Abstract
Intra-epithelial fallopian tube neoplasia is thought to be a precursor lesion to high grade serous carcinoma of the Mullerian adnexae, particularly in women with BRCA1 or BRCA2 mutations. This association has led to recommendations to assess fallopian tubes for intraepithelial atypia. However, the diagnostic reproducibility of a diagnosis of intraepithelial neoplasia is unclear. In this study, two gynecologic pathologists independently evaluated sections of fallopian tubes from a sample of women (N=198, 623 slides) undergoing salpingectomy. 101 (54%) women were undergoing risk-reducing salpingo-oophorectomy. Pathologists were blinded to patient histories and prior diagnoses. Pathologists rendered one of three diagnoses for each slide: “Negative for FTIN,” “Indeterminate for FTIN,” or “Definite for FTIN.” Cases that were considered by histology definite for fallopian tube intraepithelial neoplasia (FTIN) or suspicious for FTIN were stained with p53 and Ki67. Pathologists agreed on the diagnosis of “definite for FTIN” 61.5% of the time. There was no agreement on any cases for the diagnosis of “indeterminate for FTIN.” Fifteen “indeterminate for FTIN” and twelve “definite for FTIN” cases were stained with p53 and Ki67. Two of the “indeterminate” cases (13%) had p53-positive foci. Five of the “definite” cases had p53-positive foci. In three of the other 8 “definite” cases, there was obvious carcinoma present, but the carcinoma did not stain with p53, suggesting a possible null phenotype. We propose that immunostains should only be used to aid in the diagnosis of FTIN in cases with indeterminate histology. The use of p53 immunohistochemistry in cases that were considered “definite for FTIN” by histology was minimally helpful, and in fact often served to further confuse the diagnosis.
Keywords: fallopian tube, serous tubal intraepithelial neoplasia (STIN), serous tubal intraepithelial carcinoma (STIC), serous tubal intraepithelial lesion (STIL), ovarian carcinoma, inter-observer variability
INTRODUCTION
Our understanding of the oncogenesis of serous carcinoma of the Mullerian adnexa has changed dramatically over the last 15 years. Ovarian carcinomas were originally thought to arise from the surface epithelium of the ovary, but recent studies suggest that these neoplasms often arise from the epithelium of the fallopian tube.1,2 Intra-epithelial neoplasia of the fallopian tube is also often seen in women with “ovarian” serous carcinoma, with co-occurrence rates of up to 71%.1,3 More recent sequencing analyses suggest that intra-epithelial neoplasia is often the source of metastatic high-grade serous carcinomas.2,4 In women with BRCA1 or BRCA2 mutations, most occult high-grade intraepithelial neoplasms or microinvasive carcinomas identified at the time of risk-reducing surgery are located in the fallopian tube, not the ovary.5–9 Given these findings, intraepithelial neoplasia of the fallopian tube, particularly the distal end (fimbriae), is thought to be a precursor to serous carcinoma.10–13 This has led to recommendations to completely section and evaluate the fallopian tubes in high risk women undergoing risk-reducing surgery.14,15
Now that the fallopian tube epithelium is more closely examined, a spectrum of atypical lesions has been identified. The lesion that is most strongly associated with serous carcinoma is serous tubal intraepithelial neoplasia (STIN) (also known as serous tubal intraepithelial “carcinoma”, or STIC). STIN is considered a neoplastic process of the epithelial cells of the fallopian tube without evidence of invasion. The proposed diagnostic criteria for STIN are a discrete population of cells with marked nuclear atypia, hyperchromasia, increased nuclear-to-cytoplasmic ratios, and loss of cilia and cell polarity.20,21 In addition, Gross et al (2010)22 requires that STIN has increased expression of Ki67 and increased expression (overexpression) or complete loss of p53 (null-type expression pattern). STIN is a potential misnomer in that it has been seen in the setting of non-serous neoplasia, such as endometrioid carcinoma and carcinosarcoma.16–18 We therefore prefer and use the term “fallopian tube intraepithelial neoplas(ia/m)” or “FTIN”, which will be used in place of STIC/STIN moving forward in this manuscript.
In addition to FTIN, two other types of pathological lesions have been reported in the fallopian tube - the so-called “p53-signature” and serous tubal intraepithelial lesion (STIL). P53-signatures are contiguous patches of fallopian tube epithelial cells that appear normal histologically but have increased p53 signal on immunostaining. STIL is a poorly defined lesion; various groups report slightly different diagnostic criteria. STIL can be somewhat vaguely described as intraepithelial atypia of the fallopian tube that does not meet diagnostic criteria for FTIN. Cass et al. (2014)19 defines STIL as “mild nucleomegaly with inconspicuous nucleoli, mildly increased nuclear to cytoplasmic ratio and/or slight loss of cellular polarity.” Gross et al. (2010)20 has a broad definition of STIL that includes epithelial atypia that falls short of FTIN and normal-appearing epithelium with aberrant expression of p53 and Ki67. There is no clear evidence that STIL is a precursor to carcinoma,19 and the exact clinical implications of a diagnosis of STIL are unclear. Complicating evaluation, normal fallopian tube epithelium varies widely in its appearance, particularly in premenopausal women in which the fallopian tube responds to hormonal variation.21,22
However, it is unclear these diagnostic criteria for FTIN and STIL are reproducible. A handful of studies have noted problems with interobserver variability in making these diagnoses. Prior studies have all used κ statistics to report inter-observer agreement. Carlson et al. (2010)23 studied inter-observer agreement using hematoxylin and Eosin (H&E) in conjunction with p53 and Ki-67 immunohistochemistry (IHC), and the researchers reported κ’s of 0.333 (“fair”) to 0.453 (“moderate”). Visvanathan et al. (2011)24 found that a diagnosis of FTIN versus not FTIN had “fair” agreement (κ = 0.39) using histology alone, but improved with the use of immunohistochemical stains (κ = 0.73). This study also reported inter-observer agreement in the diagnosis of STIL based on histology alone (atypia less than FTIN): κ = 0.11 (“poor”). Using IHC, improved agreement on the diagnosis of atypia (STIL), but the κ statistic was still only “moderate” at 0.51. Vang et al. (2012)25 expanded on this work, testing their histologic and immunohistochemical definition of FTIN and STIL on pathologists who were not involved in the original study defining the diagnostic criteria. The interobserver variability had a κ of 0.67 after participants took part in training via a website. Lastly, Kuhn et al. (2012)26 report that Ki67 is statistically higher in FTIN and high-grade serous carcinoma compared to normal fallopian tube epithelium and is thus a valuable diagnostic tool. It is important to note that the kappa statistics in these studies are difficult to interpret due to the variability in the frequency of FTIN in the study sets.
