Abstract
Circadian clocks allow organisms to anticipate repetitive changes in their environment such as food availability, temperature, and predation. While they most clearly manifest at the behavioral level, driving sleep-wake cycles, for example, they also provide critical temporal regulation at the level of individual tissues. Circadian clocks within organs act to ensure that each tissue is functioning in a coordinated manner to anticipate the needs of the organism as a whole but also allow for adaptation of organs to their local environment. One critical aspect of this environment is energy availability, which is communicated at the cellular level via changes in metabolites such as ATP, calcium, and NADH. AMP-activated protein kinase (AMPK) is both sensitive to fluctuations in secondary metabolites and capable of resetting the circadian clock via destabilization of the core clock components CRY and PER. Phosphorylation of serine 71 of CRY1 by AMPK destabilizes CRY1 by decreasing its interaction with binding partner PER2, thus enabling greater association with the SCF complex substrate adaptor FBXL3. Here, we describe a transgenic mouse harboring germline mutation of CRY1 serine 71 to alanine. Unexpectedly, this mutation does not affect the steady-state level of CRY1 protein in mouse livers or quadriceps. We also did not detect changes in either behavioral or molecular circadian rhythms, but female Cry1S71A mice exhibit decreased voluntary locomotor activity compared with wild-type littermates. Together, these findings suggest that phosphorylation of CRY1 serine 71 is not required for the regulation of circadian rhythms under normal physiological conditions. However, it may be involved in responding to metabolic challenges or in other aspects of physiology that contribute to voluntary activity levels.
Keywords: circadian, CRY1, cryptochrome, AMPK, period, voluntary activity
The mammalian circadian clock is composed of a transcription-translation feedback loop (TTFL), in which the proteins BMAL1 and CLOCK heterodimerize to activate transcription of numerous target genes, including those encoding their own repressors, the PERIOD (PER1,2,3) and CRYPTOCHROME (CRY1,2) proteins (Green et al., 2008; Partch et al., 2014). While the cyclic activation and repression of BMAL1/CLOCK establishes the basic rhythm of the clock, precise timekeeping is achieved through numerous additional mechanisms, including posttranslational modifications of the core clock proteins. Functioning of the clock within the suprachiasmatic nucleus (SCN) of the mammalian brain both dictates behavioral rhythms and coordinates rhythmic functioning of peripheral tissues (Stephan and Zucker, 1972). However, for a clock to be functional, it must not only keep time but also be susceptible to resetting or “entrainment” in response to environmental timing cues. The core TTFL in peripheral organs is entrained by feeding time (Damiola et al., 2000; Stokkan et al., 2001), likely by a combination of myriad metabolic signals (Dibner and Schibler, 2015; Jordan and Lamia, 2013). One important pathway through which such signals are communicated to metabolic organs is via AMP-activated protein kinase (AMPK), which is activated under conditions of cellular ATP depletion (Jordan and Lamia, 2013) and can shift peripheral clock time (Lamia et al., 2009). AMPK phosphorylates 2 serines in CRY1 (S71 and S280); phosphorylation of S71 is sufficient to significantly destabilize overexpressed CRY1 (Lamia et al., 2009). Here we report a characterization of behavioral and peripheral tissue rhythms in mice harboring germline mutation of serine 71 to the nonphosphorylatable residue alanine.
