Abstract
Storage and quantitative analysis of small volumes of biofluids are challenging, especially when low concentrations of analytes are to be detected in the presence of complex matrices. In this study, we describe an integrated thread-based approach for stabilizing small blood volumes in the dry-state at room temperature, while also offering direct analysis capabilities via thread spray mass spectrometry. The analytical merits of this novel microsampling platform was demonstrated via the direct analysis of diazepam and cocaine in dried blood samples stored for 42 days. In-situ in-capillary blood processing from hydrophobic threads enabled limits of detection as low as parts-per-quadrillion to be reached. We validated this ultra-sensitivity by analyzing small tissue-like residues collected after pushing a thread through the sample once. The implications of this sample collection, storage and analysis platform can be extensive with direct applications in forensics and clinical studies.
Keywords: Analytical Methods, Dried Blood Storage, Mass Spectrometry, Microsampling, Thread Spray
Graphical Abstract
1. Introduction
Microsampling is essential for the rapidly changing healthcare system where the sustainable implementation of biobanks (collection and storage of biological specimens) is expected to reduce operational cost and increase access to a wider range of population groups [1–3]. This exciting new paradigm is challenged by difficulties in manipulating small sample volumes and maintaining analyte homogeneity in the microsample. For example, since there are currently no efficient methods for direct analysis of microsamples, dilution steps are deemed necessary to convert the small sample volume into a form that can be handled by traditional, large-volume analytical methods [4,5]. This dilution step does not only increase analysis time, but it also has severe consequences on the stability, storage and integrity of the sample [6,7]. The accuracy of small volume aliquots can be low, and the analysis of the diluted sample certainly requires a more sensitive instrument, one that might not be readily available in resource-limited settings. Another key challenge in microsampling, which involves the uneven analyte distribution in the collected sample, is a significant issue in dried blood spots (DBS) prepared on paper substrates [8–11]. Safeguarding homogeneity in microvolumes of liquid samples (<20 μL) is also not trivial due to (i) sample loss via potential adsorption of analyte to the wall of the container and the fact that (ii) cold storage often leads to unfavorable volume/surface ratios which results in evaporation (free-drying) after prolong storage [12].
Currently, there are multiple microsampling techniques utilized to circumvent these challenges. For example, the collection of small liquid samples via capillary microsampling (CM) [13] has significantly improved toxicological studies by reducing the number of animals required for safety assessments during drug development. After dilution, the collected blood sample is processed into plasma and stored under cold conditions for further downstream analysis in the laboratory. Remote sampling is more effectively achieved via collection platforms that allow dry-state sample storage. Lyophilization (freeze-drying) and vitrification (transformation into a “glass” state) have been used, but both techniques require vast resources and large volumes of blood [14]. Volumetric absorption microsampling (VAM) has been proposed as a microsampling technique and has recently been found to offer superior analyte recovery, stability and homogeneity compared with the traditional DBS method [15–17]. Like CM, however, direct sample analysis from VAM is not possible, requiring extensive sample preparation.
In this work, we present an integrated thread-based microsampling platform capable of (i) direct analysis of collected biological samples without dilution or pre-treatment, (ii) homogeneous distribution of the analyte within the collected microsample, and (iii) dry-state room temperature storage of blood samples, without a change in analyte integrity after prolong storage. Sample collection was achieved simply by dipping the thread substrate directly into the blood. Dried blood samples present on the thread substrates were directly analyzed by thread spray mass spectrometry (MS) [18]. Contrary to other substrate-based ambient ionization methods where the simultaneous application of spray solvent and voltage limits analyte extraction [19–22], we will show that the ability to trigger spray/ionization after a specified analyte enrichment time (via delayed extraction) during thread spray ionization experiment can represent a unique feature in ambient MS, enabling ultra-sensitive analyte detection from raw untreated blood samples. Such a platform provides a facile solution to a long-standing challenge in clinical sample analysis where extensive sample preparation often increases turnaround times and instrument requirements. With the proposed thread-based sampling and analysis technique, we demonstrate the highest level of sensitivity ever reported for any type of direct MS analysis.
2. Methods and Materials
2.1. Chemicals and Reagents
Standard solutions (1.0 mg/mL) of diazepam, benzoylecgonine, cocaine, amphetamine, and (±)-methamphetamine were obtained from Cerilliant (Round Rock, TX) and human blood was purchased from Innovative Research (Novi, MI). (3,3,3-trifiuoropropyl)-silane and acetonitrile (99.9%, HPLC grade) were purchased from Sigma-Aldrich (St. Louis, MO), including standards for ethylene glycol, dimethyl sulfoxide, quinoline, and cyclohexanol that were used in the estimation of surface energy via bracketing. 100% Cotton was purchased from a local store (JoAnn Fabrics, Columbus, OH) and Kimble 51 expansion borosilicate glass melting point capillaries (O.D. 1.5 mm) were purchased from Kimble Chase (Rockwood, TN). A 2% solution of agarose (Sigma) and McCoy’s 5A media (containing 10% fetal bovine serum (FBS) and L-glutamine) were obtained from Dr. Amanda Hummon’s laboratory, OSU.
2.2. Hydrophobic Thread Preparation
Thread (35 mm in length) was cut from the spool and placed in a plastic desiccator with 0.5 mL of silanization reagent (Trichloro(3,3,3-trifiuoropropyl) silane. Vacuum was pulled for 5 minutes, sealed, and then allowed to gas-phase react for a total of (n+5) minutes for each desired treatment time (n = 30 and 60 min). The trichloro(3,3,3-trifiuoropropyl)silane reagent was selected based on preliminary screening in prior studies [23], which indicated that it has high volatility, allowing the silanization chemistry to occur at room temperature without heating.
2.3. Mass Spectrometry
Mass spectra were acquired on a Thermo Fisher Scientific Finnigan LTQ linear ion trap mass spectrometer (San Jose, CA, U.S.A.). The tip of the thread was positioned parallel to the MS inlet via a copper alligator clip, which was connected to an external high-voltage supply (0–6 kV). The thread spray ionization method generates ions without gas assistance so a close interface distance (0.5–5 mm) between the tip and the MS inlet was used to optimize signal intensity. MS parameters used were as follows: 200 °C capillary temperature, 3 microscans, and 60% S-lens voltage. Thermo Fisher Scientific Xcalibur 2.2 SP1 software was applied for MS data collecting and processing. Tandem MS with collision-induced dissociation (CID) was utilized for analyte identification and was optimized for each analyte.
