Abstract
CML therapy has improved dramatically with the development of tyrosine kinase inhibitors (TKIs). Prior to the TKI era, we conducted two trials of alpha-interferon (IFN) for post-transplant hematologic and cytogenetic relapse. The complete cytogenetic response rate was 33% and 57% respectively. This report describes a third trial in which 40 patients with molecular relapse between 6–12 months post-transplant were treated with IFN. The projected cytogenetic relapse at 4.5 years was 12.6% compared with 42% in the historical control group. Although this data may not apply to most patients with CML today due to the availability of multiple TKIs, the effectiveness of short term IFN in post-transplant molecular relapse is supported by long-term treatment-free-survival in 75% of patients after a median follow-up of 15.6 years. This report suggests that alpha-interferon is potentially useful in the rare patient who has post-transplant molecular relapse who does not tolerate, or is resistant to TKIs.
Keywords: CML, molecular relapse, MRD, interferon, HCT
Introduction
Patients with chronic myeloid leukemia (CML) who have evidence of minimal residual disease (MRD) by polymerase chain reaction (PCR) between 6 and 12 months after allogeneic hematopoietic cell transplantation (HCT) are at high risk of subsequent disease progression. The Kaplan Meier estimate of cytogenetic or clinical relapse at 4.5 years from HCT is 42%, while only 3% of those who remain on molecular remission during the same period will relapse.1 Today CML relapse after HCT is often treated by donor lymphocyte infusion (DLI) or tyrosine kinase inhibitors (TKI).2–5 However, these options may not be always available or feasible. Currently most patients who receive HCT for CML have already failed or have not tolerated TKIs prior to HCT6–7 and, in patients with chronic graft-versus-host disease (GvHD), DLI is contraindicated.8 In some patients, DLI is not available such as in the case of umbilical cord blood HCT or when the donors are not able to provide a second cell collection for health or other reasons.8
In the pre-TKIs era, alpha-interferon (IFN) was shown to induce up to 20% complete cytogenetic responses (CCR) in patients with CML.9–10 Because CML was also one of the diseases with best evidence of the graft-versus-leukemia (GvL) effect.11–12, use of IFN to treat relapse of CML after HCT was explored as an alternative to a more toxic second HCT prior to the availability of TKIs. In hematologic relapse of CML after allogeneic HCT, IFN resulted in a 33% CCR13 while in cytogenetic relapse, the CCR rate was 57%14, suggesting that IFN is more effective in the post HCT setting when the disease burden is lower.13–16 Other data has shown that IFN had better results in recipients of grafts without T-cell depletion, underscoring the importance of donor T-cells to the GvL response.15 This historical study explored the effect of IFN on the next lower level of MRD, patients with PCR relapse 6–12 months after allogeneic HCT.
Patients and Methods
Study design and participants:
This was a phase II clinical trial conducted at Fred Hutchinson Cancer Research Center (FHCRC). We enrolled patients with CML who had molecular MRD between approximately 6 and 12 months after allogeneic HCT. We defined molecular MRD as a positive qualitative reverse transcriptase polymerase chain reaction (RT-PCR) assay for BCR-ABL mRNA, sensitivity 10(−5), in the absence of Philadelphia (Ph) chromosome in the bone marrow (BM) by conventional cytogenetics. Patients with severe cytopenias or comorbidities, poor performance status or second transplant were excluded. Of note, patients with uncontrolled GvHD were excluded but GvHD per se was not an exclusion criteria and it was not required that immunosuppression be tapered off before study entry.
Procedures:
The starting dose of IFN (alpha 2a interferon, Roche) was 1×10(6) units (U)/day given subcutaneously to be administered over 12 months. Patients were regularly monitored for toxicity by their primary oncologist and telephone calls from the research nurse (DC). Dose adjustments were made by the principal investigator (PI, CSH) based on hematologic or non-hematologic toxicity. IFN was held or discontinued for GvHD flare. Patients who progressed from molecular to cytogenetic relapse were taken off protocol. Cytogenetic relapse was defined by the presence of 2 or more Ph chromosomes within 20 counted metaphases. Toxicities were retrospectively graded based on the “Common Terminology Criteria for Adverse Events version 4·0”. GvHD flare was defined as the need to start immunosuppression or to increase the existing immunosuppressive regimen for uncontrolled GvHD activity.