MATERIALS AND METHODS
Mice
Homology arms constituting genomic regions surrounding exon 2 of the mouse Cry1 locus were cloned by HindIII restriction digest of BAC plasmid bMQ_447c17 into the pBSKS vector backbone by conventional methods. NotI and PacI sites were introduced into the pBSKS vector containing the 3′Arm, and SbfI and AscI sites were introduced into the pBSKS vector containing the 5′Arm to enable subsequent transfer to pACN. Because of difficulty in amplifying and/or mutagenizing the surrounding region by polymerase chain reaction (PCR), the S71A mutation was introduced into an overlapping genomic region in pBSKS using site-directed mutagenesis (primers: cgaaagctgaacgctcgcctcttcgtg and cgaaagctgaacgctcgcctcttcgtg). A 409-bp fragment directly surrounding either wild-type (WT) or S71A mutant exon 2 was then transferred by SwaI/(partial HincII) restriction digest into the HindIII-cloned 3′Arm in pBSKS. The resulting WT and S71A mutant homology arms were transferred to the pACN vector by directional NotI/PacI and SbfI/AscI restriction digest. The resulting targeting vector was linearized by PmeI digest prior to electroporation into c57Bl6/NJ embryonic stem (ES) cells at the Scripps Research Transgenic Core Facility. ES cell clones were screened by PCR and Southern blot, and 2 positive clones were injected into pseudopregnant females. Germline transmission was confirmed by Southern blot and PCR. Male offspring were used to breed to c57Bl6/J female mice for the first generation to ensure removal of the self-excising CRE recombinase and neomycin resistance cassette driven by a testis-specific promoter. Genotyping of the mice is performed by PCR using the following primers:
S71AF (TGAGTCGGAATTTCATGTATGCCTG)
S71AR (AGGAATCACAGCAGTAATCAAACAG)
LoxPF (ACTTCGTATAGCATACATTATACGAA)
pACN3′SJ (AAGACGGTCGCAGGACCTCAAAT)
Amplicons of 148 bp and 183 bp indicate the presence of the WT and S71A mutant alleles, respectively. The presence of the desired mutation was confirmed by PCR amplification and sequencing of the region of interest. Mice were bred with c57Bl6/J mice from the TSRI colony for ≥5 generations. Mice were entrained to a 12-h light/12-h dark cycle for at least 2 weeks prior to experiments, and all experiments were performed at 8 to 17 weeks of age. All animal care and treatments were in accordance with The Scripps Research Institute guidelines for the care and use of animals and were approved by the TSRI Institutional Animal Care and Use Committee.
Behavioral Analysis
Mice were single housed in Coulbourn Instruments (cat No. ACT-551-MS-SS) cages containing a running wheel attached to an activity monitor. Mice were entrained to a 12-h light/12-h dark cycle for 2 weeks prior to release into 24-h darkness for measurement of activity and free-running period for 5 weeks. Some mice were then reentrained to a light-dark cycle for 2 weeks and released into constant darkness again for measurement of the speed of reentrainment and of activity and free-running period at an advanced age. Running-wheel data were collected and analyzed using Actimetrics’ Clocklab Analysis Software. The free-running period was calculated from data collected in constant darkness using the chi-squared periodogram method.
Lumicycle Analysis
For analysis of circadian rhythmicity in ear fibroblasts, cells were isolated from transgenic mice in which luciferase coding sequence is fused to the C-terminus of PER2 expressed from the endogenous Per2 locus (B6.129S6-Per2tm1Jt/J; Jackson Labs strain No. 006852; Yamazaki et al., 2004). Per2Luc fibroblasts were plated at 1 × 106 cells per plate in 3.5-cm dishes. One day after plating, ear fibroblasts were exposed to media containing 50% horse serum, 44.5% Dulbecco’s Modified Eagle Medium (DMEM), 5% fetal bovine serum (FBS), and 0.5% PenStrep by volume for circadian synchronization. Following 2 h of synchronization, cells were switched to media containing 100 μM D-luciferin. Plates were sealed with vacuum grease (Dow Corning high-vacuum grease; VWR cat No. 59344–055) and glass cover slips (40CIR-1, Fisher Scientific cat No. 22038999) and placed into the Actimetrics Lumicycle 32. Background subtraction of the recorded data was performed using the running average setting and fit by least mean squares calculation to a damped sine wave to calculate the period, amplitude, and phase. Only data with at least an 80% goodness of fit were included in the analysis. Analysis of rhythmicity in tissue explants was performed similarly, except that the tissue explants were cultured in DMEM supplemented with 5% FBS and penicillin-streptomycin. Following synchronization of circadian rhythms, they were transferred to DMEM supplemented with 1% FBS, penicillin-streptomycin, and 100μM D-luciferin.