2.4. 3D Laser scanning confocal microscopy
3D surface topographic measurements of the threads were analyzed and captured using the Keyence 3D Laser Scanning Microscope VKX200 (Itasca, IL, U. S. A). Wetted thread was placed on a microscope slide onto the stage then adjusted and focused with 20 objective lenses, where the diameter of thread and sub-fibers were measured. At 20 power objective, optical laser images of the thread surfaces were recorded. 2D imaging occurs by using high-frequency XY laser as a light source with lateral resolution of 408 nm. Thread sample was then observed using 50 objective lenses, where thread topographic analysis was performed. 3D topographic imaging and surface analysis were achieved using objective lens moving in the z direction.
2.5. Doping of diazepam into agarose beads
Agarose beads were stored in a 96-well plate at 37 °C in 200 μL of McCoy’s 5A media with 10% fetal bovine serum (FBS) and L-glutamine. To spike the beads with diazepam, we removed the old, stored media and fresh excess media was doped with varying concentrations of diazepam (50, 100, and 250 ng/mL). The beads were then soaked with 200 μL of the fresh media containing FBS/L-glutamine/diazepam and left overnight before analysis. Only pink colored samples were analyzed because they indicated successful drug infusion.
3. Results and Discussion
3.1. Sampling and Analysis with Untreated Cotton Thread
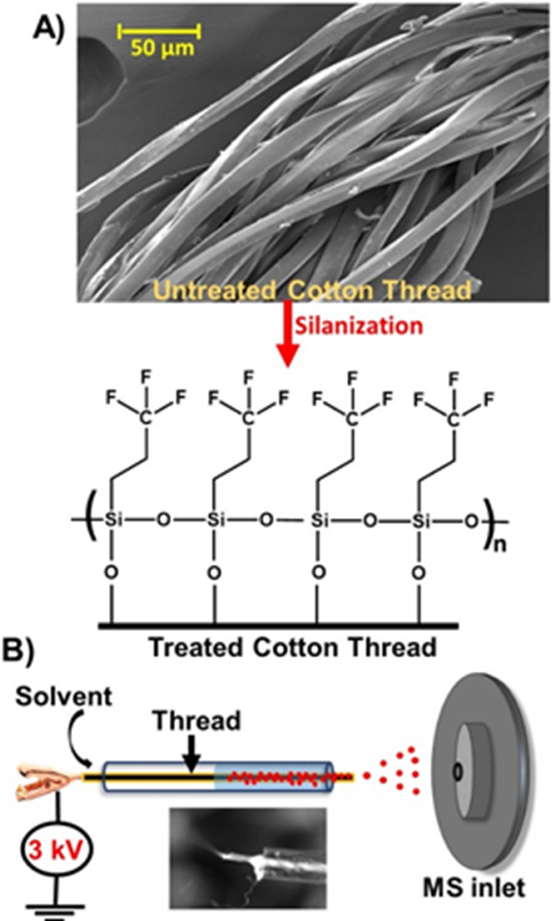
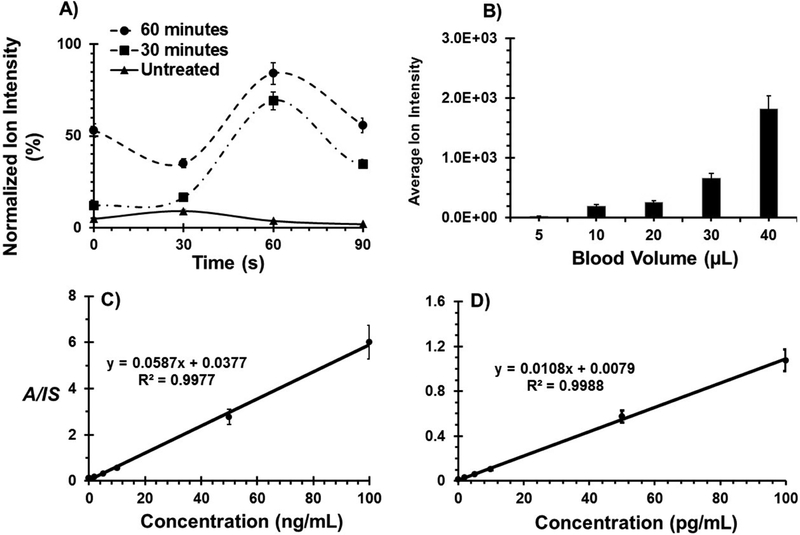
Our previous work on thread spray mass spectrometry focused largely on forensic applications and we optimized various thread types pulled from the corresponding fabrics (without treatment) for direct analysis of capsaicinoids in pepper spray [18]. In the present work, we show excellent results for direct biofluid analysis by using spooled cotton threads. The spooled thread of specific weight (30 or 50 wt, see Supplemental Figure 1) was cut (~35 mm) and used as is (hydrophilic) or after treatment with the vapor of trichloro(3,3,3-trifiuoropropyl) silane reagent, which converted the surface OH groups of the thread into hydrophobic groups. Scanning electron microscope (SEM) image of a section of spooled cotton thread is shown in Figure 1A, accompanied by an illustration of the resultant modified hydrophobic layer afforded by gas-phase silanization, which converts the surface OH groups of the thread into hydrophobic groups [23]. The vertical capillary action on thread substrates allowed for correct volume estimation for blood samples less than 10 μL (Supplemental Figure 2). Unlike paper substrates, that are anisotropic in nature, the individual fibers in thread are unidirectional providing highly controlled fluid flow. For MS analysis of samples present on the thread substrate, we place the thread in a glass capillary and an organic solvent (e.g., ethyl acetate, 20 μL), which is immiscible in water, suitable for electrospray and has high solubilizing power is applied. This selectively extracts organic analytes from the blood sample (Figure 1B), leaving behind the aqueous components present in blood and minimizing their interference in MS analysis. By fitting the threaded glass capillary in front of a mass spectrometer and applying a direct current (DC) voltage, the extracted molecules present in the organic solvent are ionized and transferred into the gas-phase via an electrospray ionization mechanism. With the thread enclosed in a small capillary, solvent evaporation is limited, and extraction time can be effectively controlled, allowing up to 84% of organic compounds such as benzoylecgonine (partition coefficient (LogP) = −0.59) to be extracted from the thread substrate within 60 s using ethyl acetate (Figures 2A; also see Supplemental Data for further discussion).
