Abstract
Aims:
Developments in genomic pathology have led to novel molecular classification schemes in gastric cancers. Two of these new subtypes, EBV-associated and MSI-H, are associated with a dominant T-cell mediated immune response. The roles of the immune modulators, Indoleamine 2,3-dioxygenase 1 (IDO1) and Tryptophanyl-tRNA Synthetase (WARS), have not been investigated in the context of this classification.
Methods and Results:
Using in situ hybridization and immunohistochemistry we subclassified 421 primary gastric adenocarcinomas into five subtypes, EBV-associated, EMT, MSI-H, p53-aberrant and p53-wildtype tumors. TILs were counted and protein expression of IDO1 and WARS was graded on tissue microarrays of these 421 tumors. High TILs as well as high expression of both IDO1 and WARS was found in EBV and MSI-H tumors. The prognostic effects of IDO1 and WARS expression were tumor subtype-dependent. While high expression levels of IDO1 and WARS were associated with poor prognosis in p53 aberrant, p53-wildtype, and all cancers combined, WARS expression was associated with better prognosis in MSI tumors.
Conclusions:
The immunomodulators, IDO1 and WARs, are upregulated and have prognostic significance in EBV associated and MSI-H tumors. Novel therapies targeting these proteins should be considered in the treatment of these patients.
Keywords: IDO1, WARS, Gastric cancer, Molecular subtype
Background
Gastric cancer is the third leading cancer-related cause of death world-wide after lung and colorectal cancers.1 Developments in molecular pathology have led to several novel classification systems. Division into the Epstein-Bar virus infection, microsatellite instability, genomic stability, chromosomal stability, epithelial to mesenchymal transition (EMT), and p53 aberrant subtypes is a leading classification scheme.2,3,4 Immunohistochemistry and in situ hybridization studies can be used to classify gastric adenocarcinoma into EBV-associated, EMT, MSI unstable, p53 mutated and p53 wildtype tumors.5,6
Studies of the tumor immune microenvironment have led to individualized cancer treatment based on the host’s immune response towards cancer.7 A recently published study analyzed over 10,000 tumors from 33 cancer types using The Cancer Genome Atlas (TCGA) data and generated six tumor subtypes based on their molecular immune landscape: wound healing, IFN-γ dominant, inflammatory, lymphocyte depleted, immunologically quiet and TGF-β dominant.8 Gastric cancer was classified into IFN-γ dominant subtype based on its high M1/M2 macrophage, strong CD8 signal, and TCR diversity.8 Among the genes that are induced by IFN-γ are two involving in tryptophan metabolism, indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophanyl-tRNA synthetase (WARS).9,10 IDO1 catalyses the first and rate-limiting step of tryptophan (Trp) catabolism during which Trp is converted into kynurenine (Kyn). IDO1 overexpression and decrease of Trp/Kyn ratio are known immune modulators which inhibit the function of natural killer (NK) cells, prevent the activation of effector T cells, and stimulate regulatory T (Treg) cell activation.11 However, the role of IDO1 in gastric tumors is still unclear. It was found that gastric tumor cells absorb amino acids selectively, resulting in a significant difference in the concentration of Trp in gastric juice between patients with gastric cancer and those with benign gastric disease.12 While IDO1 expression in gastric cancer has been shown to be associated with worse prognosis in patients with or without adjuvant chemotherapy,13,14 other studies have has showed IDO1 as a favorable prognostic marker of gastric cancer.15 WARS catalyses the aminoacylation of tRNA(trp) with tryptophan and has been known to mediate high-affinity tryptophan uptake into human cells,16 which would lead to further decrease of stromal Trp/Kyn ratio. The roles of WARS in gastric cancer progression is largely undefined. It was found to enhance tumor progression in oral cancer17 but is associated with good prognosis in colon cancer18 and triple-negative breast cancer.19
The controversial effects of IDO1 expression on patient outcome could be associated with the heterogenicity of gastric cancer. EBV-associated and MSI unstable gastric cancers are known for their strong inflammatory microenvironment and these two types of tumor tend to have higher amounts of tumor infiltrative lymphocytes than other cancer types.20 Thus, the immunomodulatory role of IDO1 and WARS in cancer may be dependent on distinct immune microenvironment among subtypes. In the present study, we assessed the expression levels of IDO1 and WARS in the tumor epithelium and stroma of the five gastric cancer subtypes and evaluated the prognostic implications of their expression.
Methods
The study was performed in accordance with the ethical guidelines and approval from the Institutional Review Boards of Seoul National University Hospital (Republic of Korea) and Rhode Island Hospital (Rhode Island, United States).
Patients
421 cases of primary gastric cancer were surgically resected at Seoul National University Hospital from 2008 to 2010. Clinicopathological data was collected from pathological reports and medical chart review. Mean follow-up time were 40.2 months (range, 0 to 60). Overall survival was used for outcome analysis. Patients lost to follow-up were censored.
Tissue microarray construction and immunohistochemical stain
Tissue array blocks were prepared as described previously.21 MSI status and EBV status (based on EBV in situ hybridization) were obtained from a previous study.22 Immunohistochemical stains were performed on 4-μm thick microarray paraffin sections on a Discovery Autostainer using DabMap Detection Kit (Ventana Medical Systems, Tucson, AZ). The antibodies used in the study are summarized in Supplementary Table 1, Supplemental Digital Content 1,http://links.lww.com/AIMM/A232.