Visvanathan et al. (2011)24 and Vang et al. (2012)25 proposed a clinical algorithm that requires evaluation of all fallopian tube epithelium by both H&E and immunohistochemical stains for p53 and Ki67, which they justified by their reported improvements in κ statistics. According to this algorithm, fallopian tube epithelium without epithelial atypia should be stained with p53 and Ki67, and if both are aberrant, this would warrant a diagnosis of “STIL.” Use of this algorithm in the clinical setting can be problematic. Aberrant expression of p53 and ki67 has a high occurrence in even low-risk populations.27–29 Ki67 is non-specific, and can be increased in reactive conditions. In addition, the fallopian tube frequently has foci of at least mild atypia.21,22 The use of immunohistochemistry to evaluate all fallopian tubes or even those with mild atypia would likely result in overdiagnosis and unnecessary worry on part of the clinician and patient.
For these reasons, we believe that the current published diagnostic guidelines may be misleading to the pathologist in diagnosis of these lesions. Based on our experience, we hypothesized that it would be beneficial to reserve the use of immunohistochemical stains for uncertain circumstances, rather than applying stains uniformly to all lesions of the fallopian tube. In the current study, we present an alternative clinical algorithm.
MATERIALS AND METHODS
We performed a cross-sectional observational study of salpingectomy specimens. Our sample consisted of 101 cases and 97 controls who had surgeries performed at our institution between 1999 and 2010. Cases were women undergoing risk-reducing salpingectomies or salpingoophorectomies for reasons including genetic predispositions, positive family histories, or personal breast cancer histories (see Table 1). Controls were women who had a salpingectomy as part of surgeries for various other benign reasons, such as leiomyomata or endometriosis. Nine patients (4.5%) had been given a diagnosis of “intraepithelial neoplasia,” “carcinoma in situ,” or “epithelial atypia” when their case had been initially evaluated. A diagnosis of FTIN was not part of the selection or exclusion criteria. Eight patients (4.0%) had been given a diagnosis of serous carcinoma. These patients were identified through our tissue bank database. This study included all patients from 1999 to 2010 who had consented to have clinical information and tissue collected, and had provided follow up data. Patients without H&E slides available for review were excluded from the study. After exclusions, slides from 198 patients were reviewed. Fallopian tubes from all 198 patients had been serially sectioned and entirely submitted, resulting in a different number of slides per patient (based on size and sections needed). Each slide was randomly assigned a three-digit identifier and reviewed independently of all other slides from the same case.
Table 1: Reasons for risk reducing surgery (note that categories are not exclusive; e.g. cases may have both a BRCA mutation and a family history of breast/ovarian cancer).
| Total Cases | 101 |
| Cases with BRCA1 or BRCA2 mutations | 61 (59.4%) |
| BRCA1 | 34 (33.7%) |
| BRCA2 | 27 (44.3%) |
| Patients with other genetic mutations associated with breast/ovarian cancer | 2 (2.0%) |
| Patients with a known family history of breast/ovarian cancer | 83 (82.2%) |
| Patients with a known personal history of breast cancer | 25 (24.8%) |
Two experienced gynecologic pathologists independently performed evaluations of the H&E -stained slides. Identifying information was removed from the slides. One of the pathologists had seen some of the cases previously at the time of initial pathology, but this study was performed after a significant amount of time had passed, and slides were reviewed out of context of the original case. Pathologists were not aware of prior diagnoses, case/control status, or the relative proportions of cases and controls. In total, 623 slides of fallopian tubes were reviewed, with multiple sections of fallopian tube on each slide. As this was a retrospective study, the SEE-Fim protocol30 was used in the risk-reduction surgeries, whereas only representative sections were taken in the control cases. To control for this, we kept the number of slides per case included in the study consistent between cases and controls. On average, 3.15 slides per case were reviewed for the cases and 3.13 slides per case were reviewed from controls. In total, approximately 3,015 sections of fallopian tube were reviewed. The pathologists reported reviewing a slightly different number of sections of fallopian tube (2,994 vs 3,036) due to disagreement as to what constituted a “section” (e.g. should a fragment count or how to interpret sections of fimbriae).