Figure 1.
A) SEM image of untreated cotton thread and schematic of silane functionalization for hydrophobic surface modification. B) Hydrophobic thread spray setup, a single piece of thread with pre-deposited sample is placed in a glass capillary and a suitable solvent applied. The inset shows Taylor cone formation, after application of onset voltage (3 kV).
Figure 2.
A) Benzoylecgonine ion yield as a function of extraction time after drying analyte onto various threads types: untreated, 30- and 60-min treated substrates. B) Average ion intensity is recorded as a function of blood volume for cocaine deposited on 60-minute treated thread. Calibration curves for diazepam in the concentration range of C) 0.2 – 100 ng/mL and D) 0.2 – 100 pg/mL in 10 μL dried whole blood on untreated and 60-minute treated cotton threads, respectively. Error bars indicate standard deviation for five replicates.
To characterize the performance of the proposed method, we chose to evaluate the sensitivity and stability of diazepam, which is important both in medicine (used for treating pediatric status epilepticus) and forensics (where it is abused with illicit drugs). First, we sampled 10 μL of blood spiked with diazepam using untreated, hydrophilic cotton thread. Direct thread spray MS analysis of these samples using 60 s extraction time resulted in excellent linearity in 0.2 – 100 ng/mL concentration range (Figure 2C). This calibration curve was constructed using isotopically labeled internal standard (IS), and monitoring analyte-to-internal standard ratio (A/IS) in tandem MS mode. Limit of quantification was determined to be 185 part per trillion (185 pg/mL). It was also observed that 10 μL blood volumes travel an average distance of 16 ± 1.7 mm along the hydrophilic thread substrates (Figure S2), which gave a basis for studying analyte distribution. To determine whether diazepam was homogeneously distributed along this distance, the thread substrate containing the blood sample was cut into two equal sections and the ion signal from each section was quantified. Comparable ion yields were recorded from both sections (Figure S3) indicating uniform analyte distribution on the thread substrate, which we attribute to the uniform fluid flow in the unidirectional thread fibers. Further analysis showed that 10X improvement in ion yield was achieved when the sample volume was increased by 4X (Figure 2B).
3.2. Sampling and Analysis with Treated Hydrophobic Threads
The above results motivated us to alter the surface properties of the thread substrate in an attempt to increase analyte availability without increasing sample volume. We adopted a hydrophobic silane treatment that is known to reduce absorption of aqueous-based samples [23]. By using a bracketing method, we characterized the surface energies of the resultant treated hydrophobic threads as <34.4 and <33.0 mN/m for 30- and 60-min silane treatment times, respectively (Figure S4). As expected, limit of quantification (LOQ) for diazepam decreased by 5X when the treated, hydrophobic threads were used for analysis, where the 30 and 60 min treated threads registered 80 and 34 part per trillion LOQs, respectively (Table 1). This increase in sensitivity is attributed to the spatial concentration of the sample at the tip of the hydrophobic thread due to the limited wetting of the blood sample. Extracted analytes do not diffuse much from their original site resulting in relatively higher analyte-to-internal standard ratios during thread spray MS. For untreated hydrophilic thread substrates, the same 10 μL sample volume yields relatively lower ion intensity because the sample is spread over a larger surface area (17 mm2, compared to 0.4 mm2 on hydrophobic thread). This allows for a higher material exchange efficiency with the solvent, and since this process occurs at markedly different positions in the glass capillary, an overall low ion signal is detected due to analyte dilution. Secondary factors contributing to ion yield may include ease of analytes’ extraction and their subsequent redistribution between the solid thread and solvent phases. The influence of both factors is exemplified by the dependence of ion signal on the amount of time the thread is allowed to sit in the extraction solvent (i.e., extraction time). Complete absorption of blood sample into the untreated hydrophilic thread substrate can introduce strong analyte/thread interactions (especially analytes located inside the core of the thread), which may limit extraction. On hydrophobic threads, however, the entire blood sample is adsorbed at the thread surface and completely accessible to solvent. Detailed on-line in-capillary dissolution experiments showed that >60% of diazepam (LogP = 2.82; Figure S5) can be desorbed from a 60-min treated thread within 60 s compared with 84% for the hydrophilic benzoylecgonine analyte (Figure 2A) present on the same 60-min treated thread. This is because the hydrophobic diazepam analyte prefers the hydrophobic medium and is preferentially retained on the hydrophobic thread substrate. Also, after dissolution, there is a higher tendency for diazepam to be redistributed back into the hydrophobic thread, reducing overall amount transferred to the mass spectrometer. Interestingly, we also observed the in-capillary, online dissolution process (for both treated and untreated cotton threads) to be more efficient than the corresponding offline, bulk-phase extraction performed using the same final sample volume (Figure S6). The online processes allow for higher ion yield presumably because of the large interfacial contact between the thread and the small solvent volume. As the thread is fully stretched in the glass capillary during the on-line experiment, the 20 μL solvent volume is able to wet the entire thread, facilitating extraction with an increased analyte-to-solvent ratio. On the other hand, for offline extractions performed in PCR tubes, it was difficult to process the thread (e.g., complete immersion) in the same 20 μL solvent; increasing the solvent volume further increased analyte dilution and hence lowered the ion yield. It should be noted that the used thread can be stored and reanalyzed (Figure S6) providing a unique opportunity to validate results and eliminate the need to increase sample volume via dilution for the same studies.
Table 1.
