TO THE EDITOR
Germline mutations in EXPH5 are associated with a recessive form of epidermolysis bullosa (EB), with skin fragility that cannot be attributed to mutations in KRT5, KRT14, or other established EB-related genes (McGrath et al., 2012, Pigors et al., 2014). Clinically identified mutations in EXPH5 result in premature truncations in the encoded protein Exophilin-5 (also referred to as Slac2-b). This is associated with mild blistering, which in some cases is accompanied by mottled hypopigmentation. EXPH5 knockout mice are not available. The normal physiologic role of Exophilin-5 in epidermis, and the mechanism by which Exophilin-5 loss contributes to skin disease are unclear.
Exophilin-5, an effector of Rab27, is implicated in intracellular vesicular trafficking and secretion (Ostrowski et al., 2010). Therefore, we hypothesized that Exophilin-5 may be required in keratinocytes for the normal vesicular trafficking of lamellar bodies (LBs) that extrude lipids into the extracellular space during normal epidermal differentiation. As LBs contain enzymes and membrane features associated with lysosomes, these secretory vesicles are also classified as Lysosome Related Organelles (LROs) (Eckhart et al., 2013, Raymond et al., 2008).
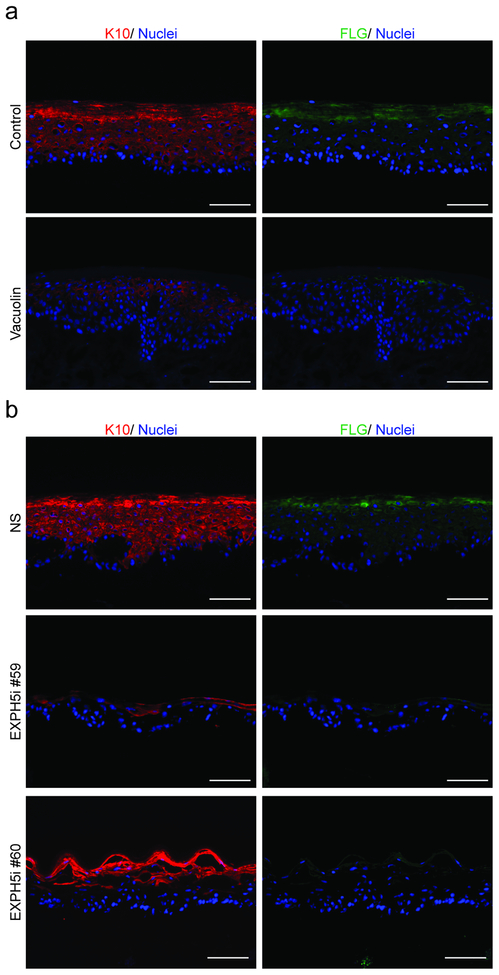
To define the role of Exophiln-5 and lysosomal exocytosis in human epidermal homeostasis, we inhibited the LRO exocytosis trafficking pathway both pharmacologically and genetically, in three-dimensional human organotypic skin cultures (OTCs) engineered using primary keratinocytes and devitalized human dermis (Ridky et al., 2010). Control tissues stratified and differentiated normally, as evidenced by the coordinated expression of keratin-10 and filaggrin (Figure 1a). However, epidermal differentiation was inhibited in the presence of vacuolin, a small molecule that blocks the fusion of secretory LROs with the plasma membrane (Figure 1a) (Supplementary Figure S1). To specifically define the role of EXPH5 in lysosomal trafficking in keratinocytes, and to test whether EXPH5 is required for normal epidermal homeostasis, we genetically depleted EXPH5 using two different shRNAs (Supplementary Figure S2). In contrast to the well-differentiated epidermis observed in the Non-Silenced (NS) shRNA control, tissues with EXPH5 depletion (EXPH5i) were poorly differentiated, as evidenced by loss of keratin-10 and filaggrin (Figure 1b, Supplementary Figure S2). Consistent with the skin fragility phenotype associated with EXPH5 germline mutation, organotypic epidermis lacking EXPH5 was also hypoproliferative, and lacked the uniform, peripheral distribution of Desmoglein-3 seen throughout the keratinocyte plasma membranes in normal control samples. Normal expression and localization of collagen VII and β1 integrin demonstrates that architecture at the dermal-epidermal junction was grossly intact in EXPH5- depleted tissue. Together, these data demonstrate that normal epidermal differentiation and proliferation depend on EXPH5, and delivery of LROs to the keratinocyte plasma membrane.
