum treatment approach107. The Barcelona Clinic Liver Cancer (BCLC) classification is currently the most commonly used standard staging system for HCC, and is the only staging system that has been prospectively validated108,109. The BCLC staging system classifies HCC into five stages and provides estimated median survival periods and recommended treatments for patients at each stage. One of the drawbacks of the BCLC system is the substantial heterogeneity of tumour burden, severity of liver dysfunction and prognosis in patients with intermediate-stage (BCLC stage B) or advanced-stage (BCLC stage C) HCC. A number of investigators have sought to refine the BCLC system by subclassifying BCLC stage B into intermediate stages. The Italian Liver Cancer Group proposed a subclassification of BCLC stage B based on the Child-Pugh Score and extent of tumour (within or beyond the up-to-seven criterion)110. Japanese investigators have proposed a further subclassification of the BCLC stage B based on the albumin-bilirubin grade, which is an indicator of the severity of hepatic dysfunction112,113. This approach has not been widely adopted as yet in routine clinical practice, and further prospective validation is needed.
The Hong Kong Liver Cancer (HKLC) staging system also provides treatment guidance in addition to prognostic classification114. The HKLC staging system classifies patients with HCC into five stages with nine substages that have distinct median survival times based on differences in the extent of tumour, presence of vascular invasion, Child-Pugh stage and Eastern Cooperative Oncology Group performance status. Several studies have shown that the HKLC staging system can accurately stratify patients with HCC into different prognostic groups107,115,116. The most important aspect of the HKLC staging system is the expansion of the criteria for potential curative therapies in patients classified as having intermediate-stage HCC using the BCLC staging system. Overall, the study performance of the HKLC staging system is promising, but further validation in prospective studies is warranted.
Several other staging systems have been proposed107,111. To minimize the subjectivity of the survival model, the Model to Estimate Survival in Ambulatory HCC patients (MESIAH) score was proposed117. The MESIAH score model incorporates age, number of tumour nodules, size of the largest nodule, vascular invasion, metastasis, and serum albumin and AFP levels in addition to the MELD (Model for End-Stage Liver Disease) score. The MESIAH score model uses the MELD score rather than the Child-Pugh score to assess the severity of liver dysfunction as the calculation of the Child-Pugh score can be subjective in the assessment of hepatic encephalopathy or ascites grade. The MESIAH model was shown to be highly discriminant with a c statistic of 0.77, which was superior to that for BCLC (0.71) (P<0.01). The model was further validated in Asian and European cohorts116–118. While the MESIAH score provides prognostication, it does not make a treatment recommendation.
Similar to the MESIAH score model, the BALAD staging system, which is based on levels of five serum markers (bilirubin, albumin, AFP-L3, AFP and DCP), has been reported to have excellent discriminative ability95,119. Bilirubin and albumin reflect the severity of liver dysfunction and AFP-L3, AFP and DCP represent tumour burden and biology. The c statistic for the BALAD score was 0.70 and discrimination was equally good regardless of the HCC treatment95.
Overall, the BCLC staging classification is currently the most commonly used standard staging system for HCC and is the only staging system with robust prospective validation despite the several limitations described above. Therefore, it is routinely used as a main stratifying factor in clinical trials.
Treatment
The management of HCC involves a complex decision-making process, taking into account not only the tumour extent and patient comorbidities but also the severity of liver dysfunction, as most treatments for HCC can exacerbate underlying liver disease. The availability of treatment options is highly variable between medical centres in different countries with various levels of expertise and resources. Thus, HCC management requires a multidisciplinary team approach to achieve the best outcome120.
Resection.
Surgical resection is a recommended treatment option in patients with resectable disease in the absence of clinically significant portal hypertension, which is defined as a hepatic venous pressure gradient ≥10 mmHg and practically assessed by the presence of ascites, oesophageal varices or a platelet count <100,000/mm3 associated with clinically significant splenomegaly121–123. The comparatively restrictive criteria of the BCLC staging system for treatment recommendation and allocation have been challenged. A multiregional cohort study of 8,656 patients with HCC showed that, in patients who were not ideal candidates for resection under the BCLC classification, surgical resection was associated with better survival than embolization (HR 1.4, 95% CI 1.3–1.6, P<0.001) or other locoregional or systemic treatments (HR 1.8, 95% CI 1.4–2.3, P<0.001)124. Similarly, a multicentre study in Korea showed that liver resection provides a survival benefit compared with nonsurgical treatment for patients with potentially resectable BCLC stage B HCC125. Surgical resection, which is potentially applicable across all resource settings, should be considered for select individuals with intermediate- or advanced-stage disease.
Surgical resection is a potentially curative treatment, but almost 70% of patients develop recurrent HCC after resection126. One of the advantages of surgical resection is the availability of the surgical histopathological specimen, which can help in predicting the risk of recurrent HCC127. The BCLC group has proposed and validated salvage transplant approaches after surgical resection in patients with high-risk histological markers such as microvascular invasion and/or satellite lesions128. Importantly, in low-resource settings, palliative surgery can be an important option for very symptomatic patients with relatively preserved liver function, who can obtain substantial relief, improved quality of life and improved survival with noncurative surgery129–131.
Transplantation.
Liver transplantation is the most definitive treatment option for HCC, as it removes not only the tumour but also the unhealthy liver that has limited functional capacity and a tendency to develop additional metachronous HCCs within the cirrhotic tissue field prone to carcinogenesis. As HCC incidence increases, HCC has become the leading indication for liver transplantation in the USA132. Liver transplantation is an excellent treatment for early-stage HCC in the setting of liver dysfunction that precludes surgical resection. The Milan criteria (one lesion <5 cm or two or three lesions each <3 cm) were first established more than two decades ago to define the optimum tumour burden for which liver transplants can achieve excellent long-term outcomes (typically >70% 5-year overall survival)133. Expanded selection criteria for HCC have been proposed to offer liver transplantation beyond the Milan criteria in patients without aggressive tumour biology who are not eligible for other potentially curative treatments134–136. Of the extended criteria for liver transplantation, the University of California San Francisco criteria (a single nodule up to 6.5 cm, or up to three lesions, the largest of which is ≥4.5 cm, with the sum of the diameters ≥8 cm) have been the most widely accepted and have been shown to have excellent post-transplant outcomes136–138. To be eligible for liver transplantation, patients who fulfil the University of California San Francisco criteria should receive local or locoregional therapies to downstage the tumours to fulfil the Milan criteria. Tumours that have been successfully downstaged have presumed favourable biology, and these patients have excellent long-term outcomes after liver transplantation139,140. A retrospective multicentre study of 187 consecutive HCC patients enrolled in the downstaging protocol at three liver transplant centres in California showed that liver transplantation was performed after successful downstaging in 58% of patients and their 5-year post-transplant survival was 80%140.
Ablation.
