Abstract
New generation, multicomponent parenteral lipid emulsions provide key fatty acids for brain growth and development, such as docosahexaenoic acid (DHA) and arachidonic acid (AA), yet the content may be suboptimal for preterm infants. Our aim was to test whether DHA and AA-enriched lipid emulsions would increase activity, growth, and neurodevelopment in preterm piglets and limit brain inflammation. Cesarean-delivered preterm pigs were given three weeks of either enteral preterm infant formula (ENT) or TPN with one of three parenteral lipid emulsions: Intralipid (IL), SMOFlipid (SMOF) or an experimental emulsion (EXP). Activity was continuously monitored and weekly blood sampling and behavioral field testing performed. At termination of the study, whole body and tissue metrics were collected. Neuronal density was assessed in sections of hippocampus (HC), thalamus, and cortex. Frontal cortex (FC) and HC tissue were assayed for fatty acid profiles and expression of genes of neuronal growth and inflammation. After 3 weeks of treatment, brain DHA content in SMOF, EXP and ENT pigs was higher (P < 0.01) in FC but not HC vs. IL pigs. There were no differences in brain weight or neuron density among treatment groups. Inflammatory cytokine TNFα and IL-1β expression in brain regions were increased in IL pigs (P < 0.05) compared to other groups. Overall growth velocity was similar among groups, but IL pigs had higher percent body fat and increased insulin resistance compared to other treatments (P <0.05). ENT pigs spent more time in higher physical activity levels compared to all TPN groups, but there were no differences in exploratory behavior among groups. We conclude that a soybean oil emulsion increased select brain inflammatory cytokines and multicomponent lipid emulsions enriched with DHA and AA in parenteral lipids results in increased cortical DHA and improved body composition without affecting short term neurodevelopmental outcomes.
Keywords: DHA, LC-PUFA, Omega-3 Fatty Acid, SMOFlipid, Preterm infant, neurodevelopment, nutrition
1. Introduction
Lipids are a key energy source for newborn infants and also provide important long-chain essential polyunsaturated fatty acids (LC-PUFAs) that are crucial for normal development of the central nervous system and other organ systems (Burrin et al., 2014; Haggarty, 2010). Formation of docosahexaenoic acid (DHA) from α-linolenic acid (ALA) is limited and highly variable, and DHA may be considered conditionally essential during early development. This is especially important for preterm infants who are born at a stage of gestation before normal placental transfer and deposition of DHA in fetal tissues are completed. This loss of fetal DHA transfer due to preterm birth may be difficult to restore by current nutritional practices and result in a “DHA gap of prematurity” (Harris and Baack, 2015). The challenge of restoring this DHA gap in premature infants is further complicated when enteral feeding is delayed and the main nutritional source of fatty acids is parenteral lipid emulsions. While there has been a high interest in the specific needs of preterm infants for LC-PUFA in relation to short and long term cognitive outcomes, there is limited evidence about the benefits of LC-PUFA given parenterally. This has become increasingly important with the availability of new generation lipid emulsions (e.g. SMOFlipid, Omegaven) that are now be used to test these benefits on neurodevelopment and growth.
The developing brain is vulnerable to external factors that induce tissue injury in the postnatal period, especially in preterm infants. These types of injury are related to impaired perfusion, uncontrolled oxygenation and excessive activation of inflammation. Brain tissue consumes a large part of the total body oxygen, especially in preterm newborns, increasing the likelihood of brain lipid peroxidation and injury due to the high abundance of brain tissue PUFAs, especially AA and DHA. Oxidative stress may lead to excessive production of neurodegenerative metabolites and is counterbalanced by the presence of antioxidants, such as vitamin E and C. Among the damaging metabolites are F2-isoprostanes, produced by non-enzymatic free radical oxidation of arachidonic acid, epoxyeicosatrienoic acid products, and hydroxyeicosatetraenoic acid products. In a recent safety study on preterm infants, F2-isoP levels were reduced in a SMOFlipid group as compared with baseline, while eicosapentanoic acid and vitamin E levels were increased (Deshpande et al., 2014).
LC-PUFAs play a central role in cellular structure and function, including the regulation of membrane fluidity, cell signaling, and protein expression. These LC-PUFAs promote and suppress tissue immune and inflammatory responses as well as organogenesis. The most well-known functions of LC-PUFAs in the developing fetus are to support brain and retina development (Martin, 2014). They are crucial for normal central nervous system development and have the potential for long-lasting effects extending beyond a period of dietary insufficiency (Lapillonne and Jensen, 2009). The absolute and relative DHA contents in fetal brain increase especially in the last trimester of pregnancy. Animal studies show that low accumulation of DHA in the retina and brain is linked to altered performance in visual, cognitive or behavioral tests. Dose-response studies in human preterm neonates indicate that DHA-enrichment of mother’s milk or formula with ≥ 1% of fatty acids as DHA with or without concomitant AA supplementation results in higher visual acuity as well as better problem-solving capability and recognition memory (Lapillonne and Jensen, 2009).
In a cohort of extremely premature infants, Martin et al. (Martin et al., 2011) measured the changes in whole blood fatty acids in the first postnatal month and showed a marked increase in LA and a decrease in AA and DHA, reflecting the composition of the parenteral soybean oil emulsion. The decline in plasma DHA and AA levels after the first week after birth plateaued, but these lower DHA and AA levels were associated with increased risk of chronic lung disease and sepsis. Given that LA products favor proinflammatory pathways, whereas DHA favors the synthesis of anti-inflammatory products, these findings suggest imply that inflammatory conditions may contribute to increased risk of neonatal diseases. Recently reported study results comparing lipid emulsions containing 10–15% fish-oil (including SMOFlipid) to soy and olive oil-based emulsions found that the fish-oil emulsions resulted in higher plasma DHA and EPA levels compared to soy-based emulsion (e.g. Intralipid), but, in early preterm infants, did not prevent the decline in DHA relative to birth levels (D’Ascenzo et al., 2011; D’Ascenzo et al., 2014; Deshpande et al., 2014; Najm et al., 2017). Isolated high intakes in DHA have been shown to result in a decrease in tissue AA, probably due to the competition of omega-3- and omega-6-LC- PUFAs. Studies of early preterm infants also showed lower levels of AA in the plasma phospholipids of infants receiving fish oil containing emulsions compared to infants receiving soybean oil (D’Ascenzo et al., 2014).
Given the critical role of DHA and AA in brain neurodevelopment we examined whether parenteral lipid emulsions with enriched LA versus DHA and AA content leads to significant enrichment in critical brain regions and if it translates into differences in functional outcomes, including brain development, inflammation and behavior. To accomplish these goals, we tested whether nutritional support with the existing commercial emulsions (SMOFlipid) and a newly developed test emulsion (EXP) can prevent the postnatal decline in plasma and RBC DHA and AA and whether they substantially enrich brain levels and influence neurodevelopment and inflammation in our preterm pig model.
2. Materials and methods
2.1. Animals and surgical procedures
Pregnant sows (Domestic pigs, species Sus scrofa domesticus) were purchased from a local swine farm and transported to the study site at least one week prior to delivery of piglets to acclimate to the Children’s Nutrition Research Center facility housing. The sires/fathers were derived from mainly Duroc and Hampshire and the dam from Yorkshire and Landrace breeds. Premature pigs were delivered 6 d preterm at 108 d gestation by cesarean section and immediately placed in stainless steel cages housed at 31–32°C with a 12-h light:dark cycle as described previously (Ng et al., 2016; Vlaardingerbroek et al., 2014). Maternal plasma (16 ml/kg intravenously during the first 24 h) was administered to pigs for passive immunological protection to compensate for the lack of colostrum. Pigs were treated every other day with prophylactic antibiotics (ampicillin, 50 mg/kg, IV) to prevent catheter related infections. All animal protocols and husbandry were approved by the Baylor College of Medicine (BCM) Institutional Animal Care and Use Committee and monitored by attending veterinary staff from the BCM Center for Comparative Medicine Program.