The pathologists categorized each fallopian tube section based on histology alone using the following classifications: negative for FTIN, indeterminate for FTIN, and definite for FTIN. “Definite for FTIN” was defined as “A discrete population of epithelial cells with moderate-to-severe nuclear atypia with stratification OR severe nuclear atypia alone.” Indeterminate for FTIN was defined as “A discrete population of cells with moderate nuclear atypia without stratification.” Negative for FTIN was defined as “Epithelium that is cytologically and architecturally normal or has only mild nuclear atypia.” Carcinoma was also identified on some slides. Diagnoses of carcinoma and FTIN were not mutually exclusive.
To further refine diagnoses and establish utility of immunohistochemical stains, cases designated indeterminate for FTIN or definite for FTIN were stained with p53 and Ki67. The immunohistochemical stains were prepared in a College of American Pathologists–accredited diagnostic immunohistochemistry laboratory using standardized protocols.31 In brief, 4-μm tissue sections cut on charged slides, were deparaffinized on a fully automated autostainer (Bond III; Leica Biosystems, Wetzlar, Germany) using a proprietary Bond dewax solution that contains no xylene. There were three applications of the Dewax solution followed by three applications of 100% ethanol and then three applications of Bond wash solution. Antigen retrieval was performed on a Bond III using either ER1 (citrate with a pH range of 5.9-6.1) or ER2 (EDTA with a pH range of 8.9-9.1) buffers for 20 minutes. After rinsing and endogenous peroxidase blocking, a post primary immunoglobulin G (IgG) linker was applied, followed by several rinses with the Bond wash solution and a deionized water rinse. The slides were incubated for 15 minutes with primary mouse antibodies and rinsed multiple times with Bond wash solution. Then, a polymer anti-mouse poly–horseradish peroxidase–IgG was applied, and slides were incubated for 8 minutes with polymer detection reagent (Bond Polymer Refine Detection kit; Leica Biosystems), then rinsed multiple times. 3,3′-Diaminobenzidine tetra hydrochloride chromogen was allowed to precipitate for 10 minutes, and then slides were counterstained with hematoxylin for 5 minutes. P53 staining was performed using the DAKO M7001 antibody, DO-7 clone, a dilution of 1:1000, and pretreatment with ER2 for 20 minutes. Ki67 staining was performed using the DAKO M7240 antibody, MIB-1 clone, a dilution of 1:100, and pretreatment with ER2 for 30 min. Positive controls were performed to evaluate for appropriate staining. All IHC slides were reviewed by two physicians, one of whom was a dedicated gynecologic pathologist and the other a senior level trainee. Intensity of staining in cells suspicious for FTIN was compared to baseline intensity of staining in the surrounding normal-appearing fallopian tube epithelium, and judgments of either “increased staining” or “no increased staining” were made by the reviewer.
One case called indeterminate for FTIN and one case called definite for FTIN could not be stained due to missing material. Following pathology evaluation, slides were unblinded and results from the same cases were grouped together. Cohen’s κ was used to measure inter-rater agreement. All analyses were performed using R v3.4.0.
RESULTS
Table 2 provides clinical demographics of cases and controls. The average age of the women in our groups at the time of surgery was 48.8 years. Of the 101 women who reported a menopausal status, 45 (44.6%) were pre-menopausal, 45 (44.6% were post-menopausal, and 11 (10.9%) were peri-menopausal. Menopausal status was only reported in 4 of the control patients (see Table 2).
Table 2: Case and control demographics and other characteristics.
| Cases | Controls | Total | |
|---|---|---|---|
| Total | 101 | 97 | 198 |
| Total Slides Reviewed | 319 (average 3.15 slides/case) | 304 (3.13) | 623 (3.15) |
| Average Age | 47.7 (33 – 70) | 50.1 (21 – 84) | 48.8 (21 – 84) |
| Definite for FTIN* | 10 (9.9%) | 0 (0.0%) | 10 (5.0%) |
| Indeterminate for FTIN* | 8 (7.9%) | 7 (7.2%) | 15 (7.6%) |
| Carcinoma present | 8 (7.9%) | 0 (0.0%) | 8 (4.0%) |
| Menopausal status (when reported) | 97 | 4 | 101 (51.0%) |
| Pre | 42 | 3 | 45 (44.6%) |
| Peri | 10 | 1 | 11 (10.9%) |
| Post | 45 | 0 | 45 (44.6%) |
This diagnosis was made by either one or both pathologists.
Twenty-seven of 623 slides were given a diagnosis of indeterminate for FTIN or definite for FTIN by at least one pathologist based on histology alone (see Figure 1 for examples). Table 2 provides those data based on case versus control designation. Diagnoses of “definite for FTIN” or carcinoma were only made (by either pathologist) in high risk women undergoing RRSO (cases), while diagnoses of “indeterminate for FTIN” were made in both cases and controls (7.9% of cases and 7.2% of controls). Six slides from 5 different women (all cases) were given a diagnosis of carcinoma. Table 3 details the number and percentage of negative, indeterminate, and definite for FTIN diagnosed by each pathologist, as well as the percent agreement between the two pathologists. Pathologists agreed on a diagnosis of “negative for FTIN” 97% of the time. They agreed on a diagnosis of “definite for FTIN” 61.5% of the time. Pathologists did not agree on any cases that were diagnosed as “indeterminate for FTIN” (0.0%). Κ statistics32 were calculated for overall agreement on negative, indeterminate for FTIN, and definite for FTIN, based on diagnoses made on individual slides. There was weak agreement (0.449) when using an unweighted κ, but using a weighted (squared) κ provided a statistic showing moderate agreement (0.657) (see Table 4). Cases with invasive neoplasm (invasive carcinoma) showed excellent inter-rater agreement (κ=0.91).
