Abstract
Background
Guidelines advise the use of antibacterials (ABs) in the management of COPD exacerbations. COPD patients often have multiple comorbidities, such as diabetes mellitus and cardiac diseases, leading to polypharmacy. Consequently, drug–drug interactions (DDIs) may frequently occur, and may cause serious adverse events and treatment failure.
Objectives
(i) To review DDIs related to frequently prescribed ABs among COPD patients from observational and clinical studies. (ii) To improve AB prescribing safety in clinical practice by structuring DDIs according to comorbidities of COPD.
Methods
We conducted a systematic review by searching PubMed and Embase up to 8 February 2018 for clinical trials, cohort and case–control studies reporting DDIs of ABs used for COPD. Study design, subjects, sample size, pharmacological mechanism of DDI and effect of interaction were extracted. We evaluated levels of DDIs and quality of evidence according to established criteria and structured the data by possible comorbidities.
Results
In all, 318 articles were eligible for review, describing a wide range of drugs used for comorbidities and their potential DDIs with ABs. DDIs between ABs and co-administered drugs could be subdivided into: (i) co-administered drugs altering the pharmacokinetics of ABs; and (ii) ABs interfering with the pharmacokinetics of co-administered drugs. The DDIs could lead to therapeutic failures or toxicities.
Conclusions
DDIs related to ABs with clinical significance may involve a wide range of indicated drugs to treat comorbidities in COPD. The evidence presented can support (computer-supported) decision-making by health practitioners when prescribing ABs during COPD exacerbations in the case of co-medication.
Introduction
COPD is a complex respiratory disorder characterized by persistent respiratory symptoms and airflow limitation.1 The chronic and progressive course of COPD is frequently aggravated by exacerbation, defined as an acute worsening of respiratory symptoms, such as increased cough, dyspnoea and production of sputum.2 Exacerbations of COPD can be triggered by respiratory tract infections; 40%–60% of exacerbations are caused by bacteria, especially Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis.3 Evidence from randomized controlled trials indicated that use of antibacterials (ABs) may reduce the frequency and severity of COPD exacerbations.4–6 Therefore, guidelines have recommended involving ABs in the therapeutic and preventive management of COPD exacerbations.1,7
Patients with COPD often suffer from multiple morbidities.8 Hence, polypharmacy is common and contributes to drug–drug interactions (DDIs). Adverse drug reactions (ADRs) or therapeutic failure may be the result of interactions between ABs and co-administered drugs. In addition, COPD is an age-related disease and the elderly are more susceptible to the effect of DDIs because of gradual physiological changes affecting pharmacokinetics and pharmacodynamics.9
The objectives of this study were to: (i) systematically review DDIs related to frequently prescribed ABs among COPD patients from observational and clinical studies; and (ii) improve AB prescribing safety in clinical practice by structuring DDIs according to comorbidities of COPD. Studies without comparison groups, and therefore with low quality of the causal evidence, such as case reports about QT-interval prolonging interactions, are not included in this review. A DDI handbook such as Stockley’s Drug Interactions and the official product information should be referred to for the clinical impact of these kinds of interaction.
Methods
Search strategy
We conducted a systematic review following the PRISMA guideline. PubMed and Embase databases were searched for related articles published in English up to 8 February 2018 using key terms ‘drug interactions’, ‘pharmacokinetics’ and ‘pharmacodynamics’, and a list of most frequently used ABs for COPD (Table 1). The ABs were selected based on two related Cochrane reviews and their prescription frequency in the University of Groningen prescription database IADB.nl (http://www.iadb.nl/) covering drug prescriptions for ∼700000 people.4,5 Additionally, we checked the primary sources of signals from Dutch DDI alert systems: G-Standard and Pharmabase.10 Reference lists from eligible studies were also tracked for additional qualified papers. Full search details are provided in the Supplementary data, available at JAC Online.
Table 1.
ABs included in the study that are frequently prescribed among COPD patientsa
| Category | Sub-category | ABs included |
|---|---|---|
| β-Lactam | penicillin | amoxicillin/clavulanic acid (co-amoxiclav), amoxicillin, flucloxacillin, pheneticillin, phenoxymethylpenicillin (penicillin V) |
| cephalosporin | cefaclor, cefuroxime, ceftriaxone, cefradine, ceftazidime | |
| Macrolide | erythromycin, clarithromycin, azithromycin, roxithromycin, clindamycin | |
| Tetracycline | tetracycline, doxycycline, minocycline | |
| Quinolone | fluoroquinolone | ciprofloxacin, moxifloxacin, levofloxacin, ofloxacin, norfloxacin |
| other quinolone | pipemidic acid | |
| Sulphonamide | sulfamethoxazole | |
| Others | nitrofurantoin, methenamine, trimethoprim |
Based on two Cochrane reviews4,5 and use within the University of Groningen (the Netherlands) prescription database, IADB.nl (http://www.iadb.nl/).
Study selection criteria
Eligible studies met the following criteria: (i) DDIs in humans; (ii) involving the targeted ABs; and (iii) being clinical trials, randomized controlled trials or cohort or case–control studies. We excluded case reports and other descriptive studies. We further excluded studies with subjects whose pharmacokinetics and pharmacodynamics were not comparable to those of general COPD patients, e.g. newborn babies, pregnant women and patients with severe renal/hepatic impairment. Other exclusion criteria were: (i) unregistered drugs (by FDA or EMA); (ii) involving three or more drug interactions; and (iii) not DDIs (food–drug or gene–drug interactions); (iv) not original studies (reviews, letters and editorials). Pharmacodynamic interactions were beyond the scope of this review and were excluded.
Data extraction and quality assessment
All records were exported to Refworks; titles and abstracts were screened by Y. W. and A. M. E. J. independently. Full-text papers were obtained for records that were considered of potential relevance by at least one of the reviewers. Final decisions were made by consensus between two reviewers according to the preset criteria. Discrepancies between reviewers were resolved by discussion; a third reviewer (E. H.) was asked if no consensus was reached. Information about names of ABs and related interacting drugs, study design, study subjects, sample size, interacting mechanism, effects of interaction and recommendation by study authors were extracted by the same reviewers (Y. W. and A. M. E. J.) and checked by another reviewer (M. A. B.). Quality of evidence was evaluated by grading 0–4 based on criteria (Table 2) used by previous studies.11,12
Table 2.
| Definition | Score |
|---|---|
Clinical research with appropriate control group and relevant pharmacokinetics and/or pharmacodynamic parameters. The studies meet all of the criteria below:
|
4 |
| Clinical research with appropriate control group and relevant pharmacokinetics and/or pharmacodynamic parameters that does not meet one or more of the pre-defined criteria above. | 3 |
| Complete observational studies with clinically relevant results. | 2 |
| Incomplete observational studies. (e.g. without controlling confounders or presence of other explanatory factors for the adverse reaction), case reports, summary of product characteristics. | 1 |
| In vitro studies, in vivo animal studies, prediction modelling studies. | 0 |
The strengths of the DDIs were classified into four levels (1, strong; 2, substantial; 3, moderate; 4, weak/no) according to preset published criteria (Table 3).12 In the cases of several studies on the same DDI combination, we categorized the DDI based on the highest level of severity. Considering that drugs with a narrow therapeutic index (NTI) are more vulnerable to DDIs, the strength of the DDI for such drugs was upgraded one level.12
Table 3.
Description of level of DDIs10
| Definition | AUC | Clearance | Scorea |
|---|---|---|---|
| Involved inhibitor | >200% ↑ | ↓ >67% | 1 |
| Involved inducer | >90% ↓ | ↑ ≥900% | 1 |
| For observational studies, RR/OR ≥10 | 1 | ||
| Involved inhibitor | 75%–200% ↑ | ↓ ≥43% to <67% | 2 |
| Involved inducer | 60%–90% ↓ | ↑ ≥150% to <900% | 2 |
| For observational studies, RR/OR 3–9 | 2 | ||
| Involved inhibitor | 25%–75% ↑ | ↓ ≥20% to <43% | 3 |
| Involved inducer | 25%–60% ↓ | ↑ ≥33% to <150% | 3 |
| For observational studies, RR/OR 1.5–2.9 | 3 | ||
| Involved inducer/inhibitor | <25% change | ↓ <20% or ↑ <33% | 4 |
| For observational studies, RR/OR <1.5 | 4 | ||
| (a) For interacting drugs with an NTI, the degree of DDIs will be improved to the level one higher | exception | ||
| (b) If the DDI level cannot be judged by the above criteria, we assessed it by discussion based on available data and evidence | exception |
RR, relative risk; OR, odds ratio; ↑, increase; ↓, decrease.
1, strong interaction; 2, substantial interaction; 3, moderate interaction; and 4, weak/no interaction.
Results
Publications identified by literature search
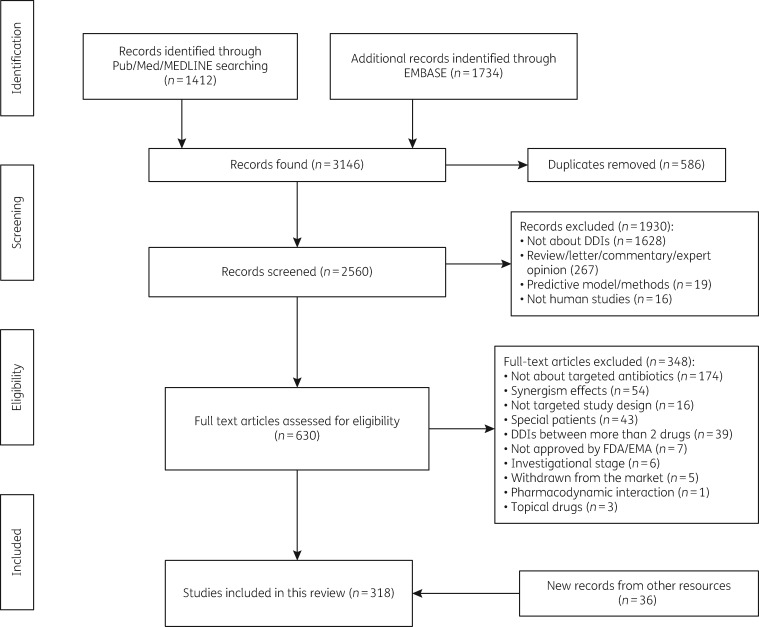
Our search yielded 1412 and 1734 studies from PubMed and Embase, respectively (Figure 1). After removing duplicates, 2560 articles were screened by title and abstract, of which 630 papers were included for full-text screening, resulting in 282 eligible articles. With 36 studies identified from other resources, we finally obtained 318 studies for assessment in this review.
Figure 1.
Flow chart of study selection.
The interacting drugs, underlying mechanisms, levels and practice recommendations for the DDIs are presented in Table 4. Details on individual studies of DDIs with a potential clinical significance (levels 1–3) are presented in Tables S1 and S2 and the data on studies with a low level of DDIs (weak or no interaction) are presented in Table S3.
Table 4.