2.2. Dietary Composition and nutritional protocol
Immediately after birth, pigs were randomized to one of four groups: control enteral diet (ENT), TPN-Intralipid (IL), TPN-SMOFlipid (SMOF), or TPN-Experimental lipid (EXP). Nutrient intake in all 4 treatment groups was isocaloric and isonitrogenous during the entire 22 day experiment as shown in Table 1. The TPN groups differed only in the lipid fatty acid (FA) composition, but SMOFlipid and EXP also contained more vitamin E (all-rac-α-tocopherol) than Intralipid. The three lipid emulsions were provided by Fresenius Kabi AG (Bad Homberg, Germany). Experimental lipid consisted of a multicomponent lipid emulsion modified to have lower content of EPA than SMOFlipid and supplemented with additional DHA and AA. Each lipid emulsion was analyzed by gas chromatography-mass spectrometry (GC-MS) to confirm FA composition and calculate actual intakes in each treatment group. The elemental nutrition solution (excluding lipid) contained a complete nutrient mixture of amino acids, glucose, electrolytes, and vitamins and trace minerals (Ng et al., 2016; Stoll et al., 2010; Vlaardingerbroek et al., 2014). The macronutrient supply during full TPN intake was 25 g/(kg•d) glucose, 13 g/(kg•d) amino acids, and 5 g/(kg•d) lipid. Pigs randomized to control enteral diet (ENT) received commercial preterm infant formula (Enfamil Premature 24 Cal, Mead Johnson Nutrition) that was fortified with whey protein, lactose and DHA (derived from the marine algae Schizochytrium sp.; life’sDHA, DSM Nutritional Products Ltd, Switzerland) to match the DHA intake in SMOFlipid group. Electrolytes and micronutrients were also added to the enteral formula in order to match the intake of the SMOFlipid group.
Table 1.
Daily dietary nutrient intake in the four nutritional groups.
| ENT n = 11 |
IL n = 12 |
SMOF n = 11 |
EXP n = 11 |
|
|---|---|---|---|---|
| Protein (g/kg) | 13 | 13 | 13 | 13 |
| Carbohydrate (g/kg) | 25 | 25 | 25 | 25 |
| Fat/Lipid (g/kg) | 5 | 5 | 5 | 5 |
| DHA (mg/kg) | 110 | 12.7 | 148 | 270 |
| AA (mg/kg) | 26.4 | 9.55 | 16.2 | 225 |
| EPA (mg/kg) | 0 | 0 | 359 | 49 |
| LA (mg/kg) | 1216 | 2567 | 925 | 1050 |
| C8:0 (mg/kg) | 760 | 0.22 | 145 | 130 |
| C10:0 (mg/kg) | 388 | 0.16 | 115 | 100 |
These values are based on published ingredient compositions of formula and TPN solutions, with the exception of lipid composition which was measured directly as described below in methods. Commercial preterm infant formula (Enfamil Premature 24 Cal, Mead Johnson Nutrition). The infant formula contains the following sources of protein (60% whey, 40% casein), carbohydrate (60% corn syrup solids, 40% lactose), and fat (40% MCT oil, 30% soy oil, 27% high oleic vegetable oil, 3% single-cell oil blend rich in docosahexaenoic acid, DHA and arachidonic acid, ARA). The infant formula was fortified with whey protein, lactose and DHA to match the macronutrient and DHA level in SMOFlipid group. Electrolytes and micronutrients also were matched to the intake of the SMOFlipid group.
An overview of the study design and timeline is shown in Supplemental Fig 1. All pigs were implanted with central catheters and given TPN solutions continuously starting at a rate of 5 ml/(kg•h). Within the first 6 study days, the rate of TPN infusion was gradually increased to 10 ml/kg/hr and maintained for the remaining days (22 d total, 240 ml/(kg•d)). Piglets in the enteral formula group were implanted with an orogastric tygon feeding tube (6 French) to enable enteral formula feeding. For the insertion of the orogastric feeding tube, a small puncture wound on the left cheek was made and the tube advanced 15–20 cm into the stomach. The feeding tube was then secured to the left cheek and head with non-absorbable sutures. The TPN-fed pigs received a sham surgical procedure, but not the indwelling orogastric feeding tube. All pigs in ENT and TPN groups had indwelling jugular catheters that were attached to tether and harness apparatus to allow pigs freedom of movement around the cages. Pigs were housed after surgery until 14 days of age in stainless steel cages with Plexiglas dividers (cage dimension: 61cm length × 30 cm width × 61 cm height), and thereafter from 15–22 days dividers were removed to create a larger cage area configuration (cage dimension: 61 cm length × 61 cm width × 61 cm height) to allow for greater range of movement. Plexiglas cage dividers had multiple 3 cm holes to allow nose contact between the pig in the adjacent cage.
The enterally fed pigs received TPN immediately after birth similar to the TPN treatment groups and the lipid emulsion provided was SMOFlipid. Enteral formula feeding was introduced gradually via orogastric feeding tube in equal feeding volumes every three hours beginning on the day after surgery. Enteral feeding volumes were increased in the first 6 study days while the TPN infusion volume was decreased proportionally, such that by study day 7, formula-fed piglets received the full feeding volume enterally (240 ml fluid/(kg•d) and TPN was stopped. Between study days 5–10, enterally fed pigs were trained to drink liquid formula from a stainless steel bowl and then on study day 10, pigs were transitioned to every four hour feedings.
2.3. Acquisition of basic motor skills, home cage activity, and exploratory behavior
Timing of acquisition of neuromuscular control after birth (eyelid opening, first stand and first walk) was observed and recorded. Physical activity was measured for each piglet over the course of the study. We defined physical activity as the piglet’s movement over time within its enclosure. Actigraph wGT3X-BT activity monitors (ActiGraph, Pensacola, Florida, U.S.A.) were used to measure the activity of each piglet during the study and only removed temporarily during scheduled field tests and DXA body scans. The monitors were installed immediately following the surgery for catheter placement on the first day of life. Activity monitors were secured dorsally to the right of the piglet’s spine with the use of tubular elastic net dressing (SeProNet, Medical Action Industries Inc., Brentwood, New York, U.S.A.). The orientation of the activity monitor on the piglet’s body was consistent for each pig in the study.
At completion of the study, activity data was downloaded from accelerometers and analyzed using the ActiLife 6 Data Analysis program. Data output was computed from horizontal, vertical, and perpendicular axes and provided as vector magnitude (units of gravity (g or m/s2), at a rate of 30 Hz) sums across minute long epochs. Results were generated for total activity and further categorized into levels of activity: 0–100g (Sedentary), 101–425g (Low level activity), 426–750g (Mid-level activity), and values greater than 750g (High level activity). Categories were determined based on standard cut off limits and confirmed upon review of a selection of piglets’ graphical output in ActiLife 6 software. Activity categorization was computed in Mathworks’ MATLAB program using the previously stated limits. Additional algorithms were applied to each piglet’s output data set to remove the effect of handling (post-operative cares and feeding) and provide an estimate of unprovoked or undisturbed spontaneous activity.
On study days 7, 14, and 20, selected behavioral and functional domains were assessed in individual piglets were using an open field arena test. The open field arena (1.2 × 1.2 m) had black walls and black rubber flooring to assess coordination and open field behavior. A Novel Object Recognition (NOR) test was conducted to asses both short term memory and specific exploratory behavior using the same test area as for the open field evaluations. The test consisted of a 3 min sample phase without the novel object in the field and then a 3 min test phase with the novel object lowered into the field, separated by a 4 min inter-trial interval (ITI). After the field test, the piglets were returned to their home cages. During the field testing, piglets were simultaneously recorded by video cameras mounted from the ceiling (bird view to assess distance travelled and pattern) and from the side of the arena (side view for coordination assessments). Tracking analyses of the open field video recordings were done using a commercially available software (EthoVision XT10, Noldus Information Technology, Wageningen, The Netherlands) described previously (Andersen et al., 2016). This analysis provided quantitative information on distance travelled (locomotion), movement pattern within the arena (general exploratory behavior) and duration of stays in border and center zones, respectively.
2.4. Body composition and tissue collection
On day 20 pigs were subjected to body composition analysis using DXA as described previously (Stoll et al., 2010). Scan results provided values for total body bone mineral content (BMC), bone mineral density (BMD), non-bone lean tissue, and total body fat mass. Summing BMC and lean tissue values provided a measure of fat-free mass (FFM). Following the scan, pigs were returned to their home cage and monitored for recovery. At the end of the protocol, day 22, all pigs were euthanized for plasma/serum and tissue collection and analysis. Brain, liver, spleen, small intestine, and lungs were dissected, weighed and frozen for later analysis. The frontal cortex and hippocampus from the right hemisphere of the brain were sampled and frozen tissue and the left hemisphere was fixed in formalin.
2.5. Blood and tissue analysis
Blood samples were collected after catheter placement during surgery (Day 0) and on study days 7, 14 and 22. Blood samples were processed to yield serum, plasma and RBC. Whole blood cell counting analysis was done in EDTA-treated whole blood immediately. Serum, plasma, and RBC were stored at −80°C until analysis. Cholesterol profile, insulin, glucose, and amino acid concentrations were measured in final plasma samples (d22) as described previously (Stoll et al., 2000; Stoll et al., 2010; Vlaardingerbroek et al., 2014). Plasma and RBC (d0, d7, d14, d22), as well as brain tissue (d22) were analyzed for total fatty acid composition using GC-MS as described previously (Mohammad and Haymond, 2013).