Limits of detection (LOD) and quantification (LOQ) of illicit and pharmaceutical drugs in 10 μL dried blood samples.
| LOD (LOQ) in pg/mL | ||||
|---|---|---|---|---|
| Diazepam (LogP 2.82) | Amphetamine (LogP 1.76) | Methamphetamine (LogP 2.07) | Cocaine (LogP 2.30) | |
| Untreated | 131 (185) | 37 (65) | 43 (107) | 99 (142) |
| 30 minutes | 43 (80) | 28 (44) | 31 (50) | 44 (67) |
| 60 minutes | 17 (34) | 13 (34) | 16 (22) | 14 (22) |
For the purposes of comparing with other direct ionization methods (e.g., paper spray), and the fact that diazepam is often abused with other illicit drugs, the limits of detection (LOD) and limits of quantification (LOQ) for cocaine, amphetamine and methamphetamine were also determined using the thread spray MS methodology. In all cases, treated hydrophobic threads offered lower LODs and LOQs (Table 1) than direct analysis from untreated thread. Relative standard deviations less than 10% were obtained at all concentrations tested for both treated and untreated threads, and excellent linearity (R2 > 0.999) and reproducibility (as indicated by error bars in the calibrations curves, Figures S7 and S8) were also recorded for all analytes. LODs as low as 13 pg/mL were calculated for amphetamine (Table 1) compared to 60 ng/mL previously determined using hydrophobic paper spray MS [23]. When using hydrophobic paper, analyte dilution due to spreading of aqueous-based samples via capillary action is minimal. Therefore, we attribute the observed increase in sensitivity to the delayed extraction capabilities in thread spray allowing more analytes to be extracted and detected. That is, on planar hydrophobic paper substrates, the ethyl acetate organic spray solvent spread and evaporate very quickly necessitating the DC voltage to be applied simultaneously with the solvent. The concomitant application of spray voltage and solvent, and the continuous MS analysis, lead to low extraction efficiency and an overall reduced signal intensity, compared with the proposed thread spray experiment in which extraction and MS analysis can be decoupled by an optimized 60 s enrichment time.
While the coupling of a separate solid-phase extraction (SPE) process/device with ambient ionization has resulted in improved sensitivity, this combination cannot be used for microsamples since large volumes of biofluid (10 – 250 μL) are required for the SPE step [24–27]. It is important to point out that the SPE-based approaches have not been applied to analyze raw blood; it often utilizes less complex biofluids, such as urine and plasma. The 60 s enrichment time employed here for thread spray ionization experiments can be considered as a form of SPE where the ethyl acetate spray solvent selectively transfers the extracted organic compounds to the mass spectrometer, leaving the bulk of the blood matrix immobilized on the thread substrate. The reduced matrix effects resulted in a very high ion yield (Figures S7 and S8; 60-min treatment – results summarized in Table 1), suggesting that the sensitivity of the proposed thread-based method may be well below the typical parts-per-billion concentrations. Therefore, we sought to characterize the analytical performance of the thread spray MS methodology in a low parts-pertrillion concentration range. Figure 2D shows the calibration curve constructed using 2 – 100 pg/mL standard solutions of diazepam spiked separately in 10 μL of whole blood. This analysis was performed using 60-min treated hydrophobic thread substrates, which provided an unprecedented 25 parts-per-quadrillion detection limit for diazepam (LOQ is 52 fg/mL) without sacrificing linearity, precision and reproducibility. This establishes thread spray as an ultrasensitive ambient ionization technique enabling direct analysis of microsamples by mass spectrometry. The in-capillary sample processing is highly efficient in eliminating matrix effects and yielding results that are comparable to the most sensitive SPE surfaces but without the use of extra washing steps, large sample volumes or specialized accessories like cartridges [22,25,28–30]. It is important to note that the silane self-assembly chemistry used for thread treatment represents one of the surface modification procedures recently developed by us and others to improve the sensitivity of substrate-based ambient ionization techniques without prior sample preparation. These include metal oxide deposition, wax-printing, and direct coating with silica, carbon nanotubes or metal organic frameworks [31–36]. Similar to results observed with treated thread substrates, most of the reported surface treatment methods afforded enhanced sensitivity for complex mixture analysis.
3.3. Dry- State Blood Storage on Thread Substrates
