Abstract
Adverse childhood experiences have been associated with more negative coupling between the ventromedial prefrontal cortex (vmPFC) and amygdala, a brain network involved in emotion regulation in both children and adults. This pattern may be particularly likely to emerge in individuals exposed to threatening experiences during childhood (e.g., exposure to child abuse), although this has not been examined in prior research. We collected functional magnetic resonance imaging data on 57 adolescents during an emotion regulation task. Greater negative functional connectivity between vmPFC and amygdala occurred during viewing of negative compared to neutral images. This vmPFC-amygdala task-related functional connectivity was more negative in adolescents exposed to physical, sexual, or emotional abuse than those without a history of maltreatment and was associated with abuse severity. This pattern of more negative functional connectivity was associated with higher levels of externalizing psychopathology concurrently and 2 years later. Greater negative connectivity in the vmPFC-amygdala network during passive viewing of negative images may reflect disengagement of regulatory responses from vmPFC in situations eliciting strong amygdala reactivity, potentially due to stronger appraisals of threat in children exposed to early threatening environments. This pattern may be adaptive in the short term but place adolescents at higher risk of psychopathology later in life.
Keywords: child abuse, psychopathology, adolescents, negative emotions, adverse childhood experiences
Adverse childhood experiences are potent risk factors for psychopathology, with approximately one third of all mental disorders in the population associated with exposure to childhood adversity (Cohen, Brown, & Smaile, 2001; Green et al., 2010; McLaughlin et al., 2012). Study of neurodevelopmental changes following adversity may reveal mechanisms which explain this increased risk. A functional brain network encompassing the ventromedial prefrontal cortex (vmPFC) and amygdala plays a role in threat discrimination and emotion regulation (Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Milad & Quirk, 2012). This network has been shown to differ structurally in individuals exposed to childhood adversity (Gold et al., 2016), and individual differences in functional connectivity in this network during an emotional processing task have been associated with anxiety among children exposed to some forms of adversity (Gee, Gabard-Durnam, et al., 2013). Traumatic events experienced early in development that are characterized by a high degree of threat—such as violence exposure—may be particularly likely to influence this circuitry due to its role in threat discrimination (McLaughlin & Lambert, 2017). However, existing studies have not examined the association between threatening events and functional connectivity in this network during emotional processing. Here, we examine whether exposure to child abuse is associated with vmPFC-amygdala functional connectivity during a passive emotional processing task. We further explore whether variation in task-related functional connectivity is associated with psychopathology 2 years later.
Brain Networks Involved in Emotion Processing
The vmPFC is centrally involved in regulating responses to emotional stimuli. The vmPFC serves a regulatory function over emotional responses through projections to the amygdala, including to amygdala subregions which both excite (e.g., central nucleus) and inhibit (e.g., intercalated nuclei) amygdala output (Vertes, 2004). A recent theoretical model argues that the vmPFC is specifically involved in appraising and assigning value to a wide range of internal and external stimuli during emotional processing (see Dixon, Thiruchselvam, Todd, & Christoff, 2017, for review). For example, vmPFC has been implicated in inhibiting fear-related amygdala activity by supporting recall of fear extinction memories (Milad & Quirk, 2012; Phelps, Delgado, Nearing, & LeDoux, 2004). In this context, vmPFC may allow for appraisals of the extinction context as separate from the context in which fear was acquired (Dixon et al., 2017). Negative functional connectivity between vmPFC and amygdala (i.e., increased amygdala activation associated with decreased vmPFC activation or vice versa) has been observed in other types of emotional processing tasks. For example, during passive viewing of threat-related stimuli (e.g., fearful faces), negative functional connectivity emerges around the age of 10 years, with younger children instead showing a pattern of positive connectivity between vmPFC and amygdala (Gee, Humphreys, et al., 2013).
The vmPFC is comprised of functionally distinct subregions (Dixon et al., 2017; Price & Drevets, 2010). Two subregions of vmPFC that are frequently recruited during emotional processing tasks are medial orbitofrontal cortex (mOFC) and subgenual anterior cingulate cortex (sgACC), a small cortical region below the genu of the corpus collosum. These regions are anatomically distinct: mOFC tissue shows higher densities of granular cells than sgACC, and sgACC has a higher density of projections to the amygdala than mOFC (Dixon et al., 2017; Price & Drevets, 2010). While functional differentiation of these areas is an ongoing area of research, a recent theoretical account suggests that they may preferentially make appraisals of the value of specific types of stimuli (Dixon et al., 2017). Specifically, mOFC is thought to play a role in appraising episodic memories and imagined events, whereas sgACC is thought to be involved in appraising visceromotor signals and making predictions about future physiological needs in order to regulate arousal. These areas may work together to coordinate autonomic and neuroendocrine responses given prior experience and current context (Dixon et al., 2017).
Childhood Adversity and Emotion Processing Networks
The function of the vmPFC-amygdala circuit may be altered by exposure to childhood adversity. Exposure to adverse experiences in childhood has been associated with reduced resting-state connectivity between these regions in adolescents (Herringa et al., 2013; Thomason et al., 2015). Some of these changes may be adaptive: a study of resting-state connectivity in adult women previously exposed to threatening early-life experiences showed that increased variability in amygdala-sgACC connectivity over time was associated with improved mood and less blunting of the stress response associated with early adversity (Kaiser et al., 2018). With regard to task-related functional connectivity, childhood institutional rearing has been associated with an earlier shift from positive to negative connectivity between vmPFC and amygdala during passive viewing of threat-related stimuli (fearful faces), such that children exposed to institutionalization exhibit a more mature negative connectivity pattern (Gee, Gabard-Durnam, et al., 2013). Another study examining medial prefrontal cortex (mPFC)-amygdala functional connectivity during a passive viewing task also found differences related to childhood adversity (defined using a composite of family-related adversities not including abuse) but in more dorsal regions of the mPFC (Herringa et al., 2016). In a more complex task combining emotional processing with cognitive control, adolescents exposed to trauma exhibited a reversal of the negative functional connectivity between vmPFC and amygdala and instead exhibited positive connectivity between these regions (Marusak, Martin, Etkin, & Thomason, 2015). Together, existing studies clearly suggest that vmPFC-amygdala connectivity is sensitive to early experience and may develop differently in children who have experienced adversity. However, existing findings differ both in direction of connectivity differences described and in which brain regions were primarily affected. This divergence could be explained by variability across studies in sample age, emotion processing task, and, importantly, the type of adversity examined.