Tissue Preparation for Western Blot Analysis
Quadriceps muscles were collected from sedentary male mice of the given genotype synchronized to a 12-h light/12-h dark cycle. Following collection, both quadriceps muscles were rinsed briefly in phosphatebuffered saline prior to nuclear fractionation as described (Dimauro et al., 2012). Nuclear lysates were collected and equilibrated prior to Western blot analysis.
Western Blots
Total cell lysates (30–50 μg) or nuclear extracts (3–5 μg) were separated by sodium dodecyl sulfate– polyacrylamide gel electrophoresis and transferred to polyvinylidine difluoride membranes. Proteins were detected by standard Western blotting procedures. Antibodies used recognized anti-Cry1-CT and anti-Cry2-CT as described (Lamia et al., 2011); LaminA antibody (L1293) was purchased from Sigma.
Statistics
All data were analyzed by analysis of variance (ANOVA) with post hoc testing using GraphPad Prism 7 software. In Figure 1, male and female free-running period and voluntary activity data were analyzed by 2-way ANOVA. In Figure 2, measurements of free-running period were analyzed by Student t test. In Figure 3, period and total activity were analyzed by multiple 1-way ANOVA. All p values were adjusted to correct for multiple comparisons.
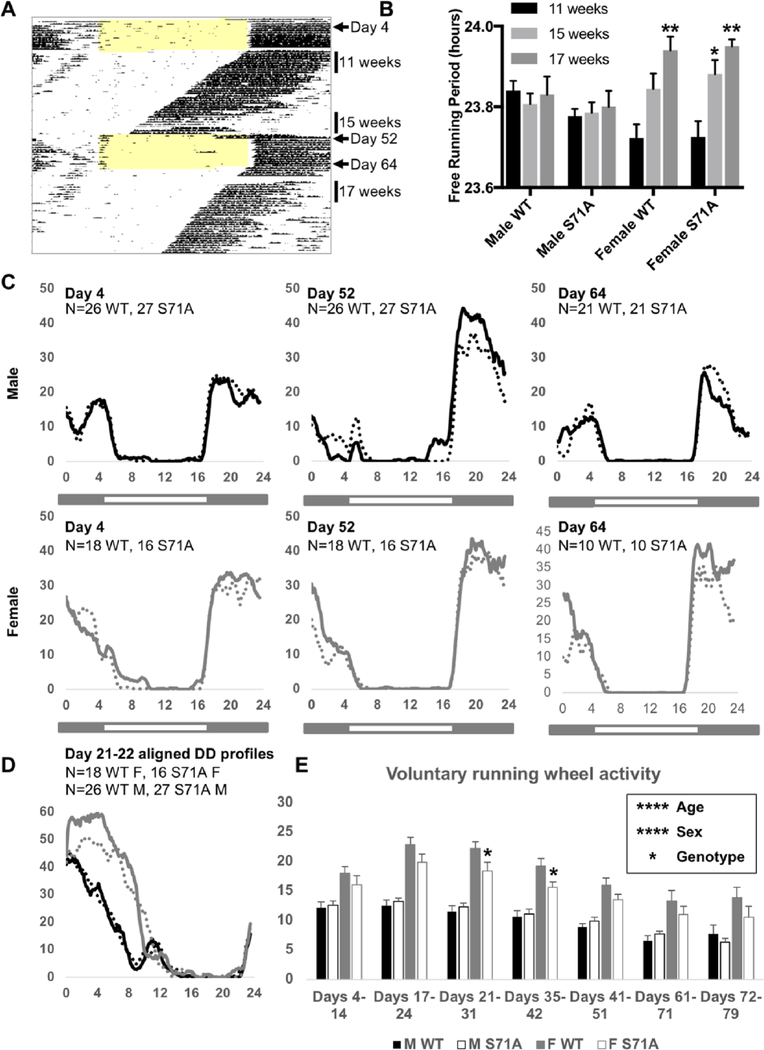
Figure 1.