Absence, nuclear or attenuated E-cadherin stains were considered as aberrant expression. Absence of expression or expression in >95% of tumor cell nuclei was considered as aberrant p53 staining pattern. Expression of IDO1 and WARS was evaluated in the epithelium and surrounding stroma and was scored based on intensity and extent. Intensity was scored as mild (identifiable at 20×, score 1), moderate (identifiable at 10×, score 2) and strong (identifiable at 4×, score 3). The extent was evaluated by percent of tumor cells positive; <5% was negative, 5% to <25% score 1, 25% to −50% score 2, and >50% score 3. Summation of intensity and extent scores of 4 or more was considered positive for epithelial staining of IDO, WARS and stromal staining of IDO. WARS stromal staining was considered positive if more than 30% of the stroma stained was present (identifiable at 4×). CD3 positive cells were counted based on four consecutive high-power fields (HPF) (40×) in the area with the most TILs (Olympus BX53 microscope with UPlanFL N objective, total area equal to 0.952 mm2). Mean TIL count/HPF for each tumor was calculated by dividing the total number of TILs by four. All cut-offs were determined and scored by a single observer (LJW) to minimize inter-observer variability.
Statistical methods
Chi-square analysis was applied to evaluate associations between categorical variables. Fisher’s test was used to replace Chi-square analysis when appropriate. T-test or analysis of variance (ANOVA) was used to compare between continuous variables. Tukey’s HSD was used for pairwise comparison. For time-to-event measures, the Kaplan–Meier method was used to estimate the empirical survival and log-rank estimates were used. Cox proportional hazards model was used for multivariate analysis. All tests were 2-sided using a p-value of 0.05 as threshold for statistical significance. All analyses were performed using JMP Pro 13 (SAS, Cary, NC).
Results
Gastric cancer subtyping and clinicopathological features of each subtype
Following the algorithm proposed by Ahn et al 2017, 421 gastric cancers were classified into 5 groups. Representative immunostains used for subtyping are summarized in Figure 1. Thirty-two (7.6%) cases of EBV positive tumor (EBV subtype), 34 (8.1%) cases of MSI-H (MSI subtype) and 74 (17.6%) cases with aberrant E-cadherin expression (EMT subtype) were identified. Based on their p53 staining pattern, the remaining cases were divided into 180 (42.8%) cases of p53-mutant subtype and 98 (23.3%) cases of p53-wildtype subtype (Table 1). All the EBV tumors were MSI stable and none of the MSI tumors were EBV positive. A minor proportion of EBV (3/27) and MSI (5/31) tumors exhibited aberrant E-cadherin expression. The majority of EBV (23/27), MSI-H (17/31), and EMT (41/69) tumors exhibited wild-type p53 expression pattern (Table 1).
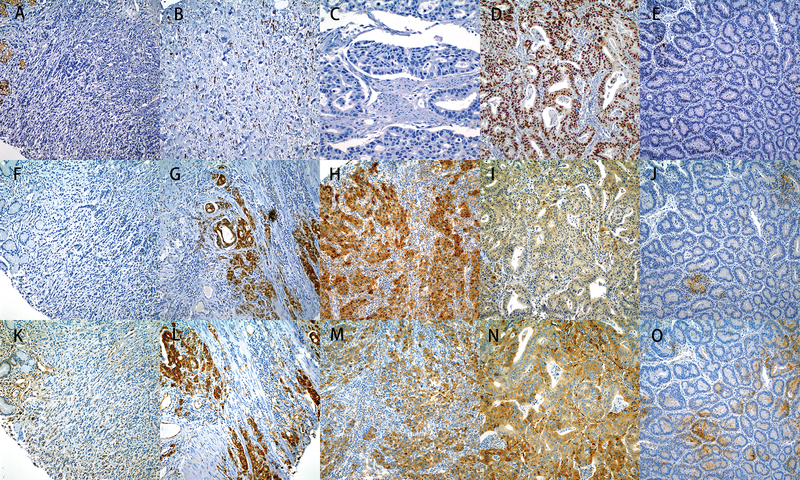
Figure 1.
Immunohistochemical stains of IDO1 and WARS in gastric tumor subgroups. A-E. Subtypes of gastric carcinoma: Aberrant E-cadherin expression (EMT tumor) was determined on the basis of absence or reduced membranous expression of the tumor (A). The membranous expression was present on the normal gastric mucosa at left upper corner of (A) as well as a E-cadherin positive tumor (B). Tumors that were completely negative for p53 expression (C) or strong p53 staining (D) were considered as aberrant p53 expression, while weak and patchy nuclear staining was considered as wild type (E). F-J. IHC for IDO1: Absence of expression of IDO1 in the tumor epithelium and the stromal cells of EMT tumor (F), strong expression in the epithelium and moderate expression in some stromal cells of MSI tumor (G), strong expression in the epithelium and in the stromal cells of EBV tumor (H), focal weakly expression in the epithelium and stromal cells of P53 mutated tumor (I) and negative staining of both tumor and stromal cells of p53 wild type tumor (J). K-O. IHC for WARS: Weak expression of Wars in the endothelial and inflammatory cells in the stroma and negative staining for tumor epithelium of EMT tumor (K), strong diffuse cytoplasmic expression in epithelium and moderate expression in stromal cells of MSI tumor (L), strong expression in the epithelium and in the stromal cells of EBV tumor (M), strong diffuse cytoplasmic expression in the epithelium and negative staining in the stromal cells of p53 mutated tumor (N) moderate expression in the stromal cells and weakly staining in the epithelium of p53 wild type (O). All subtype tumors are from the same cases (A, F and K for EMT’s, G and L for MSI’s; H and M for EBV’s; D, I and N for p53 aberrant and E, J, O for p53 wild type).