Figure 1:
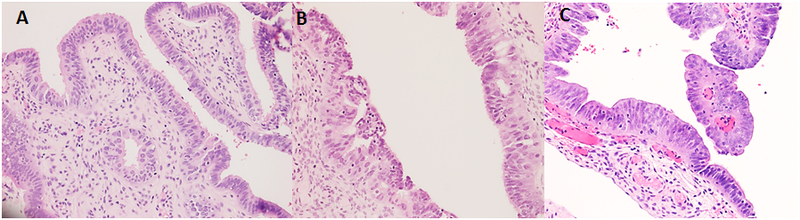
Examples of cases interpreted as (A) negative for FTIN (with mild atypia), (B) indeterminate for FTIN, and (C) indefinite for FTIN.
Table 3: Diagnoses by pathologist based on histology.
This table shows the percent of slides interpreted at “Negative for FTIN,” “Indeterminate for FTIN” and “Definite for FTIN” by each pathologist, as well as how many cases were interpreted as invasive carcinoma, and how many cases had no intraepithelial nuclear atypia. The right-most column shows the number percent of slides in which the pathologists made the same diagnosis, as well as the percent agreement (Percent agreement is the number of slides in which pathologists agreed on that diagnosis divided by total number of slides given that diagnosis by at least one pathologist, which is given in column 3). Also note that patients can have both carcinoma and intraepithelial neoplasia, hence the columns do not add to 100%.
| Pathologist 1 | Pathologist 2 | Total | Percent Agreement | |
|---|---|---|---|---|
| N(%) | N(%) | N | % (N) | |
| Negative for FTIN | 601(96.5%) | 608(97.6%) | 613 | 97.2% (596) |
| Indeterminate for FTIN | 10 (1.6%) | 6 (1.0%) | 16 | 0.0% (0) |
| Definite for FTIN | 12 (1.9%) | 9 (1.4%) | 13 | 61.5% (8) |
| Carcinoma | 5 (0.8%) | 6 (1.0%) | 6 | 83.3% (5) |
Table 4: Overall inter-rater agreement based on histology alone and based on histology combined with IHC, weighted and unweighted kappa statistics.
| Agreement based on histology alone |
Agreement based on histology and IHC |
|||
|---|---|---|---|---|
| Overall diagnosis | Kappa | |||
| Unweighted | 0.45 | Weak agreement | 0.79 | Good agreement |
| Weighted, equal | 0.57 | Weak agreement | 0.84 | Very good agreement |
| Weighted, squared | 0.66 | Moderate agreement | 0.87 | Very good agreement |
Slides from cases that had been called either “indeterminate for FTIN” or “definite for FTIN” by at least one pathologist were stained with p53 and Ki67 (25 slides in total). Fifteen of these cases were called indeterminate for FTIN by at least one pathologist, and twelve cases were called definite for FTIN by at least one pathologist. In two cases, one pathologist rendered a diagnosis of definite for FTIN and the other rendered a diagnosis of indeterminate for FTIN (this accounts for the discrepancy in the sum). In all cases of “indeterminate for FTIN,” there was no concordance between the pathologists. Of the 12 “definite for FTIN” cases, the pathologists agreed on 8 cases.
Of the indeterminate for FTIN cases, corresponding p53+ foci were identified in two cases (13%), consistent with a diagnosis of FTIN. (See Table 5.) Of the definite for FTIN cases, corresponding p53+ foci were identified in 5 of the blocks (42%), supporting a diagnosis of FTIN. In 4 of these 5 cases, Ki67 staining was increased, further supporting the diagnosis. It is important to note that diagnoses of carcinoma and FTIN were not mutually exclusive. Slides could contain separate foci of carcinoma and FTIN and both were reported. Four of the 12 blocks with diagnoses of “definite for FTIN” had, in addition, obvious carcinoma that, interestingly, did not stain strongly positive for p53 (see Figure 3), raising the possibility of neoplasms with p53 null-type expression patterns. In 3 of these 4 cases, Ki67 was at least mildly increased in the regions of histologic atypia. It was difficult, if not impossible, to identify p53 null FTIN in all cases due to the nature of the stain and small size of the lesions. Thus, 9 of the 12 (75%) “definite for FTIN” cases either had p53+ foci (5 of 8) or possible p53 null-type foci (4 of 8). Ki67 staining supported this diagnosis in 7 of 9 cases. However, Ki67 was also focally increased in one additional case with no suspicious p53+ foci or evidence of null phenotype. This case had originally been considered “indeterminate for FTIN,” and we suspect this increased Ki67 is reactive.
Table 5: Results of immunohistochemical stains (p53 and Ki67) performed on cases called “Indeterminate for FTIN” or “Definite for FTIN”.
| Indeterminate for FTIN | Definite for FTIN | |
|---|---|---|
| Slides stained | 15* | 12* |
| Concordance between Pathologists | 0 | 8 |
| Increased Ki67 | 2 (13%) | 5 (42%) |
| Increased P53 or possible null | 2 (13%) | 9(75%) |
| Increased P53 | 2 | 5 |
| Possible null | 0 | 4 |
Two blocks were missing and thus IHC could not be performed (one Indeterminate case and one Definite case)
Figure 3:
Example of case with obvious carcinoma on H&E (A), but with absence of strong p53 staining in the carcinoma (B). P53 staining is weak and non-specific, similar to the staining on non-neoplastic tissues in this block. In this case, it is difficult to use p53 staining of an intraepithelial lesion to support a diagnosis of FTIN.