CRY1S71A mice have a normal free-running period and slightly decreased overall activity levels. Representative actogram (A) depicting voluntary wheel-running activity as black tick marks for which the height represents the amount of voluntary activity in 6-min bins across the 24-h cycle. Each horizontal line represents 1 day. Light colored shading indicates when lights were on. The data used to calculate free-running periods at 11 weeks, 15 weeks, and 17 weeks of age and to generate temporal activity profiles on day 4, day 52, and day 64 are indicated by vertical bars to the right of the actogram. Free-running period in constant darkness (B), temporal activity profiles smoothed over 1-h windows in standard light-dark cycles (C), or in constant darkness (D), and voluntary running-wheel activity per day in WT and Cry1S71A male and female mice. In (C, D), black and gray lines represent male and female mice, respectively; solid and dashed lines represent WT and Cry1S71A mice, respectively. Data represent the mean for n = 10 to 27 mice per group. In (B, E), error bars represent SEM. In (B), *p < 0.05, **p < 0.01 versus 11 weeks. In (E), *p < 0.05 versus wild-type. p values were determined by post hoc testing after a significant main effect for the factor of interest was revealed by 2-way analysis of variance.
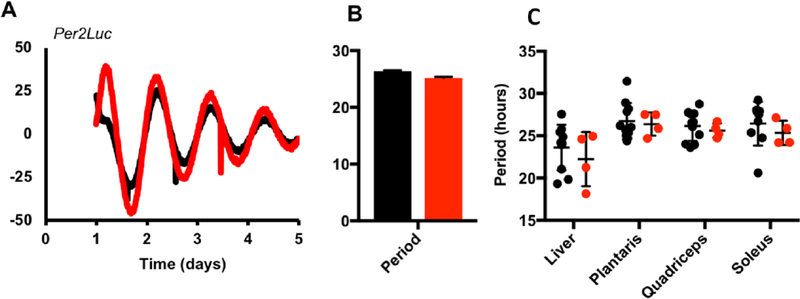
Figure 2.
Cry1S71A fibroblasts and tissue explants have normal circadian periods. (A) Circadian rhythms of luciferase activity recorded from Per2Luc-expressing ear fibroblasts isolated from wild-type (WT) and Cry1S71A mice. (B, C) Average period of luminescence rhythms measured in (WT, black) or mutant (Cry1S71A/S71A, grey) fibroblasts (B) or explants of the indicated tissue type (C). Data in (A, B) are representative of 2 independent experiments using cells derived from 2 different WT and Cry1S71A littermate pairs. In (B, C), each data point represents 1 plate of cells or 1 tissue explant from a unique animal. Lines and error bars represent the mean ± SEM of 8 replicates per cell line (A) line or 4 to 11 replicates per explant (B).
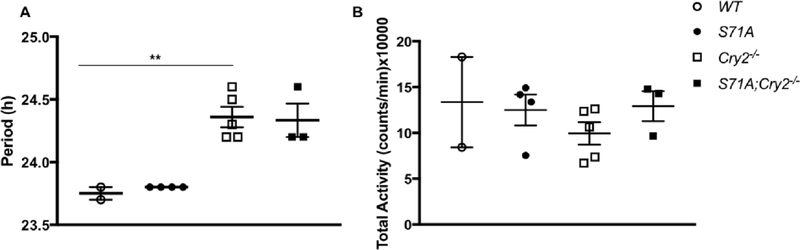
Figure 3.
Period and running-wheel activity of Cry2−/− mice are not affected by the S71A mutation. (A) Period of WT, Cry1S71A, Cry2−/−, and Cry1S71A;Cry2−/− mice in 24-h darkness (DD) at 8 weeks of age. (B) Total running-wheel activity of indicated genotypes at 8 weeks of age. Data shown represent activity during 2 weeks of standard 12 h:12 h (LD) and 5 weeks of (DD). Data represent the mean ± SEM for n = 2 to 5 male mice per genotype. **p < 0.01 versus wild-type by multiple 1-way analysis of variance.
