Abstract
Hydralazine, an antihypertensive agent used during pregnancy, undergoes N-acetylation primarily via N-acetyltransferase 2 (NAT2) to form 3-methyl-1,2,4-triazolo[3,4-a]phthalazine (MTP). To characterize the steady-state pharmacokinetics (PK) of hydralazine during pregnancy and evaluate the effects of NAT2 genotype on hydralazine and MTP PK during pregnancy, 12 pregnant subjects received oral hydralazine (5–25 mg every 6 hours) in mid- (n=5) and/or late-pregnancy (n=8). Serial blood samples were collected over one dosing interval, and steady-state non-compartmental PK parameters were estimated. Subjects were classified as either rapid acetylators (RA, n=6) or slow acetylators (SA, n=6) based on NAT2 genotype. During pregnancy, when compared to the SA group, the RA group had faster weight-adjusted hydralazine apparent oral clearance (70.0 ± 13.6 vs 20.1 ± 6.9 L/h, p < .05), lower dose-normalized area under the concentration-time curve (AUC) (1.5 ± 0.8 vs 5.9 ± 3.7 ng*h/mL, p < .05), lower dose-normalized peak concentrations (0.77 ± 0.51 vs 4.04 ± 3.18 ng/mL, p < .05), and larger weight-adjusted apparent oral volume of distribution (302 ± 112 vs 116 ± 45 L/kg, p < .05). Furthermore, the MTP/hydralazine AUC ratio was ~10-fold higher in the RA group (78 ± 30 vs 8 ± 3, p < .05) than in the SA group. No gestational age or dose-dependent effects were observed, possibly due to the small sample size. This study describes for the first time, the PK of oral hydralazine and its metabolite, MTP, during pregnancy, and confirmed that the PK of oral hydralazine is NAT2 genotype dependent during pregnancy.
Keywords: hydralazine; 3-methyl-1,2,4-triazolo[3,4-a]phthalazine; pregnancy; NAT2; hypertension; pharmacokinetics
Introduction
Hypertensive disorders during pregnancy include gestational hypertension, chronic hypertension, preeclampsia, and chronic hypertension with superimposed preeclampsia. These disorders have been on the rise over the past two decades and affect up to 10% of pregnant women1–3. Some of the complications associated with severe hypertensive disorders in pregnancy includes placental abruption, kidney injury, liver injury, cerebral hemorrhage, seizures, fetal growth restriction, preterm birth, and in some cases maternal or fetal demise4. Treatment initiation criteria and hypertension management approaches vary4–7. Among other drugs, hydralazine is one of the antihypertensive drugs recommended by The American College of Obstetricians and Gynecologists for pregnant women due to its efficacy in lowering total peripheral resistance (TPR) and relative safety for the mother and fetus4.
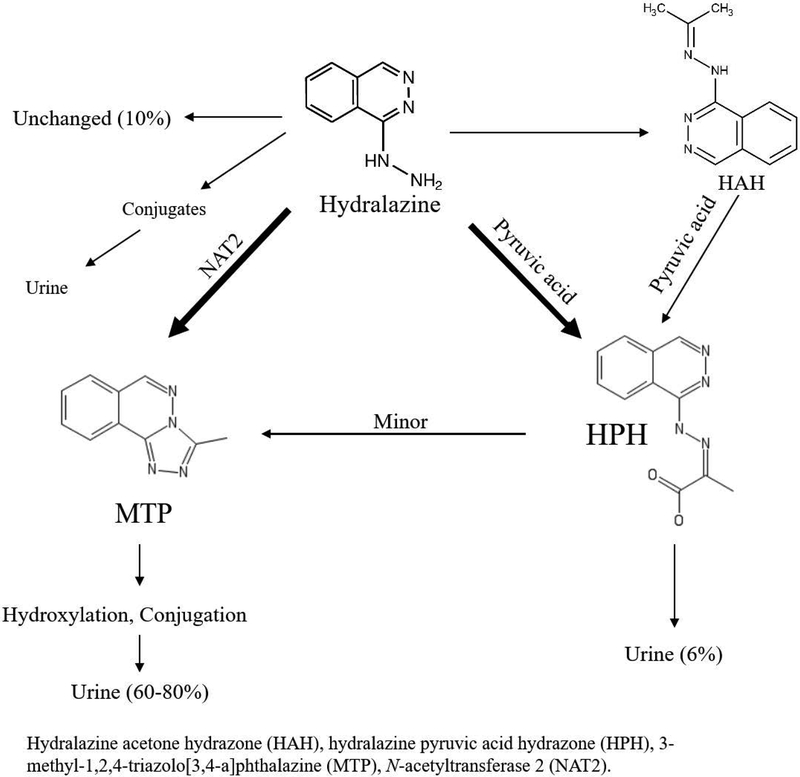
The parent compound, hydralazine, is responsible for the significant decrease in systemic vascular resistance, mean arterial pressure, compensatory increase in cardiac output, and stroke volume8. It is extensively metabolized and only 10% of unchanged drug is recovered in the urine9. Hydralazine is highly protein bound and unstable in blood under physiological conditions10. The predominate circulating form (>90%) is the acid-labile inactive metabolite hydralazine pyruvic acid hydrazone (HPH)11–13. However, only 6% of an oral dose is recovered in the urine as HPH14. In addition, hydralazine readily forms another acetone hydrazone (HAH) that is reportedly equipotent as the parent compound15,16. Iwaki et al. reported that HAH is capable of reacting with pyruvic acid and forming HPH17. Despite being pharmacologically active, HAH does not circulate abundantly due to its instability in vivo and thus generally considered a minor contributor to the overall anti-hypertensive effect9,17,18. Descriptions of hydralazine PK have varied over time due to lack of specificity of early hydralazine assays, which failed to avoid the acid-catalyzed hydrolysis of HPH to release hydralazine during derivatization reactions; as a result, earlier quantification of plasma hydralazine concentrations were, in reality, a combination of the parent compound and the acid-labile hydrazones11,12. This was later referred to as “apparent” hydralazine. The majority of the dose was recovered in the urine as the inactive sequential metabolites of 3-methyl-1,2,4-triazolo[3,4-a]phthalazine (MTP)9. MTP formation occurs via N-acetyltransferase 2 (NAT2)19,20. MTP is further hydroxylated and eliminated as glucuronide conjugates. A summary of the metabolic pathway schematic is shown in Figure 1. NAT2 is a genetically determined polymorphic enzyme. In non-pregnant subjects, hydralazine plasma concentrations reportedly varied up to 15-fold between slow and rapid acetylator phenotypes21–25. Higher frequency of hydralazine induced adverse effects, including systemic lupus erythematosus, has been reported in non-pregnant NAT2 slow acetylators compared to rapid acetylators22. In the same study, NAT2 rapid acetylators failed to achieve adequate blood pressure reduction with hydralazine22. Both increased risk of adverse effects in slow acetylators and reduced efficacy in rapid acetylators suggest a significant role of NAT2 genotype in hydralazine PK and variation in response.
Figure 1 –
Hydralazine metabolic pathway.
As a result of NAT2-mediated polymorphic first-pass metabolism, the systemic oral bioavailability of parent drug is ~16% for rapid acetylators and ~35% for slow acetylators in non-pregnant individuals26. This difference in first-pass metabolism can be explained by genetically determined differential expression of NAT2 in the intestines and liver27–29. In non-pregnant adults, the peak plasma concentration of hydralazine occurs within an hour after dose, and the elimination half-life typically ranges from 1 to 4 hours13,30,31.
Earlier studies of hydralazine exposure during gestation were limited to blood samples collected at the time of delivery or postpartum32,33. These studies demonstrated trans-placental fetal exposure to hydralazine with mean umbilical cord-to-maternal plasma concentration ratio of 2 ± 2.5 at the time of birth. Postpartum studies have reported the presence of hydralazine in the breast milk with mean breast milk-to-maternal plasma concentration ratio of 0.8 ± 0.532,33. Rigorous intensive sampling PK studies of hydralazine and its metabolites during pregnancy have not been reported. Although the impact of NAT2 genotype on hydralazine PK has been studied in the non-pregnant population, little is known regarding the effect of NAT2 on hydralazine and its metabolites during pregnancy. The main objective of our study was to describe the PK of hydralazine during pregnancy and evaluate the effects of NAT2 genotype on steady-state PK of hydralazine and its metabolite MTP during pregnancy.