As previously stated, in the 12 “definite for FTIN” cases, there was discordance between pathologists in 4 cases, and agreement in 8 cases. Of the 8 concordant “definite for FTIN” cases, 7 (88%) had corresponding p53-positive foci or were potentially null-type. In the one case in which there was consensus on the diagnosis, but no p53-positive foci or null-type findings, the H&E recut that was made at the time of the IHC stains was reviewed. A “definite for FTIN” lesion was no longer identified on the H&E recut, and thus was presumably lost on the IHC slides as well.
Κ statistics were re-calculated after final diagnoses based on immunohistochemical stains were made. The unweighted κ was 0.788. The weighted κ (equal) was 0.842, and the weighted κ (squared) was 0.871 (see Table 4).
DISCUSSION
In the literature, it has been proposed that FTIN, STIL, and the p53-signature are part of a biologic spectrum that progress from normal fallopian tube epithelium to invasive carcinoma.15,28,33 Crum et al. (2007)15 suggests that the progression begins with p53 mutations causing a p53 signature, continues to STIL, then FTIN, and finally to invasive carcinoma. For this reason, it has been suggested that pathologists should look for p53 signatures, STIL, and FTIN in fallopian tubes. However, the clinical significance and diagnostic reproducibility of these diagnoses are unclear.
Regarding the clinical significance of these diagnoses, the proposed biologic continuum15 is a plausible theory, but there are limitations. First of all, p53 signature lesions are much more common than would be expected. A large percentage of women (26-47%) with and without known BRCA mutations have been found to have p53-signatures in the fallopian tubes.19,33,34 The high frequency of p53 signatures in women at low risk for ovarian carcinoma suggest that if it is a precursor lesion, p53 signatures rarely progress to a neoplastic lesion. Additionally, not all so-called STIL’s overexpress p53, so the natural progression is somewhat muddled. While most agree that high grade FTIN is a precursor to invasive carcinoma, the biological significance of p53-signature and STIL is not yet fully understood.
Studies suggest that the prognosis of a diagnosis of FTIN is good. While it is generally accepted that FTIN is a precursor to high grade serous carcinoma, there is evidence that patients with isolated FTIN have favorable outcomes,35–37 though the number of patients with long-term follow up is limited, and some have been treated with chemotherapy.38 There is a growing consensus that treatment of isolated FTIN does not require adjuvant chemotherapy, particularly in patients with negative cytology and complete resection and pathologic evaluation of both fallopian tubes.39 However, studies are still mixed.40,41
Regarding the diagnostic reproducibility, the diagnosis of so-called “STIL” and “FTIN” of the epithelium of the fallopian tube is challenging. Clinical algorithms using p53 and Ki67 immunohistochemistry have been proposed to aid in the diagnosis.24,25 The previous studies on interobserver variability by Vang, Visvanthan, and Carlson23–25 reported diagnostic reproducibility using κ statistics, and demonstrated κs ranging from 0.11 to 0.73. One of the limitations of our current study and prior studies is the use of a κ statistic in evaluating a rare event. The κ statistic is best-suited for frequent events and is artificially low when dealing with rare events, as is the case in our study. However, we used κ statistics to be comparable to prior literature, and additionally, we report the percentage of agreement, providing additional evidence of agreement. A second limitation of both our study and prior studies is the lack of ground truth in diagnosing FTIN. There is no independent means to identify FTIN, and thus no way to determine if either pathologist is accurate in their diagnoses. However, the fact that FTIN was only identified in the high-risk group and not in any of the average-risk women lends credibility to our diagnoses, as it would be rather unlikely to identify FTIN in a sample of 98 average-risk women. Regardless, we hope we have demonstrated in this study the significant inter-pathologist variability in diagnosing FTIN.
In our study, based on histology alone, pathologists had relatively good concordance in making a diagnosis of definite for FTIN. Criteria used to identify cases that were definite for FTIN included severe nuclear atypia and moderate nuclear atypia with associated nuclear stratification. Conversely, there was no agreement between pathologists on what should be considered “indeterminate for FTIN.” In support of the reliability of the FTIN diagnosis, FTIN was only identified in women with at increased risk of ovarian carcinoma based on genetic results or family history while atypia was identified in both cases and controls. Although, the κ statistics improved following the use of immunostains, we find that they have limited utility. Immunostains served to change diagnoses of indeterminate for FTIN to negative for FTIN, and confused diagnoses in cases that were definite for FTIN by histology and agreed upon by both pathologists. For this reason, at our institution, we use an alternative diagnostic algorithm for atypical lesions in the fallopian tube epithelium.
Our proposed algorithm is summarized in Figure 4. Lesions that meet criteria for FTIN by histology (based on nuclear atypia and nuclear stratification) are given a diagnosis of FTIN and do not require additional studies (IHC). Cases that are considered indeterminate for FTIN are stained with p53 and Ki67. Strong p53 staining is supportive of a diagnosis of FTIN. Ki67 staining can help support the diagnosis, but absence of increased Ki67 staining should not exclude a diagnosis of FTIN. Cases that show no increased p53 or Ki67 staining are considered non-neoplastic in our algorithm. However, it is important to note lack of increased p53 staining does not truly exclude a diagnosis of FTIN; we simply do not have diagnostic evidence of the presence of FTIN and would not want to unnecessarily treat a patient for FTIN. Similarly, in the one case with increased Ki67 staining, but no increase in p53 staining or evidence of null phenotype, we are unable to deliver a diagnosis of FTIN. The increased Ki67 in these kinds of cases is non-specific and could be indicative of a reactive, not neoplastic, lesion. In these cases, we render a diagnosis of indeterminate for FTIN, but favor a reactive etiology.