DDIs of antibacterials (ABs) for COPD exacerbation and other drugs for treating its comorbidities
| Comorbidity | Medication | Interacting AB | Mechanism | Management suggestions | Level of interactiona | Reference |
|---|---|---|---|---|---|---|
| Diabetes Antidiabetic medication | glipizide, glyburide | TMP/SMX | Inhibition of CYP2C9. | Consider alternative or adjusted dose of substrate or use cautiously by monitoring patient's blood glucose. | 2 | 16–19 |
| glyburide | clarithromycin | Inhibition of P-gp. | ||||
| glipizide, glyburide | levofloxacin | Inhibition of P-gp. | Monitor patient's blood glucose and if necessary adjust dose of substrate. | 3 | 16 , 20–26 | |
| tolbutamide | clarithromycin | Inhibition of CYP3A4 and P-gp. | ||||
| TMP/SMX | Inhibition of CYP2C9. | |||||
| glipizide, repaglinide | clarithromycin | Inhibition of CYP3A4. | ||||
| repaglinide, rosiglitazone | TMP/SMX | Inhibition of CYP2C8. | ||||
| metformin | TMP/SMX | Inhibition of OCT2 and MATE1. | ||||
| Heart and circulatory system diseases | ||||||
| Antihypertensive agents | spironolactone | TMP/SMX | Inhibition of potassium secretion. | Avoid combination or adjust dose of substrates and closely monitor potassium plasma levels. | 1 | 32 |
| calcium channel blocker | erythromycin, clarithromycin | Inhibition of CYP3A4. | Consider alternative or adjusted dose of substrate, or use cautiously by monitoring side effects. | 2 | 27–29 | |
| azithromycin | Inhibition of CYP3A4. | Monitor side effects and if necessary adjust dose of substrate. | 3 | 27 | ||
| Lipid-lowering drugs | simvastatin | erythromycin | Inhibition of CYP3A4. | Avoid combination or adjust dose of substrates and closely monitor side effects. | 1 | 34 |
| atorvastatin | clarithromycin | Inhibition of CYP3A4. | Consider alternative or adjusted dose of substrate, or use cautiously by monitoring side effects. | 2 | 35 | |
| erythromycin | Inhibition of CYP3A4. | Monitor side effects and if necessary adjust dose of substrate. | 3 | 36 , 204 | ||
| rosuvastatin/pravastatin/fluvastatin | clarithromycin | Inhibition of OAT. | ||||
| Oral anticoagulants | warfarin, phenprocoumon /acenocoumarol | TMP/SMX | Inhibition of CYP2C9. | Avoid combination or closely monitor the change of INR routinely and adjust dose if needed. | 1 | 39–58 |
| amoxicillin/co- amoxiclav, ceftriaxone | Alterations in normal gut flora. | Choose alternative AB or, if not possible, monitor the change of INR routinely. | 2 | |||
| clarithromycin, azithromycin, ciprofloxacin, levofloxacin, ofloxacin, doxycycline | Inhibition of CYP3A4 or alterations in normal gut flora. | |||||
| edoxaban, dabigatran, rivaroxaban | erythromycin, clarithromycin | Inhibition of CYP3A4 and/or P-gp. | Consider alternative or adjusted dose of substrate or monitor signs of excessive anticoagulant effect. | 2 | 62 , 63 | |
| warfarin | moxifloxacin | Inhibition of CYP3A4 or alterations in normal gut flora. | Monitor the change of INR routinely. | 3 | 41 | |
| Antiarrhythmic agents | digoxin | clarithromycin | Inhibition of P-gp. | Avoid combination or perform TDM and if necessary adjust dose of substrate. | 1 | 68–71 |
| quinidine, lignocaine | erythromycin | Inhibition of CYP3A4. | Consider alternative or perform TDM and if necessary adjust dose of substrate. | 2 | 64–67 | |
| procainamide | TMP | Inhibition of tubular secretion. | ||||
| pindolol, digoxin | TMP/SMX | Inhibition of tubular secretion. | Perform TDM and if necessary adjust dose of substrate. | 3 | 72–75 | |
| procainamide | levofloxacin, ofloxacin | Inhibition of OCT. | ||||
| Respiratory diseases | ||||||
| Medication for obstructive airways diseases | methylprednisolone, montelukast | clarithromycin | Inhibition of CYP3A4 and P-gp. | Consider alternative or adjusted dose of substrate or use cautiously by monitoring side effects. For theophylline, perform TDM. | 2 | 78–85 , 90–97 |
| theophylline | erythromycin | Inhibition of CYP3A4. | ||||
| ciprofloxacin | Inhibition of CYP1A2. | |||||
| loratadine | erythromycin, clarithromycin | Inhibition of CYP3A4. | Monitor side effects and if necessary adjust dose of substrate. | 3 | 86 , 87 | |
| roflumilast | erythromycin | Inhibition of CYP3A4. | ||||
| Anti-TB drugs | rifabutin | clarithromycin | Inhibition of CYP3A4. | Avoid combination. | 1 | 101 , 110 , 111 |
| rifampicin, rifabutin | clarithromycin | Induction of CYP3A4. | Consider alternative AB for COPD | 2 | 100 , 101 | |
| rifampicin, rifabutin | TMP/SMX, doxycycline | Induction of CYP3A4/CYP2C9. | Consider alternative AB for COPD or monitor the effectiveness of AB and if necessary adjust dose of AB. | 3 | 102–104 , 106–109 | |
| rifampicin | TMP/SMX | Inhibition of mixed oxidases. | ||||
| moxifloxacin | Induction of phase II enzymes. | |||||
| Neurological disorders | ||||||
| Anti-Parkinson’s agents | bromocriptine | erythromycin | Inhibition of CYP3A4. | Avoid combination or adjust dose of substrates and closely monitor side effects. | 1 | 112 |
| cabergoline | clarithromycin | Inhibition of CYP3A4 and P-gp. | Consider alternative or adjusted dose of substrate or use cautiously by monitoring side effects. | 2 | 113 | |
| Antiepileptic drugs | carbamazepine, phenytoin | doxycycline | Induction of CYP3A4. | Consider alternative or perform TDM. | 2 | 116 , 117 |
| carbamazepine | ciprofloxacin | Inhibition of CYP3A4/1A2. | Consider alternative or perform TDM. | 2 | 118 | |
| phenytoin | TMP/SMX | Inhibition of CYP2C8. | Consider alternative or perform TDM. | 2 | 116 , 119 | |
| phenobarbital | doxycycline | Induction of CYP3A4. | Monitor side effects and if necessary adjust dose of substrate. | 3 | 115 | |
| Depression and psychiatric disorders | ||||||
| Antidepressant, anxiolytic and antipsychotic agents | buspirone | erythromycin | Inhibition of CYP3A4. | Avoid combination or adjust dose of substrates and closely monitor side effects. | 1 | 125 |
| quetiapine | erythromycin | Inhibition of CYP3A4. | Consider alternative or adjusted dose of substrate, or use cautiously by monitoring side effects. For clozapine, perform TDM. | 2 | 122–124 , 129 | |
| pimozide, trazodone | clarithromycin | Inhibition of CYP3A4. | ||||
| clozapine | ciprofloxacin | Inhibition of CYP1A2. | ||||
| diazepam | ciprofloxacin | Inhibition of CYP3A4. | Monitor side effects and if necessary adjust the dose of substrate. | 3 | 127 | |
| Dyspepsia | ||||||
| Antidyspepsia medications | aluminium hydroxide, sucralfate | quinolone, tetracyclines | Complex formation. | Avoid combination or administer quinolone at least 2 h before or 6 h after co-agents. | 1 | 131–142 |
| lansoprazole | clarithromycin | Inhibition of CYP3A4. | Consider alternative or adjusted dose of substrate or use cautiously by monitoring side effects. | 2 | 147 | |
| calcium carbonate | quinolone, tetracyclines | Complex formation. | Avoid co-administration or administer at interval of at least 2 h. | 2 | 131 , 139 | |
| bismuth subsalicylate | quinolone, tetracyclines | Complex formation. | Administration interval of at least 2 h. | 3 | 143 , 205 | |
| HIV | ||||||
| Anti-HIV drugs | didanosine | ciprofloxacin | Complex formation. | Avoid combination or administer quinolone at least 2 h before or 6 h after co-agents. | 1 | 149 , 150 |
| saquinavir | erythromycin | Inhibition of CYP3A4. | Consider alternative or adjusted dose of substrate or use cautiously by monitoring side effects. | 2 | 151 | |
| lamivudine, didanosine | TMP/SMX | Inhibition of tubular secretion. | Monitor side effects and if necessary adjust dose of substrate. | 3 | 152 , 153 | |
| Other | ||||||
| Pulmonary arterial hypertension medications | bosentan | clarithromycin | Inhibition of CYP3A4 and P-gp. | Avoid combination or adjust dose of substrates and closely monitor side effects. | 1 | 206 |
| ambrisentan | clarithromycin | Inhibition of CYP3A4 and P-gp. | Monitor side effects and if necessary adjust dose of substrate. | 3 | 207 | |
| Insomnia medications | brotizolam, triazolam, zopiclone | erythromycin | Inhibition of CYP3A4. | Consider an alternative AB or other hypnotic drugs (not a CYP3A4 substrate). | 2 | 208–210 |
| zolpidem | ciprofloxacin | Inhibition of CYP3A4. | Monitor side effects and if necessary choose alternative AB or other hypnotic drugs (not a CYP3A4 substrate). | 3 | 211 | |
| Antifungal agents | voriconazole | erythromycin | Inhibition of CYP3A4. | Consider alternative or adjusted dose of substrate, or use cautiously by monitoring side effects. For voriconazole, perform TDM and adjust dose if needed. | 2 | 154 , 155 |
| itraconazole | ciprofloxacin | Inhibition of CYP3A4. | ||||
| Antineoplastic drugs | vinorelbine | clarithromycin | Inhibition of CYP3A4 and P-gp. | Avoid combination or adjust dose of substrates and closely monitor side effects. | 1 | 179 |
| Anti-gout drugs | colchicine | clarithromycin | Inhibition of CYP3A4. | Avoid combination or perform TDM and adjust dose if needed. | 1 | 180 |
| azithromycin | Inhibition of CYP3A4. | Consider alternative or adjusted dose of substrate or use cautiously by monitoring side effects. | 2 | 180 | ||
| probenecid | ciprofloxacin | Inhibition of OAT. | Monitor side effects and if necessary adjust dose of substrate. | 3 | 194 , 195 | |
| Anaesthesia drugs | midazolam | clarithromycin, erythromycin | Inhibition of CYP3A4. | Avoid combination or adjust dose of substrates and closely monitor side effects. | 1 | 156–160 |
| ketamine | clarithromycin | Inhibition of CYP3A4. | Consider alternative or perform TDM and adjust dose if needed. | 2 | 161 | |
| alfentanil | erythromycin | Inhibition of CYP3A4. | Monitor side effects and if necessary adjust dose of substrate. | 3 | 162–166 | |
| ropivacaine | clarithromycin | Inhibition of CYP3A4. | ||||
| ciprofloxacin | Inhibition of CYP1A2. | |||||
| midazolam | roxithromycin | Inhibition of CYP3A4. | ||||
| Analgesics | oxycodone | clarithromycin | Inhibition of CYP3A4. | Avoid combination or adjust dose of substrates and closely monitor side effects. | 1 | 167 |
| Immunosuppressant drugs | cyclosporine | erythromycin | Inhibition of CYP3A4. | Avoid combination or adjust dose of substrates and perform TDM. | 1 | 168 , 169 , 181 , 182 |
| everolimus | erythromycin | Inhibition of CYP3A4 and/ P-gp. | ||||
| tacrolimus | levofloxacin | Inhibition of CYP3A4 or P-gp. | Consider alternative or adjusted dose of substrate, or use cautiously by monitoring side effects. | 2 | 170 | |
| cyclosporine | ciprofloxacin | Inhibition of CYP3A4. | Monitor side effects and, if necessary adjust dose of substrate. | 3 | 171 , 172 | |
| Vasoactive agents | sildenafil | clarithromycin, erythromycin, ciprofloxacin | Inhibition of CYP3A4. | Consider alternative or adjusted dose of substrate, or use cautiously by monitoring side effects. | 2 | 173 , 174 |
| Appetite suppressants | sibutramine | clarithromycin | Inhibition of CYP3A4 and P-gp. | Avoid combination or adjust dose of substrates and closely monitor side effects. | 1 | 175 , 176 |
| Emergency birth control | ulipristal acetate | erythromycin | Inhibition of CYP3A4. | Avoid combination or adjust dose of substrates and closely monitor side effects. | 1 | 178 |
| Antimalarial agents | halofantrine | tetracycline | Probably by CYP3A4 inhibition. | Avoid combination or perform TDM and adjust dose if needed. | 1 | 177 |
| Muscle relaxants | tizanidine | ciprofloxacin | Inhibition of CYP1A2. | Avoid combination or perform TDM and adjust dose if needed. | 1 | 183 |
| Anti-diarrhoeals | loperamide | TMP/SMX | Inhibition of CYP2C8. | Consider alternative or adjusted dose of substrate, or use cautiously by monitoring side effects. | 2 | 186 |
| Anaemia medications | iron supplements | quinolone, tetracyclines | Complex formation. | Avoid co-administration or administer at interval of at least 2 h. | 2 | 212–220 |
| Other metal cations | zinc sulfate | quinolone, tetracyclines | Complex formation. | Avoid co-administration or administer at interval of at least 2 h. | 2 | 144 , 188 , 189 |
| calcium acetate, calcium carbonate, calcium polycarbophil, patiromer, lanthanum carbonate, sevelamer | quinolone, tetracyclines | Complex formation. | Administer at interval of at least 2 h. | 3 | 139 , 190–193 | |
| Other ABs | linezolid | clarithromycin | Inhibition of P-gp. | Consider alternative or perform TDM and adjust dose if needed. | 2 | 196 |
| dapsone | trimethoprim | Inhibition of CYP2C8. | Monitor side effects and if necessary adjust dose of substrate. | 3 | 187 | |
| neomycin | penicillin V | NA | Consider alternative or adjust dose of penicillin. | 3 | 221 | |
All detailed supporting information about each DDI is available in Tables S1 and S2.