Table 1.
Clinicopathologic parameters of gastric cancer subgroups.
| Parameters | total | Subtype | P-value | ||||
|---|---|---|---|---|---|---|---|
| EBV | MSI | EMT | p53-ab | p53-wt | |||
| Sex | 0.0002 | ||||||
| female | 112 | 2 | 13 | 25 | 36 | 36 | |
| male | 309 | 30 | 21 | 49 | 147 | 62 | |
| Age* | 57.5±0.6 | 56.8±2.0 | 67.8±2.1 | 56.2±1.4 | 59.5±0.9 | 52.8±1.3 | <0.0001 |
| WHO | <0.0001 | ||||||
| well diff | 32 | 2 | 4 | 0 | 21 | 5 | |
| mod diff | 147 | 4 | 16 | 8 | 91 | 28 | |
| poorly diff | 147 | 27 | 13 | 27 | 53 | 29 | |
| signet ring cell | 73 | 0 | 0 | 28 | 11 | 34 | |
| others | 22 | 1 | 1 | 11 | 7 | 2 | |
| Lauren | <0.0001 | ||||||
| intestinal | 176 | 9 | 18 | 52 | 107 | 31 | |
| diffuse | 170 | 14 | 9 | 11 | 42 | 53 | |
| mixed | 72 | 9 | 7 | 10 | 32 | 14 | |
| undetermined | 3 | 0 | 0 | 0 | 2 | 0 | |
| EBV | <0.0001 | ||||||
| negative | 381 | 0 | 34 | 72 | 179 | 96 | |
| positive | 32 | 32 | 0 | 0 | 0 | 0 | |
| MSI | <0.0001 | ||||||
| stable | 387 | 32 | 0 | 74 | 183 | 98 | |
| unstable | 34 | 0 | 34 | 0 | 0 | 0 | |
| E-cadherin | <0.0001 | ||||||
| aberrant | 82 | 3 | 5 | 74 | 0 | 0 | |
| normal | 331 | 24 | 26 | 0 | 183 | 98 | |
| p53 status | |||||||
| mutant | 229 | 4 | 14 | 28 | 180 | 0 | <0.0001 |
| wildtype | 179 | 23 | 17 | 41 | 0 | 98 | |
| T stage** | 0.0037 | ||||||
| T1 | 112 | 12 | 5 | 11 | 47 | 37 | |
| T2 | 64 | 3 | 11 | 9 | 25 | 16 | |
| T3 | 151 | 10 | 15 | 34 | 65 | 27 | |
| T4 | 94 | 7 | 3 | 20 | 46 | 18 | |
| N stage** | 0.2168 | ||||||
| N0 | 176 | 18 | 15 | 25 | 69 | 49 | |
| N1 | 58 | 4 | 6 | 7 | 27 | 14 | |
| N2 | 58 | 5 | 5 | 12 | 24 | 12 | |
| N3 | 129 | 5 | 8 | 30 | 63 | 23 | |
| TNM stage** | 0.0110 | ||||||
| I | 141 | 13 | 9 | 16 | 57 | 46 | |
| II | 94 | 9 | 12 | 17 | 38 | 18 | |
| III | 133 | 5 | 12 | 31 | 60 | 25 | |
| IV | 53 | 5 | 1 | 10 | 28 | 9 | |
p53-wt: p53 wildtype; p53-ab: p53 aberrant
Age is presented as mean ± SEM (standard error of mean)
Based on AJCC seventh edition.