Objectives:
The primary objective of this study was to explore the potential efficacy of IFN decreasing cytogenetic or clinical relapse in comparison to historical control data for untreated patients with molecular MRD between 6 and 12 months after HCT. The treatment was considered efficacious if the KM estimate of relapse of PCR-positive patients was decreased from 40% to 20% at 4·5 years from HCT. The other study objectives included evaluation of IFN toxicities and rate of molecular response. In addition, treatment-free-survival (TFS), overall survival (OS), treatment related mortality (TRM), non-relapse mortality (NRM) and GvHD activity were analyzed. Molecular response was defined as no evidence of BCR-ABL by qualitative RT-PCR after initiation of IFN.1 TFS was defined as time from IFN to further CML therapies or death. TRM was defined as non-relapse death during IFN or within 4 months of its discontinuation. GvHD flare was defined as the need to start immunosuppression or to increase the existing immunosuppressive regimen for uncontrolled GvHD activity.
Statistical analysis:
The data for this study were abstracted from each subject’s research records and the FHCRC clinical database. The estimate of cytogenetic or clinical relapse at 4.5 years was defined based on KM method.17 Using a two-sided significance level of 0·10, 32 patients would allow detection of the difference of 40 to 20% in the relapse at 4·5 years, with 80% power (normal approximation to the binomial distribution). The survival curves (TFS and OS) were calculated using the KM method. Molecular disease free survival (DFS) could not be accurately calculated due to the lack of longitudinal PCR data, however, TFS is assumed to be an indicator of DFS. Comparisons of rate of molecular response and cytogenetic relapse among binary subgroups were based on Fisher’s Exact Test. The statistical analysis and graphics were performed using SAS software version 9·4. An informed consent was obtained from all enrolled patients and this study was approved by the FHCRC Institutional Review Board (IRB).
Results
Forty subjects enrolled and were treated with INF between 1995 and 1999, median age 42 years, (interquartile range [IQR] 34–47 years). Table 1 depicts the characteristics of the study population. None had previously received TKI therapy and 19 (47·5%) had received IFN prior to the HCT for CML. At HCT, 27 (67·5%) subjects were in chronic phase (CP), 10 (25%) in accelerated phase (AP), and 3 (7·5%) were in complete hematologic remission (CHR) after blast crisis (BC), chronic phase 2 (CP2). All subjects received a myeloablative conditioning HCT and methotrexate with calcineurin inhibitors (CIN) for GvHD prophylaxis. Four patients (10%) also received in vivo T-cell depletion with anti-thymocyte globulin (ATG) for GvHD prophylaxis. There were 18 (45%) HLA-matched related, 13 (32·5%) HLA-matched unrelated, and 9 (22·5%) HLA-mismatched donors. The graft source was BM in 95% and mobilized peripheral blood stem cells in 5% of the subjects. The median time from HCT to molecular MRD was 6 months (IQR 6–9).
Table 1:
Patient characteristics
| Patients, n (%) | 40 (100) |
|---|---|
| Age at study entry, median (range) | 42 (16–67) |
| Gender, female, n (%) | 20 (50) |
| Phase of chronic myeloid leukemia at HCT, n (%) | |
| Chronic | 27 (67.5) |
| Accelerated | 10 (25) |
| Blast crisis in complete hematologic remission | 3 (7.5) |
| Donor type, n (%) | |
| Matched related | 18 (45) |
| Matched unrelated | 13 (32.5) |
| Mismatched related | 4 (10) |
| Mismatched unrelated | 5 (12.5) |
| Graft source, n (%) | |
| Bone Marrow | 38 (95) |
| Peripheral blood stem cells | 2 (5) |
| Conditioning regimen, n (%) | |
| Total body irradiation + cyclophosphamide | 22 (55) |
| Busulfan + cyclophosphamide | 18 (45) |
| GvHD prophylactic regimen, n (%) | |
| Methotrexate + calcineurin inhibitor | 40 (100) |
| Anti-thymocyte globulin | 4 (10) |
| Months from HCT to molecular relapse, median (range) | 6.3 (5.6–13.6) |
| Complete cytogenetic remission at enrollment, n (%) | 40 (100) |
| Use of TKIs prior to enrollment, n (%) | 0 |
| Use of IFN prior to transplant, n (%) | 19 (47.5) |
The median time from transplant to start of IFN was 9 months (IQR 8–11) and the median length of IFN treatment was 6 months (IQR 3–12). The median maximum tolerated IFN daily dose was 1×10(6) units. Of the 40 subjects, 16 (40%) completed the planned one year of treatment with IFN. Reasons for early discontinuation of IFN in 24 are grade 1/2 adverse effect in 11 (27·5%), GvHD in 5 (12·5%), grade 3/4 adverse events in 4 (10%), disease progression in 3 (7·5%) and unknown in 1 case (2·5%).