Different forms of adversity may be associated with distinct patterns of function in the vmPFC-amygdala circuit. This possibility is in line with prior work demonstrating distinct neural correlates of different forms of adversity (see McLaughlin & Lambert, 2017; McLaughlin & Sheridan, 2016). Here, we focus on children exposed to abuse—a form of adversity characterized by a high degree of threat. Children exposed to events involving harm or threat of harm may adapt to their environment through alterations to brain systems involved in threat detection and response, including the vmPFC-amygdala circuit (McLaughlin & Lambert, 2017). Children exposed to threatening experiences (e.g., interpersonal violence) show a pattern of increased perceptual sensitivity and behavioral response to potentially threatening stimuli (Heleniak, Jenness, Stoep, McCauley, & McLaughlin, 2016; Shackman & Pollak, 2014) as well as correspondingly increased amygdala reactivity to threat cues (McCrory et al., 2013; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015). Chronic hyperactivity of the amygdala could lead to a more negative pattern of task-related functional connectivity to vmPFC. This pattern has arisen in previously institutionalized children, where amygdala hyperactivity has been theorized to drive early development of structural vmPFC-amygdala connections, resulting in a more mature (i.e., negative) pattern of connectivity at an earlier age (Gee, Gabard-Durnam, et al., 2013). Alternately, negative task-related functional connectivity could arise in response to threatening experiences as part of a pattern of increased response to threat cues characterized by greater amygdala activity coupled with weaker top-down regulatory responding from vmPFC. This latter hypothesis is consistent with existing data showing reductions in resting-state connectivity in this network in children from a normative community sample with higher reported maltreatment experiences (Herringa et al., 2013). Although there are strong theoretical reasons to expect that early experiences of threat may produce developmental adaptations to threat processing involving vmPFC-amygdala circuitry, scant research has examined this question. Here, we extend this literature by examining the association of child abuse—a form of adversity characterized by a high degree of threat—with vmPFC-amygdala functional connectivity during a passive emotional processing task in adolescence.
Disrupted Emotion Processing and Mental Health
Differences in functional connectivity could have important implications for the mental health of adolescents previously exposed to childhood adversity. Prior studies have explored models where functional connectivity in the vmPFC-amygdala network moderates the association of childhood adversity with psychopathology. For example, Gee and colleagues (2013) found that children exposed to early institutionalization who exhibited an earlier shift to “adultlike” negative connectivity between vmPFC and amygdala when viewing fearful faces had reduced levels of separation anxiety, although they still had higher separation anxiety than children who had never experienced adversity. Herringa and colleagues (2016) found that adolescents with prior exposure to family-related adversity and higher levels of internalizing psychopathology showed more negative functional connectivity during an emotion processing task. However, we are unaware of prior work examining the association of vmPFC-amygdala functional connectivity during passive emotional processing with psychopathology in adolescents previously exposed to child abuse. Functional connectivity in this network may reflect altered threat processing and emotion regulation following child abuse, and this alteration—while potentially adaptive when being raised in a threatening environment—may contribute to higher levels of psychopathology later in life. Elevated emotional reactivity and difficulties with emotion regulation have been previously shown to mediate the relationship between child abuse and later psychopathology (Heleniak et al., 2016; J. Kim & Cicchetti, 2010). Given the importance of this circuit to emotional responding, functional connectivity during passive emotional processing may mediate the association between abuse and later psychopathology. This pattern has also been suggested by prior studies examining early-life stress and resting-state functional connectivity in this circuit in adolescents (Burghy et al., 2012; Herringa et al., 2013) as well as studies showing an association between higher levels of anxiety and reduced resting-state functional and structural connectivity in adults (M. J. Kim, Gee, Loucks, Davis, & Whalen, 2011; M. J. Kim & Whalen, 2009).
The present study investigates whether exposure to child abuse influences functional connectivity between the vmPFC and amygdala during a passive emotional processing task among adolescents. In addition, we evaluate whether patterns of task-related vmPFC-amygdala functional connectivity are associated with internalizing and externalizing psychopathology 2 years later and mediate the association of child abuse with later psychopathology. We hypothesized that adolescents who had been exposed to abuse would show a more negative pattern of task-related functional connectivity between vmPFC and amygdala, that this pattern would be associated with higher levels of internalizing and externalizing psychopathology, and that this negative pattern of task-related functional connectivity would mediate the relationship between child abuse and later psychopathology.
Method
Participants
Participants were recruited as part of a longitudinal study of child maltreatment. Initial recruitment took place in neighborhoods with high rates of community violence and poverty in order to recruit a sample with variability in violence exposure. At Wave 2, neuroimaging data were collected on 59 youth including participants with prior exposure to child abuse and controls matched on age, sex, and handedness. Two participants were dropped due to motion. At Wave 3 (mean time to followup = 23.1 months), 49 Wave 2 participants completed a follow-up clinical interview. Participants who did not return for Wave 3 did not differ from those who were assessed at Wave 3 on exposure to adversity, age, sex, or psychopathology (all p > .11). All participants provided informed consent prior to inclusion in the study. Experimental procedures were approved by the institutional review boards of Boston Children’s Hospital and Harvard University. The final sample included 24 adolescents with previous exposure to child abuse and 33 control participants. Sample recruitment details are included in the Online Supplemental Material.
Measures
Child abuse and adverse experiences.
A composite variable assessing exposure to physical, sexual, or emotional abuse was constructed using the Childhood Experiences of Care and Abuse interview (Bifulco, Brown, & Harris, 1994) and the Childhood Trauma Questionnaire (CTQ; Bernstein, Ahluvalia, Pogge, & Handelsman, 1997). The distribution of CTQ abuse scores is described in Table 1. Community violence was assessed as an additional indicator of early threat exposure using the Screen for Adolescent Violence Exposure (Hastings & Kelley, 1997). Parental socioeconomic status (SES) was assessed via parent report of highest educational attainment of either parent. We control for parent education in all analyses examining functional connectivity as a function of child abuse. Details on validity and coding of adversity measures are presented in the Online Supplemental Material.
Table 1.
