Abstract
The role of stem cells in normal and neoplastic hematopoiesis is well established. However, neither normal nor neoplastic hematopoietic stem cells (HSCs) develop in isolation and accumulating evidence indicates that a critical developmental role is played by the perivascular “niche.” The cellular, humoral, and cell surface contacts that provide the proper environment for HSC survival, proliferation, and differentiation are becoming increasingly better understood. A number of studies have established that endothelial cells (ECs), several types of perivascular stromal cells, and megakaryocytes (MKs) provide several cell surface and secreted molecules required for HSC development. Accumulating evidence also indicates that the normal stem cell niche is altered in patients with hematological neoplasms and that the “neoplastic niche” plays an important role in promoting malignant and suppressing normal blood cell development in such patients. To explore this concept in the myeloproliferative neoplasms (MPNs), we employed a murine model to determine the effects of Jak2V617F, an oncogene found in a majority of such patients, in marrow ECs and MKs and their effect on promoting neoplastic and suppressing normal hematopoiesis. We found that Jak2V617F has profound effects on both cell types, which together are critical for the growth advantage and radioresistance shown by Jak2V617F-bearing HSCs. Such findings should provide new approaches to the treatment of patients with MPNs.
Each day, the adult human produces approximately 5 × 1011 blood cells, a number that can increase 10- to 20-fold in times of heightened demand. This remarkably prolific and adaptable system arises from a relatively small pool of hematopoietic stem cells (HSCs), rare marrow cells that have the capacity, not only to proliferate and differentiate into multiple distinct types of blood cells, but also to self-renew and repopulate all of hematopoiesis. Although there remains some controversy over the precise pathway that any given stem cell takes to become one or more of the mature types of blood cells, there is little doubt that the cells require the proper environment (the hematopoietic vascular niche) to perform these functions and, should a single HSC acquire one of a variety of somatic mutations, it can ultimately give rise to a hematological malignancy. As an illustrative example, this review will focus on the myeloproliferative neoplasms (MPNs) polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), three disorders in which an activating Jak2 kinase mutation, Jak2V617F, plays a critical, pathogenetic role in from 50–100% of patients with these disorders. It is the central theme of this review that Jak2V617F affects both stem cells and vascular cells. At the risk of providing the punch line before the story, evidence is presented that the development of MPNs requires both an abnormal, mutation-bearing HSC and an abnormal, mutation-bearing stem cell niche.
Much has been written about the hematopoietic niche. Stem cell niches were first postulated to exist in the 1970s based on the discovery of clonal (i.e., single-cell derived) splenic colonies of multiple cell types upon transplantation of marrow cells into lethally irradiated mice [1]. Studies 35–40 years later suggested that there were two anatomically defined stem cell niches, the periosteal niche adjacent to bony trabeculae of the marrow and the vascular niche adjacent to the capillary endothelial cells of marrow sinusoids [2]. Most current evidence favors the latter site as critical, and much literature focuses on the contributions of three distinct cell types to the stem cell niche, endothelial cell (ECs), and perivascular stromal cells, some of which express nestin and leptin receptors [2]. Evidence indicates that marrow megakaryocytes (MKs) must be added to this cadre of nurturing niche cells. One such line of evidence comes from a seemingly simple, control experiment.
Murine model of a myeloproliferative neoplasm
Several investigators have created murine models of myeloproliferative neoplasms (MPNs) by introducing a transgene for Jak2V617F into the germline and then activating the mutation with a specific Cre recombinase. Based on work in the Skoda laboratory, we employed a murine model of MPN possessing an activatable Jak2V617F transgene (FF1) [3] and two Cre recombinases: Tie2 Cre, which is expressed in HSCs and ECs [4] and when crossed to FF1 mice was expected to create a robust MPN, and platelet factor 4 (PF4) Cre, which is expressed exclusively in MKs and platelets [5] and expected to produce, at most, modest thrombocytosis, serving as a control. Although the transgene present in the FF1 mouse contains nine copies of the human Jak2V617F cassette, in our study, the level of human Jak2V617F expressed in murine hematopoietic cells is only a fraction of the level of their endogenous, wild-type (WT) murine Jak2.