2.6. Histology, neuronal density, and gene expression
Tissue sections of the left hemisphere of the brain (frontal cortex, hippocampus) were fixed in 10% buffered formalin for 24 h, then transferred to 70% ethanol and stored until staining for analysis. Morphometric analysis to quantify neuron cell numbers by staining with NeuN in brain tissue and quantifying total number of neurons visualized per high power field (HPF) at 20× magnification. For consistency, neuronal density was measured at the temporal cortex, parietal cortex, CA3 region of the hippocampus and the nucleus ventralis posterior thalamus in all samples with preserved tissue after processing. Brain tissues were assayed by qRT-PCR for mRNA of neurodevelopmental growth factor genes (NGF, BDNF, c-fos, and Egr1) and proinflammatory genes (TNF-α, IL-6, Il-1β, and NFкB). The relative quantification of target mRNA expression was calculated and normalized to beta-actin expression using the 2-ΔΔCT-method and expressed as fold change relative to the Intralipid or enteral group.
2.7. Statistical analysis
For each outcome, in order to accommodate the repeated measures on each pig, a mixed effects linear model with a first order autoregressive covariance structure was fit with the independent variables lipid emulsion/diet group, day of study (d0, d7, d14, d22) and the study group × day of study interaction term. Differences between treatment groups were determined by analysis of variance with Dunnet’s test for post-hoc analysis. Data from open-field behavior and activity measurements were analyzed by either Kruskal Wallis for ordinal score data or one-way ANOVA/Dunnet’s for continuous data. The primary endpoints in the study were plasma, RBC and brain fatty acid profiles (molar %). Secondary endpoints were structural, histological and functional measures of brain growth and development and overall animal growth and metabolic profile. All variables were tested with an α-value of 5% and missing values were not replaced. In most cases, continuous endpoint variables were expressed as mean ± standard error of mean. In some cases statistical output was expressed as percentages of a particular treatment group, such as Enteral Formula or TPN-IL.
3. Results
3.1. Fatty acid intake and red blood cell, plasma and tissue fatty acid composition
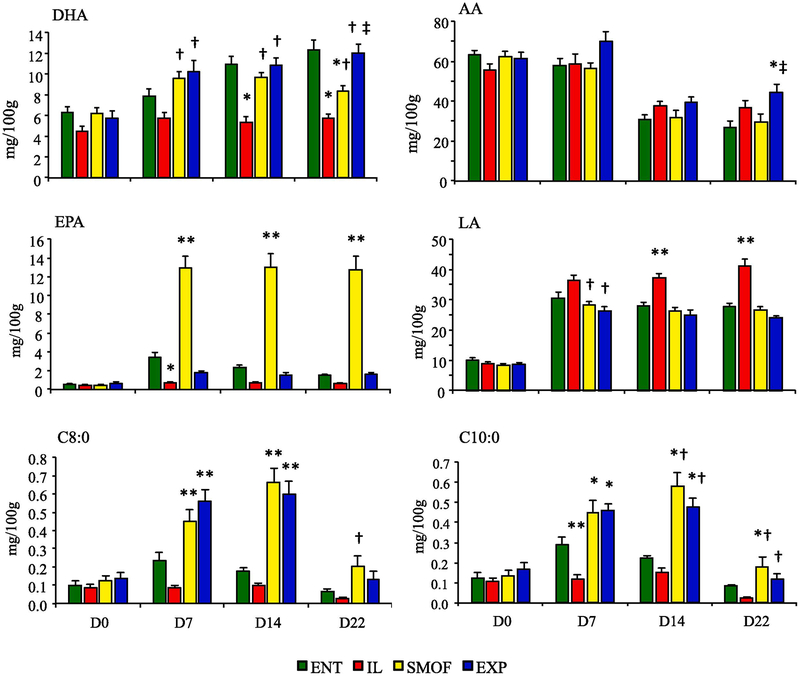
The measured fatty acid intakes of each treatment group are shown in Tables 1 and 2. There were large several-fold differences in DHA, ARA and EPA intakes between the IL group and enteral, SMOF and EXP groups. The results for key FA content of RBC membranes at 0, 7, 14, and 22 days treatment are shown in Fig. 1. Table 3 provides the complete FA profile of each treatment group at completion of the study. There were no statistically significant differences in fatty acid levels among the treatment groups on day 0. On day 7, DHA (C22:6) levels were higher in the SMOF and EXP groups compared to ENT or IL (P < 0.01). On Day 14, ENT piglets had significantly more DHA than IL piglets (P < 0.01) and surpassed SMOF piglets by the end of treatment (P < 0.01). Levels of AA (C20:4) were equivalent among groups at 0, 7 and 14 days, and increased in EXP compared to ENT (P < 0.01) or SMOF (P = 0.04) piglets at day 22. AA levels declined in all treatments between day 0 and 22 (P < 0.01). SMOF piglets had higher levels of EPA (C20:5) than all other groups by day 7 (P < 0.01) and persisted through completion of the study protocol. ENT piglets had higher EPA than IL piglets at day 7 (P = 0.02), but not at days 14 and 22. Levels of LA (C18:2) and ALA (C18:3)(not shown) were higher in IL than SMOF or EXP groups (P < 0.01) by day 7, but not different from ENT group until day 14 (P < 0.01). Plasma fatty acid profiles generally followed the pattern of changes observed in RBC and thus values are not shown.
Table 2.
Fatty acid composition (mg/ml) of enteral formula and parenteral lipid emulsions.
| ENT | IL | SMOF | EXP | |
|---|---|---|---|---|
| C8:0 | 3.17 | 0.04 | 15.64 | 18.70 |
| C10:0 | 1.62 | 0.03 | 14.89 | 14.46 |
| C12:0 | 0.12 | 0.05 | 0.13 | 0.07 |
| C14:0 | 0.08 | 0.09 | 1.80 | 0.04 |
| C16:0 | 0.16 | 26.81 | 21.09 | 17.72 |
| C16:1 | 0.01 | 0.19 | 3.94 | 0.73 |
| C18:0 | 0.16 | 9.18 | 7.34 | 6.98 |
| C18:1 | 5.05 | 59.03 | 58.09 | 57.03 |
| C18:2 LA | 5.07 | 102.7 | 52.45 | 51.55 |
| C18:3 ALA | 0.49 | 14.98 | 6.94 | 4.29 |
| C20:0 | 0.08 | 0.75 | 0.54 | 0.63 |
| C20:4 AA | 0.11 | 0.38 | 0.64 | 6.59 |
| C20:5 EPA | 0.00 | 0.00 | 14.36 | 1.97 |
| C22:0 | 0.00 | 0.85 | 0.36 | 0.81 |
| C22:5 | 0.01 | 0.01 | 0.30 | 0.39 |
| C22:6 DHA | 0.46 | 0.51 | 5.92 | 10.82 |
| Sum | 16.58 | 215.23 | 204.41 | 192.78 |
Daily intake was 240 mL/kg enteral formula and 25 ml/kg parenteral lipid emulsions. Values are based on analysis of lipid emulsions and complete mixed enteral formula described in methods.
Fig. 1.
Fatty acid content in RBC membrane at study day 0, 7, 14, and 22 in dietary treatment groups. Values shown are mg/100g RBC (ENT n = 11, IL n = 12, SMOF n = 11, EXP n = 11). Data are presented as mean ± SEM. * P = 0.02 v ENT, † P <0.01 v IL, ‡ P < 0.01 v SMOF, ** P < 0.05 v all groups
Table 3.