There was no treatment related mortality associated with IFN. Table 2 shows all grades of hematological and non-hematological adverse events observed in the study. Most common grade 3/4 hematologic adverse events was uncomplicated neutropenia in 7 of the 11 cases, with none developing grade 4 neutropenia or neutropenic fever. Of the 3 subjects who required transfusion support, 1 had immune thrombocytopenic purpura and 1 had auto-immune hemolytic anemia. The most common non-hematologic adverse events were grade 1/2 fatigue (62·5%), gastrointestinal symptoms (60%) and febrile symptoms including fevers, chills or night sweats (37·5%). There were 3 cases of grade 3/4 non-hematologic adverse events including one case of idiopathic pneumonitis, and 2 cases of community acquired pneumonia that required hospital admission. One of these patients also had grade 3 elevation of liver enzymes. One patient developed nephrotic syndrome that was attributed to GvHD.
Table 2:
Adverse events
|
Adverse event |
CTCAEv4 Grade |
Total |
|
|---|---|---|---|
| 1–2 | 3–4 | ||
| n (%) | |||
| Non-Hematologic | |||
| Fatigue | 25 (62.5) | 0 | 25 (62.5) |
| Gastrointestinal | 24 (60) | 0 | 24 (60) |
| Fevers/Chills | 15 (37.5) | 0 | 15 (37.5) |
| MSK pain | 11 (27.5) | 0 | 11 (27.5) |
| Depression | 6 (15) | 0 | 6 (15) |
| Weight loss | 6 (15) | 0 | 6 (15) |
| Pneumonia | 2 (5) | 2 (5) | 4 (10) |
| Liver | 2 (5) | 1 (2.5) | 3 (7.5) |
| Pneumonitis | 0 | 1 (2.5) | 1 (2.5) |
| Hematologic | |||
| Anemia | 7 (17.5) | 3 (7.5) | 10 (25) |
| Neutropenia | 13 (32.5) | 7 (17.5) | 20 (50) |
| Thrombocytopenia | 22 (55) | 2 (5) | 24 (60) |
Characteristics of GvHD and immunosuppression treatment (IS), before and during treatment with INF, are summarized in Table 3. At enrollment, 35 patients (87·5%), were receiving immunosuppression for prophylaxis or treatment of prior GvHD. During the study, 24 of the 35 patients (68·6%) were able to be tapered off IS. Nine patients (22·5%) developed GvHD during IFN treatment that required additional or increased dose of IS for either new diagnosis of GvHD (n=5) or exacerbation of prior GvHD (n=4).
Table 3:
GvHD and Immunosuppression (IS) characteristics before and during interferon
| Characteristics | |
|---|---|
| Prior history of grade II-IV acute GvHD, n (%) | 29 (73) |
| Prior history of chronic GvHD requiring IS, n (%) | 14 (35) |
| Receiving IS at time of molecular relapse, n (%) | 35 (87) |
| With corticosteroids > 0.5 mg/kg/day | 3 (7) |
| With corticosteroids ≤ 0.5 mg/kg/day | 14 (35) |
| Without corticosteroids | 18 (45) |
| Patients tapered off IS during IFN, n (%) | 24/35 (69) |
| New GvHD requiring IS during IFN, n (%) | 5/26 (19) |
| Exacerbation of prior GvHD requiring IS, n (%) | 4/14 (29) |
| Receiving IS during IFN, n (%) | 26 (65) |
| Highest level of IS at any point on INF, n (%) | |
| With corticosteroids > 0.5 mg/kg | 5 (12) |
| With corticosteroids ≤ 0.5 mg/kg | 13 (32) |
| Without corticosteroids | 8 (20) |
A swimmer plot shown in Figure 1 depicts major disease outcomes for each of the 40 study participants. The KM estimate cytogenetic or clinical relapse at 4·5 years after HCT was 12·6%, a relative decrease of 70% when compared to the historical data, p<0·01. The cumulative incidence of cytogenetic and clinical relapse is shown in Figure 2. With a median follow-up of 15·6 years, 4 patients (10%) developed cytogenetic-only relapse and 6 (15%) hematologic relapse. Only 10 patients (25%) required further CML therapy. Among these, 2 (5%) received a second course of IFN, 5 (12.5%) TKIs, 1 (2.5%) DLI and 1 (2.5%) a second HCT. During follow-up, relapse related mortality was 12·5% and non-relapse related mortality was 15%. Median TFS and OS were not reached as shown in Figure 3. Molecular remission was achieved by 30 patients (75%) after IFN. Without any further CML therapy after IFN, 27 of the subjects (67·5%) did not have measurable residual disease by PCR at the end of follow-up. Regarding recipients of in vivo T-cell depleted grafts by ATG, 2/4 patients (50%), experienced further cytogenetic relapse. Among patients at AP or CP2 at transplant, 6/13 (46.2%) had further cytogenetic relapse, while only 4/27 (14.8%) on CP, p = 0.052.