As predicted, driving Jak2V617F expression in HSCs and ECs produced a robust MPN, with peripheral blood neutrophilia and thrombocytosis, massive splenomegaly, and HSC expansion. When Jak2V617F expression was restricted to MKs, modest thrombocytosis ensued, with platelet counts approximately twofold, marrow CD41+ cells threefold, and MK colony-forming cells twofold normal levels [6]. What was unexpected was the myeloproliferative effect of Jak2V617F-expressing MKs. Granulocyte–macrophage and erythroid progenitor cells (CFU-GM and BFU-E, respectively) were each elevated twofold and the number of CD45+/CD201+/CD150+/CD48− (E-SLAM positive) marrow cells, a population of cells highly enriched in HSCs, threefold that seen in the marrow of PF4Cre control mice. When an equal mixture of PF4Cre/FF1 or PF4Cre (CD45.2) marrow cells were transplanted together with CD45.1 WT competitor marrow cells into lethally irradiated, normal recipient mice, the number of blood cells derived from the PF4Cre/FF1 donor was threefold higher than blood cells derived from the control, proving a threefold increase in functional HSCs in mice expressing Jak2V617F restricted to MKs. Rigorous, extremely sensitive reverse transcription polymerase chain reaction (PCR) assays eliminated the possibility that HSCs in PF4Cre/FF1 mice express the mutant kinase. Together with the studies of others using alternate experimental strategies [7,8], as well as the perivascular location of many marrow MKs, the MK is now an established component of the HSC niche.
Fifty-four years before the identification of Jak2V617F, William Dameshek postulated that three MPNs—PV, ET, and PMF—were pathogenetically related [9]. In 1974, Jeff Prchal identified that marrow cells from patients with PV could form erythroid cells in cultures without the addition of exogenous growth-promoting factors [10]. Until that time, a myriad of blood cell types could be grown in tissue culture, but only upon the addition of growth factors derived from certain tissue-culture-conditioned medium or spleen-conditioned medium or, later, with purified erythropoietin or other hematopoietic growth factors. Such “endogenous erythroid colonies” (EECs) were pathognomonic of PV and, later, the same phenomenon was shown to be true for marrow and blood cells from patients with ET and PMF [11,12]. Hypersensitivity to growth factors, or autocrine growth, was postulated to account for the finding of EECs and the presence of EECs was considered definitive evidence of an MPN.
Another major milestone in the pathogenesis of MPNs (and another hematological conundrum, thrombopoiesis) came in 1992, when Vigon et al. [13] reported that the cellular proto-oncogene related to the murine myeloproliferative leukemia virus (MPLV) had all of the hallmarks of a hematopoietic growth factor receptor. Because there was no obvious corresponding ligand, c-Mpl was termed an orphan cytokine receptor. Two years later, several groups nearly simultaneously reported that the c-Mpl ligand was the long sought after stimulator of platelet production, thrombopoietin (TPO) [14,15]. Subsequent studies by us and others, as well as both murine knock-out and human studies, established that the c-Mpl/TPO receptor/cytokine system was critical for HSC survival and expansion [16,17]. The first link between c-Mpl and MPNs came in 1996, when Li et al. [18] reported that antisense oligonucleotide-mediated knock-down of c-Mpl expression in marrow cells from patients with MPNs reduced endogenous colony formation. Together with the presence of c-Mpl on HSCs [17], this finding led us, and eventually others, to suggest that c-Mpl was necessary for the formation of MPNs [19].