Materials and Methods
Subjects.
The study was approved by the institutional review board at the University of Washington and was conducted in accordance with its guidelines. Written informed consent was obtained from all subjects. We examined steady-state, whole blood hydralazine and plasma MTP PK in 12 pregnant women receiving hydralazine for therapeutic reasons. Women who had hematocrits less than 28% were excluded from the study.
Dosing regimen.
Oral hydralazine hydrochloride (5 – 25 mg four times daily) was prescribed to women with hypertension, and dosages titrated according to clinical need without regard to the study. As previously described, a validated, noninvasive, Doppler technique (Ultracom Cardiac Output Monitor, Laurence Medical, Redmond WA) was utilized to measure hemodynamics prior to and during oral hydralazine treatment for clinical titration of antihypertensive agents34. Hemodynamic measurements were collected for clinical purposes. The timing relative to dosing was variable and not specified. The oral hydralazine tablets [Teva Pharmaceutical Industries (Petah Tikva, Israel)] were provided by the investigators for the 3 days prior to the study day and dosing times were recorded on a calendar during this time period. Calendar checks and pill counts were conducted to assess adherence. Hydralazine was taken every 6 hours for the 3 days prior to the study. As food is known to increase absorption of hydralazine35–39, subjects were asked to fast for at least 5 hours prior to study dose administration on the day of the PK study, and for another hour post-dosing to minimize food interaction effects. Participants were encouraged to drink clear liquids during the fasting period.
Sample Collection and Analysis.
Serial blood samples were collected from an indwelling venous catheter over one dosing interval (pre-dose, then 0.5, 1, 1.5, 2, 3, 4 and 6 hours after dosing) to determine hydralazine and MTP steady-state concentrations. Five subjects completed mid- (22–26 weeks gestation) and 8 subjects completed late-pregnancy (30–38 weeks gestation) study days. Of these participants, one subject completed both mid- and late-pregnancy studies. Concomitant medications were also recorded. One subject’s samples had to be collected over three dosing intervals due to the subject’s clinical situation, but maintained every 6 hour dosing throughout the study period.
Semple et al. previously reported an HPLC-UV assay for hydralazine in blood as its stable p-nitrobenzaldehyde hydrazone with the distinct advantages of avoiding hydrolysis of acid-labile hydrazone metabolites by achieving rapid and quantitative derivatization at physiological pH, and having a stable hydrazine derivative that allowed derivatized blood samples to be stored at −80 ℃ for long periods of time pending further processing and analysis40. We improved the specificity and sensitivity of the assay by deploying mass spectrometric detection. Furthermore, all blood samples were derivatized immediately upon their collection to minimize instability of hydralazine in blood. The N-acetylated metabolite MTP in plasma was analyzed by adapting the procedure described by Reece et al. to an LC-MS/MS method. Handling, processing and analysis of blood and plasma samples for the respective measurement of hydralazine and MTP are briefly described below41.
Whole blood was collected in EDTA vacutainer tubes, which stabilizes the chemical integrity of hydralazine, and placed into wet ice. Immediately after collection, 1 mL of the whole blood was added to a 15 mL polypropylene centrifuge tube containing 1 mL of 0.1 M sodium citrate (pH 6) and 75 μL of 0.1 mg/mL p-nitrobenzaldehyde in dioxane40. Duplicate tubes were prepared. Internal standard (40 ng 1-hydrazino-4-methyl phthalazine in acidified methanol) was added to each tube. The samples were then vortexed vigorously, placed on a horizontal rocker-shaker and mixed gently at room temperature for 45 min. The samples were then immediately frozen on dry ice. Samples were stored at −80° C for 4–14 months for 9 subjects and 2.5–3 years for 4 subjects until extraction and analysis. Stability testing over two years showed minimal to no degradation. Remaining whole blood was centrifuged at 1500 × g for 5 min at 4° C. Calibration and quality assurance samples were prepared by spiking plasma with MTP (5 – 250 ng/mL) and treating in the same fashion as a subject sample.
Buccal swab samples were collected from each subject and frozen at −80° C for 2–6 years until DNA extraction and analysis.
Sample Extraction.
For hydralazine, 5 mL of 98.5:1.5 heptane:isoamyl alcohol was added to each calibration, quality assurance and subject sample. They were placed on a reciprocating shaker set at 250 cycles per minute for 20 minutes at room temperature, and centrifuged at 2000 × g for 5 minutes. Tubes were then frozen on dry ice, and organic layer decanted to 12 × 75 mm culture tubes. Another 3 mL of 98.5:1.5 heptane:isoamyl alcohol was added to the frozen aqueous layer, allowed to thaw either at room temperature or in a tepid water bath, and the extraction procedure was repeated. Combined heptane:isoamyl alcohol extracts were evaporated under a gentle stream of air at 35–40° C, and reconstituted in 100 μL acetonitrile. Reconstituted extracts were transferred to 96-well plate, and 2 μL was injected onto the high-performance liquid chromatography (LC) system with mass spectrometry (MS) detection.
For MTP, internal standard (40 ng 3-trifluoromethyl-s-triazolo[3,4α]phthalazine or MTP-F3) in 0.25 mL of 0.1 M borate buffer (pH 8.9) and 3 mL ethyl acetate was added to calibration, quality assurance, and subject samples. Samples were extracted in a similar manner as with hydralazine, but the residue reconstituted in 125 μL of 50:50 0.1% formic acid in water:methanol. The extract was transferred to a 0.5 mL micro-centrifuge tube and centrifuged at 14,000 × g for 10 minutes at 4° C. The extract was then transferred to 96-well plate, and 2 μL was injected onto the LC-MS/MS.
Whole Blood Hydralazine and Plasma MTP LC-MS Analysis.
Hydralazine concentrations and internal standards were detected and quantified as p-nitrobenzaldehyde hydrazones, and analyzed using an AB Sciex QTRAP 6500 LC-MS/MS (Concord, ON Canada). HPLC separation was achieved on an Agilent SB-Phenyl column (150mm × 2.1mm × 5 μm; Agilent Technologies, Santa Clara, CA) maintained at 30° C. The mobile phase consisted of A) 30% 20 mM ammonium formate (pH 3.3), and B) 70% methanol delivered isocratically in a proportion of 30:70; flow rate was set at 0.3 mL/min. Total run time was 6 minutes, and sample tray was kept at 4° C. The mass spectrometer was operated in positive API-ES mode. Multiple reaction monitoring transitions for hydralazine and internal standard (1-hydrazino-4-methyl phthalazine) were m/z 294.0 > 145.1 and m/z 308.2 > 158.9, respectively. Mass spectrometer conditions were optimized to the following conditions: 350° C drying gas temperature, 100° C quadrupole temperature, 10 L/min nitrogen drying gas flow rate, 35 psi nebulizer pressure, 3200 V capillary voltage. Fragmentor voltage was 135 V for hydralazine and 125 V for the internal standard. Collision energy was 20 V for hydralazine and 24 V for the internal standard. Dwell time was 100 msec for both. Retention time for hydralazine was 3.7 min and for internal standard was 4.0 min. The relationship between peak area ratio for hydralazine to 1-hydrazino-4-methyl phthalazine and hydralazine concentration was analyzed by second order polynomial regression that included origin and 1/x weighting to determine the respective coefficients of the calibration curve. The limit of detection was 0.02 ng/mL and limit of quantification was 0.04 ng/mL. The coefficient of variance for both were under 20% at respective signal-to-noise ratio of 24:1 and 49.2:1.