RBC fatty acid profile of preterm piglets on day 22 of life.
| Fatty Acid (mg/100 g) |
ENT | IL | SMOF | EXP | ANOVA P-value |
|---|---|---|---|---|---|
| C4:0 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.003 | 0.08 ± 0.01 | 0.94 |
| C6:0 | 0.02 ± 0.003 | 0.03 ± 0.004 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.95 |
| C8:0 | 0.07 ± 0.01 | 0.03 ± 0.01 | 0.20 ± 0.06b | 0.13 ± 0.04 | <0.01 |
| C10:0 | 0.08 ± 0.01 | 0.02 ± 0.01 | 0.18 ± 0.05ab | 0.12 ± 0.03b | <0.01 |
| C12:0 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.52 |
| C14:0 | 0.97 ± 0.05 | 0.85 ± 0.01c | 1.44 ± 0.12a | 0.83 ± 0.05c | <0.01 |
| C15:0 | 0.33 ± 0.02 | 0.30 ± 0.03c | 0.48 ± 0.04a | 0.33 ± 0.02c | <0.01 |
| C16:0 | 76.9 ± 3.24 | 89.2 ± 4.74 | 87.8 ± 2.57 | 84.9 ± 2.21 | 0.07 |
| C16:1 | 2.41 ± 0.17 | 2.71 ± 0.18c | 4.07 ± 0.27a | 2.59 ± 0.12c | <0.01 |
| C18:0 | 54.0 ± 2.96 | 53.6 ± 3.65 | 57.7 ± 4.48 | 48.5 ± 2.34 | 0.33 |
| C18:1 | 74.2 ± 3.16 | 100 ± 4.89a | 74.9 ± 2.64b | 64.8 ± 1.96b | <0.01 |
| C18:2 LA | 27.8 ± 0.86 | 41.1 ± 2.26a | 26.6 ± 1.11b | 24.0 ± 0.63b | <0.01 |
| C18:3 ALA | 2.00 ± 0.09 | 3.05 ± 0.24a | 1.90 ± 0.11b | 1.57 ± 0.10b | <0.01 |
| C20:0 | 0.56 ± 0.06 | 0.51 ± 0.06 | 0.53 ± 0.06 | 0.50 ± 0.06 | 0.91 |
| C20:1 | 1.03 ± 0.11 | 0.77 ± 0.09 | 0.87 ± 0.10 | 0.83 ± 0.06 | 0.23 |
| C20:2 | 2.48 ± 0.22 | 4.64 ± 0.55a | 2.03 ± 0.26b | 2.43 ± 0.13b | <0.01 |
| C20:3 | 9.39 ± 1.14 | 10.3 ± 0.97 | 11.8 ± 1.26 | 9.72 ± 0.77 | 0.39 |
| C20:4 AA | 26.7 ± 3.37 | 36.5 ± 3.81 | 29.4 ± 4.09 | 44.4 ± 3.74ac | <0.01 |
| C20:5 EPA | 1.50 ± 0.15 | 0.63 ± 0.07c | 12.8 ± 1.39a | 1.60 ± 0.17c | <0.01 |
| C22:0 | 2.38 ± 0.12 | 2.89 ± 0.28 | 2.95 ± 0.16 | 2.82 ± 0.18 | 0.19 |
| C22:1 | 0.11 ± 0.02 | 0.06 ± 0.02 | 0.12 ± 0.02 | 0.11 ± 0.02 | 0.12 |
| C22:2 | 0.27 ± 0.04 | 0.84 ± 0.09a | 0.33 ± 0.06b | 0.36 ± 0.04b | <0.01 |
| C22:4 | 5.18 ± 0.78 | 12.0 ± 1.11a | 4.05 ± 0.47b | 9.51 ± 0.92ac | <0.01 |
| C22:6 DHA | 12.3 ± 0.95 | 5.71 ± 0.38a | 8.35 ± 0.49ab | 12.1 ± 0.80bc | <0.01 |
| C24:0 | 3.67 ± 0.17 | 3.63 ± 0.39 | 3.68 ± 0.19 | 3.64 ± 0.20 | 1.00 |
| C24:1 | 4.05 ± 0.39 | 2.92 ± 0.34 | 4.03 ± 0.20 | 3.53 ± 0.23 | 0.04 |
| Total FA | 309 ± 16.0 | 373 ± 18.3 | 336 ± 17.2 | 319 ± 9.79 | 0.13 |
Mean ± SEM, ANOVA with Tukey;
P < 0.05 vs. ENT,
P < 0.05 vs. IL,
P < 0.05 vs. SMOF,
P < 0.05 vs. EXP
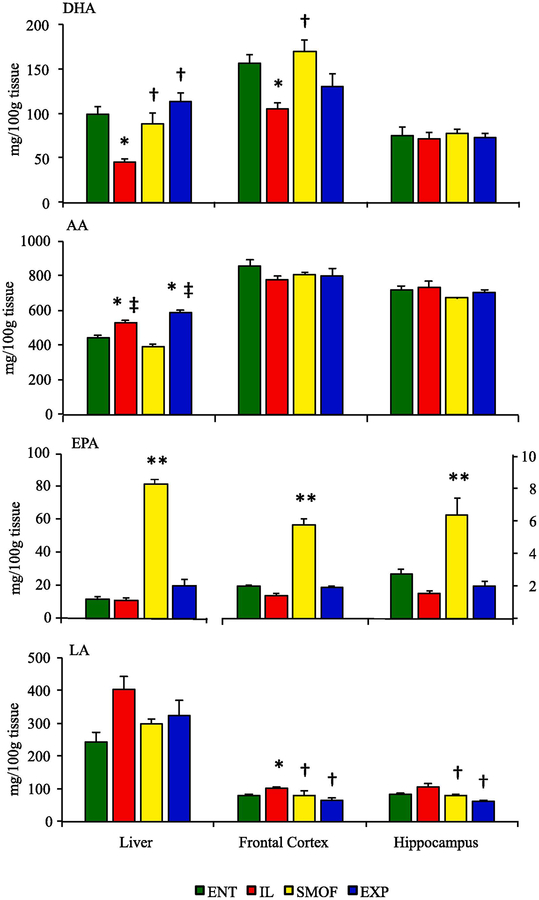
Tissue fatty acid profiles are shown in Fig. 2 and Supplemental Tables 1–3. Analysis of fatty acid content of liver tissues revealed lower levels of DHA and higher levels of ALA in IL piglets compared to all other groups (P < 0.01). Additionally, SMOF reared piglets had greater accumulation of EPA in liver tissue than other groups (P < 0.01). AA levels were higher in IL and EXP than in ENT or SMOF groups (P < 0.01). While LA levels were higher on average in IL piglets, this was not significant. In frontal cortex samples, DHA levels were lower in IL compared to ENT or SMOF groups (P < 0.01). SMOF reared piglets had greater accumulation of EPA than in all other groups (P < 0.01). IL piglets had higher levels of LA than ENT (P = 0.02) or either SMOF and EXP piglets (P < 0.01). Both IL and SMOF groups had higher levels of ALA compared to ENT (P = 0.03 v IL, P < 0.01 v SMOF) and EXP (P < 0.01) groups. No differences were found in AA levels among groups. DHA and AA levels in hippocampus samples were not different among groups. SMOF reared piglets had greater accumulation of EPA in than all other groups (P < 0.01). LA was higher in IL piglets compared to SMOF or EXP (P < 0.01) but not statistically different from ENT piglets. Levels of ALA were highest in IL piglets compared to ENT or EXP groups (P < 0.01), with greater accumulation also in SMOF compared to EXP piglets (P = 0.04).
Fig. 2.
Fatty acid content in liver, frontal cortex and hippocampus tissue in dietary treatment groups at the end of study. Values shown are mg/100g tissue (ENT n = 11, IL n = 12, SMOF n = 11, EXP n = 11). Data are presented as mean ± SEM. * P < 0.05 v ENT, † P <0.05 v IL, ‡ P < 0.05 v SMOF, ** P < 0.05 v all groups
3.2. Body growth rates and organ weights
As shown in Table 4, there were no significant differences among groups with regards to birthweight at beginning of study or growth rate throughout the study. DEXA scanning at end of study revealed a higher percent body fat mass and lower percent lean mass in the Intralipid (IL) group (P < 0.05) compared to either the enteral group or parenteral lipid emulsion groups. Piglets reared on SMOFlipid (SMOF) and Experimental (EXP) lipid had similar body composition to enterally reared piglets. The final organ weights showed that piglets fed an enteral diet had higher small intestine mass (P < 0.05) compared to parenterally fed piglets. Liver mass was found to be greater in all parenteral groups compared to enterally fed piglets (P < 0.05) with evidence that SMOF and EXP lipids reduced the degree of hepatomegaly compared to IL (P < 0.05).
Table 4.