Lymphocyte infiltrates in the tumor epithelium and stroma
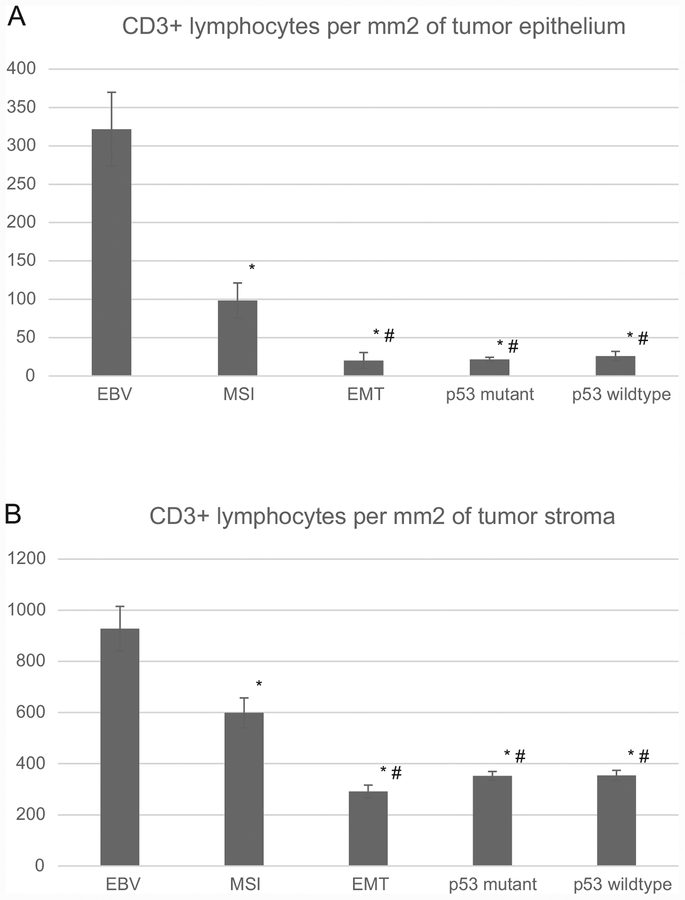
The mean TIL count in the tumor epithelium was 321.7/mm2 in EBV tumors and was significantly higher than all other subtypes (P<0.0001). The mean TIL count in MSI tumor epithelium was 98.5/mm2 and was significantly higher than those in EMT (20.7), p53 aberrant (21.7) or p53 wildtype (25.9) subtypes (all Ps <0.0001). No difference was seen in the numbers of TILs among EMT, p53 aberrant and p53 wildtype tumors (Figure 2A).
Figure 2.
CD3-positive lymphocytes in tumor epithelium (A) and stroma (B) of gastric cancer subgroups (error bar: standard error of mean); * significantly different from EBV subgroup; # significantly different from MSI subgroup.
Mean stromal lymphocyte count was 928.1/mm2 in the EBV subtype which was significantly higher than all other subtypes (P<0.0001). The stromal lymphocyte count in MSI tumors was 598.9/mm2 which was significantly higher than those in EMT (291.6/mm2), p53 aberrant (352.1) and p53 wildtype (354.4) subtypes (all Ps <0.0001) (Figure 2). No difference was seen in the stromal lymphocyte counts among EMT, p53 aberrant and p53 wildtype tumors (Figure 2B).
IDO1 and WARS expression in gastric cancer subtypes
Epithelial and stromal IDO1 was expressed in 66.7% and 32.4% of the tumors and epithelial and stromal WARS was expressed in 35.1% and 30.3% of the tumors, respectively (Table 2, Figure 1). Epithelial and stromal IDO1 expression was more likely to be positive in EBV (66.7% and 90.3%) and MSI subtypes (67.8% and 71%) as compared to other subtypes (P<0.0001 and P=0.0008). Similarly, EBV (85.2% and 92.6%) and MSI (74.2% and 81.2%) subtypes had more epithelial and stromal WARS expression than other subtypes (both P<0.0001) (Table 2).
Table 2.
IDO1 and WARS expression in gastric cancer subgroups.
| N (%) | Total* | Subtype | P-value | ||||
|---|---|---|---|---|---|---|---|
| EBV | MSI | EMT | p53-ab | p53-wt | |||
| IDO1-t | <0.0001 | ||||||
| neg | 275 (67.6%) | 9 (33.3%) | 10 (32.2%) | 58 (81.7%) | 130 (71.4%) | 68 (70.8%) | |
| pos | 132 (32.4%) | 18 (66.7%) | 21 (67.8%) | 13 (18.3%) | 52 (28.6%) | 28 (29.2%) | |
| IDO1-s | 0.0008 | ||||||
| neg | 173 (41.9%) | 3 (10.7%) | 9 (29%) | 38 (52.1%) | 80 (43.7%) | 43 (43.8%) | |
| pos | 240 (58.1%) | 25 (89.3%) | 22 (71%) | 35 (47.9%) | 103 (56.3%) | 55 (56.1%) | |
| WARS-t | <0.0001 | ||||||
| neg | 263 (64.9%) | 4 (14.8%) | 8 (25.8%) | 54 (78.3%) | 128 (70.7%) | 69 (71.1%) | |
| pos | 142 (35.1%) | 23 (85.2%) | 23 (74.2%) | 15 (21.7%) | 53 (29.3%) | 28 (28.9%) | |
| WARS-s | <0.0001 | ||||||
| pos | 281 (69.7%) | 2 (7.4%) | 6 (18.8%) | 58 (85.3%) | 140 (77.8%) | 75 (78.1%) | |
| neg | 122 (30.3%) | 25 (92.6%) | 26 (81.2%) | 10 (14.7%) | 40 (22.8%) | 21 (21.9%) | |
p53-wt: p53 wildtype; p53-ab: p53 aberrant; IDO1-t: IDO1 tumor; IDO1-s: IDO1 stroma; WARS-t: WARS tumor; WARS-s: WARS stroma
A small number of cores were lost during the staining process.
