Abstract
The purpose of this chapter is to provide an introduction to the mechanisms for the regulation of endocannabinoid signaling through CB1 cannabinoid receptors in the central nervous system. The processes involved in the synthesis and degradation of the two most well studied endocannabinoids, 2-arachidonoylglycerol and N-arachidonylethanolamine are outlined along with information regarding the regulation of the proteins involved. Signaling mechanisms and pharmacology of the CB1 cannabinoid receptor are outlined, as is the paradigm of endocannabinoid/CB1 receptor regulation of neurotransmitter release. The reader is encouraged to appreciate the importance of the endocannabinoid/CB1 receptor signaling system in the regulation of synaptic activity in the brain.
Keywords: endocannabinoid, CB1 cannabinoid receptor, synaptic plasticity, synthesis, degradation
Introduction
Cannabis sativa has been used by humans for thousands of years as a medicinal agent and for its euphoric and relaxing properties. The active principal of cannabis sativa was isolated and identified as Δ9-tetrahydrocannabinol (THC; Gaoni & Mechoulam, 1964], a landmark discovery that lead to the ultimate uncovering of the endocannabinoid system [ECS) which modulates virtually every brain region and thereby contributes to nearly every function of the CNS.
Using levo-nantradol, a structural analog of THC (Milne, Koe, & Johnson, 1979), Howlett and colleagues provided evidence that THC had biochemical effects consistent with activation of a G protein coupled receptor (GPCR) (Howlett, 1984, 1985; Howlett & Fleming, 1984; Howlett, Qualy, & Khachatrian, 1986). Another THC analog, [3H]CP55940, was used to demonstrate that a high affinity receptor for THC and structural analogs was present at high density throughout the brain (Devane, Dysarz, Johnson, Melvin, & Howlett, 1988). In a clever set of studies utilizing autoradiography in cerebellar neuron mutant mice, cannabinoid receptor was found to be enriched in axon terminals, which hinted that their activation would modulate neurotransmitter release (Herkenham, Groen, Lynn, De Costa, & Richfield, 1991). Molecular cloning of the cannabinoid receptor followed shortly after, officially introducing the CB1 cannabinoid receptor (CB1R) gene and its protein product to the scientific world (Matsuda, Lolait, Brownstein, Young, & Bonner, 1990). Subsequent studies have added considerably to our understanding of the ECS; however, none of these would have been possible without these and other seminal observations made between 1965 and 1990.
As the other chapters in this issue describe, there is strong evidence that dysregulation of the ECS contributes to many human maladies, including pain, psychiatric disorders, neurodegenerative diseases, and inflammation. Thus, therapies that alter the ECS could have usefulness as treatments for diseases and disorders that can significantly reduce quality of life. The purpose of this chapter is to introduce the ECS and review what is currently known about its regulation and role in synaptic function.
The endocannabinoids
Definitions
The definition of endocannabinoid used in this chapter is “an endogenous molecule that activates CB1R signaling”. This definition is somewhat arbitrary, since receptors in addition to the CB1R are activated by phytocannabinoids, and the endocannabinoids can bind to other receptors that are not activated by phytocannabinoids.
The first endocannabinoid identified is the very low abundance brain lipid, N-arachidonylethanolamine (also called anandamide; AEA) (Devane et al., 1992). AEA is one of a family of N-acylethanolamines (NAEs), first identified by Udenfriend (Bachur, Masek, Melmon, & Udenfriend, 1965; Bachur & Udenfriend, 1966; Colodzin, Bachur, Weissbach, & Udenfriend, 1963) and studied in depth by Schmid and colleagues (Schmid, Schmid, & Natarajan, 1990; Schmid et al., 1995). Shortly after the identification of AEA, two laboratories simultaneously and independently reported that a high abundance 2-monoacyl glycerol, 2-arachidonoylglycerol (2-AG), also bound and activated CB1R (Mechoulam et al., 1995; Sugiura et al., 1995). Other endogenous lipids that can bind CB1R include O-arachidonylethanolamine (virodamine), which is a weak, partial agonist (Porter et al., 2002) and noladin ether, the ether of arachidonic acid (AA) and glycerol (Hanus et al., 2001). There is far less known about the roles of these lipids in the regulation of the ECS than is known about AEA and 2-AG.
In vitro assays demonstrated that the peptide, hemopressin, a nonapeptide derived from the α chain of hemoglobin, binds to the CB1R with high affinity and signals as an inverse agonist (Heimann et al., 2007). More recent studies suggest that hemopressin is not likely the primary signaling molecule but is a cleavage product of RVD-hemopressin (Bomar & Galande, 2013). RVD-hemopressin and several other hemopressin peptides have activity as negative allosteric modulators of the CB1R (Bomar & Galande, 2013).
The focus of this chapter will be on the lipid endocannabinoids, AEA and 2-AG, given the large amount of data supporting their role in brain function and as endocannabinoids.
Mechanisms of AEA biosynthesis
Precursor synthesis
Available evidence indicates that the primary mechanisms for AEA synthesis (and NAE synthesis in general) involve hydrolysis of a minor phospholipid class, N-acyl phosphatidylethanolamines (NAPEs; Figure 1). NAPEs can be synthesized by an N-acyl transferase (NAT) that catalyzes transfer and formation of an amide bond between the fatty acyl moiety at the sn-1 position of a donor phospholipid and the ethanolamine of phosphatidylethanolamine (PE; Cadas, di Tomaso, & Piomelli, 1997; Schmid, Schmid, & Natarajan, 1996; Sugiura et al., 1996) or plasmenylethanolamine (Hansen, Moesgaard, Hansen, & Petersen, 2000). NAT activity is dependent on high micromolar concentrations of calcium and is present in membranes harvested from brain (Cadas et al., 1997; Cadas, Gaillet, Beltramo, Venance, & Piomelli, 1996; Schmid et al., 1990; Schmid, Schmid, & Natarajan., 1990).
Figure 1.
AEA biosynthesis and degradation pathways. The pathways described in the text are shown here specifically for the endocannabinoid, AEA. Abbreviations: AA: arachidonic acid; Abhd4: Alpha beta hydrolase domain protein 4; AEA: N-arachidonylethanolamine; EA: ethanolamine; FAAH: fatty acid amide hydrolase; GDE1: glycerophosphodiesterase 1; GP-AEA: glycerophospho-N-arachidonylethanolamine; NAAA: N-acylethanolamine-hydrolyzing acid amidase; NAPE-PLD: N-acyl phosphatidylethanolamine-specific phospholipase D; NAT: N-acyl transferase; PC: phosphatidylcholine; PE: phosphatidylethanolamine; PLA/AT: phospholipase A/acyltransferase; PLC: phospholipase C; PTPN22: protein tyrosine phosphatase, non-receptor type 22; SHIP1: Src homology 2-containing inositol phosphatase-1. Scheme is a modification of figure 2 from [Rahman, Tsuboi, Uyama, & Ueda, 2014).
The phospholipase A/acyltransferase (PLA/AT) family of enzymes also carries out the acyl transfer reaction needed to form the NAPEs (Golczak et al., 2012; Shinohara et al., 2011; Uyama et al., 2013). One member of this family, PLA/AT-1, is expressed in brain of human, mouse and rat; and catalyzes the same reaction as NAT, but is calcium independent. Cellular overexpression of PLA/AT-1 results in significantly increased concentrations of NAPE, while its silencing in endogenously expressing cells leads to reduced NAPE (Uyama et al., 2013).
Since acyl groups in the sn-1 position are retained in the NAEs, and there is very little AA in this position, these mechanisms would be expected to produce very little N-arachidonvlPE. the precursor of AEA. Indeed, the concentrations of N-arachidonylPE and AEA are very low compared to the saturated substrate and product pairs, such as N-palmitoylPE and palmitoylethanolamide (PEA; Ueda, Tsuboi, & Uyama, 2010).
NAPE conversion to NAE: NAPE-PLD
Multiple pathways have been described for the conversion of NAPE into NAEs (Figure 1). The first is a phosphodiesterase of the phospholipase D (PLD) family (NAPE-PLD) that converts NAPEs into phosphatidic acid and NAE (Schmid et al., 1990). NAPE-PLD does not exhibit selectivity for the N-acyl moiety, suggesting that it regulates formation of the entire family of NAEs (Wang et al., 2006). The majority of available evidence demonstrates a lack of calcium dependence by NAPE-PLD (Rahman et al., 2014), but little else is known about the mechanisms that regulate its activity. There are a few studies investigating NAPE-PLD mRNA expression. The transcription factor Sp1 regulates basal NAPE-PLD in macrophages, while endotoxin decreases its expression, supporting a link between NAE synthesis and inflammatory state (Zhu et al., 2011). NAPE-PLD expression in the human endometrium changes across the menstrual cycle, suggesting hormonal regulation of expression (Scotchie, Savaris, Martin, & Young, 2015).
Genetic deletion of NAPE-PLD in mice (NAPE-PLD−/−) results in a significant increase in the concentrations of the NAPEs, evidence that NAPE-PLD-mediated hydrolysis plays an important role in the regulation of NAPE concentrations (Leung, Saghatelian, Simon, & Cravatt, 2006; Tsuboi et al., 2011). Concentrations of saturated NAEs are significantly reduced, but not completely absent, in brains from NAPE-PLD−/− mice (Leung et al., 2006) and brain homogenates of NAPE-PLD−/− mice incubated with N-arachidonylPE exhibit only 25% of the conversion to AEA of wild type mice (Liu et al., 2008). However, brain concentrations of AEA and other polyunsaturated NAEs in NAPE-PLD−/− are not significantly different from wild type mice, which suggests that other pathways are important in the biosynthesis of AEA and that these pathways could be up-regulated in the NAPE-PLD−/− mice.
NAPE-PLD is associated with intracellular membranes (Okamoto, Morishita, Tsuboi, Tonai, & Ueda, 2004). In the ventral pallidum, NAPE-PLD is in presynaptic terminals that are opposed to other axon terminals that express the CB1R, suggesting that AEA could contribute to axo-axonal regulation of CB1R signaling (Pickel, Shobin, Lane, & Mackie, 2012). Studies in the hippocampus also demonstrate presynaptic distribution of NAPE-PLD in CB1R-negative, glutamatergic terminals (Nyilas et al., 2008). NAPE-PLD is found in both axons and dendrites in the hypothalamus (Reguero et al., 2014).
NAPE conversion to NAE: multi-enzyme pathways
Two alternative pathways have been discovered that can convert NAPE to NAE (Figure 1). NAPE can be deacylated to lysoNAPE via phospholipase A2 family members (Sun et al., 2004) and by alpha-beta hydrolase domain-containing protein 4 (Abhd4; Simon & Cravatt, 2006). Abhd4 is expressed in mouse brain and can also deacylate lysoNAPE, resulting in the formation of glycerophospho-N-acylethanolamine (GP-NAE; Figure 1).
GP-NAE is a substrate for glycerophosphodiester phosphodiesterase 1 (GDE1) which catalyzes its hydrolysis to glycerol-3-phosphate and NAE (Figure 1; (Simon & Cravatt, 2008). GDE1 is widely distributed in mammalian tissues, including brain and spinal cord. GDE1−/− mice have been used to examine the role of the Abhd4-GDE1 pathway in the synthesis of NAEs in vivo (Simon & Cravatt, 2010). Brains from GDE1−/− mice exhibit no detectable conversion of GP-NAE to NAE; however, brain concentrations of the NAEs, including AEA, are not different from wild type mice indicating that GDE1 is not essential for NAE synthesis in vivo. GDE1−/−/NAPE-PLD−/− double knock out mice demonstrate no conversion of NAPE to NAE in brain homogenates; however, cultured neurons from these mice can convert NAPE to NAE (Simon & Cravatt, 2010). These data indicate that there are mechanisms other than NAPE-PLD and Abhd4-GDE1 that can convert NAPE to NAE in an intact cell that are not operative when brain tissue is homogenized.
An additional, multi-step pathway that generates NAE from NAPE which involves phospholipase C (PLC) has also been described (Figure 1). This pathway, elucidated in macrophages, involves PLC-mediated conversion of NAPE to diacylglycerol (DAG) and phospho-NAE, which is subsequently dephosphorylated by several phosphatases, including protein tyrosine phosphatase, non-receptor type 22 (PTPN22) and Src homology 2-containing inositol phosphatase-1 (SHIP-1; Liu et al., 2008; Liu et al., 2006). The PLC/phosphatase pathway was shown to be essential for endotoxin-induced synthesis of AEA in macrophages (Liu et al., 2006).
Inhibitor studies suggest both the Abhd4/GDE-1 and PLC/phosphatase pathways contribute to AEA synthesis in NAPE-PLD−/− mice (Liu et al., 2008). Interestingly, the kinetics of these pathways differ; the PLC/phosphatase pathway is active during the first 1–10 min after the addition of NAPE, and the Abhd4/GDE-1 pathway contributes to AEA accumulation only at later times of NAPE incubation (Liu et al., 2008). Phospho-AEA can be detected in brain and its concentration is significantly increased in the presence of vanadate, which provides further support for the possibility that the PLC/phosphatase pathway is involved in brain NAE formation (Liu et al., 2008).
AEA synthesis from AA
A synthetic pathway for AEA has been identified in mammalian tissues that does not involve NAPE as a precursor (Figure 1). In this pathway, the NAE hydrolyzing enzyme, fatty acid amide hydrolase (FAAH), acts in “reverse” as an NAE synthase to directly couple AA and ethanolamine to form AEA (Arreaza et al., 1997; Katayama et al., 1997; Kurahashi, Ueda, Suzuki, Suzuki, & Yamamoto, 1997). This mechanism generates AEA during severe hepatic damage (Mukhopadhyay et al., 2011) and in post mortem brain (Patel et al., 2005), conditions that are characterized by large concentrations of ethanolamine, which is necessary to drive the synthase function of the enzyme.
Summary
The synthesis of AEA occurs by a number of possible routes, but it is not clear whether any of the known mechanisms contribute to stimulation of AEA production for the purpose of activating ECS. The answer to this question has not been provided by genetic deletion of possible enzymes, suggesting that the multiple mechanisms can compensate for one another. The development of effective and selective inhibitors of the various synthetic pathways are needed to delineate these processes.