OCT, organic cation transporter; OAT, organic anion transporter; MATE1, multidrug and toxin extrusion 1; TMP/SMX, trimethoprim/sulfamethoxazole; NA, not available yet.
1, strong interaction; 2, substantial interaction; 3, moderate interaction; 4, weak or no interaction.
We present a step-by-step approach to AB prescribing in COPD: (i) check whether co-morbidity is present; (ii) a quick overview of the AB and its interacting medication, possible interaction mechanism, level of interaction, and practical recommendations is provided in Table 4; and (iii) detailed explanation about related interacting mechanisms and recommendations for the management of related DDIs are provided in the main text.
Mechanisms of DDI
An AB can act as an inhibitor/inducer and/or a substrate, producing moderate to strong DDI with other co-administered medication. There are two scenarios: (i) the co-administered drug alters the pharmacokinetic parameters of the AB; and (ii) the AB influences the pharmacokinetic parameters of the co-administered medication. The main mechanisms of these DDIs are complex formation, inhibition/induction of drug-metabolizing enzymes and alteration of drug transporters (Table 4). The ability to inhibit CYP3A4 makes ABs prone to interaction with many different drugs as CYP3A4 metabolizes >50% of clinically prescribed drugs.13
Information structured according to drugs for comorbidities
The presentation of information on potential clinically significant DDIs with a moderate to strong level of interaction is according to the most frequent comorbidities that have been reported in COPD patients.8,14 Potential mechanisms of DDIs and actionable recommendations to manage the DDIs are provided in Table 4.
Diabetes
Patients with COPD have a 50% higher risk of developing diabetes than persons without COPD.15 Some antidiabetic drugs are substrates of enzymes such as CYP3A4 (glipizide, tolbutamide), CYP2C9 (glipizide, glyburide) and CYP2C8 (repaglinide), and substrates of drug transporter-like P-glycoprotein (P-gp) transporter (glipizide, glyburide).16–26 ABs such as clarithromycin (CYP3A4 and P-gp inhibitor), trimethoprim/sulfamethoxazole (CYP2C8/2C9 inhibitor) and levofloxacin (P-gp inhibitor) may inhibit the function of these metabolic enzymes and transporters. These ABs can potentially increase the blood concentration of the antidiabetic agents mentioned above.16–26 Consequently, patients may develop hypoglycaemia. Therefore, it is suggested that these combinations should be avoided by substituting a related AB or adjusting the dose of antidiabetic agents as well as monitoring the patients’ blood glucose.
Heart and circulatory system diseases
Antihypertensive agents
Hypertension is associated with COPD with a relative risk of 1.6.15 Antihypertensive calcium channel blockers (CCBs) such as diltiazem and verapamil are CYP3A4 substrates.27–29 Therefore, macrolides (CYP3A4 inhibitors) can enhance the pharmacological activity of CCBs.30 Avoiding the combination by replacement of macrolides or CCBs with another group of drugs or adjusting the dose of CCBs while monitoring blood pressure is recommended. Erythromycin and clarithromycin are the most potent CYP3A4 inhibitors, while azithromycin and roxithromycin are weak inhibitors.30,31 Hence, if prescribing macrolides, choosing macrolides with minimal inhibitory capacity to be co-prescribed with CCBs may minimize the risk of DDI.
Spironolactone, a potassium-sparing diuretic, is used to lower blood pressure. Combination of spironolactone with trimethoprim/sulfamethoxazole may produce hyperkalaemia because both drugs can inhibit renal excretion of potassium.32 Therefore, avoiding this combination (by selecting an alternative AB) or adjusting the dose of spironolactone and closely monitoring potassium plasma levels is strongly recommended.
Lipid-lowering drugs
Lipid metabolism problems are among the most prevalent comorbidities in COPD patients.14 The main pharmacological approach to the management of blood cholesterol levels is statin therapy.33 Some ABs increase the plasma concentration of statins by several mechanisms. Statins such as simvastatin and atorvastatin are biodegraded by CYP3A4.34,35 Therefore, potent CYP3A4 inhibitors (erythromycin and clarithromycin) increase the risk of statin-related side effects such as rhabdomyolysis.34,35 Other statins, such as rosuvastatin, pravastatin and fluvastatin, are not CYP3A4 substrates.36 However, the hepatic clearance of these statins is facilitated by anion-transporting polypeptides.37 These influx transporters facilitate the transport of statins from systemic blood to liver cells to be metabolized or subsequently delivered into the bile for elimination.37 Clarithromycin and erythromycin have been reported to be inhibitors of these transporters.38 Therefore, replacing erythromycin and clarithromycin with other ABs, temporarily stopping statins or adjusting the dose of statins while monitoring statin-related side effects is recommended.
Oral anticoagulants
Both coumarins and direct oral anticoagulants (DOACs) may interact with ABs. Multiple studies reported that DDIs between ABs and coumarins (warfarin, phenprocoumon, acenocoumarol) led to increased risks of haemorrhage.39–58 Several interaction mechanisms were proposed.59,60 One mechanism is by disruption of intestinal flora that synthesizes vitamin K, as many ABs could alter the balance of gut flora.59 Another mechanism is that ABs (e.g. trimethoprim/sulfamethoxazole and macrolides) alter coumarin metabolism, which mainly involves CYP2C9 (trimethoprim/sulfamethoxazole) and CYP3A4 (macrolides).60 Therefore, to choose alternative ABs or, if not possible, to monitor international normalized ratio (INR) values and adjust the dose of coumarins is recommended.
DOACs are regarded as a safe alternative to replace coumarins.61 However, since some DOACs (edoxaban, rivaroxaban, dabigatran) are substrates of CYP3A4 and/or the P-gp transporter, their AUC values can be increased by ABs such as macrolides.62,63 Therefore, when macrolides and DOACs are required in combination, careful monitoring of signs of bleeding is needed, and adjusting the dose of DOACs should be done if necessary.
Antiarrhythmic agents
Some antiarrhythmic agents, such as digoxin, quinidine, lignocaine and procainamide, potentially interact with ABs.64–75 Quinidine and lignocaine are CYP3A4 substrates and therefore macrolides may inhibit their degradation and increase their bioavailabilities.64,65 The renal clearance of procainamide and digoxin is inhibited by trimethoprim.66,67,72,73 The mechanism of interaction is inhibition of tubular secretion via inhibition of the renal organic cation transporter because they are substrates of the transporter.66,67,72,73 Consequently, blood concentrations of these drugs are increased.66,67,72,73 Digoxin is a substrate of the P-gp transporter.68–71 Clarithromycin could elevate the AUC of digoxin, which may cause toxicities.68–71 Since quinidine, lignocaine, digoxin and procainamide are drugs with an NTI, avoiding ABs that can lead to DDIs with these drugs is recommended.76,77 However, if their co-prescription is necessary, therapeutic drug monitoring (TDM) of these antiarrhythmic agents is strongly recommended.77
Respiratory diseases
Medication for obstructive airways diseases
One of the most prevalent comorbidities in COPD is asthma.14 Some anti-asthma drugs, such as methylprednisolone, montelukast, loratadine, roflumilast and theophylline, are substrates of CYP3A4 and/or the P-gp transporter and have been shown to interact with macrolides.78–87 Hence, one might consider other ABs for combination with asthma drugs, or closely monitor patients, especially in the case of theophylline, which is an NTI drug.88 As theophylline is also metabolized by CYP1A2,89 ciprofloxacin (a CYP1A2 potent inhibitor) should be avoided.90–97
Antimycobacterial agents
Tuberculosis and COPD share comparable risk factors and therefore can co-occur in individuals, particularly elderly patients.98 Rifampicin and rifabutin (antimycobacterial agents) work as potent inducers of hepatic and intestinal CYP enzymes.99 They can markedly reduce the activities of clarithromycin, doxycycline and trimethoprim/sulfamethoxazole by causing their rapid elimination.100–104 Since rifampicin also exhibits other AB properties, such as activity against MRSA in combination with other drugs, rationalizing antimicrobial therapy should be considered accordingly.105 Alternative ABs for treating COPD are also recommended to reduce the risk of treatment failures.
Moxifloxacin might be an alternative AB for clarithromycin, doxycycline and trimethoprim/sulfamethoxazole owing to its moderate or weak interaction with rifampicin.106–109 Moxifloxacin is not metabolized by CYP450 and its interacting mechanisms with rifampicin might be facilitated by induction of other enzymes, such as uridine diphosphate-glucuronosyltransferases and sulfotransferases.106–109
Rifabutin and rifampicin are CYP substrates. Rifabutin is a CYP3A4 substrate, and therefore macrolides may increase its serum concentration and enhance the risk of related ADR.101,110,111 Another study reported that rifampicin concentrations in blood are moderately elevated by co-trimoxazole.104 It was assumed that the interaction was facilitated by inhibition of mixed-function oxidases, which are responsible for metabolizing rifampicin.104 Thus, considering alternative ABs or monitoring the clinical and biochemical parameters for rifampicin-related hepatotoxicity is suggested when rifampicin and co-trimoxazole are combined.
It should be mentioned that not all the drugs for atypical Mycobacterium spp. were included in this review because selection was limited to ABs that are used frequently among COPD patients. For drugs outside the scope of this review, other references (e.g. statements of product characteristics) need to be considered.
Neurological disorders
Anti-Parkinson’s drugs
Bromocriptine and cabergoline (dopamine agonists) are substrates of CYP3A4 and/or the P-gp transporter.112,113 Co-prescription of these drugs with clarithromycin and erythromycin may produce major interactions and therefore might lead to toxicities.112,113 Thus, avoiding such combinations is recommended. However, if this is not possible, adjusting the dose of these Parkinson’s medications and closely monitoring side effects are needed.
Antiepileptic drugs
Carbamazepine, phenytoin and phenobarbital can stimulate the activity of a variety of CYP (CYP1A2/2C9/3A4) and glucuronyl transferase enzymes, which results in multiple DDIs with other substrates for these enzymes.114–116 Carbamazepine and phenytoin were reported to reduce the half-life of doxycycline by stimulating the hepatic metabolism of doxycycline.117 It is suggested that an alternative AB should be considered or that the dose of antiepileptic drugs should be adjusted while monitoring the AB activity of doxycycline.
Carbamazepine and phenytoin are substrates of CYP1A2/3A4 and CYP2C8, respectively. A CYP1A2/3A4 inhibitor (ciprofloxacin) and a CYP2C8 inhibitor (trimethoprim) were reported to increase the bioavailability of carbamazepine and phenytoin, respectively.116–119 Moreover, phenytoin is an NTI drug and therefore avoiding using trimethoprim concomitantly or performing TDM of phenytoin is recommended when this DDI is not avoidable.120
Ciprofloxacin was reported to increase the AUC of carbamazepine by >50%.118 Although it is not clear whether carbamazepine can be considered to be an NTI drug, a rising carbamazepine plasma concentration because of this DDI needs special caution.121 Dose adjustment and TDM of carbamazepine are suggested to diminish potential toxicities.