Associations between TILs and IDO1/WARS expression
Irrespective of the tumor subtype, significantly higher stromal and epithelial lymphocyte counts were observed in tumors with positive IDO1 and WARS expression (all Ps < 0.001) (Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/AIMM/A232). In EBV tumors, significantly higher tumor TILs were seen in IDO1 positive epithelium and in WARS positive stroma. In EMT tumors, significantly higher TILs were seen in IDO1 positive and WARS positive stroma. In MSI tumors, significantly higher TILs were seen in WARS positive epithelium and stroma. In p53 aberrant tumors, significantly higher TILs were seen in IDO1 and WARS positive stroma. In p53 wildtype tumors, significantly higher TILs were seen in WARS positive stroma (Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/AIMM/A232).
The overall correlations between IDO1 and WARS expression were statistically significant. P values were < 0.0001 for epithelial IDO1 versus epithelial WARS and stromal IDO1 versus epithelial WARS. P values were 0.0078 and < 0.0001 for epithelial versus stromal IDO1 and epithelial versus stromal WARS, respectively. P values were 0.0005 and 0.0008 for epithelial IDO1 versus stromal WARS and stromal IDO1 versus epithelial WARS, respectively.
Prognostic utilities of IDO1 and WARS in gastric cancer subtypes
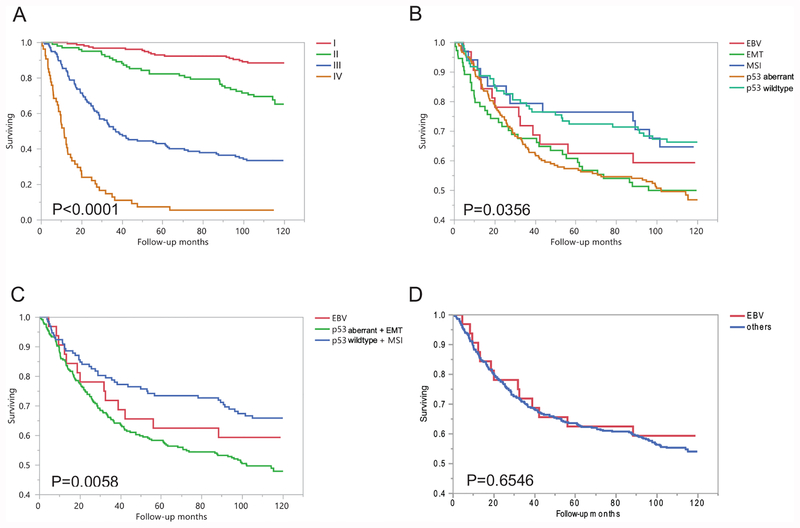
The patient outcome was tumor stage dependent with a clear-cut stepwise decrease of overall survival from stage 1 to stage 4 (P < 0.0001, Figure 3A). The best prognosis was seen in p53 wildtype and MSI tumor patients, whereas the worst prognosis was seen in p53 aberrant and EMT tumor patients. The outcome of patients with EBV tumor was in between with two groups stated above. (Figure 3B–D).
Figure 3.
A. Overall survival analysis of all gastric tumors in respect to TNM stage. B. Overall survival analysis of all gastric tumors in respect of 5 subgroups. C. Overall survival analysis of all gastric tumors in respect of 3 subgroups. D. Overall survival analysis of EBV subgroups versus other tumors.
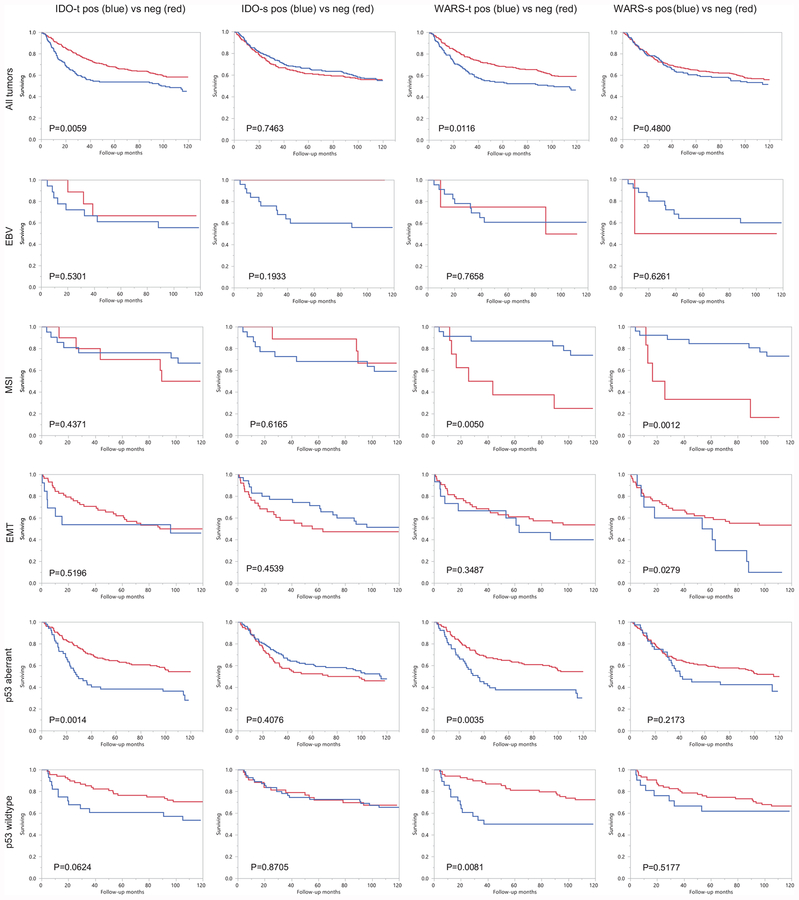
Expression of IDO1 and WARS within the epithelial components of the tumor was a significant poor prognostic factor in all tumors, while stromal expression of IDO1 and WARS was not significantly associated with prognosis. A similar trend was detected in p53 wildtype tumor patients and p53 aberrant tumor patients. This pattern was not seen in EBV and EMT tumors likely due to limited case size. However, WARS expression in both the epithelial and stromal components was associated with better prognosis in MSI tumor patients (Figure 4).