Percutaneous local ablation is a potentially curative treatment used in patients with early-stage HCC. The two most commonly used modalities are radiofrequency ablation and microwave ablation. Microwave ablation is an increasingly used local ablation modality141. Both approaches induce tumour necrosis by delivering heat directly into tumours, but microwave ablation has some advantages over radiofrequency ablation owing to its higher thermal efficiency through its different mechanism of heat generation. Microwave ablation is less susceptible to the heat sink effect from large vessels adjacent to the tumour, is more efficacious for ablation of larger tumours 3–4 cm in size, and requires less ablation time than radiofrequency ablation142–144. A randomized controlled, single-blinded phase II trial at four tertiary university centres in France and Switzerland compared the efficacy of microwave ablation (n = 76) with that of radiofrequency ablation (n = 76) in patients with HCC with up to three lesions of 4 cm or smaller size who were not eligible for surgery. At 2 years, six (6%) of 98 lesions had local tumour progression in the microwave ablation group compared with 12 (12%) of 104 in the radiofrequency ablation group (risk ratio 1.6, 95% CI 0.7–3.9, P = 0.27). Although the local progression rate was numerically lower with microwave ablation, it did not reach statistical significance, likely due to an underpowered study design145. Percutaneous ethanol injection into HCC tumours was frequently used in the past as an ablation technique and is still commonly used in resource-limited settings12. Ethanol injection can be a preferred treatment in high-resource countries when the tumour nodule is adjacent to large intrahepatic vessels or bile ducts to avoid heat injury to these organs by radiofrequency ablation or microwave ablation. In addition, cryoablation has been shown to be as effective as radiofrequency ablation and is occasionally used in high-resource settings146–148. Local ablation is usually used in patients who are not candidates for liver transplantation or surgical resection owing to medical comorbidities or hepatic dysfunction. Local ablation can also be used as bridge therapy to liver transplantation149. The best management approach for small tumours that are eligible for both resection and local ablation has been controversial. Based on a meta-analysis of three RCTs, the most recent AASLD guidelines recommend that adults with Child-Pugh score class A cirrhosis and resectable small tumours should undergo resection rather than ablation150–153.
Transarterial embolization and radiotherapy.
Transarterial chemoembolization (TACE) is an effective treatment option in patients with intermediate-stage HCC. TACE involves two main steps — intra-arterial infusion of cytotoxic chemotherapeutic agents and delivery of embolization particles into the tumour-feeding artery, causing ischaemic necrosis of the tumour. The most common drugs used during conventional TACE are doxorubicin, epirubicin or cisplatin154. TACE is the most commonly used locoregional treatment in patients listed for liver transplantation to prevent tumour progression. TACE was shown to improve overall survival in patients with nonresectable HCC in RCTs performed in Europe and Asia155,156. The additional use of chemotherapeutic agents in TACE compared with the use of bland embolization with plastic beads alone, referred to as transarterial embolization (TAE), has been challenged as there is some evidence that most of the antitumour effect achieved by TACE is via the ischaemia induced by occluding the vascular supply to the tumour. An RCT of 101 patients (51 treated with TAE and 50 treated with doxorubicin-eluting bead TACE) showed no difference in treatment response, progression-free survival, overall survival or adverse events between the two groups, suggesting no additional benefit of chemotherapy in TACE157. In this RCT, median overall survival in both arms was only 21 months, which was shorter than expected as other reports show a median survival of up to 30–40 months following TACE treatment109,158. The survival of patients following TACE is dependent on the tumour extent and severity of liver dysfunction and shows substantial variability between different regions of the world. A prospective study of 173 patients with HCC from Greece who were not suitable for curable treatments showed a mean overall survival of 43 months following TACE using drug-eluting beads loaded with doxorubicin158. The 5-year overall survival rate was 23% in Child-Pugh class A patients compared with 13% in Child-Pugh class B patients (P = 0.03)158.
As the evidence of a lack of benefit from the chemotherapeutic agent in TACE when compared with TAE is from a single-centre study with a comparatively small number of patients, further external validation is needed before changing clinical practice. With the promising efficacy and approval of newer systemic treatments for advanced-stage HCC, the potential for using newer systemic treatments rather than conventional chemotherapeutic agents for TACE also needs to be further investigated in future studies.
Transarterial radioembolization (TARE) is another form of locoregional treatment that is useful as a primary treatment for nonresectable HCC as a downstaging treatment before liver transplantation or to produce lobar ablation (radiation lobectomy), which induces compensatory hypertrophy of the untreated, uninvolved lobe and facilitates surgical resection. TARE is a form of intratumoural brachytherapy that delivers radioactive microspheres loaded with β-emitting yttrium-90 isotope into the arteries that feed HCC tumours, potentially achieving radiation doses that are higher than those achievable with external beam radiation. In contrast to TACE, TARE has minimal embolic effects in the hepatic artery distribution and can, therefore, be used in patients with portal vein thrombosis or tumour invasion159. The efficacy and safety of TARE have been extensively evaluated over the past decade160–165. A meta-analysis that included 284 patients with TACE and 269 with TARE showed no statistically significant difference in survival between the two groups (HR 1.06, 95% CI 0.81–1.46, P = 0.57)164. Patients treated with TACE had more posttreatment pain than those treated with TARE (RR 0.51, 95% CI 0.36–0.72, P<0.01), but less subjective fatigue (RR 1.68, 95% CI 1.08–2.62, P<0.01)164. TARE has been proposed as a treatment option in patients with locally advanced HCC. RCTs from Europe and the Asian Pacific have evaluated the safety and efficacy of TARE in comparison with sorafenib in patients with locally advanced HCC and neither trial showed a survival benefit of TARE over sorafenib166,167.
Stereotactic body radiation therapy and proton beam therapy have also demonstrated efficacy in HCC, and early results suggest outcomes equivalent to those of TACE with a reduced adverse-effect profile168–175. However, high-quality data on stereotactic body radiation therapy and proton beam therapy are lacking and additional studies are needed to define the optimal treatment selection process for locoregional therapy.
Systemic pharmacological treatment.
Sorafenib is a small-molecule multikinase inhibitor that targets the vascular endothelial growth factor receptors VEGFR1, VEGFR2 and VEGFR3, platelet-derived growth factor receptor-β (PDGFRβ) and the Raf family kinases (predominantly C-Raf rather than B-Raf). Sorafenib was the first drug approved for first-line systemic treatment of patients with advanced-stage HCC and is the first systemic treatment that has been shown to prolong the survival of patients with advanced-stage HCC in phase III RCTs, with an improvement in median overall survival of 2–3 months (median overall survival of 10.7 months in the sorafenib group versus 7.9 months in the placebo group in the SHARP trial, and median survival of 6.5 months in the sorafenib group versus 4.2 months in the placebo group in the Asia-Pacific trial)176,177. However, in phase III RCTs, sorafenib was found to be ineffective as an adjuvant treatment after curative resection (STORM trial)178, or as concurrent treatment with TACE (SPACE trial)179.
Lenvatinib, a multikinase inhibitor targeting VEGFR1–3, fibroblast growth factor receptors FGFR1–4, PDGFRα, RET and KIT was shown to be noninferior to sorafenib in the phase III REFLECT trial180. This trial was conducted in 954 eligible patients with HCC at 154 sites in 20 countries from the Asian Pacific, European and North American regions who were randomly assigned to lenvatinib (n = 478) or sorafenib (n = 476). The study had strict exclusion criteria including 50% or higher involvement of the liver by tumour, invasion of the bile duct or the main portal vein, and previous systemic therapy for HCC. The median overall survival with lenvatinib was 13.6 versus 12.3 months for sorafenib (HR 0.92, 95% CI 0.79–1.06). Progression-free survival, time to progression and objective response rate were also improved with lenvatinib compared with sorafenib. Lenvatinib was approved for use in the USA, European Union and most Asian countries as a first-line systemic therapy for HCC in 2018.