Depression and psychiatric disorders
Depression and psychiatric disorders are common among COPD patients.14 Some antidepressant (trazodone), anxiolytic (buspirone) and antipsychotic (quetiapine, and pimozide) drugs are CYP3A4 substrates and therefore might trigger clinically relevant DDIs with ABs.122–125 Erythromycin and clarithromycin increased the AUCs of these drugs substantially.122–125 Considering alternative ABs or adjusting the dose of substrates and monitoring related side effects is the way to control potential ADR.
CYP3A4 is also responsible for metabolizing diazepam, in addition to CYP2C19.126 Ciprofloxacin was reported to decrease diazepam clearance moderately by inhibiting CYP3A4 activity.127 Monitoring diazepam-related side effects can therefore be considered when this combination is prescribed.
Ciprofloxacin is also a potent CYP1A2 inhibitor.128 Therefore, metabolism of an atypical antipsychotic, clozapine, a CYP1A2 substrate with an NTI, can be altered by ciprofloxacin, which produces a significant increase in clozapine serum concentration.129,130 Replacing ciprofloxacin or TDM of clozapine is an option that can be chosen in managing this DDI.
Dyspepsia
Drugs containing metal cations (e.g. antacids, sucralfate and bismuth salts) produced chemical interactions with some ABs, such as oral tetracyclines (e.g. tetracycline, doxycycline) and fluoroquinolones (e.g. ciprofloxacin, moxifloxacin).131–144 Tetracyclines have a strong tendency to form chelates due to their structural features, which include many chelation sites.145 Meanwhile, fluoroquinolones have two main sites of metal chelation: 4-keto oxygen and 3-carboxylic acid groups.146
The formation of metal ion chelation complexes decreases absorption of tetracycline and fluoroquinolones, and this reduced bioavailability may lead to ineffectiveness of these ABs.131–144 Therefore, it is recommended that their combination should be avoided by replacing tetracyclines and fluoroquinolones with another AB, e.g. amoxicillin or amoxicillin/clavulanic acid. It was reported that antacids did not affect the bioavailability of amoxicillin and amoxicillin/clavulanic acid when they were co-administered.136 If replacement of the AB is not possible, replacement of antacids, sucralfate or bismuth salts with a proton pump inhibitor (PPI) is also favoured. Another alternative is to separate administration by using quinolone or tetracycline at least 2 h before or 6 h after the dyspepsia drugs.
When considering a PPI, lansoprazole may not be the best alternative as it is partly metabolized by CYP3A4 and has been found to interact with clarithromycin.147
HIV
HIV-positive patients have an ∼50% higher risk of developing COPD than HIV-negative patients.148 Thus, the risk of co-prescriptions for treating these chronic conditions may also be high. A protease inhibitor (saquinavir) and NRTIs (didanosine and lamivudine) were found to clinically interact with ABs.149–153
Didanosine is very acid sensitive, and therefore didanosine formulations are supplemented with buffering mixtures containing magnesium hydroxide, dihydroxyaluminium sodium carbonate and sodium citrate to prevent hydrolysis by gastric acid.149 These metal ions may form chelation complexes with quinolones and reduce their serum concentration.149,150 Two studies confirmed the didanosine and ciprofloxacin interaction, and recommended that when co-administration cannot be avoided, ciprofloxacin must be given at least 2 h before didanosine.149,150
Trimethoprim/sulfamethoxazole may inhibit clearances of didanosine and lamivudine by competitively hindering their renal secretion.152,153 Consequently, AUCs of didanosine and lamivudine are elevated moderately.152,153 Monitoring of the presumed side effects should be performed.
Saquinavir is metabolized by CYP3A4 and the presence of erythromycin increased its AUC by almost 100%.151 Choosing an alternative AB or adjusting the dose of saquinavir while monitoring toxicities can be considered as a means of managing this DDI.
Other potential clinically significant DDIs
Some other drugs that have indications for comorbidities in COPD patients were found to interact with ABs. Some individual drugs of different classes (e.g. voriconazole and vinorelbine) are metabolized by CYP3A4.154–182 Therefore, their metabolism is interfered with by CYP3A4 inhibitors (macrolides).154–182 Other drugs are CYP1A2 substrates (e.g. ropivacaine and tizanidine) and therefore potent inhibitors of CYP1A2, such as quinolones, significantly alter their metabolism and elevate their bioavailabilities.164,183–185 Others are CYP2C8 substrates (e.g. loperamide for diarrhoea) and therefore trimethoprim (a potent CYP2C8 inhibitor) inhibits their clearance and increases their AUC values.186,187 Some drugs containing metal cations (e.g. Fe, Zn, Ca) should be avoided or administered with a separation of at least 2 h from quinolones and tetracyclines.139,144,188–193 Other interactions were facilitated by drug transporters. A uricosuric agent (probenecid) interacts moderately with ciprofloxacin via competitive inhibition of organic anion transporters in renal tubules.194,195 Moreover, linezolid, which is a substrate of the P-gp transporter, can potentially produce clinically significant interaction with P-gp inhibitors (macrolides).196
DDIs related to NTIs
Some ABs may interact with NTI drugs and therefore can produce serious ADRs. The NTI drugs in this review include CYP3A4 substrates (theophylline, ketamine, everolimus, tacrolimus, halofantrine, lignocaine, quinidine, voriconazole, carbamazepine, warfarin, cyclosporine, colchicine, phenprocoumon/acenocoumarol); CYP1A2 substrates (theophylline, carbamazepine, clozapine, tizanidine); CYP2C9 substrates and drugs sensitive to alterations in the normal gut flora (warfarin, phenprocoumon/acenocoumarol); a CYP2C8 substrate (phenytoin); substrates of the P-gp transporter (digoxin, linezolid); and a substrate of the organic cation transporter (procainamide).76,77,88,120,197
Discussion
Included articles
This study outlines the possible DDIs related to frequently prescribed ABs in COPD patients from clinical and observational studies. We only included well-designed studies (≥2 points) since they provide more valid evidence than studies without a control or comparison group (0 or 1 point). DDIs based on case reports or hypotheses may lead to unnecessary warnings if these are not confirmed by well-designed studies. One classic example of this point is ABs and oral contraceptive interactions; many cases of unintended pregnancies were reported after ABs were prescribed to women on oral contraceptives, which attracted much attention from health practitioners.198,199 After scientific evidence from clinical and pharmacokinetic studies has consistently and repeatedly failed to support such interaction, the warning about DDIs between hormonal contraception and non-rifampicin ABs has finally been cancelled in related guidelines.199
Mechanisms of DDI
A DDI of potential clinical significance between an AB and a co-administered medication may occur in two situations: (i) the co-administered drug influences the absorption, distribution, metabolism and elimination (ADME) of the AB; and (ii) the AB influences the ADME of the co-administered medication. When the AB acted as a substrate, some co-administered drugs reduced the blood concentration of the AB and led to failure of the AB treatment to reduce COPD exacerbations. Other co-administered drugs increased the blood concentration of the AB, which could result in the termination of AB use because of an ADR, and therefore acted against the control of infection. Acting as inhibitors, ABs could also increase the blood concentrations of co-administered drugs, which may also produce an ADR and lead to termination of co-administered drugs, and therefore may lead to failure of treatment of comorbidities. Thus, DDIs related to ABs may hinder effective infection control and exacerbation management among COPD patients as well as treatment of comorbidities in COPD.
Comorbidities among COPD patients
The impact of comorbidities on quality of life in COPD patients is well reported; however, potential drug interactions between drugs for these comorbidities and ABs used for COPD have received little specific attention. From this review, we found that many drugs (e.g. those used for heart and circulatory system diseases) should not be co-administered with related ABs, and other actions are necessary, such as dose adjustment, choosing an alternative drug and monitoring ADRs. These drug interactions may not only influence treatment options for clinical practitioners but also influence treatment effects for both COPD and comorbidities.
Information collected in this review can be used as input to improve the sensitivity and specificity of DDI alert systems. Moreover, this study may also be attractive for researchers in this field who may take into account the availability of high-quality studies when evaluating the evidence for many potential interactions.
Special warning for NTI drugs
We found that some NTI drugs might potentially interact with ABs. Because of the narrow separation between effective and toxic dosing of these drugs, a small alteration of their pharmacokinetic parameters can produce fatal consequences.88,120 Therefore, combination with particular ABs that have an ability to inhibit their clearance pathways should be avoided if possible. However, if the benefits of combination outweigh the potential side effects, dose adjustment and performing TDM of the NTI drugs are strongly recommended.
Limitations
Some limitations of this review are worth mentioning. First, although we reviewed a significant part of the literature, we did not include all sources that might indicate relevant DDIs, such as case reports, summary of product characteristics and theoretical hypotheses. As a result, we did not find some DDIs that are considered serious and clinically highly relevant, such as QT-interval prolonging interactions for combinations of macrolides with other QT-prolonging drugs or the risk of pseudotumour cerebri in the case of combinations of doxycycline with vitamin A analogues.200,201 Such interactions are commonly found as case reports, as it is unethical to design studies to confirm these serious risks in clinical studies. However, for some DDIs it is possible to study the clinical manifestation of a potential DDI in an observational study using real-world drug utilization data.202 Secondly, selection of the ABs included in this review was based on their frequent use in COPD and therefore information for other ABs used for COPD comorbidities, such as atypical Mycobacterium spp., is limited, and this may restrict the scope of application of this review. Thirdly, due to limited comparative analyses for several specific DDIs included in this review, it may be difficult to make recommendations for a specific situation. Our classification of DDI levels only offers a general consideration. The specific impact of a DDI is determined by many variables, such as different doses and formulations and the comorbidities of patients. Therefore, case-by-case analysis is important in clinical practice and a drug interaction handbook such as Stockley’s Drug Interactions203 further expands on these issues.
Conclusions
Clinically significant DDIs related to ABs may involve a wide range of indicated drugs to treat comorbidities in COPD. Clinicians should pay attention to these drug interactions when prescribing ABs in order to reduce the frequency and severity of exacerbations in COPD patients and take necessary actions to ensure therapeutic effect and safety of patients. This study may contribute to better prescribing of ABs to COPD patients with comorbidities where potentially interacting drug combinations may be used. Furthermore, the information may highlight gaps in scientific knowledge about potential adverse effects from DDIs.
Funding
This study was supported by internal funding. Y. W. received a scholarship (file number: 201506010259) from China Scholarship Council (CSC; http://www.csc.edu.cn/) for her PhD studies at the University of Groningen, Groningen, the Netherlands. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Transparency declarations
None to declare.