Figure 4.
Overall survival analysis of gastric subgroups in respect to IDO1 and WARS expression in tumor and stroma. IDO1-t: IDO1 expression in tumor epithelium, IDO1-s: IDO1 expression in tumor stroma. WARS-t: WARS expression in tumor epithelium, WARS-s: WARS expression in tumor stroma.
Overall survival data was further analyzed by Cox proportional hazards model including age, tumor subtype, TNM stage, and expression of IDO1 and WARS. The multivariate analysis revealed age and TNM stage as independent prognostic factors (P = 0.0079 and P < 0.0001, respectively), while tumoral and stromal expression of IDO1 and WARS failed to demonstrate similar predictive power (P-values ranged from 0.0663 to 0.5069) (Table 3).
Table 3.
Multivariate analysis of IDO1 and WARS expression
| Parameter | Risk Ratio | Lower 95% | Upper 95% | P-value |
|---|---|---|---|---|
| Age | 2.78* | 1.31 | 5.92 | 0.0079 |
| Subtype | 0.1423 | |||
| EBV/p53-wt+MSI | 1.03 | 0.51 | 2.07 | 0.9324 |
| p53-ab+EMT/EBV | 1.42 | 0.71 | 2.83 | 0.3198 |
| TNM stage | <0.0001 | |||
| T2/T1 | 1.55 | 0.73 | 3.29 | 0.2544 |
| T3/T2 | 3.34 | 1.85 | 6.03 | <0.0001 |
| T4/T3 | 2.15 | 1.56 | 2.97 | <0.0001 |
| IDO1-t pos/neg | 1.20 | 0.86 | 1.65 | 0.2816 |
| IDO1-s pos/neg | 1.11 | 0.81 | 1.53 | 0.5069 |
| WARS-t pos/neg | 1.41 | 0.98 | 2.04 | 0.0663 |
| WARS-s pos/neg | 0.82 | 0.55 | 1.23 | 0.3386 |
Per change in regressor over entire range
Discussion
Our study revealed that EBV tumors had ~10 times more TILs than EMT, p53 aberrant and wildtype tumors in the epithelium and ~3 times more stromal TILs, whereas MSI tumors had ~3 times more TILs in the epithelium and ~ 2 times more stromal TILs. Other studies have also revealed this trend of increased TILs in EBV-associated gastric.23 In a recent study on CD8+ TILs in gastric cancer, marked infiltrates of CD8 T cells was present in 95.3% of EBV cancers followed by MSI cancers (81%) and EBV-/MSI stable cancers (40.8%).24 In a recent meta-analysis including 4942 gastric cancer patients from 29 studies revealed that high density of intratumoral CD3 and CD8 T cells were associated with better overall survival.25
This is the first study to investigate the association between CD3+ TILs and IDO1 and WARS expression in the five gastric tumor molecular subtypes. Expression of IDO1 and WARS was associated with significantly more TILs in both the epithelium and stroma in all tumors and higher percentage of tumors with elevated expression of IDO1 and WARS was seen in EBV and MSI tumors. Another gastric cancer study without tumor subtyping found that higher IDO1 expression correlated with lower percentages of CD4+ memory TILs and CD8+ memory TILs while the higher IDO1 expression correlated with higher percentages of CD8+ central memory T cells in the tumor environment.26 In our recent study of colon cancer, IDO1 was found to be significantly higher expressed in medullary type and MSI unstable cancer, both harboring high amounts of TILs.27 In another recent study on gastric gastrointestinal stromal tumors (GISTs), no association was found between TILs and IDO1 and WARS expression.28 These findings suggest the correlation between IDO1 and WARS expression and TILs may be tumor type dependent.
In this study, gastric tumors with higher epithelial expression of IDO1 were associated with worse overall patient survival (Figure 4). A similar pattern was also seen in p53 aberrant and wild-type tumors. These findings are consistent with published studies investigating IDO1 expression in gastric cancer.13,14,29 Higher expression of IDO1 has also been shown to be associated with poor patient outcome of tumors from colon, lung, prostate, oesophagus and uterus.30
As to WARS, only one study was published regarding WARS expression in gastric cancer and it found that neither epithelial nor stromal WARS expression had any prognostic values in a group of unclassified gastric cancers.31 Its prognostic role in other cancer types appears disease-dependent, associated with good outcome in colon cancer and triple negative breast cancer but with poor prognosis in oral cancer.17,18,19 In our studies, WARS expression was a poor prognostic factor and appeared to be subtype-dependent (Figure 4). The prognosis of MSI tumors was better if epithelial or stromal expression was present while p53 aberrant and wildtype tumors showed similar patterns as overall tumors.