RESULTS
Generation of Cry1S71A Mice
To generate CRY1S71A mice, we designed a targeting construct containing a self-excising CRE recombinase and neomycin resistance gene flanked by sequences homologous to the genomic region surrounding exon 2 of mouse Cry1. Expression of CRE recombinase in ES cells led to the replacement of the serine 71 codon with an alanine codon as well as a residual loxP site upstream of exon 2 (Fig. 4A). Successful mutation of S71 was confirmed in ES cells via Southern blot prior to host blastocyst injection (Fig. 4B). Genomic DNA from the resulting mice was sequenced to confirm the S71A mutation (Fig. 4C).
Figure 4.
Generation of Cry1S71A mice. (A) Design of the targeting strategy: a self-excising CRE recombinase and neomycin resistance cassette was used to generate animals in which serine 71 is replaced with alanine in the Cry1 genomic locus. A single loxP site remains upstream of exon 2. (B) Southern blot demonstrating proper targeting of embryonic stem (ES) cells used for blastocyst injection (S71A/+). (C) Sequencing of genomic DNA from littermate animals harboring wild-type or Cry1S71A targeted locus.
Cry1S71A Mice have a Normal Free-Running Period and Decreased Voluntary Activity
In mice, loss of Cry1 or Cry2 leads to shorter or longer free-running periods of behavioral rhythms in constant darkness, respectively (Vitaterna et al., 2002). We anticipated that the S71A mutation, which is expected to stabilize CRY1, would lead to changes in circadian rhythms. To examine behavioral rhythms, homozygous Cry1S71A mice and control WT littermates were placed in running-wheel cages and acclimated to a standard 12 h:12 h light-dark cycle for 2 weeks followed by 5 weeks of constant darkness; this pattern was repeated to enable measurement of voluntary activity levels and free-running period in young and middle-aged mice (Fig. 1A,B). Both age and sex can influence the free-running period (Davis et al., 2017; Weitzman et al., 1982). Here, we found that from 11 to 17 weeks of age, the free-running period of male mice remained relatively constant, while it increased steadily with age in females (Fig. 1B). In contrast, the Cry1S71A mutation did not significantly affect the free-running period in either male or female mice (Fig. 1B). Voluntary running-wheel activity was much higher in female mice of both genotypes, as reported by others (Garland et al., 2011), and is robustly affected by age, with sexually dimorphic patterns (Figs. 1C–E). Although there was no change in circadian behavioral rhythmicity or the temporal profile of activity (Figs. 1C,D), the Cry1S71A mutation may slow reentrainment to a light-dark cycle (Fig. 1C, day 52) and decreases voluntary activity in female mice (Figs. 1D,E).
Cry1S71A Tissues Do Not have Altered Circadian Rhythms
While overt behavioral rhythms in mammals are driven by the clock in the SCN (Welsh et al., 2010), clocks in peripheral tissues can be uncoupled from the master oscillator through temporal restriction of feeding (Damiola et al., 2000; Stokkan et al., 2001). We therefore sought to determine if the circadian clock in peripheral tissues was altered, independent of a lack of genotype effect on central clock function, as revealed by behavioral rhythms. Cry1S71A mice were bred with mice expressing luciferase as a fusion protein with endogenous PER2 (Per2LucTg) to generate Cry1+/+;Per2 LucTg/+ and Cry1S71A/S71A;Per2LucTg/+ littermates. Circadian rhythms recorded from ear fibroblasts isolated from these mice had period and amplitude indistinguishable from controls (Fig. 2A,B). To more fully address whether rhythms in key metabolic organs were altered, we measured luciferase activity in tissue explants from the liver and muscle (quadriceps, soleus, and plantaris) of WT and S71A mice. None of the tissues examined exhibited a significant difference in period due to genotype (Fig. 2C).