Mechanisms of AEA hydrolysis
Fatty acid amide hydrolase (FAAH)
Early studies identified an enzymatic activity in liver microsomes that hydrolyzed NAEs to free fatty acid and ethanolamine (Schmid, Zuzarte-Augustin, & Schmid, 1985). The activity identified was likely due to the enzyme identified molecularly by Cravatt and colleagues and given the name fatty acid amide hydrolase, or FAAH (Cravatt et al., 1996). In addition to saturated and unsaturated, long and short chain NAEs, FAAH also hydrolyzes oleamide (a primary amine) and N-acyltaurines, which are very significantly increased brain tissue from FAAH−/− mice (Saghatelian & Cravatt, 2005). FAAH can also function as an esterase and hydrolyze 2-AG in vitro (Patricelli & Cravatt, 1999), although genetic deletion of FAAH in mice does not affect brain 2-AG contents (Patel et al., 2005), suggesting that FAAH does not play a prominent role in 2-AG hydrolysis.
FAAH is an integral membrane protein, present primarily on endoplasmic reticulum (Hillard, Wilkison, Edgemond, & Campbell, 1995) and mitochondria (Gulyas et al., 2004). FAAH is localized primarily in large, outflow neurons and is ubiquitously expressed throughout the brain (Tsou et al., 1998). FAAH is active over a wide range of pH values and its activity is unaffected by either the addition or removal of divalent cations (Hillard et al., 1995; Schmid et al., 1985).
FAAH is constitutively active and several studies suggest that its activity is can be regulated by post-translational processes. For example, follicle stimulating hormone treatment increases FAAH activity through a mechanism that requires increased protein kinase A (PKA) activity, which appears to phosphorylate an accessory protein, not FAAH itself (Grimaldi, Rossi, Catanzaro, & Maccarrone, 2009). Activation of receptors for corticotropin releasing factor receptor 1 (CRF1) by corticotropin releasing hormone (CRH) in vivo results in increased FAAH activity ex vivo through a mechanism consistent with a post-translational modification (Gray et al., 2015). Given that CRF1 receptors can also couple to activation of PKA (Pollandt et al., 2006), it is possible that similar mechanisms are involved in the actions of both follicle stimulating hormone and CRH.
The FAAH promoter has been analyzed; its mRNA is transcribed from multiple transcription start sites that lack a TATA box element (Puffenbarger, Kapulina, Howell, & Deutsch, 2001). The promoter region contains several estrogen receptor binding elements (Grimaldi et al., 2012; Waleh, Cravatt, Apte-Deshpande, Terao, & Kilduff, 2002); and estrogen (Grimaldi et al., 2012) and the xenoestrogen, bisphenol A (Vermeer, Gregory, Winter, McCarson, & Berman, 2014) increase FAAH gene transcription. Progesterone also increases FAAH expression in T lymphocytes through the transcription factor, Ikaros (Maccarrone, Bari, Di Rienzo, Finazzi-Agro, & Rossi, 2003). Glucocorticoid receptor binding sites are present in the FAAH promoter and reporter studies demonstrate that glucocorticoid receptors regulate FAAH expression in a negative manner (Waleh et al., 2002). However, glucocorticoid administration in the drinking water results in increased FAAH activity without any effect of mRNA expression in brain (Bowles et al., 2012), suggesting that this process may not occur in vivo.
In vitro treatment with endotoxin reduces FAAH expression at a transcriptional level in human peripheral lymphocytes (Maccarrone et al., 2001) and in peripheral blood monocytes of mice (Wolfson et al., 2013). Recent data indicate that treatment of lymphocytes with IL6 results in increased FAAH expression and that a cAMP response element in the FAAH promoter is likely involved in the regulatory mechanism (Gasperi et al., 2014). Leptin receptors activate the FAAH promoter in T lymphocytes through STAT3; perhaps also acting via a cAMP-responsive element (Maccarrone, Di Rienzo, Finazzi-Agro, & Rossi, 2003; Maccarrone, Gasperi, Fezza, Finazzi-Agro, & Rossi, 2004).
A single nucleotide polymorphism (SNP) has been identified in the coding region of the human FAAH gene that results in a missense mutation changing a conserved proline residue to threonine (Sipe, Chiang, Gerber, Beutler, & Cravatt, 2002). The amino acid change does not alter FAAH kinetics, but results in reduced protein amounts, likely as a result of protein instability (Chiang, Gerber, Sipe, & Cravatt, 2004). Recently, a transgenic mouse line was established in which a nucleotide substitution homologous to the rare allele in humans was introduced (Dincheva et al., 2015). The transgenic mice exhibit reduced FAAH protein amounts and higher brain AEA contents.
FAAH−/− mice exhibit 10 fold increases in basal concentrations of AEA and other NAEs in brain tissue (Cravatt et al., 2001), suggesting that FAAH activity is essential for the regulation of NAE concentrations in brain. FAAH−/− mice have been used in a large number of behavioral paradigms and changes are generally ascribed to increased AEA tone. However, the other NAEs are increased to an even greater extent than AEA by FAAH deletion and these lipids have non-CB1R targets, most significantly, the peroxisome proliferation activating receptors (PPARs; Panlilio, Justinova, & Goldberg, 2013). In addition, the N-acyl taurine family of lipids is very significantly increased in FAAH−/− mice (Saghatelian & Cravatt, 2005) can affect TRPV channel function (Saghatelian, McKinney, Bandell, Patapoutian, & Cravatt, 2006).
There are several widely used pharmacological inhibitors of FAAH that are very effective in vivo, including URB597 (Tarzia et al., 2003), PF-3845 (Ahn et al., 2009) and JNJ5003 (Hill et al., 2013). One FAAH inhibitor (PF-04457845) is currently in phase 2 clinical trials for the treatment of post-traumatic stress disorder (http://www.pfizer.com/sites/default/files/product-pipeline/Februarv_27_2015_Pipeline_Update_Fina12.pdf).
NAE-hydrolyzing acid amidase (NAAA): A peripheral AEA hydrolase
A second amidohydrolase, NAAA, has been identified in peripheral tissues (Ueda, Yamanaka, & Yamamoto, 2001). NAAA is present in lysosomes, active at acidic pH, and prefers PEA as a substrate over AEA. Several inhibitors of NAAA have been developed and shown to increase endogenous concentrations of PEA (Rahman et al., 2014). There are no effects of NAAA inhibitors on AEA concentrations and no overlap or substitution of NAAA for FAAH-mediated hydrolysis of AEA in brain (Rahman et al., 2014).
Mechanisms of 2-AG Biosynthesis
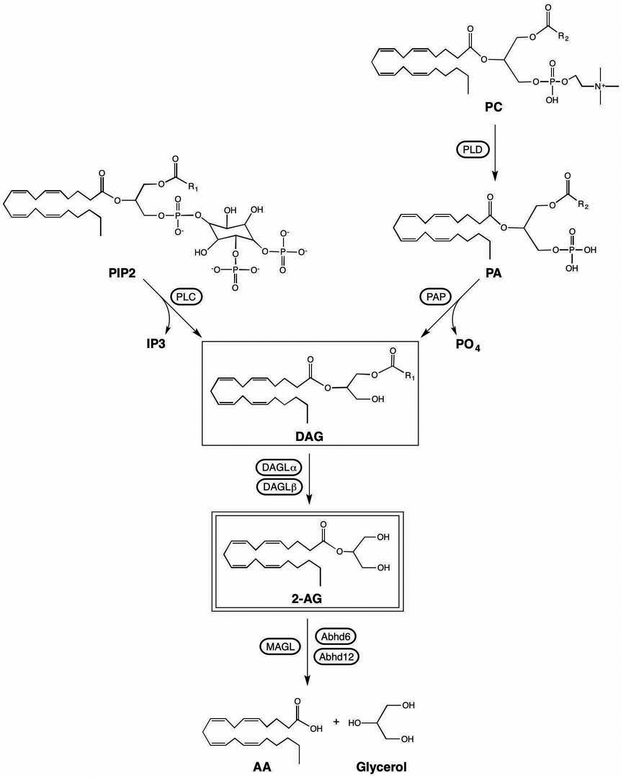
Diacylglycerol lipase
2-AG is present in brain tissue and microdialysates at nanomolar concentrations (Buczynski & Parsons, 2010). A well-supported mechanism for the synthesis of 2-AG involves hydrolysis of the ester bond at the sn-1 position of diacylglycerol (DAG) by diacylglycerol lipase (DAGL; Figure 2). Two isotypes of DAGL are expressed in mammals, DAGLα and DAGLβ, that share a transmembrane domain of 4 loops, coupled to a catalytic domain and regulatory loop (Bisogno et al., 2003; Reisenberg, Singh, Williams, & Doherty, 2012). DAGLα has an additional, large C terminal tail. Both isotypes are expressed in brain; however, DAGLα is present in high density at perisynaptic regions of dendrites in many brain regions (Katona et al., 2006; Matyas et al., 2008; Uchigashima et al., 2007; Yoshida et al., 2006), which is consistent with a prominent role for this isoform in ECS-mediated regulation of synaptic plasticity (described below).
Figure 2.
Biosynthesis and catabolism of 2-AG. Abbreviations: AA: arachidonic acid; abhd6: alpha-beta hydrolase domain containing protein 6; abhd12: alpha-beta hydrolase domain containing protein 12; 2-AG: 2-arachidonoylglycerol; DAG: diacylglycerol; DAGL: diacylglycerol lipase; IP3: inositol triphosphate; MAGL: monoacylglycerol lipase; PA: phosphatidic acid; PAP: phosphatidic acid phosphatase; PC: phosphatidylcholine; PIP2: phosphatidylinositol 4,5 bisphosphate; PLC: phospholipase C; PLD: phospholipase D.
Mass spectrometry studies demonstrate that both isoforms of DAGL are phosphorylated within the catalytic and regulatory domains; and identify many phosphor-residues in the C terminal tail of DAGLα (Reisenberg et al., 2012). There is some evidence that both PKA and protein kinase C (PKC) can stimulate DAGL activity (Malcher-Lopes et al., 2006; Rosenberger, Farooqui, & Horrocks, 2007; Vellani et al., 2008). DAGLα is phosphorylated on two serines in the C terminal region by calcium/calmodulin dependent kinase II (CamKII); this phosphorylation inhibits DAGLα activity and thereby possibly provides a mechanism by which calcium can produce a feedback inhibition of 2-AG synthesis (Shonesy et al., 2013).
Proteomic analyses demonstrate that both DAGL isoforms can be palmitoylated (Kang et al., 2008; Martin & Cravatt, 2009; Yang, Di Vizio, Kirchner, Steen, & Freeman, 2010). Although both DAGLs are membrane intrinsic proteins, palmitoylation could contribute to membrane localization, particularly to specific membrane regions in which other palmitoylated proteins are localized. However, no studies that specifically explore the role of palmitoylation in localization or activity of DAGL have been published.
The catalytic activities of DAGLα and DAGLβ do not differ (Bisogno et al., 2003), so the additional C terminal domain of DAGLα is apparently not important for enzymatic activity. In addition to being a target for phosphorylation, the DAGLα C terminus contains a consensus motif for binding to Homer proteins [Jung et al., 2007), which are a family of adaptor proteins that can tether synaptic proteins, such as metabotropic glutamate receptors (mGluRs) to the post synaptic density (Gao, Tronson, & Radulovic, 2013). Although interaction between DAGLα and Homer proteins was found to be important for DAGLα association with the plasma membrane in a cell line, this interaction was not required for 2-AG synthesis (Jung et al., 2007). On the other hand, several studies have demonstrated that Homer is required for ECS-mediated changes in synaptic transmission (Fourgeaud et al., 2004; Roloff, Anderson, Martemyanov, & Thayer, 2010; Won, Puhl, & Ikeda, 2009), suggesting that DAGLα location within the cell is regulated by Homer proteins and that this process is required for efficient activation of 2-AG-mediated signaling.
Several DAGL inhibitors have been identified and used to implicate 2-AG synthesis in cellular and physiological function. Tetrahydrolipstatin (THL; orlistat) is a high affinity inhibitor of DAGL at concentrations that are without effect on monoacylglycerol lipase (MAGL) or NAPE-PLD (Bisogno et al., 2006; Lee, Kraemer, & Severson, 1995) and is a potent inhibitor of 2-AG in vitro (Hashimotodani, Ohno-Shosaku, Maejima, Fukami, & Kano, 2008; Won et al., 2009). However, THL inhibits a broad range of gastric and pancreatic lipases and is irreversible (Guerciolini, 1997), which limits its use in vivo. RHC 80267 is also a DAGL inhibitor, but it interferes with the CB1R-mediated effects of exogenously administered 2-AG, suggesting direct effects on CB1R signaling (Hashimotodani et al., 2008). OMDM-188, a THL analog (Ortar et al., 2008), is also a selective and potent inhibitor of DAGL in vitro (Hashimotodani et al., 2013; Min et al., 2010). LEI105 is a reversible inhibitor of both DAGLα and DAGLβ; is without effect on the known 2-AG catabolic enzymes or FAAH; and reduces 2-AG but not AEA concentrations in Neuro2A cells (Baggelaar et al., 2015). If OMDM-188 and LEI105 have in vivo efficacy and selectivity, they will be important additions to the ECS pharmacological toolbox.
Mechanisms of DAG synthesis
The DAG substrate for DAGL can be generated in multiple ways (Figure 2). The PLC family of enzymes, particularly PLCβ and PLCγ isoforms, act on phosphatidylinositol 4,5 bisphosphate (PIP2) to produce DAG and inositol-trisphosphate (IP3). This pathway links the regulation of 2-AG synthesis to GPCRs, since the Gαq family of G proteins activate PLCβ. The group I mGluRs are Gαq coupled and their activation in brain slices has been shown to increase 2-AG concentrations (Jung et al., 2005) through a mechanism that requires DAGLα (Jung et al., 2007). Other Gαq -activating GPCRs that have been shown to elevate 2-AG include muscarinic (Straiker & Mackie, 2007; Uchigashima et al., 2007); orexin 1 (Ho et al., 2011); angiotensin (Turu et al., 2007); alpha 1 adrenergic (Turu et al., 2009); vasopressin (Turu et al., 2009); bradykinin (Turu et al., 2009); and neurotensin (Kortleven, Bruneau, & Trudeau, 2012) receptors. Studies in the cerebellum demonstrate a close spatial relationship between PLCβ1, mGluRs and DAGLα in dendritic spines (Fukaya et al., 2008), supporting the synthetic pathway outlined and specifically placing these components together in the perisynaptic region.
Like PLCβ, PLCγ isoforms also act on PIP2 and generate DAG, but are downstream of tyrosine kinase linked, growth factor receptors, suggesting that 2-AG synthesis can also be regulated by that receptor class. In support of this notion, brain derived neurotrophic factor induces 2-AG synthesis through a trkB receptor/PLCγ-requiring mechanism (Zhao & Levine, 2014). Thus 2-AG synthesis can potentially be mediated by a wide variety of receptor types, including GPCRs and growth factor receptors. The proximity of DAGL determines whether 2-AG will be produced from DAG when PLC activating receptors are engaged.