Jak2V617F signaling kinase
Postulations regarding the pathogenetic relatedness of the various MPNs, first suggested in 1951 by Dameshek [9], were definitively established in 2005 with the finding of the presence of the constitutively active mutant kinase Jak2V617F in virtually all patients with PV and about half of patients with ET and PMF [20—22]. This finding potentially called into question a role for c-Mpl in the MPNs because introduction of a Jak2V617F-bearing transgene in marrow cells produced polycythemia in mice [20]. However, because all members of the Jak family of signaling kinases act by binding to the cytoplasmic domain of one or more cytokine receptors such as c-Mpl, and because c-Mpl is expressed in the cell of origin of all MPNs, the HSC, it remained possible that c-Mpl served as the vital scaffold upon which Jak2V617F acts. To test this hypothesis, as noted previously (Murine Model of a Myeloproliferative Neoplasm), a murine model of MPN was generated by crossing Jak2V617F FF mice with mice bearing a Tie2-Cre, in which the recombinase is expressed in both HSCs and ECs [23]. The rationale for including EC expression in our murine model of MPNs was based on findings from Sozer et al. [24], later corroborated and expanded by Rosti and colleagues [25], that Jak2V617F is expressed in several types of endothelium of patients with MPNs. As predicted, introduction of Jak2V617F into HSCs and ECs produced a robust MPN characterized by profound thrombocytosis and neutrophilia, splenomegaly, and marrow with greatly expanded numbers of MKs, erythroid and leukocyte progenitors, and transplantable HSCs. These mice eventually developed myelosclerosis at about age 8–12 months. We then eliminated the expression of c-Mpl in these mice through extensive back-crossing. All signs of disease were eliminated, and the marrow and blood resembled that seen in c-Mpl-null mice [26]. Others have reported a similar dependence of MPN on c-Mpl through alternate experimental approaches (e.g., in PMF) [27].
Central role of c-Mpl in Jak2V617F-induced and other MPNs
As noted, the presence of Jak2V617F accounts for approximately 75% of all patients with an MPN. What of the remainder? Since the discovery of Jak2V617F, several additional mutations have been identified in “Jak2V617F-negative” patients with an MPN. Approximately 5–10% of these patients have a mutation in c-Mpl, some have an alternate activating mutation in Jak2 in a different exon, also likely working through c-Mpl, but the most common Jak2V617F-negative MPN mutation, first reported in 2013, is in the C-terminus of the calreticulin (CALR) gene [28]. Although there are numerous reported mutations, all are in the final coding exon of the gene and all are predicted to eliminate the C-terminus endoplasmic reticulum anchor sequence, thereby releasing the truncated gene product to interact with other cellular proteins. Several investigators have now shown that the pathogenesis of CALR-mutation-induced MPNs is dependent on its interaction with c-Mpl [29]. Therefore, it takes two (i.e., either Jak2V617F or CALR, along with c-Mpl) to “tango” (i.e., develop an MPN).
Role of endothelial cell Jak2V617F in MPNs
The second example of “taking two to tango” comes from our Tie2Cre/Jak2V617F model of MPN. As noted previously, ECs make up a critical part of the HSC niche and ECs in patients with Jak2V617F-bearing MPNs express the mutant kinase in their ECs [24,25]. Therefore, one of the hypotheses that we hoped to address in our Tie2/Jak2V617F MPN is whether the niche is an important part of a Jak2V617F-induced MPN.
The concept that the HSC niche is altered in hematological malignancies was developed in the late 1990s, when marrow vascular density was found to be greatly increased in patients with hematological malignancies [30,31]. Subsequent studies demonstrated that genetic changes in the niche can initiate and propagate hematopoietic malignancies and, conversely, malignant cells from both animal models and humans with leukemia alter niche cell functions to reinforce hematopoietic-cell-based diseases. In fact, remodeling of the BM microenvironment has emerged as an important event in the development of blood malignancies and is involved in controlling the maintenance and activity of disease-initiating leukemic stem cells and their progeny [32].