Figure 1: Delivery of Lysosome Related Organelles to the Plasma Membrane is essential for normal keratinocyte differentiation.
(a) Control organotypic human epidermis differentiated properly, including the spatially coordinated expression of keratin-10 (red) and filaggrin (green), nuclei (blue). Differentiation was inhibited when lysosome-mediated trafficking was blocked with Vacuolin (10 μM) (b) shRNA knockdown of Exophilin-5 (EXPH5i) resulted in similarly diminished epidermal differentiation, keratin-10 (red) and filaggrin (green), nuclei (blue). (Scale Bars = 100um).
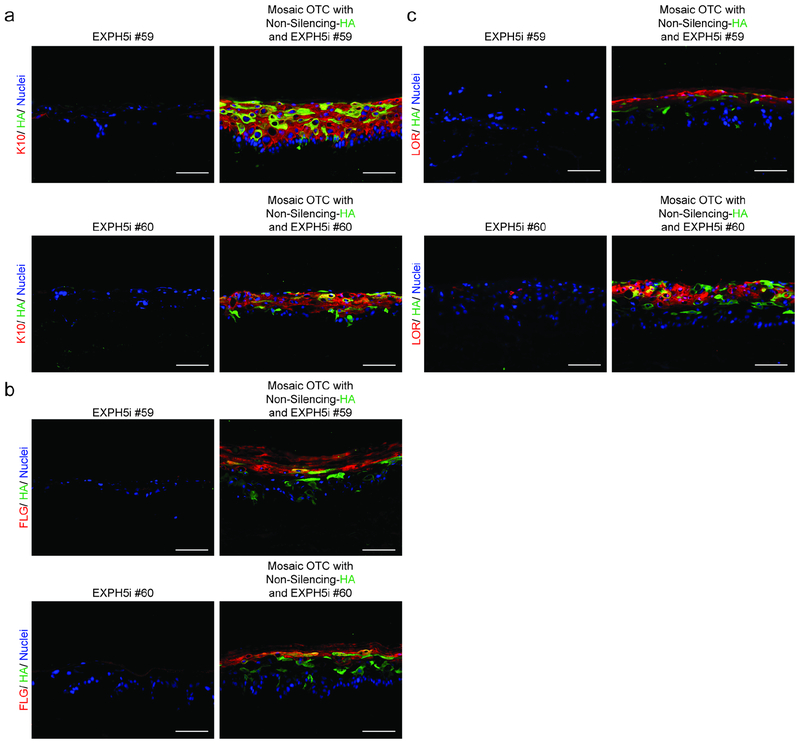
To determine whether secreted LROs from differentiating keratinocytes communicate with adjacent cells to promote differentiation, we assembled mosaic OTCs containing and equal mixture of EXPH5i and Non-Silenced (NS) keratinocytes. Epidermal differentiation was rescued in EXPH5i keratinocytes when they were co-cultured with NS keratinocytes (tagged with K14- HA), as evidenced by the expression of both early and late differentiation proteins including keratin-10, filaggrin, and loricrin (Figure 2, Supplementary Figure S4). To test whether this ability of normal keratinocytes to rescue differentiation in trans was specific to EHPH5 deficiency, we pharmacologically inhibited epidermal differentiation using Lys05, a lysosome inhibitor that we previously determined inhibits differentiation (Monteleon et al., 2018). As we observed with EXPH5 depletion, differentiation was also restored in Lys05 treated keratinocytes when co-cultured with an equal number of normal keratinocytes (Supplementary Figure S5).
Figure 2: The differentiation defect in keratinocytes with lysosomal defects is rescued in trans by co-culture with normal keratinocytes.
(a) Organotypic epidermis engineered with EXPH5i keratinocytes yielded undifferentiated tissue lacking keratin 10 (red). In contrast, mosaic tissue engineered using an equal mixture of EXPH5i keratinocytes and control NS keratinocytes (tagged with K14-HA, green) expressed keratin 10 (red) in both normal and EXPH5i keratinocytes. (b, c) Expressions of filaggrin and loricrin are also restored in EXPH5i- keratinocytes when co-cultured with NS-keratinocytes. (Scale Bars = 100um).