Supplementary Material
References
- 1. Vogelmeier CF, Criner GJ, Martinez FJ. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 2017; 195: 557–82. [DOI] [PubMed] [Google Scholar]
- 2. Decramer M, Janssens W, Miravitlles M.. Chronic obstructive pulmonary disease. Lancet 2012; 379: 1341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sethi S, Murphy TF.. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 2355–65. [DOI] [PubMed] [Google Scholar]
- 4. Vollenweider DJ, Jarrett H, Steurer-Stey CA. et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; issue 12: CD010257.. [DOI] [PubMed] [Google Scholar]
- 5. Herath SC, Poole P.. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2013; issue 12: CD009764. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Zijp TR, Bahar MA. et al. Effects of prophylactic antibiotics on patients with stable COPD: a systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2018; 73: 3231–43. [DOI] [PubMed] [Google Scholar]
- 7. Wedzicha JA, Calverley PMA, Albert RK. et al. Prevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2017; 50: 1602265.. [DOI] [PubMed] [Google Scholar]
- 8. Chetty U, McLean G, Morrison D. et al. Chronic obstructive pulmonary disease and comorbidities: a large cross-sectional study in primary care. Br J Gen Pract 2017; 67: e321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mangoni AA, Jackson SH.. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 2004; 57: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bahar MA, Hak E, Bos JH. et al. The burden and management of cytochrome P450 2D6 (CYP2D6)-mediated drug–drug interaction (DDI): co-medication of metoprolol and paroxetine or fluoxetine in the elderly. Pharmacoepidemiol Drug Saf 2017; 26: 752–65. [DOI] [PubMed] [Google Scholar]
- 11. van Roon EN, Flikweert S, le Comte M. et al. Clinical relevance of drug–drug interactions: a structured assessment procedure. Drug Saf 2005; 28: 1131–9. [DOI] [PubMed] [Google Scholar]
- 12. Bahar MA, Setiawan D, Hak E. et al. Pharmacogenetics of drug–drug interaction and drug-drug-gene interaction: a systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenomics 2017; 18: 701–39. [DOI] [PubMed] [Google Scholar]
- 13. Zhou S, Chan E, Li X. et al. Clinical outcomes and management of mechanism-based inhibition of cytochrome P450 3A4. Ther Clin Risk Manag 2005; 1: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Divo M, Cote C, de Torres JP. et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 155–61. [DOI] [PubMed] [Google Scholar]
- 15. Mannino DM, Thorn D, Swensen A. et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32: 962–9. [DOI] [PubMed] [Google Scholar]
- 16. Schelleman H, Bilker WB, Brensinger CM. et al. Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin Pharmacol Ther 2010; 88: 214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lilja JJ, Niemi M, Fredrikson H. et al. Effects of clarithromycin and grapefruit juice on the pharmacokinetics of glibenclamide. Br J Clin Pharmacol 2007; 63: 732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan A, Holmes HM, Kuo YF. et al. Coadministration of co-trimoxazole with sulfonylureas: hypoglycemia events and pattern of use. J Gerontol A Biol Sci Med Sci 2015; 70: 247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kradjan WA, Witt DM, Opheim KE. et al. Lack of interaction between glipizide and co-trimoxazole. J Clin Pharmacol 1994; 34: 997–1002. [DOI] [PubMed] [Google Scholar]
- 20. Jayasagar G, Dixit AA, Kishan V. et al. Effect of clarithromycin on the pharmacokinetics of tolbutamide. Drug Metabol Drug Interact 2000; 16: 207–15. [DOI] [PubMed] [Google Scholar]
- 21. Wing LM, Miners JO.. Cotrimoxazole as an inhibitor of oxidative drug metabolism: effects of trimethoprim and sulphamethoxazole separately and combined on tolbutamide disposition. Br J Clin Pharmacol 1985; 20: 482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niemi M, Neuvonen PJ, Kivisto KT.. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther 2001; 70: 58–65. [DOI] [PubMed] [Google Scholar]
- 23. Niemi M, Kajosaari LI, Neuvonen M. et al. The CYP2C8 inhibitor trimethoprim increases the plasma concentrations of repaglinide in healthy subjects. Br J Clin Pharmacol 2004; 57: 441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hruska MW, Amico JA, Langaee TY. et al. The effect of trimethoprim on CYP2C8 mediated rosiglitazone metabolism in human liver microsomes and healthy subjects. Br J Clin Pharmacol 2005; 59: 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grun B, Kiessling MK, Burhenne J. et al. Trimethoprim-metformin interaction and its genetic modulation by OCT2 and MATE1 transporters. Br J Clin Pharmacol 2013; 76: 787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller F, Pontones CA, Renner B. et al. N(1)-methylnicotinamide as an endogenous probe for drug interactions by renal cation transporters: studies on the metformin-trimethoprim interaction. Eur J Clin Pharmacol 2015; 71: 85–94. [DOI] [PubMed] [Google Scholar]
- 27. Wright AJ, Gomes T, Mamdani MM. et al. The risk of hypotension following co-prescription of macrolide antibiotics and calcium-channel blockers. CMAJ 2011; 183: 303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gandhi S, Fleet JL, Bailey DG. et al. Calcium-channel blocker-clarithromycin drug interactions and acute kidney injury. JAMA 2013; 310: 2544–53. [DOI] [PubMed] [Google Scholar]
- 29. Fraser LA, Shariff SZ, McArthur E. et al. Calcium channel blocker-clarithromycin drug interaction did not increase the risk of nonvertebral fracture: a population-based study. Ann Pharmacother 2015; 49: 185–8. [DOI] [PubMed] [Google Scholar]
- 30. Westphal JF. Macrolide-induced clinically relevant drug interactions with cytochrome P-450A (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin. Br J Clin Pharmacol 2000; 50: 285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamazaki H, Shimada T.. Comparative studies of in vitro inhibition of cytochrome P450 3A4-dependent testosterone 6β-hydroxylation by roxithromycin and its metabolites, troleandomycin, and erythromycin. Drug Metab Dispos 1998; 26: 1053–7. [PubMed] [Google Scholar]
- 32. Antoniou T, Hollands S, Macdonald EM. et al. Trimethoprim-sulfamethoxazole and risk of sudden death among patients taking spironolactone. CMAJ 2015; 187: E138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parhofer KG. The treatment of disorders of lipid metabolism. Dtsch Arztebl Int 2016; 113: 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kantola T, Kivisto KT, Neuvonen PJ.. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther 1998; 64: 177–82. [DOI] [PubMed] [Google Scholar]
- 35. Amsden GW, Kuye O, Wei GC.. A study of the interaction potential of azithromycin and clarithromycin with atorvastatin in healthy volunteers. J Clin Pharmacol 2002; 42: 444–9. [PubMed] [Google Scholar]
- 36. Li DQ, Kim R, McArthur E. et al. Risk of adverse events among older adults following co-prescription of clarithromycin and statins not metabolized by cytochrome P450 3A4. CMAJ 2015; 187: 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kalliokoski A, Niemi M.. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 2009; 158: 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seithel A, Eberl S, Singer K. et al. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos 2007; 35: 779–86. [DOI] [PubMed] [Google Scholar]
- 39. Glasheen JJ, Fugit RV, Prochazka AV.. The risk of overanticoagulation with antibiotic use in outpatients on stable warfarin regimens. J Gen Intern Med 2005; 20: 653–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lane MA, Zeringue A, McDonald JR.. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med 2014; 127: 657.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghaswalla PK, Harpe SE, Tassone D. et al. Warfarin-antibiotic interactions in older adults of an outpatient anticoagulation clinic. Am J Geriatr Pharmacother 2012; 10: 352–60. [DOI] [PubMed] [Google Scholar]
- 42. Mergenhagen KA, Olbrych PM, Mattappallil A. et al. Effect of azithromycin on anticoagulation-related outcomes in geriatric patients receiving warfarin. Clin Ther 2013; 35: 425–30. [DOI] [PubMed] [Google Scholar]
- 43. McCall KL, Anderson HG Jr, Jones AD.. Determination of the lack of a drug interaction between azithromycin and warfarin. Pharmacotherapy 2004; 24: 188–94. [DOI] [PubMed] [Google Scholar]
- 44. Fischer HD, Juurlink DN, Mamdani MM. et al. Hemorrhage during warfarin therapy associated with cotrimoxazole and other urinary tract anti-infective agents: a population-based study. Arch Intern Med 2010; 170: 617–21. [DOI] [PubMed] [Google Scholar]
- 45. Washington C, Hou SY, Hughes NC. et al. Ciprofloxacin prolonged-release tablets do not affect warfarin pharmacokinetics and pharmacodynamics. J Clin Pharmacol 2007; 47: 1320–6. [DOI] [PubMed] [Google Scholar]
- 46. Israel DS, Stotka J, Rock W. et al. Effect of ciprofloxacin on the pharmacokinetics and pharmacodynamics of warfarin. Clin Infect Dis 1996; 22: 251–6. [DOI] [PubMed] [Google Scholar]
- 47. Bianco TM, Bussey HI, Farnett LE. et al. Potential warfarin-ciprofloxacin interaction in patients receiving long-term anticoagulation. Pharmacotherapy 1992; 12: 435–9. [PubMed] [Google Scholar]
- 48. Abdel-Aziz MI, Ali MA, Hassan AK. et al. Warfarin-drug interactions: an emphasis on influence of polypharmacy and high doses of amoxicillin/clavulanate. J Clin Pharmacol 2016; 56: 39–46. [DOI] [PubMed] [Google Scholar]
- 49. Schelleman H, Bilker WB, Brensinger CM. et al. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther 2008; 84: 581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baillargeon J, Holmes HM, Lin YL. et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med 2012; 125: 183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Reilly RA, Motley CH.. Racemic warfarin and trimethoprim-sulfamethoxazole interaction in humans. Ann Intern Med 1979; 91: 34–6. [DOI] [PubMed] [Google Scholar]
- 52. Mercadal Orfila G, Gracia Garcia B, Leiva Badosa E. et al. Retrospective assessment of potential interaction between levofloxacin and warfarin. Pharm World Sci 2009; 31: 224–9. [DOI] [PubMed] [Google Scholar]
- 53. Liao S, Palmer M, Fowler C. et al. Absence of an effect of levofloxacin on warfarin pharmacokinetics and anticoagulation in male volunteers. J Clin Pharmacol 1996; 36: 1072–7. [DOI] [PubMed] [Google Scholar]
- 54. Stroud LF, Mamdami MM, Kopp A. et al. The safety of levofloxacin in elderly patients on warfarin. Am J Med 2005; 118: 1417.. [DOI] [PubMed] [Google Scholar]
- 55. Beest P-v, van Meegen E, Rosendaal FR. et al. Drug interactions as a cause of overanticoagulation on phenprocoumon or acenocoumarol predominantly concern antibacterial drugs. Clin Pharmacol Ther 2001; 69: 451–7. [DOI] [PubMed] [Google Scholar]
- 56. Jobski K, Behr S, Garbe E.. Drug interactions with phenprocoumon and the risk of serious haemorrhage: a nested case–control study in a large population-based German database. Eur J Clin Pharmacol 2011; 67: 941–51. [DOI] [PubMed] [Google Scholar]
- 57. Abbas S, Ihle P, Harder S. et al. Risk of bleeding and antibiotic use in patients receiving continuous phenprocoumon therapy. A case–control study nested in a large insurance- and population-based German cohort. Thromb Haemost 2014; 111: 912–22. [DOI] [PubMed] [Google Scholar]