For MTP LC-MS/MS analysis, an Agilent Technologies (Santa Clara, CA) 1100 series HPLC coupled to a model G1946D mass spectrometer was used. Agilent Chemstation (version B.03.02) was used for instrument control. HPLC was performed on an Advanced Chromatography Technologies (Aberdeen, Scotland) ACE C8 column (150 mm × 2.1 mm × 3 μm) maintained at 3° C. The mobile phase consisted of A) 0.1% formic acid in water and B) methanol delivered isocratically at a 50:50 proportion. The flow rate was 0.25 mL/min, and the autosampler tray was kept at 4° C. Total run time was 8.5 min. The mass spectrometer was operated in positive electrospray mode and selected ion monitoring (SIM) was used in unit resolution. The transition ions monitored for MTP and its internal standard (MTP-F3) were m/z 185.0 and m/z 239.0, respectively. Mass spectrometer conditions were identical to that of hydralazine analysis method, except that capillary voltage was 1000 V. The fragmentor voltage for MTP was 160 V and for internal standard was 60 V. Dwell time was 289 msec for both. Retention time for MTP was 3.1 min, and for MTP-F3 was 5.1 min. Quantification of MTP was performed in the same fashion as hydralazine. Limit of detection and quantification for MTP was 1.5 ng/mL, with coefficient of variance under 5% at respective signal-to-noise ratio of 6.6:1 and 19.8:1.
NAT2 Genotyping Analysis and Inferred Phenotype.
Buccal cell DNA was isolated using QIAGEN DNeasy Blood & Tissue Kit (Hilden, Germany) according to manufacturer’s instructions. Single nucleotide polymorphisms (SNP) in the NAT2 coding region and their corresponding haplotypes were determined using four-SNP assays, i.e., rs181280, c.341T>C; rs1799930, c.590G>A; rs1799931, c.857G>A; rs1801279, c.191G>A, with TaqMan Assays from Thermo Fisher Scientific (Waltham, MA). Subjects were classified as slow acetylators (SA) if they had 2 reduced-activity NAT2 alleles (*5, *6, *7, or *14), or rapid acetylators (RA) if they carried 1 reduced-activity allele and 1 fully functional allele (*4), or 2 fully functional alleles42.
Pharmacokinetic Analysis.
Non-compartmental, steady-state PK parameters were estimated using Phoenix WinNonlin (Princeton, NJ). Steady-state area under the concentration-time curve (AUCτss) was calculated using linear trapezoidal method for ascending concentrations and log trapezoidal method for descending concentrations over one dosing interval (0–6 hours). Apparent oral clearance was determined by CL/F = dose/AUCτss and apparent oral volume of distribution by Vz/F = (CL/F)/kel, in which kel was the elimination rate constant estimated using log-linear regression of terminal decline in concentration and F was bioavailability. The terminal elimination half-life was determined by t1/2,z = ln(2)/kel. Tmax and Cmax were determined directly from the concentration-time profiles. The metabolite to parent area under the concentration-time curve ratio was calculated using AUCτss(MTP)/AUCτss(hydralazine). Actual body weight for each participant was used for weight adjusted parameter estimates. For comparison purposes, AUC and Cmax were dose-normalized to 10-mg every 6 hours. For one subject with a measured concentration just below the limit of quantification, group PK analyses were performed including and excluding the data point. Because inclusion of the measured concentration below limit of quantification did not alter group PK means, we included this data point in the final analysis.
Statistical Analysis.
Mann-Whitney U tests were performed to compare PK parameter estimates between RAs and SAs. Linear regression with multiple variables was used to analyze the relationship between PK estimates and the subjects’ NAT2 status, gestational age and dose. Only one subject had repeat measurements in mid- and late- pregnancy. Her PK estimates were considered independently for gestational age-dependent PK comparisons. In addition, the average of both of her mid- and late- pregnancy PK estimates was used for NAT2 genotype-dependent PK comparison. All data analyses were performed with GraphPad Prism (La Jolla, CA) and R43. Results are reported as means ± SD unless otherwise stated, and p < .05 was considered significant.
Results
A total of 13 pregnant women consented, but only 12 (7 non-Hispanic White, 3 Asian, and 2 Native Hawaiian/other Pacific Islander) completed at least 1 study day. Among the 12 subjects, 6 were identified as RAs (NAT2 genotypes *4/*5 in 2, *4/*6 in 3, and *4/*7 in 1) and 6 were identified as SAs (NAT2 genotypes *5/*5 in 1, *5/*6 in 2, *5/*7 in 1, and *6/*6 in 2). Five subjects were mid-pregnancy at the time of study, and eight were late-pregnancy. Table 1 presents the demographics for subjects studied in mid- and late-pregnancy. There were no significant differences in age, height, weight, or wrist circumferences between RAs and SAs. There was no significant difference between mid-pregnancy and late-pregnancy in body weight (122 ± 41 kg vs 108 ± 38 kg) or age (32 ± 5.9 yrs vs 35 ± 8.3 yrs). The average height, weight and wrist circumference of all subjects were 163 ± 6 cm, 113 ± 38 kg and 6.3 ± 0.8 inches, respectively. The hydralazine dosage ranged from 5 to 25 mg orally every 6 hours. There was no statistical difference between doses taken by RAs versus SAs (10.7 ± 6.7 mg vs 17.5 ± 8.2 mg, p = .1). All subjects were also taking atenolol as a part of their hypertension management. Similarly, there was no difference in atenolol dosage taken by RAs compared to SAs, when considered alone or in combination with hydralazine dose (p = .7 and p = 1, respectively) (see Table 1 for doses). None of the concomitant medication or supplements taken by the subjects had known drug interactions with hydralazine PK, and a list of concomitant medications are included in Supplemental Table 1. No significant difference was seen based on acetylator status in total peripheral resistance at baseline (RA: 1074 ± 192 dyne•sec•cm−5 vs SA: 1232 ± 253 dyne•sec•cm−5, p = .3) or with hydralazine (RA: 949 ± 237 dyne•sec•cm−5 vs SA: 1209 ± 295 dyne•sec•cm−5, p = .1). There was also no difference in the change in TPR with hydralazine (RA: −125 ± 272 dyne•sec•cm−5 vs SA: −23 ± 130 dyne•sec•cm−5, p = .5). No significant difference was seen based on acetylator status in mean arterial pressure (MAP) at baseline (RA: 101 ± 9 mm Hg vs SA: 100 ± 8 mm Hg, p = .8) or with hydralazine (RA: 92 ± 6 mm Hg vs SA: 99 ± 10 mm Hg, p = .2). There was also no difference in change in MAP with hydralazine (RA: −9 ± 12 mm Hg vs SA: −1 ± 9 mm Hg, p = .3). No difference was observed based on acetylator status in heart rate (HR) at baseline (RA: 77 ± 4 BPM vs SA: 73 ± 8 BPM, p = .4) or with hydralazine (RA: 69 ± 9 BPM vs SA: 77 ± 6 BPM, p = .1). There was also no significant difference in change in HR with hydralazine (RA: −8 ± 10 BPM vs SA: 5 ± 13 BPM, p = .1).
Table 1.