LRO-lamellar bodies contain complex cargo, and it is unlikely that rescue in trans results from trafficking of a single factor. Lysosome-associated enzymes contribute to the biosynthesis of ceramide, formation of the cornified envelope, and the proteolytic degradation that facilitates corneocyte desquamation (Egberts et al., 2004). It has also been demonstrated that secretory cargo initiates signaling back to keratinocytes through pathways that are not well understood (Appelqvist et al., 2013, Conus et al., 2012, Kovalenko et al., 2009). Interestingly, the signals that stimulate lysosomes to traffic as exocytic vesicles (Appelqvist et al., 2013, Jans et al., 2004), principally the sustained elevation of cytoplasmic calcium and increased oxidative stress, are the same factors responsible for initiating keratinocyte differentiation, and therefore may be inextricably linked.
Many different physical, chemical, and genetic factors can lead to dysfunction in LB/LRO assembly or trafficking in keratinocytes. Disruption of normal LB secretion compromises the lipid barrier, which renders skin more susceptible to dehydration, mechanical stress, and infiltration by microbes, and contributes to functional barrier defects in skin disorders including eczema and ichthyosis (Elias and Wakefield, 2014, Milner et al., 1992, Rizzo et al., 2010, Werner et al., 1986). LROs are also necessary for packaging and trafficking of melanin, which may be partially responsible for the mottled pigmentation in patients with EXPH5-EB (Turcan et al., 2016).
This current work furthers our understanding of epidermal homeostasis, and suggests that keratinocyte differentiation within epidermis may not be a purely cell-autonomous process. Specifically, cell-cell communication via lysosome-mediated exocytosis may contribute to both early and late differentiation. The capacity for normal keratinocytes to rescue epidermal differentiation in trans in adjacent keratinocytes with defective lysosomal exocytosis suggests that future gene therapy approaches for some genetic skin disorders may require correction of only a subset of keratinocytes, rather than the entire epidermal keratinocyte population.
MATERIALS AND METHODS
Cell culture
All experiments were conducted using primary keratinocytes. Cells were isolated from normal human skin by previously described methods (Ridky et al., 2010). Keratinocytes were cultured in a 1:1 mixture of Gibco Keratinocytes-SFM medium + L-glutamine + EGF + BPE and Gibco Cascade Biologics 154 medium with 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA). Transduced keratinocytes were fully puromycin selected before the commencement of each experiment. Lys05 (Gift from R. Amavaradi at U. of Pennsylvania) was used at 2 μM. Vacuolin (Sigma, St. Louis, MO) was used at 2 μM. Ionomycin (Sigma) was used at 30 μM. LysoSensor (Molecular Probes, Carlsbad, CA) was used at 1 μM.
Lentiviral and Retroviral Production and Transduction
293T or 293T phoenix cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 5% FBS containing Antibiotic/Antimycotic. Lentiviral shRNA (OpenBiosystems, Lafayette, CO) particles were generated according to Thermoscientific specifications and as described previously (Ridky et al., 2010). For the production of viral particles, lentiviral constructs were co-transfected with viral packaging plasmids pCMVΔR8.91 and pUC-MDG into 293T cells using Fugene 6 Transfection Reagent (Promega, Fitchburg, WI). Retroviral particles, made from a phoenix cells carrying a LZRS viral vector expressing human K14 with a c-terminal HA tag, were used to transduce keratinocytes in order to label with HA.
Organotypic cultures
Organotypic skin cultures were established using parental or genetically engineered keratinocytes. For each culture, between 8.0 × 105 and 1.0 × 106 keratinocytes were suspended in 80 μL KGM or high calcium (1.2uM CaCl2) growth media, and seeded onto devitalized human dermis, according to previously established methods (Ridky et al., 2010). Unless otherwise indicated, small molecule and other chemical treatments were begun at seeding. OTCs were maintained at 37 °C at an air-liquid interface for 4–12 days.
Immunofluorescence microscopy
Whole mount cryosections were prepared for immunofluorescence microscopy as previously described (Ridky et al., 2010). In short, slides were fixed in 4% paraformaldehyde or −20°C methanol, permeabilized as required and blocked with 10% horse serum/PBS, followed by incubation with primary antibodies and secondary antibodies conjugated to fluorophores. Slides were mounted with Prolong Gold Antifade Reagent with DAPI (Life Technologies, Grand Island, NY). The primary antibodies used in this study were collagen-VII (Millipore, Burlington, MA), ß1-integrin (Abcam, Cambridge, MA), ki67, desmoglein-3 (Thermo Fisher Scientific, Carlsbad, CA), HA, keratin-10, keratin-5, loricrin, and filaggrin (Covance, Dedham, MA). To quantify differentiation, the ratio of the area in pixels of keratin-10 positive epidermis to area in pixels of total epidermis was measured as a percentage in ImageJ. This analysis was based previously reported methods (Billings et al., 2015, Natale et al., 2018). Results are the mean of at least 3 technical replicates across at least three biologic replicates from individual donors (± s.d.). Significance was assessed by Welch’s t-test across the biological replicates.