Body and tissue weights of preterm piglets after 22 days.
| ENT n = 11 |
IL n = 12 |
SMOF n = 11 |
EXP n = 11 |
ANOVA P-value |
|
|---|---|---|---|---|---|
| Body Weight | |||||
| Day Initial (g) | 1158 ± 62.1 | 1161 ± 75.3 | 1202 ± 104 | 1106 ± 73.6 | 0.87 |
| Day Final (g) | 3678 ± 173 | 3420 ± 215 | 3769 ± 288 | 3615 ± 166 | 0.69 |
| Growth Rate g/(kg·d) | 47.6 ± 1.29 | 45.6 ± 1.75 | 47.7 ± 1.23 | 48.9 ± 0.99 | 0.37 |
| Lean tissue, g | 3265 ± 150 | 2948 ± 171 | 3357 ± 240 | 3139± 147 | 0.41 |
| Lean tissue, % | 93.0 ± 0.45 | 90.6 ± 0.39a | 93.6 ± 0.51 | 93.2 ± 0.44b | <0.01 |
| Fat tissue, g | 201 ± 19.6 | 260 ± 17.5 | 182 ± 21.6b | 182 ± 16.3b | 0.01 |
| Fat tissue, % | 5.66 ± 0.43 | 8.03 ± 0.38a | 4.95 ± 0.46b | 5.41 ± 0.43b | <0.01 |
| BMC, g | 46.5 ± 2.50 | 47.1 ± 4.14 | 52.4 ± 4.66 | 47.6 ± 2.76 | 0.66 |
| BMC, g/cm2 | 0.155 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.70 |
| Tissue Weights (g/kg body weight) | |||||
| Liver | 40.3 ± 0.73 | 59.5 ± 1.89a | 49.7 ± 1.84a,b | 47.7 ± 1.42a,b | <0.01 |
| Spleen | 4.28 ± 0.60 | 5.79 ± 0.45 | 6.98 ± 0.70 | 7.28 ± 1.00a | 0.02 |
| Small Intestine | 48.7 ± 1.53 | 22.3 ± 1.40a | 24.6 ± 1.00a | 25.3 ± 0.98a | <0.01 |
| Lung | 14.3 ± 0.34 | 17.2 ± 0.73a | 17.1 ± 0.72a | 16.8 ± 0.56a | <0.01 |
| Brain | 11.1 ± 0.48 | 12.8 ± 0.62 | 11.8 ± 0.91 | 11.8 ± 0.49 | 0.34 |
Mean ± SEM, ANOVA with Tukey;
P < 0.05 vs. ENT;
P < 0.05 vs. IL
3.3. Plasma and tissue metabolite analysis
The plasma concentrations of glucose and insulin are shown in Table 5. Glucose levels increased overtime (P < 0.001) without a significant difference among treatment groups, with exception of lower values in IL group at day 22. Insulin levels analyzed on final plasma samples at day 22 were higher in IL group compared to other treatment groups (P < 0.05). We calculated the homeostatic model assessment insulin resistance (HOMA-IR) values using basal (fasting) glucose and insulin concentrations as an estimate for assessing β-cell function and insulin resistance (IR). HOMA-IR values were also higher in IL vs EXP group suggestive of insulin resistance. Total cholesterol levels increased in groups over time of study (P < 0.001) and were lower in EXP group compared to ENT (P = 0.02) or IL (P = 0.045). Triglyceride levels increased in all groups over time (P = 0.002) were higher levels in ENT compared to parenteral groups (P = 0.02 v IL; P < 0.01 v SMOF or EXP).
Table 5.
Metabolic profile in preterm piglets after 22 days.
| ENT | IL | SMOF | EXP | ANOVA P-value |
|
|---|---|---|---|---|---|
| Plasma glucose (uM) | 7.61 ± 0.39 | 5.46 ± 0.56a | 6.17 ± 0.62 | 5.61 ± 0.60 | 0.04 |
| Plasma insulin (pmol/L) | 44.3 ± 7.26 | 101 ± 18.7a | 41.2 ± 7.97b | 33.6 ± 6.02b | <0.01 |
| HOMA-IR | 2.5 ± 0.5 | 3.8 ± 0.9 | 1.9 ± 0.6 | 1.4 ± 0.9b | 0.03 |
| Cholesterol (mM) | 1.86 ± 0.32 | 1.79 ± 0.71 | 1.38 ± 0.33 | 1.25 ± 0.41ab | <0.01 |
| Triglyceride (mM) | 0.44 ± 0.17 | 0.26 ± 0.13a | 0.23 ± 0.10a | 0.20 ± 0.13a | <0.01 |
Mean ± SEM, ANOVA with Tukey;
P < 0.05 vs. ENT;
P < 0.05 vs. IL;
P < 0.05 vs. SMOF
Plasma amino acid profiles are reported in Supplemental Table 1. The most notable differences were the low arginine, ornithine and citrulline concentrations in the three TPN groups compared to enteral pigs, likely due to the low intestinal mass. There were also higher concentrations of histidine, glutamine, glycine, phenylalanine, serine and lower concentrations of isoleucine, lysine, taurine, threonine, and tryptophan among the TPN groups compared to enteral pigs.
3.4. Brain weight, neuronal morphometry
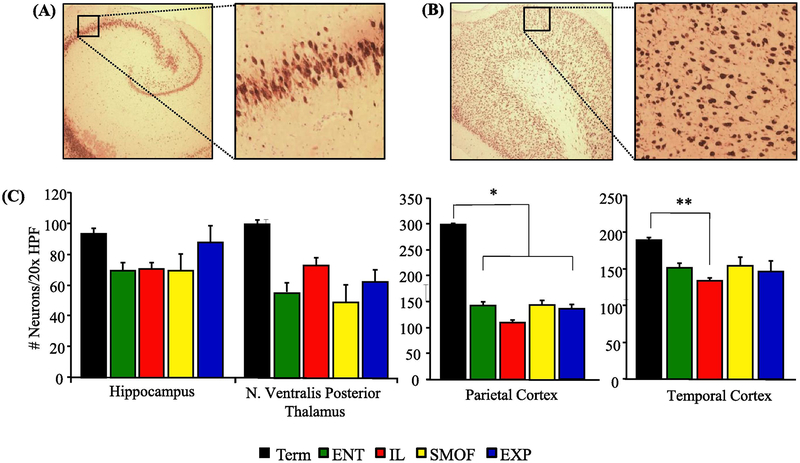
These results are shown in Table 4 and Fig. 3. At 22 days of study there were no difference in either absolute or relative brain weight (g and g/kg body weight) among the four treatment groups. Brain weight (g) of preterm piglets after 22 days treatment were greater compared to newborn 6 day preterm, 4 day preterm and term piglets sacrificed at birth (Supplemental Fig. 2). There were no significant differences in neuronal density between treatments groups in any of the sampled cerebral regions. When compared to term controls, all groups had fewer neurons per HPF in the parietal cortex (P < 0.01) and the IL piglets had fewer neurons in the temporal cortex (P < 0.05).
Fig. 3.
NeuN staining to quantify neuronal density in brain tissue in dietary treatment groups at the end of the study. (A) Sample NeuN staining of preterm hippocampus (HC) at 4× and 20× magnification of CA3 section. (B) Sample NeuN staining of preterm parietal cortex (PC) at 4× and 20× magnification. (C) Comparison of visualized neurons per 20× HPF between term piglet and four preterm piglet study groups. The number of tissue sections analyzed in the hippocampus, nucleus ventralis posterior thalamus, parietal cortex and temporal cortex were as follows: Term n = 2–4, ENT n = 3–10, IL n = 8–11, SMOF n = 6–10, EXP n= 5–10. Data are presented as mean ± SEM. * P < 0.01 Term v preterm, ** P = 0.02 Term v IL
3.5. Activity and behavior measurements
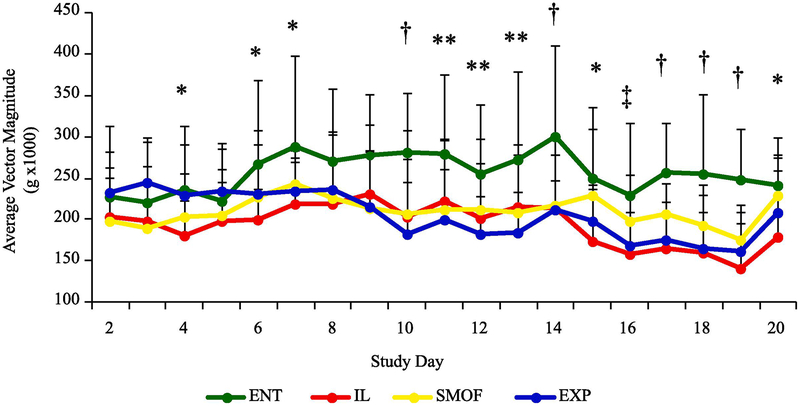
These results are in Fig. 4 and Table 6. Continuous total activity levels were observed over the course of the treatment protocol. There were higher mean values in total activity and high level activity in ENT vs all TPN pigs, even when activity data for handling and feeding times were removed. ENT piglets had increased total daily activity compared to IL reared piglets by day 4 (P < 0.05), and compared to all parenteral groups on day 7 (P < 0.05). This difference persisted through until day 22. When adjusted for periods of handling and feeding, ENT piglets had significantly higher levels of total daily activity compared to parenteral groups by day 10 (P < 0.05). Activity was further categorized into sedentary vs mild, moderate and high activity. All pigs spent a majority of time sedentary, but ENT pigs had an increased amount of time spent in high-level activity compared to parenteral groups (P < 0.01) (Supplemental Fig. 2). There were no significant treatment differences over time in behavior field test measurements including distance traveled (cm), velocity (cm/s), or number of snout-object events (Table 6).