Subject Characteristics.
| Weight | Height | Wrist Circ. | NAT2 Inferred Phenotype1 | Hydralazine dose every 6 hours (mg) |
Atenolol dose twice daily (mg) |
|
|---|---|---|---|---|---|---|
| (kg) | (cm) | (inch) | ||||
| Mid-pregnancy (22–26 weeks) | 102.8 | 161.0 | 6.4 | Slow | 25 | 50 |
| 183.4 | 168.5 | 6.6 | Rapid | 10 | 25 | |
| 75.0 | 160.0 | 6.0 | Rapid | 10 | 25 | |
| 113.3 | 166.5 | 6.5 | Slow | 10 | 50 | |
| 133.9 | 173.3 | 8.0 | Slow | 10 | 25 | |
| Mean (SD) | 121.7 (40.5) | 165.9 (5.5) | 6.7 (0.8) | 13.0 (6.7) | 35 (14) | |
| Late-pregnancy (30–38 weeks) | 87.8 | 157.7 | 6.5 | Rapid | 10 | 100 |
| 92.4 | 163.9 | 5.9 | Slow | 25 | 12.5 | |
| 114.8 | 163.1 | 6.9 | Rapid | 5 | 12.5 | |
| 79.5 | 151.5 | 6.1 | Rapid | 25 | 25 | |
| 77.0 | 160.0 | 4.5 | Slow | 25 | 25 | |
| 192.6 | 167.0 | 6.6 | Rapid | 10 | 25 | |
| 118.8 | 173.3 | 7.0 | Rapid | 5 | 25 | |
| 98.8 | 162.4 | 6.8 | Slow | 10 | 12.5 | |
| Mean (SD) | 107.7 (37.5) | 162.4 (6.4) | 6.3 (0.8) | 14.4 (9.0) | 30 (30) |
Rapid acetylators consists of subjects with genotypes *4/*5, *4/*6, or *4/*7; slow acetylators consists of subjects with genotypes *5/*5, *5/*6, *5/*7, or *6/*6.
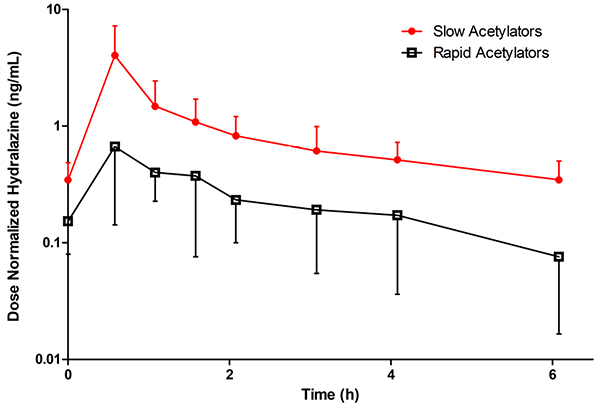
Mean steady-state, concentration-time profiles for hydralazine comparing RAs and SAs are depicted in Figure 2, and all PK parameter estimates are summarized in Table 2. In SAs, mean hydralazine dose-normalized AUCτss was 408% larger (p < .05), dose-normalized Cmax 525% higher (p < .05), weight-adjusted CL/F 71% slower (p < .05), and weight-adjusted Vz/F was 68% smaller (p < .05) than in RAs.
Figure 2 –
Mean (± SD), dose normalized, steady-state, whole blood oral hydralazine concentration versus time profiles for N-acetyltransferase 2 rapid (NAT2 genotypes *4/*5, *4/*5, *4/*6, *4/*6, *4/*6, *4/*7) and slow (NAT2 genotypes *5/*5, *5/*6, *5/*6, *5/*7, *6/*6, *6/*6) acetylators in pregnant women. Concentrations were dose normalized to oral hydralazine 10 mg every 6 hours. Closed circles and red line indicate slow acetylators. Open squares and black line indicate rapid acetylators.
Table 2.
Estimated whole blood hydralazine and plasma MTP steady-state pharmacokinetic parameters throughout gestation in subjects treated with 5 – 25 mg of oral hydralazine every 6 hours.
| Parameter | Hydralazine | MTP | ||
|---|---|---|---|---|
| Rapid Acetylators (n = 6) | Slow Acetylators (n = 6) | Rapid Acetylators (n = 6) | Slow Acetylators (n = 6) | |
| AUCdose-normalized (ng•h/mL) | 1.5 ± 0.8 | 5.9 ± 3.7 (p < .05) | 118.1 ± 64.9 | 56.4 ± 40.7 (p < .05) |
| Tmax (h) | 0.8 ± 0.4 | 0.6 ± 0.1 (N.S.) | 1.2 ± 0.6 | 0.7 ± 0.2 (N.S.) |
| Cmax, dose-normalized (ng/mL) | 0.8 ± 0.5 | 4.0 ± 3.2 (p < .05) | 32.3 ± 11.7 | 15.0 ± 8.8 (p < .05) |
| CL/F (L/h) | 8999 ± 4981 | 2129 ± 883 (p < .05) | N.A. | N.A. |
| CL/F (L/h/kg) | 70.01 ± 13.55 | 20.1 ± 6.9 (p < .05) | N.A. | N.A. |
| Vz/F (L) | 39483 ± 26369 | 12617 ± 6353 (p < .05) | N.A. | N.A. |
| Vz/F (L/kg) | 302 ± 112 | 116 ± 45 (p < .05) | N.A. | N.A. |
| Elimination half-life (h) | 2.96 ± 0.92 | 4.0 ± 1.1 (N.S.) | 2.6 ± 1.1 | 3.2 ± 1.4 (N.S.) |
| Elimination rate constant (1/h) | 0.3 ± 0.1 | 0.2 ± 0.1 (N.S.) | 0.3 ± 0.1 | 0.3 ± 0.1 (N.S.) |
| MTP/hydralazine AUC ratio | 78 ± 30 | 8 ± 3 (p < .05) | ||
Estimated pharmacokinetic parameters compared between NAT2 rapid and slow acetylators. Area under the concentration-time curve (AUCdose-normalized) and maximal concentration (Cmax, dose-normalized) are dose-normalized to 10 mg for comparison purposes. CL/F=apparent oral clearance, Vz/F=apparent oral volume of distribution, Tmax=time to peak concentration, Cmax=peak concentration, N.S. = not significant and N.A. = not applicable, Data reported as mean ± SD.
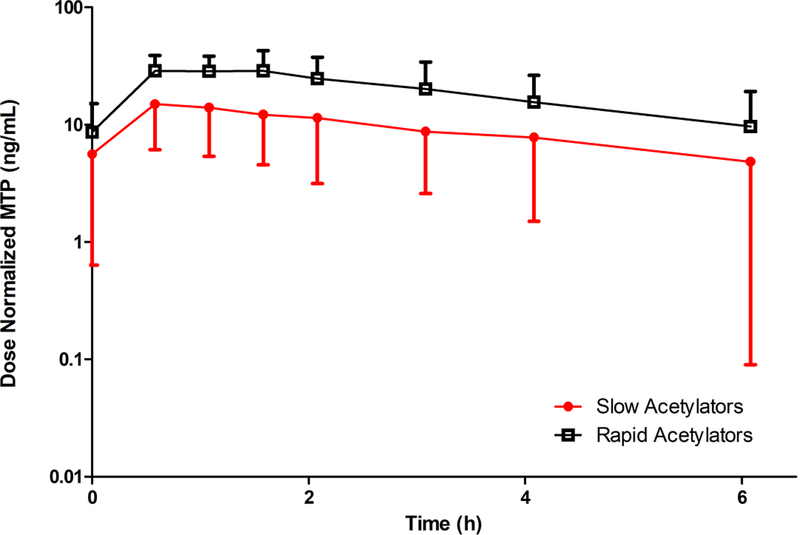
Figure 3 depicts the mean, dose-normalized, steady-state plasma MTP concentration-time profiles for RAs and SAs. In SAs, mean MTP dose-normalized Cmax was 53% lower (p < .05), and steady-state, dose-normalized AUC was 52% smaller (p < .05) than in RAs. Mean MTP/hydralazine AUC ratio among the SAs was 10% that of the RAs (p < .05). Terminal half-life was similar in SAs and RAs (p = .6), and comparable to that of parent hydralazine.
Figure 3 –
Mean (± SD), dose normalized, steady-state, plasma MTP concentration versus time profiles for N-acetyltransferase 2 rapid (NAT2 genotypes *4/*5, *4/*5, *4/*6, *4/*6, *4/*6, *4/*7) and slow (NAT2 genotypes *5/*5, *5/*6, *5/*6, *5/*7, *6/*6, *6/*6) acetylators in pregnant women. Concentrations were dose normalized to oral hydralazine 10 mg every 6 hours. Closed circles and red line indicate slow acetylators. Open squares and black line indicate rapid acetylators.