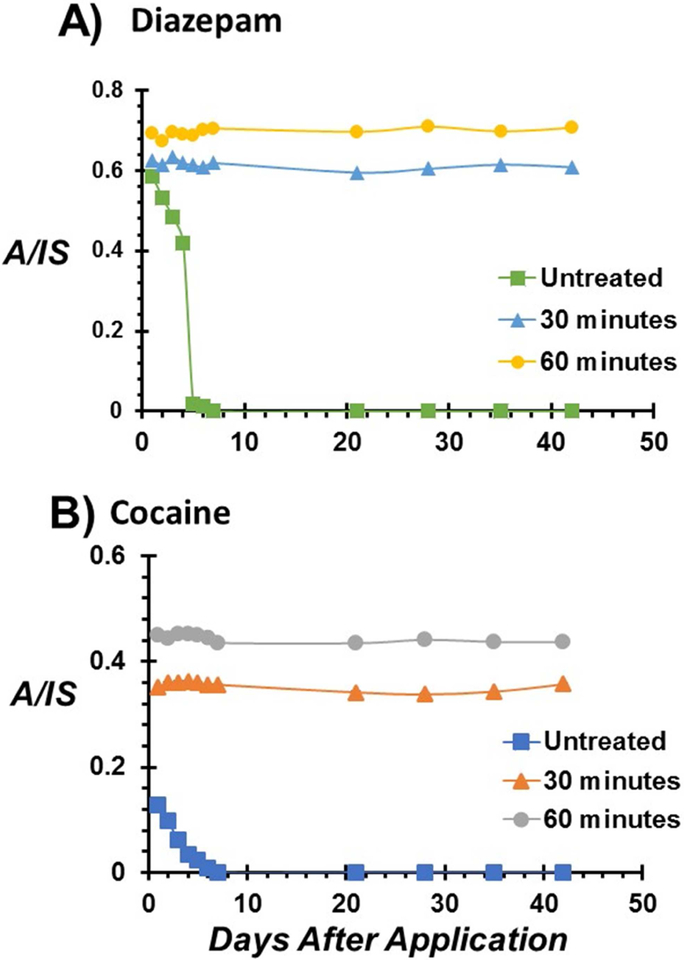
Storage is the main determining factor governing the stability of diazepam in blood. While varied diazepam stability has been reported under freezer (–20°C) storage conditions [37], most studies agree on severe degradation when stored at room temperature [38–40]. Therefore, we investigated diazepam stability in blood after storage on treated and untreated thread substrates. As already indicated, the rapid flow of aqueous samples on untreated, hydrophilic thread (radius 175 μm) results in the distribution of the 10 μL blood over a surface area of 17 mm2 compared with 0.4 mm2 for treated, hydrophobic threads (see Supporting Information for details, Scheme S1). This increased surface area-to-volume ratio predisposes majority of labile diazepam analytes present on the untreated thread substrate to oxidation. Diazepam degraded in less than 5 days after blood storage at room temperature on the untreated hydrophilic thread (Figure 3A). On the contrary, the spatial concentration of 10 μL blood to a small area, as a blood spheroid, when stored on hydrophobic threads provided improved analyte stability (Figure 3A). In this case, near-surface molecules provide transient passivation leading to limited thermal and/or oxidant flux into the sample and thus mitigating oxidation of analyte within the core of the stored blood over the entire six week period. Similar stability profiles were observed for cocaine in whole blood storage on untreated, hydrophilic versus treated, hydrophobic thread substrates (Figure 3B). In all cases, the relative ion signal (A/IS) derived from the 60-min treated threads was higher than signal from the 30 min treated thread substrates. This is likely due to the higher ionization efficiency from the more hydrophobic thread substrate. However, the marked reduction (~57%) in the cocaine signal after the first day of storage on hydrophilic thread is consistent with rapid degradation in a storage environment without a protective surface layer, as observed in dried blood spot samples [6,41]. The ability to detect diazepam and cocaine in biofluid samples over an extended period without sacrificing sensitivity or the integrity of the sample has important implications for forensic and clinical applications. In addition, the thread-based sampling and storage methodology uses small sample volumes and does not require special storage conditions, making it ideal for field studies.
Figure 3.
Stability profiles of A) diazepam and B) cocaine in 10 μL dried whole blood on untreated, 30 minutes treated, and 60 minutes treated thread substrates stored over a six-week period under ambient conditions. Error bars represent five replicates.
3.4. Tissue Sampling with Treated Hydrophobic Thread
Diazepam is also commonly analyzed in postmortem biochemical investigations due to its role in accidental overdoses. The concentration of diazepam changes rapidly after death due to decomposition and redistribution phenomena so sensitive analytical methods for tissue samples can serve to complement biofluid analyses. To demonstrate this concept, we used soft tissue-mimicking agarose beads (5 mm, ID) that exhibit high water uptake and controllable permeation for oxygen and nutrients [42–45]. They were stored in a 96-well plate at 37 °C in 200 μL of McCoy’s 5A media with 10% FBS and L-glutamine, which we removed to enable diazepam infusion. For spiking of the analyte into agarose beads, excess media was doped with varying concentrations of diazepam (50, 100, and 250 ng/mL) before adding 200 μL to each well. This drug-doped media was infused into the soft, porous agarose beads overnight before taking a threaded needle to punch through each sample (Figure 4A). Pink colored samples were deemed viable for analysis because they reflected the pink color of the media, suggesting that diffusion of the analyte into the beads was successful, and were the only samples used for analysis. Small agarose bead residues collected on the 60-min thread, after punching through the bead sample, were analyzed directly by thread spray MS.
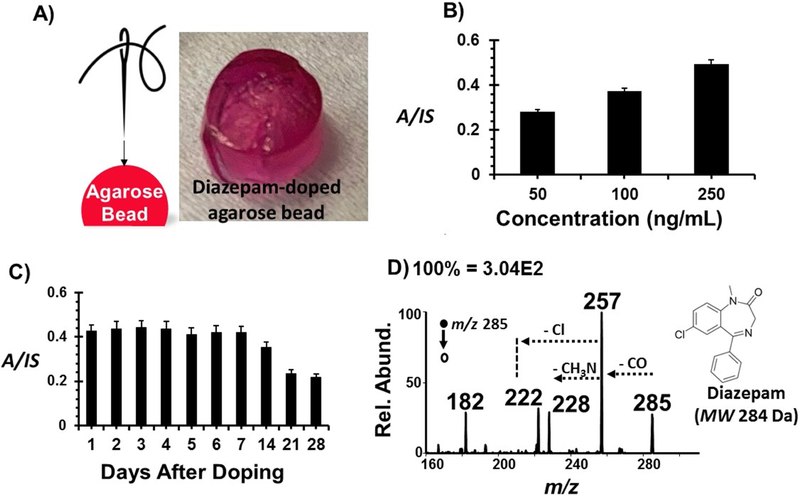
Figure 4.
A) Photograph showing a viable tissue-mimicking agarose bead soaked with McCoy’s 5A media with 10% FBS and L-glutamine. Tissues sampling occurred via 60 minutes treated thread suspended on a needle. B) Concentration dependence of diazepam in agarose beads. The ratio of diagnostic ions for diazepam and its isotopically-labelled species were plotted. Error bars represent three replicates. C) Stability profile of agarose beads infused with 250 ng/mL diazepam over a four-week period. Error bars represent five replicates. D) Representative tandem MS spectrum of 100 ng/mL diazepam doped agarose bead sample.