There is evidence in N18TG2 cells that 2-AG synthesis can be evoked by calcium without a requirement for PLC (Bisogno, Melck, De Petrocellis, & Di Marzo, 1999). A combination of inhibitor studies and analysis of intermediates indicates that calcium evokes production of DAG via a two enzyme pathway in this cellular model: phospholipase D resulting in production of phosphatidic acid (PA), followed by removal of the phosphate group of PA via PA phosphatase (Figure 2).
Mechanisms of 2-AG Catabolism
Monoacylglycerol lipase
2-AG is catabolized by hydrolysis of the ester bond between the AA backbone and glycerol through the actions of several enzymes (Figure 2). MAGL is responsible for more than 85% of 2-AG hydrolysis by brain homogenates (Blankman, Simon, & Cravatt, 2007) and is considered the dominant mechanism for the inactivation of 2-AG in its role as CB1R agonist in neurons (Murataeva, Straiker, & Mackie, 2014). MAGL is a serine hydrolase that hydrolyzes both 1(3)- and 2 -monoacylglycerols with little ability to hydrolyze triacylglycerols or diacylglycerols (Tornqvist & Beifrage, 1976). MAGL is ubiquitously distributed throughout the body, including brain. Interestingly, mouse and rat brain MAGL (but not that of other tissues) appear as a doublet on Western blot (one at the predicted molecular weight of 33 and a second that migrates at approximately 35 kDa; Dinh et al., 2002; Karlsson et al., 2001). The reason for this is not known; it is possible that the proteins have the same amino acid sequence but differ in post translational modification. Alternatively, previous studies have shown that multiple mRNA species are present for mouse MAGL that could give rise to proteins of varying amino acid length (Karlsson et al., 2001). How this would occur in a tissue-specific manner is not clear.
In situ hybridization has been used to assess the distribution of MAGL mRNA expression in the brain, showing good agreement of brain regions expressing the CB1R and MAGL (Dinh et al., 2002). MAGL is found in presynaptic terminals (Gulyas et al., 2004; Horvath et al., 2014; Suarez et al., 2008) and enzymatic activity is enriched in synaptosomal preparations (Farooqui & Horrocks, 1997; Vyvoda & Rowe, 1973). A recent study provides evidence that MAGL is expressed in astrocytes as well as neurons; and astrocytic MAGL contributes to overall regulation of brain 2-AG content (Viader et al., 2015).
Although MAGL does not contain canonical transmembrane domains, subcellular fractionation studies find MAGL enzymatic activity in particulate (i.e. membrane) as well as cytosolic fractions (Bisogno et al., 1997; Di Marzo et al., 1999; Goparaju, Ueda, Taniguchi, & Yamamoto, 1999; Sakurada & Noma, 1981). Although there are no detectable differences in catalytic activity between cytosolic and membrane associated MAGL (Goparaju et al., 1999), recent studies using nanodisk models of phospholipid bilayers demonstrate that interactions of MAGL with the bilayer hold MAGL in a conformation that facilitates substrate access to the catalytic region (Nasr et al., 2013). This, together with the likelihood that 2-AG will partition to membranes rather than cytosol, provides evidence that MAGL associated with the plasma membrane is more important for termination of 2-AG action at the CB1R than cytosolic MAGL. There are several studies demonstrating compartment-selective changes in MAGL protein or activity. Cytosolic MAGL activity was found to be reduced by 50% in adipocytes from fasted rats while particulate MAGL activity was not changed (Sakurada & Noma, 1981). Importantly for its function in regulating brain ECS, chronic stress selectively reduced MAGL protein detected in membrane but not cytosolic fractions of the basolateral amygdala (Sumislawski, Ramikie, & Patel, 2011).
Several studies demonstrate decreased MAGL mRNA expression in tissues that are inflamed (Engeli et al., 2014; Lappas, 2014; Mai et al., 2015). Sustained elevation of neuregulin-1 increases MAGL expression in hippocampal slices, likely through activation of the ErbB4 receptor (Du, Kwon, & Kim, 2013). The transcription factor and tumor suppressor Prdm5 is a repressor of MAGL expression and its loss acts synergistically with WNT pathway activation to increase MAGL expression and to increase adenoma formation (Galli et al., 2014). Genetic deletion of PLCβ1 results in reduced MAGL expression, suggesting that its expression is regulated by 2-AG concentrations (Filis, Kind, & Spears, 2013). MAGL protein is degraded in developing cholinergic neurons through a process that involves nerve growth factor upregulation of the E3 ubiquitin ligase, BRCA1 (Keimpema et al., 2013).
Although there are no studies to date examining the role of posttranslational modifications in the regulation of MAGL activity, one study showing rapid changes in MAGL activity during ischemia is consistent with phosphorylation or other short term regulatory mechanisms (Strosznajder, Singh, & Horrocks, 1984).
Several inhibitors of MAGL have been developed. URB602 is a relatively weak inhibitor of recombinant MAGL in vitro (King et al., 2007). URB602 increases 2-AG concentrations when injected into the periaqueductal gray (Hohmann et al., 2005) and significantly increases depolarization-induced increases in 2-AG in microdialysates of rat nucleus accumbens (Wiskerke et al., 2012). JZL184 is a more potent inhibitor of mouse MAGL (Long, Li, et al., 2009), but is not as an effective inhibitor of rat MAGL (Long, Nomura, & Cravatt, 2009; Pan et al., 2009). Microdialysis studies in nucleus accumbens of rat and mice confirm that JZL184 is effective at elevation of 2-AG in mice but not rats (Wiskerke et al., 2012). Although these findings could suggest that JZL184 is not a good indirect agonist of CB1R signaling in rat, it has been shown to increase CB1R-mediated behavioral effects in rats without affecting tissue 2-AG concentrations (eg. Woodhams et al., 2012). Since both URB602 and JZL184 show evidence of being efficacious in vivo in spite of relatively poor inhibition of MAGL in vitro, it is likely that even modest reductions of MAGL activity can significantly enhance 2-AG actions. There are several recent reports of other MAGL inhibitors that have not been used as widely as JZL184 and URB602 (Ignatowska-Jankowska et al., 2014; Kapanda et al., 2012; Tuccinardi et al., 2014).
Although acute inhibition of MAGL activity potentiates CB1R signaling (Pan et al., 2009), genetic deletion (Chanda et al., 2010) and chronic pharmacological inhibition (Schlosburg et al., 2010) of MAGL both result in functional reductions in CB1R signaling rather than activation. Interestingly, the MAGL−/− mice have increased tonic CB1R activity (Pan et al., 2011), but exhibit region-specific desensitization of CB1R agonist-induced activation of G proteins (Navia-Paldanius et al., 2015). Given that both genetic deletion and chronic inhibition of MAGL produce very large increases in brain 2-AG contents, these data are consistent with agonist (i.e. 2-AG)-induced desensitization of CB1R signaling. The profound effects of reduced MAGL activity on 2-AG suggest that 2-AG homeostasis is regulated more by its degradation than synthesis. In support of this hypothesis, recent data demonstrate that 2-AG is generated continuously at hippocampal GABA synapses and that MAGL activity is critical for opposing this steady stream of 2-AG to maintain the CB1R in a low ligand state (Lee et al., 2015).
Other enzymes that hydrolyze 2-AG in the brain
Abhd6 and Abhd12 were identified as potential 2-AG hydrolases using a functional proteomic approach (Blankman et al., 2007). Subsequent studies have demonstrated that Abhd6 is expressed by neurons in postsynaptic compartments and its activity can regulate 2-AG-mediated activation of CB1R (Marrs et al., 2010). Given the postsynaptic distribution, Abhd6 has been proposed to regulate 2-AG concentrations at the site of synthesis (Savinainen, Saario, & Laitinen, 2012), which complements the role of MAGL to regulate 2-AG concentrations in the axon terminal. As was argued above, this function of Abhd6 could play a very important role in basal CB1R activity by controlling the amount of 2-AG that survives to exit the postsynaptic terminal.
Studies in brain homogenates suggest that Abhd12 accounts for approximately 9% of brain 2-AG hydrolysis (Blankman et al., 2007). Abhd12 is mutated in the human neurodegenerative disorder, PHARC (Blankman, Long, Trauger, Siuzdak, & Cravatt, 2013). There is little information regarding the role of Abhd12 in brain 2-AG homeostasis; however, its mRNA is enriched in microglia, suggesting it may be important in the termination of 2-AG-mediated CB2 receptor activation (Fiskerstrand et al., 2010).
Contribution of 2-AG toAA concentrations
Early studies in platelets demonstrated that free AA was increased by the metabolism of DAG and 2-AG (Bell, Kennerly, Stanford, & Majerus, 1979; Prescott & Majerus, 1983). MAGL−/− mice exhibit significantly lower brain tissue concentrations of AA in addition to elevated 2-AG (Schlosburg et al., 2010). This finding, together with data that acute inhibition of MAGL abolishes endotoxin-induced increases in brain AA and prostaglandin E2 (Nomura et al., 2011), strongly indicate that 2-AG is a biologically significant precursor for AA and that MAGL is a critical enzyme in the provision of free AA for further metabolism. A recent study using cell specific MAGL deletion suggests that astrocyte MAGL is mainly responsible for converting 2-AG to inflammatory prostaglandins in brain (Viader et al., 2015). Similar roles for 2-AG and MAGL have been demonstrated in hepatic injury (Cao et al., 2013) and for the synthesis of vasodilatory arachidonates in coronary arteries (Gauthier et al., 2005). Studies carried out using vascular tissue suggest that the AEA/FAAH pair can serve a parallel function in the synthesis of vasoactive AA metabolites (Pratt, Hillard, Edgemond, & Campbell, 1998).
These and other studies demonstrate integration and synergism between the endocannabinoid and AA signaling systems. This conclusion is further supported by the findings discussed immediately below that AEA and 2-AG can also serve as substrates for enzymes that metabolize AA.
Other inactivation mechanisms for AEA and 2-AG
Uptake, accumulation and sequestration
The addition of labeled AEA to the outside of cells results in its cellular association in a manner that is consistent with accumulation and in some cases, intracellular sequestration (Hillard & Jarrahian, 2003). However, details of the mechanisms involved in this “uptake” process remain unclear. FAAH-mediated catabolism of AEA can maintain the concentration gradient and thereby enhance AEA uptake into the cells by either passive or facilitated diffusion and many inhibitors of AEA uptake also inhibit FAAH-mediated catabolism of AEA (Deutsch et al., 2001; Glaser et al., 2003; Kaczocha, Hermann, Glaser, Bojesen, & Deutsch, 2006). However, AEA is also accumulated by cells that express very low or no FAAH (Hillard, Edgemond, Jarrahian, & Campbell, 1997; Hillard & Jarrahian, 2005; Nicolussi, Chicca, et al., 2014), suggesting that other processes can contribute to accumulation. Indeed, other intracellular proteins have been identified that bind AEA and could thereby serve as sequestration sites. These include fatty acid binding proteins (FABPs; Kaczocha, Glaser, & Deutsch, 2009); heat shock proteins (Oddi et al., 2009) and sterol carrier protein 2 (Liedhegner, Vogt, Sem, Cunningham, & Hillard, 2014). In addition, AEA associates with membrane lipid rafts (McFarland et al., 2004; McFarland, Terebova, & Barker, 2006), which could also serve as a sequestration site following uptake. Importantly, it is likely that AEA uptake occurs via different processes in different cells (Hillard & Jarrahian, 2005)
There are several experiments that indirectly support a protein transporter that can translocate AEA across the plasma membrane in a bidirectional manner (Chicca, Marazzi, Nicolussi, & Gertsch, 2012; Hillard et al., 1997; Ligresti et al., 2004; Ronesi, Gerdeman, & Lovinger, 2004). These data suggest that an endocannabinoid transporter could participate in both inactivation (as a first step in a cellular sequestration process) and in the release of AEA. FLAT, a variant of FAAH that is without catalytic activity, has been suggested as a putative AEA transport protein (Fu et al., 2012). However, other studies dispute these findings (Leung, Elmes, Glaser, Deutsch, & Kaczocha, 2013). Although fewer experiments have been done, inhibitor studies suggest that 2-AG could also be subjected to similar regulation by uptake and/or sequestration (Bisogno et al., 2001; Nicolussi & Gertsch, 2015).
There are several inhibitors of the cellular accumulation of the endocannabinoids, although the lack of complete understanding of the processes involved has made it difficult to ascribe mechanisms to the inhibitors. Some inhibitors of AEA uptake, including AM404, VDM11, AM1172 and LY2183240, also inhibit or are substrates for FAAH (Alexander & Cravatt, 2006; Fowler, Tiger, Ligresti, Lopez-Rodriguez, & Di Marzo, 2004; Glaser et al., 2003; Vandevoorde & Fowler, 2005) and can compete with endocannabinoids for binding to intracellular proteins (Kaczocha, Vivieca, Sun, Glaser, & Deutsch, 2012; Liedhegner et al., 2014). Recent reports have identified the natural product, guineensine, as a potent inhibitor of AEA uptake in several cell types (Nicolussi, Viveros-Paredes, et al., 2014) and a series of N-alkylcarbamates as extremely potent inhibitors of both AEA uptake and FAAH activity (Nicolussi, Chicca, et al., 2014). Characteristics of the inhibitory effects of guineensine and the N-alkylcarbamates support the notion that FAAH activity contributes to AEA uptake, but it is not sufficient to explain all forms of uptake in all cell types. Development and study of inhibitors with novel structures will undoubtedly move our understanding of the mechanisms involved in AEA and 2-AG uptake further.
In vivo treatment with uptake inhibitors AM404 and UCM707 increase brain tissue concentrations of AEA and, to a lesser extent, 2-AG (de Lago et al., 2005; Di et al., 2005); however, AM404 only affects 2-AG, and UCM707 is ineffective at elevating either AEA or 2-AG in brain extracellular space, measured using microdialysis (Wiskerke et al., 2012). Since effective inhibitors of FAAH and MAGL have very significant effects on microdialysate AEA and 2-AG, respectively, these findings suggest that the processes of endocannabinoid uptake and accumulation may contribute only a small amount to their overall clearance. On the other hand, small changes in clearance, particularly of 2-AG, could result in a significant increase in CB1R activation and might even be a very useful approach because it should avoid inducing receptor desensitization.