- 58. Beest P-v, Koerselman J, Herings RM.. Risk of major bleeding during concomitant use of antibiotic drugs and coumarin anticoagulants. J Thromb Haemost 2008; 6: 284–90. [DOI] [PubMed] [Google Scholar]
- 59. Juurlink DN. Drug interactions with warfarin: what clinicians need to know. CMAJ 2007; 177: 369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ufer M. Comparative pharmacokinetics of vitamin K antagonists—warfarin, phenprocoumon and acenocoumarol. Clin Pharmacokinet 2005; 44: 1227–46. [DOI] [PubMed] [Google Scholar]
- 61. Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med (Lond) 2014; 14: 165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parasrampuria DA, Mendell J, Shi M. et al. Edoxaban drug–drug interactions with ketoconazole, erythromycin, and cyclosporine. Br J Clin Pharmacol 2016; 82: 1591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gouin-Thibault I, Delavenne X, Blanchard A. et al. Interindividual variability in dabigatran and rivaroxaban exposure: contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J Thromb Haemost 2017; 15: 273–83. [DOI] [PubMed] [Google Scholar]
- 64. Damkier P, Hansen LL, Brosen K.. Effect of diclofenac, disulfiram, itraconazole, grapefruit juice and erythromycin on the pharmacokinetics of quinidine. Br J Clin Pharmacol 1999; 48: 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Isohanni MH, Neuvonen PJ, Olkkola KT.. Effect of erythromycin and itraconazole on the pharmacokinetics of oral lignocaine. Pharmacol Toxicol 1999; 84: 143–6. [DOI] [PubMed] [Google Scholar]
- 66. Kosoglou T, Rocci ML Jr, Vlasses PH.. Trimethoprim alters the disposition of procainamide and N-acetylprocainamide. Clin Pharmacol Ther 1988; 44: 467–77. [DOI] [PubMed] [Google Scholar]
- 67. Vlasses PH, Kosoglou T, Chase SL. et al. Trimethoprim inhibition of the renal clearance of procainamide and N-acetylprocainamide. Arch Intern Med 1989; 149: 1350–3. [PubMed] [Google Scholar]
- 68. Zapater P, Reus S, Tello A. et al. A prospective study of the clarithromycin-digoxin interaction in elderly patients. J Antimicrob Chemother 2002; 50: 601–6. [DOI] [PubMed] [Google Scholar]
- 69. Chan AL, Wang MT, Su CY. et al. Risk of digoxin intoxication caused by clarithromycin-digoxin interactions in heart failure patients: a population-based study. Eur J Clin Pharmacol 2009; 65: 1237–43. [DOI] [PubMed] [Google Scholar]
- 70. Tanaka H, Matsumoto K, Ueno K. et al. Effect of clarithromycin on steady-state digoxin concentrations. Ann Pharmacother 2003; 37: 178–81. [DOI] [PubMed] [Google Scholar]
- 71. Rengelshausen J, Goggelmann C, Burhenne J. et al. Contribution of increased oral bioavailability and reduced nonglomerular renal clearance of digoxin to the digoxin-clarithromycin interaction. Br J Clin Pharmacol 2003; 56: 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Petersen P, Kastrup J, Bartram R. et al. Digoxin-trimethoprim interaction. Acta Med Scand 1985; 217: 423–7. [DOI] [PubMed] [Google Scholar]
- 73. Bauer LA, Black DJ, Lill JS. et al. Levofloxacin and ciprofloxacin decrease procainamide and N-acetylprocainamide renal clearances. Antimicrob Agents Chemother 2005; 49: 1649–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Martin DE, Shen J, Griener J. et al. Effects of ofloxacin on the pharmacokinetics and pharmacodynamics of procainamide. J Clin Pharmacol 1996; 36: 85–91. [DOI] [PubMed] [Google Scholar]
- 75. Ujhelyi MR, Bottorff MB, Schur M. et al. Aging effects on the organic base transporter and stereoselective renal clearance. Clin Pharmacol Ther 1997; 62: 117–28. [DOI] [PubMed] [Google Scholar]
- 76. Raebel MA, Carroll NM, Andrade SE. et al. Monitoring of drugs with a narrow therapeutic range in ambulatory care. Am J Manag Care 2006; 12: 268–75. [PubMed] [Google Scholar]
- 77. Campbell T, Williams K.. Therapeutic drug monitoring: antiarrhythmic drugs. Br J Clin Pharmacol 2001; 52: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fost DA, Leung DY, Martin RJ. et al. Inhibition of methylprednisolone elimination in the presence of clarithromycin therapy. J Allergy Clin Immunol 1999; 103: 1031–5. [DOI] [PubMed] [Google Scholar]
- 79. Hegazy SK, Mabrouk MM, Elsisi AE. et al. Effect of clarithromycin and fluconazole on the pharmacokinetics of montelukast in human volunteers. Eur J Clin Pharmacol 2012; 68: 1275–80. [DOI] [PubMed] [Google Scholar]
- 80. Branigan TA, Robbins RA, Cady WJ. et al. The effects of erythromycin on the absorption and disposition of kinetics of theophylline. Eur J Clin Pharmacol 1981; 21: 115–20. [DOI] [PubMed] [Google Scholar]
- 81. Reisz G, Pingleton SK, Melethil S. et al. The effect of erythromycin on theophylline pharmacokinetics in chronic bronchitis. Am Rev Respir Dis 1983; 127: 581–4. [DOI] [PubMed] [Google Scholar]
- 82. Prince RA, Wing DS, Weinberger MM. et al. Effect of erythromycin on theophylline kinetics. J Allergy Clin Immunol 1981; 68: 427–31. [DOI] [PubMed] [Google Scholar]
- 83. May DC, Jarboe CH, Ellenburg DT. et al. The effects of erythromycin on theophylline elimination in normal males. J Clin Pharmacol 1982; 22: 125–30. [DOI] [PubMed] [Google Scholar]
- 84. Maddux MS, Leeds NH, Organek HW. et al. The effect of erythromycin on theophylline pharmacokinetics at steady-state. Chest 1982; 81: 563–5. [DOI] [PubMed] [Google Scholar]
- 85. Pfeifer HJ, Greenblatt DJ, Friedman P.. Effects of three antibiotics on theophylline kinetics. Clin Pharmacol Ther 1979; 26: 36–40. [DOI] [PubMed] [Google Scholar]
- 86. Carr RA, Edmonds A, Shi H. et al. Steady-state pharmacokinetics and electrocardiographic pharmacodynamics of clarithromycin and loratadine after individual or concomitant administration. Antimicrob Agents Chemother 1998; 42: 1176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brannan MD, Reidenberg P, Radwanski E. et al. Loratadine administered concomitantly with erythromycin: pharmacokinetic and electrocardiographic evaluations. Clin Pharmacol Ther 1995; 58: 269–78. [DOI] [PubMed] [Google Scholar]
- 88. Blix HS, Viktil KK, Moger TA. et al. Drugs with narrow therapeutic index as indicators in the risk management of hospitalised patients. Pharm Pract (Granada) 2010; 8: 50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ha HR, Chen J, Freiburghaus A. et al. Metabolism of theophylline by cDNA-expressed human cytochromes P-450. Br J Clin Pharmacol 1995; 39: 321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nix DE, DeVito JM, Whitbread MA. et al. Effect of multiple dose oral ciprofloxacin on the pharmacokinetics of theophylline and indocyanine green. J Antimicrob Chemother 1987; 19: 263–9. [DOI] [PubMed] [Google Scholar]
- 91. Wijnands WJ, Vree TB, van Herwaarden CL.. The influence of quinolone derivatives on theophylline clearance. Br J Clin Pharmacol 1986; 22: 677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Loi CM, Parker BM, Cusack BJ. et al. Individual and combined effects of cimetidine and ciprofloxacin on theophylline metabolism in male nonsmokers. Br J Clin Pharmacol 1993; 36: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Robson RA, Begg EJ, Atkinson HC. et al. Comparative effects of ciprofloxacin and lomefloxacin on the oxidative metabolism of theophylline. Br J Clin Pharmacol 1990; 29: 491–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Prince RA, Casabar E, Adair CG. et al. Effect of quinolone antimicrobials on theophylline pharmacokinetics. J Clin Pharmacol 1989; 29: 650–4. [DOI] [PubMed] [Google Scholar]
- 95. Raoof S, Wollschlager C, Khan FA.. Ciprofloxacin increases serum levels of theophylline. Am J Med 1987; 82: 115–8. [PubMed] [Google Scholar]
- 96. Antoniou T, Gomes T, Mamdani MM. et al. Ciprofloxacin-induced theophylline toxicity: a population-based study. Eur J Clin Pharmacol 2011; 67: 521–6. [DOI] [PubMed] [Google Scholar]
- 97. Batty KT, Davis TM, Ilett KF. et al. The effect of ciprofloxacin on theophylline pharmacokinetics in healthy subjects. Br J Clin Pharmacol 1995; 39: 305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Inghammar M, Ekbom A, Engström G. et al. COPD and the risk of tuberculosis—a population-based cohort study. PLoS One 2010; 5: e10138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Baciewicz AM, Chrisman CR, Finch CK. et al. Update on rifampin, rifabutin, and rifapentine drug interactions. Curr Med Res Opin 2013; 29: 1–12. [DOI] [PubMed] [Google Scholar]
- 100. Wallace RJ Jr, Brown BA, Griffith DE. et al. Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. intracellulare infection. J Infect Dis 1995; 171: 747–50. [DOI] [PubMed] [Google Scholar]
- 101. Hafner R, Bethel J, Power M. et al. Tolerance and pharmacokinetic interactions of rifabutin and clarithromycin in human immunodeficiency virus-infected volunteers. Antimicrob Agents Chemother 1998; 42: 631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Colmenero JD, Fernandez-Gallardo LC, Agundez JA. et al. Possible implications of doxycycline-rifampin interaction for treatment of brucellosis. Antimicrob Agents Chemother 1994; 38: 2798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Winter HR, Trapnell CB, Slattery JT. et al. The effect of clarithromycin, fluconazole, and rifabutin on sulfamethoxazole hydroxylamine formation in individuals with human immunodeficiency virus infection (AACTG 283). Clin Pharmacol Ther 2004; 76: 313–22. [DOI] [PubMed] [Google Scholar]
- 104. Bhatia RS, Uppal R, Malhi R. et al. Drug-interaction between rifampicin and cotrimoxazole in patients with tuberculosis. Hum Exp Toxicol 1991; 10: 419–21. [DOI] [PubMed] [Google Scholar]
- 105. Garske L, Kidd T, Gan R. et al. Rifampicin and sodium fusidate reduces the frequency of methicillin-resistant Staphylococcus aureus (MRSA) isolation in adults with cystic fibrosis and chronic MRSA infection. J Hosp Infect 2004; 56: 208–14. [DOI] [PubMed] [Google Scholar]
- 106. Weiner M, Burman W, Luo CC. et al. Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob Agents Chemother 2007; 51: 2861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nijland HM, Ruslami R, Suroto AJ. et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis 2007; 45: 1001–7. [DOI] [PubMed] [Google Scholar]
- 108. Manika K, Chatzika K, Papaioannou M. et al. Rifampicin-moxifloxacin interaction in tuberculosis treatment: a real-life study. Int J Tuberc Lung Dis 2015; 19: 1383–7. [DOI] [PubMed] [Google Scholar]
- 109. Naidoo A, Chirehwa M, McIlleron H. et al. Effect of rifampicin and efavirenz on moxifloxacin concentrations when co-administered in patients with drug-susceptible TB. J Antimicrob Chemother 2017; 72: 1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Apseloff G, Foulds G, LaBoy-Goral L. et al. Comparison of azithromycin and clarithromycin in their interactions with rifabutin in healthy volunteers. Eur Respir J 1998; 38: 830–5. [PubMed] [Google Scholar]
- 111. Jordan MK, Polis MA, Kelly G. et al. Effects of fluconazole and clarithromycin on rifabutin and 25-O-desacetylrifabutin pharmacokinetics. J Clin Pharmacol 2000; 44: 2170–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nelson MV, Berchou RC, Kareti D. et al. Pharmacokinetic evaluation of erythromycin and caffeine administered with bromocriptine. Clin Pharmacol Ther 1990; 47: 694–7. [DOI] [PubMed] [Google Scholar]