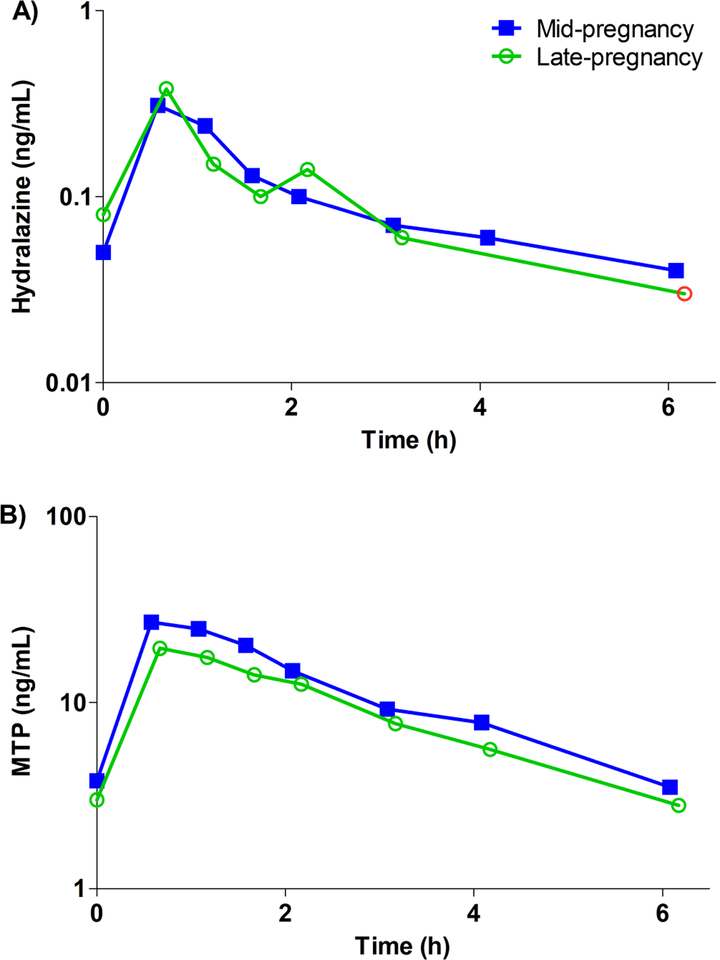
Blood or plasma concentration-time curves for the subject that completed mid- and late-pregnancy study days are depicted in Figure 4A for hydralazine and in Figure 4B for MTP. This subject took the same hydralazine dose (10 mg every 6 hours) during both mid- and late-pregnancy studies. Hydralazine apparent oral clearance adjusted for weight in mid- and late pregnancy were 85.4 and 81.3 L/h/kg, respectively. Hydralazine area under the concentration-time curve was 0.6 ng•h/mL for both mid- and late pregnancy. Elimination half-life was 3.7 and 3.0 h, and metabolite-to-parent ratio was 100 and 76 for mid- and late pregnancy, respectively.
Figure 4 –
Steady-state A) whole blood hydralazine concentration and B) plasma MTP concentration versus time profile of the subject that completed both mid- (closed squares, blue line) and late-pregnancy (open circles, green line) study days. Red open circle indicates measured concentration, which was just below the limit of quantification. A 10 mg dose every 6 hours was administered during both study days. This subject was identified as a NAT2 rapid acetylator.
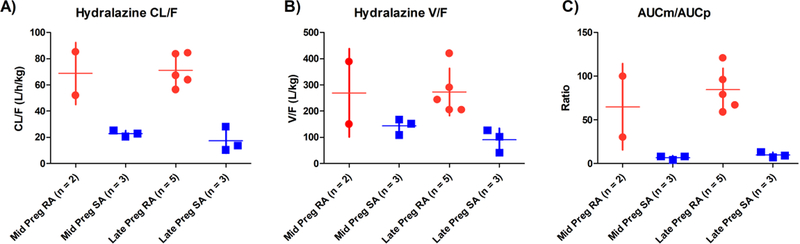
The influence of NAT2 genotype and gestational age on weight-adjusted apparent oral clearance (Figure 5A), apparent oral volume of distribution (Figure 5B), and MTP/hydralazine AUC ratio (Figure 5C) were analyzed by multiple linear regression. There was a significant effect of NAT2 genotype on weight-adjusted CL/F (β = −50.3, SE = 6.6, p < .001), weight-adjusted Vz/F, (β = −191.2, SE = 52.1, p < .01) and MTP/hydralazine AUC ratio (β = −67.8, SE = 13, p < .001).
Figure 5 –
The effects of NAT2 status and gestational age on A) apparent oral clearance, B) apparent volume of distribution, and C) metabolite to parent AUC ratio. Red circle indicates rapid acetylators and blue squares indicate slow acetylators. Mid-pregnancy was defined as 22 – 26 weeks gestation, and late-pregnancy as 30 – 38 weeks gestation. NAT2 acetylation status was categorized as rapid (RA, NAT2 *4/*5, *4/*6, *4/*7) and slow (SA, NAT2 *5/*5, *5/*6, *5/*7, *6/*6) acetylators based on genotype. No gestational age effects were observed on these key pharmacokinetic parameters after adjusting for subjects’ NAT2 phenotype.
However, gestational age did not affect weight-adjusted CL/F (β = 1.9, SE = 6.7, p = .8), weight-adjusted Vz/F (β = 26.2, SE = 53.3, p = .6) or MTP/hydralazine AUC ratio (β = −11.0, SE = 13.3, p = .4). In addition, dose did not significantly affect any of these parameters (CL/F, β = −0.8, SE = 0.4, p = 0.09; Vz/F, β = −2.9, SE = 3.6, p = .4; and AUCMTP/AUChydralzine, β = −0.6, SE = 0.9, p = .5).
Discussion
Hydralazine is an antihypertensive agent commonly prescribed during pregnancy. However, its PK and impact of NAT2 genotype during gestation have not been studied. In this study we characterized for the first time the PK of oral hydralazine and its metabolite MTP during pregnancy. Similar to previous reports in the non-pregnant population, oral hydralazine PK during pregnancy exhibited dependence upon NAT2 genotype13,30,44,45. Pregnant subjects with NAT2 RA genotypes exhibited significantly higher apparent oral clearance and apparent oral volume of distribution of hydralazine compared to women with SA genotypes. In addition, MTP-to-hydralazine AUC ratio was significantly higher in pregnant subjects with NAT2 RA genotypes.
The marked distinction in PK between NAT2 rapid and slow acetylators is likely in part due to difference in the oral bioavailability of hydralazine as a result of disparity in rate and extent of acetylation during first pass. Oral bioavailability (F) of a drug is governed by the percentage of parent drug that passes from the gut lumen into the mucosa (Fa), through the gut mucosa into the vasculature (Fg), and finally through the liver (Fh) into systemic circulation. NAT2 is known to be abundantly expressed in the intestine and liver29. In non-pregnant subjects, the bioavailability of hydralazine was reportedly 2.2-fold lower in RA than SA subjects26. We report a 2.5-fold difference in apparent oral volume of distribution (V/F), and nearly 3.5-fold difference in apparent oral clearance (CL/F) between RA and SA pregnant subjects. Small changes in NAT2 activity during pregnancy have been described46. Furthermore, the ~10-fold difference in MTP/hydralazine AUC ratio in pregnant subjects matched that of a previous study that found a 10-fold difference in urinary MTP/hydralazine ratio between RA and SA non-pregnant subjects47. This result is consistent with the current knowledge that MTP is primarily formed by N-acetylation via NAT248. While it is possible that there are compensatory mechanisms that would increase elimination of hydralazine by other pathways, the effect is expected to be minor as metabolism to MTP is the dominant pathway for hydralazine elimination9,49.