Oxygenation of the arachidonate backbone
The acyl chain of AA can be modified by cyclooxygenases, lipoxygenases and cytochrome P450s, resulting in the production of prostaglandins, leukotrienes, and epoxyeicosatrienoic acids, respectively. Subtypes of each of these enzyme classes can also utilize AEA and 2-AG as substrates, resulting in the formation of ethanolamide and glycerol ester analogs of the arachidonates (see Urquhart, Nicolaou, & Woodward, 2015 for an excellent recent review). The result of these processes are a large number of lipid mediators, some of which have been shown to have their own targets. On the other hand, metabolism of AEA and 2-AG along any of these pathways is likely to reduce affinity for CB1R, thus they are inactivation mechanisms for the CB1R signaling roles of these lipids.
In particular, 2-AG is an excellent substrate for COX-2, having Km and kcat values that are very similar to those of AA (Rouzer & Marnett, 2011). Based upon structure function studies showing that 2-AG and AA bind differentially to COX-2, substrate-specific inhibitors of COX-2 that selectively reduce 2-AG metabolism while preserving prostaglandin formation have been designed (Hermanson, Gamble-George, Marnett, & Patel, 2014). The in vivo effects of these inhibitors support the hypothesis that COX-2 mediated metabolism of 2-AG contributes in a significant manner to the regulation of CB1R signaling (Hermanson et al., 2013).
Endocannabinoid Receptors
Introduction
The focus of this review is on the CB1R. However, other receptors have been identified that can bind the endocannabinoids AEA and 2-AG and it is highly likely that these receptors contribute to the biological effects of the lipids. Both AEA and 2-AG bind to CB2 cannabinoid receptors (CB2R; Gonsiorek et al., 2000), which are GPCRs (Munro, Thomas, & Abu-Shaar, 1993). Although 2-AG has the characteristics of a full CB2R agonist, AEA does not induce CB2R-mediated GDP/GTP exchange (Gonsiorek et al., 2000; Hillard et al., 1999) and is likely a weak partial agonist of the CB2R. CB2R are expressed in circulating immune cells (Bouaboula et al., 1993), spleen (Galiegue et al., 1995) and tissue resident macrophage populations, including microglial cells (Carlisle, Marciano-Cabral, Staab, Ludwick, & Cabral, 2002). CB2R are also expressed by some neuronal populations, although the expression levels are far lower than CB1R (Van Sickle et al., 2005; Zhang et al., 2014).
AEA is an agonist of the vanilloid type 1 receptor, also called TRPV1 (Kim et al., 2007; Ross et al., 2001; Saghatelian et al., 2006). TRPV1 is a nonselective cation channel expressed widely in the CNS. Intracellular AEA induces opening of the channel and this function contributes to many of the non-CB1 mediated effects of AEA. AEA and other NAEs are agonists of PPARs, particularly PPARα (Bouaboula et al., 2005; Fu et al., 2003; Lo Verme et al., 2005). Recent data suggests that PPARα mediated changes could contribute to the effects of FAAH inhibition (Panlilio et al., 2013).
CB1 cannabinoid receptors
CB1R are heterogeneously expressed throughout the CNS (Hu & Mackie, 2015). CB1R are present at extremely high density in the cingulate gyrus, frontal cortex, hippocampus, cerebellum and the basal ganglia. Moderate receptor densities are found in the basal forebrain, amygdala, nucleus accumbens, periaqueductal grey and hypothalamus; and low density is seen in the midbrain, pons and medulla. Relatively little receptor is found in primary motor cortex or thalamus. In the forebrain, CB1R mRNA is expressed at very high density in a restricted number of neurons (Marsicano & Lutz, 1999). These CB1R-expressing neurons project widely, resulting in a dense network of CB1R positive processes. Double-labeling studies demonstrate that these highly expressing cells are GABAergic interneurons that also express the neuropeptide cholecystokinin (CCK; Katona et al., 1999). Other neurons express the CB1R at lower densities; these neurons are more heterogenous and consist of both non-CCK, GABAergic interneurons and glutamatergic terminals (Hu & Mackie, 2015).
The CB1R is also expressed by non-neuronal cells in the CNS, including astrocytes (Navarrete & Araque, 2008; Salio, Doly, Fischer, Franzoni, & Conrath, 2002), oligodendrocytes (Molina-Holgado et al., 2002) and by endothelial (Golech et al., 2004) and smooth muscle cells (Gebremedhin, Lange, Campbell, Hillard, & Harder, 1999) of the cerebral circulation.
CB1R signaling
Activation of the CB1R results in inhibition of adenylyl cyclase activity in most tissues and cells via activation of Gαi-mediated signaling (Howlett, 1985; Howlett & Fleming, 1984). As is the case for most GPCRs that couple to Gαi, CB1R also engage Gαo-mediated signaling (Glass & Northup, 1999) which results in inhibition of the opening of voltage-dependent calcium channels (VDCCs) through the release of associated βγ subunits (Caulfield & Brown, 1992; Mackie & Hille, 1992). This mechanism is likely the primary process by which CB1R regulate short-term changes in neurotransmitter release (described below). Signaling through Gαi/o also results in activation of inward rectifying potassium channels (Henry & Chavkin, 1995). There is some evidence that CB1R agonists can exhibit bias toward activation of specific G protein alpha subtypes (Turu & Hunyady, 2010), suggesting that they can selectively engage inhibition of calcium influx versus adenylyl cyclase, for example. If Gαi/o proteins are unavailable, CB1R will couple to Gas and thereby enhance adenylyl cyclase activity (Glass & Felder, 1997).
CB1R activation is coupled to activation of p42/p44 and p38 mitogen activated kinases and Jun N-terminal kinase through a variety of signaling mechanisms, including G proteins (Turu & Hunyady, 2010) and β-arrestin (Ahn, Mahmoud, & Kendall, 2012). CB1R activation has also been linked to the activation of PLC (Lograno & Romano, 2004) and Akt signaling in some cells (Gomez et al., 2011); through these pathways CB1R activation can influence intracellular calcium concentrations, protein kinase activities and other signaling cascades that regulate cell growth and differentiation.
There is evidence that the CB1R exhibits considerable constitutive activity, meaning that it can activate signaling cascades in the absence of agonist binding (Lee et al., 2015; Nie & Lewis, 2001; Savinainen, Saario, Niemi, Jarvinen, & Laitinen, 2003).
CB1R pharmacology
The endocannabinoids 2-AG and AEA are arachidonates that bind to the CB1R with affinities in the mid to high nanomolar range. 2-AG acts as a full agonist at the CB1R, at least with respect to G protein activation, while AEA is a partial agonist (Hillard, 2000). Ethanolamides of several other long chain, unsaturated fatty acids are also agonists of the CB1R, including the ethanolamide of docosahexaenoic acid (Hillard & Campbell, 1997). Two synthetic arachidonates, N-arachidonyl-2-chloroethylamide and arachidonylcyclopropylamide that have 500–1000 times greater affinity for the CB1R than CB2R have been developed (Hillard et al., 1999) and are the only CB1R-selective ligands in wide use. Many other synthetic agonists for the CB1R have been synthesized and characterized. These include derivatives of THC, such as levo-nantradol, CP55940 and HU210; and the aminoalkylindole, WIN55212–2 (Pertwee, 2008).
The identification of a CB1R antagonist occurred much later than the synthesis of the agonists. The first antagonist discovered was SR141716A, later named rimonabant (Rinaldi-Carmona et al., 1994). Rimonabant and a structural analog, AM251, are both inverse agonists of the CB1R (Bouaboula et al., 1997; Savinainen et al., 2003), so will reduce both constitutive and endocannabinoid-activated CB1R signaling. Several neutral antagonists have been designed that can be used to differentiate these possible mechanisms (Kirilly, Gonda, & Bagdy, 2012).
Recent studies have identified multiple allosteric ligands that interact with the CB1R. ORG27569 was originally reported to function as a negative allosteric modulator of the CB1R (Price et al., 2005). Subsequent studies found that this compound is a biased allosteric modulator, inhibiting CB1R agonist activation of G protein signaling (Baillie et al., 2013) while increasing high affinity orthosteric agonist binding sites, beta-arrestin 1 recruitment, and ERK activation (Ahn et al., 2012). ORG27569 attenuates CB1R-mediated signaling in hippocampal neurons in culture (Straiker, Mitjavila, Yin, Gibson, & Mackie, 2015): however, is not effective in vivo, perhaps because of a poor pharmacokinetic profile (Gamage et al., 2014).
PSNCBAM-1 is a negative allosteric modulator of CB1R-mediated activation of G protein signaling (Horswill et al., 2007). This compound exhibits in vivo efficacy to reduce body weight and feeding in a rat model, findings that are consistent with reduced CB1R activation. Further in vitro studies have demonstrated that PSNCBAM-1 reduces the effects of CP55940 to modulate synaptic CB1R signaling, but not the effects of WIN55212–2 (Wang, Horswill, Whalley, & Stephens, 2011). PSNCBAM-1 inhibits endocannabinoid-mediated activation of CB1R in cultured hippocampal neurons, suggesting that it negatively modulates 2-AG activation (Straiker et al., 2015).
Two endogenous compounds have been reported to function as CB1R negative allosteric modulators: a family of hemopressin-related peptides, particularly pepcan 12 (Bauer et al., 2012) which are widely expressed in brain (Hofer et al., 2015); and the steroid, pregnenolone (Vallee et al., 2014). While pepcan 12 is effective at reducing endogenous CB1R signaling in cultured hippocampal neurons, pregnenolone is not (Straiker et al., 2015). However, pregnenolone might be more effective at reducing the signaling of THC, and therefore could reduce its adverse effects (Vallee et al., 2014).
The phytocannabinoid, cannabidiol (CBD) was reported to act as a negative allosteric modulator for CB1R-mediated beta-arrestin 2 recruitment and ERK signaling (Laprairie, Bagher, Kelly, & Denovan-Wright, 2015). This is an interesting observation, given that CBD can dampen many of the effects of THC, including its anxiogenic effects.
Two positive allosteric modulators of the CB1R have been described. The first is the naturally occurring arachidonate, lipoxin A4 (Pamplona et al., 2012) and the second is a synthetic, ZCZ011 (Ignatowska-Jankowska et al., 2015). ZCZOll potentiates binding of [3H]CP55,940 to the CB1R and increases AEA-stimulated [35S]GTPγS binding in mouse brain membranes and β-arrestin recruitment and ERK phosphorylation in CB1R expressing cells. Lipoxin A4 also increases AEA signaling as well as its affinity for the CB1R.
CB1R and retrograde regulation of synaptic activity
The basic paradigms
Short term depression of synaptic transmission
Three simultaneous reports in 2001 described endocannabinoid/CB1R signaling as a mediator of retrograde inhibition of neurotransmitter release (Kreitzer & Regehr, 2001; Ohno-Shosaku, Maejima, & Kano, 2001; Wilson & Nicoll, 2001). Many studies in multiple brain regions carried out since demonstrate that modulation of synaptic plasticity is a major mechanism by which CB1R signaling affects the brain (Freund, Katona, & Piomelli, 2003; Kano, 2014; Patel & Hillard, 2009). In this mechanism, endocannabinoids are mobilized from the postsynaptic neuron in response to triggers, such as depolarization and activation of NMDA receptors, which increase calcium, and/or receptors that couple to an increase in PLC. These triggers result in the synthesis of 2-AG which diffuses from the postsynaptic neuron and binds to CB1R on the presynaptic terminal. The result of presynaptic CB1R activation is inhibition of neurotransmitter release; in the case of short term modulation, the CB1R signaling mechanisms include inhibition of the opening of VDCCs and perhaps, also increased activation of potassium channels, resulting in hyperpolarization. In the case where depolarization of the post synaptic neuron is the trigger and CB1R activation inhibits GABA release, the plasticity is called depolarization-induced suppression of inhibition (DSI). Evidence supporting the role of the CB1R in DSI include data that DSI is both mimicked and occluded by a CB1R agonist; and that CB1R agonist and DSI increase the paired-pulse ratio and decrease spontaneous, calcium-dependent, miniature IPSPs, both indicative of a reduction in presynaptic vesicular release probability.
Endocannabinoid/CB1R signaling also produces suppression of glutamate release by a parallel mechanism. Specifically, depolarization of CA1 pyramidal neurons results in transient suppression of excitatory postsynaptic potential amplitude that is dependent upon CB1R activation (Ohno-Shosaku et al., 2002). DSE is also dependent upon presynaptic CB1R availability and is the result of activation of CB1R on glutamatergic terminals (Ohno-Shosaku et al., 2002; Ruehle et al., 2013).
DSI is not affected by botulinum toxin applied to the postsynaptic neuron, indicating that the retrograde messenger does not utilize a synaptic release mechanism. This finding is consistent with an “on-demand” synthesis of the endocannabinoids (Freund et al., 2003). Further studies have demonstrated that acute inhibition of DAGL suppresses (Hashimotodani et al., 2013) while acute inhibition of MAGL prolongs (Pan et al., 2009) hippocampal DSI. DSI/DSE are completely absent in slices from hippocampus, cerebellum, striatum and prefrontal cortex mice in which DAGLα has been genetically deleted (Gao et al., 2010; Tanimura et al., 2010; Yoshino et al., 2011). These findings, together with a lack of effect of FAAH inhibition on DSI (Pan et al., 2009), indicate that 2-AG is the endocannabinoid subserving this type of synaptic plasticity.
The primary triggers for the synthesis of endocannabinoid to produce short term plasticity are increased post synaptic calcium and activation of receptors that increase PLC activity (Kano, 2014). Receptor-driven, endocannabinoid mediated plasticity was first demonstrated for the group I mGluRs (Maejima, Hashimoto, Yoshida, Aiba, & Kano, 2001), which couple to Gαq proteins and therefore increase PLCβ activity and generate DAG as described above. Subsequent studies demonstrated that many receptors that activate PLC can also evoke endocannabinoid-dependent short term suppression of neurotransmitter release. Endocannabinoid signaling can be induced by strong Gαq linked receptor activation alone, or by a combination of sub-threshold Gαq activation with a simultaneous increase in postsynaptic calcium (Kim, Isokawa, Ledent, & Alger, 2002). It is not completely clear how increased postsynaptic calcium concentrations results in an increase in 2-AG; a two enzyme pathway for DAG synthesis involving PLD and PAP is calcium sensitive and could serve this function (Bisogno et al., 1999). There is evidence that different DAGLα pools could be responsible for 2-AG generation form DAG synthesized in response to calcium versus PLC activation (Zhang, Wang, Bisogno, Di Marzo, & Alger, 2011).