Most patients treated with sorafenib eventually show disease progression. RCTs of agents for second-line treatment after sorafenib failure had been negative until a phase III multicentre RCT of regorafenib, a multikinase inhibitor chemically related to sorafenib, showed improved overall survival for regorafenib versus placebo (median survival 10.6 months versus 7.8 months)181. Regorafenib was approved for treatment of HCC after prior sorafenib therapy in 2017 in the USA, Europe and most Asian countries.
In 2018, the phase III CELESTIAL trial showed that treatment with cabozantinib, an inhibitor of tyrosine kinases including MET, AXL and VEGF receptors, resulted in a statistically significant and clinically meaningful improvement in median overall survival compared with placebo in patients with advanced HCC who had previously received sorafenib182. In a total of 707 patients randomly assigned in a 2:1 ratio to receive cabozantinib or placebo, the median overall survival was 10.2 months with cabozantinib and 8.0 months with placebo (HR 0.76, 95% CI 0.63–0.92, P = 0.005). Grade 3 or grade 4 adverse events occurred in 68% of patients in the cabozantinib group and 36% in the placebo group. It was interesting to note that in the subgroup analysis the hazard ratio for death was 0.69 in patients with HCC infected with HBV and 1.11 in patients with HCC infected with HCV, suggesting the possibility of effect modification of cabozantinib according to the underlying aetiology of liver disease and that cabozantinib could be an effective second-line treatment option particularly in patients with HBV infection. This aspect should be further investigated in future studies.
In 2019, the antiangiogenic VEGFR2 antagonist ramucirumab was shown to improve overall survival in patients with advanced HCC and serum AFP levels ≥400 ng/ml183. Ramucirumab blocks the activation of VEGFR2 by blocking the binding of the VEGF receptor ligands VEGFA, VEGFC and VEGFD184. Ramucirumab is the first biomarker-based systemic treatment for HCC and could be an excellent second-line treatment option in the subset of patients with advanced HCC and AFP levels ≥400 ng/ml. This was the first positive enrichment clinical trial in HCC and new trials should consider enrolling selected groups of patients with HCC with similar tumour biology to maximize the treatment efficacy in future studies. Ramucirumab was approved by the FDA in May 2019 as a single agent for treatment of HCC in patients previously treated with sorafenib who have an AFP level ≥400 ng/ml.
Immune checkpoint inhibitors have emerged as a promising treatment option for advanced-stage HCC185. A multicentre phase I/II, open-label, dose-escalation and dose-expansion trial published in 2017 showed promising efficacy of nivolumab, a human anti-PD-1 monoclonal antibody, for the treatment of patients with advanced HCC (n = 262)185. The objective response rate was 15% in the dose-escalation phase and 20% in patients treated with 3 mg/kg nivolumab in the dose-expansion phase. Nivolumab had an acceptable adverse effect profile regardless of underlying HCC aetiology185. Based on these results, in September 2017 the FDA granted approval for the use of nivolumab as a second-line treatment for advanced HCC in patients previously treated with sorafenib. A phase III RCT of nivolumab monotherapy compared with sorafenib in the first-line setting for advanced HCC is ongoing, with an estimated study completion date of June 2019 (NCT02576509)186. Similarly, in November 2018, the FDA granted accelerated approval to pembrolizumab for patients with HCC previously treated with sorafenib based on the results of KEYNOTE 224 (NCT02702414), a single-arm, phase II, multicentre trial of 104 patients with disease progression on or after sorafenib or who were intolerant to sorafenib. The overall response rate was 17% (95% CI 11–26%)187.
For patients with advanced HCC who are not amenable to treatments that can improve survival, providing a range of palliative and supportive care options is crucial. In those with intractable pain, partial tumour resection and low-dose external beam radiation protocols can be very useful131.
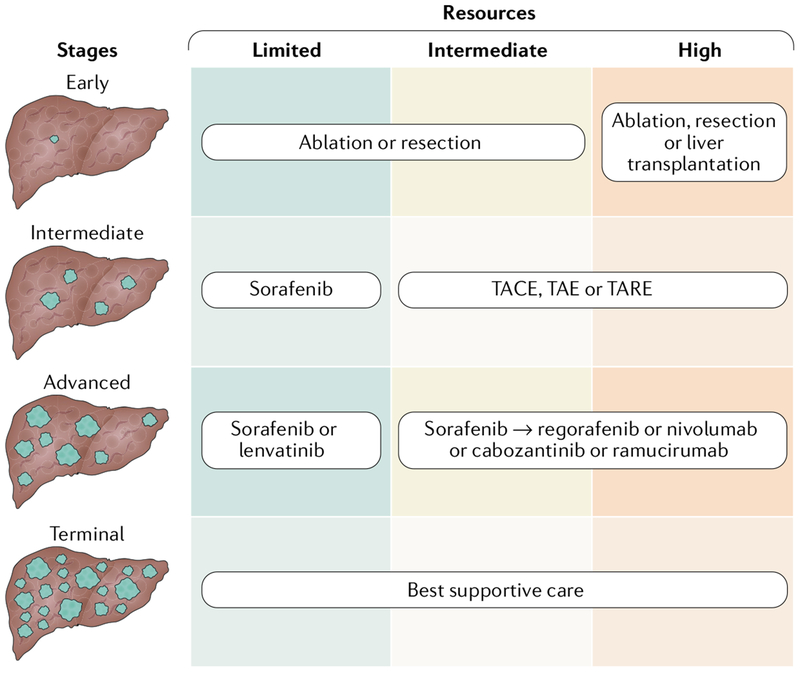
HCC management at different resource levels.
The proposed overall approach for HCC management in different resource settings is summarized in FIG. 3. The optimal treatment should be considered even in intermediate-resource or low-resource countries if the treatment option is available; however, not all forms of treatment are equally cost-effective and equally years-of-life-saving in every setting, regardless of their cost. For early-stage HCC, potentially curative treatments (namely, ablation, resection or liver transplantation) should be considered. For intermediate-stage HCC, locoregional treatments such as TACE, TAE or TARE should be offered. However, these therapeutic modalities are resource-intensive, and sorafenib could be considered as an alternative option in countries with limited resources. For advanced-stage HCC, targeted receptor tyrosine kinase inhibitors or immunotherapy should be considered regardless of the level of resources. Reducing the cost of medication will be critical to enabling the successful use of these medications, particularly in resource-limited countries. Best supportive care should be provided for patients with terminal-stage HCC.
Fig. 3 |. Strategy for HCC treatment in countries with different resource levels.
The optimal-treatment for hepatocellular carcinoma (HCC) should be considered even in intermediate-resource or low-resource countries if the treatment option is available; however, not all forms of treatment are equally cost-effective and years-of-life-saving in every setting, regardless of their cost. For early-stage HCC, potentially curative treatment should be considered whereas locoregional treatment would be the first-line treatment for intermediate-stage HCC. However, these therapeutic modalities are resource-intensive and sorafenib could be considered as an alternative option in countries with limited resources. For advanced-stage HCC, targeted or immunotherapy should be considered regardless of resource level. Best supportive care should be provided in patients with terminal-stage HCC. TACE, transarterial chemoembolization; TAE, transarterial embolization; TARE, transarterial radioembolization.