Myeloproliferation is dependent on Jak2V617F expression in both HSCs and ECs
To test the effect of Jak2V617F-bearing ECs on normal and Jak2V617F-bearing HSCs, we conducted both in vitro (tissue culture) and in vivo (mixed transplantations) experiments. WT or JAK2V617F marrow line-age-negative/c-Kit positive (Lin−/cKit+) cells were cultured on a monolayer of normal or JAK2V617F ECs; no difference between WT and JAK2V617F Lin−/Kit+ cell proliferation was noted when co-cultured on WT ECs. In contrast, JAK2V617F Lin−/cKit+ displayed a relative growth advantage over the corresponding WT Lin−/cKit+ cells when co-cultured on JAK2V617F-bearing ECs [33].
To test for a competitive growth advantage in vivo, we performed competitive marrow transplantation experiments. Donor marrow cells from CD45.2 Tie2+/FF1+ mice were injected intravenously together with an equal number of CD45.1 WT marrow cells into either lethally irradiated CD45.2 Tie2+/FF1+ mice (i.e., ECs were Jak2V617F-positive) or CD45.2 WT (i.e., ECs were Jak2 WT) recipients. During a 4-month follow up, Tie2+/FF1+ recipients displayed a twofold greater level of peripheral blood JAK2V617F-bearing cells than WT cells, whereas WT recipients displayed an equal number of JAK2V617F and WT blood cells. Moreover, JAK2V617F recipients developed an MPN phenotype with neutrophilia, thrombocytosis, and moderate splenomegaly [34]. In contrast, no apparent disease developed in the WT recipients of a mixture of the mutant and WT donor cells. Consistent with these results, quantitative evaluation of the marrow 4 months after transplantation revealed significant increases in JAK2V617F mutant-bearing CD150+CD48− cells, a population of cells highly enriched in HSCs (~20% display long-term repopulating capacity), in Tie2+/FF1+ recipients compared with WT recipients. Therefore, the JAK2V617F-bearing vascular niche promoted the expansion of JAK2V617F HSCs but not WT cells.
Jak2V617F expression in the niche leads to HSC radioresistance
Relapse following myeloablative, radiation-based transplantation of patients with MPNs is a common occurrence. We hypothesized that this relative radioresistance of MPNs could be due to the JAK2V617F-bearing vascular niche. To investigate the effects of the JAK2V617F-bearing vascular niche on the response of HSCs to irradiation, we transplanted CD45.1 WT marrow cells directly into lethally irradiated (950 cGy, a dose that we have found virtually always results in full donor engraftment) CD45.2 Tie2+/FF1+ mice or CD45.2 WT recipients. During a 3-month follow up, whereas all WT recipients displayed full donor (CD45.1) engraftment, ~60% of recipients with a JAK2V617F-bearing vascular niche displayed mixed donor/recipient chimerism 10 weeks after irradiation. Therefore, resident JAK2V617F-bearing HSCs were relatively protected in the JAK2V617F-bearing vascular niche compared with a WT vascular niche from an otherwise lethal dose of irradiation administered during marrow cell transplantation. Because this result could have been due to relative radioresistance of the JAK2V617F-bearing HSCs or to an effect of the JAK2V617F-bearing vascular niche on HSCs, we next tested posttransplantation WT marrow cells for radiation sensitivity in a WT recipient compared with JAK2V617F-bearing marrow cells transplanted into a WT mouse. We found that, compared with WT cells in a WT niche, JAK2V617F hematopoietic cell apoptosis following 300 cGy irradiation was significantly greater in a WT niche (Figure 2B [35]). In contrast, when WT marrow cells were transplanted into a WT recipient or a JAK2V617F-bearing recipient and then irradiated following engraftment, marrow cell apoptosis was reduced by 40% in JAK2V617F recipients compared with WT recipients. Therefore, intrinsic radioresistance of JAK2V617F-bearing marrow cells did not seem to account for the radiation resistance of JAK2V617F HSCs in JAK2V617F recipients; rather, it is the niche that counts.