Figure 4:
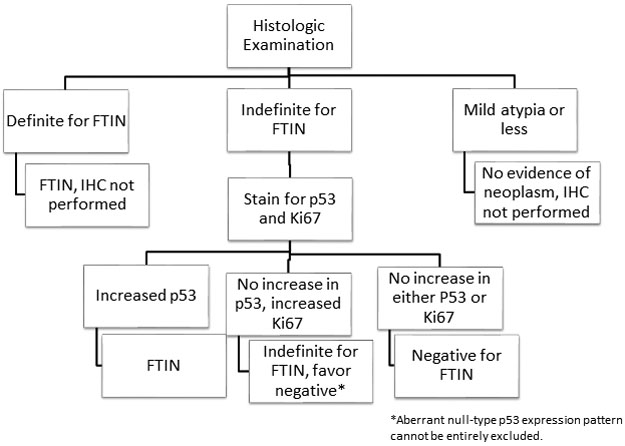
Clinical algorithm used at our institution for diagnosing FTIN
After using immunostains in cases of indeterminate for FTIN and definite for FTIN and following our proposed clinical algorithm, the κ statistics improved. This improvement was due to clarification of diagnosis in the indeterminate category, which prior to immunostains had no agreement between pathologists. Immunostains for p53 and Ki67 performed on cases that were diagnosed as definite for FTIN either supported the histologic diagnosis (41.7% of cases) or were suggestive of a possible null-type p53 phenotype (25% of cases), which only served to further confuse the diagnosis. Immunostains on cases that were worrisome, but not definite for FTIN, identified a p53+ FTIN lesion in 13% of cases. However, as was seen in the “definite for FTIN” cases, a negative stain (p53 null-type phenotype neoplasms) is challenging to evaluate in small foci (common in fallopian tube intraepithelial lesions). Thus, in our experience, the absence of p53-positive foci cannot exclude a diagnosis FTIN. Overall, the usefulness of p53 and Ki67 in this small sample of lesions was relatively limited. By using our algorithm and only considering immunostains in the case of “indeterminate for FTIN” cases, we reached a κ of 0.79, which was better than the κ’s cited in previous literature.
There were additional limitations to this study beyond the use of κ statistics. One challenge is in distinguishing diagnoses of FTIN from metastases masquerading as intraepithelial neoplasia.42 Secondly, the significance of detecting FTIN in the setting of uterine serous carcinoma in unclear.43 These are difficult issues in actual practice and are not addressed in our study. Another limitation was having only two pathologists review the cases. However, although there were only two pathologists reviewing the cases, these dedicated gynecologic pathologists each had over 10 and 20 years of experience. It is likely that equally experienced and less-experienced pathologists would have the same difficulty in diagnosing this entity. Additionally, this study design does not mirror actual real-life practice. The reviewing pathologists were not able to order levels and they couldn’t show challenging cases around to colleagues. The slides were each looked at independently rather than as part of a case. In practice, we expect pathologists to show slides to colleagues in cases that they are concerned may be FTIN. In fact, we strongly recommend this practice, as poor concordance between pathologists was common in cases that did not receive a diagnosis of FTIN when IHC stains were performed. Lastly, we did not test intra-observer variability, so we cannot address the issue of internal reproducibility.
In summary, we propose an alternative clinical algorithm based predominantly on histologic evaluation (see Figure 4). In our model, fallopian tubes with foci of severe nuclear atypia or moderate nuclear atypia with nuclear stratification are given a diagnosis of FTIN. In cases with moderate nuclear atypia but without nuclear stratification (i.e. indeterminate for FTIN), p53 and Ki67 immunostains may be performed, with the caveat that absence of strong p53 staining (overexpression of p53) does not exclude FTIN. Diagnoses of “STIL” are not clinically actionable in our system and therefore are not included in our algorithm. Further evaluation of the biologic behavior of these lesions (STIL) is needed.
Figure 2:
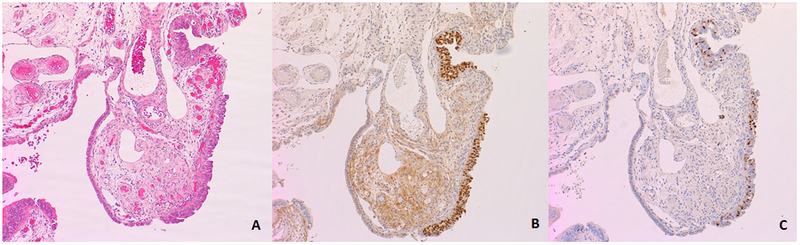
Examples of immunohistochemical staining for (B) p53 and (C) Ki67. In this example, both p53 and Ki67 are increased in the region of epithelial atypia.
Acknowledgments
Funding: This study was funded by the National institute of Health 1R01CA131965 to EMS.
