Abstract
BACKGROUND
The role of assessment of myocardial viability in identifying patients with ischemic cardiomyopathy who might benefit from surgical revascularization remains controversial. Furthermore, although improvement in left ventricular function is one of the goals of revascularization, its relationship to subsequent outcomes is unclear.
METHODS
Among 601 patients who had coronary artery disease that was amenable to coronaryartery bypass grafting (CABG) and who had a left ventricular ejection fraction of 35% or lower, we prospectively assessed myocardial viability using single-photonemission computed tomography, dobutamine echocardiography, or both. Patients were randomly assigned to undergo CABG and receive medical therapy or to receive medical therapy alone. Left ventricular ejection fraction was measured at baseline and after 4 months of follow-up in 318 patients. The primary end point was death from any cause. The median duration of follow-up was 10.4 years.
RESULTS
CABG plus medical therapy was associated with a lower incidence of death from any cause than medical therapy alone (182 deaths among 298 patients in the CABG group vs. 209 deaths among 303 patients in the medical-therapy group; adjusted hazard ratio, 0.73; 95% confidence interval, 0.60 to 0.90). However, no significant interaction was observed between the presence or absence of myocardial viability and the beneficial effect of CABG plus medical therapy over medical therapy alone (P = 0.34 for interaction). An increase in left ventricular ejection fraction was observed only among patients with myocardial viability, irrespective of treatment assignment. There was no association between changes in left ventricular ejection fraction and subsequent death.
CONCLUSIONS
The findings of this study do not support the concept that myocardial viability is associated with a long-term benefit of CABG in patients with ischemic cardiomyopathy. The presence of viable myocardium was associated with improvement in left ventricular systolic function, irrespective of treatment, but such improvement was not related to long-term survival. (Funded by the National Institutes of Health; STICH ClinicalTrials.gov number, .)
Ischemic heart disease is the most common and most lethal cause of heart failure.1,2 It is also the only cause of left ventricular dysfunction that is amenable to the benefit of surgical revascularization. The Surgical Treatment for Ischemic Heart Failure (STICH) trial3 and its extension study (STICHES)4 showed that after a median follow-up of approximately 10 years, patients with ischemic cardiomyopathy who underwent coronary artery bypass grafting (CABG) and received medical therapy had better outcomes than those who received medical therapy alone.
Approximately half the patients enrolled in the STICH trial underwent prospective, protocol-driven evaluation of myocardial viability. In the initial report of the myocardial viability substudy,5 with a median follow-up of 5.1 years, the presence of myocardial viability was not associated with a survival benefit from CABG. These findings were inconsistent with the previously accepted concept — based on retrospective studies — that assessment of myocardial viability is crucial for the identification of patients who would be most likely to benefit from revascularization6–8 and resulted in considerable debate.9,10
Improvement in systolic function has been accepted as the reference standard for the assessment of myocardial viability, as one of the therapeutic goals of revascularization, and as the mechanism that leads to improved prognosis in patients with ischemic cardiomyopathy.9–12 Accordingly, the current study was conducted to more definitively assess the relationship between myocardial viability and the long-term treatment effect of CABG in patients with ischemic cardiomyopathy. In addition, we sought to determine the relationship between the presence of myocardial viability and changes in left ventricular ejection fraction during the early stages of follow-up and the effect of myocardial viability on the subsequent long-term prognosis of these patients.
METHODS
STUDY POPULATION
The STICH trial was a prospective, multicenter, randomized, nonblinded trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. In this trial, 1212 patients with coronary artery disease who had a left ventricular ejection fraction of 35% or lower were enrolled during the period from 2002 through 2007. The trial was performed to test the hypothesis that CABG in combination with appropriate medical therapy would result in better survival outcomes than appropriate medical therapy alone. A complete list of inclusion and exclusion criteria is provided in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org. Patients were randomly assigned to undergo CABG and receive medical therapy (CABG group) or to receive medical therapy alone (medical-therapy group). The NHLBI and the ethics committee at each participating center approved the trial protocol, available at NEJM.org. All the patients provided written informed consent. The design of the STICH trial and the results of the analysis of the primary hypothesis have been reported previously.3,4,13 Before the treatment-group assignments were revealed and before any intermediate-term results were reported, the protocol was amended to extend the follow-up period by an additional 5 years. The final clinical assessment of surviving patients was performed during the 6-month period before November 30, 2015, which was the data cutoff date for the extended follow-up.
Although tests to assess myocardial viability were initially mandated as part of the STICH protocol, this requirement was discontinued because of difficulties in patient enrollment. Hence, only a proportion of patients enrolled in the STICH trial were included in the myocardial viability substudy. Specifically, patients who underwent either single-photon-emission tomography (SPECT), dobutamine echocardiography, or both within 90 days before or after randomization and before initiation of therapy were included in the myocardial viability substudy.