Sample Characteristics by Group.
| Control (n = 33) | Maltreated (n = 24) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Value | n | % | n | % | n | % | χ2 | p |
| Sex | Male | 14 | 42.4 | 8 | 33.3 | 22 | 38.6 | 0.18 | .67 |
| Female | 19 | 57.6 | 16 | 66.7 | 35 | 61.4 | |||
| Race | White | 12 | 36.4 | 4 | 16.7 | 16 | 28.1 | 6.70 | .15 |
| Black | 9 | 27.3 | 8 | 33.3 | 17 | 29.8 | |||
| Latino | 6 | 18.2 | 5 | 20.8 | 11 | 19.3 | |||
| Asian | 4 | 12.1 | 1 | 4.2 | 5 | 8.8 | |||
| Other | 2 | 6.1 | 6 | 25.0 | 8 | 14.0 | |||
| Parent education | High school or less | 4 | 12.1 | 4 | 16.7 | 8 | 14.0 | 4.71 | .19 |
| Some college | 5 | 15.2 | 8 | 33.3 | 13 | 22.8 | |||
| College degree | 15 | 45.5 | 5 | 20.8 | 20 | 35.1 | |||
| Graduate education | 8 | 24.2 | 7 | 29.2 | 15 | 26.3 | |||
| Continuous measures | M | SD | M | SD | M | SD | t | p |
|---|---|---|---|---|---|---|---|---|
| Age at scan | 17.05 | 1.48 | 16.77 | 1.46 | 16.93 | 1.46 | 0.71 | .482 |
| Physical abuse | 5.27 | 0.76 | 9.88 | 4.73 | 7.21 | 3.84 | −4.72 | <.001** |
| Sexual abuse | 5.00 | 0.00 | 9.67 | 6.04 | 6.97 | 4.52 | −3.79 | .001** |
| Emotional abuse | 6.24 | 1.44 | 12.88 | 4.46 | 9.04 | 4.50 | −7.03 | <.001** |
| Combined abuse | 16.52 | 1.75 | 32.42 | 10.71 | 23.21 | 10.56 | −7.20 | <.001** |
| Community violence | 45.18 | 11.53 | 48.67 | 12.11 | 46.65 | 11.80 | −1.09 | .279 |
| Internalizing (Wave 2) | 14.03 | 8.17 | 25.71 | 12.83 | 18.95 | 11.81 | −3.92 | <.001** |
| Externalizing (Wave 2) | 9.82 | 6.73 | 18.00 | 10.11 | 13.26 | 9.19 | −3.45 | .001** |
| Internalizing (Wave 3) | 12.54 | 10.11 | 22.71 | 9.07 | 16.90 | 10.85 | −3.70 | .001** |
| Externalizing (Wave 3) | 9.25 | 6.28 | 16.48 | 6.48 | 12.35 | 7.26 | −3.92 | <.001** |
p < .05.
p < .001.
Psychopathology.
Psychopathology in Waves 2 and 3 was measured using the Diagnostic Interview Schedule for Children, Version IV (DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). Internalizing and externalizing scores were calculated by summing symptom counts of relevant disorders (see Online Supplemental Material). The DISC-IV is a highly structured clinical interview that assesses a wide range of psychopathology and was administered by trained research assistants.
Emotion Regulation Task
Participants completed a task widely used to measure event-related markers of neural reactivity and regulation in adults and children (Buhle et al., 2014; Ochsner, Silvers, & Buhle, 2012). Participants viewed neutral, negative, and positive images from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2005). Prior to presentation of these pictures, participants were cued to use cognitive strategies to either “look” at the image without trying to modify their emotional response or to regulate their emotional response using cognitive reappraisal strategies they learned prior to the scan (see McLaughlin et al., 2015). Here, we focus only on the look trials, given our interest in automatic or implicit forms of emotion processing in response to negatively valenced stimuli as examined in prior studies on pre-frontal-amygdala circuitry following early-life adversity (Gee, Humphreys, et al., 2013; Herringa et al., 2016). We present information about connectivity during regulation in the Online Supplemental Material. We did not analyze trials where participants viewed positive images. Participants completed 26 trials where they were asked to view negative images and 26 trials where they were asked to view neutral images across 4 scanning runs. Length of emotional stimulus presentation and intertrial interval were jittered to reduce model autocorrelation (see Figure S2).
Image Acquisition and Preprocessing
Scanning was performed on a 3-T Siemens Trio scanner using a 32-channel head coil. Anatomical scans were acquired for co-registration with functional magnetic resonance imaging (fMRI). Blood oxygenation level-dependent (BOLD) signal during functional runs was acquired using a gradient-echo T2*-weighted echo planar imaging sequence.
Preprocessing and analysis steps were implemented within GNU Make (v4.0), a software tool that can be used to create neuroimaging workflows incorporating multiple software packages (Askren et al., 2016). Preprocessing included simultaneous motion and slice timing correction, followed by skull stripping, de-spiking, and smoothing using a 6-mm full-width half-max kernel. High-motion volumes (of one voxel or greater) or volumes where the derivative of variance in BOLD signal (DVARS) across the brain exceeded the upper fence (above 75th percentile + 1.5 × interquartile range) or the change in signal intensity exceeded 3 SD were considered outliers and excluded from analysis by regressing these volumes out of person-level models. No significant differences were found between abused and control participants on any motion parameter (all p < .21). Following estimation of person-level models, the resulting contrast images were registered to standard space of the Montreal Neurological Institute template. Detailed acquisition parameters and preprocessing steps are available in the Online Supplemental Material.
fMRI Analysis
Event-related regressors were created by convolving a boxcar function of phase, duration, and amplitude one with the standard (double-γ) hemodynamic response function for each phase (cue, stimulus, rating) of the task separately for each trial type (look, decrease, and increase) and valence (negative, neutral, positive). A general linear model was constructed for each participant. We examined a contrast designed to isolate neural recruitment related to passively viewing negative relative to neutral images (look negative > look neutral). Individual-level estimates of BOLD activity were submitted to group-level random effects models using FMRIB’s Local Analysis of Mixed Effects (FLAME) 1 from the FMRIB Software Library (FSL; v5.0).
Task-related functional connectivity.
The look negative > look neutral contrast from whole-brain analysis in the entire sample was thresholded at z = 3.72 (p =.001) and interacted with anatomical masks for left and right amygdala defined by the Harvard-Oxford Subcortical Atlas (Desikan et al., 2006; 50% threshold) to construct seed regions for the right and left amygdala. The time series was extracted from these seed regions for each participant. Psychophysiological interaction (PPI) models were constructed by entering the time series extracted from each amygdala seed, an event-related regressor (look negative > look neutral), and their interaction into the person-level models. Covariates were entered for nuisance factors (e.g., frame-wise displacement, physiological noise within ventricles and white matter) as well as task-related events of no interest (e.g., positive stimuli, rating screens, cues). In this model, the interaction term represents an estimate of event-related functional connectivity to the amygdala seed during trials involving negative relative to neutral stimuli, over and above connectivity during other parts of the task (O’Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012). These individual-level estimates of task-related functional connectivity were then submitted to group-level random effects models. Clusters from these models were identified using a threshold of z > 2.32 and corrected for family-wise error using Gaussian random-field theory in FSL at p < .05.