Global strategies for the management of HCC
Four main areas need to be improved to decrease the burden of HCC worldwide: preventing HBV and HCV infection; treating chronic hepatitis B, hepatitic C and liver disease; mitigating exposure to dietary and metabolic risk factors; and improving liver cancer detection, diagnosis and therapy. Strict estimates of the effects of specific measures are lacking, but it should be considered that none of these measures on their own will be sufficient to considerably decrease the number of deaths through liver cancer in the next decades. These measures need to be combined into an organized and structured action plan for a realistic chance of counterbalancing the increase in liver cancer cases owing to population expansion and ageing. In BOX 1 we propose a framework of global recommendations, taking into account progress to date as well as new actions that seem both necessary and possible on the basis of the latest advances in HCC prevention and therapy188. This framework includes primary prevention, including universal HBV vaccine coverage, controlling chronic viral hepatitis with antiviral treatment, and reducing environmental- and lifestyle-related risk factors, secondary prevention, including early detection via HCC surveillance programmes, and tertiary prevention through universal access to the most appropriate treatments in different resource settings, which are further discussed in detail below.
Box 1 |. Framework recommendations for global reduction of HCC burden.
Achieving universal HBV vaccine coverage
Roll out sustainable HBV neonatal vaccination programmes
Monitor long-term protection (vaccine effectiveness)
Study interactions with other infections
Assess the usefulness of administration of hepatitis B immunoglobulin to pregnant mothers for preventing mother-to-child transmission
Inform and educate populations and stakeholders, address changing perceptions towards vaccination
Controlling HCV-related and HBV-related diseases
Promote HBV and HCV screening in high-risk populations
Promote universal access to pharmacological treatment of chronic HBV and HCV infections
Reducing environmental and lifestyle exposures
Promote substitute crops with less contamination by aflatoxin
Enforce stricter control on aflatoxin contamination levels
Promote healthy diet and physical exercise for prevention of metabolic syndrome, NAFLD and NASH
Promote alcohol and smoking cessation
Control exposure to liver carcinogens and toxicants in the environment and in the workplace
Improving early detection, staging and management
Develop nationwide programmes for HCC surveillance of at-risk individuals
Develop and roll out improved early detection strategies using noninvasive biomarkers and imaging
Address the need for early detection in low-resource settings where HCC often develops early without pre-existing cirrhosis
Promote universal access to best-available treatment options irrespective of resource context, including access to targeted therapies and immunotherapies
Develop awareness of liver disease, support to patients and families and access to palliation
HCC, hepatocellular carcinoma.
To date, only infant HBV vaccination has been rolled out as a structured worldwide programme, as part of the Expanded Programme of Immunization, using multivalent vaccines that are usually administered beginning at 6–8 weeks after birth. This is primarily due to the efforts of the WHO (World Health Organization), the UNICEF (United Nations Children’s Fund) and Gavi, the Vaccine Alliance, a public-private global health partnership founded by the Bill and Melinda Gates Foundation with the shared goal of improving access to vaccines for children living in the world’s poorest countries, in collaboration with local national health systems. However, population coverage of the Expanded Programme of Immunization remains <75% in around one-third of these countries, particularly those countries with limited medical resources69. Thus, despite much hope for universal HBV vaccination, the number of deaths from HCC will continue to grow in the next decades unless better coverage and other prevention measures are efficiently implemented. In particular, the lack of effort to prevent mother-to-child transmission by broad introduction of the birth dose of HBV vaccine to newborn babies within the first 24 h of life, as well as by identification and treatment of the hepatitis B ‘e’ antigen-positive pregnant women who are most likely to transmit HBV infection to infants, is a missed opportunity in many regions of low-income and middle-resource countries69. In addition to HBV vaccination, HBV and HCV screening in high-risk populations and universal access to pharmacological treatment of chronic HBV and HCV infections for those who have acquired infection will reduce the global burden of HCC.
Active mitigation of aflatoxin exposure requires a multilevel action plan, but passive reduction will spontaneously occur through diversification of the diet as many low-resource countries are moving from low-income to middle-income status188. However, economic growth and dietary diversification are also expected to cause many changes that might lead to an increase in the incidence of liver cancer. A switch towards Westernized hypercaloric diets and sedentary lifestyles, occurring in many traditionally underdeveloped areas, is causing a rapid surge in population obesity, metabolic syndrome and diabetes189. This development increases the prevalence of risk factors for liver cancer in both HBV carriers and noncarriers, including individuals in whom carriage has been prevented by neonatal vaccination75. Monitoring these trends will be essential to distinguish positive and negative effects of the changes associated with development. Thus, curbing the liver cancer epidemic will require careful and rational management of global ecosystems, taking into account economic growth, changes in agricultural and dietary practices, and reduction of endemic conditions, such as viral hepatitis and obesity and lifestyle-related risk factors such as alcohol and cigarette smoking.
Access to diagnosis, treatment and palliation is dramatically constrained by limited economic resources in most HCC endemic countries. However, the global trajectory of liver disease and cancer indicate that an excellent window of opportunity exists for screening, early detection and early intervention190. Technology-intensive treatments, such as liver transplantation or catheter-based locoregional treatments, are often not available in countries with low-resource or middle-resource levels. Thus, best alternative treatments should be provided depending on the stage of HCC. The positive results of trials with kinase inhibitors and immune checkpoint inhibitors in the past few years are likely to spur increased access and reductions in cost of systemic therapy worldwide. For all these interventions, improved medical access and clinical expertise in affected countries and regions is crucial. Efforts might be particularly successful in the case of local ablation and surgical resection, for which it is feasible to provide comparably low-cost solutions and transfer the needed knowledge for hepatobiliary surgery, anaesthesia and perioperative care to enable relatively safe and curative management options.
All aspects of the effort to decrease the burden of liver cancer worldwide urgently require advocacy at the local, governmental and regional levels. The main areas include the development and deployment of effective screening and surveillance programmes and improvement of access to affordable diagnostics and therapies. In addition, adoption of transformative new technologies needs to be promoted, including eHealth or mobile health applications, new low-cost device platforms for improving viral hepatitis screening and new technologies for HCC surveillance, including molecular assays, cell-phone or hand-held device-based technology for liver ultrasonography or other forms of liver imaging, such as for neoangiogenesis191.
The growth of national, regional and global advocacy networks focusing on addressing the epidemic of viral hepatitis and liver cancer has been one of the most important developments in the past 10 years. These networks have stimulated the development of new guidelines for hepatitis B and hepatitis C by the WHO, the adoption of resolutions recognizing chronic viral hepatitis as a major cause of morbidity and mortality worldwide at the World Health Assembly and the addition of the elimination of viral hepatitis worldwide to the global Sustainable Development Goals192. The work of these networks, which have coalesced in the World Hepatitis Alliance, is highlighted by the organization of the World Hepatitis Summit meetings in 2015 and 2017. These meetings have gathered key stakeholders and resulted in the development of action plans for achieving the global elimination of viral hepatitis by the year 2030 (REF.13). A major characteristic of these initiatives that distinguishes them from previous efforts to combat HIV infection worldwide is the recognition that efforts to eliminate viral hepatitis must include broad-based efforts to strengthen health system infrastructures in low-income and middle-income countries where the vast majority of those infected live. The launch of the global Fund for the Elimination of Viral Hepatitis in November 2017 is a key step and commitment by the global philanthropic sector to mobilize resources towards the achievement of these goals.