The S71A Mutation Does Not Alter CRY1 Steady-State Protein Level in Liver or Muscle of Mice
Phosphorylation of CRY1 on serines 71 and 280 by AMPK destabilizes CRY1 in cultured fibroblasts, and deletion of the AMPK-activating kinase LKB1 stabilizes CRY1 in mouse liver nuclei throughout the day (Lamia et al., 2009). Furthermore, mutation of serine 71 in mouse CRY1 to the nonphosphorylatable amino acid alanine significantly increases the stability of overexpressed CRY1 (Lamia et al., 2009). Although Cry1S71A mice exhibit no apparent alteration in central circadian function measured by overt behavioral rhythms, we hypothesized that peripheral circadian clocks would be more likely affected by the loss of an AMPK-mediated signal. We therefore investigated molecular circadian rhythms in key metabolic tissues of Cry1S71A and littermate control mice. In liver nuclei prepared from both WT and Cry1S71A littermates at 6 zeitgeber times (ZTs; hours after lights-on), we observed the expected oscillation of CRY1 and CRY2 abundance, with no apparent impact of genotype on CRY1 protein levels (Fig. 5A). Next, we examined CRY1 protein abundance at ZT0 and ZT12 in quadriceps muscle of WT and S71A mice, as these times were representative of high and low CRY abundance, respectively. While we detected the expected difference between ZTs, CRY1 protein level was unaffected by Cry1S71A genotype (Fig. 5B).
Figure 5.
CRY1 and CRY2 protein rhythms are normal in Cry1S71A mouse liver and quadriceps muscle. CRY1, CRY2, and LAMIN A detected by Western blot in mouse liver nuclei (A) or quadriceps muscle nuclei (B) collected at the indicated zeitgeber times (ZT, hours after lights-on).
The S71A Mutation Does Not Alter Behavioral Rhythms in Cry2-Deficient Mice
Despite a high level of sequence conservation, mammalian CRY1 and CRY2 are not redundant in controlling the timing of the clock. Indeed, studies in mice have revealed that while Cry1−/− animals display a shorter period compared with WT littermates, Cry2−/− animals have a longer period (Hotz Vitaterna et al., 1999). While precise tuning and resetting of the clock relies on a large suite of posttranslational modifications regulating the stability of individual clock components (Gallego and Virshup, 2007), the relative ratio of the cryptochrome proteins, rather than direct stability, seems to be important for setting the circadian period (Li et al., 2016). We therefore investigated whether the S71A mutation conferred additional changes to the circadian period in Cry2-null mice. WT, Cry1S71A, Cry2−/−, and Cry1S71A;Cry2−/− male littermates were acclimated for 2 weeks in a standard 12 h:12 h light-dark cycle prior to being released into complete darkness for 5 weeks, during which time the circadian period and total running-wheel activity were recorded. As expected, Cry2−/− animals displayed a significantly longer period compared with WT controls (Fig. 3A). The Cry1S71A mutation, however, did not confer any additional change in period length compared with Cry2−/− animals. No significant difference in activity was found for any of the genotypes examined, although this experiment lacks statistical power to detect subtle changes because of the small number of animals studied (Fig. 3B).
DISCUSSION
Here we report the effects of germline mutation of CRY1 serine 71 to alanine in a genetically engineered mouse model. While the S71A mutation stabilizes CRY1 protein when overexpressed in cultured cells (Lamia et al., 2009), we sought to characterize the effect of this mutation on protein stability in vivo within several key metabolic tissues. Surprisingly, it does not significantly affect the steady-state level of CRY1 protein at any of the zeitgeber times examined in mouse liver, muscle, or fibroblasts. Nor does it appear to affect behavioral rhythms in mice. We cannot exclude the possibility that CRY1S71A protein stability is enhanced but other mechanisms compensate in vivo to maintain normal molecular and behavioral circadian rhythmicity. Also, Cry1S71A mice may exhibit altered behavioral responses to photic stimuli that were not examined here.