Quantitative RT/PCR
RNA was extracted from cells and tissues according to the RNeasy Mini Kit protocol (Qiagen, Valencia, CA), and reverse transcribed to cDNA using the High Capacity RNA-to cDNA kit (Applied Biosystems, Grand Island, NY). Quantitative PCR of resulting cDNA was conducted using Power SYBR Green Master Mix (Applied Biosystems, Grand Island, NY) and gene-specific primers, with three technical replicates on a ViiA 7 Real-Time PCR System (Life Technologies, Grand Island, NY). Relative expression was determined using the 2-[delta][delta] Ct method. Results are the average from at least two individual donors (± s.d.).
DATA AVAILABILITY STATEMENT
Datasets related to this article can be found at http://dx.doi.org/10.17632/82zk6kdx6c.1, hosted at Mendeley (Lee, In Young (2019), “Role of Exophilin-5 in keratinocyte differentiation”, Mendeley Data, v1).
Supplementary Material
Supplementary Figure S1: Vacuolin inhibits epidermal differentiation, the expression of differentiation markers, and lysosome-mediated traffic to the plasma membrane. (a) Epidermal keratinocyte differentiation in control and vacuolin treated organotypic cultures was determined by quantifying the area of keratin-10 positive epidermis relative to that of the total epidermis. Inhibition of lysosome-mediated exocytosis diminished epidermal differentiation (* indicates significance from Control, p <0.05). (b) Quantitative PCR shows that vacuolin inhibits the calcium-induced expression of keratin-1 and filaggrin. (Data represents three independent experiments repeated with duplicates. *** indicates significance from control, p<0.001. **** indicates significance from control, p<0.0001.) (c) 2 μM Vacuolin, for 24-hours, blocked ionomycin-induced trafficking of lysosomes, shown with LysoSensor (green), to the plasma membrane (white arrows). (Scale Bars = 50um)
Supplementary Figure S2: shRNA mediated Exophilin-5 knockdown diminishes epidermal differentiation and lysosome-mediated traffic to the plasma membrane. (a) Differentiation in control and EXPH5i organotypic cultures was determined by quantifying the area of keratin-10 positive epidermis relative to the total epidermal area. Expohilin-5 depletion inhibited epidermal differentiation (* indicates significance from NS, p <0.05 and ** indicates significance from NS, p<0.01). (b) Exophilin-5 knockdown, and the associated decrease in calcium-induced Keratin-1 expression, was determined by qPCR. (Data represents at least two independent experiments repeated with duplicates. *** indicates significance from NS, p<0.001). (c) EXPH5 depletion diminished ionomycin-induced peripheral lysosome trafficking (LysoSensor, green), to plasma membrane (white arrows). (Scale Bars = 50um)
Supplementary Figure S3: Exophilin-5 depletion in organotypic epidermis inhibits proliferation and desmoglein localization, but does not grossly disrupt the basement membrane. (a) The epidermal architecture in NS and EXPH5i organotypic cultures was evaluated by examining Keratin-5 (red), collagen-VII (green), (b) ß1-integrin (red), collagen-VII (green), and (c) desmoglein-3 (green), and ki67(red). Representative fields were analyzed for percentage of ki67 positive basal cells (* indicates significance from NS, p <0.05).
Supplementary Figure S4: Expression of keratin-10 is restored in EXPH5i-keratinocytes co-cultured with normal NS control keratinocytes. (a) Organotypic epidermis engineered with non-silenced keratinocytes (tagged with K14-HA, green) differentiated normally (keratin-10, red). (b, c) EXPH5i OTCs were undifferentiated, lacking keratin-10 (red). (d, e) Mosaic tissues engineered from an equal mixture of control NS keratinocytes and EXPH5i keratinocytes expressed Keratin-10 (red) in both normal and EXPH5i keratinocytes. (Scale Bars = 100um).