- 113. Nakatsuka A, Nagai M, Yabe H. et al. Effect of clarithromycin on the pharmacokinetics of cabergoline in healthy controls and in patients with Parkinson’s disease. J Pharmacol Sci 2006; 100: 59–64. [DOI] [PubMed] [Google Scholar]
- 114. Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 2006; 61: 246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Neuvonen PJ, Penttila O.. Interaction between doxycycline and barbiturates. Br Med J 1974; 1: 535–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hansen JM, Kampmann JP, Siersbaek-Nielsen K. et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl 1979; 624: 106–10. [DOI] [PubMed] [Google Scholar]
- 117. Penttila O, Neuvonen PJ, Aho K. et al. Interaction between doxycycline and some antiepileptic drugs. Br Med J 1974; 2: 470–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shahzadi A, Javed I, Aslam B. et al. Therapeutic effects of ciprofloxacin on the pharmacokinetics of carbamazepine in healthy adult male volunteers. Pak J Pharm Sci 2011; 24: 63–8. [PubMed] [Google Scholar]
- 119. Antoniou T, Gomes T, Mamdani MM. et al. Trimethoprim/sulfamethoxazole-induced phenytoin toxicity in the elderly: a population-based study. Br J Clin Pharmacol 2011; 71: 544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Greenberg RG, Melloni C, Wu H. et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol 2016; 39: 232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bialer M, Rene'H L, Perucca E.. Does carbamazepine have a narrow therapeutic plasma concentration range? Ther Drug Monit 1998; 20: 56–9. [DOI] [PubMed] [Google Scholar]
- 122. Li KY, Li X, Cheng ZN. et al. Effect of erythromycin on metabolism of quetiapine in Chinese suffering from schizophrenia. Eur J Clin Pharmacol 2005; 60: 791–5. [DOI] [PubMed] [Google Scholar]
- 123. Desta Z, Kerbusch T, Flockhart DA.. Effect of clarithromycin on the pharmacokinetics and pharmacodynamics of pimozide in healthy poor and extensive metabolizers of cytochrome P450 2D6 (CYP2D6). Clin Pharmacol Ther 1999; 65: 10–20. [DOI] [PubMed] [Google Scholar]
- 124. Farkas D, Volak LP, Harmatz JS. et al. Short-term clarithromycin administration impairs clearance and enhances pharmacodynamic effects of trazodone but not of zolpidem. Clin Pharmacol Ther 2009; 85: 644–50. [DOI] [PubMed] [Google Scholar]
- 125. Kivisto KT, Lamberg TS, Kantola T. et al. Plasma buspirone concentrations are greatly increased by erythromycin and itraconazole. Clin Pharmacol Ther 1997; 62: 348–54. [DOI] [PubMed] [Google Scholar]
- 126. Dean L. Diazepam therapy and CYP2C19 genotype In: Pratt V, McLeod H, Rubinstein W. et al. , eds. Medical Genetics Summaries. Bethesda, USA: National Center for Biotechnology Information, 2012. https://www.ncbi.nlm.nih.gov/books/NBK379740/. [PubMed] [Google Scholar]
- 127. Kamali F, Thomas SH, Edwards C.. The influence of steady-state ciprofloxacin on the pharmacokinetics and pharmacodynamics of a single dose of diazepam in healthy volunteers. Eur J Clin Pharmacol 1993; 44: 365–7. [DOI] [PubMed] [Google Scholar]
- 128. Fuhr U, Anders EM, Mahr G. et al. Inhibitory potency of quinolone antibacterial agents against cytochrome P450IA2 activity in vivo and in vitro. Antimicrob Agents Chemother 1992; 36: 942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Raaska K, Neuvonen PJ.. Ciprofloxacin increases serum clozapine and N-desmethylclozapine: a study in patients with schizophrenia. Eur J Clin Pharmacol 2000; 56: 585–9. [DOI] [PubMed] [Google Scholar]
- 130. Meyer JM, Proctor G, Cummings MA. et al. Ciprofloxacin and clozapine: a potentially fatal but underappreciated interaction. Case Rep Psychiatry 2016; 2016: 5606098.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nix DE, Wilton JH, Ronald B. et al. Inhibition of norfloxacin absorption by antacids. Antimicrob Agents Chemother 1990; 34: 432–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Garty M, Hurwitz A.. Effect of cimetidine and antacids on gastrointestinal absorption of tetracycline. Clin Pharmacol Ther 1980; 28: 203–7. [DOI] [PubMed] [Google Scholar]
- 133. Van Slooten AD, Nix DE, Wilton JH. et al. Combined use of ciprofloxacin and sucralfate. DICP 1991; 25: 578–82. [DOI] [PubMed] [Google Scholar]
- 134. Parpia SH, Nix DE, Hejmanowski LG. et al. Sucralfate reduces the gastrointestinal absorption of norfloxacin. Antimicrob Agents Chemother 1989; 33: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lehto P, Kivisto KT.. Effect of sucralfate on absorption of norfloxacin and ofloxacin. Antimicrob Agents Chemother 1994; 38: 248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Deppermann KM, Lode H, Hoffken G. et al. Influence of ranitidine, pirenzepine, and aluminum magnesium hydroxide on the bioavailability of various antibiotics, including amoxicillin, cephalexin, doxycycline, and amoxicillin-clavulanic acid. Antimicrob Agents Chemother 1989; 33: 1901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nix DE, Watson WA, Lener ME. et al. Effects of aluminum and magnesium antacids and ranitidine on the absorption of ciprofloxacin. Clin Pharmacol Ther 1989; 46: 700–5. [DOI] [PubMed] [Google Scholar]
- 138. Stass H, Bottcher MF, Ochmann K.. Evaluation of the influence of antacids and H2 antagonists on the absorption of moxifloxacin after oral administration of a 400 mg dose to healthy volunteers. Clin Pharmacokinet 2001; 40 Suppl 1: 39–48. [DOI] [PubMed] [Google Scholar]
- 139. Frost RW, Lasseter KC, Noe AJ. et al. Effects of aluminum hydroxide and calcium carbonate antacids on the bioavailability of ciprofloxacin. Antimicrob Agents Chemother 1992; 36: 830–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Motoya T, Shimozono T, Yamaguchi T. et al. Effects of milk and aluminum hydroxide on the absorption of norfloxacin, ciprofloxacin and tosufloxacin in healthy volunteers. J Appl Ther 1997; 1: 213–7. [Google Scholar]
- 141. Nguyen VX, Nix DE, Gillikin S. et al. Effect of oral antacid administration on the pharmacokinetics of intravenous doxycycline. Antimicrob Agents Chemother 1989; 33: 434–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nix DE, Watson WA, Handy L. et al. The effect of sucralfate pretreatment on the pharmacokinetics of ciprofloxacin. Pharmacotherapy 1989; 9: 377–80. [DOI] [PubMed] [Google Scholar]
- 143. Albert KS, Welch RD, DeSante KA. et al. Decreased tetracycline bioavailability caused by a bismuth subsalicylate antidiarrheal mixture. J Pharm Sci 1979; 68: 586–8. [DOI] [PubMed] [Google Scholar]
- 144. Mapp RK, McCarthy TJ.. The effect of zinc sulphate and of bicitropeptide on tetracycline absorption. S Afr Med J 1976; 50: 1829–30. [PubMed] [Google Scholar]
- 145. Chopra I, Roberts M.. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 2001; 65: 232.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kamberi M, Nakashima H, Ogawa K. et al. The effect of staggered dosing of sucralfate on oral bioavailability of sparfloxacin. Br J Clin Pharmacol 2000; 49: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Miura M, Tada H, Yasui-Furukori N. et al. Effect of clarithromycin on the enantioselective disposition of lansoprazole in relation to CYP2C19 genotypes. Chirality 2005; 17: 338–44. [DOI] [PubMed] [Google Scholar]
- 148. Crothers K, Butt AA, Gibert CL. et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006; 130: 1326–33. [DOI] [PubMed] [Google Scholar]
- 149. Sahai J, Gallicano K, Oliveras L. et al. Cations in the didanosine tablet reduce ciprofloxacin bioavailability. Clin Pharmacol Ther 1993; 53: 292–7. [DOI] [PubMed] [Google Scholar]
- 150. Knupp CA, Barbhaiya RH.. A multiple-dose pharmacokinetic interaction study between didanosine (Videx) and ciprofloxacin (Cipro) in male subjects seropositive for HIV but asymptomatic. Biopharm Drug Dispos 1997; 18: 65–77. [DOI] [PubMed] [Google Scholar]
- 151. Grub S, Bryson H, Goggin T. et al. The interaction of saquinavir (soft gelatin capsule) with ketoconazole, erythromycin and rifampicin: comparison of the effect in healthy volunteers and in HIV-infected patients. Eur J Clin Pharmacol 2001; 57: 115–21. [DOI] [PubMed] [Google Scholar]
- 152. Srinivas NR, Knupp CA, Batteiger B. et al. A pharmacokinetic interaction study of didanosine coadministered with trimethoprim and/or sulphamethoxazole in HIV seropositive asymptomatic male patients. Br J Clin Pharmacol 1996; 41: 207–15. [DOI] [PubMed] [Google Scholar]
- 153. Moore KH, Yuen GJ, Raasch RH. et al. Pharmacokinetics of lamivudine administered alone and with trimethoprim-sulfamethoxazole. Clin Pharmacol Ther 1996; 59: 550–8. [DOI] [PubMed] [Google Scholar]
- 154. Shi HY, Yan J, Zhu WH. et al. Effects of erythromycin on voriconazole pharmacokinetics and association with CYP2C19 polymorphism. Eur J Clin Pharmacol 2010; 66: 1131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sriwiriyajan S, Samaeng M, Ridtitid W. et al. Pharmacokinetic interactions between ciprofloxacin and itraconazole in healthy male volunteers. Biopharm Drug Dispos 2011; 32: 168–74. [DOI] [PubMed] [Google Scholar]
- 156. Olkkola KT, Aranko K, Luurila H. et al. A potentially hazardous interaction between erythromycin and midazolam. Clin Pharmacol Ther 1993; 53: 298–305. [DOI] [PubMed] [Google Scholar]
- 157. Yeates RA, Laufen H, Zimmermann T.. Interaction between midazolam and clarithromycin: comparison with azithromycin. Int J Clin Pharmacol Ther 1996; 34: 400–5. [PubMed] [Google Scholar]
- 158. Quinney SK, Haehner BD, Rhoades MB. et al. Interaction between midazolam and clarithromycin in the elderly. Br J Clin Pharmacol 2008; 65: 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zimmermann T, Yeates RA, Laufen H. et al. Influence of the antibiotics erythromycin and azithromycin on the pharmacokinetics and pharmacodynamics of midazolam. Arzneimittelforschung 1996; 46: 213–7. [PubMed] [Google Scholar]
- 160. Mattila MJ, Vanakoski J, Idanpaan HJJ.. Azithromycin does not alter the effects of oral midazolam on human performance. Eur J Clin Pharmacol 1994; 47: 49–52. [DOI] [PubMed] [Google Scholar]
- 161. Hagelberg NM, Peltoniemi MA, Saari TI. et al. Clarithromycin, a potent inhibitor of CYP3A, greatly increases exposure to oral S-ketamine. Eur J Pain 2010; 14: 625–9. [DOI] [PubMed] [Google Scholar]
- 162. Backman JT, Aranko K, Himberg JJ. et al. A pharmacokinetic interaction between roxithromycin and midazolam. Eur J Clin Pharmacol 1994; 46: 551–5. [DOI] [PubMed] [Google Scholar]
- 163. Bartkowski RR, Goldberg ME, Larijani GE. et al. Inhibition of alfentanil metabolism by erythromycin. Clin Pharmacol Ther 1989; 46: 99–102. [DOI] [PubMed] [Google Scholar]
- 164. Jokinen MJ, Olkkola KT, Ahonen J. et al. Effect of ciprofloxacin on the pharmacokinetics of ropivacaine. Eur J Clin Pharmacol 2003; 58: 653–7. [DOI] [PubMed] [Google Scholar]
- 165. Jokinen MJ, Ahonen J, Neuvonen PJ. et al. Effect of clarithromycin and itraconazole on the pharmacokinetics of ropivacaine. Pharmacol Toxicol 2001; 88: 187–91. [DOI] [PubMed] [Google Scholar]
- 166. Jokinen MJ, Ahonen J, Neuvonen PJ. et al. The effect of erythromycin, fluvoxamine, and their combination on the pharmacokinetics of ropivacaine. Anesth Analg 2000; 91: 1207–12. [DOI] [PubMed] [Google Scholar]