While there was a marked difference in hydralazine AUC between NAT2 genotypes, we did not find an association with genotype and hydralazine dosage needed for therapeutic reasons. In fact, contrary to what we expected, we saw a non-significant but numberically higher average dosage in the SA group. We explored gestational age, body weights, demographics, baseline hemodynamics and concomitant medication dosage, but did not find an explanation for this unexpected finding. A study in healthy non-pregnant volunteers demonstrated that higher hydralazine dose was needed for NAT2 RAs to achieve comparable hydralazine AUC as with the SAs50. A large study in non-pregnant subjects showed that RAs did not achieve adequate therapeutic effect with oral hydralazine, suggesting that NAT2 acetylation status might be important in pharmacodynamic response to oral hydralazine22. This too was not observed in our study. The lack of difference in dosage as well as pharmacodynamic response seen in our study between NAT2 RAs and SAs is likely multifactorial. First, this study was not adequately powered to assess these outcomes. Second, the hemodynamic measurements reported in this study were not performed for research purposes and not controlled for timing relative to dosing. Therefore, the ability to interpret these outcomes is confounded. Third, the patients participating in this study had their hydralazine titrated based on clinical response without regard to the study. Physician discretion on target endpoint for blood pressure control likely contributed to variability in dosage and pharmacodynamic response. Fourth, hydralazine was administered as part of combination therapy, which included atenolol for blood pressure management. Therefore, interpretation of hemodynamic changes were confounded by concomitant treatment. Lastly, intersubject variability likely contributed to our inability to detect differences.
Nevertheless, the increased exposure of hydralazine among pregnant SAs may still be of clinical concern. Earlier studies in the non-pregnant population demonstrate that N-acetylation is a saturable process for hydralazine and other NAT2 drug substrates (e.g., procainamide and isoniazid)9,51–53. Although we did not see a significant dose-dependent effect in our study, our sample size was relatively small and dosage range was limited to 5–25 mg. Previous hydralazine PK studies reported nonlinearity with single doses higher than 100–150 mg9. Since both pharmacological activity as well as toxicity are attributed to parent drug exposure, caution is suggested with high-dose hydralazine during pregnancy54–56. Adverse effects and complications when parenteral hydralazine is needed during pregnancy include placental abruption, increased risk of surgical delivery, maternal oliguria, altered fetal heart rate, and low Apgar scores at one minute57. NAT2 SAs, especially in female patients, have been found to have increased risk of developing severe adverse events (e.g. systemic lupus erythematosus) with high-dose hydralazine (> 200 mg/day)54. While the current study did not address the extent of fetal exposure to hydralazine, previous studies have reported trans-placental passage of hydralazine through measurement of umbilical cord hydralazine concentrations at the time of delivery32,33. Thus, high inter-individual variability in maternal hydralazine PK during pregnancy due to NAT2 polymorphism may not only affect maternal efficacy with low concentrations in RAs, but in SAs there may be increased maternal and fetal exposure to hydralazine and potential toxicity. Furthermore, NAT2 protein and activity were detected in term placentas, suggesting potential for placental metabolism of hydralazine58. Fetal metabolism of hydralazine has not been shown, but a pediatric study using isoniazid (another NAT2 substrate) reported lower NAT2 activity in neonates compared to older children59. While fetal metabolism contribution to overall maternal hydralazine PK is expected to be small, fetal metabolism can play a role in enhancing toxicity or minimizing adverse effects of drugs and toxins in the fetus. Although published data is conflicting60, Shi et al. reported that following in utero exposure to maternal smoking, there was a decreased risk of orofacial clefts in fetuses that expressed low activity NAT2 variants61. Additional studies are needed to explore hydralazine maternal and fetal safety in NAT2 SAs.
Since pregnancy results in gestational age dependent effects on numerous drug-metabolizing enzymes, we explored the effects of gestational age on hydralazine PK while accounting for NAT2 genotype. Among our subjects, we did not observe any significant difference in hydralazine PK (apparent oral clearance, apparent volume of distribution and MTP/hydralazine AUC ratio) between mid- and late pregnancy. In the one subject who completed both mid- and late-pregnancy study days, the subject’s hydralazine concentration-time profiles were very similar during both gestational windows. Previous work utilizing caffeine as a probe for NAT2 activity reported a 13% lower NAT2 activity in early pregnancy, which returned to baseline by mid-pregnancy. There was no difference in NAT2 activity between mid-pregnancy, late-pregnancy, or postpartum46.
In evaluating previously publish studies on hydralazine PK, there are some key technical problems. For years, quantification of hydralazine and its acid-labile metabolites has been a challenge for researchers due to chemical instability of hydralazine and potential interference from its pyruvate hydrazone metabolite. The present study adapted a previously developed derivatization technique at pH 6 that avoids release of apparent hydralazine from the acid-labile hydrazine metabolites (mainly HPH) and minimized hydralazine instability by performing the derivatization reaction immediately after blood collection40,62. Therefore, the present study measured the pharmacologically active parent hydralazine concentrations.
In non-pregnant subjects, the elimination half-life of parent hydralazine was reported in the literature to be 0.7 ± 0.1 h in SA and 0.3 ± 0.1 h in RA after oral administration of hydralazine in solution44; this is rather different from what we found in pregnant RA and SA subjects (3.0 ± 0.9 vs 4.0 ± 1.1, p = .1). The discrepancy is not surprising because the previous study by Shepherd et al. only sampled blood for 3 hours after hydralazine administration. Furthermore, their assay had limited sensitivity (LOQ of 2 ng/mL vs 0.04 ng/mL in our study). IV hydralazine exhibits multi-compartmental pharmacokinetics63. It is likely that, with rapid oral absorption from a solution formulation and brief duration of blood sampling, the short half-life observed by Shepherd et al. reflected the distribution phase of hydralazine disposition. By comparison, previously reported hydralazine Cmax and apparent oral clearance in non-pregnant subjects were close to what we found in our study in pregnant women. In non-pregnant RAs, the dose-normalized (to 10 mg) Cmax range was 4 – 5 ng/mL, and in SAs the range was 7 – 50 ng/mL, compared to the range of 0.3 – 1.8 ng/mL in pregnant RAs, and 1.4 – 9.4 ng/mL in pregnant SAs that we reported in our study44,64,65. Similarly, earlier studies report hydralazine CL/F ranging from 18,750 – 37,500 L/h in RAs and 3260 – 7500 L/h in SAs in non-pregnant subjects, whereas we report CL/F ranging from 3903 – 16,292 L/h in RAs and 800 – 2849 L/h in SA pregnant subjects44,64. It should be noted these two publications reported plasma hydralazine concentrations instead of whole blood concentrations; hence, we adjusted reported dose-normalized Cmax and CL/F values by the known whole blood-to-plasma ratio of 1.6563. Comparison of apparent oral volume of distribution (V/F) is not meaningful as calculation of V/F is dependent on an estimate of the elimination rate constant. As stated previously, earlier studies were not able to capture the true terminal elimination half-life. Based on aforementioned PK parameter estimates, hydralazine PK during pregnancy is reasonably comparable to that of non-pregnant subjects considering differences in study design, study population, assay methodologies, and hydralazine formulation (tablet vs. solution).
Lastly, we were not able to quantify the many known hydralazine metabolites in urine to establish a mass-balance recovery of the dose administered. We expect that future studies will overcome the formidable analytical challenges and allow for a more comprehensive understanding of hydralazine’s complex metabolic pathways and fraction metabolized by each metabolic enzyme.
Conclusions
In conclusion, we describe, for the first time, the impact of NAT2 genotype on oral hydralazine PK during mid- and late pregnancy. We report significantly higher hydralazine exposure, lower apparent oral clearance, and lower MTP-to-hydralazine ratio in subjects identified as slow acetylators compared to rapid acetylators. Our results did not show evidence of significant effect of dose or gestational age on the pharmacokinetics of hydralazine, possibly due to the small sample size. Clinicians should be mindful of the substantial variations in oral hydralazine exposure in pregnant women. Larger studies are needed to assess whether the PK differences leads to differences in safety or efficacy of hydralazine during pregnancy.