Similar to what we have seen with blood, there is an increase in signal intensity as the concentration increases (Figure 4B), suggesting this method is capable of detecting varying analyte concentrations with statistical significance. Note that, unlike blood analysis where the whole sample is subjected to extraction, here only a small fraction of the agarose bead is collected, and yet intense ions are detected for diazepam via tandem MS (Figure 4D). In this case, collision-induced dissociation was employed producing a diagnostic fragment ion at m/z 257 via CO (MW 28) neutral loss, which further dissociated to give ions at 228 and 222 through the elimination of nitrine (CH3N; MW 29) and chlorine (Cl; MW 35) species. To further explore sensitivity for this application, a stability test, in dry conditions, was performed. Fresh agarose beads were doped with diazepam (250 ng/mL) as described above and left overnight for analyte infusion. After the initial 24-hour period, diazepam-doped media was removed, and the dry agarose beads were stored at 37°C for four weeks. Removal of the media was done to mimic post-mortem conditions for tissue storage [37,46], where proper nutrients are not available to keep them viable. Analysis for this study included daily sampling in the first week followed by weekly analyses for the subsequent weeks. Diazepam signal was stable in the first week of storage suggesting the agarose samples stayed viable. There was a visible change from a pink to brown color after the second week, which may indicate the onset of oxidation of the agarose beads. This fact was reflected in the thread spray MS signal, where a noticeable drop in ion yield was continuously detected after this point, (Figure 4C). This gradual degradation of the agarose samples, and in turn the analyte, was expected due to the porous nature of the agarose beads and, with the steady detection and identification of diazepam in these samples, further validates thread spray as a sensitive technique that could be used for post-mortem analyses.
4. Conclusion.
In conclusion, through the hydrophobic treatment of cotton threads and subsequent encapsulation in glass capillary – enabling controlled solvent evaporation and extraction – we have established thread spray mass spectrometry as ultrasensitive platform for quantitative analysis of small blood volumes. Most importantly, this study shows that cotton threads can be used as an all-in-one substrate for sample collection, storage, and direct analysis. The observed parts-per-quadrillion detection limit make it an attractive alternative to other substrate-based ambient methods with unique features as demonstrated in the tissue-like sampling for postmortem biochemical investigations. Both untreated, hydrophilic and treated, hydrophobic thread substrates are found viable for biological fluid analyses. The advantages of surface modifications, uniform diffusion, and online analyte enrichment capabilities directly influence the analytical performance of the proposed method. We expect these to benefit both biomedical and translational research.
Supplementary Material
Highlights.
Functionalized cotton thread is proposed for blood sampling, storage and analysis.
Thread-based microsampling platform enables extended dry-state blood storage.
Threads act as solid-phase extraction media for ultra-sensitive detection.
Threads enable tissue sampling and quantitative analysis by mass spectrometry.
ACKNOWLEDGMENT
This research was supported by NIH grant R35-GM-133647, research award from the American Society for Mass spectrometry and young investigator award by Eli Lilly and Company. The authors thank Dr. Amanda Hummon’s lab at The Ohio State University for assistance with diazepam infusion into agarose beads.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website. Details on ionization efficiency, thread diffusion, surface energies, comparison of ion yield from different thread surfaces, and calibration curves. (.docx)
Declaration of Interest Statement
The authors declare no competing financial interest.
REFERENCES
- [1].De Souza YG; Greenspan JS Biobanking Past, Present and Future: Responsibilities and Benefits. AIDS Lond. Engl 2013, 27 (3), 303–312. 10.1097/QAD.0b013e32835c1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kinkorová J Biobanks in the Era of Personalized Medicine: Objectives, Challenges, and Innovation. EPMA J. 2016, 7 (1). 10.1186/s13167-016-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peakman T; Elliott P Current Standards for the Storage of Human Samples in Biobanks. Genome Med. 2010, 2 (10), 72 10.1186/gm193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gómez-Ríos GA; Pawliszyn J Development of Coated Blade Spray Ionization Mass Spectrometry for the Quantitation of Target Analytes Present in Complex Matrices. Angew. Chem. Int. Ed 2014, 53 (52), 14503–14507. 10.1002/anie.201407057. [DOI] [PubMed] [Google Scholar]
- [5].Piri-Moghadam H; Lendor S; Pawliszyn J Development of a Biocompatible In-Tube Solid-Phase Microextraction Device: A Sensitive Approach for Direct Analysis of Single Drops of Complex Matrixes. Anal. Chem 2016, 88 (24), 12188–12195. 10.1021/acs.analchem.6b03160. [DOI] [PubMed] [Google Scholar]