Region of interest (ROI) analysis.
Person-level estimates of task-related functional connectivity were extracted from several functionally defined ROIs. Whole-brain analysis of task-related functional connectivity in the entire sample was used to identify regions of the vmPFC that exhibited significant differences in connectivity with the left and right amygdala, separately, in the look negative relative to look neutral condition. These images were thresholded at z > 2.32 to construct a mask of task-related functional connectivity in the entire sample. We then constructed anatomical masks from two sub-regions of vmPFC, mOFC and sgACC, based on functional parcellations of vmPFC (Dixon et al., 2017). Anatomical masks for mOFC and sgACC were extracted from the Harvard Cortical Atlas regions “Frontal Medial Cortex” and “Frontal Orbital Cortex,” respectively (20% threshold). Each anatomical mask was then interacted with the functional mask to define medial PFC ROIs where we observed significant task-related functional connectivity. This produced four specific ROIs that we use in all further analysis (see Figure 1). To obtain person-level estimates of task-related functional connectivity, we extracted the mean parameter estimate (β) for the interaction of each participant’s amygdala activity with the task parameter of interest (look negative > look neutral) contrasted with baseline activity from first-level models within each ROI. We then used these person-level estimates to examine associations with abuse and future psychopathology.
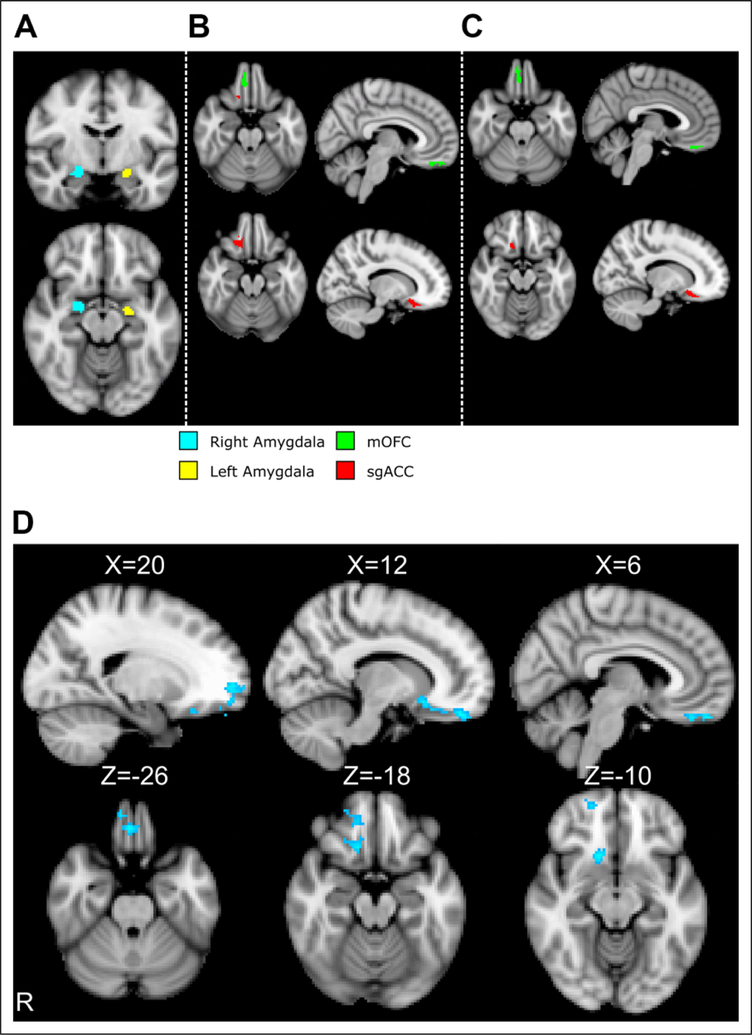
Figure 1.
(A) Seed regions from left and right amygdala entered into psychophysiological interaction analysis. (B) Regions of interest generated for mOFC and sgACC based on connectivity to left amygdala. (C) Regions of interest based on connectivity to right amygdala. A one-voxel spatially outlying cluster was removed from the right amygdala × sgACC mask. (D) Task-related functional connectivity (Look Negative > Look Neutral) to the left amygdala seed, controlling for race and parent education, in the whole sample.
Brain-Behavior Associations
We examined associations of abuse with task-related functional connectivity within each ROI, using both dichotomous measures of exposure to abuse as well as a continuous variable reflecting the severity of abuse. To examine effects of different forms of early adversity, we also examined the association of exposure to community violence and SES on task-related functional connectivity. Effects of dichotomous and continuous measures of exposure were tested using analysis of variance (ANOVA) and linear regression, respectively. We additionally examined associations of task-related functional connectivity with internalizing and externalizing psychopathology assessed concurrently with the fMRI scan (Wave 2) and at the 2-year follow-up (Wave 3), controlling for Wave 2 psychopathology. Finally, mediation was tested in models where direct effects were observed between abuse and psychopathology, abuse and connectivity, and connectivity and psychopathology. Mediation analysis was performed using nonparametric bootstrapping implemented in the “mediation” package in R (Tingley, Yamamoto, Hirose, Keele, & Imai, 2014). All models controlled for age, sex, parental education, and race/ethnicity.
Results
Affect Ratings
A 2 × 2 (Emotional Valence × Abuse Exposure) ANOVA was run on participant’s ratings of their emotional response. Negative images were rated as more emotionally intense than neutral images, F(1, 54) = 418.58, p < .001. Adolescents exposed to child abuse reported affect .26 higher on average than controls across condition, F(1, 48) = 5.74, p =.021. There was no Group × Valence interaction, F(1, 54) = 0.11, p =.74, meaning that abused and control adolescents showed similar differences in their ratings of negative relative to neutral images.
BOLD Response
BOLD response was previously reported in a subsample of 42 participants from the current study, including children exposed to physical and sexual abuse (see McLaughlin et al., 2015). Of relevance to the current study, numerous regions in the salience network were more active in the look negative versus look neutral contrast, including bilateral amygdala, thalamus, anterior insula, putamen, and vmPFC. Maltreated adolescents exhibited greater activation than controls in several of these areas in this contrast, including the bilateral putamen, thalamus, amygdala, and anterior insula.