Fig. 4.
Total physical activity per day of study in dietary treatment groups. Values are average vector magnitude (g × 1000) and were adjusted to remove periods of scheduled handling, feeding and field testing. Data are presented as mean ± SEM. * P < 0.05 ENT v IL, ** P < 0.05 ENT v EXP, † P <0.05 ENT v IL, SMOF, EXP, ‡ P < 0.05 ENT v IL, EXP
Table 6.
Exploratory behavior field test observations in preterm piglets.
| ENT n = 11 |
IL n = 12 |
SMOF n = 11 |
EXP n = 10 |
ANOVA P-value |
|
|---|---|---|---|---|---|
| Day of life 7 | |||||
| Distance moved (cm) | 1005 ± 132 | 1084 ± 113 | 797 ± 148 | 889 ± 92.6 | 0.37 |
| Velocity (cm/s) | 8.4 ± 1.1 | 9.0 ± 1.0 | 6.6 ± 1.2 | 7.4 ± 0.8 | 0.38 |
| Novel object explorations (n) | 3.1 ± 0.5 | 2.5 ± 0.7 | 1.5 ± 0.6 | 1.3 ± 0.5 | 0.15 |
| Day of life 14 | |||||
| Distance moved (cm) | 1058 ± 150 | 1079 ± 163 | 863 ± 143 | 773 ± 114 | 0.39 |
| Velocity (cm/s) | 8.8 ± 1.3 | 9.0 ± 1.4 | 7.2 ± 1.2 | 6.4 ± 1.0 | 0.39 |
| Novel object explorations (n) | 3.6 ± 0.5 | 3.7 ± 0.5 | 2.9 ± 0.6 | 2.2 ± 0.7 | 0.24 |
| Day of life 20 | |||||
| Distance moved (cm) | 738 ± 190 | 463 ± 91.0 | 734 ± 149 | 693 ± 143 | 0.47 |
| Velocity (cm/s) | 6.1 ± 1.6 | 3.9 ± 0.8 | 6.1 ± 1.2 | 5.8 ± 1.2 | 0.47 |
| Novel object explorations (n) | 2.6 ± 0.7 | 2.3 ± 0.8 | 2.3 ± 0.7 | 1.7 ± 0.7 | 0.84 |
Mean ± SEM
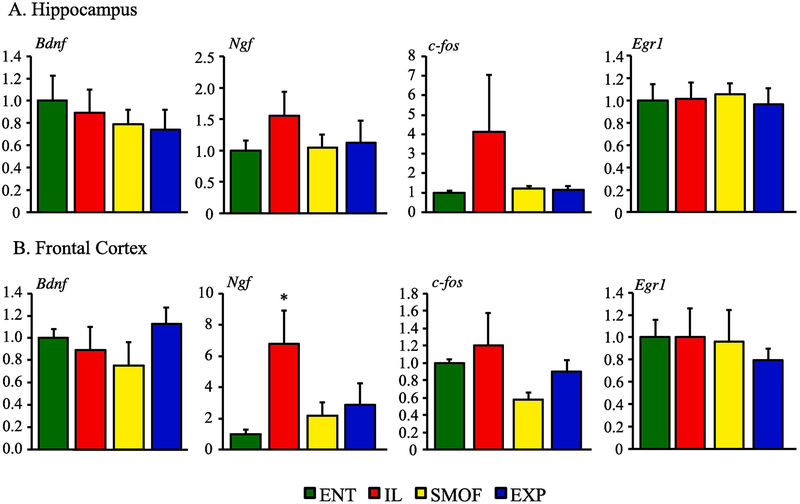
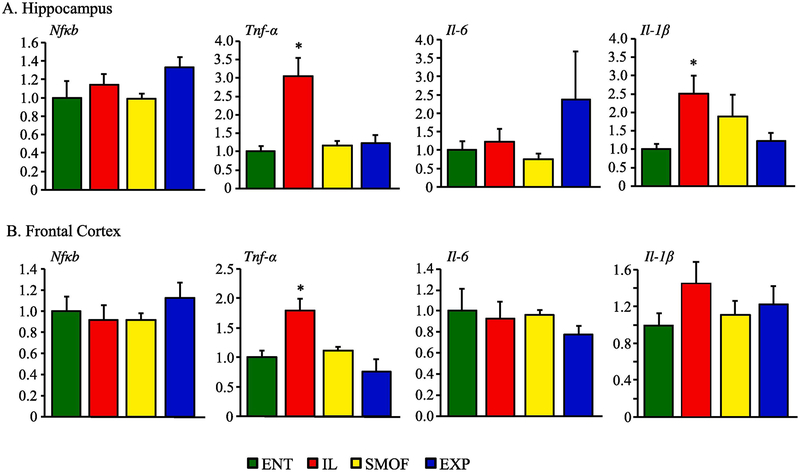
3.6. Neuronal growth and inflammation gene expression
Alterations in expression of pro-growth and proinflammatory genes in HC and FC tissues are shown in Fig. 5 and 6. There was higher expression of NGF in IL vs. other groups in frontal cortex tissue. Otherwise, there were no significant difference in the expression of BDNF, NGF, c-Fos, or Egr1 among the treatment groups in either hippocampus or frontal cortex tissue. There was higher TNF-α expression in IL vs all other groups in both hippocampus and frontal cortex tissue and IL- 1β was increased in IL vs ENT in thehippocampus.
Fig. 5.
Pro-growth gene expression in neuronal tissues expressed a fold change of mRNA level compared to ENT control. (A) Hippocampus levels of bdnf, ngf, c-fos, and egr1 after 22 days treatment with ENT, IL, SMOF or EXP diet. (B) Frontal cortex levels of bdnf, ngf, c-fos, and egr1 after 22 days treatment with ENT, IL, SMOF or EXP diet. Data are presented as mean ± SEM. * P < 0.05 ENT v IL
Fig. 6.
Proinflammatory gene expression in neuronal tissues expressed a fold change of mRNA level compared to ENT control. (A) Hippocampus levels of nfκb, tnf-α, il-6 and il-1-β after 22 days treatment with ENT, IL, SMOF or EXP diet. (B) Frontal cortex levels of nfκb, tnf-α, il-6 and il-1-β after 22 days treatment with ENT, IL, SMOF or EXP diet. Data are presented as mean ± SEM. * P < 0.05 ENT v IL
4. Discussion
The aim of this study was to test if DHA and AA enrichment in parenteral lipid emulsions would lead to a significant fatty acid enrichment in critical brain regions and further translate into improved neurodevelopment, behavioral and inflammatory outcomes. Our data showed that increased parenteral lipid fatty acid intake of DHA and AA resulted in enriched RBC DHA and AA content and in the frontal cortex but not in hippocampus, whereas brain AA profiles were not affected. Additionally, short-term measurements of neurodevelopmental outcomes did not differ among TPN groups. Our results showed a positive effect whereby new generation lipid emulsions (SMOFlipid and Experimental emulsion) prevented the increased body fat mass and insulin resistance and reduced the markers of inflammation in the brain regions observed in pigs given Intralipid. Importantly, we also show novel evidence that parenteral nutrition, regardless of lipid emulsion, reduced total activity compared to enteral nutrition support.
Extreme premature birth deprives infants of late gestation accretion of key LC-PUFA, especially DHA that may be vital for neurodevelopment and maturation. In preterm infant studies, parenteral lipid emulsions containing fish oil, have been shown to improve serum DHA content compared to soy-based emulsions, but was unable to prevent the decline in blood DHA in the first weeks of life (D’Ascenzo et al., 2011; D’Ascenzo et al., 2014; Najm et al., 2017). Our results in preterm pigs show that new generation lipid emulsions, particularly the EXP lipid, produced a more favorable blood and tissue fatty acid profile compared to soy-based Intralipid. Further we show that supplementation of DHA in the EXP lipid normalized blood RBC levels to that of the enteral control group. Despite our favorable blood findings, frontal cortex DHA content was higher in SMOF and ENT pigs compared to IL but not the EXP group. This finding confirms our previous report in preterm pigs (Guthrie et al., 2016) and others in term TPN-fed pigs (Turner 2015) where SMOFlipid increased retinal DHA content. In contrast to the brain, the increased delivery of DHA in both SMOF and EXP emulsions was readily incorporated into liver tissue suggesting differential fatty acid uptake between organs.