Supplementary Figure S5: Lys05-induced block in epidermal differentiation is rescued in trans. (Scale Bars = 100um). Keratinocytes were pre-treated with Lys05 (Monteleon et al., 2018) for 48 hours and then incorporated into OTCs, where they were incapable of differentiating as shown by the lack of (a) keratin-10, (b) filaggrin, and (c) loricrin expression. In contrast, mosaic OTCs engineered using Lys05-pretreated keratinocytes mixed with normal keratinocytes (tagged with HA, green), differentiated normally.
Acknowledgements
The authors thank the University of Pennsylvania Skin Biology and Diseases Resource-based Center (SBDRC), funded by 1P30AR069589–01 (Millar) for technical assistance. Primary cells were obtained through the SBDRC core from deidentified discarded material though an IRB approved protocol. This work was supported by The National Institutes of Health (NIH) (R01CA163566, T.W.R.), CLM was partially supported by an NIH/NIAMS training grant (T32AR007465).
Abbreviations:
- EB
Epidermolysis Bullosa
- EXPH5
Exophilin-5
- LBs
lamellar bodies
- OTC
Organotypic Culture
- NS
Non-silenced
- LRO
Lysosome Related Organelle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT
Datasets related to this article can be found at http://dx.doi.org/10.17632/82zk6kdx6c.1, hosted at Mendeley (Lee, In Young (2019), “Role of Exophilin-5 in keratinocyte differentiation”, Mendeley Data, v1).
Conflict of Interest
The authors have no conflict of interest.
REFERENCES
- Appelqvist H, Wäster P, Eriksson I, Rosdahl I, Öllinger K. Lysosomal exocytosis and caspase-8-mediated apoptosis in UVA-irradiated keratinocytes. J Cell Sci 2013;126(24):5578–84. [DOI] [PubMed] [Google Scholar]
- Conus S, Pop C, Snipas SJ, Salvesen GS, Simon H-U. Cathepsin D primes caspase-8 activation by multiple intra-chain proteolysis. Journal of Biological Chemistry 2012;287(25):21142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2013;1833(12):3471–80. [DOI] [PubMed] [Google Scholar]
- Egberts F, Heinrich M, Jensen JM, Winoto-Morbach S, Pfeiffer S, Wickel M, et al. Cathepsin D is involved in the regulation of transglutaminase 1 and epidermal differentiation. J Cell Sci 2004;117(Pt 11):2295–307. [DOI] [PubMed] [Google Scholar]
- Elias PM, Wakefield JS. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol 2014;134(4):781–91 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans R, Sartor M, Jadot M, Poumay Y. Calcium entry into keratinocytes induces exocytosis of lysosomes. Archives of dermatological research 2004;296(1):30–41. [DOI] [PubMed] [Google Scholar]
- Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. The Journal of experimental medicine 2009;206(10):2161–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JA, Stone KL, Begum R, Simpson MA, Dopping-Hepenstal PJ, Liu L, et al. Germline mutation in EXPH5 implicates the Rab27B effector protein Slac2-b in inherited skin fragility. The American Journal of Human Genetics 2012;91(6):1115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner ME, O’Guin WM, Holbrook KA, Dale BA. Abnormal lamellar granules in harlequin ichthyosis. J Invest Dermatol 1992;99(6):824–9. [DOI] [PubMed] [Google Scholar]
- Monteleon CL, Agnihotri T, Dahal A, Liu M, Rebecca VW, Beatty GL, et al. Lysosomes Support the Degradation, Signaling, and Mitochondrial Metabolism Necessary for Human Epidermal Differentiation. J Invest Dermatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature cell biology 2010;12(1):19–30. [DOI] [PubMed] [Google Scholar]
- Pigors M, Schwieger-Briel A, Leppert J, Kiritsi D, Kohlhase J, Bruckner-Tuderman L, et al. Molecular heterogeneity of epidermolysis bullosa simplex: contribution of EXPH5 mutations. Journal of Investigative Dermatology 2014;134(3):842–5. [DOI] [PubMed] [Google Scholar]
- Raymond A-A, de Peredo AG, Stella A, Ishida-Yamamoto A, Bouyssie D, Serre G, et al. Lamellar Bodies of Human Epidermis Proteomics Characterization by High Throughput Mass Spectrometry and Possible Involvement of CLIP-170 in their Trafficking/Secretion. Molecular & Cellular Proteomics 2008;7(11):2151–75. [DOI] [PubMed] [Google Scholar]