This observation of prognostic effect of WARS only on MSI tumors is intriguing: MSI tumors have a unique immune microenvironment, harboring numerous different neoantigens from defect DNA mismatch repair machinery. The neoantigen-induced tumor immune microenvironment may be different from that driven by EBV associated changes. In a gene profiling study that compared MSI and EBV gastric tumors, mutations providing proliferative and survival advantage were more frequently seen in MSI tumor while EBV+ tumors showed a downregulation of genes involved in mitotic pathways.32 Further studies are needed to investigate these unique features of MSI tumors.
In the current study, the majority of EBV and MSI tumors exhibited epithelial and stromal expression of IDO1 and WARS, while most EMT, p53-ab and p53-wt tumors did not. Abundant stromal IDO1 expression in EBV tumors is likely directly associated with EBV infection. EBV infection has been shown to induce IDO1 mRNA, protein, and enzymatic activity in human monocyte-derived macrophages33 which constitute an important fraction of active immune cells in the microenvironment.
IDO1 overexpression and associated decreased Trp/Kyn ratio exert an immunosuppressive effect in the tumor microenvironment in multiple cancer types.11 Many clinical trials have been initiated using IDO1 inhibitors along with other modalities.30 Although IDO1 inhibitors combined with PD-L1 inhibitors (Keytruda) showed no statistically difference from treatment with PD-L1 inhibitor alone in treating melanoma in terms of progression-free survival and overall survival,34 clinical trials evaluating IDO1 inhibitors on gastric cancer treatment are still ongoing.
The effectiveness of IDO1 inhibitor treatment of gastric cancer could be subtype-specific. Given the high expression of IDO1 in EBV and MSI tumor seen in this study, better clinical outcomes may likely be seen in these two types of gastric cancer.
In summary, the current study investigated the expression of IDO1 and WARS expression in five molecular gastric subtypes and confirmed the high expression levels of IDO1 and WARS in EBV and MSI tumors. High expression levels of IDO1 and WARS were associated with increased TILs in epithelial and stromal components. The prognostic effects of IDO1 and WARS expression were tumor subtype-dependent. While high expression levels of IDO1 and WARS in tumor epithelium and stroma was associated with poor prognosis in p53 aberrant, p53-wildtype, and all cancers combined, WARS expression in tumor epithelium and stroma was associated with better prognosis in MSI tumors.
Supplementary Material
Funding:
Research funding was provided by grant No. 02-2013-064 from the SNUBH research fund (Republic of Korea) and the Molecular Pathology Core of the COBRE Center for Cancer Research Development funded by the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM103421 (United States)
Footnotes
Ethics approval and consent to participate:
The study was performed in accordance with the ethical guidelines and approval from the Institutional Review Boards of Seoul National University Hospital (Republic of Korea) and Rhode Island Hospital (Rhode Island, United States). The study was performed in accordance with the Declaration of Helsinki.
Consent for publication:
Not applicable.
Availability of data and materials:
Original stained tissue microarray slides, raw readouts of the immunostains, and original survival data are available upon request via the corresponding author.
Conflict of Interest:
The authors declare no conflict of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; e-pub ahead of print 12 September 2018; doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, et al. Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cristescu R, Nebozhyn M, Kim K, Ting J, Wong S, Liu J, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–456. [DOI] [PubMed] [Google Scholar]
- 4.Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, et al. Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project. Clin Cancer Res 2017;23;4441–4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn S, Lee S, Kim Y, Kim A, Shin N, Choi K, et al. High-throughput protein and mRNA expression-based classification of gastric cancers can identify clinically distinct subtypes, concordant with recent molecular classifications. Am J Surg Pathol 2017;41:106–115. [DOI] [PubMed] [Google Scholar]
- 6.Birkman EM, Mansuri N, Kurki S, Ålgars A, Lintunen M, Ristamäki R, et al. Gastric cancer: immunohistochemical classification of molecular subtypes and their association with clinicopathological characteristics. Virchows Arch 2018;472:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The Immune Landscape of Cancer. Immunity 2018;48:812–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 1991;5:2516–2522. [PubMed] [Google Scholar]
- 10.Fleckner J, Martensen PM, Tolstrup AB, Kjeldgaard NO, Justesen J. Differential regulation of the human, interferon inducible tryptophanyl-tRNA synthetase by various cytokines in cell lines. Cytokine 1995;7:70–77. [DOI] [PubMed] [Google Scholar]