To more directly assess radiation sensitivity, Lin− marrow cells were isolated from WT or primary JAK2V617F mice and cultured on primary EC monolayers derived from WT or JAK2V617F-bearing murine lungs. The co-cultures were then irradiated with 300 cGy and the hematopoietic cells were counted and then placed in colony-forming assays 24 hours after irradiation. We observed higher total hematopoietic cell numbers and colony-forming progenitors from JAK2V617F marrow cells cultured on JAK2V617F-bearing ECs compared with those cultured on WT ECs, again indicating that the JAK2V617F-bearing vascular niche contributed directly to JAK2V617F-mutant marrow cell radioprotection. Taken together, these data indicate that the JAK2V617F-bearing vascular niche contributes directly to HSC radioprotection, which could be responsible for the high incidence of disease relapse in patients undergoing allogeneic stem cell transplantation for MPNs.
To determine the cellular basis of how JAK2V617F promotes radioresistance and supports the neoplastic JAK2V617F-bearing HSCs, we compared the activity of JAK2V617F ECs with those of WT in several assays. We found that the presence of JAK2V617F enhanced the proliferation of ECs twofold, reduced radiation-induced apoptosis 2.5-fold, and enhanced tube formation (as a measure of in vitro angiogenesis) [33], indicating that the mutant kinase could act to expand the vascular niche in JAK2V617F-positive mice, findings that also characterize the marrow of patients with MPNs [36].
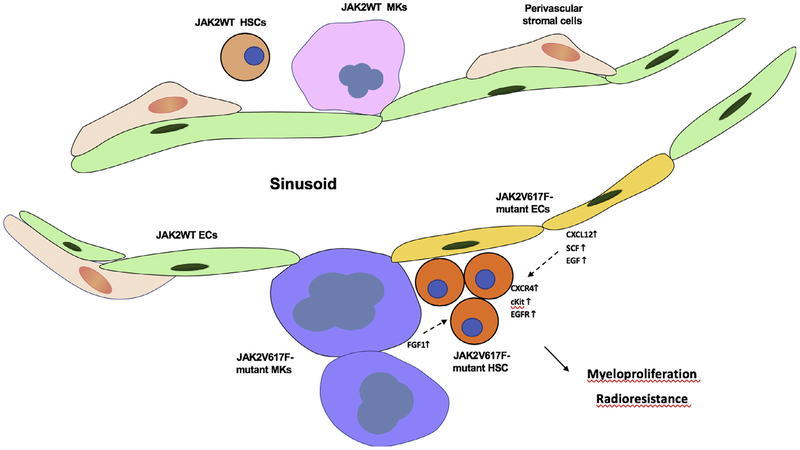
Next, to determine the molecular basis for the enhanced malignant HSC support provided by Jak2V617F ECs, we assessed two signaling systems known to play an important role in HSC biology, CXCL12/CXCR4 and SCF/c-Kit [37–40]. We found that the expression of CXCL12 and SCF were both modestly increased in freshly isolated marrow ECs from Tie2Cre/Jak2V617F-bearing mice compared with WT mice [34] Figure 1. Moreover, and perhaps more importantly, the proportion of primitive hematopoietic cells (CD150+CD48−) expressing the CXCL12 receptor CXCR4 or the SCF receptor c-Kit were increased twofold and 10-fold, respectively, in the marrow of JAK2V617F compared with WT mice. Therefore, the increased CXCL12 and SCF levels in the JAK2V617F-bearing ECs could contribute to the clonal expansion of JAK2V617F HSCs via the upregulated CXCR4 and c-Kit receptors over that seen in normal animals. To determine whether this same mechanism might be at least in part responsible for the relative radioresistance seen in Tie2Cre/Jak2V617F-bearing mice, the expression levels of CXCL12 and two additional growth factors important for HSCs, epidermal growth factor and pleiotrophin, were also upregulated in irradiated JAK2V617F ECs compared with WT ECs [35].
Figure 1.
JAK2V617F-bearing MKs and ECs promote JAK2V617F-mutant HSC expansion and radioresistance in myeloproliferative neoplasms. Shown are the cytokines and cytokine receptors that are altered in JAK2V617F-expressing HSCs, MKs, and ECs in the cited experimental studies proposed to contribute to MPNs.