Figure 1:
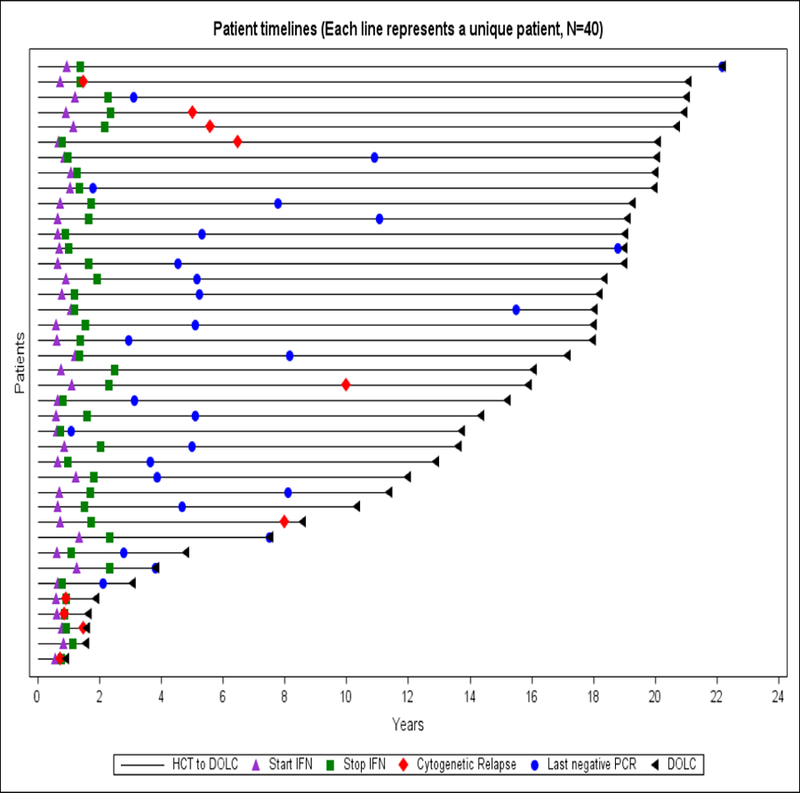
Individual disease status after study enrollment:
Figure 2:
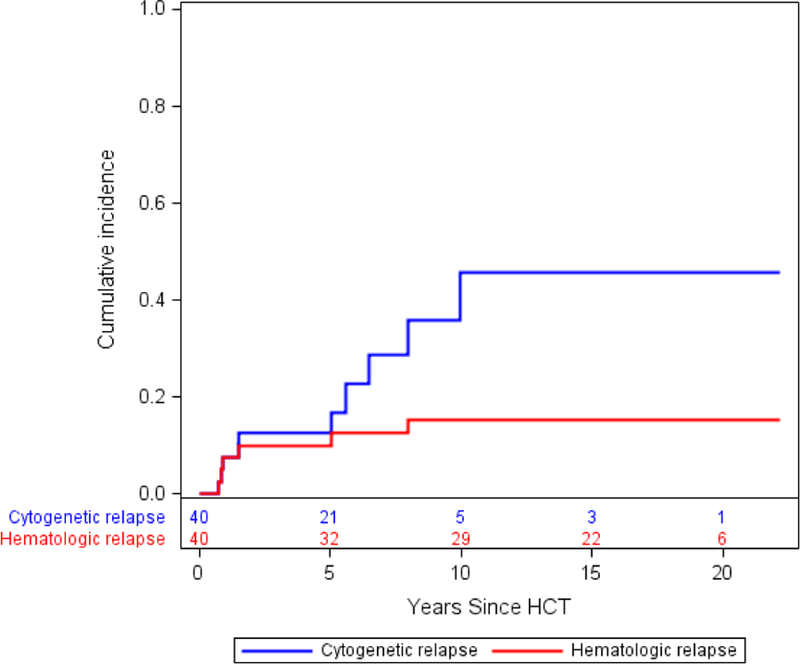
Cumulative incidence of cytogenetic relapse by Kaplan-Meier:
Figure 3:
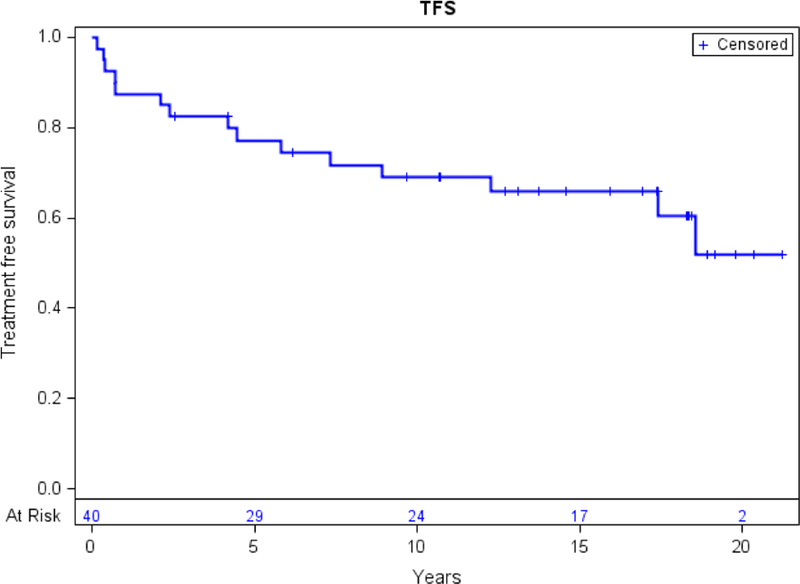
Treatment free survival:
Discussion
Since this study was designed and executed, the development of TKIs targeting bcr-abl have made a major impact on the treatment of patients with CML. Prior to that time, HCT was the only possible curative therapy. However, today most patients are treated with TKI therapy and never need HCT. Nonetheless, the data clearly demonstrate the principle that the GvL effect of IFN is best when there is molecular MRD compared to either cytogenetic or hematologic relapse after HCT. The activity and long-term follow-up of patients treated with IFN in this setting has never been reported.
For this high risk CML population with MRD detected between 6 and 12 months after HCT who received IFN treatment in this study, the 4.5 year estimate of subsequent relapse was 12.6%. This finding contrasts with our historical previously reported estimate of relapse of 42% at 4.5 years in a similar high risk population who received no intervention despite MRD during the same window of time as the current study.1 Another remarkable outcome of this clinical trial was that only 25% of the patients required further CML therapies like TKI (12.5%) or DLI (2.5%) over a very long median follow-up of 15.6 years, suggesting that IFN prevented disease progression and de facto cured 75% of the patients.
Although this clinical trial was conducted 20 years ago, treatment with interferon to prevent or treat malignancy after HCT via optimizing the graft-versus-leukemia (GVL) effect remains a great interest, especially as we gain better understanding of the power of the immune system and new immune modulation therapies are emerging to eradicate malignancies.18–19 Of note, Mo et al reported that patients with AML who had MRD after HCT who were treated with IFN decreased relapse rate from 57 to 30% at 2 years, similar to treatment with DLI, but with less GvHD exacerbation.20–21
Treatment with IFN less than one year after HCT was associated with significant side effects in our study resulting in early discontinuation of treatment before 1 year in 50% of the patients. Nonetheless, there were no deaths related to IFN treatment and most of the early treatment discontinuation was due to grade 1/2 toxicities. Of note, major toxicities seen in this study such as ITP, AIHA, pneumonia and pneumonitis are common complications in the post-transplant setting even without IFN therapy.