- Ridky TW, Chow JM, Wong DJ, Khavari PA. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat Med 2010;16(12):1450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo WB, S’Aulis D, Jennings MA, Crumrine DA, Williams ML, Elias PM. Ichthyosis in Sjogren-Larsson syndrome reflects defective barrier function due to abnormal lamellar body structure and secretion. Arch Dermatol Res 2010;302(6):443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcan I, Pasmooij AM, Van den Akker PC, Lemmink H, Sinke RJ, Jonkman MF. Association of Epidermolysis Bullosa Simplex With Mottled Pigmentation and EXPH5 Mutations. JAMA Dermatol 2016;152(10):1137–41. [DOI] [PubMed] [Google Scholar]
- Werner Y, Lindberg M, Forslind B. Membrane-coating granules in” dry” non-eczematous skin of patients with atopic dermatitis. A quantitative electron microscopic study. Acta dermato-venereologica 1986;67(5):385–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Vacuolin inhibits epidermal differentiation, the expression of differentiation markers, and lysosome-mediated traffic to the plasma membrane. (a) Epidermal keratinocyte differentiation in control and vacuolin treated organotypic cultures was determined by quantifying the area of keratin-10 positive epidermis relative to that of the total epidermis. Inhibition of lysosome-mediated exocytosis diminished epidermal differentiation (* indicates significance from Control, p <0.05). (b) Quantitative PCR shows that vacuolin inhibits the calcium-induced expression of keratin-1 and filaggrin. (Data represents three independent experiments repeated with duplicates. *** indicates significance from control, p<0.001. **** indicates significance from control, p<0.0001.) (c) 2 μM Vacuolin, for 24-hours, blocked ionomycin-induced trafficking of lysosomes, shown with LysoSensor (green), to the plasma membrane (white arrows). (Scale Bars = 50um)
Supplementary Figure S2: shRNA mediated Exophilin-5 knockdown diminishes epidermal differentiation and lysosome-mediated traffic to the plasma membrane. (a) Differentiation in control and EXPH5i organotypic cultures was determined by quantifying the area of keratin-10 positive epidermis relative to the total epidermal area. Expohilin-5 depletion inhibited epidermal differentiation (* indicates significance from NS, p <0.05 and ** indicates significance from NS, p<0.01). (b) Exophilin-5 knockdown, and the associated decrease in calcium-induced Keratin-1 expression, was determined by qPCR. (Data represents at least two independent experiments repeated with duplicates. *** indicates significance from NS, p<0.001). (c) EXPH5 depletion diminished ionomycin-induced peripheral lysosome trafficking (LysoSensor, green), to plasma membrane (white arrows). (Scale Bars = 50um)
Supplementary Figure S3: Exophilin-5 depletion in organotypic epidermis inhibits proliferation and desmoglein localization, but does not grossly disrupt the basement membrane. (a) The epidermal architecture in NS and EXPH5i organotypic cultures was evaluated by examining Keratin-5 (red), collagen-VII (green), (b) ß1-integrin (red), collagen-VII (green), and (c) desmoglein-3 (green), and ki67(red). Representative fields were analyzed for percentage of ki67 positive basal cells (* indicates significance from NS, p <0.05).
Supplementary Figure S4: Expression of keratin-10 is restored in EXPH5i-keratinocytes co-cultured with normal NS control keratinocytes. (a) Organotypic epidermis engineered with non-silenced keratinocytes (tagged with K14-HA, green) differentiated normally (keratin-10, red). (b, c) EXPH5i OTCs were undifferentiated, lacking keratin-10 (red). (d, e) Mosaic tissues engineered from an equal mixture of control NS keratinocytes and EXPH5i keratinocytes expressed Keratin-10 (red) in both normal and EXPH5i keratinocytes. (Scale Bars = 100um).
Supplementary Figure S5: Lys05-induced block in epidermal differentiation is rescued in trans. (Scale Bars = 100um). Keratinocytes were pre-treated with Lys05 (Monteleon et al., 2018) for 48 hours and then incorporated into OTCs, where they were incapable of differentiating as shown by the lack of (a) keratin-10, (b) filaggrin, and (c) loricrin expression. In contrast, mosaic OTCs engineered using Lys05-pretreated keratinocytes mixed with normal keratinocytes (tagged with HA, green), differentiated normally.
Data Availability Statement
Datasets related to this article can be found at http://dx.doi.org/10.17632/82zk6kdx6c.1, hosted at Mendeley (Lee, In Young (2019), “Role of Exophilin-5 in keratinocyte differentiation”, Mendeley Data, v1).