Long-term depression of transmission (LTD)
The ECS has also been implicated in more persistent forms of synaptic plasticity. Stimulation protocols which induce LTP of excitatory synapses onto CA1 pyramidal neurons also produce long-term depression of GABAergic inputs to the same neurons; a phenomenon called iLTD (Chevaleyre & Castillo, 2003). High frequency stimulation (HFS) of inputs to CA1 pyramidal neurons depresses GABAergic transmission onto these neurons for almost 1 hour, an effect that is presynaptic in nature, and requires activation of mGluR and CB1R (Chevaleyre & Castillo, 2003). Induction of iLTD is blocked by PLC and DAGL inhibition, supporting a role for 2-AG in the initiation of the plasticity (Edwards, Kim, & Alger, 2006). HFS of cortical afferents to the striatum induce LTD of glutamatergic transmission, an effect absent in CB1R−/− mice and mice treated with CB1R antagonist (Gerdeman, Ronesi, & Lovinger, 2002). Interestingly, CB1R antagonist application 10 minutes after HFS does not block LTD, suggesting that the ECS is required for the induction of LTD, but not its long-term maintenance (Ronesi et al., 2004). LTD at this synapse is dependent upon increases in post-synaptic intracellular calcium (Gerdeman et al., 2002).
The triggers for endocannabinoid-mediated LTD are the same as those for short term synaptic suppression; i.e. increased post-synaptic calcium and increased PLC activity. What differentiates short and long term changes in neurotransmitter release are the presynaptic signaling mechanisms evoked by CB1R activation. For ECS-mediated LTD, inhibition of PKA is critical (Castillo, Younts, Chavez, & Hashimotodani, 2012) and synergistic changes in the presynaptic terminal are likely required, such as increased calcium concentrations or co-activation of other GPCRs (Kano, 2014).
There is evidence that AEA can also produce changes in synaptic activity at a more limited number of synapses and through TRPV1 signaling. For example, AEA-mediated post-synaptic TRPV1 activation produces LTD in the hippocampus (Chavez, Chiu, & Castillo, 2010), nucleus accumbens (Grueter, Brasnjo, & Malenka, 2010) and extended amygdala (Puente et al., 2011). These effects do not involve CB1R signaling.
Summary
The ECS is a beautiful and fascinating neuromodulatory system that allows for moment to moment, synapse-specific regulation of neurotransmission. The basic building blocks of the proteins involved in endocannabinoid synthesis and degradation together with the receptor targets are utilized throughout the brain, but in very diverse ways depending upon their relative locations and in relationship to other ongoing signaling events. The ECS modulates the functions of the major excitatory and inhibitory neurotransmitters, serving as a break on their release (described above). CB1R can also inhibit the release of biogenic amines (Haring, Guggenhuber, & Lutz, 2012) and neuropeptides (Hirasawa et al., 2004), thereby modulating the modulators! The ECS is likely the mechanism by which steroid hormones such as glucocorticoids (Di, Malcher-Lopes, Halmos, & Tasker, 2003) and estrogen (Huang & Woolley, 2012) alter synaptic plasticity. Recent evidence that a cycle of 2-AG → AA is tightly regulated by MAGL activity (discussed above) adds another layer of complexity to the role of 2-AG homeostasis to brain function. It is not surprising that increasing evidence indicates that dysregulation of ECS contributes to many forms of brain dysfunction, including psychopathology, developmental problems and plays a role in neurodegenerative diseases as well.
Acknowledgements
I was supported during the writing of this review by NIH grants DA038663; DA026996 and MH102838 and by the Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin. I am extremely grateful to Margaret Beatka for making the figures.
Abbreviations
- AA
arachidonic acid
- Abhd
Alpha beta hydrolase domain protein
- AEA
N-arachidonylethanolamine
- 2-AG
2-arachidonoylglycerol
- CB1R
cannabinoid receptor subtype 1
- CBD
cannabidiol
- CCK
cholecystokinin
- DAG
diacylglycerol
- DAGL
diacylglycerol lipase
- DSE
depolarization-induced suppression of excitation
- DSI
depolarization-induced suppression of inhibition
- EA
ethanolamine
- ECS
endocannabinoid system
- FAAH
fatty acid amide hydrolase
- GDE1
glycerophosphodiesterase 1
- GP-AEA
glycerophospho-N-arachidonylethanolamine
- GP-NAE
glycerophospho-N-acylethanolamine
- GPCR
G protein coupled receptor
- HFS
high frequency stimulation
- IP3
inositol triphosphate
- LTD
long-term depression
- MAGL
monoacylglycerol lipase
- mGluR
metabotropic glutamate receptor
- NAAA
N-acylethanolamine-hydrolyzing acid amidase
- NAE
N-acylethanolamine
- NAPE
N-acyl phosphatidylethanolamine
- NAPE-PLD
N-acyl phosphatidylethanolamine-specific phospholipase D
- NAT
N-acyl transferase; PC: phosphatidylcholine
- PA
phosphatidic acid
- PAP
phosphatidic acid phosphatase
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PIP2
phosphatidylinositol 4,5 bisphosphate
- PKA
protein kinase A
- PKC
protein kinase C
- PLA/AT
phospholipase A/acyl transferase
- PLC
phospholipase C
- PLD
phospholipase D
- PPAR
peroxisome proliferation activating receptor
- PTPN22
protein tyrosine phosphatase, non-receptor type 22
- SHIP1
Src homology 2-containing inositol phosphatase-1
- THC
Δ9-tetrahydrocannabinol
- THL
tetrahydrolipstatin
- VDCC
voltage- dependent calcium channel
Bibliography
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, … Cravatt BF (2009). Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol, 16(4), 411–420. doi: 10.1016/j.chembiol.2009.02.013blan [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn KH, Mahmoud MM, & Kendall DA (2012). Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J Biol Chem, 287(15), 12070–12082. doi: 10.1074/jbc.Mlll.316463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JP, & Cravatt BF (2006). The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. J Amer Chem Soc, 128(30), 9699–9704. doi: 10.1021/ja062999h [DOI] [PubMed] [Google Scholar]
- Arreaza G, Devane WA, Omeir DL, Sajnani G, Kunz J, B.F. C, & Deutsch DG (1997). The cloned rat hydrolytic enzyme responsible for the breakdown of anandamide also catalyzes its formation via the condensation of arachidonic acid and ethanolamine. Neurosci Letts, 234, 59–62. [DOI] [PubMed] [Google Scholar]
- Bachur NR, Masek K, Melmon KL, & Udenfriend S (1965). Fatty acid amides of ethanolamine in mammalian tissues. J Biol Chem, 240,1019–1024. [PubMed] [Google Scholar]
- Bachur NR, & Udenfriend S (1966). Microsomal synthesis of fatty acid amides. J Biol Chem, 241 (6), 1308–1313. [PubMed] [Google Scholar]
- Baggelaar MP, Chameau PJ, Kantae V, Hummel J, Hsu KL, Janssen F, … van der Stelt M (2015). Highly selective, reversible inhibitor identified by comparative chemoproteomics modulates diacylglycerol lipase activity in neurons. J Am Chem Soc, 137(27), 8851–8857. doi: 10.1021/jacs.5b04883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie GL, Horswill JG, Anavi-Goffer S, Reggio PH, Bolognini D, Abood ME, … Ross RA (2013). CB1 receptor allosteric modulators display both agonist and signaling pathway specificity. Molec Pharmacol, 83(2), 322–338. doi: 10.1124/mol.112.080879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Chicca A, Tamborrini M, Eisen D, Lerner R, Lutz B, … Gertsch J (2012). Identification and quantification of a new family of peptide endocannabinoids (pepcans] showing negative allosteric modulation at CB1 receptors. J Biol Chem, 287(44), 36944–36967. doi: 10.1074/jbc.M112.382481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Kennedy DA, Stanford N, & Majerus PW (1979). Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U SA, 76(7), 3238–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Cascio MG, Saha B, Mahadevan A, Urbani P, Minassi A, … Di Marzo V (2006). Development of the first potent and specific inhibitors of endocannabinoid biosynthesis. Biochim Biophys Acta, 1761(2), 205–212. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, … Doherty P (2003). Cloning of the first snl-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol, 163(3), 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Maccarrone M, De Petrocellis L, Jarrahian A, Finazzi-Agro A, Hillard C, & Di Marzo V (2001). The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur J Biochem 268(7), 1982–1989. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Melck D, De Petrocellis L, & Di Marzo V (1999). Phosphatidic acid as the biosynthetic precursor of the endocannabinoid 2-arachidonylglycerol in intact mouse neuroblastoma cells stimulated with ionomycin. J Neurochem, 72,2113–2119. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, & Di Marzo V (1997). Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J, 322(Pt 2), 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Long JZ, Trauger SA, Siuzdak G, & Cravatt BF (2013). ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc Natl Acad Sci U SA, 110(4), 1500–1505. doi: 10.1073/pnas.1217121110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, & Cravatt BF (2007). A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol, 14(12), 1347–1356. doi: 10.1016/j.chembiol.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomar MG, & Galande AK (2013). Modulation of the cannabinoid receptors by hemopressin peptides. Life Sci, 92(8–9), 520–524. doi: 10.1016/j.lfs.2012.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, & Casellas P (2005). Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. EurJ Pharmacol, 517(3), 174–181. doi: 10.1016/j.ejphar.2005.05.032 [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, … Casellas P [1997). A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem, 272(35), 22330–22339. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, … Casellas P (1993). Cannabinoid-receptor expression in human leukocytes. Eur J Biochem, 214(1), 173–180. [DOI] [PubMed] [Google Scholar]
- Bowles NP, Hill MN, Bhagat SM, Karatsoreos IN, Hillard CJ, & McEwen BS (2012). Chronic, noninvasive glucocorticoid administration suppresses limbic endocannabinoid signaling in mice. Neuroscience, 204, 83–89. doi: 10.1016/j.neuroscience.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, & Parsons LH (2010). Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Brit J Pharmacol, 160(3), 423–442. doi: BPH787[pii] 10.1111/j.1476-5381.2010.00787.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadas H, di Tomaso E, & Piomelli D (1997). Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci, 17(4), 1226–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadas H, Gaillet S, Beltramo M, Venance L, & Piomelli D (1996). Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci, 16(12), 3934–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Mulvihill MM, Mukhopadhyay P, Xu H, Erdelyi K, Hao E, … Pacher P (2013). Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology, 144,808–817. doi: 10.1053/j.gastro.2012.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, & Cabral GA (2002). Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol, 2(1), 69–82. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, & Hashimotodani Y (2012). Endocannabinoid signaling and synaptic function. Neuron, 76(1), 70–81. doi: 10.1016/j.neuron.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP, & Brown DA (1992). Cannabinoid receptor agonists inhibit Ca current in NG108–15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Brit J Pharmacol, 106, 231–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda PK, Gao Y, Mark L, Btesh J, Strassle BW, Lu P, … Samad TA (2010). Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Molec Pharmacol, 78(6), 996–1003. doi: mol.110.068304[pii] 10.1124/mol.H0.068304 [DOI] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, & Castillo PE (2010). TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci, 13(12),1511–1518. doi: nn.2684[pii] 10.1038/nn.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, & Castillo PE (2003). Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron, 38(3), 461–472. [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, & Cravatt BF (2004). Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet, 13(18), 2113–2119. [DOI] [PubMed] [Google Scholar]
- Chicca A, Marazzi J, Nicolussi S, & Gertsch J (2012). Evidence for bidirectional endocannabinoid transport across cell membranes. J Biol Chem, 287(41), 34660–34682. doi: 10.1074/jbc.M112.373241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colodzin M, Bachur NR, Weissbach H, & Udenfriend S (1963). Enzymatic formation of fatty acid amides of ethanolamine by rat liver microsomes. Biochem Biophys Res Commun, 10(2), 165–170. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, & Lichtman AH (2001). Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA, 98(16), 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, & Gilula NB (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature, 384(6604), 83–87. [DOI] [PubMed] [Google Scholar]
- de Lago E, Petrosino S, Valenti M, Morera E, Ortega-Gutierrez S, Fernandez-Ruiz J, & Di Marzo V (2005). Effect of repeated systemic administration of selective inhibitors of endocannabinoid inactivation on rat brain endocannabinoid levels. Biochem Pharmacol, 70(3), 446–452. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Glaser ST, Howell JM, Kunz JS, Puffenbarger RA, Hillard CJ, & Abumrad N (2001). The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J Biol Chem, 276(10), 6967–6973. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, & Howlett AC (1988). Determination and characterization of a cannabinoid receptor in rat brain. Molec Pharmacol, 84(5), 605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, … Mechoulam R (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science, 258(5090), 1946–1949. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, & Kunos G (1999). Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. EurJ Biochem 264(1), 258–267. [DOI] [PubMed] [Google Scholar]
- Di S, Boudaba C, Popescu IR, Weng FJ, Harris C, Marcheselli VL, … Tasker JG (2005). Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus. J Physiol, 569(Pt 3), 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Haimos KC, & Tasker JG (2003). Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci, 23(12), 4850–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, … Lee FS (2015). FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature Comm, 6, 6395. doi: 10.1038/ncomms7395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona L, Sensi SL, … Piomelli D (2002). Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA, 99(16), 10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Kwon IK, & Kim J (2013). Neuregulin-1 impairs the long-term depression of hippocampal inhibitory synapses by facilitating the degradation of endocannabinoid 2-AG. J Neurosci, 35(38), 15022–15031. doi: 10.1523/JNEUROSCI.5833-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, Kim J, & Alger BE (2006). Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol, 95(1), 67–75. [DOI] [PubMed] [Google Scholar]
- Engeli S, Lehmann AC, Kaminski J, Haas V, Janke J, Zoerner AA, … Jordan J (2014). Influence of dietary fat intake on the endocannabinoid system in lean and obese subjects. Obesity (Silver Spring), 22(5), E70–76. doi: 10.1002/oby.20728 [DOI] [PubMed] [Google Scholar]
- Farooqui AA, & Horrocks LA (1997). Nitric oxide synthase inhibitors do not attenuate diacylglycerol or monoacylglycerol lipase activities in synaptoneurosomes. Neurochem Res, 22(10), 1265–1269. [DOI] [PubMed] [Google Scholar]