Development of or flare of previous GvHD during treatment with INF was observed in 22.5% of patients and no patient died as a consequence of this complication. The GvHD rates in our study are similar to those previously reported with post-transplant interferon for hematological relapse.13–16 The cumulative incidence of GvHD reported after DLI is 40%, higher than that observed after INF, albeit lower rates of GvHD after DLI are possible using lower initial cell dose.8, 22
IFN at the dose of 3 × 106 U/M2/day is often associated with significant toxicity.13–16 In this study, a substantially lower dose of IFN 1MU/day was not only effective but also safe, even in such close proximity to HCT. In spite of using a lower dose of IFN and a shorter duration of therapy than originally planned, IFN resulted in a higher rate of complete molecular response, 75%, than that previously reported for patient treated for hematologic and cytogenetic relapse after HCT.13–16 In Mo’s study of IFN treatment of post-HCT MRD in AML, IFN was administered at 3×10(6) units 2–3 times per week rather than daily, and the median duration of treatment was only 35 days.20–21
Both TKI and DLI represent alternative strategies for prevention or treatment of post-transplant relapse of CML.2–5, 23 The largest report of TKI in this clinical scenario of molecular MRD was published by Hess et al, where 15 of 18 patients (83.3%) achieved complete molecular remission22–24, compared to 75% seen with IFN in this study. Nonetheless, the rates of post-transplant TKI intolerance are not insignificant, 31–38%.25–26 Other potential restrictions to TKIs are pregnancy, significant cardiac dysfunction, and lack of worldwide availability of ponatinib for patients harboring T135I mutation.27–29 Radujkovic et al showed that DLI resulted in failure free survival of 68% at 5 years in 80 patients with molecular relapse and Chalandon et al saw a 59% of molecular remission at 5 years in 85 subjects.30–31 Neither of these studies restricted the timing of molecular relapse and therefore their outcomes may be inflated due to the inclusion of those who became PCR positive after 12 months who have a very low relapse rate.1, 32 The 67.5% of molecular remission observed in this study includes only those at high risk. The rate of new onset GvHD at Radujkovic et al was at least 28%, while our data with IFN was only 12.5%.30 Chalandon et al reported 81% of OS and non-relapse mortality of 11% at 5 years, compared to 85% and 5% in our study respectively.31 Both Chalandon et al and Radukkovic et al also showed that there are no differences in outcome when DLI is given for molecular versus cytogenetic relapse while we have consistently shown that the magnitude of response to IFN is related to disease burden.13–16
The current study has several limitations. First, the study was conducted 20 years ago prior to the advent of TKI’s and with limited DLI reports. Likewise, the technology for identifying PCR positivity and cytogenetic relapse has improved over time. However, these limitations might be overcome by the fact that the historical control data and our trial used the same older technologies. Another limitation is the formulation of interferon used in our study is no longer available, thus extrapolation of dosing of other interferons may be difficult. However, modern formulations of IFN (eg pegylated) may be better tolerated.
Our results are aligned with Talpaz et al who suggest that IFN may still be a treatment option for CML in selected cases.33–34 For instance, most CML patients who currently undergo allogeneic HCT have either failed or not tolerated treatment with TKIs. In addition, after HCT, patients often relapse with the same TKI resistance profile as they had before transplant.35 For such patients, treatment of post-transplant relapse of CML with TKIs may be problematic. Given that DLI has higher rates of complications than does IFN and that DLI has the same outcomes when given either in molecular or cytogenetic relapse, the few patients who progress to cytogenetic relapse on IFN could theoretically still be salvaged by DLI without worse outcomes. While this study of IFN does not examine its performance after prior TKI therapy and HCT, IFN should still be effective if the mechanism of action is related to GvL rather than direct cytotoxic effect.
Acknowledgements
The authors thank all patients who enrolled on this study and acknowledge the staff of the “Long Term Follow-up” clinic at FHCRC who assisted with the subject’s follow-up. This study was funded in part by an unrestricted grant from Dr. Judith A. Prestifilippo at Hoffman LaRoche. M.E.F. was funded in part by a grant from the National Institutes of Health, National Cancer Institute (CA118953).
Footnotes
Data sharing statement
All individual participant data collected during this trial will be available after de-identification. The study protocol and the consent forms will also be available. The data will be made available from publication through the following 36 months. The data will be provided to researchers who require it to achieve a methodological proposal. Proposal should be addressed to the corresponding author.