Finally, an action network addressing HCC screening, diagnosis and treatment in the low-resource setting is urgently needed. Efforts are underway through the African and International Hepatobiliary Pancreatic Associations to enhance training in liver surgery and through the formation of the Gastroenterology and Hepatology Association of Sub-Saharan Africa193. Anticipated collaborations with the African Organization for Research and Training in Cancer and local, regional and international professional and advocacy organizations will be needed to achieve meaningful progress in this area.
Conclusions
HCC is a major contributor to the cancer-related disease burden in many regions of the world, particularly in African and Asian countries where medical and social care resources are often limited. Prevention and treatment of viral hepatitis and minimizing aflatoxin exposure are crucial elements in decreasing the global burden of HCC. HCC surveillance leads to early detection of cancer and improves overall survival. Thus, implementation of programmes for viral hepatitis screening, HCC surveillance in high-risk patients and effective treatment of HCC are important to improve the current dismal outcomes of patients with this disease. The marked increase of community, health advocacy, public health and civil society efforts in the past few years gives hope for the prospect of substantially decreasing the global burden of illness and death from HCC within the next few decades.
Supplementary Material
Key points.
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death worldwide; >80% of HCC cases occur in low-resource and middle-resource countries, particularly in Eastern Asia and sub-Saharan Africa, where medical and social care resources are often constrained.
Prevention and treatment of viral hepatitis and mitigation of exposure to aflatoxin and aristolochic acid, the main risk factors in high-incidence regions, are critical for decreasing the global burden of HCC.
HCC surveillance enables early detection and increases the chance of potentially curative treatment; therefore, broad implementation of HCC surveillance in high-risk patients is essential to reduce the high mortality from HCC.
Early-stage HCC is amenable to potentially curative treatment, which includes local ablation, surgical resection and liver transplantation.
Catheter-based locoregional treatment is indicated in patients with intermediate-stage disease; kinase and immune checkpoint inhibitors have been shown to be effective treatment options in patients with advanced-stage HCC.
Global reduction of HCC burden can be achieved by universal HBV vaccination, control of chronic viral hepatitis, avoiding environmental and lifestyle risk factors, and improving early detection and management.
Acknowledgements
The authors are supported by grant numbers T32 DK07198 from the National Institute of Diabetes and Digestive and Kidney Diseases (J.D.Y.), CA165076, CA186566, CA 221205 and CA 210964 from the National Cancer Institute (L.R.R.) and the French National Research Agency ‘Investissements d’avenir’ program (ANR-15-IDEX-02) (P.H.). The contents of this Review are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Glossary
- Years of life lost
An estimate of the average years a person would have lived if he or she had not died prematurely.
- Age-standardized incidence rate (ASIR).
The incidence rate after accounting for the differences in the age structure of the populations.
- Sociodemographic index
A summary measure of geographical sociodemographic development, determined based on average income per person, educational attainment and total fertility rate.
- Surveillance, Epidemiology, and End Results (SEER)
A National Cancer Institute programme that provides information on the incidence of cancer and survival from cancer in the USA.
- Adjusted odds ratio (AOR)
The ratio of the odds of the presence of an antecedent in those with a positive outcome to the Odds in those with a negative outcome after adjusting for Other factors that can affect the outcome.
- Population-attributable fraction
The proportion of incidents in the population that are attributable to the risk factor.
- Relative risk
The ratio of the probability of an outcome in an exposed group to the probability of an outcome in an unexposed group.
- Absolute risk
Tile risk of developing the outcome of interest.
- Direct-acting antiviral (DAA)
A new class of medication that acts directly to target specific steps in the HCV life-cycle.
- Sustained virologic response (SVR)
An undetectable viral titre at least 12 weeks after completing treatment.
- Primary prevention
Preventive interventions that are applied before there is any evidence of disease or injury.
- Hepatitis B immunoglobulin
A human immunoglobulin that is used to prevent the transmission of HBV infection.
- Enterosorbents
An adsorbent for binding toxic substances in the gastrointestinal tract.
- Secondary prevention
Preventive interventions that try to detect a disease early and prevent it from getting worse.
- Child–Pugh score
A classification system for the severity of cirrhosis.
- Up-to-seven criterion
The sum of the largest tumour size in centimetres and the number of tumours ≤7.
- Eastern Cooperative Oncology Group performance status
A performance status score used to assess the ability of a patient to tolerate cancer treatment.
- MELD
A scoring system for assessing the severity of chronic liver disease which is now used by the United Network for Organ Sharing and Eurotransplant for prioritizing allocation of liver transplants.
- Embolization
A treatment that blocks blood vessels to prevent blood flow to the tumour.
- Heat sink effect
The cooling effect of blood flow leading to incomplete thermal ablation of liver tumours near large blood vessels.
- Bridge therapy
Hepatocellular carcinoma treatment during the waiting time prior to transplantation to prevent tumour progression.
- Lobar ablation
Delivery of high-dose radiation to one lobe of the liver, causing hypotrophy of the treated lobe of the liver.
- Brachytherapy
A form of radiotherapy where a sealed radiation source is placed inside or next to the area requiring treatment.
- Stereotactic body radiation therapy
A focused radiation treatment using several beams of various intensities aimed at different angles to precisely target the tumour.
- Objective response rate
The proportion of patients with a reduction in tumour burden of a predefined amount.
- Immune checkpoint inhibitors
A form of immunotherapy that works by releasing a natural brake on the immune system so that T cells can recognize and attack tumours.
- Sustainable Development Goals
A collection of 17 global goals set by the United Nations General Assembly in 2015 for the year 2030 to end poverty, protect the planet and ensure that all people enjoy peace and prosperity.
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41575-019-0186-y.