Task-Related Functional Connectivity During Emotional Processing
In our whole-brain analysis, task-related functional connectivity to left amygdala was more strongly negative during trials involving negative as compared to neutral stimuli in a cluster located in the right vmPFC, encompassing mOFC and sgACC (668 voxels; peak activation at X = 12, Y = 48, Z = −22; z = −3.82, p =.0104; see Figure 1). No clusters survived cluster correction for task-related functional connectivity to the right amygdala. No clusters survived cluster correction testing for maltreatment-related differences in task-related functional connectivity in a whole-brain analysis. All remaining analysis of maltreatment-related group differences focuses on mPFC ROIs.
Average task-related functional connectivity from left and right amygdala to right mOFC and sgACC (see Figure 1) was extracted for ROI analysis. Connectivity to left and right amygdala was negative while viewing emotionally negative as compared to neutral stimuli in both ROIs (all t < −2, p < .039) across the entire sample.
Abuse and Task-Related Functional Connectivity
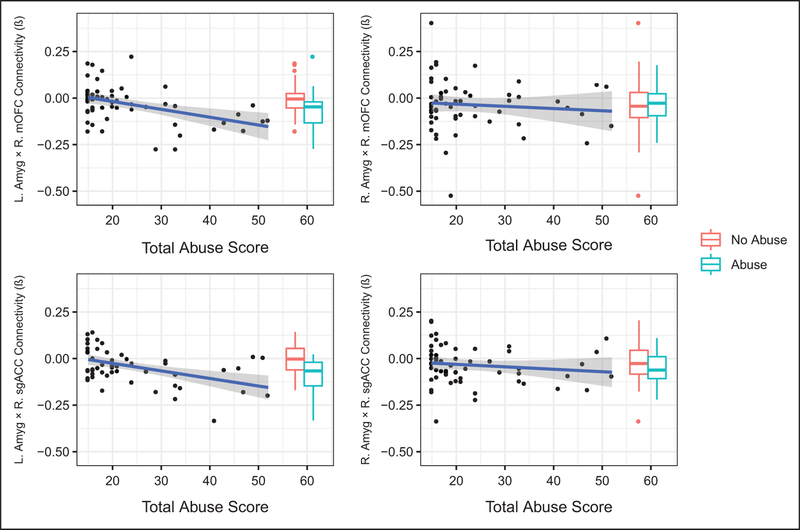
Exposure to abuse was associated with more negative task-related functional connectivity of left amygdala with both vmPFC clusters (mOFC and sgACC) as compared to control participants (see Table 2). A post hoc analysis suggested that this effect was driven both by more negative connectivity in abused adolescents versus controls when viewing negative images (contrasted with baseline) and more positive connectivity in abused adolescents versus controls when viewing neutral images (contrasted with baseline; see Online Supplemental Material for details). Similarly, abuse severity was negatively associated with task-related functional connectivity of left amygdala with both mOFC and sgACC, such that adolescents who had experienced more severe forms of abuse had more negative task-related functional connectivity when viewing negative versus neutral images (see Figure 2). Association between abuse and task-related functional connectivity between left amygdala and sgACC remained significant after controlling for concurrent internalizing and externalizing symptoms (see Online Supplemental Material for details).
Table 2.
Association of Child Abuse and Parental Education With Functional Connectivity.
| Left Amygdala × mOFC | Right Amygdala × mOFC | Left Amygdala × sgACC | Right Amygdala × sgACC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | η2 | p | F | η2 | p | F | η2 | p | F | η2 | p | |
| Community violence | 0.63 | .01 | .431 | 2.08 | .04 | .156 | 1.42 | .03 | .239 | 2.24 | .04 | .141 |
| β | p | β | p | β | p | β | p | |||||
| Community violence severity | −.21 | .297 | −.35 | .075 | −.28 | .145 | −.28 | .152 | ||||
| SES | F | η2 | p | F | η2 | p | F | η2 | p | F | η2 | p |
| Less than college degree | .38 | .006 | .541 | .17 | .003 | .680 | 2.59 | .038 | .114 | .77 | .015 | .384 |
| β | p | β | p | β | p | β | p | |||||
| Parent education | .12 | .382 | −.03 | .834 | .174 | .201 | .09 | .558 | ||||
Note. All models controlled for age, race, and sex. Threat models controlled for parent education and vice versa. mOFC = medial orbitofrontal cortex; sgACC = subgenual anterior cingulate cortex; SES = socioeconomic status.
p < .05.
p < .001.
Figure 2.
Task-related functional connectivity (β) and abuse severity. Task-related functional connectivity by group is also depicted as offset boxplots.
Other Forms of Adversity and Task-Related Functional Connectivity
Tests for association of community violence exposure and SES with task-related functional connectivity were not statistically significant in any of our regions of interest (all p > .075; see Table 2).
Task-Related Functional Connectivity and Psychopathology
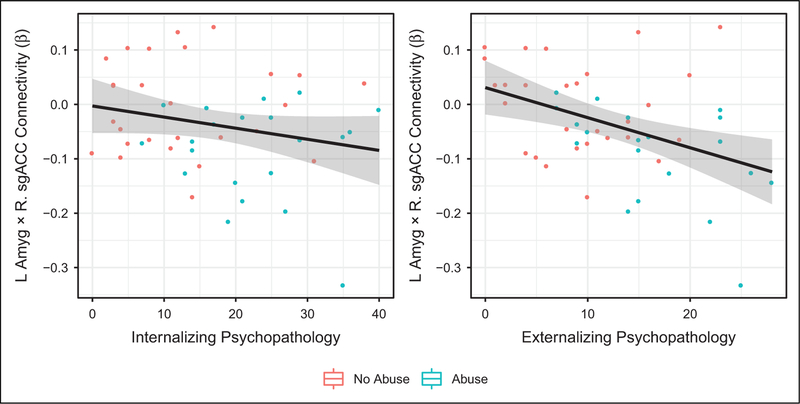
Both exposure to abuse and abuse severity were associated with increased internalizing and externalizing psychopathology concurrently and 2 years after the scan (see Table 3). More negative task-related functional connectivity of left amygdala with both mOFC and sgACC was associated with higher levels of concurrent externalizing psychopathology, and task-related functional connectivity between left amygdala and mOFC was associated with concurrent internalizing symptoms. Functional connectivity between left amygdala and sgACC also predicted externalizing psychopathology 2 years later, after controlling for baseline externalizing symptoms (β = −.322, p =.014; see Table 3; Figure 3). Given these associations, we examined whether sgACC-amygdala task-related functional connectivity mediated the relationship between abuse severity and externalizing psychopathology. No indirect effect of abuse on externalizing psychopathology was detected (p =.39).