Supplementary Material
Acknowledgements
We thank Maggie Leahy, RN and Debra Brateng, RN for their assistance with the study, and Laura Shireman, Ph.D. for her guidance in statistical analysis. We also thank Nina Isoherranen, Ph.D., Lindsay Czuba, Ph.D., and Sara Shum, M.S., for their assistance and guidance in NAT2 genotyping.
Funding:
This research was supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health & Human Development through support of the Obstetric-Fetal Pharmacology Research Unit Network # U10HD047892, the National Center for Advancing Translational Sciences of the National Institutes of Health # UL1TR002319 and the National Institute of General Medical Sciences of the National Institutes of Health # R01GM124264. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, the National Institute of General Medical Sciences, the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Data Accessibility:
Readers with data sharing requests should contact Mary F. Hebert, PharmD, FCCP at mhebert@uw.edu.
Disclosures:
LWH: No financial conflicts of interest at the time of this study.
RR: No financial conflicts of interest at the time of this study.
MC: No financial conflicts of interest at the time of this study.
TRE: No financial conflicts of interest at the time of this study.
BRP: No financial conflicts of interest at the time of this study.
LJR: No financial conflicts of interest at the time of this study.
DS: No financial conflicts of interest at the time of this study.
MFH: No financial conflicts of interest at the time of this study.
References
- 1.Folk DM. Hypertensive Disorders of Pregnancy: Overview and Current Recommendations. J Midwifery Womens Health. May 2018. [DOI] [PubMed] [Google Scholar]
- 2.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol. 2013;25(2):124–132. [DOI] [PubMed] [Google Scholar]
- 3.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive Disorders and Pregnancy-Related Stroke. Obstet Gynecol. 2015;125(1):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- 5.Redman CWG. Hypertension in pregnancy: the NICE guidelines. Heart. 2011;97(23):1967–1969. [DOI] [PubMed] [Google Scholar]
- 6.Hypertension in pregnancy: diagnosis and management clinical guideline. National Institute for Health and Care Excellence. [PubMed] [Google Scholar]
- 7.Easterling TR. Pharmacological management of hypertension in pregnancy. Semin Perinatol. 2014;38(8):487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Easterling TR, Benedetti TJ, Schmucker BC, Carlson KL. Antihypertensive therapy in pregnancy directed by noninvasive hemodynamic monitoring. Am J Perinatol. 1989;6(1):86–89. [DOI] [PubMed] [Google Scholar]
- 9.Talseth T Clinical Pharmacokinetics of Hydrallazine. Clin Pharmacokinet. 1977;2(5):317–329. [DOI] [PubMed] [Google Scholar]
- 10.Lesser JM, Israili ZH, Davis DC, Dayton PG. Metabolism and Disposition of Hydralazine-14C in Man and Dog. Drug Metab Dispos. 1974;2(4). [PubMed] [Google Scholar]
- 11.Zak SB, Lukas G, Gilleran TG. Plasma levels of real and “apparent” hydralazine in man and rat. Drug Metab Dispos. 5(2):116–121. [PubMed] [Google Scholar]
- 12.Reece PA, Stanley PE, Zacest R. Interference in assays for hydralazine in humans by a major plasma metabolite, hydralazine pyruvic acid hydrazone. J Pharm Sci. 1978;67(8):1150–1153. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd AM, Ludden TM, Haegele KD, Talseth T, McNay JL. Pharmacokinetics of hydralazine, apparent hydralazine and hydralazine pyruvic acid hydrazone in humans. Res Commun Chem Pathol Pharmacol. 1979;26(1):129–144. [PubMed] [Google Scholar]
- 14.Wagner J, Faigle JW, Imhof P, Liehr G. Metabolism of hydralazine in man. Arzneimittelforschung. 1977;27(12):2388–2395. [PubMed] [Google Scholar]
- 15.Barron K, Carrier O, Haegele KD, McLean AJ, McNay JL, Du Souich P. Comparative evaluation of the in vitro effects of hydralazine and hydralazine acetonide on arterial smooth muscle. Br J Pharmacol. 1977;61(3):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLean AJ, du Souich P, Barron K, Carrier O, Haegele KD, McNay JL. Study of in vitro effects of hydralazine metabolites--comparative evaluation of products of hydroxylation, hydrolysis and conjugation. Arch Int Pharmacodyn Ther. 1978;235(1):19–25. [PubMed] [Google Scholar]
- 17.Iwaki M, Ogiso T, Ito Y. In vitro kinetic studies of the reaction of hydralazine and its acetone hydrazone with pyruvic acid. J Pharm Sci. 1988;77(3):280–283. [DOI] [PubMed] [Google Scholar]
- 18.Reece PA. Hydralazine and related compounds: chemistry, metabolism, and mode of action. Med Res Rev. 1981;1(1):73–96. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer H, Glaser R, Kokosa J, Garteiz DA, Hess EV, Litwin A. 3-Hydroxymethyl-s-triazolo[3,4-a]phthalazine, a novel urinary hydralazine metabolite in man. J Med Chem. 1975;18(10):1031–1033. [DOI] [PubMed] [Google Scholar]
- 20.Dubois JP, Schmid K, Riess W, Hanson A, Henningsen NC, Andersson OK. Metabolism of hydralazine in man. Part II: Investigation of features relevant to drug safety. Arzneimittelforschung. 1987;37(2):189–193. [PubMed] [Google Scholar]
- 21.Shepherd AM, McNay JL, Ludden TM, Lin MS, Musgrave GE. Plasma concentration and acetylator phenotype determine response to oral hydralazine. Hypertens (Dallas, Tex 1979). 3(5):580–585. [DOI] [PubMed] [Google Scholar]
- 22.Spinasse LB, Santos AR, Suffys PN, Muxfeldt ES, Salles GF. Different phenotypes of the NAT2 gene influences hydralazine antihypertensive response in patients with resistant hypertension. Pharmacogenomics. 2014;15(2):169–178. [DOI] [PubMed] [Google Scholar]
- 23.Lemke LE, McQueen CA. Acetylation and its role in the mutagenicity of the antihypertensive agent hydralazine. Drug Metab Dispos. 1995;23(5):559–565. [PubMed] [Google Scholar]
- 24.Timbrell JA, Harland SJ, Facchini V. Polymorphic acetylation of hydralazine. Clin Pharmacol Ther. 1980;28(3):350–355. [DOI] [PubMed] [Google Scholar]
- 25.Allen CE, Doll MA, Hein DW. N-Acetyltransferase 2 Genotype-Dependent N-Acetylation of Hydralazine in Human Hepatocytes. Drug Metab Dispos. 2017;45(12):1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardman J, Limbird L, Gilman A. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. McGraw Hill; 2001. [Google Scholar]
- 27.Jenne JW. Studies of human patterns of isoniazid metabolism using an intravenous fall-off technique with a chemical method. Am Rev Respir Dis. 1960;81(1P1):1–8. [DOI] [PubMed] [Google Scholar]
- 28.Evans DA, White TA. Human Acetylation Polymorphism. J Lab Clin Med. 1964;63:394–403. [PubMed] [Google Scholar]
- 29.Windmill KF, Gaedigk A, de la M. Hall P, Samaratunga H, Grant DM, McManus ME. Localization of N-Acetyltransferases NAT1 and NAT2 in Human Tissues. Toxicol Sci. 2000;54(1):19–29. [DOI] [PubMed] [Google Scholar]
- 30.Shen DD, Hosler JP, Schroder RL, Azarnoff DL. Pharmacokinetics of hydralazine and its acid-labile hydrazone metabolites in relation to acetylator phenotype. J Pharmacokinet Biopharm. 1980;8(1):53–68. [DOI] [PubMed] [Google Scholar]