References
- 1.Global Burden of Disease Cancer, C. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 3, 524–548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB & Rudolph KL Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 (2007). [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142, 1264–1273 e1261 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang A, Hallouch O, Chernyak V, Kamaya A & Sirlin CB Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom. Radiol. (NY) 43, 13–25 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Altekruse SF, McGlynn KA & Reichman ME Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol 27, 1485–1491 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajarizadeh B, Grebely J & Dore GJ Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol 10, 553–562 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Park JW et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the bridge study. Liver Int 35, 2155–2166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang JD et al. Hepatocellular carcinoma occurs at an earlier age in Africans, particularly in association with chronic hepatitis B. Am. J. Gastroenterol 110, 1629–1631 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Yang JD, Altekruse SF, Nguyen MH, Gores GJ & Roberts LR Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer 123, 81–89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JD et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol. Hepatol 2, 103–111 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Kudo M Management of hepatocellular carcinoma in Japan as a world-leading model. Liver Cancer 7, 134–147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JD & Roberts LR Hepatocellular carcinoma: a global view. Nat. Rev. Gastroenterol. Hepatol 7, 448–458 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. WHO; https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ (2016). [Google Scholar]
- 14.Heffernan A, Cooke GS, Nayagam S, Thursz M & Hallett TB Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet 393, 1319–1329 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JD et al. Diabetes mellitus heightens the risk of hepatocellular carcinoma except in patients with hepatitis c cirrhosis. Am. J. Gastroenterol 111, 1573–1580 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chayanupatkul M et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J. Hepatol 66, 355–362 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Younossi ZM et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 64, 1577–1586 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Younossi ZM et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Younossi ZM et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 62, 1723–1730 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Yang JD et al. Recent trends in the epidemiology of hepatocellular carcinoma in Olmsted county, Minnesota: a US population-based study. J. Clin. Gastroenterol 51,742–748 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welzel TM et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am. J. Gastroenterol 108, 1314–1321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittal S et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol 14, 124–131 e121 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Serag HB, Hampel H & Javadi F The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol 4, 369–380 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Huang SF et al. Metabolic risk factors are associated with non-hepatitis B non-hepatitis C hepatocellular carcinoma in Taiwan, an endemic area of chronic hepatitis B. Hepatol. Commun 2, 747–759 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balkwill F & Mantovani A Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Hirosumi J et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Hui L, Zatloukal K, Scheuch H, Stepniak E & Wagner EF Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J. Clin. Invest 118, 3943–3953 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norat T, Aune D, Navarro D & Abar L Diet, nutrition, physical activity and liver cancer. World Cancer Research Fund International https://www.wcrf.org/sites/default/files/Liver-Cancer-2015-Report.pdf (2015).
- 29.West J, Card TR, Aithal GP & Fleming KM Risk of hepatocellular carcinoma among individuals with different aetiologies of cirrhosis: a population- based cohort study. Aliment. Pharmacol. Ther 45, 983–990 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Jepsen P, Ott P, Andersen PK, Sorensen HT & Vilstrup H Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis: a Danish nationwide cohort study. Ann. Intern. Med 156, 841–847, W295 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Mancebo A et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin. Gastroenterol. Hepatol 11,95–101 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Wild CP, Miller JD & Groopman JD Mycotoxin Control in Low- and Middle-Income Countries Vol. 9 (IARC, 2015). [PubMed] [Google Scholar]
- 33.Gouas D, Shi H & Hainaut P The aflatoxin-induced TP53 mutation at codon 249 (R249S): biomarker of exposure, early detection and target for therapy. Cancer Lett 286, 29–37 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Weng MW et al. AFB1 hepatocarcinogenesis is via lipid peroxidation that inhibits DNA repair, sensitizes mutation susceptibility and induces aldehyde-DNA adducts at p53 mutational hotspot codon 249. Oncotarget 8, 18213–18226 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helleday T, Eshtad S & Nik-Zainal S Mechanisms underlying mutational signatures in human cancers. Nat. Rev. Genet 15, 585–598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen HM & Ong CN Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat. Res 366, 23–44 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Ross RK et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 339, 943–946 (1992). [DOI] [PubMed] [Google Scholar]
- 38.Kew MC Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int 23, 405–409 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Arlt VM, Stiborova M & Schmeiser HH Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis 17, 265–277 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Rosenquist TA & Grollman AP Mutational signature of aristolochic acid: clue to the recognition of a global disease. DNA Repair (Amst.) 44, 205–211 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Ng AWT et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci. Transl Med 9, eaan6446 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Hsieh SC, Lin IH, Tseng WL, Lee CH & Wang JD Prescription profile of potentially aristolochic acid containing Chinese herbal products: an analysis of National Health Insurance data in Taiwan between 1997 and 2003. Chin. Med 3, 13 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen CJ et al. Herbal medicine containing aristolochic acid and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. In. J. Cancer, 143, 1578–1587 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Bravi F, Tavani A, Bosetti C, Boffetta P & La Vecchia C Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur. J. Cancer Prev 26, 368–377 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Singh S, Singh PP, Singh AG, Murad MH & Sanchez W Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 144, 323–332 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Zhou YY et al. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci. Rep 6, 33743 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahasrabuddhe VV et al. Nonsteroidal antiinflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J. Natl Cancer Inst 104, 1808–1814 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol 69, 182–236 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Kim WR et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer 121, 3631–3638 (2015). [DOI] [PubMed] [Google Scholar]
- 50.van der Meer AJ et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 308, 2584–2593 (2012). [DOI] [PubMed] [Google Scholar]
- 51.El-Serag HB, Kanwal F, Richardson P & Kramer J Risk of hepatocellular carcinoma after sustained virological response in veterans with hepatitis C virus infection. Hepatology 64, 130–137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanwal F et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 153, 996–1005 e1001 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Reig M et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol 65, 719–726 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Conti F et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol 65, 727–733 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Yang JD et al. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. J. Hepatol 65, 859–860 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Ravi S et al. Unusually high rates of hepatocellular carcinoma after treatment with direct-acting antiviral therapy for hepatitis c related cirrhosis. Gastroenterology 152, 911–912 (2017). [DOI] [PubMed] [Google Scholar]
- 57.ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J. Hepatol 65, 734–740 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Petta S et al. Hepatocellular carcinoma recurrence in patients with curative resection or ablation: impact of HCV eradication does not depend on the use of interferon. Aliment. Pharmacol. Ther 45, 160–168 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Singal AG et al. Direct-acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multicenter North American cohort study. Gastroenterology 156, 1683–1692 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nahon P et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology 155, 1436–1450 e1436 (2018). [DOI] [PubMed] [Google Scholar]
- 61.The Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341 e1323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulze K et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet 47, 505–511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoshida Y et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res 69, 7385–7392 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amaddeo G et al. Integration of tumour and viral genomic characterizations in HBV-related hepatocellular carcinomas. Gut 64, 820–829 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boyault S et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 45, 42–52 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Calderaro J et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol 67, 727–738 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Harding JJ et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next- generation sequencing for matching patients to targeted and immune therapies. Clin. Cancer Res 25, 2116–2126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang MH et al. Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology 151,472–480 (2016). [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization. WHO-UNICEF estimates of HepB3 coverage. WHO; http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragehepb3.html (2019). [Google Scholar]