Our inability to detect increased CRY1 protein or changes in circadian rhythms in Cry1S71A mice is consistent with another study that did not detect a difference in circadian period between Cry1−/−;Cry2−/− fibroblasts, in which rhythmicity was rescued by transiently expressing either WT CRY1 or CRY1S71A (Ode et al., 2017), but contrasts with observed effects on CRY1 stability in livers of mice following genetic or pharmacological manipulation of AMPK activity (Lamia et al., 2009). Multiple factors could contribute to this apparent discrepancy. First, phosphorylation of either serine 71 or 280 may be sufficient for AMPK to destabilize CRY1 in vivo. Indeed, mutation of either serine 71 or serine 280 to the phospho-serine mimetic aspartic acid destabilizes CRY1 in cultured cells (Lamia et al., 2009), and transient expression of CRY1S71D in Cry1−/−;Cry2−/− fibroblasts results in a significantly lengthened circadian period compared with that of the same cells expressing WT CRY1 (Ode et al., 2017). In addition, AMPK can destabilize PER2 (Jee et al., 2007), which could indirectly contribute to CRY1 destabilization. Thus, S71 phosphorylation may be redundant with other pathways for modulating CRY1 stability and circadian rhythms. Finally, given the importance of AMPK in relaying information pertaining to energy abundance to the clock, we cannot rule out the possibility that Cry1S71A mice may exhibit altered circadian or other functions under conditions of metabolic stress, such as fasting or intense exercise.
AMPK phosphorylation of CRY1 increases its interaction with FBXL3 (Lamia et al., 2009). We recently demonstrated that both CRY1 and CRY2 can act as co-factors to recruit additional proteins for turnover via SCFFBXL3 (Correia et al., 2019; Huber et al., 2016). Phosphorylation of CRY1 by AMPK and its increased association with FBXL3 may thereby transmit signals pertaining to energy status not only to the circadian clock but to other pathways as well. Mutation of serine 71 to alanine in Cry1S71A mice might therefore result in decreased turnover of undefined protein targets. The decreased voluntary activity levels of female Cry1S71A mice indicate a role of CRY1 serine 71 phosphorylation in the regulation of voluntary activity. Intriguingly, voluntary running-wheel activity in female rats is modified by expression of protein kinase inhibitor alpha (Roberts et al., 2014; Grigsby et al., 2019), which inhibits cyclic AMP signaling. CRY1 has been shown to regulate cyclic AMP signaling (Zhang et al., 2010; Narasimamurthy et al., 2012), so this may contribute to the observed difference in voluntary runningwheel activity observed here. Alternatively, reduced voluntary running-wheel activity may reflect a change in metabolism. Further characterization of this mouse model is needed to understand the impact of the S71A mutation on CRY1 function and downstream effects on physiology.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health to K.A.L. (DK090188, DK097164, and DK112927), a Searle Scholars award to K.A.L. from the Kinship Foundation, and by fellowships from Deutsche Forschungsgemeinschaft and the American Heart Association (AHA fellowship number 15POST22510020) to S.D.J. We thank Anne-Laure Huber, Emma Henriksson, Stephanie Papp Correia, Anna Kriebs, Carsten Merkwirth, Henriette Uhlenhaut, Satchidananda Panda, Ronald Evans, and Reuben Shaw for helpful discussions, sharing reagents, and/or critical reading of the manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Correia SP, Chan AB, Vaughan M, Zolboot N, Perea V, Huber A-L, Kriebs A, Moresco JJ, Yates JR, and Lamia KA (2019) The circadian E3 ligase complex SCFFBXL3+CRY targets TLK2. Sci Rep 9(1):1–9. 10.1038/s41598-018-36618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F, Le Minli N, Preitner N, Kornmann B, Fleury-Olela F, and Schibler U (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14(23):2950–2961. 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FC, Darrow JM, and Menaker M (2017). Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol 244(1):R93–R105. 10.1152/ajpregu.1983.244.1.r93. [DOI] [PubMed] [Google Scholar]
- Dibner C and Schibler U (2015) Circadian timing of metabolism in animal models and humans. J Intern Med 277(5):513–527. 10.1111/joim.12347. [DOI] [PubMed] [Google Scholar]