Table 3.
Association of Internalizing and Externalizing Psychopathology With Abuse, Abuse Severity, and Functional Connectivity.
| Psychopathology Measure | Abuse Severity | Abuse | FC (L.Amyg. × mOFC) | FC (L.Amyg. × sgACC) | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | F | p | β | p | β | p | |
| Wave 2 psychopathology | ||||||||
| Internalizing | .56** | <.001 | 9.94* | .003 | −.34* | .009 | −.16 | .232 |
| Externalizing | .44** | <.001 | 9.36* | .004 | −.38** | .001 | −.29* | .013 |
| Wave 3 psychopathology | ||||||||
| Internalizing | .38* | .009 | 6.92* | .012 | −.11 | .445 | −.22 | .113 |
| Externalizing | .38* | .008 | 11.06* | .002 | −.20 | .135 | −.44** | .001 |
| Wave 3 psychopathology,controlling for Wave 2 psychopathology | ||||||||
| Internalizing | .07 | .676 | 1.17 | .286 | .15 | .252 | −.01 | .423 |
| Externalizing | .24 | .156 | 4.84* | .034 | −.01 | .950 | −.32* | .014 |
Note. All models controlled for age, race, sex, and parent education. FC = frontal cortex; L.Amyg. = left amygdala; mOFC = medial orbitofrontal cortex; sgACC = subgenual anterior cingulate cortex.
p < .05.
p < .001.
Figure 3.
Task-related functional connectivity (β) and Wave 3 psychopathology.
Discussion
The present study evaluated whether exposure to abuse was associated with functional connectivity between the amygdala and vmPFC during a passive emotional processing task. As expected, we observed more negative task-related functional connectivity between vmPFC and amygdala during passive viewing of negative versus neutral emotional stimuli. This finding is consistent with extensive evidence indicating negative coupling of vmPFC and amygdala in tasks that involve implicit or passive forms of emotional processing and emotion regulation (Etkin, Egner, & Kalisch, 2011; Gee, Humphreys, et al., 2013). This negative connectivity was stronger in youth who experienced abuse and was correlated with abuse severity. The association between connectivity and abuse persisted after adjustment for current psychopathology. More negative task-related functional connectivity was associated with internalizing and externalizing psychopathology concurrently and externalizing psychopathology 2 years later. However, greater negative task-related connectivity did not mediate the association between abuse and later psychopathology. These findings add to a growing body of evidence that early experiences of adversity can alter prefrontal-amygdala circuitry involved in emotion processing.
Task-Related Functional Connectivity in Abused Adolescents
We extend prior work on other forms of adversity by demonstrating that youth with a history of physical, sexual, or emotional abuse—forms of adversity characterized by a high degree of threat—exhibit more negative functional coupling of the vmPFC and amygdala during passive viewing of negative images. More frequent engagement of emotional processing circuitry is a likely consequence of child abuse, due to frequent exposure to threatening environmental circumstances that elicit strong emotional reactions. A similar pattern might also occur in children exposed to institutional rearing, who lack a caregiver to help them modulate arousal and emotional reactivity. Indeed, heightened amygdala reactivity to threat cues has been observed in both previously institutionalized (Gee, Gabard-Durnam, et al., 2013) and abused children (McCrory et al., 2013; McLaughlin et al., 2015). The presence of a caregiver buffers this amygdala reactivity in young children and leads to improved affect regulation (Gee et al., 2014). In children exposed to institutional rearing, a more mature pattern of functional connectivity is observed during childhood, potentially reflecting accelerated development in the absence of a caregiver to buffer amygdala reactivity and promote adaptive emotion regulation (Gee et al., 2013). Differences in prefrontal-amygdala connectivity as a function of institutional rearing disappear by adolescence, however (Gee et al., 2014). In contrast, we observe greater negative task-related functional connectivity between vmPFC and amygdala persisting into late adolescence among youth who were exposed to child abuse.
The persistence of more negative task-related functional connectivity in adolescents exposed to child abuse, but not previous institutionalization, may reflect divergent effects of different forms of adversity across development. Accelerated development of prefrontal-amygdala networks in previously institutionalized children could reflect earlier development of neural connections between vmPFC and amygdala which normally develop later in childhood (producing a negative connectivity pattern between vmPFC and amygdala at an earlier age). These differences in connectivity appear to remit by adolescence as typically developing children undergo the same developmental changes that occurred earlier in previously institutionalized children. In contrast, greater negative connectivity between vmPFC and amygdala (i.e., reduced regulatory response from vmPFC when amygdala activity is high) may reflect an adaptive response to living in an environment characterized by danger. For children exposed to abuse, dampening amygdala response to threat cues may be less adaptive given the legitimate threats present in their early environments (McLaughlin & Lambert, 2017). Given greater amygdala activity in adolescents exposed to child abuse (McLaughlin et al.,2015) and the role of vmPFC in making attributions of the emotional relevance of stimuli given prior experiences (Dixon et al., 2017), negative connectivity in this sample might reflect a reduced tendency to bring vmPFC online to modulate arousal given prior experiences of threat. Although functional parcellations of vmPFC are still in development, this interpretation can also be explained with respect to the specific vmPFC subregions examined. The sgACC may be involved in appraising visceromotor signals and making predictions about future physiological needs in order to regulate arousal given prior experience and current context (Dixon et al., 2017). For children exposed to abuse, stimuli that elicit memories of threat may dampen sgACC modulation of amygdala as these situations may be appraised as ones in which high arousal and physiological resources are required to promote safety (i.e., removing the “brakes” on amygdala reactivity when a perceived threat is present). The mOFC is thought to play a role in appraising relevance and meaning of episodic memories linked to internal and external cues (Dixon et al., 2017). For children exposed to abuse, negative images are likely to rapidly generate memories of past experiences of danger, potentially leading to quick disengagement of vmPFC when amygdala reactivity is high. In contrast to previously institutionalized children, these changes in vmPFC-amygdala connectivity may be an adaptive change that persists into adolescence and beyond.
As both institutionalization and child abuse involve varying degrees of exposure to threat, differences in the age and chronicity of exposure could also explain the persistence of more negative connectivity in our sample into adolescence. Previously institutionalized children in the aforementioned study were placed in high-quality care environments at an average age of 28 months (Gee et al., 2013), whereas adolescents in our sample often experienced child abuse at later ages. Continued differences in connectivity in our adolescent sample may reflect later exposure to abuse and/or greater heterogeneity in the quality of caregiving and environmental experiences during adolescence. For example, adolescents in our study might have more recent fear learning experiences and fewer opportunities for extinction learning than children removed from unsafe environments early in development, resulting in more threatening appraisals of negative images because of the relative recency of their adverse experiences. Future longitudinal studies are needed to examine the effects of exposure to adversity at different points in development and of different types of exposure in order to disentangle these effects.