As noted previously, elimination of the c-Mpl receptor abrogated Jak2V617F-mediated MPN in our murine model [26]. Because elimination of c-Mpl greatly reduces marrow MKs [16] and marrow MKs contributes to the marrow vascular niche [41], we sought to determine whether c-Mpl might also exert niche function through its expression on ECs [42,43].
We measured EC c-Mpl expression by quantitative PCR and found that, compared with WT cells, the receptor was upregulated in JAK2V617F-bearing lung and marrow ECs [32,34]. We also examined the effect of Tpo on EC function in vitro and found that the cytokine significantly stimulated EC cell migration and enhanced the expression of the EC junction molecules ZO-1 and PECAM1 (platelet endothelial cell adhesion molecule or CD31) in a dose-dependent fashion [6].
To test directly whether EC c-Mpl affects Jak2V617F-bearing HSC expansion through soluble factors in the perivascular niche, we designed an in vitro competitive growth assay in which Lin−/cKit+ cells from either WT or JAK2V617F were cultured together (1:1 mixture) in the presence of EC conditioned medium (ECCM) collected from either WT or c-Mpl−/− murine lung ECs. At the end of the 6-day culture, WT and JAK2V617F cells remained 1:1 when cultured in WT ECCM compared with a 4:1 output of WT cells over JAK2V617F cells when grown in the presence of Mpl−/− ECCM. [32]. This observation suggests that growth factors secreted from ECs affect JAK2V617F clonal expansion in MPNs and that c-Mpl signaling is critical for this EC function. In support of this hypothesis, we found that CXCL12 expression was reduced by half in c-Mpl−/− marrow ECs compared with WT ECs. Because CXCL12 is important in localizing MKs and HSCs to the perivascular niche, the decrease in chemokine expression in c-Mpl−/− marrow ECs could impair the interactions between MKs and ECs in the vascular niche, reducing HSC expansion.
Over the past several years, much has been learned of the role of the vascular niche on both normal and neoplastic HSC survival, function, and expansion. As has been shown for acute leukemias and marrow-based lymphoid malignancies (e.g., myeloma), the marrow microenvironmental niche is significantly altered in chronic MPNs [30,31,36]. In this review, we have summarized changes seen in the vascular niche in a murine model of an aggressive MPN produced by HSC and EC expression of Jak2V617F, the most common genetic alteration found in patients with acquired PV, ET, and PMF. In this model, two primary cell types of the vascular niche (EC and MK) carry the Jak2V617F mutation, as is seen in patients with MPNs (although EC expression is 100% in our model, less so in patients) and each altered cell type contributes to the differential survival, growth, and radioresistance of Jak2V617F-bearing cells over that of the residual WT cells. In essence, it takes two to tango, an abnormal stem cell and an abnormal environment, to produce a disease sufficient to jeopardize the lives of our patients.
Others have come to very similar conclusions using several different experimental conditions: it takes the niche and the stem cells and communication between the two partners to produce an MPN. For example, Walkley et al. [44] reported that Mx1-Cre-mediated deletion of the retinoblastoma (Rb) gene in both hematopoietic and stromal elements leads to a widespread MPN-like disease with splenomegaly, mobilization of cells from the BM, and eventual loss of HSCs, but neither the sole inactivation of Rb in HSC nor in stromal cells results in a myeloid disorder. The importance of interplay between genetic alterations in stem cells and their environment was also highlighted in a recent Perspective in Experimental Hematology. Migliaccio [45] stressed a potential role for inflammation, mediated by tumor necrosis factor, to alter the effects of tet2 deficiency (one of the non-Jak2V617F mutations associated with MPNs) on stem cell biology. The role of inflammation and MPNs was mechanistically explored further by Lu et al. [46], who concluded that LCN2 derived from neoplastic leukocytes is an additional inflammatory cytokine that contributes to the predominance of the neoplastic clone of cells in PMF and to a dysfunctional niche. Arranz and colleagues [47] have focused in recent studies on both sympathetic nerve fibers and Nestin+ mesenchymal stromal cell reductions in the marrow of patients with MPNs and in mice expressing JAK2V617F in the HSCs to further illustrate the importance of the communication between HSCs and their niche. It is hoped that such studies of these and other models of MPNs will lead to better, more comprehensive approaches to the treatment of patients with life-threatening PV, ET, and IMF. Our patients deserve nothing less.