- 11.Hornyák L, Dobos N, Koncz G, Karányi Z, Páll D, Szabó Z, et al. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front Immunol 2018;9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lian W, Ma DJ, Xu X, Chen Y, Wu YL. Rapid high-performance liquid chromatography method for determination of tryptophan in gastric juice. J Dig Dis 2012;13:100–106. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Shen Z, Wang Z, Wang X, Zhang H, et al. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci Rep 2016;6:21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishi M, Yoshikawa K, Higashijima J, Tokunaga T, Kashihara H, Takasu C, et al. The Impact of Indoleamine 2,3-dioxygenase (IDO) Expression on Stage III Gastric Cancer. Anticancer Res 2018;38:3387–3392. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH, Kim SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2016;19:42–52. [DOI] [PubMed] [Google Scholar]
- 16.Miyanokoshi M, Yokosawa T, Wakasugi K. Tryptophanyl-tRNA synthetase mediates high-affinity tryptophan uptake into human cells. J Biol Chem 2018;293:8428–8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CW, Chang KP, Chen YY, Liang Y, Hsueh C, Yu JS, et al. Overexpressed tryptophanyl-tRNA synthetase, an angiostatic protein, enhances oral cancer cell invasiveness. Oncotarget 2015;6:21979–21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghanipour A, Jirström K, Pontén F, Glimelius B, Påhlman L, Birgisson H. The prognostic significance of tryptophanyl-tRNA synthetase in colorectal cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2949–2956. [DOI] [PubMed] [Google Scholar]
- 19.Campone M, Valo I, Jézéquel P, Moreau M, Boissard A, Campion L, et al. Prediction of Recurrence and Survival for Triple-Negative Breast Cancer (TNBC) by a Protein Signature in Tissue Samples. Mol Cell Proteomics 2015;14:2936–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rosa S, Sahnane N, Tibiletti MG, Magnoli F, Vanoli A, Sessa F, et al. EBV⁺ and MSI Gastric Cancers Harbor High PD-L1/PD-1 Expression and High CD8⁺ Intratumoral Lymphocytes. Cancers (Basel) 2018;10:E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HE, Han N, Kim MA, Lee HS, Yang HK, Lee BL, et al. DNA damage response-related proteins in gastric cancer: ATM, Chk2 and p53 expression and their prognostic value. Pathobiology 2014;81:25–35. [DOI] [PubMed] [Google Scholar]
- 22.Han N, Kim MA, Lee HS, Kim WH. Loss of ARID1A Expression is Related to Gastric Cancer Progression, Epstein-Barr Virus Infection, and Mismatch Repair Deficiency. Appl Immunohistochem Mol Morphol 2016;24:320–325. [DOI] [PubMed] [Google Scholar]
- 23.Kang BW, Seo AN, Yoon S, Bae HI, Jeon SW, Kwon OK, et al. Prognostic value of tumor-infiltrating lymphocytes in Epstein-Barr virus-associated gastric cancer. Ann Oncol 2016;27:494–501. [DOI] [PubMed] [Google Scholar]
- 24.Cho J, Chang YH, Heo YJ, Kim S, Kim NK, Park JO, et al. Four distinct immune microenvironment subtypes in gastric adenocarcinoma with special reference to microsatellite instability. ESMO Open 2018;3:e000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu PC, Long D, Liao CC, Zhang S. Association between density of tumor-infiltrating lymphocytes and prognoses of patients with gastric cancer. Medicine (Baltimore) 2018;97:e11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Liu H, Li F, Li H, Yu J, et al. The correlation between the subsets of tumor infiltrating memory T cells and the expression of indoleamine 2,3-dioxygenase in gastric cancer. Dig Dis Sci 2013;58:3494–3502. [DOI] [PubMed] [Google Scholar]
- 27.Friedman K, Brodsky AS, Lu S, Wood S, Gill AJ, Lombardo K, et al. Medullary carcinoma of the colon: a distinct morphology reveals a distinctive immunoregulatory microenvironment. Mod Pathol 2016;29:528–541. [DOI] [PubMed] [Google Scholar]
- 28.Blakely AM, Matoso A, Patil PA, Taliano R, Machan JT, Miner TJ, et al. Role of immune microenvironment in gastrointestinal stromal tumors. Histopathology 2018;72:405–413. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Huang J, Li S, Li H, Yu J, Ren X, et al. The subsets of dendritic cells and memory T cells correspond to indoleamine 2,3-dioxygenase in stomach tumor microenvironment. Tumor Biol 2014;35:8691–8698. [DOI] [PubMed] [Google Scholar]
- 30.Zhai L, Ladomersky E, Lenzen A, Nguyen B, Patel R, Lauing KL, et al. IDO1 in cancer: a Gemini of immune checkpoints. Cell Mol Immunol 2018;15:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patil PA, Blakely AM, Lombardo KA, Machan JT, Miner TJ, Wang LJ, et al. Expression of PD-L1, indoleamine 2,3-dioxygenase and the immune microenvironment in gastric adenocarcinoma. Histopathology 2018;73:124–136. [DOI] [PubMed] [Google Scholar]
- 32.Gullo I, Carvalho J, Martins D, Lemos D, Monteiro AR, Ferreira M, et al. The Transcriptomic Landscape of Gastric Cancer: Insights into Epstein-Barr Virus Infected and Microsatellite Unstable Tumors. Int J Mol Sci 2018;19: E2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood 2007;109:3351–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loveman C, DPhil MB, Eisele P, Sell E, Loxam T, DeCarbo M. Incyte and Merck provide update on Phase 3 study of epacadostat in combination with KEYTRUDA® (pembrolizumab) in patients with unresectable or metastatic melanoma. https://www.businesswire.com/news/home/20180406005141/en/Incyte-Merck-Provide-Update-Phase-3-Study (2018) Accessed on 6/26/2018
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.