- 167. Liukas A, Hagelberg NM, Kuusniemi K. et al. Inhibition of cytochrome P450 3A by clarithromycin uniformly affects the pharmacokinetics and pharmacodynamics of oxycodone in young and elderly volunteers. J Clin Psychopharmacol 2011; 31: 302–8. [DOI] [PubMed] [Google Scholar]
- 168. Gupta SK, Bakran A, Johnson RW. et al. Cyclosporin-erythromycin interaction in renal transplant patients. Br J Clin Pharmacol 1989; 27: 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Freeman DJ, Martell R, Carruthers SG. et al. Cyclosporin-erythromycin interaction in normal subjects. Br J Clin Pharmacol 1987; 23: 776–8. [PMC free article] [PubMed] [Google Scholar]
- 170. Federico S, Carrano R, Capone D. et al. Pharmacokinetic interaction between levofloxacin and ciclosporin or tacrolimus in kidney transplant recipients—ciclosporin, tacrolimus and levofloxacin in renal transplantation. Clin Pharmacokinet 2006; 45: 169–75. [DOI] [PubMed] [Google Scholar]
- 171. Wrishko RE, Levine M, Primmett DR. et al. Investigation of a possible interaction between ciprofloxacin and cyclosporine in renal transplant patients. Transplantation 1997; 64: 996–9. [DOI] [PubMed] [Google Scholar]
- 172. Tan KK, Trull AK, Shawket S.. Co-administration of ciprofloxacin and cyclosporin: lack of evidence for a pharmacokinetic interaction. Br J Clin Pharmacol 1989; 28: 185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hedaya MA, El-Afify DR, El-Maghraby GM.. The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers. Biopharm Drug Dispos 2006; 27: 103–10. [DOI] [PubMed] [Google Scholar]
- 174. Muirhead GJ, Faulkner S, Harness JA. et al. The effects of steady-state erythromycin and azithromycin on the pharmacokinetics of sildenafil in healthy volunteers. Br J Clin Pharmacol 2002; 53 Suppl 1: 37S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Shinde DD, Kim HS, Choi JS. et al. Different effects of clopidogrel and clarithromycin on the enantioselective pharmacokinetics of sibutramine and its active metabolites in healthy subjects. J Clin Pharmacol 2013; 53: 550–8. [DOI] [PubMed] [Google Scholar]
- 176. Pan W, Bae SK, Shim EJ. et al. Effects of clopidogrel and clarithromycin on the disposition of sibutramine and its active metabolites M1 and M2 in relation to CYP2B6*6 polymorphism. Xenobiotica 2013; 43: 211–8. [DOI] [PubMed] [Google Scholar]
- 177. Bassi PU, Onyeji CO, Ukponmwan OE.. Effects of tetracycline on the pharmacokinetics of halofantrine in healthy volunteers. Br J Clin Pharmacol 2004; 58: 52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Pohl O, Osterloh I, Gotteland JP.. Effects of erythromycin at steady-state concentrations on the pharmacokinetics of ulipristal acetate. J Clin Pharm Ther 2013; 38: 512–7. [DOI] [PubMed] [Google Scholar]
- 179. Yano R, Tani D, Watanabe K. et al. Evaluation of potential interaction between vinorelbine and clarithromycin. Ann Pharmacother 2009; 43: 453–8. [DOI] [PubMed] [Google Scholar]
- 180. Terkeltaub RA, Furst DE, Digiacinto JL. et al. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum 2011; 63: 2226–37. [DOI] [PubMed] [Google Scholar]
- 181. Kovarik JM, Beyer D, Bizot MN. et al. Effect of multiple-dose erythromycin on everolimus pharmacokinetics. Eur J Clin Pharmacol 2005; 61: 35–8. [DOI] [PubMed] [Google Scholar]
- 182. Kovarik JM, Hsu CH, McMahon L. et al. Population pharmacokinetics of everolimus in de novo renal transplant patients: impact of ethnicity and comedications. Clin Pharmacol Ther 2001; 70: 247–54. [DOI] [PubMed] [Google Scholar]
- 183. Granfors MT, Backman JT, Neuvonen M. et al. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther 2004; 76: 598–606. [DOI] [PubMed] [Google Scholar]
- 184. Tan KK, Allwood MC, Shawket S.. Effect of ciprofloxacin on the pharmacokinetics of antipyrine in healthy volunteers. J Clin Pharm Ther 1990; 15: 151–4. [DOI] [PubMed] [Google Scholar]
- 185. Ludwig E, Szekely E, Csiba A. et al. The effect of ciprofloxacin on antipyrine metabolism. J Antimicrob Chemother 1988; 22: 61–7. [DOI] [PubMed] [Google Scholar]
- 186. Kamali F, Huang ML.. Increased systemic availability of loperamide after oral administration of loperamide and loperamide oxide with cotrimoxazole. Br J Clin Pharmacol 1996; 41: 125–8. [DOI] [PubMed] [Google Scholar]
- 187. Lee BL, Medina I, Benowitz NL. et al. Dapsone, trimethoprim, and sulfamethoxazole plasma levels during treatment of pneumocystis pneumonia in patients with the acquired immunodeficiency syndrome (AIDS). Evidence of drug interactions. Ann Intern Med 1989; 110: 606–11. [DOI] [PubMed] [Google Scholar]
- 188. Piccolo ML, Toossi Z, Goldman M.. Effect of coadministration of a nutritional supplement on ciprofloxacin absorption. Am J Hosp Pharm 1994; 51: 2697–9. [PubMed] [Google Scholar]
- 189. Penttila O, Hurme H, Neuvonen PJ.. Effect of zinc sulphate on the absorption of tetracycline and doxycycline in man. Eur J Clin Pharmacol 1975; 9: 131–4. [DOI] [PubMed] [Google Scholar]
- 190. Kays MB, Overholser BR, Mueller BA. et al. Effects of sevelamer hydrochloride and calcium acetate on the oral bioavailability of ciprofloxacin. Am J Kidney Dis 2003; 42: 1253–9. [DOI] [PubMed] [Google Scholar]
- 191. Kato R, Ueno K, Imano H. et al. Impairment of ciprofloxacin absorption by calcium polycarbophil. J Clin Pharmacol 2002; 42: 806–11. [DOI] [PubMed] [Google Scholar]
- 192. Lesko LJ, Offman E, Brew CT. et al. Evaluation of the potential for drug interactions with patiromer in healthy volunteers. J Cardiovasc Pharmacol Ther 2017; 22: 434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. How PP, Fischer JH, Arruda JA. et al. Effects of lanthanum carbonate on the absorption and oral bioavailability of ciprofloxacin. Clin J Am Soc Nephrol 2007; 2: 1235–40. [DOI] [PubMed] [Google Scholar]
- 194. Landersdorfer CB, Kirkpatrick CM, Kinzig M. et al. Competitive inhibition of renal tubular secretion of ciprofloxacin and metabolite by probenecid. Br J Clin Pharmacol 2010; 69: 167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Jaehde U, Sorgel F, Reiter A. et al. Effect of probenecid on the distribution and elimination of ciprofloxacin in humans. Clin Pharmacol Ther 1995; 58: 532–41. [DOI] [PubMed] [Google Scholar]
- 196. Bolhuis MS, van Altena R, van Soolingen D. et al. Clarithromycin increases linezolid exposure in multidrug-resistant tuberculosis patients. Eur Respir J 2013; 42: 1614–21. [DOI] [PubMed] [Google Scholar]
- 197. Mitchell PB. Therapeutic drug monitoring of psychotropic medications. Br J Clin Pharmacol 2000; 49: 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Dickinson BD, Altman RD, Nielsen NH. et al. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol 2001; 98: 853–60. [DOI] [PubMed] [Google Scholar]
- 199. Taylor J, Pemberton MN.. Antibiotics and oral contraceptives: new considerations for dental practice. Br Dent J 2012; 212: 481–3. [DOI] [PubMed] [Google Scholar]
- 200. Albert RK, Schuller JL, Network C.. Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med 2014; 189: 1173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Tabibian JH, Gutierrez MA.. Doxycycline-induced pseudotumor cerebri. South Med J 2009; 102: 310–1. [DOI] [PubMed] [Google Scholar]
- 202. Berger FA, Monadian N, Groot N. et al. QTc prolongation during ciprofloxacin and fluconazole combination therapy: prevalence and associated risk factors. Br J Clin Pharmacol 2018; 84: 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Baxter K, Preston C.. Stockley's Drug Interactions . London: Pharmaceutical Press, 2010. [Google Scholar]
- 204. Siedlik PH, Olson SC, Yang BB. et al. Erythromycin coadministration increases plasma atorvastatin concentrations. J Clin Pharmacol 1999; 39: 501–4. [PubMed] [Google Scholar]
- 205. Ericsson CD, Feldman S, Pickering LK. et al. Influence of subsalicylate bismuth on absorption of doxycycline. JAMA 1982; 247: 2266–7. [PubMed] [Google Scholar]
- 206. Markert C, Schweizer Y, Hellwig R. et al. Clarithromycin substantially increases steady-state bosentan exposure in healthy volunteers. Br J Clin Pharmacol 2014; 77: 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Markert C, Hellwig R, Burhenne J. et al. Interaction of ambrisentan with clarithromycin and its modulation by polymorphic SLCO1B1. Eur J Clin Pharmacol 2013; 69: 1785–93. [DOI] [PubMed] [Google Scholar]
- 208. Tokairin T, Fukasawa T, Yasui-Furukori N. et al. Inhibition of the metabolism of brotizolam by erythromycin in humans: in vivo evidence for the involvement of CYP3A4 in brotizolam metabolism. Br J Clin Pharmacol 2005; 60: 172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Phillips JP, Antal EJ, Smith RB.. A pharmacokinetic drug interaction between erythromycin and triazolam. J Clin Psychopharmacol 1986; 6: 297–9. [PubMed] [Google Scholar]
- 210. Aranko K, Luurila H, Backman JT. et al. The effect of erythromycin on the pharmacokinetics and pharmacodynamics of zopiclone. Br J Clin Pharmacol 1994; 38: 363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Vlase L, Popa A, Neag M. et al. Pharmacokinetic interaction between zolpidem and ciprofloxacin in healthy volunteers. Eur J Drug Metab Pharmacokinet 2011; 35: 83–7. [DOI] [PubMed] [Google Scholar]
- 212. Polk RE, Healy DP, Sahai J. et al. Effect of ferrous sulfate and multivitamins with zinc on absorption of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother 1989; 33: 1841–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kara M, Hasinoff BB, McKay DW. et al. Clinical and chemical interactions between iron preparations and ciprofloxacin. Br J Clin Pharmacol 1991; 31: 257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Lehto P, Kivisto KT, Neuvonen PJ.. The effect of ferrous sulphate on the absorption of norfloxacin, ciprofloxacin and ofloxacin. Br J Clin Pharmacol 1994; 37: 82–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Gothoni G, Neuvonen PJ, Mattila M. et al. Iron-tetracycline interaction: effect of time interval between the drugs. Acta Med Scand 1972; 191: 409–11. [PubMed] [Google Scholar]
- 216. Leyden JJ. Absorption of minocycline hydrochloride and tetracycline hydrochloride. Effect of food, milk, and iron. J Am Acad Dermatol 1985; 12: 308–12. [DOI] [PubMed] [Google Scholar]
- 217. Neuvonen PJ, Penttila O.. Effect of oral ferrous sulphate on the half-life of doxycycline in man. Eur J Clin Pharmacol 1974; 7: 361–3. [DOI] [PubMed] [Google Scholar]
- 218. Neuvonen PJ, Turakka H.. Inhibitory effect of various iron salts on the absorption of tetracycline in man. Eur J Clin Pharmacol 1974; 7: 357–60. [DOI] [PubMed] [Google Scholar]
- 219. Venho VM, Salonen RO, Mattila MJ.. Modification of the pharmacokinetics of doxycycline in man by ferrous sulphate or charcoal. Eur J Clin Pharmacol 1978; 14: 277–80. [DOI] [PubMed] [Google Scholar]
- 220. Stass H, Kubitza D.. Effects of iron supplements on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in humans. Clin Pharmacokinet 2001; 40 Suppl 1: 57–62. [DOI] [PubMed] [Google Scholar]
- 221. Cheng SH, White A.. Effect of orally administered neomycin on the absorption of penicillin V. N Engl J Med 1962; 267: 1296–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.