- [6].Liu G; Ji QC; Jemal M; Tymiak AA; Arnold ME Approach To Evaluating Dried Blood Spot Sample Stability during Drying Process and Discovery of a Treated Card To Maintain Analyte Stability by Rapid On-Card PH Modification. Anal. Chem 2011, 83 (23), 9033–9038. 10.1021/ac2023876. [DOI] [PubMed] [Google Scholar]
- [7].Xie I; Xu Y; Anderson M; Wang M; Xue L; Breidinger S; Goykhman D; Woolf EJ; Bateman KP Extractability-Mediated Stability Bias and Hematocrit Impact: High Extraction Recovery Is Critical to Feasibility of Volumetric Adsorptive Microsampling (VAMS) in Regulated Bioanalysis. J. Pharm. Biomed. Anal 2018, 156, 58–66. 10.1016/j.jpba.2018.04.001. [DOI] [PubMed] [Google Scholar]
- [8].Iv JHM; Poston PA; Rutan SC; H TK An On-Card Approach for Assessment of Hematocrit on Dried Blood Spots Which Allows for Correction of Sample Volume. J. Anal. Bioanal. Tech 2013, 4 (1). 10.4172/2155-9872.1000162. [DOI] [Google Scholar]
- [9].Demirev PA Dried Blood Spots: Analysis and Applications. Anal. Chem 2013, 85 (2), 779–789. 10.1021/ac303205m. [DOI] [PubMed] [Google Scholar]
- [10].Tanna S; Lawson G Analytical Methods Used in Conjunction with Dried Blood Spots. Anal. Methods 2011, 3 (8), 1709–1718. 10.1039/C1AY05160A. [DOI] [Google Scholar]
- [11].Yannell KE; Kesely KR; Chien HD; Kissinger CB; Cooks RG Comparison of Paper Spray Mass Spectrometry Analysis of Dried Blood Spots from Devices Used for In-Field Collection of Clinical Samples. Anal. Bioanal. Chem 2017, 409 (1), 121–131. 10.1007/s00216-016-9954-5. [DOI] [PubMed] [Google Scholar]
- [12].Malm J; Fehniger TE; Danmyr P; Végvári Á; Welinder C; Lindberg H; Appelqvist R; Sjödin K; Wieslander E; Laurell T; et al. Developments in Biobanking Workflow Standardization Providing Sample Integrity and Stability. Spec. Issue Stand. Qual. Control Proteomics 2013, 95, 38–45. 10.1016/j.jprot.2013.06.035. [DOI] [PubMed] [Google Scholar]
- [13].Lapierre F; Gooley A; Breadmore M Principles around Accurate Blood Volume Collection Using Capillary Action. Langmuir 2017, 33 (50), 14220–14225. 10.1021/acs.langmuir.7b02825. [DOI] [PubMed] [Google Scholar]
- [14].Hubel A; Aksan A; Skubitz APN; Wendt C; Zhong X State of the Art in Preservation of Fluid Biospecimens. Biopreservation Biobanking 2011, 9 (3), 237–244. 10.1089/bio.2010.0034. [DOI] [PubMed] [Google Scholar]
- [15].Denniff P; Spooner N Volumetric Absorptive Microsampling: A Dried Sample Collection Technique for Quantitative Bioanalysis. Anal. Chem 2014, 86 (16), 8489–8495. 10.1021/ac5022562. [DOI] [PubMed] [Google Scholar]
- [16].Verougstraete N; Lapauw B; Van Aken S; Delanghe J; Stove C; Stove V Volumetric Absorptive Microsampling at Home as an Alternative Tool for the Monitoring of HbA1c in Diabetes Patients. Clin. Chem. Lab. Med 2017, 55 (3), 462–469. 10.1515/cclm-2016-0411. [DOI] [PubMed] [Google Scholar]
- [17].Kovač J; Panic G; Neodo A; Meister I; Coulibaly JT; Schulz JD; Keiser J Evaluation of a Novel Micro-Sampling Device, Mitra™, in Comparison to Dried Blood Spots, for Analysis of Praziquantel in Schistosoma Haematobium-Infected Children in Rural Côte d’Ivoire. J. Pharm. Biomed. Anal 2018, 151, 339–346. 10.1016/j.jpba.2018.01.030. [DOI] [PubMed] [Google Scholar]
- [18].Jackson S; J. Swiner D; C. Capone P; Badu-Twaiah A Thread Spray Mass Spectrometry for Direct Analysis of Capsaicinoids in Pepper Products. Anal. Chim. Acta 2018. 10.1016/j.aca.2018.04.008. [DOI] [PubMed] [Google Scholar]
- [19].Tang S; Zhang H; Lee HK Advances in Sample Extraction. Anal. Chem 2016, 88 (1), 228–249. 10.1021/acs.analchem.5b04040. [DOI] [PubMed] [Google Scholar]
- [20].van Hout MW; Hofiand CM; Niederländer HA; de Jong GJ On-Line Coupling of Solid-Phase Extraction with Mass Spectrometry for the Analysis of Biological Samples. II. Determination of Clenbuterol in Urine Using Multiple-Stage Mass Spectrometry in an Ion-Trap Mass Spectrometer. Rapid Commun. Mass Spectrom. RCM 2000, 14 (22), 2103–2111. . [DOI] [PubMed] [Google Scholar]
- [21].Fang L; Deng J; Yang Y; Wang X; Chen B; Liu H; Zhou H; Ouyang G; Luan T Coupling Solid-Phase Microextraction with Ambient Mass Spectrometry: Strategies and Applications. TrAC Trends Anal. Chem 2016, 85, Part A, 61–72. 10.1016/j.trac.2016.05.025. [DOI] [Google Scholar]
- [22].Zhang C; Manicke NE Development of a Paper Spray Mass Spectrometry Cartridge with Integrated Solid Phase Extraction for Bioanalysis. Anal. Chem 2015, 87 (12), 6212–6219. 10.1021/acs.analchem.5b00884. [DOI] [PubMed] [Google Scholar]
- [23].Damon DE; Davis KM; Moreira CR; Capone P; Cruttenden R; Badu-Tawiah AK Direct Biofluid Analysis Using Hydrophobic Paper Spray Mass Spectrometry. Anal. Chem 2016, 88 (3), 1878–1884. 10.1021/acs.analchem.5b04278. [DOI] [PubMed] [Google Scholar]
- [24].Jian W; Romm MV; Edom RW; Miller VP; LaMarr WA; Weng N Evaluation of a High-Throughput Online Solid Phase Extraction–Tandem Mass Spectrometry System for In Vivo Bioanalytical Studies. Anal. Chem 2011, 83 (21), 8259–8266. 10.1021/ac202017c. [DOI] [PubMed] [Google Scholar]
- [25].Wachs T; Henion J A Device for Automated Direct Sampling and Quantitation from Solid-Phase Sorbent Extraction Cards by Electrospray Tandem Mass Spectrometry. Anal. Chem 2003, 75 (7), 1769–1775. 10.1021/ac020501y. [DOI] [PubMed] [Google Scholar]
- [26].Kennedy JH; Aurand C; Shirey R; Laughlin BC; Wiseman JM Coupling Desorption Electrospray Ionization with Solid-Phase Microextraction for Screening and Quantitative Analysis of Drugs in Urine. Anal. Chem 2010, 82 (17), 7502–7508. 10.1021/ac101295g. [DOI] [PubMed] [Google Scholar]