- 31.Ludden TM, Shepherd AM, McNay JL, Lin MS. Effect of intravenous dose on hydralazine kinetics after administration. Clin Pharmacol Ther. 1983;34(2):148–152. [DOI] [PubMed] [Google Scholar]
- 32.Liedholm H, Wåhlin-Boll E, Hanson A, Ingemarsson I, Melander A. Transplacental passage and breast milk concentrations of hydralazine. Eur J Clin Pharmacol. 1982;21(5):417–419. [DOI] [PubMed] [Google Scholar]
- 33.Lamont RF, Elder MG. Transfer of hydralazine across the placenta and into breast milk. J Obstet Gynaecol (Lahore). 1986;7(1):47–48. [DOI] [PubMed] [Google Scholar]
- 34.Hebert MF, Carr DB, Anderson GD, et al. Pharmacokinetics and Pharmacodynamics of Atenolol During Pregnancy and Postpartum. J Clin Pharmacol. 2005;45(1):25–33. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd AM, Irvine NA, Ludden TM. Effect of food on blood hydralazine levels and response in hypertension. Clin Pharmacol Ther. 1984;36(1):14–18. [DOI] [PubMed] [Google Scholar]
- 36.Liedholm H, Wåhlin-Boll E, Hanson A, Melander A. Influence of food on the bioavailability of "real" and "apparent" hydralazine from conventional and slow-release preparations. Drug Nutr Interact. 1982;1(4):293–302. [PubMed] [Google Scholar]
- 37.Melander A, Danielson K, Hanson A, et al. Enhancement of hydralazine bioavailability by food. Clin Pharmacol Ther. 1977;22(1):104–107. [DOI] [PubMed] [Google Scholar]
- 38.Melander A, Liedholm H, McLean A. Concomitant food intake does enhance the bioavailability and effect of hydralazine. Clin Pharmacol Ther. 1985;38(4):475–476. [DOI] [PubMed] [Google Scholar]
- 39.Walden RJ, Hernandez R, Witts D, Graham BR, Prichard BN. Effect of food on the absorption of hydralazine in man. Eur J Clin Pharmacol. 1981;20(1):53–58. [DOI] [PubMed] [Google Scholar]
- 40.Semple HA, Tam YK, Tin S, Coutts RT. Assay for hydralazine as its stable p-nitrobenzaldehyde hydrazone. Pharm Res. 1988;5(6):383–386. [DOI] [PubMed] [Google Scholar]
- 41.Reece PA, Cozamanis I, Zacest R. Selective high-performance liquid chromatographic assays for hydralazine and its metabolites in plasma of man. J Chromatogr. 1980;181(3–4):427–440. [DOI] [PubMed] [Google Scholar]
- 42.Hein DW, Doll MA. Accuracy of various human NAT2 SNP genotyping panels to infer rapid, intermediate and slow acetylator phenotypes. Pharmacogenomics. 2012;13(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 44.Shepherd AM, Ludden TM, McNay JL, Lin MS. Hydralazine kinetics after single and repeated oral doses. Clin Pharmacol Ther. 1980;28(6):804–811. [DOI] [PubMed] [Google Scholar]
- 45.Talseth T Kinetics of hydralazine elimination. Clin Pharmacol Ther. 1977;21(6):715–720. [DOI] [PubMed] [Google Scholar]
- 46.Tsutsumi K, Kotegawa T, Matsuki S, et al. The effect of pregnancy on cytochrome P4501A2, xanthine oxidase, and N -acetyltransferase activities in humans. Clin Pharmacol Ther. 2001;70(2):121–125. [DOI] [PubMed] [Google Scholar]
- 47.Rashid JR, Kofi-Tsepko, Juma FD. Acetylation status using hydralazine in African hypertensives at Kenyatta National Hospital. East Afr Med J. 1992;69(7):406–408. [PubMed] [Google Scholar]
- 48.Reece PA, Stafford I, Prager RH, Walker GJ, Zacest R. Synthesis, formulation, and clinical pharmacological evaluation of hydralazine pyruvic acid hydrazone in two healthy volunteers. J Pharm Sci. 1985;74(2):193–196. [DOI] [PubMed] [Google Scholar]
- 49.Haegele KD, Talseth T, Skrdlant HB, Shepherd AM, Huff SL. Determination of hydralazine pyruvic acid hydrazone and its correlation with "apparent" hydralazine. Arzneimittelforschung. 1981;31(2):357–362. [PubMed] [Google Scholar]
- 50.Garcés-Eisele SJ, Cedillo-Carvallo B, Reyes-Núñez V, et al. Genetic selection of volunteers and concomitant dose adjustment leads to comparable hydralazine/valproate exposure. J Clin Pharm Ther. 2014;39(4):368–375. [DOI] [PubMed] [Google Scholar]
- 51.Drucker MM, Blondheim SH, Wislicki L. Factors affecting the acetylation in-vivo of para-aminobenzoic acid by human subjects. Clin Sci. 1964;27:133–141. [PubMed] [Google Scholar]
- 52.Talseth T, Landmark KH. Polymorphic acetylation of sulphadimidine in normal and uraemic man. Eur J Clin Pharmacol. 1977;11(1):33–36. [DOI] [PubMed] [Google Scholar]
- 53.Graffner C Elimination rate of N-acetylprocainamide after a single intravenous dose of procainamide hydrochloride in man. J Pharmacokinet Biopharm. 1975;3(2):69–76. [DOI] [PubMed] [Google Scholar]
- 54.Cameron HA, Ramsay LE. The lupus syndrome induced by hydralazine: a common complication with low dose treatment. Br Med J (Clin Res Ed). 1984;289(6442):410–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hofstra AH. Metabolism of Hydralazine: Relevance to Drug-Induced Lupus. Drug Metab Rev. 1994;26(3):485–505. [DOI] [PubMed] [Google Scholar]
- 56.Iyer P, Dirweesh A, Zijoo R. Hydralazine Induced Lupus Syndrome Presenting with Recurrent Pericardial Effusion and a Negative Antinuclear Antibody. Case Rep Rheumatol. 2017;2017:5245904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magee LA, Cham C, Waterman EJ, Ohlsson A, von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327(7421):955–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smelt VA, Mardon HJ, Sim E. Placental Expression of Arylamine N-Acetyltransferases: Evidence for Linkage Disequilibrium between NAT1 * 10 and NAT2 * 4 Alleles of the Two Human Arylamine N-Acetyltransferase Loci NAT1 and NAT2. Pharmacol Toxicol. 1998;83(4):149–157. [DOI] [PubMed] [Google Scholar]
- 59.Rogers Z, Hiruy H, Pasipanodya JG, et al. The Non-Linear Child: Ontogeny, Isoniazid Concentration, and NAT2 Genotype Modulate Enzyme Reaction Kinetics and Metabolism. EBioMedicine. 2016;11:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lammer EJ, Shaw GM, Iovannisci DM, Van Waes J, Finnell RH. Maternal smoking and the risk of orofacial clefts: Susceptibility with NAT1 and NAT2 polymorphisms. Epidemiology. 2004;15(2):150–156. [DOI] [PubMed] [Google Scholar]
- 61.Shi M, Christensen K, Weinberg CR, et al. Orofacial Cleft Risk Is Increased with Maternal Smoking and Specific Detoxification-Gene Variants. Am J Hum Genet. 2007;80(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ludden TM, Goggin LK, McNay JL, Haegele KD, Shepherd AM. High-pressure liquid chromatographic assay for hydralazine in human plasma. J Pharm Sci. 1979;68(11):1423–1425. [DOI] [PubMed] [Google Scholar]
- 63.Ludden TM, Shepherd AM, McNay JL, Lin MS. Hydralazine kinetics in hypertensive patients after intravenous administration. Clin Pharmacol Ther. 1980;28(6):736–742. [DOI] [PubMed] [Google Scholar]
- 64.Ludden TM, McNay JL, Shepherd AM, Lin MS. Variability of plasma hydralazine concentrations in male hypertensive patients. Arthritis Rheum. 1981;24(8):987–993. [DOI] [PubMed] [Google Scholar]
- 65.Ludden TM, Rotenberg KS, Ludden LK, Shepherd AM, Woodworth JR. Relative bioavailability of immediate- and sustained-release hydralazine formulations. J Pharm Sci. 1988;77(12):1026–1032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.