Acknowledgments
The authors thank the anonymous reviewer who pointed out that the title “It takes two to tango” has been used in the biomedical literature 158 prior times!
The work reviewed in this manuscript was supported by grants from the National Institutes of Health (DK R01 49855 to KK and R01 HL134970 to HZ) and the Department of Veterans Affairs (IK2BX001559 to HZ).
Footnotes
Conflict of interest disclosure
The authors declare no competing financial interests.
References
- 1.Schofield R The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. [DOI] [PubMed] [Google Scholar]
- 3.Tiedt R, Hao-Shen H, Sobas MA, et al. Ratio of mutant JAK2V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–3940. [DOI] [PubMed] [Google Scholar]
- 4.Kisanuki YY, Hammer RE, Miyazaki J, et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. [DOI] [PubMed] [Google Scholar]
- 5.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Lin CHS, Kaushansky K, Zhan H. JAK2V617F megakaryocytes promote hematopoietic stem/progenitor cell expansion in mice through thrombopoietin/MPL signaling. Stem Cells. 2018;36:1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinho S, Marchand T, Yang E, et al. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev Cell. 2018;44:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamad SF, Xu L, Ghosh J, et al. Osteomacs interact with megakaryocytes and osteoblasts to regulate murine hematopoietic stem cell function. Blood Adv. 2017;1:2520–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dameshek W Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–375. [PubMed] [Google Scholar]
- 10.Prchal JF, Axelrad AA. Bone-marrow responses in polycythemia vera. N Engl J Med. 1974;290:1382. [DOI] [PubMed] [Google Scholar]
- 11.Florensa L, Besses C, Woessner S, et al. Endogenous megakaryocyte and erythroid colony formation from blood in essential thrombocythaemia. Leukemia. 1995;9:271–273. [PubMed] [Google Scholar]
- 12.Reid CD. The significance of endogenous erythroid colonies (EEC) in haematological disorders. Blood Rev. 1987;1:133–140. [DOI] [PubMed] [Google Scholar]
- 13.Vigon I, Mornon JP, Cocault L, et al. Molecular cloning and characterization of MPL, the human homolog of the v mpl oncogene: Identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci U S A. 1992;89:5640–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushansky K, Lok S, Holly RD, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–571. [DOI] [PubMed] [Google Scholar]
- 15.de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369:533–538. [DOI] [PubMed] [Google Scholar]
- 16.Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c mpl-deficient mice. Science. 1994;265:1445–1447. [DOI] [PubMed] [Google Scholar]
- 17.Sitnicka E, Lin N, Priestley GV, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87:4998–5005. [PubMed] [Google Scholar]
- 18.Li Y, Hetet G, Kiladjian JJ, et al. Proto-oncogene c mpl is involved in spontaneous megakaryocytopoiesis in myeloproliferative disorders. Br J Haematol. 1996;92:60–66. [DOI] [PubMed] [Google Scholar]
- 19.Kaushansky K The role of the MPL receptor in myeloproliferative disorders. Leukemia. 1998;12(Suppl. 1):S47–S50. [PubMed] [Google Scholar]
- 20.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. [DOI] [PubMed] [Google Scholar]
- 21.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. [DOI] [PubMed] [Google Scholar]
- 22.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. [DOI] [PubMed] [Google Scholar]
- 23.Etheridge SE, Roh ME, Cosgrove ME, et al. JAK2V617F-positive endothelial cells contribute to clotting abnormalities in myeloproliferative neoplasms. Proc Nat Acad Sci U S A. 2014;111:2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sozer S, Fiel MI, Schiano T, et al. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009;113:5246–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosti V, Villani L, Riboni R, et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood. 2013;121:360–368. [DOI] [PubMed] [Google Scholar]