Arachidonic acid is another LC-PUFA that is critically important in perinatal growth and development of neuronal and retinal tissues. Recent studies in preterm infants have demonstrated that SMOFlipid reduced the serum concentrations of AA but markedly increased EPA (Najm et al., 2017; Nilsson et al., 2018). These studies reveal a paradoxical decline in serum AA despite increased parenteral AA delivery with SMOFlipid which may be due to the competitive effect of markedly increased EPA on membrane fatty acid incorporation and desaturation pathways (Martin, 2014). In the current study, we observed a decline in AA in both IL and SMOF groups, but a marked increase in EPA with SMOF; these changes in AA and EPA are similar to our previous report in preterm TPN fed pigs given SMOF and pure fish oil (Omegaven) emulsions (Guthrie et al., 2016). Importantly, we show that the EXP lipid resulted in low RBC and tissue EPA and modest increase in AA content in RBC and liver but not brain by the third week of treatment.
We observed a remarkable suppression of physical activity in pigs fed parenterally compared to those fed an enteral formula that was evident after one week of treatment. However, there was no difference in activity among the TPN groups and no differences among all groups in exploratory behavior. This finding supports the emerging evidence of poorly developed motor function reported in preterm pigs and the positive effect of enteral feeding. Andersen et al. (Andersen et al., 2016) described a decrease in activity, exploratory behavior and learning in preterm compared to term piglets. Further studies in preterm pigs showed that enteral feeding of either formula or bovine colostrum increased physical activity, whereas the onset of NEC in formula-fed pigs suppressed activity (Cao et al., 2016; Cao et al., 2015). Deficits in motor proficiency have been reported in infants with intestinal failure (So et al., 2018; So et al., 2016 The structural and functional connection between the brain and gut are well established and select neurotransmitters, such as serotonin may be key players (Martin et al., 2018). In this study, we observed a two-fold greater small intestinal mass in formula-fed compared to parenterally-fed pigs. In this context, enteral feeding is an important stimulus for gut growth and the secretion of serotonin from enteroendocrine cells. Collectively these findings point to an important role of early enteral nutrition in stimulating the development of the gut-brain axis which may be especially critical in the premature infant (Keunen et al., 2015).
Contrary to our initial predictions, IL reared pigs had the highest relative brain weight even when compared to enterally reared pigs. Absolute brain weight and observed neuronal density did not differ among treatment groups. These findings are similar to that of Sun et al. (Sun et al., 2018) who recently reported the effects of inflammation associated with necrotizing enterocolitis on neuronal growth. This study showed that while total neuron counts were equivalent among groups, there was a significant increase in neurite outgrowth and microglial activation in the pigs that expressed higher inflammation and worsened bowel disease. Likewise, we also observed greater expression of trophic and inflammatory genes in brain tissue from IL-reared pigs. The increased proinflammatory cytokine expression (e.g. TNF-α and IL-1β) support the idea that lipid emulsions high in the N-6 LC-PUFA, especially linoleic acid and arachidonic acid, promote inflammation (Martin, 2014). Its notable that for TNF-α and to some extent IL-1β in SMOF and EXP groups were not increased compared to enteral feeding suggesting a possible preventive effect. The effects of these changes on brain morphology and inflammatory gene expression will be important to examine in longer term settings. In comparison to term pigs, analysis of brain tissue of all our preterm pig showed significantly lower neuron density at the end of the study. This finding may be important as it suggests that the brain growth of preterm pigs does not catch up to that of term pigs, even after 3 weeks of nutritional support, either enteral or parenteral.
Another key finding was the improvement in body composition and insulin sensitivity in pig given parenteral new generation compared to soybean lipid emulsions. As in our previous, the current study showed that TPN-fed pigs given IL develop insulin resistance when compared to SMOFlipid and enteral nutrition groups (Guthrie et al., 2016; Stoll et al., 2010; Stoll et al., 2012). Important in this study was that both SMOF and EXP emulsions normalized body composition similar to enteral formula feeding. In these preterm pigs, body composition is dominated by lean tissue and fat represents only slightly more than 5% of body mass, but the absolute increase in fat mass in IL pigs compared to all other groups is proportionally large (260 vs 189 g). It is not clear whether the association between insulin resistance and fat mass in mechanistically linked. However, our previous study showed an increase in plasma TNF-α and IL-6 and several proinflammatory cytokines in liver tissue in TPN-fed pigs given Intralipid (Stoll et al., 2010). The n-3 LC-PUFAs, DHA and EPA, both have been shown to reduced inflammation, adiposity and insulin resistance and are enriched in SMOF and EXP compared to IL emulsion (Kalupahana et al., 2011; Pinel et al., 2016). This finding warrants further study to test whether these effects of parenteral lipid emulsions persist and if they modulate tissue proinflammatory pathways given that premature infants are predisposed to long-term metabolic disease and increased adiposity.
In summary, our findings in preterm pigs demonstrate the benefit of new generation lipid emulsions on tissue fatty acid profiles and metabolic function. This work shows that multicomponent lipid emulsions (SMOF and EXP) prevent the postnatal depletion of DHA in blood and frontal cortex, prevent the increase in proinflammatory brain cytokines observed with the soybean emulsion and result in normalized body composition in preterm pigs. We show that these new generation lipid emulsions do not enhance neurodevelopment outcome during a three week period. We also show additional new evidence that parenteral nutrition delays or suppresses physical activity compared to enteral formula feeding. This benefit of enteral feeding may be due to stimulation of the gut-brain axis and warrants further study. With the increased use of new generation lipid emulsions in Europe and North America, these findings may have important implications for the nutritional support of preterm infants.
Supplementary Material
Supplemental Fig. 1. Diagram of study protocol. Cesarean delivery performed at 108d gestation (6 days preterm). Jugular catheter placed in all piglets and orogastric tube placed on ENT piglets on study day 0. Blood sampling performed on study days 0, 7, 14 and 22. Behavioral field testing completed on study days 7, 14 and 21. DEXA scan completed on study day 21. Tissues collected at completion of study on day 22.
Supplemental Fig. 2. Measurement of brain weight as a function of gestation and dietary treatment. Brain weight is shown as total mass (g) and relative to body weight (g/kg). (Preterm − 6d n = 7, preterm −4d n = 3, term n = 4, ENT n = 11, IL n = 12, SMOF n = 11, EXP n = 11). Data are presented at mean ± SEM. * P < 0.05 ENT v IL
Supplemental Fig. 3. Total activity per day of study including handling and feeding times. Values are average vector magnitude (g × 1000) including all times. Data are presented as mean ± SEM. * P < 0.05 ENT v IL, ** P < 0.05 ENT v EXP, † P <0.05 ENT v IL, SMOF, EXP, ‡ P < 0.05 ENT v IL, EXP
Supplemental Fig. 4. Accelerometer measurements categorized by level of activity, sedentary and high activity as described in methods section. Average minutes per day spent in different activity level shown each in dietary treatment groups. Data are presented as mean ± SEM. * P < 0.001 ENT v IL, SMOF, EXP
HIGHLIGHTS.
Modification of parenteral lipid profile alters cortical fatty acid (FA) profiles, but did not promote brain growth or improve short term neurodevelopment outcomes.
Soybean lipid emulsion increased select markers brain inflammation
Parenteral nutrition dampens physical activity compared to piglets given an enteral diet.
Omega-3 enriched emulsions improve body composition and insulin sensitivity.
Source of funding
This work was supported in part by federal funds from the USDA, Agricultural Research Service under Cooperative Agreement Number 3092-51000-060-01, and grants from the University of Copenhagen, Fresenius Kabi, the Whitlock Foundation, National Institutes of Health Grant DK-094616 (D.G.B), and the Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338). Lee Call was supported by training fellowships from the National Institutes of Health Grant T32-GM088129 and Gulf Coast Consortia, NLM Training Program in Biomedical Informatics (T15-LM007093).