References
- 1.Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 2013;24(suppl 10):x16–x21. doi: 10.1093/annonc/mdt463 [DOI] [PubMed] [Google Scholar]
- 2.Labidi-Galy SI, Papp E, Hallberg D, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8(1):1093. doi: 10.1038/s41467-017-00962-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial Carcinoma of the Fimbria and Pelvic Serous Carcinoma: Evidence for a Causal Relationship. Am J Surg Pathol. 2007;31(2):161–169. doi: 10.1097/01.pas.0000213335.40358.47 [DOI] [PubMed] [Google Scholar]
- 4.Eckert MA, Pan S, Hernandez K, et al. Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov 2016;6:1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carcangiu ML, Radice P, Manoukian S, et al. Atypical Epithelial Proliferation in Fallopian Tubes in Prophylactic Salpingo-oophorectomy Specimens from BRCA1 and BRCA2 Germline Mutation Carriers. Int J Gynecol Pathol. 2004;23(1):35–40. doi: 10.1097/01.pgp.0000101082.35393.84 [DOI] [PubMed] [Google Scholar]
- 6.Lamb JD, Garcia RL, Goff BA, et al. Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. Am J Obstet Gynecol 2006;194:1702–1709. [DOI] [PubMed] [Google Scholar]
- 7.Mingels MJJM, Roelofsen T, van der Laak JAWM, et al. Tubal epithelial lesions in salpingo-oophorectomy specimens of BRCA-mutation carriers and controls. Gynecol Oncol. 2012;127(1):88–93. doi: 10.1016/j.ygyno.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 8.Conner JR, Meserve E, Pizer E, et al. Outcome of unexpected adnexal neoplasia discovered during risk reduction salpingo-oophorectomy in women with germ-line BRCA1 or BRCA2 mutations. Gynecol Oncol. 2014;132:280–286. doi: 10.1016/j.ygyno.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agoff SN, Mendelin JE, Grieco VS, Garcia RL. Unexpected gynecologic neoplasms in patients with proven or suspected BRCA-1 or −2 mutations: implications for gross examination, cytology, and clinical follow-up. Am J Surg Pathol. 2002;26(2):171–178. http://www.ncbi.nlm.nih.gov/pubmed/11812938. Accessed November 7, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Vang R, Shih I-M, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology. 2013;62(1):44–58. doi: 10.1111/his.12046 [DOI] [PubMed] [Google Scholar]
- 11.Lim D, Oliva E. Precursors and pathogenesis of ovarian carcinoma. Pathology. 2013;45(3):229–242. doi: 10.1097/PAT.0b013e32835f2264 [DOI] [PubMed] [Google Scholar]
- 12.Reade CJ, McVey RM, Tone AA, et al. The fallopian tube as the origin of high grade serous ovarian cancer: review of a paradigm shift. J Obstet Gynaecol Can. 2014;36(2):133–140. doi: 10.1016/S1701-2163(15)30659-9 [DOI] [PubMed] [Google Scholar]
- 13.Rabban JT, Garg K, Crawford B, Chen L, Zaloudek CJ. Early Detection of High-grade Tubal Serous Carcinoma in Women at Low Risk for Hereditary Breast and Ovarian Cancer Syndrome by Systematic Examination of Fallopian Tubes Incidentally Removed During Benign Surgery. Am J Surg Pathol. 2014;38(6):729–742. doi: 10.1097/PAS.0000000000000199 [DOI] [PubMed] [Google Scholar]
- 14.Mehrad M, Ning G, Chen EY, Mehra KK, Crum CP. A Pathologistʼs Road Map to Benign, Precancerous, and Malignant Intraepithelial Proliferations in the Fallopian Tube. Adv Anat Pathol. 2010;17(5):293–302. doi: 10.1097/PAP.0b013e3181ecdee1 [DOI] [PubMed] [Google Scholar]
- 15.Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol 2007;19:3–9. [DOI] [PubMed] [Google Scholar]
- 16.McDaniel AS, Stall JN, Hovelson DH, et al. Next-Generation Sequencing of Tubal Intraepithelial Carcinomas. JAMA Oncol. 2015;1(8):1128. doi: 10.1001/jamaoncol.2015.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman MS, Huberman R. Carcinosarcoma Arising from Serous Tubal Intraepithelial Carcinoma. J Gynecol Surg. 2017;33(5):205–206. doi: 10.1089/gyn.2017.0012 [DOI] [Google Scholar]
- 18.Brustmann H Ovarian carcinosarcoma associated with bilateral tubal intraepithelial carcinoma: a case report. Int J Gynecol Pathol. 2013;32(4):384–389. doi: 10.1097/PGP.0b013e318264aece [DOI] [PubMed] [Google Scholar]
- 19.Cass I, Walts AE, Barbuto D, Lester J, Karlan B. A cautious view of putative precursors of serous carcinomas in the fallopian tubes of BRCA mutation carriers. Gynecol Oncol. 2014;134(3):492–497. doi: 10.1016/j.ygyno.2014.07.084 [DOI] [PubMed] [Google Scholar]
- 20.Gross AL, Kurman RJ, Vang R, Shih I-M, Visvanathan K. Precursor Lesions of High-Grade Serous Ovarian Carcinoma: Morphological and Molecular Characteristics. J Oncol. 2010;2010:1–9. doi: 10.1155/2010/126295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clement PB, Young RH. Atlas of Gynecologic Surgical Pathology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2008. [Google Scholar]
- 22.Rosai J, Ackerman LV. Rosai and Ackerman’s Surgical Pathology. 9th ed. St. Louis, MO: London: Mosby; 2004. [Google Scholar]