References:
- 1.Radich JP, Gehly G, Gooley T, et al. Polymerase Chain Reaction Detection of the BCR-ABL Fusion Transcript After Allogeneic Marrow Transplantation for Chronic Myeloid Leukemia: Results and Implications in 346 Patients. Blood 1995; 85: 2632–2638. [PubMed] [Google Scholar]
- 2.Dazzi F, Szydlo RM, Cross NC, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood 2000; 96: 2712–2716. [PubMed] [Google Scholar]
- 3.Kantarjian HM, O’Brien S, Cortes JE, et al. Imatinib mesylate therapy for relapse after allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood 2002; 100: 1590–1595. [PubMed] [Google Scholar]
- 4.Zeidner JF, Zahurak M, Rosner GL, Gocke CD, Jones RJ, Smith BD. The evolution of treatment strategies for patients with chronic myeloid leukemia relapsing after allogeneic bone marrow transplantation: Can tyrosine kinase inhibitors replace donor lymphocyte infusions? Leuk Lymphoma 2015; 56(1): 128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanavas M, Messner HA, Kamel-Reid S, et al. A Comparison of Long-Term Outcomes of Donor Lymphocyte Infusions and Tyrosine Kinase Inhibitors in Patients With Relapsed CML After Allogeneic Hematopoietic Cell Transplantation. Clinical Lymphoma, Myeloma & Leukemia 2014; 14: 87–92. [DOI] [PubMed] [Google Scholar]
- 6.Nair AP, Barnett MJ, Broady RC, et al. Allogeneic Hematopoietic Stem Cell Transplantation Is an Effective Salvage Therapy for Patients with Chronic Myeloid Leukemia Presenting with Advanced Disease or Failing Treatment with Tyrosine Kinase Inhibitors. Biology of Blood and Marrow Transplantation 2015; 21: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 7.Nicolini FE, Basak GW, Soverini S, et al. Allogeneic stem cell transplantation for patients harboring T315I BCR-ABL mutated leukemias. Blood 2011; 118: 5697–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castagna L, Sarina B, Bramanti S, Perseghin P, Mariotti J, Morabito L. Donor lymphocyte infusion after allogeneic stem cell transplantation. Transfusion and Apheresis Science 2016; 54: 345–355. [DOI] [PubMed] [Google Scholar]
- 9.Talpaz M, Kantarjian HM, McCredie K, Trujillo JM, Keating MJ, Gutterman JU. Hematologic remission and cytogenetic improvement induced by recombinant human interferon alpha A in chronic myelogenous leukemia. New Engl J Med 1986; 314: 1065–1069. [DOI] [PubMed] [Google Scholar]
- 10.Talpaz M, Kantarjian H, Kurzrock R, Trujillo JM, Gutterman JU. Interferon-alpha produces sustained cytogenetic responses in chronic myelogenous leukemia. Philadelphia chromosome-positive patients. Ann Intern Med 1991; 114(7): 532–538 [DOI] [PubMed] [Google Scholar]
- 11.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus- leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995; 86: 2041–2050. [PubMed] [Google Scholar]
- 12.Porter DL, Roth MS, McGarigle C, Ferrara JL, Antin JH. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. New England Journal of Medicine 1994; 330: 100–106. [DOI] [PubMed] [Google Scholar]
- 13.Higano CS, Raskind WH, Singer JW. Use of α Interferon for the Treatment of Relapse of Chronic Myelogenous Leukemia in Chronic Phase After Allogeneic Bone Marrow Transplantation. Blood 1992; 80: 1437–1442. [PubMed] [Google Scholar]
- 14.Higano CS, Chielens D, Raskind W, et al. Use of α−2a-Interferon to Treat Cytogenetic Relapse of Chronic Myeloid Leukemia After Marrow Transplantation. Blood 1997; 90: 2549–2554. [PubMed] [Google Scholar]
- 15.Steegmann JL, Casado LF, Toma’s JF, et al. Interferon alpha for chronic myeloid leukemia relapsing after allogeneic bone marrow transplantation. Bone Marrow Transplantation 1999; 23: 483–488. [DOI] [PubMed] [Google Scholar]
- 16.Pigneux A, Devergie A, Pochitaloff M et al. Recombinant alpha-interferon as treatment for chronic myelogenous leukemia in relapse after allogeneic bone marrow transplantation: a report from the Societe Francaise de Greffe de Moelle. Bone Marrow Transplant 1995; 15: 819–824. [PubMed] [Google Scholar]
- 17.Kaplan EI, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457. [Google Scholar]