- Dimauro I, Pearson T, Caporossi D, and Jackson MJ (2012) A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res Notes 5:513 10.1186/1756-0500-5-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego M and Virshup DM (2007) Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8(2):139–148. 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Garland T Jr, Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, Van Cleave SL, and Middleton KM (2011) How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proc Biol Sci 278:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, and Bass J (2008) The meter of metabolism. Cell 134(5):P728–P742. 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby KB, Ruegsegger GN, Childs TE, and Booth FW (2019) Overexpression of protein kinase inhibitor alpha reverses rat low voluntary running behavior. Mol Neurobiol 56:1782–1797. [DOI] [PubMed] [Google Scholar]
- Hotz Vitaterna M, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, et al. (1999) Differential regulation of mammalian Period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A 96(21):12114–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AL, Papp SJ, Chan AB, Henriksson E, Jordan SD, Kriebs A, Nguyen M, Wallace M, Li Z, Metallo CM, et al. (2016). CRY2 and FBXL3 cooperatively degrade c-MYC. Mol Cell 64(4):774–789. 10.1016/j.molcel.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee HU, Yang S, Yamazaki S, Kang H, Viollet B, Foretz M, and Chung JH (2007) Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iε (CKIε)-dependent degradation of clock protein mPer2. J Biol Chem 282:20794–20798. 10.1074/jbc.C700070200. [DOI] [PubMed] [Google Scholar]
- Jordan SD and Lamia KA (2013) AMPK at the cross-roads of circadian clocks and metabolism. Mol Cell Endocrinol 366(2):163–169. 10.1016/j.mce.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan K-A, Yamazaki S, Tei H, Sakaki Y, and Menaker M (2001) Entrainment of the circadian clock in the liver by feeding. Science 291(5503):490–493. 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, and Evans RM (2011) Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480:552–556. 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, Di Tacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, et al. (2009) AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326(5951):437–440. 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiong W, and Zhang EE (2016) The ratio of intracellular CRY proteins determines the clock period length. Biochem Biophys Res Commun. 472(3):531–538. 10.1016/j.bbrc.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, and Verma IM (2012) Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A 109: 12662–12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ode KL, Ukai H, Susaki EA, Narumi R, Matsumoto K, Hara J, Koide N, Abe T, Kanemaki MT, Kiyonari H, et al. (2017). Knockout-rescue embryonic stem cell-derived mouse reveals circadian-period control by quality and quantity of CRY1. Mol Cell 2017;65(1):176–190. 10.1016/j.molcel.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Partch CL, Green CB, and Takahashi JS (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24(2):90–99. 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Toedebusch RG, Wells KD, Company JM, Brown JD, Cruthirds CL, Heese AJ, Zhu C, Rottinghaus GE, Childs TE, et al. (2014) Nucleus accumbens neuronal maturation differences in young rats bred for low versus high voluntary running behaviour. J Physiol 592:2119–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK and Zucker I (1972) Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci 69(6):1583–1586. 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, and Menaker M (2001) Entrainment of the circadian clock in the liver by feeding. Science 291(5503):490–493. 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, et al. (2002) Differential regulation of mammalian Period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A 96(21):12114–12119. 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman ED, Moline ML, Czeisler CA, and Zimmerman JC (1982) Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging 3(4):299–309. 10.1016/0197-4580(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, and Kay SA (2010) Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 72(1):551–577. 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Hong H-K, Yoo S-H, Oh WJ, Buhr ED, Yoo OJ, Takahashi JS, et al. (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339–5346. 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, et al. (2010) Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 16:1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]