- 70.Jourdain G et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N. Engl. J. Med 378, 911–923 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eke AC, Eleje GU, Eke UA, Xia Y & Liu J Hepatitis B immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis B virus. Cochrane Database Syst. Rev 2, CD008545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith BD et al. Hepatitis C virus testing of persons born during 1945–1965: recommendations from the centers for disease control and prevention. Ann. Intern. Med 157, 817–822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papatheodoridis GV, Lampertico P, Manolakopoulos S & Lok A Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J. Hepatol 53, 348–356 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Thoms DL Global elimination of chronic hepatitis. N. Engl. J. Med 380, 2041–2050 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Patel NS et al. Weight loss decreases magnetic resonance elastography estimated liver stiffness in nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol 15, 463–464 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petrick JL et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the liver cancer pooling project. Br. J. Cancer 118, 1005–1012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y, Chang CC, Marsh GM & Wu F Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur. J. Cancer 48, 2125–2136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner PC et al. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in West Africa: a community-based intervention study. Lancet 365, 1950–1956 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Chen JG et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. CancerPrev. Res 6, 1038–1045 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marrero JA et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 68, 723–750 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Papatheodoridis G et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in caucasians with chronic hepatitis B on 5-year antiviral therapy. J. Hepatol 64, 800–806 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Singal AG, Pillai A & Tiro J Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLOS Med 11, e1001624 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.National Cancer Institute (ed.) PDQ Cancer Information Summaries, 2019. https://www.ncbi.nlm.nih.gov/books/NBK65785/ (NCBI, 2019). [Google Scholar]
- 84.Poustchi H et al. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology 54, 1998–2004 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Tan CH, Low SC & Thng CH APASL and AASLD consensus guidelines on imaging diagnosis of hepatocellular carcinoma: a review. Int. J. Hepatol 2011,519783 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Santi V et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J. Hepatol 53, 291–297 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Trinchet JC et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 54, 1987–1997 (2011). [DOI] [PubMed] [Google Scholar]
- 88.Chang TS et al. Alpha-fetoprotein measurement benefits hepatocellular carcinoma surveillance in patients with cirrhosis. Am. J. Gastroenterol 110, 836–845 (2015). [DOI] [PubMed] [Google Scholar]
- 89.McMahon BJ et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology 32, 842–846 (2000). [DOI] [PubMed] [Google Scholar]
- 90.Yang JD et al. Improved performance of serum alpha-fetoprotein for hepatocellular carcinoma diagnosis in HCV cirrhosis with normal alanine transaminase. Cancer Epidemiol. Biomarkers Prev 26, 1085–1092 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El-Serag HB, Kanwal F, Davila JA, Kramer J & Richardson P A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology 146, 1249–1255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tayob N, Lok AS, Do KA & Feng Z Improved detection of hepatocellular carcinoma by using a longitudinal alpha-fetoprotein screening algorithm. Clin. Gastroenterol. Hepatol 14, 469–475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marrero JA et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 137, 110–118 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lok AS et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology 138, 493–502 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berhane S et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin. Gastroenterol. Hepatol 14, 875–886 (2016). [DOI] [PubMed] [Google Scholar]
- 96.Johnson PJ et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol. Biomarkers Prev 23, 144–153 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Yang JD et al. GALAD score for hepatocellular carcinoma detection in comparison with liver ultrasound and proposal of GALADUS score. Cancer Epidemiol. Biomarkers Prev 28, 531–538 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin XJ et al. A serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol 16, 804–815 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Zhao Y et al. Genome-wide methylation profiling of the different stages of hepatitis B virus-related hepatocellular carcinoma development in plasma cell- free DNA reveals potential biomarkers for early detection and high-risk monitoring of hepatocellular carcinoma. Clin. Epigenet 6, 30 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liao W et al. Value of quantitative and qualitative analyses of circulating cell-free DNA as diagnostic tools for hepatocellular carcinoma: a meta-analysis. Medicine 94, e722 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shang S et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology 55, 483–490 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kisiel JB et al. Hepatocellular carcinoma detection by plasma methylated DNA: discovery, phase I pilot, and phase II clinical validation. Hepatology 69, 1180–1192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Atiq O et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 65, 1196–1205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rich NE, Parikh ND & Singal AG Overdiagnosis: an understudied issue in hepatocellular carcinoma surveillance. Semin. Liver Dis 37, 296–304 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mitchell DG, Bruix J, Sherman M & Sirlin CB LI-RADS (liver imaging reporting and data system): summary, discussion, and consensus of the LI-RADS management working group and future directions. Hepatology 61, 1056–1065 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Tang A et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology 286, 29–48 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu PH et al. Prognosis of hepatocellular carcinoma: assessment of eleven staging systems. J. Hepatol 64, 601–608 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Llovet JM, Bru C & Bruix J Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis 19, 329–338 (1999). [DOI] [PubMed] [Google Scholar]
- 109.Forner A, Reig M & Bruix J Hepatocellular carcinoma. Lancet 391, 1301–1314 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Giannini EG et al. Application of the intermediate- stage subclassification to patients with untreated hepatocellular carcinoma. Am. J. Gastroenterol 111, 70–77 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Kudo M et al. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology 40, 1396–1405 (2004). [DOI] [PubMed] [Google Scholar]
- 112.Hiraoka A et al. Proposed new sub-grouping for intermediate-stage hepatocellular carcinoma using albumin-bilirubin grade. Oncology 91, 153–161 (2016). [DOI] [PubMed] [Google Scholar]
- 113.Johnson PJ et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—the ALBI grade. J. Clin. Oncol 33, 550–558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yau T et al. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 146, 1691–1700 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Sohn JH et al. Validation of the Hong Kong liver cancer staging system in determining prognosis of the North American patients following intra-arterial therapy. Clin. Gastroenterol. Hepatol 15, 746–755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu L, Bartlett A, Plank L & McCall J Validation of the Hong Kong liver cancer staging system in hepatocellular carcinoma patients treated with curative intent. J. Hepatol 64, 978–979 (2016). [DOI] [PubMed] [Google Scholar]
- 117.Yang JD et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology 56, 614–621 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Farinati F et al. Development and validation of a new prognostic system for patients with hepatocellular carcinoma. PLOS Med 13, e1002006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Toyoda H et al. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin. Gastroenterol. Hepatol 4, 1528–1536 (2006). [DOI] [PubMed] [Google Scholar]
- 120.Serper M et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology 152, 1954–1964 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berzigotti A, Reig M, Abraldes JG, Bosch J & Bruix J Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 61, 526–536 (2015). [DOI] [PubMed] [Google Scholar]
- 122.Boleslawski E et al. Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. Br. J. Surg 99, 855–863 (2012). [DOI] [PubMed] [Google Scholar]
- 123.Teh SH et al. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J. Gastrointest. Surg 9, 1207–1215 (2005). [DOI] [PubMed] [Google Scholar]
- 124.Roayaie S et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 62, 440–451 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Kim H et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br. J. Surg 104, 1045–1052 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Tabrizian P, Jibara G, Shrager B, Schwartz M & Roayaie S Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann. Surg 261,947–955 (2015). [DOI] [PubMed] [Google Scholar]