- 18.Salter A, Pont M, Riddell S. Chimeric antigen receptor modified T cells: CD19 and road beyond. Blood 2018; doi: 10.1182/blood-2018-01-785840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlardot A, Bargou R. Bispecific antibodies in hematologic malignancies. Cancer Treatment Reviews 2018; doi: 10.1016/j.ctrv.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Mo X, Zhang X, Xu L, et al. Interferon-a: A Potentially Effective Treatment for Minimal Residual Disease in Acute Leukemia/Myelodysplastic Syndrome after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 2015; 21: 1939–1947. [DOI] [PubMed] [Google Scholar]
- 21.Mo X, Zhang X, Xu L, et al. IFN-α Is Effective for Treatment of Minimal Residual Disease in Patients with Acute Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Registry Study. Biol Blood Marrow Transplant 2017; 23: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 22.Bar M, Sandmaier BM, Inamoto Y, et al. Donor lymphocyte infusion for relapsed hematological malignancies after allogeneic hematopoietic cell transplantation: prognostic relevance of the initial CD3+ T cell dose. Biol Blood Marrow Transplant 2013; 19: 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilleece MH and Dazzi F. Donor Lymphocyte Infusions for Patients with Relapse After Allogeneic Stem Cell Transplantation for Chronic Myeloid Leukemia. Leukemia and Lymphoma 2003; 44: 23–28. [DOI] [PubMed] [Google Scholar]
- 24.Hess G, Bunjes D, Siegert W, et al. Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for chronic myelogenous leukemia: results of a prospective phase II open-label multicenter study. Journal of Clinical Oncology 2005; 23: 7583–7593. [DOI] [PubMed] [Google Scholar]
- 25.DeFilipp Z, Langston AA, Chen Z, et al. Does Post-Transplant Maintenance Therapy with Tyrosine Kinase Inhibitors Improve Outcomes of Patients with High-Risk Philadelphia Chromosome-Positive Leukemia? Clin Lymphoma Myeloma Leuk. 2016; 16(8):466–471. [DOI] [PubMed] [Google Scholar]
- 26.Shimoni A, Volchek Y, Koren-Michowitz M, et al. Phase 1/2 study of nilotinib prophylaxis after allogeneic stem cell transplantation in patients with advanced chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2015; 121(6):863–71 [DOI] [PubMed] [Google Scholar]
- 27.Balsat M, Etienne M, Elhamri M, et al. Successful pregnancies in patients with BCR-ABL-positive leukemias treated with interferon-alpha therapy during the tyrosine kinase inhibitors era. Eur J Haematol 2018; doi: 10.1111/ejh.13167 [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Almaguer D, Cantú-Rodríguez OG, Gutiérrez-Aguirre CH, et al. The treatment of CML at an environment with limited resources. Hematology 2016; 21(10):576–582 [DOI] [PubMed] [Google Scholar]
- 29.Moslehi JJ, Deininger M. Tyrosine kinase inhibitor associated cardiovascular toxicity in chronic myeloid leukemia. Journal of Clinical Oncology 2015; 33(35): 4210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radujkovic A, Guglielmi C, Bergantini S, et al. Donor Lymphocyte Infusions for Chronic Myeloid Leukemia Relapsing after Allogeneic Stem Cell Transplantation: May We Predict Graft-versus-Leukemia Without Graft-versus-Host Disease? Biology of Blood and Marrow Transplantation 2015; 21: 1230–1236. [DOI] [PubMed] [Google Scholar]
- 31.Chalandon Y, Passweg JR, Guglielmi C, et al. Early administration of donor lymphocyte infusions upon molecular relapse after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia: a study by the Chronic Malignancies Working Party of the EBMT. Haematologica 2014; 99: 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamura K, Tahara T, Tanimoto M, et al. Long persistent bcr-abl positive transcript detected by polymerase chain reaction after marrow transplant for chronic myelogenous leukemia without clinical relapse: a study of 64 patients. Blood 1993; 81: 1089–1093. [PubMed] [Google Scholar]
- 33.Talpaz M, Mercer J, Hehlmann R. The interferon-alpha revival in CML. Ann Hematol 2015; 94(Suppl 2): S195–S207. [DOI] [PubMed] [Google Scholar]
- 34.Talpaz M, Hehlmann R, Quintás-Cardama A, Mercer J, Cortes J. Re-emergence of interferon-α in the treatment of chronic myeloid leukemia. Leukemia 2013; April;27(4): 803–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egan DN, Beppu L, Radich JP. Patients with Philadelphia-positive leukemia with BCR-ABL kinase mutations prior to allogeneic transplantation predominantly relapse with the same mutation. Biology Blood Marrow Transplant 2015; 21: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]