LIST OF ABBREVIATIONS
- AA
Arachidonic acid
- ALA
Alpha-linolenic acid
- BDNF
Brain-derived neurotrophic factor
- BMC
Bone mineral content
- BMD
Bone mineral density
- DXA
Dual-energy X-ray absorptiometry
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FFM
Fat-free mass
- H&E
Hematoxylin and eosin
- HPF
High power field
- HPLC
High performance liquid chromatography
- IFNg
Interferon gamma
- IL-1b
Interleukin 1 beta
- IL-6
Interleukin 6
- LA
Linoleic acid
- LC-PUFAs
Long-chain polyunsaturated fatty acids
- NFkB
Nuclear factor kappa B
- NGF
Nerve Growth Factor
- PUFAs
Polyunsaturated fatty acids
- qRT-PCR
Quantitative real-time polymerase chain reaction
- RBC
Red blood cell
- TNFα
Tumor necrosis factor alpha
- TPN
Total parenteral nutrition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen AD, Sangild PT, Munch SL, van der Beek EM, Renes IB, Ginneken C, Greisen GO, Thymann T, 2016. Delayed growth, motor function and learning in preterm pigs during early postnatal life. Am J Physiol Regul Integr Comp Physiol 310, R481–492. [DOI] [PubMed] [Google Scholar]
- Burrin DG, Ng K, Stoll B, Saenz De Pipaon M, 2014. Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition-associated liver disease. Advances in nutrition 5, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Andersen AD, Li Y, Thymann T, Jing J, Sangild PT, 2016. Physical Activity and Gastric Residuals as Biomarkers for Region-Specific NEC Lesions in Preterm Neonates. Neonatology 110, 241–247. [DOI] [PubMed] [Google Scholar]
- Cao M, Andersen AD, Van Ginneken C, Shen RL, Petersen SO, Thymann T, Jing J, Sangild PT, 2015. Physical activity level is impaired and diet dependent in preterm newborn pigs. Pediatr Res 78, 137–144. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo R, D’Egidio S, Angelini L, Bellagamba MP, Manna M, Pompilio A, Cogo PE, Carnielli VP, 2011. Parenteral nutrition of preterm infants with a lipid emulsion containing 10% fish oil: effect on plasma lipids and long-chain polyunsaturated fatty acids. J Pediatr 159, 33–38 e31. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo R, Savini S, Biagetti C, Bellagamba MP, Marchionni P, Pompilio A, Cogo PE, Carnielli VP, 2014. Higher Docosahexaenoic acid, lower Arachidonic acid and reduced lipid tolerance with high doses of a lipid emulsion containing 15% fish oil: A randomized clinical trial. Clinical nutrition. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Simmer K, Deshmukh M, Mori TA, Croft KD, Kristensen J, 2014. Fish Oil (SMOFlipid) and olive oil lipid (Clinoleic) in very preterm neonates. J Pediatr Gastroenterol Nutr 58, 177–182. [DOI] [PubMed] [Google Scholar]
- Guthrie G, Kulkarni M, Vlaardingerbroek H, Stoll B, Ng K, Martin C, Belmont J, Hadsell D, Heird W, Newgard CB, Olutoye O, van Goudoever J, Lauridsen C, He X, Schuchman EH, Burrin D, 2016. Multi-omic profiles of hepatic metabolism in TPN-fed preterm pigs administered new generation lipid emulsions. Journal of lipid research 57, 1696–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty P, 2010. Fatty acid supply to the human fetus. Annu Rev Nutr 30, 237–255. [DOI] [PubMed] [Google Scholar]
- Harris WS, Baack ML, 2015. Beyond building better brains: bridging the docosahexaenoic acid (DHA) gap of prematurity. J Perinatol 35, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalupahana NS, Claycombe KJ, Moustaid-Moussa N, 2011. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Advances in nutrition 2, 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keunen K, van Elburg RM, van Bel F, Benders MJ, 2015. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr Res 77, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapillonne A, Jensen CL, 2009. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot.Essent.Fatty Acids 81, 143–150. [DOI] [PubMed] [Google Scholar]
- Martin CR, 2014. Fatty acid requirements in preterm infants and their role in health and disease. Clin Perinatol 41, 363–382. [DOI] [PubMed] [Google Scholar]
- Martin CR, Dasilva DA, Cluette-Brown JE, Dimonda C, Hamill A, Bhutta AQ, Coronel E, Wilschanski M, Stephens AJ, Driscoll DF, Bistrian BR, Ware JH, Zaman MM, Freedman SD, 2011. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr 159, 743–749 e741–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CR, Osadchiy V, Kalani A, Mayer EA, 2018. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol 6, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MA, Haymond MW, 2013. Regulation of lipid synthesis genes and milk fat production in human mammary epithelial cells during secretory activation. Am J Physiol Endocrinol Metab 305, E700–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm S, Lofqvist C, Hellgren G, Engstrom E, Lundgren P, Hard AL, Lapillonne A, Savman K, Nilsson AK, Andersson MX, Smith LEH, Hellstrom A, 2017. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: A randomized controlled trial. Clin Nutr ESPEN 20, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K, Stoll B, Chacko S, Saenz de Pipaon M, Lauridsen C, Gray M, Squires EJ, Marini J, Zamora IJ, Olutoye OO, Burrin DG, 2016. Vitamin E in New-Generation Lipid Emulsions Protects Against Parenteral Nutrition-Associated Liver Disease in Parenteral Nutrition-Fed Preterm Pigs. JPEN J Parenter Enteral Nutr 40, 656–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson AK, Lofqvist C, Najm S, Hellgren G, Savman K, Andersson MX, Smith LEH, Hellstrom A, 2018. Influence of Human Milk and Parenteral Lipid Emulsions on Serum Fatty Acid Profiles in Extremely Preterm Infants. JPEN J Parenter Enteral Nutr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel A, Pitois E, Rigaudiere JP, Jouve C, De Saint-Vincent S, Laillet B, Montaurier C, Huertas A, Morio B, Capel F, 2016. EPA prevents fat mass expansion and metabolic disturbances in mice fed with a Western diet. Journal of lipid research 57, 1382–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So S, Patterson C, Evans C, Wales PW, 2018. Motor Proficiency and Generalized Self-Efficacy towards Physical Activity in Children with Intestinal Failure. J Pediatr Gastroenterol Nutr. [DOI] [PubMed] [Google Scholar]
- So S, Patterson C, Gold A, Rogers A, Kosar C, de Silva N, Burghardt KM, Avitzur Y, Wales PW, 2016. Early neurodevelopmental outcomes of infants with intestinal failure. Early Hum Dev 101, 11–16. [DOI] [PubMed] [Google Scholar]
- Stoll B, Chang X, Fan MZ, Reeds PJ, Burrin DG, 2000. Enteral nutrient intake level determines intestinal protein synthesis and accretion rates in neonatal pigs. Am.J Physiol Gastrointest.Liver Physiol 279, G288–G294. [DOI] [PubMed] [Google Scholar]
- Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA, Burrin DG, 2010. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr. 140, 2193–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B, Puiman PJ, Cui L, Chang X, Benight NM, Bauchart-Thevret C, Hartmann B, Holst JJ, Burrin DG, 2012. Continuous parenteral and enteral nutrition induces metabolic dysfunction in neonatal pigs. JPEN J.Parenter.Enteral Nutr. 36, 538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Pan X, Christiansen LI, Yuan XL, Skovgaard K, Chatterton DEW, Kaalund SS, Gao F, Sangild PT, Pankratova S, 2018. Necrotizing enterocolitis is associated with acute brain responses in preterm pigs. J Neuroinflammation 15, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaardingerbroek H, Ng K, Stoll B, Benight N, Chacko S, Kluijtmans LA, Kulik W, Squires EJ, Olutoye O, Schady D, Finegold ML, van Goudoever JB, Burrin DG, 2014. New generation lipid emulsions prevent PNALD in chronic parenterally fed preterm pigs. Journal of lipid research 55, 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Diagram of study protocol. Cesarean delivery performed at 108d gestation (6 days preterm). Jugular catheter placed in all piglets and orogastric tube placed on ENT piglets on study day 0. Blood sampling performed on study days 0, 7, 14 and 22. Behavioral field testing completed on study days 7, 14 and 21. DEXA scan completed on study day 21. Tissues collected at completion of study on day 22.
Supplemental Fig. 2. Measurement of brain weight as a function of gestation and dietary treatment. Brain weight is shown as total mass (g) and relative to body weight (g/kg). (Preterm − 6d n = 7, preterm −4d n = 3, term n = 4, ENT n = 11, IL n = 12, SMOF n = 11, EXP n = 11). Data are presented at mean ± SEM. * P < 0.05 ENT v IL
Supplemental Fig. 3. Total activity per day of study including handling and feeding times. Values are average vector magnitude (g × 1000) including all times. Data are presented as mean ± SEM. * P < 0.05 ENT v IL, ** P < 0.05 ENT v EXP, † P <0.05 ENT v IL, SMOF, EXP, ‡ P < 0.05 ENT v IL, EXP
Supplemental Fig. 4. Accelerometer measurements categorized by level of activity, sedentary and high activity as described in methods section. Average minutes per day spent in different activity level shown each in dietary treatment groups. Data are presented as mean ± SEM. * P < 0.001 ENT v IL, SMOF, EXP