Our results are also consistent with resting-state studies finding reduced connectivity between amygdala and vmPFC in adolescents who have experienced child abuse (e.g., Herringa et al., 2013; Thomason et al., 2015). Weaker resting-state functional connectivity could reflect a pattern wherein vmPFC and amygdala coactivate less frequently over time in individuals exposed to child abuse, producing weaker structural connectivity between these areas. During exposure to negative stimuli, this could produce disengagement of vmPFC areas in response to amygdala reactivity, producing a more negative pattern of connectivity as shown here.
An alternate explanation for our findings is that more negative connectivity between vmPFC and amygdala reflects an increase in vmPFC regulation of amygdala, although this interpretation seems less plausible here. In this view, abused adolescents could have shown more negative connectivity because they were regulating more than controls in response to stronger baseline reactivity. However, were this the case we would expect to see stronger reactivity from vmPFC when viewing negative versus neutral images in adolescents exposed to abuse—no such differences emerged in this sample (McLaughlin et al., 2015). This interpretation is also less consistent with the resting-state connectivity literature previously discussed. Weaker resting-state connectivity would not be likely to result from a pattern, wherein adolescents who have experienced abuse are recruiting vmPFC more frequently than unexposed adolescents. Instead, this pattern would lead to stronger structural connections between the amygdala and vmPFC, resulting in a more positive pattern of resting-state functional connectivity; this is inconsistent with current evidence suggesting weaker resting-state functional connectivity between these regions (Birn, Patriat, Phillips, Germain, & Herringa, 2014; Herringaetal., 2013; VanderWerffetal., 2013). Nevertheless, future studies should investigate this alternate explanation.
We also examined association of task-related functional connectivity with two other forms of adversity: community violence exposure and low SES. Neither exposure was associated with differences in task-related functional connectivity, although some associations with community violence were present at a trend level. This suggests that threat exposure may need to reach a relatively severe threshold to influence amygdala-vmPFC connectivity. Consistent with conceptual models arguing that distinct forms of childhood adversity may have divergent neurodevelopmental consequences (McLaughlin & Sheridan, 2016), we found no associations of low SES with amygdala-vmPFC connectivity. This interpretation is consistent with studies of less severe forms of family-related adversity, which have not observed differences in amygdala-vmPFC connectivity during a similar emotional processing task (Herringa et al., 2016).
Task-Related Functional Connectivity and Psychopathology
Adolescents with more negative vmPFC-amygdala task-related connectivity had higher levels of internalizing and externalizing psychopathology, regardless of exposure to child abuse. This is consistent with convergent evidence demonstrating increased anxiety and PTSD symptoms associated with structural and functional differences in vmPFC (Herringa et al., 2013; M. J. Kim & Whalen, 2009; Simmons et al., 2008). Our findings suggest that functional connectivity in this network may also be associated with externalizing psychopathology, consistent with models wherein emotion regulation disruptions frequently associated with child abuse represent transdiagnostic risk factors for numerous forms of psychopathology (Hele-niak et al., 2016; McLaughlin & Lambert, 2017). Importantly, task-related functional connectivity was also associated with externalizing symptoms 2 years following the scan even after controlling for concurrent psychopathology, suggesting that individuals with this connectivity pattern were more likely to experience increases in externalizing psychopathology later in adolescence. Interestingly, the association of negative connectivity of vmPFC with amygdala and psychopathology was in a different direction than suggested by studies in previously institutionalized children, who showed lower levels of developmentally normal separation anxiety associated with negative connectivity in this circuit (Gee, Gabard-Durnam, et al.,2013). The protective role of negative vmPFC-amygdala connectivity in previously institutionalized youth may be specific to separation anxiety, a type of anxiety strongly related to children’s relationship with their caregiver. This is consistent with findings that presence of a caregiver is an important moderator of the neural development of this circuit as well as the finding that connectivity reductions in this circuit partially mediate developmentally normal reductions in separation anxiety with age (Gee, Humphreys, et al., 2013).
Although child abuse was associated with task-related functional connectivity and task-related functional connectivity was associated with later psychopathology, task-related functional connectivity did not mediate the relationship between abuse and later psychopathology. Child abuse clearly increases risk of psychopathology through a variety of mechanisms other than task-related functional connectivity between amygdala and vmPFC. These mechanisms may still involve emotion regulation-related brain networks: Although vmPFC-amygdala connectivity has been theorized to be important to specific appraisal processes related to emotion regulation (Dixon et al., 2017), emotion regulation is a complex construct encompassing a variety of explicit and implicit emotion regulation strategies both adaptive (i.e., reappraisal) and maladaptive (i.e., rumination; see Aldao, Nolen-Hoeksema, & Schweizer, 2010, for review). Although emotion regulation has strong support as a transdiagnostic risk factor mediating the relationship between child maltreatment and psychopathology (Heleniak et al.,2016), we would not expect that the vmPFC-amygdala network would be principally involved in all aspects of regulation. Child abuse might create broader disruptions in emotion regulation-related networks in the brain including the vmPFC-amygdala network, but other disruptions may more readily explain the link between child abuse and psychopathology. Child abuse may also effect psychopathology through other mechanisms not necessarily related to emotion regulation (e.g., altered social information processing; Dodge, Bates, & Pettit, 1990; Pollak & Sinha, 2002).
Limitations
Some limitations of the present study should be considered when interpreting our results. Although comparable to other neuroimaging studies of adversity, our sample size was small considering that PPI analysis has reduced power relative to a standard fMRI analysis, particularly in event-related designs such as this one. This increases the likelihood of false negatives in our analysis. Task-related functional connectivity cannot measure direct connectivity or provide evidence for direction of activation. Correlated brain activation may be caused or mediated by activity in other areas. The cluster correction threshold used in the PPI analysis was relatively lenient, which increases the likelihood of false positives. Although data on timing of exposure were collected, high levels of missing data precluded considering them in our analysis. This may obscure important moderators of the described effects. As adolescents do not mature at the same rate, our results may be confounded by systematic differences in pubertal timing between groups. Future studies should incorporate measures of pubertal timing such as tanner stage or hormonal data. Additionally, we assessed psychopathology using symptom counts rather than by formal diagnosis; associations with psychopathology may be affected by variation in symptoms within a normal (adaptive) range. Finally, although our analysis examined only passive viewing trials, there was no way to verify that subjects were not voluntarily using the more active strategies employed in other trials or that they were doing so at different rates between groups. Further research using other imaging and behavioral methods and taking into account time of exposure will be necessary to verify these results.