- 23.Carlson JW, Jarboe EA, Kindelberger D, Nucci MR, Hirsch MS, Crum CP. Serous Tubal Intraepithelial Carcinoma: Diagnostic Reproducibility and its Implications. Int J Gynecol Pathol. 2010;29(4):310–314. doi: 10.1097/PGP.0b013e3181c713a8 [DOI] [PubMed] [Google Scholar]
- 24.Visvanathan K, Vang R, Shaw P, et al. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. Am J Surg Pathol. 2011;35(12):1766–1775. doi: 10.1097/PAS.0b013e31822f58bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vang R, Visvanathan K, Gross A, et al. Validation of an algorithm for the diagnosis of serous tubal intraepithelial carcinoma. Int J Gynecol Pathol. 2012;31(3):243–253. doi: 10.1097/PGP.0b013e31823b8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn E, Kurman RJ, Sehdev AS, Shih I-M. Ki-67 Labeling Index as an Adjunct in the Diagnosis of Serous Tubal Intraepithelial Carcinoma. Int J Gynecol Pathol. 2012;31(5):416–422. doi: 10.1097/PGP.0b013e31824cbeb4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishida N, Murakami F, Higaki K. Detection of serous precursor lesions in resected fallopian tubes from patients with benign diseases and a relatively low risk for ovarian cancer. Pathol Int. 2016;66(6):337–342. doi: 10.1111/pin.12419 [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. doi: 10.1002/path.2091 [DOI] [PubMed] [Google Scholar]
- 29.Chene G, Urvoas S, Moret S, et al. Opportunistic Salpingectomy at the Time of Benign Laparoscopic Hysterectomy: Assessment of Possible Complications and Histopathological p53-Signatures. Geburtshilfe Frauenheilkd. 2018;78(06):605–611. doi: 10.1055/a-0611-5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koc N, Ayas S, Arinkan SA. Comparison of the Classical Method and SEE-FIM Protocol in Detecting Microscopic Lesions in Fallopian Tubes with Gynecological Lesions. J Pathol Transl Med. 2018;52(1):21–27. doi: 10.4132/jptm.2016.06.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andeen NK, Bowman R, Baullinger T, Brooks JM, Tretiakova MS. Epitope Preservation Methods for Tissue Microarrays. Am J Clin Pathol. 2017;148(5):380–389. doi: 10.1093/ajcp/aqx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHugh ML. Interrater reliability: the kappa statistic. Biochem Medica. 2012;22(3):276–282. doi: 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folkins AK, Jarboe EA, Saleemuddin A, et al. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. 2008;109(2):168–173. doi: 10.1016/j.ygyno.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norquist BM, Garcia RL, Allison KH, et al. The molecular pathogenesis of hereditary ovarian carcinoma: alterations in the tubal epithelium of women with BRCA1 and BRCA2 mutations. Cancer. 2010;116(22):5261–5271. doi: 10.1002/cncr.25439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trabert B, Coburn SB, Mariani A, et al. Reported Incidence and Survival of Fallopian Tube Carcinomas: A Population-Based Analysis From the North American Association of Central Cancer Registries. J Natl Cancer Inst. 2018;110(7):750–757. doi: 10.1093/jnci/djx263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wethington SL, Park KJ, Soslow RA, et al. Clinical Outcome of Isolated Serous Tubal Intraepithelial Carcinomas (STIC). Int J Gynecol Cancer. 2013;23(9):1603–1611. doi: 10.1097/IGC.0b013e3182a80ac8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patrono MG, Iniesta MD, Malpica A, et al. Clinical outcomes in patients with isolated serous tubal intraepithelial carcinoma (STIC): A comprehensive review. Gynecol Oncol. 2015;139(3):568–572. doi: 10.1016/j.ygyno.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 38.Powell CB, Swisher EM, Cass I, et al. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecol Oncol. 2013;129(2):364–371. doi: 10.1016/j.ygyno.2013.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinberger V, Bednarikova M, Cibula D, Zikan M. Serous tubal intraepithelial carcinoma (STIC) – clinical impact and management. Expert Rev Anticancer Ther. 2016;16(12):1311–1321. doi: 10.1080/14737140.2016.1247699 [DOI] [PubMed] [Google Scholar]
- 40.Van der Hoeven NMA, Van Wijk K, Bonfrer SE, et al. Outcome and Prognostic Impact of Surgical Staging in Serous Tubal Intraepithelial Carcinoma: A Cohort Study and Systematic Review. Clin Oncol. 2018;30(8):463–471. doi: 10.1016/j.clon.2018.03.036 [DOI] [PubMed] [Google Scholar]
- 41.PATRONO MG, CORZO C, INIESTA M, RAMIREZ PT. Management of Preinvasive Lesions. Clin Obstet Gynecol. 2017;60(4):771–779. doi: 10.1097/GRF.0000000000000316 [DOI] [PubMed] [Google Scholar]
- 42.Singh R, Cho KR. Serous Tubal Intraepithelial Carcinoma or Not? Metastases to Fallopian Tube Mucosa Can Masquerade as In Situ Lesions. Arch Pathol Lab Med. 2017;141(10):1313–1315. doi: 10.5858/arpa.2017-0231-RA [DOI] [PubMed] [Google Scholar]
- 43.Kommoss F, Faruqi A, Gilks CB, et al. Uterine Serous Carcinomas Frequently Metastasize to the Fallopian Tube and Can Mimic Serous Tubal Intraepithelial Carcinoma. Am J Surg Pathol. 2017;41(2):161–170. doi: 10.1097/PAS.0000000000000757 [DOI] [PubMed] [Google Scholar]