- 26.Sangkhae V, Etheridge SE, Kaushansky K, Hitchcock IS. The thrombopoietin receptor, c-MPL, is critical for development of JAK2V617F-positive MPNs. Blood. 2014;124:3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zingariello M, Sancillo L, Martelli F, et al. The thrombopoietin/MPL axis is activated in the Gata1low mouse model of myelofibrosis and is associated with a defective RPS14 signature. Blood Cancer J. 2017;7:e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. [DOI] [PubMed] [Google Scholar]
- 29.Chachoua I, Pecquet C, El-Khoury M, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127:1325–1335. [DOI] [PubMed] [Google Scholar]
- 30.Jakob C, Sterz J, Zavrski I, et al. Angiogenesis in multiple myeloma. Eur J Cancer. 2006;42:1581–1590. [DOI] [PubMed] [Google Scholar]
- 31.Albitar M Angiogenesis in acute myeloid leukemia and myelodysplastic syndrome. Acta Haematol. 2001;106:170–176. [DOI] [PubMed] [Google Scholar]
- 32.Schepers K, Campbell TB, Passegué E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16:254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CH, Kaushansky K, Zhan H. JAK2V617F-mutant vascular niche contributes to JAK2V617F clonal expansion in myeloproliferative neoplasms. Blood Cells Mol Dis. 2016;62:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan H, Lin CHS, Segal Y, Kaushansky K. The JAK2V617F-bearing vascular niche promotes clonal expansion in myeloproliferative neoplasms. Leukemia. 2018;32:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin CHS, Zhang Y, Kaushansky K, Zhan H. JAK2V617F-bearing vascular niche enhances malignant hematopoietic regeneration following radiation injury. Haematologica. 2018;103: 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boveri E, Passamonti F, Rumi E, et al. Bone marrow microvessel density in chronic myeloproliferative disorders: a study of 115 patients with clinicopathological and molecular correlations. Br J Haematol. 2008;140:162–168. [DOI] [PubMed] [Google Scholar]
- 37.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foudi A, Jarrier P, Zhang Y, et al. Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironment in CXCR4−/− chimeric mice. Blood. 2006;107:2243–2251. [DOI] [PubMed] [Google Scholar]
- 40.Broxmeyer HE, Cooper S, Kohli L, et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol. 2003;170:421–429. [DOI] [PubMed] [Google Scholar]
- 41.Zhan H, Ma Y, Lin CH, Kaushansky K. JAK2V617F-mutant megakaryocytes contribute to hematopoietic stem/progenitor cell expansion in a model of murine myeloproliferation. Leukemia. 2016;30:2332–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardier JE, Dempsey J. Thrombopoietin and its receptor, c-mpl, are constitutively expressed by mouse liver endothelial cells: evidence of thrombopoietin as a growth factor for liver endothelial cells. Blood. 1998;91:923–929. [PubMed] [Google Scholar]
- 43.Brizzi MF, Battaglia E, Montrucchio G, et al. Thrombopoietin stimulates endothelial cell motility and neoangiogenesis by a platelet-activating factor-dependent mechanism. Circ Res. 1999;84:785–796. [DOI] [PubMed] [Google Scholar]
- 44.Walkley CR, Shea JM, Sims NA, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Migliaccio AR. A vicious interplay between genetic and environmental insults in the etiology of blood cancers. Exp Hematol. 2018;59:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu M, Xia L, Liu YC, et al. Lipocalin produced by myelofibrosis cells affects the fate of both hematopoietic and marrow microenvironmental cells. Blood. 2015;126:972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arranz L, Sánchez-Aguilera A, Martín-Pérez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. [DOI] [PubMed] [Google Scholar]