Conclusion
Adolescents exposed to child abuse exhibit more negative task-related functional connectivity between the vmPFC and amygdala in response to potentially threatening versus neutral stimuli. This pattern could reflect chronic disengagement of regulatory responses from vmPFC in situations of strong amygdala response to perceived environmental threats. This pattern may reflect a greater readiness to make threatening appraisals of environmental stimuli and higher levels of physiological arousal in response to such stimuli. This finding is consistent with theoretical models in which child abuse leads to adaptations that facilitate the rapid identification of threats in the environment, leading to increased emotional reactivity to potentially threatening stimuli and greater difficulty modulating those responses (McLaughlin & Lambert, 2017; McLaughlin et al., 2015). Regardless of abuse history, adolescents who displayed greater negative connectivity in this circuit had higher levels of internalizing and externalizing psychopathology concurrently and externalizing psychopathology at a later point in development. Although we did not find significant indirect effects through vmPFC-amygdala connectivity here, this pathway remains a plausible mechanism linking child abuse with the onset of psychopathology that warrants further investigation in future research.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from the National Institutes of Health (K01-MH092526, K01-MH092555, R01-MH103291, and R01-MH106482), the Brain and Behavior Foundation NARSAD Early Investigator Award, an Early Career Research Fellowship from the Jacobs Foundation, and a Child Health Young Investigator Award from the Charles H. Hood Foundation. These funders provided support for all data collection and analysis. In addition, this research was supported in part by the Intramural Research Program of the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- Aldao A, Nolen-Hoeksema S, & Schweizer S (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30, 217–237. [DOI] [PubMed] [Google Scholar]
- Askren MK, McAllister-Day TK, Koh N, Mestre Z, Dines JN, Korman BA, … Madhyastha TM (2016). Using Make for reproducible and parallel neuroimaging workflow and quality-assurance. Frontiers in Neuroinformatics, 10, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, & Handelsman L (1997). Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 340–348. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, & Harris TO (1994). Childhood Experience of Care and Abuse (CECA): A retrospective interview measure. Journal of Child Psychology and Psychiatry, 35, 1419–1435. [DOI] [PubMed] [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, & Herringa RJ (2014). Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depression and Anxiety, 31, 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex (New York, N.Y.: 1991), 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, … Birn RM (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15, 1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Brown J, & Smaile E (2001). Child abuse and neglect and the development of mental disorders in the general population. Development and Psychopathology, 13, 981–999. [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Thiruchselvam R, Todd R, & Christoff K (2017). Emotion and the prefrontal cortex: An integrative review. Psychological Bulletin, 143, 1033–1081. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, & Pettit GS (1990). Mechanisms in the cycle of violence. Science, 250, 1678–1683. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, & Hirsch J (2006). Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–882. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, 110, 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, … Tottenham N (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25, 2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala-pre-frontal circuitry. The Journal of Neuroscience, 33, 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert HK, Alves S, … McLaughlin KA (2016). Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. Journal of Child Psychology and Psychiatry, 57, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TL, & Kelley ML (1997). Development and validation of the Screen for Adolescent Violence Exposure (SAVE). Journal of Abnormal Child Psychology, 25, 511–520. [DOI] [PubMed] [Google Scholar]
- Heleniak C, Jenness JL, Stoep AV, McCauley E, & McLaughlin KA (2016). Childhood maltreatment exposure and disruptions in emotion regulation: A transdiagnostic pathway to adolescent internalizing and externalizing psychopathology. Cognitive Therapy and Research, 40, 394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, … Essex MJ (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences of the United States of America, 110, 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, & Essex MJ (2016). Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biological Psychiatry:Cognitive Neuroscience and Neuroimaging, 1, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G, … Pizzagalli DA (2018). Childhood stress, grown-up brain networks: Corticolimbic correlates of threat-related early life stress and adult stress response. Psychological Medicine, 48, 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, & Cicchetti D (2010). Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51, 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, & Whalen PJ (2011). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex (New York, NY), 21, 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, & Whalen PJ (2009). The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2005). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: NIMH, Center for the Study of Emotion & Attention. [Google Scholar]
- Marusak HA, Martin KR, Etkin A, & Thomason ME(2015). Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology, 40, 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, Brito SAD, Kelly PA, Bird G, Sebastian CL, Mechelli A, … Viding E (2013). Amygdala activation in maltreated children during pre-attentive emotional processing. The British Journal of Psychiatry, 202, 269–276. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of adolescents. Archives of General Psychiatry, 69, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Lambert HK (2017). Child trauma exposure and psychopathology: Mechanisms of risk and resilience. Current Opinion in Psychology, 14, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Sheridan MA (2016). Beyond cumulative risk: A dimensional approach to childhood adversity. Current Directions in Psychological Science, 25, 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2012). Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology, 63, 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, & Johansen-Berg H (2012). Tools of the trade: Psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7, 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, & LeDoux JE (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron, 43, 897–905. [DOI] [PubMed] [Google Scholar]
- Pollak SD, & Sinha P (2002). Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology, 38, 784–791. [DOI] [PubMed] [Google Scholar]
- Price JL, & Drevets WC (2010). Neurocircuitry of Mood Disorders. Neuropsychopharmacology, 35, 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman JE, & Pollak SD (2014). Impact of physical maltreatment on the regulation of negative affect and aggression. Development and Psychopathology, 26, 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 28–38. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Feinstein JS, Hitchcock C, Paulus MP, & Stein MB (2008). Anxiety vulnerability is associated with altered anterior cingulate response to an affective appraisal task. Neuroreport, 19, 1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, & Rosenberg DR (2015). Altered amygdala connectivity in urban youth exposed to trauma. Social Cognitive and Affective Neuroscience, 10, 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, & Imai K (2014). Mediation: R Package for Causal Mediation Analysis. Journal of Statistical Software, 59, 1–38.26917999 [Google Scholar]
- Van der Werff SJA, Pannekoek JN, Veer IM, van Tol M-J, Aleman A, Veltman DJ, … van der Wee NJA (2013). Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychological Medicine, 43, 1825–1836. [DOI] [PubMed] [Google Scholar]
- Vertes RP (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51, 32–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.