- 127.Hoshida Y et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N. Engl. J. Med 359, 1995–2004 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferrer-Fabrega J et al. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology 63, 839–849 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Hammad AY et al. Palliative interventions for hepatocellular carcinoma patients: analysis of the National Cancer Database. Ann. Palliat. Med 6, 26–35 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Hawkins MA & Dawson LA Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer 106, 1653–1663 (2006). [DOI] [PubMed] [Google Scholar]
- 131.Hayashi S, Tanaka H & Hoshi H Palliative external- beam radiotherapy for bone metastases from hepatocellular carcinoma. World J. Hepatol 6, 923–929 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang JD et al. Hepatocellular carcinoma is the most common indication for liver transplantation and placement on the waitlist in the United States. Clin. Gastroenterol. Hepatol 15, 767–775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mazzaferro V et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med 334, 693–699 (1996). [DOI] [PubMed] [Google Scholar]
- 134.Yao FY et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl 11, 1505–1514 (2005). [DOI] [PubMed] [Google Scholar]
- 135.Mazzaferro V et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 10, 35–43 (2009). [DOI] [PubMed] [Google Scholar]
- 136.Yao FY et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33, 1394–1403 (2001). [DOI] [PubMed] [Google Scholar]
- 137.Yao FY et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl 8, 765–774 (2002). [DOI] [PubMed] [Google Scholar]
- 138.Yao FY et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am. J. Transplant 7, 2587–2596 (2007). [DOI] [PubMed] [Google Scholar]
- 139.Yao FY et al. Excellent outcome following downstaging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 48, 819–827 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mehta N et al. Excellent outcomes of liver transplantation following down-staging of hepatocellular carcinoma to within Milan criteria: a multicenter study. Clin. Gastroenterol. Hepatol 16, 955–964 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shiina S et al. Percutaneous ablation for hepatocellular carcinoma: comparison of various ablation techniques and surgery. Can. J. Gastroenterol. Hepatol 2018, 4756147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu J et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut 66, 1172–1173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu W et al. Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Aliment. Pharmacol. Ther 48, 671–681 (2018). [DOI] [PubMed] [Google Scholar]
- 144.Kang TW, Lim HK & Cha DI Percutaneous ablation for perivascular hepatocellular carcinoma: refining the current status based on emerging evidence and future perspectives. World. J. Gastroenterol 24, 5331–5337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vietti Violi N et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol. Hepatol 3, 317–325 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Kim GM et al. Cryoablation of hepatocellular carcinoma with high-risk for percutaneous ablation: safety and efficacy. Cardiovasc. Intervent. Radiol 39, 1447–1454 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Littrup PJ et al. Percutaneous cryoablation of hepatic tumors: long-term experience of a large U.S. series. Abdom. Radiol 41,767–780 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Xu Y et al. Microwave ablation for the treatment of hepatocellular carcinoma that met up-to-seven criteria: feasibility, local efficacy and long-term outcomes. Eur. Radiol 27, 3877–3887 (2017). [DOI] [PubMed] [Google Scholar]
- 149.Lee MW et al. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: a 10-year intention-to-treat analysis. Hepatology 65, 1979–1990 (2017). [DOI] [PubMed] [Google Scholar]
- 150.Heimbach JK et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67, 358–380 (2018). [DOI] [PubMed] [Google Scholar]
- 151.Huang J et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg 252, 903–912 (2010). [DOI] [PubMed] [Google Scholar]
- 152.Feng K et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol 57, 794–802 (2012). [DOI] [PubMed] [Google Scholar]
- 153.Chen MS et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg 243, 321–328 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lencioni R, de Baere T, Soulen MC, Rilling WS & Geschwind JF Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology 64, 106–116 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Lo CM et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35, 1164–1171 (2002). [DOI] [PubMed] [Google Scholar]
- 156.Llovet JM et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359, 1734–1739 (2002). [DOI] [PubMed] [Google Scholar]
- 157.Brown KT et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J. Clin. Oncol 34, 2046–2053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Malagari K et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc. Intervent. Radiol 35, 1119–1128 (2012). [DOI] [PubMed] [Google Scholar]
- 159.Kulik LM et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 47, 71–81 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Sangro B et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 54, 868–878 (2011). [DOI] [PubMed] [Google Scholar]
- 161.Salem R et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 140, 497–507 e492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mazzaferro V et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 57, 1826–1837 (2013). [DOI] [PubMed] [Google Scholar]
- 163.Salem R et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 151, 1155–1163 e1152 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lobo L et al. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc. Intervent. Radiol 39, 1580–1588 (2016). [DOI] [PubMed] [Google Scholar]
- 165.Carr BI, Kondragunta V, Buch SC & Branch RA Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer 116, 1305–1314 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chow PKH et al. SIRveNIB: selective internal radiation therapy versus sorafenib in asia-pacific patients with hepatocellular carcinoma. J. Clin. Oncol 36, 1913–1921 (2018). [DOI] [PubMed] [Google Scholar]
- 167.Vilgrain V et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 18, 1624–1636 (2017). [DOI] [PubMed] [Google Scholar]
- 168.Bush DA et al. Randomized clinical trial comparing proton beam radiation therapy with transarterial chemoembolization for hepatocellular carcinoma: results of an interim analysis. Int. J. Radiat. Oncol. Biol. Phys 95, 477–482 (2016). [DOI] [PubMed] [Google Scholar]
- 169.Fukuda K et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci 108, 497–503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hong TS et al. Multi-institutional phase ii study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Clin. Oncol 34, 460–468 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Igaki H et al. A systematic review of publications on charged particle therapy for hepatocellular carcinoma. Int. J. Clin. Oncol 23, 423–433 (2018). [DOI] [PubMed] [Google Scholar]
- 172.Sapir E et al. Stereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys 100, 122–130 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sapisochin G et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J. Hepatol 67, 92–99 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Teraoka Y et al. Clinical outcomes of stereotactic body radiotherapy for elderly patients with hepatocellular carcinoma. Hepatol. Res 48, 193–204 (2018). [DOI] [PubMed] [Google Scholar]
- 175.Zeng ZC et al. Consensus on stereotactic body radiation therapy for small-sized hepatocellular carcinoma at the 7th Asia-Pacific primary liver cancer expert meeting. Liver Cancer 6, 264–274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Llovet JM et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med 359, 378–390 (2008). [DOI] [PubMed] [Google Scholar]
- 177.Cheng AL et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase iii randomised, double-blind, placebo-controlled trial. Lancet Oncol 10, 25–34 (2009). [DOI] [PubMed] [Google Scholar]
- 178.Bruix J et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo- controlled trial. Lancet Oncol 16, 1344–1354 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Lencioni R et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J. Hepatol 64, 1090–1098 (2016). [DOI] [PubMed] [Google Scholar]
- 180.Kudo M et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018). [DOI] [PubMed] [Google Scholar]
- 181.Bruix J et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017). [DOI] [PubMed] [Google Scholar]
- 182.Abou-Alfa GK et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med 379, 54–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhu AX et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20, 282–296 (2019). [DOI] [PubMed] [Google Scholar]
- 184.Spratlin JL et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J. Clin. Oncol 28, 780–787 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.El-Khoueiry AB et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02576509 (2019). [DOI] [PubMed]
- 187.Zhu AX et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 19, 940–952 (2018). [DOI] [PubMed] [Google Scholar]
- 188.Hainaut P & Boyle P Curbing the liver cancer epidemic in Africa. Lancet 371,367–368 (2008). [DOI] [PubMed] [Google Scholar]
- 189.Goh LY & Goh KL Obesity: an epidemiological perspective from Asia and its relationship to gastrointestinal and liver cancers. J. Gastroenterol. Hepatol 28, Suppl 4, 54–58, (2013). [DOI] [PubMed] [Google Scholar]
- 190.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA & Rosenberg PS Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J. Clin. Oncol 34, 1787–1794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kjesbu IE et al. Feasibility and diagnostic accuracy of point-of-care abdominal sonography by pocket-sized imaging devices, performed by medical residents. J. Ultrasound Med 36, 1195–1202 (2017). [DOI] [PubMed] [Google Scholar]
- 192.Waheed Y Transition from millennium development goals to sustainable development goals and hepatitis. Pathog. Glob. Health 109, 353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kenya Outreach Mission. IHPBA http://www.ihpba.org/includes/moxiemanager/data/files/IHPBA%20Outreach%20Kenya%202017%20Leaflet%20WEBSITE.pdf (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.