- Filis P, Kind PC, & Spears N (2013). Implantation failure in mice with a disruption in Phospholipase C beta 1 gene: lack of embryonic attachment, aberrant steroid hormone signalling and defective endocannabinoid metabolism. Molec Hum Rep, 19(5), 290–301. doi: 10.1093/molehr/gas067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskerstrand T, H’Mida-Ben Brahim D, Johansson S, M’Zahem A, Haukanes BI, Drouot N, … Knappskog PM (2010). Mutations in ABHD12 cause the neurodegenerative disease PHARC: An inborn error of endocannabinoid metabolism. Am J Hum Genet, 87(3), 410–417. doi: S0002-9297(10)00414-3[pii] 10.1016/j.ajhg.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, & Manzoni OJ (2004). A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci, 24(31), 6939–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G, Ligresti A, Lopez-Rodriguez ML, & Di Marzo V (2004). Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis-a difficult issue to handle. Eur J Pharmacol, 492(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, & Piomelli D (2003). Role of endogenous cannabinoids in synaptic signaling. Physiol Rev, 83(3), 1017–1066. [DOI] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, … Piomelli D (2012). A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci, 15(1), 64–69. doi: 10.1038/nn.2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, … Piomelli D (2003). Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature, 425(6953), 90–93. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Uchigashima M, Nomura S, Hasegawa Y, Kikuchi H, & Watanabe M (2008). Predominant expression of phospholipase Cbetal in telencephalic principal neurons and cerebellar interneurons, and its close association with related signaling molecules in somatodendritic neuronal elements. Eur J Neurosci, 28(9), 1744–1759. doi: EJN6495[pii] 10.1111/j.1460-9568.2008.06495.x [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, … Casellas P (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232(1), 54–61. [DOI] [PubMed] [Google Scholar]
- Galli GG, Multhaupt HA, Carrara M, de Lichtenberg KH, Christensen IB, Linnemann D, … Lund AH (2014). Prdm5 suppresses Apc(Min)-driven intestinal adenomas and regulates monoacylglycerol lipase expression. Oncogene, 33(25), 3342–3350. doi: 10.1038/onc.2013.283 [DOI] [PubMed] [Google Scholar]
- Gamage TF, Ignatowska-Jankowska BM, Wiley JL, Abdelrahman M, Trembleau L, Greig IR, … Lichtman AH (2014). In-vivo pharmacological evaluation of the CBl-receptor allosteric modulator Org-27569. Behav Pharmacol, 25(2), 182–185. doi: 10.1097/FBP.0000000000000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Tronson NC, & Radulovic J (2013). Modulation of behavior by scaffolding proteins of the post-synaptic density. Neurobio Learn Mem, 105, 3–12. doi: 10.1016/j.nlm.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, … Doherty P (2010). Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci, 30(6), 2017–2024. doi: 30/6/2017[pii] 10.1523/JNEUROSCI.5693-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, & Mechoulam R (1964). Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc, 86,1646–1647. [Google Scholar]
- Gasperi V, Ceci R, Tantimonaco M, Talamonti E, Battista N, Parisi A, … Maccarrone M (2014). The fatty acid amide hydrolase in lymphocytes from sedentary and active subjects. Med Sci Sports Exerc, 46(1), 24–32. doi: 10.1249/MSS.0b013e3182a10ce6 [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Baewer DV, Hittner S, Hillard CJ, Nithipatikom K, Reddy DS, … Campbell WB (2005). Endothelium-derived 2-arachidonylglycerol: an intermediate in vasodilatory eicosanoid release in bovine coronary arteries. Am J Physiol Heart Circ Physiol, 288(3), H1344–1351. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, & Harder DR (1999). Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol Heart Circ Physiol, 276(6 Pt 2), H2085–H2093. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, & Lovinger DM (2002). Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci, 5(5), 446–451. [DOI] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, & Deutsch DG (2003). Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci USA, 100(7), 4269–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, & Felder CC (1997). Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci, 17,5327–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, & Northup JK (1999). Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Moecl Pharmacol, 56(6), 1362–1369. [DOI] [PubMed] [Google Scholar]
- Golczak M, Kiser PD, Sears AE, Lodowski DT, Blaner WS, & Palczewski K (2012). Structural basis for the acyltransferase activity of lecithin:retinol acyltransferase-like proteins. J Biol Chem, 287(28), 23790–23807. doi: 10.1074/jbc.M112.361550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, … Spatz M (2004). Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res; 132(1), 87–92. [DOI] [PubMed] [Google Scholar]
- Gomez O, Sanchez-Rodriguez A, Le M, Sanchez-Caro C, Molina-Holgado F, & Molina-Holgado E (2011). Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. Brit J Pharmacol, 163(7), 1520–1532. doi: 10.1111/j.1476-5381.2011.01414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, & Hipkin RW (2000). Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Molec Pharmacol, 57(5), 1045–1050. [PubMed] [Google Scholar]
- Goparaju SK, Ueda N, Taniguchi K, & Yamamoto S (1999). Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochem Pharmacol, 57(4), 417–423. [DOI] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, … Hill MN (2015). Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci, 35(9), 3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi P, Pucci M, Di Siena S, Di Giacomo D, Pirazzi V, Geremia R, & Maccarrone M (2012). The faah gene is the first direct target of estrogen in the testis: role of histone demethylase LSD1. Cell Mol Life Sci, 69(24), 4177–4190. doi: 10.1007/s00018-012-1074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi P, Rossi G, Catanzaro G, & Maccarrone M (2009). Modulation of the endocannabinoid-degrading enzyme fatty acid amide hydrolase by follicle-stimulating hormone. Vitam Horm, 81, 231–261. doi: S0083-6729(09)81010-8 [pii] 10.1016/S0083-6729(09)81010-8 [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, & Malenka RC (2010). Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci, 13(12), 1519–1525. doi: nn.2685[pii] 10.1038/nn.2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerciolini R (1997). Mode of action of orlistat. Int J Obes Relat Metab Disord, 21 Suppl 3, S12–23. [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, & Freund TF (2004). Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci, 20(2), 441–458. [DOI] [PubMed] [Google Scholar]
- Hansen HS, Moesgaard B, Hansen HH, & Petersen G (2000). N-Acylethanolamines and precursor phospholipids - relation to cell injury. Chem Phys Lipids, 108(1–2), 135–150. [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, … Mechoulam R (2001). 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA, 98(7), 3662–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M, Guggenhuber S, & Lutz B (2012). Neuronal populations mediating the effects of endocannabinoids on stress and emotionality. Neuroscience, 204, 145–158. doi: 10.1016/j.neuroscience.2011.12.035 [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, & Kano M (2008). Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology, 54, 58–67. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tanimura A, Kita Y, Sano Y, Shimizu T, … Kano M (2013). Acute inhibition of diacylglycerol lipase blocks endocannabinoid-mediated retrograde signalling: evidence for on-demand biosynthesis of 2-arachidonoylglycerol. J Physiol, 591 (Pt 19), 4765–4776. doi: 10.1113/jphysiol.2013.254474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, … Devi LA (2007). Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci USA, 104(51), 20588–20593. doi: 10.1073/pnas.0706980105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, & Chavkin C (1995). Activation of inwardly rectifying potassium channels (GIRK1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neurosci Letts, 186, 91–94. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Groen BG, Lynn AB, De Costa BR, & Richfield EK (1991). Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res, 552(2), 301–310. [DOI] [PubMed] [Google Scholar]
- Hermanson DJ, Gamble-George JC, Marnett LJ, & Patel S (2014). Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci, 85(7), 358–367. doi: 10.1016/j.tips.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson DJ, Hartley ND, Gamble-George J, Brown N, Shonesy BC, Kingsley PJ, … Patel S (2013). Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat Neurosci, 16(9), 1291–1298. doi: 10.1038/nn.3480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, … McEwen BS (2013). Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psych, 18,1125–1135. doi: 10.1038/mp.2012.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ (2000). Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Médiat, 61(1–2), 3–18. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, & Campbell WB (1997). Biochemistry and pharmacology of arachidonylethanolamide, a putative endogenous cannabinoid. J Lipid Res, 88(12), 2383–2398. [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Jarrahian A, & Campbell WB (1997). Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem, 69(2), 631–638. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, & Jarrahian A (2003). Cellular accumulation of anandamide: consensus and controversy. Brit J Pharmacol, 140(3), 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, & Jarrahian A (2005). Accumulation of anandamide: Evidence for cellular diversity. Neuropharmacology, 48(8), 1072–1078. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, … Campbell WB (1999). Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J Pharmacol Exp Ther, 289(3), 1427–1433. [PubMed] [Google Scholar]
- Hillard CJ, Wilkison DM, Edgemond WS, & Campbell WB (1995). Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim Biophys Acta, 1257(3), 249–256. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, & Pittman QJ (2004). Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol, 559(Pt 2), 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, … Chiou LC (2011). Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci, 31 (41), 14600–14610. doi: 10.1523/JNEUROSCI.2671-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SC, Ralvenius WT, Gachet MS, Fritschy JM, Zeilhofer HU, & Gertsch J (2015). Localization and production of peptide endocannabinoids in the rodent CNS and adrenal medulla. Neuropharmacology, doi: 10.1016/j.neuropharm.2015.03.021 [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, … Piomelli D (2005). An endocannabinoid mechanism for stress-induced analgesia. Nature, 435(7045), 1108–1112. [DOI] [PubMed] [Google Scholar]
- Horswill JG, Bali U, Shaaban S, Keily JF, Jeevaratnam P, Babbs AJ, … Wong Kai In P (2007). PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB(1) receptors with hypophagic effects in rats. BrJ Pharmacol, 152(5),805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath E, Woodhams SG, Nyilas R, Henstridge CM, Kano M, Sakimura K, … Katona I (2014). Heterogeneous presynaptic distribution of monoacylglycerol lipase, a multipotent regulator of nociceptive circuits in the mouse spinal cord. Eur J Neurosci, 39(3), 419–434. doi: 10.1111/ejn.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC (1984). Inhibition of neuroblastoma adenylate cyclase by cannabinoid and nantradol compounds. Life Sci, 35,1803–1810. [DOI] [PubMed] [Google Scholar]
- Howlett AC (1985). Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Molec Pharmacol, 27,429–436. [PubMed] [Google Scholar]
- Howlett AC, & Fleming RM (1984). Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response of neuroblastoma cell membranes. Molec Pharmacol, 26, 532–538. [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, & Khachatrian LL (1986). Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Molec Pharmacol, 29, 307–313. [PubMed] [Google Scholar]
- Hu SS, & Mackie K (2015). Distribution of the Endocannabinoid System in the Central Nervous System. Handb Exp Pharmacol, 231, 59–93. doi: 10.1007/978-3-319-20825-1_3 [DOI] [PubMed] [Google Scholar]
- Huang GZ, & Woolley CS (2012). Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron, 74(5), 801–808. doi: 10.1016/j.neuron.2012.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, … Ross RA (2015). A Cannabinoid CB Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacol doi: 10.1038/npp.2015.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, … Lichtman AH (2014). In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Brit J Pharmacol, 171(6), 1392–1407. doi: 10.1111/bph.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, & Piomelli D (2007). A key role for diacylglycerol lipase-{alpha) in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Molec Pharmacol, 72(3), 612–621. [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, … Piomelli D (2005). Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Molec Pharmacol, 68,1196–1202. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, & Deutsch DG (2009). Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci USA, 106(15), 6375–6380. doi: 0901515106 [pii] 10.1073/pnas.0901515106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Hermann A, Glaser ST, Bojesen IN, & Deutsch DG (2006). Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J Biol Chem, 281 (14), 9066–9075. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Vivieca S, Sun J, Glaser ST, & Deutsch DG (2012). Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem, 287(5), 3415–3424. doi: 10.1074/jbc.M111.304907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, … El-Husseini A (2008). Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature, 456(7224), 904–909. doi: 10.1038/nature07605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M (2014). Control of synaptic function by endocannabinoid-mediated retrograde signaling. Proc Jpn Acad Ser B Phys Biol Sci, 90(7), 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanda CN, Masquelier J, Labar G, Muccioli GG, Poupaert JH, & Lambert DM (2012). Synthesis and pharmacological evaluation of 2,4-dinitroaryldithiocarbamate derivatives as novel monoacylglycerol lipase inhibitors. J Med Chem, 55(12), 5774–5783. doi: 10.1021/jm3006004 [DOI] [PubMed] [Google Scholar]
- Karlsson M, Reue K, Xia YR, Lusis AJ, Langin D, Tornqvist H, & Holm C (2001). Exon-intron organization and chromosomal localization of the mouse monoglyceride lipase gene. Gene, 272(1–2), 11–18. [DOI] [PubMed] [Google Scholar]
- Katayama K, Ueda N, Kurahashi Y, Suzuki H, Yamamoto S, & Kato I (1997). Distribution of anandamide amidohydrolase in rat tissues with special reference to small intestine. Biochim Biophys Acta, 1347(2–3), 212–218. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, & Freund TF (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci, 19(11), 4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, … Freund TF (2006). Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci, 26(21), 5628–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keimpema E, Tortoriello G, Alpar A, Capsoni S, Arisi I, Calvigioni D, … Harkany T (2013). Nerve growth factor scales endocannabinoid signaling by regulating monoacylglycerol lipase turnover in developing cholinergic neurons. Proc Natl Acad Sci USA 110(5), 1935–1940. doi: 10.1073/pnas.1212563110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, & Alger BE (2002). Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci, 22(23), 10182–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Chung YC, Chung ES, Park KW, Won SY, Bok E, … Jin BK (2007). Roles of transient receptor potential vanilloid subtype 1 and cannabinoid type 1 receptors in the brain: neuroprotection versus neurotoxicity. Molec Neurobiol, 35(3), 245–254. [DOI] [PubMed] [Google Scholar]