- [27].Poole CF New Trends in Solid-Phase Extraction. TrAC Trends Anal. Chem 2003, 22 (6), 362–373. 10.1016/S0165-9936(03)00605-8. [DOI] [Google Scholar]
- [28].Vasiljevic T; Gómez-Ríos GA; Pawliszyn J Single-Use Poly(Etheretherketone) Solid-Phase Microextraction–Transmission Mode Devices for Rapid Screening and Quantitation of Drugs of Abuse in Oral Fluid and Urine via Direct Analysis in Real-Time Tandem Mass Spectrometry. Anal. Chem 2018, 90 (1), 952–960. 10.1021/acs.analchem.7b04005. [DOI] [PubMed] [Google Scholar]
- [29].Cai Y-Q; Jiang G-B; Liu J-F; Zhou Q-X Multi-Walled Carbon Nanotubes Packed Cartridge for the Solid-Phase Extraction of Several Phthalate Esters from Water Samples and Their Determination by High Performance Liquid Chromatography. Anal. Chim. Acta 2003, 494 (1), 149–156. 10.1016/j.aca.2003.08.006. [DOI] [Google Scholar]
- [30].Huck CW; Bonn GK Recent Developments in Polymer-Based Sorbents for Solid-Phase Extraction. J. Chromatogr. A 2000, 885 (1), 51–72. 10.1016/S0021-9673(00)00333-2. [DOI] [PubMed] [Google Scholar]
- [31].Colletes TC; Garcia PT; Campanha RB; Abdelnur PV; Romão W; Coltro WKT; Vaz BG A New Insert Sample Approach to Paper Spray Mass Spectrometry: A Paper Substrate with Paraffin Barriers. Analyst. 2016, 141 (5), 1707–1713. 10.1039/C5AN01954K. [DOI] [PubMed] [Google Scholar]
- [32].Basuri P; Baidya A; Pradeep T Sub-Parts-per-Trillion Level Detection of Analytes by Superhydrophobic Preconcentration Paper Spray Ionization Mass Spectrometry (SHPPSI MS). Anal. Chem 2019, 91 (11), 7118–7124. 10.1021/acs.analchem.9b00144. [DOI] [PubMed] [Google Scholar]
- [33].Damon DE; Maher YS; Yin M; Jjunju FPM; Young IS; Taylor S; Maher S; Badu-Tawiah AK 2D Wax-Printed Paper Substrates with Extended Solvent Supply Capabilities Allow Enhanced Ion Signal in Paper Spray Ionization. Analyst 2016, 141 (12), 3866–3873. 10.1039/C6AN00168H. [DOI] [PubMed] [Google Scholar]
- [34].Wang Q; Zheng Y; Zhang X; Han X; Wang T; Zhang Z A Silica Coated Paper Substrate: Development and Its Application in Paper Spray Mass Spectrometry for Rapid Analysis of Pesticides in Milk. Analyst. 2015, 140 (23), 8048–8056. 10.1039/C5AN01823D. [DOI] [PubMed] [Google Scholar]
- [35].Wang X; Zheng Y; Wang T; Xiong X; Fang X; Zhang Z Metal–Organic Framework Coated Paper Substrates for Paper Spray Mass Spectrometry. Anal. Methods 2016, 8 (45), 8004–8014. 10.1039/C6AY02123A. [DOI] [Google Scholar]
- [36].Narayanan R; Sarkar D; Cooks RG; Pradeep T Molecular Ionization from Carbon Nanotube Paper. Angewandte Chemie International Edition. 2014, 53 (23), 5936–5940. 10.1002/anie.201311053. [DOI] [PubMed] [Google Scholar]
- [37].Gottwald MD; Akers LC; Liu P-K; Orsulak PJ; Corry MD; Bacchetti P; Fields SM; Lowenstein DH; Alldredge BK Prehospital Stability of Diazepam and Lorazepam. Am. J. Emerg. Med 1999, 17 (4), 333–337. 10.1016/S0735-6757(99)90079-7. [DOI] [PubMed] [Google Scholar]
- [38].Atanasov VN; Stoykova S; Runiov A; Dimitrova T; Aleksandrova D; Tsakovski S; Mitewa M Stability of Diazepam in Blood Samples at Different Storage Conditions and in the Presence of Alcohol. 48th Annu. Meet. Int. Assoc. Forensic Toxicol. TIAFT Jt. Meet. Soc. Toxicol. Forensic Chem. GTFCh 2012, 215 (1), 159–163. 10.1016/j.forsciint.2011.04.005. [DOI] [PubMed] [Google Scholar]
- [39].Mata DC Stability of 26 Sedative Hypnotics in Six Toxicological Matrices at Different Storage Conditions. J. Anal. Toxicol 2016, 40 (8), 663–668. 10.1093/jat/bkw084. [DOI] [PubMed] [Google Scholar]
- [40].Wu F; Marin SJ; McMillin GA Stability of 21 Cocaine, Opioid and Benzodiazepine Drug Analytes in Spiked Meconium at Three Temperatures. J. Anal. Toxicol 2017, 41 (3), 196–204. 10.1093/jat/bkw113. [DOI] [PubMed] [Google Scholar]
- [41].Damon DE; Yin M; Allen DM; Maher YS; Tanny CJ; Oyola-Reynoso S; Smith BL; Maher S; Thuo MM; Badu-Tawiah AK Dried Blood Spheroids for Dry-State Room Temperature Stabilization of Microliter Blood Samples. Anal. Chem 2018. 10.1021/acs.analchem.8b01962. [DOI] [PubMed] [Google Scholar]
- [42].Campos F; Bonhome-Espinosa AB; García-Martínez L; Durán JDG; López-López MT; Alaminos M; Sánchez-Quevedo MC; Carriel V Characterization of a Novel Tissue-like Cross-Linked Fibrin-Agarose Hydrogel for Tissue Engineering Applications. Biomed. Mater 2016, 11 (5), 055004-. 10.1088/1748-6041/11/5/055004. [DOI] [PubMed] [Google Scholar]
- [43].Chen Z-J; Gillies GT; Broaddus WC; Prabhu SS; Fillmore H; Mitchell RM; Corwin FD; Fatouros PP A Realistic Brain Tissue Phantom for Intraparenchymal Infusion Studies. J. Neurosurg 2004, 101 (2), 314–322. 10.3171/jns.2004.101.2.0314. [DOI] [PubMed] [Google Scholar]
- [44].Levine B; Blanke R; Valentour J Postmortem Stability of Benzodiazepines in Blood and Tissues. Postmortem Stab. Benzodiazepines Blood Tissues 1983. [PubMed] [Google Scholar]
- [45].Shiota H; Nakashima M; Terazono H; Sasaki H; Nishida K; Nakamura J; Taniyama K Postmortem Changes in Tissue Concentrations of Triazolam and Diazepam in Rats. Leg. Med 2004, 6 (4), 224–232. 10.1016/j.legalmed.2004.05.006. [DOI] [PubMed] [Google Scholar]
- [46].Petković S; Simić M; Vujić D Postmortem Production of Ethanol in Different Tissues Under Controlled Experimental Conditions. Postmortem Prod. Ethanol Differ. Tissues Control. Exp. Cond 2005. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.