- King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, … Piomelli D (2007). URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol, 14(12), 1357–1365. doi: 10.1016/j.chembiol.2007.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly E, Gonda X, & Bagdy G (2012). CB1 receptor antagonists: new discoveries leading to new perspectives. Acta Physiol, 205(1), 41–60. doi: 10.1111/j.1748-1716.2012.02402.x [DOI] [PubMed] [Google Scholar]
- Kortleven C, Bruneau LC, & Trudeau LE (2012). Neurotensin inhibits glutamate-mediated synaptic inputs onto ventral tegmental area dopamine neurons through the release of the endocannabinoid 2-AG. Neuropharmacol, 63(6), 983–991. doi: 10.1016/j.neuropharm.2012.07.037 [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, & Regehr WG (2001). Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron, 29(3), 717–727. [DOI] [PubMed] [Google Scholar]
- Kurahashi Y, Ueda N, Suzuki H, Suzuki M, & Yamamoto S (1997). Reversible hydrolysis and synthesis of anandamide demonstrated by recombinant rat fatty-acid amide hydrolase. Biochem Biophys Res Commun, 237(3), 512–515. [DOI] [PubMed] [Google Scholar]
- Lappas M (2014). Effect of pre-existing maternal obesity, gestational diabetes and adipokines on the expression of genes involved in lipid metabolism in adipose tissue. Metabol, 63(2), 250–262. doi: 10.1016/j.metabol.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, & Denovan-Wright EM (2015). Cannabidiol is a negative allosteric modulator of the type 1 cannabinoid receptor. Brit J Pharmacol, doi: 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, Kraemer FB, & Severson DL (1995). Characterization of a partially purified diacylglycerol lipase from bovine aorta. Biochim Biophys Acta, 1254(3), 311–318. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ledri M, Toth B, Marchionni I, Henstridge CM, Dudok B, … Katona I (2015). Multiple forms of endocannabinoid and endovanilloid signaling regulate the tonic control of GABA release. J Neurosci, 35(27), 10039–10057. doi: 10.1523/JNEUROSCI.4112-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, & Cravatt BF (2006). Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochem, 45(15), 4720–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K, Eimes MW, Glaser ST, Deutsch DG, & Kaczocha M (2013). Role of FAAH-like anandamide transporter in anandamide inactivation. PLoS ONE, 8(11), e79355. doi: 10.1371/journal.pone.0079355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedhegner ES, Vogt CD, Sem DS, Cunningham CW, & Hillard CJ (2014). Sterol carrier protein-2: binding protein for endocannabinoids. Molec Neurobiol, 50,149–158. doi: 10.1007/s12035-014-8651-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A, Morera E, Van Der Stelt M, Monory K, Lutz B, Ortar G, & Di Marzo V (2004). Further evidence for the existence of a specific process for the membrane transport of anandamide. Biochem J, 380(Pt 1), 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, … Kunos G (2008). Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacol, 54(1), 1–7. doi: S0028-3908(07)00157-8 [pii] 10.1016/j.neuropharm.2007.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, … Kunos G (2006). A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA, 103(36), 13345–13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, & Piomelli D (2005). The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Molec Pharmacol, 67(1), 15–19. doi: mol.104.006353 [pii] 10.1124/mol.104.006353 [DOI] [PubMed] [Google Scholar]
- Lograno MD, & Romano MR (2004). Cannabinoid agonists induce contractile responses through Gi/o-dependent activation of phospholipase C in the bovine ciliary muscle. Eur J Pharmacol, 494(1), 55–62. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, … Cravatt BF (2009). Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol, 5(1), 37–44. doi: nchembio.129 [pii] 10.1038/nchembio.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, & Cravatt BF (2009). Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol, 16(7), 744–753. doi: S1074-5521(09)00176-8 [pii] 10.1016/j.chembiol.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agro A, & Rossi A (2003). Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J Biol Chem, 278(35), 32726–32732. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, De Petrocellis L, Bari M, Fezza F, Salvati S, Di Marzo V, & Finazzi-Agro A (2001). Lipopolysaccharide downregulates fatty acid amide hydrolase expression and increases anandamide levels in human peripheral lymphocytes. Arch Biochem Biophys, 393(2), 321–328. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Di Rienzo M, Finazzi-Agro A, & Rossi A (2003). Leptin activates the anandamide hydrolase promoter in human T lymphocytes through STAT3. J Biol Chem, 278(15), 13318–13324. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Gasperi V, Fezza F, Finazzi-Agro A, & Rossi A (2004). Differential regulation of fatty acid amide hydrolase promoter in human immune cells and neuronal cells by leptin and progesterone. Eur J Biochem, 271(23–24), 4666–4676. doi: 10.1111/j.1432-1033.2004.04427.x [DOI] [PubMed] [Google Scholar]
- Mackie K, & Hille B (1992). Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA, 89, 3825–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, & Kano M (2001). Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron, 31(3), 463–475. [DOI] [PubMed] [Google Scholar]
- Mai P, Yang L, Tian L, Wang L, Jia S, Zhang Y, … Li L (2015). Endocannabinoid system contributes to liver injury and inflammation by activation of bone marrow-derived monocytes/macrophages in a CBl-dependent manner. J Immunol, 195(7), 3390–3401. doi: 10.4049/jimmunol.1403205 [DOI] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, & Tasker JG (2006). Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci, 26(24), 6643–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, … Stella N (2010). The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci, 13(8), 951–957. doi: nn.2601 [pii] 10.1038/nn.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, & Lutz B (1999). Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci, 11(12), 4213–4225. [DOI] [PubMed] [Google Scholar]
- Martin BR, & Cravatt BF (2009). Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods, 6(2), 135–138. doi: 10.1038/nmeth.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, & Bonner TI (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature, 546(6284), 561–564. [DOI] [PubMed] [Google Scholar]
- Matyas F, Urban GM, Watanabe M, Mackie K, Zimmer A, Freund TF, & Katona I (2008). Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology,54(1), 95–107. doi: S0028-3908(07)00170-0 [pii] 10.1016/j.neuropharm.2007.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland MJ, Porter AC, Rakhshan FR, Rawat DS, Gibbs RA, & Barker EL (2004). A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J Biol Chem, 279(40), 41991–41997. [DOI] [PubMed] [Google Scholar]
- McFarland MJ, Terebova EA, & Barker EL (2006). Detergent-resistant membrane microdomains in the disposition of the lipid signaling molecule anandamide. AAPSJ, 8(1), E95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, … et al. (1995). Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50(1), 83–90. [DOI] [PubMed] [Google Scholar]
- Milne GM, Koe BK, & Johnson MR (1979). Stereospecific and potent analgetic activity for nantradol—a structurally novel, cannabinoid-related analgetic. NIDA Res Monogr, 27, 84–92. [PubMed] [Google Scholar]
- Min R, Testa-Silva G, Heistek TS, Canto CB, Lodder JC, Bisogno T, … Mansvelder HD (2010). Diacylglycerol lipase is not involved in depolarization-induced suppression of inhibition at unitary inhibitory connections in mouse hippocampus. J Neurosci, 30(7), 2710–2715. doi: 30/7/2710 [pii] 10.1523/JNEUROSCI.BC-3622-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, & Guaza C (2002). Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci, 22(22), 9742–9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay B, Cinar R, Yin S, Liu J, Tam J, Godlewski G, … Kunos G (2011). Hyperactivation of anandamide synthesis and regulation of cell-cycle progression via cannabinoid type 1 (CB1) receptors in the regenerating liver. Proc Natl Acad Sci US A, 108(15), 6323–6328. doi: 1017689108 [pii] 10.1073/pnas.1017689108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, & Abu-Shaar M (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365,61–65. [DOI] [PubMed] [Google Scholar]
- Murataeva N, Straiker A, & Mackie K (2014). Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Brit J Pharmacol, 171(6), 1379–1391. doi: 10.1111/bph.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr ML, Shi X, Bowman AL, Johnson M, Zvonok N, Janero DR, … Makriyannis A (2013). Membrane phospholipid bilayer as a determinant of monoacylglycerol lipase kinetic profile and conformational repertoire. Prot Sci 22(6), 774–87. doi: 10.1002/pro.2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, & Araque A (2008). Endocannabinoids mediate neuron-astrocyte communication. Neuron, 57(6), 883–893. doi: S0896-6273(08)00116-5 [pii] 10.1016/j.neuron.2008.01.029 [DOI] [PubMed] [Google Scholar]
- Navia-Paldanius D, Aaltonen N, Lehtonen M, Savinainen JR, Taschler U, Radner FP, … Laitinen JT (2015). Increased tonic cannabinoid CB1R activity and brain region-specific desensitization of CB1R Gi/o signaling axis in mice with global genetic knockout of monoacylglycerol lipase. EurJ Pharm Sci,77,180–188. doi: 10.1016/j.ejps.2015.06.005 [DOI] [PubMed] [Google Scholar]
- Nicolussi S, Chicca A, Rau M, Rihs S, Soeberdt M, Abels C, & Gertsch J (2014). Correlating FAAH and anandamide cellular uptake inhibition using N-alkylcarbamate inhibitors: from ultrapotent to hyperpotent. Biochem Pharmacol, 92(4), 669–689. doi: 10.1016/j.bcp.2014.09.020 [DOI] [PubMed] [Google Scholar]
- Nicolussi S, & Gertsch J (2015). Endocannabinoid transport revisited. Vitam Horm, 98, 441–485. doi: 10.1016/bs.vh.2014.12.011 [DOI] [PubMed] [Google Scholar]
- Nicolussi S, Viveros-Paredes JM, Gachet MS, Rau M, Flores-Soto ME, Blunder M, & Gertsch J (2014). Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice. Pharmacol Res, 80, 52–65. doi: 10.1016/j.phrs.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Nie J, & Lewis DL (2001). Structural domains of the CB1 cannabinoid receptor that contribute to constitutive activity and G-protein sequestration. J Neurosci, 21(22), 8758–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, … Cravatt BF (2011). Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science, 334(6057), 809–813. doi: 10.1126/science.1209200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyilas R, Dudok B, Urban GM, Mackie K, Watanabe M, Cravatt BF, … Katona I (2008). Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J Neurosci, 28(5), 1058–1063. doi: 28/5/1058 [pii] 10.1523/JNEUROSCI.5102-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddi S, Fezza F, Pasquariello N, D’Agostino A, Catanzaro G, De Simone C, … Maccarrone M (2009). Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chem Biol, 16(6), 624–632. doi: S1074-5521(09)00151-3 [pii] 10.1016/j.chembiol.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, & Kano M (2001). Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron, 29(3), 729–738. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, & Kano M (2002). Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci, 22(10), 3864–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, & Ueda N (2004). Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem, 279(7), 5298–5305. [DOI] [PubMed] [Google Scholar]
- Ortar G, Bisogno T, Ligresti A, Morera E, Nalli M, & Di Marzo V (2008). Tetrahydrolipstatin analogues as modulators of endocannabinoid 2-arachidonoylglycerol metabolism. J Med Chem, 51,6970–6979. doi: 10.1021/jm800978m [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Ferreira J, Menezes de Lima O Jr., Duarte FS, Bento AF, Forner S, … Takahashi RN (2012). Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci USA, 109(51), 21134–21139. doi: 10.1073/pnas.1202906109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, & Liu QS (2009). Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther, 331(2), 591–597. doi: jpet.109.158162 [pii] 10.1124/jpet.109.158162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, & Liu QS (2011). Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci, 31(38), 13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, & Goldberg SR (2013). Inhibition of FAAH and activation of PPAR: New approaches to the treatment of cognitive dysfunction and drug addiction. Pharmacol Ther 138(1), 84–102. doi: 10.1016/j.pharmthera.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, … Hillard CJ (2005). The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res, 46(2), 342–349. [DOI] [PubMed] [Google Scholar]
- Patel S, & Hillard CJ (2009). Endocannabinoids as modulators of synaptic signaling In Reggio PH (Ed.), The Cannabinoid Receptors (pp. 281–308). New York: Humana Press. [Google Scholar]
- Patricelli MP, & Cravatt BF (1999). Fatty acid amide hydrolase competitively degrades bioactive amides and esters through a nonconventional catalytic mechanism. Biochem, 38,14125–14130. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2008). Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol, 13(2), 147–159. doi: ADB108 [pii] 10.1111/j.1369-1600.2008.00108.x [DOI] [PubMed] [Google Scholar]
- Pickel VM, Shobin ET, Lane DA, & Mackie K (2012). Cannabinoid-1 receptors in the mouse ventral pallidum are targeted to axonal profiles expressing functionally opposed opioid peptides and contacting N-acylphosphatidylethanolamine-hydrolyzing phospholipase D terminals. Neuroscience, 227C, 10–21. doi: 10.1016/j.neuroscience.2012.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, & Shinnick-Gallagher P (2006). Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci, 24(6), 1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x [DOI] [PubMed] [Google Scholar]
- Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, … Felder CC (2002). Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther, 301 (3), 1020–1024. [DOI] [PubMed] [Google Scholar]
- Pratt PF, Hillard CJ, Edgemond WS, & Campbell WB (1998). N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Physiol, 274(1 Pt 2), H375–381. [DOI] [PubMed] [Google Scholar]
- Prescott SM, & Majerus PW (1983). Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem, 258(2), 764–769. [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, … Ross RA (2005). Allosteric modulation of the cannabinoid CB1 receptor. Molec Pharmacol, 68(5), 1484–1495. [DOI] [PubMed] [Google Scholar]
- Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, … Manzoni OJ (2011). Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci, 14(12), 1542–1547. doi: 10.1038/nn.2974 [DOI] [PubMed] [Google Scholar]
- Puffenbarger RA, Kapulina O, Howell JM, & Deutsch DG (2001). Characterization of the 5’-sequence of the mouse fatty acid amide hydrolase. Neurosci Lett, 314(1–2), 21–24. [DOI] [PubMed] [Google Scholar]
- Rahman IA, Tsuboi K, Uyama T, & Ueda N (2014). New players in the fatty acyl ethanolamide metabolism. Pharmacol Res 86,1–10. doi: 10.1016/j.phrs.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Reguero L, Puente N, Elezgarai I, Ramos-Uriarte A, Gerrikagoitia I, Bueno-Lopez JL, … Grandes P (2014). Subcellular localization of NAPE-PLD and DAGL-alpha in the ventromedial nucleus of the hypothalamus by a preembedding immunogold method. Histochem Cell Biol, 141(5), 543–550. doi: 10.1007/s00418-013-1174-x [DOI] [PubMed] [Google Scholar]
- Reisenberg M, Singh PK, Williams G, & Doherty P (2012). The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci,367(1607), 3264–3275. doi: 10.1098/rstb.2011.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, … et al. (1994). SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett, 350(2–3), 240–244. [DOI] [PubMed] [Google Scholar]
- Roloff AM, Anderson GR, Martemyanov KA, & Thayer SA (2010). Homer la gates the induction mechanism for endocannabinoid-mediated synaptic plasticity. J Neurosci, 30(8), 3072–3081. doi: 30/8/3072 [pii] 10.1523/JNEUROSCI.4603-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, & Lovinger DM (2004). Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci, 24(7), 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger TA, Farooqui AA, & Horrocks LA (2007). Bovine brain diacylglycerol lipase: substrate specificity and activation by cyclic AMP-dependent protein kinase. Lipids, 42(3), 187–195. doi: 10.1007/s11745-007-3019-7 [DOI] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, … Pertwee RG (2001). Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Brit J Pharmacol, 132(3), 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer CA, & Marnett LJ (2011). Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev, 111(10), 5899–5921. doi: 10.1021/cr2002799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehle S, Remmers F, Romo-Parra H, Massa F, Wickert M, Wortge S, … Lutz B (2013). Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J Neurosci, 33(25), 10264–10277. doi: 10.1523/JNEUROSCI.4171-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelian A, & Cravatt BF (2005). Discovery metabolite profiling - forging functional connections between the proteome and metabolome. Life Sci, 77(14), 1759–1766. [DOI] [PubMed] [Google Scholar]
- Saghatelian A, McKinney MK, Bandell M, Patapoutian A, & Cravatt BF (2006). A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochem, 45(30), 9007–9015. doi: 10.1021/bi0608008 [DOI] [PubMed] [Google Scholar]
- Sakurada T, & Noma A (1981). Subcellular localization and some properties of monoacylglycerol lipase in rat adipocytes. J Biochem (Tokyo), 90(5), 1413–1419. [DOI] [PubMed] [Google Scholar]
- Salio C, Doly S, Fischer J, Franzoni MF, & Conrath M (2002). Neuronal and astrocytic localization of the cannabinoid receptor-1 in the dorsal horn of the rat spinal cord. Neurosci Lett, 329(1), 13–16. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, & Laitinen JT (2012). The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol, 204(2), 267–276. doi: 10.11ll/j.1748-1716.2011.02280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, Niemi R, Jarvinen T, & Laitinen JT (2003). An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. Br J Pharmacol 140(8), 1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, … Cravatt BF (2010). Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci, 13(9), 1113–1119. doi: nn.2616 [pii] 10.1038/nn.2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid HH, Schmid PC, & Natarajan V (1990). N-acylated glycerophospholipids and their derivatives. Prog Lipid Res, 29(1), 1–43. [DOI] [PubMed] [Google Scholar]
- Schmid HH, Schmid PC, & Natarajan V (1996). The N-acylation-phosphodiesterase pathway and cell signalling. Chem Phys Lipids, 80(1–2), 133–142. [DOI] [PubMed] [Google Scholar]
- Schmid HHO, Schmid PC, & Natarajan V (1990). N-Acylated glycerophospholipids and their derivatives. Prog Lipid Res, 29,1–43. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Krebsbach RJ, Perry SR, Dettmer TM, Maasson JL, & Schmid HH (1995). Occurrence and postmortem generation of anandamide and other long-chain N-acylethanolamines in mammalian brain. FEBS Lett, 375(1–2), 117–120. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Zuzarte-Augustin ML, & Schmid HHO (1985). Properties of rat liver N-acylethanolamine amidohydrolase. J Biol Chem, 260,14145–14149. [PubMed] [Google Scholar]
- Scotchie JG, Savaris RF, Martin CE, & Young SL (2015). Endocannabinoid regulation in human endometrium across the menstrual cycle. Reprod Sci, 22(1), 113–123. doi: 10.1177/1933719114533730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N, Uyama T, Jin XH, Tsuboi K, Tonai T, Houchi H, & Ueda N (2011). Enzymological analysis of the tumor suppressor A-Cl reveals a novel group of phospholipid-metabolizing enzymes. J Lipid Res, 52(11), 1927–1935. doi: 10.1194/jlr.M015081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Wang X, Rose KL, Ramikie TS, Cavener VS, Rentz T, … Colbran RJ (2013). CaMKII regulates diacylglycerol lipase-alpha and striatal endocannabinoid signaling. Nat Neurosci, 16(A), 456–463. doi: 10.1038/nn.3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, & Cravatt BF (2006). Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for {alpha}/beta-hydrolase 4 in this pathway. J Biol Chem, 281(36), 26465–26472. [DOI] [PubMed] [Google Scholar]
- Simon GM, & Cravatt BF (2008). Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem, 283, 9341–9349. doi: M707807200 [pii] 10.1074/jbc.M707807200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, & Cravatt BF (2010). Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol Biosyst, 6(8), 1411–1418. doi: 10.1039/c000237b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, & Cravatt BF (2002). A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA, 99(12), 8394–8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, & Mackie K (2007). Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol, 578(Pt 3), 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mitjavila J, Yin D, Gibson A, & Mackie K (2015). Aiming for allosterism: Evaluation of allosteric modulators of CB1 in a neuronal model. Pharmacol Res, 99, 370–376. doi: 10.1016/j.phrs.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosznajder J, Singh H, & Horrocks LA (1984). Monoacylglycerol lipase. Regulation and increased activity during hypoxia and ischemia. Neurochem Pathol, 2(2), 139–147. [DOI] [PubMed] [Google Scholar]
- Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, & de Fonseca FR (2008). Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol, 509(4), 400–421. doi: 10.1002/cne.21774 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, … Waku K (1995). 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun, 215(1), 89–97. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, … Waku K (1996). Transacylase-mediated and phosphodiesterase-mediated synthesis of N-arachidonoylethanolamine, an endogenous cannabinoid-receptor ligand, in rat brain microsomes. Comparison with synthesis from free arachidonic acid and ethanolamine. Eur J Biochem, 240(1), 53–62. [DOI] [PubMed] [Google Scholar]
- Sumislawski JJ, Ramikie TS, & Patel S (2011). Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsyehopharmacol, 36(13), 2750–2761. doi: 10.1038/npp.2011.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, & Ueda N (2004). Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A 2 and lysophospholipase D. Biochem J 380(Pt 3), 749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, … Kano M (2010). The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron, 65(3), 320–327. doi: S0896-6273(10)00047-4 [pii] 10.1016/j.neuron.2010.01.021 [DOI] [PubMed] [Google Scholar]
- Tarzia G, Duranti A, Tontini A, Piersanti G, Mor M, Rivara S, … Piomelli D (2003). Design, synthesis, and structure-activity relationships of alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase inhibitors. J Med Chem, 46(12), 2352–2360. [DOI] [PubMed] [Google Scholar]
- Tornqvist H, & Beifrage P (1976). Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J Biol Chem, 251 (3), 813–819. [PubMed] [Google Scholar]
- Tsou K, Nogueron MI, Muthian S, Sanudo-Pena MC, Hillard CJ, Deutsch DG, & Walker JM (1998). Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci Lett, 254(3), 137–140. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Okamoto Y, Ikematsu N, Inoue M, Shimizu Y, Uyama T, … Ueda N (2011). Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase D-dependent and -independent pathways. Biochim BiophysActa, 1811(10), 565–577. doi: S1388-1981(11)00134-X [pii] 10.1016/j.bbalip.2011.07.009 [DOI] [PubMed] [Google Scholar]
- Tuccinardi T, Granchi C, Rizzolio F, Caligiuri I, Battistello V, Toffoli G, … Martinelli A (2014). Identification and characterization of a new reversible MAGL inhibitor. Bioorg Med Chem, 22(13), 3285–3291. doi: 10.1016/j.bmc.2014.04.057 [DOI] [PubMed] [Google Scholar]
- Turu G, & Hunyady L (2010). Signal transduction of the CB1 cannabinoid receptor. J Mol Endocrinol, 44(2), 75–85. doi: JME-08-0190 [pii] 10.1677/JME-08-0190 [DOI] [PubMed] [Google Scholar]
- Turu G, Simon A, Gyombolai P, Szidonya L, Bagdy G, Lenkei Z, & Hunyady L (2007). The role of diacylglycerol lipase in constitutive and angiotensin ATI receptor-stimulated cannabinoid CB1 receptor activity. J Biol Chem, 282(11), 7753–7757. [DOI] [PubMed] [Google Scholar]
- Turu G, Varnai P, Gyombolai P, Szidonya L, Offertaler L, Bagdy G, … Hunyady L (2009). Paracrine Transactivation of the CB1 Cannabinoid Receptor by ATI Angiotensin and Other Gq/11 Protein-coupled Receptors. J Biol Chem, 284,16914–16921. doi: M109.003681 [pii] 10.1074/jbc.M109.003681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, & Watanabe M (2007). Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci, 27(14), 3663–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, & Uyama T (2010). Enzymological studies on the biosynthesis of N-acylethanolamines. Biochim Biophys Acta, 1801(12), 1274–1285. doi: S1388-1981(10)00188-5 [pii] 10.1016/j.bbalip.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Ueda N, Yamanaka K, & Yamamoto S (2001). Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J Biol Chem, 276(38), 35552–7. [DOI] [PubMed] [Google Scholar]
- Urquhart P, Nicolaou A, & Woodward DF (2015). Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases. Biochim Biophys Acta, 1851(4), 366–376. doi: 10.1016/j.bbalip.2014.12.015 [DOI] [PubMed] [Google Scholar]
- Uyama T, Inoue M, Okamoto Y, Shinohara N, Tai T, Tsuboi K, … Ueda N (2013). Involvement of phospholipase A/acyltransferase-1 in N-acylphosphatidylethanolamine generation. Biochim Biophys Acta, 1831(12), 1690–1701. doi: 10.1016/j.bbalip.2013.08.017 [DOI] [PubMed] [Google Scholar]
- Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, … Piazza PV (2014). Pregnenolone can protect the brain from cannabis intoxication. Science, 343(6166), 94–98. doi: 10.1126/science.1243985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, … Sharkey KA (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science, 310(5746), 329–332. [DOI] [PubMed] [Google Scholar]
- Vandevoorde S, & Fowler CJ (2005). Inhibition of fatty acid amide hydrolase and monoacylglycerol lipase by the anandamide uptake inhibitor VDM11: evidence that VDM11 acts as an FAAH substrate. Brit J Pharmacol, 145(7), 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Petrosino S, De Petrocellis L, Valenti M, Prandini M, Magherini PC, … Di Marzo V (2008). Functional lipidomics. Calcium-independent activation of endocannabinoid/endovanilloid lipid signalling in sensory neurons by protein kinases C and A and thrombin. Neuropharmacol, 55,1274–1279. doi: S0028-3908(08)00034-8 [pii] 10.1016/j.neuropharm.2008.01.010 [DOI] [PubMed] [Google Scholar]
- Vermeer LM, Gregory E, Winter MK, McCarson KE, & Berman NE (2014). Exposure to bisphenol A exacerbates migraine-like behaviors in a multibehavior model of rat migraine. Toxicol Sci, 137(2), 416–427. doi: 10.1093/toxsci/kft245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viader A, Blankman JL, Zhong P, Liu X, Schiosburg JE, Joslyn CM, … Cravatt BF (2015). Metabolic interplay between astrocytes and neurons regulates endocannabinoid action. Cell Rep, 12(5), 798–808. doi: 10.1016/j.celrep.2015.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyvoda OS, & Rowe CE (1973). Glyceride lipases in nerve endings of guinea-pig brain and their stimulation by noradrenaline, 5-hydroxytryptamine and adrenaline. Biochem J, 132(2), 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleh NS, Cravatt BF, Apte-Deshpande A, Terao A, & Kilduff TS (2002). Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene, 291 (1–2), 203–210. [DOI] [PubMed] [Google Scholar]
- Wang J, Okamoto Y, Morishita J, Tsuboi K, Miyatake A, & Ueda N (2006). Functional analysis of the purified anandamide-generating phospholipase D as a member of the metallo-beta-lactamase family. J Biol Chem, 281 (18), 12325–12335. doi: 10.1074/jbc.M512359200 [DOI] [PubMed] [Google Scholar]
- Wang X, Horswill JG, Whalley BJ,& Stephens GJ (2011). Effects of the allosteric antagonist l-(4-Chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea (PSNCBAM-1) on CB1 receptor modulation in the cerebellum. Molec Pharmacol, 79(4), 758–767. doi: mol.110.068197 [pii] 10.1124/mol.110.068197 [DOI] [PubMed] [Google Scholar]
- Wilson RI, & Nicoll RA (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature, 410(6828), 588–592. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Irimia C, Cravatt BF, De Vries TJ, Schoffelmeer AN, Pattij T, & Parsons LH (2012). Characterization of the effects of reuptake and hydrolysis inhibition on interstitial endocannabinoid levels in the brain: an in vivo microdialysis study. ACS Chem Neurosci, 3(5), 407–417. doi: 10.1021/cn300036b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson ML, Aisemberg J, Salazar AI, Dominguez Rubio AP, Vercelli CA, & Franchi AM (2013). Progesterone reverts LPS-reduced FAAH activity in murine peripheral blood mononuclear cells by a receptor-mediated fashion. Mol Cell Endocrinol, 381(1–2), 97–105. doi: 10.1016/j.mce.2013.07.020 [DOI] [PubMed] [Google Scholar]
- Won YJ, Puhl HL 3rd, & Ikeda SR (2009). Molecular reconstruction of mGluR5a-mediated endocannabinoid signaling cascade in single rat sympathetic neurons. J Neurosci, 29(43), 13603–13612. doi: 29/43/13603 [pii] 10.1523/JNEUROSCI.2244-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams SG, Wong A, Barrett DA, Bennett AJ, Chapman V, & Alexander SP (2012). Spinal administration of the monoacylglycerol lipase inhibitor JZL184 produces robust inhibitory effects on nociceptive processing and the development of central sensitization in the rat. Brit J Pharmacol 167(8):1609–19.. doi: 10.1111/j.1476-5381.2012.02179.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Di Vizio D, Kirchner M, Steen H, & Freeman MR (2010). Proteome scale characterization of human S-acylated proteins in lipid raft-enriched and non-raft membranes. Mole Cell Proteom 9(1), 54–70. doi: 10.1074/mcp.M800448-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, & Watanabe M (2006). Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci, 26(18), 4740–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, Pedicord D, … Gonzalez-Burgos G (2011). Postsynaptic diacylglycerol lipase alpha mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J Physiol, doi: jphysiol.2011.212225 [pii] 10.1113/jphysiol.2011.212225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, … Xi ZX (2014). Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U SA, 111 (46), E5007–5015. doi: 10.1073/pnas.1413210111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang M, Bisogno T, Di Marzo V, & Alger BE (2011). Endocannabinoids generated by Ca2+ or by metabotropic glutamate receptors appear to arise from different pools of diacylglycerol lipase. PLoS ONE, 6(1), e16305. doi: 10.1371/journal.pone.0016305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, & Levine ES (2014). BDNF-endocannabinoid interactions at neocortical inhibitory synapses require phospholipase C signaling. J Neurophysiol, 111(B), 1008–1015. doi: 10.1152/jn.00554.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Solorzano C, Sahar S, Realini N, Fung E, Sassone-Corsi P, & Piomelli D (2011). Proinflammatory stimuli control N-acylphosphatidylethanolamine-specific phospholipase D expression in macrophages. Molec Pharmacol, 79(4), 786–792. doi: 10.1124/mol.110.070201 [DOI] [PMC free article] [PubMed] [Google Scholar]