STUDY DESIGN AND OVERSIGHT
The current study was designed by the first author and was approved by the policy and publications committee of the STICH trial. The clinical and outcomes data were collected by personnel at the participating sites and were sent directly to the data coordinating center at the Duke Clinical Research Institute. The images obtained for myocardial viability and other noninvasive studies were sent from the participating sites to the respective independent core laboratories, where they were evaluated; the resultant data were then sent to the data coordinating center. The data coordinating center stored the data, and the statisticians at the Duke Clinical Research Institute (the second and third authors) wrote the statistical analysis plan and conducted the data analyses. All the authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol. The first draft of the manuscript was written by the first author, and all the authors reviewed and revised subsequent drafts. The manuscript was reviewed and approved for submission by the policy and publications committee of the STICH trial and by the NHLBI.
ASSESSMENT OF MYOCARDIAL VIABILITY
Data collection for and analysis of the SPECT and dobutamine echocardiographic studies were coordinated at independent core laboratories that were funded by the NHLBI; investigators at each laboratory were unaware of the treatment assignments and the individual characteristics of the patients. Details of the imaging protocols used to identify and quantify viable myocardium have been published previously.5,14 In brief, thresholds of the extent of viable myocardium were prespecified to classify patients in a binary fashion as either having or not having substantial myocardial viability. For SPECT, patients with viability were defined as those with 11 or more viable segments on the basis of relative tracer activity. For dobutamine echocardiography, patients with viability were defined as those with 5 or more segments with abnormal resting systolic function but manifesting contractile reserve during dobutamine administration. In addition, we analyzed myocardial viability as a continuous variable by computing the proportion of viable segments relative to the number of segments analyzed for each individual patient.5,14
ASSESSMENT OF LEFT VENTRICULAR FUNCTION
The study protocol specified that patients had to undergo echocardiography, radionuclide scanning, or cardiac magnetic resonance imaging at baseline and at 4 months after randomization. Left ventricular function was quantified on the basis of the left ventricular ejection fraction, which was derived from volume measurements with the following equation: end-diastolic volume minus the end-systolic volume, divided by the end-diastolic volume. Details of the methods of assessment of left ventricular volume at each independent core laboratory have been reported previously.15 When more than one imaging method was used for a patient at a single time point, we used a prespecified hierarchical algorithm that was based on the quality of each of the imaging studies to determine the best available method for measurement of the left ventricular ejection fraction.15 For the purpose of this study, and because of variations across imaging methods, changes in left ventricular ejection fraction were determined only in patients who were assessed with the same imaging method at each time point (“paired imaging”).
FOLLOW-UP AND OUTCOMES
After enrollment, patients were followed every 4 months during the first year and every 6 months thereafter. The primary outcome was death from any cause. Secondary end points were death from cardiovascular causes and a composite of death from any cause or hospitalization for cardiovascular causes. Definitions of the trial end points have been reported previously.13 The comparisons of outcomes that were related to treatment were based on intention-to-treat analyses.
STATISTICAL ANALYSIS
Comparisons of the incidence of death from any cause between patients who underwent myocardial viability testing and those who did not and between the two randomly assigned treatment groups among all patients included in the myocardial viability substudy were performed with the use of multivariable Cox proportional-hazards models16 that were adjusted for relevant baseline covariates, and survival curves were generated with the use of Kaplan-Meier estimates.17 Competing-risk models and cumulative incidence functions based on the method of Fine and Gray were used to evaluate the risk of death from cardiovascular causes in patients who did and those who did not undergo viability testing.18
Other assessments included the difference between patients who had myocardial viability and those who did not with respect to the incidence of death from any cause and the incidence of death from cardiovascular causes; the interaction between treatment assignment and myocardial viability status with respect to death from any cause, death from cardiovascular causes, and a composite of death from any cause or hospitalization for cardiovascular causes; the difference between patients who had improvement in left ventricular ejection fraction and those who did not, among patients who were alive at 4 months, with respect to subsequent death from any cause and death from cardiovascular causes; and the interaction between improvement in left ventricular ejection fraction and myocardial viability status with respect to death from any cause and death from cardiovascular causes. Cox proportional-hazards models and Kaplan-Meier estimates were used in the analyses of death from any cause, and competing-risk regression and cumulative incidence functions were used in the analyses of death from cardiovascular causes.
A generalized linear model was used to assess differences in the change in left ventricular ejection fraction in various subgroups of patients. All generalized linear models included the baseline value of left ventricular ejection fraction as a covariate. Least-squares means, standard errors, and 95% confidence intervals were calculated for each comparison. Linear regression was then used to examine the strength and direction of the relationship between the change in left ventricular ejection fraction and myocardial viability, measured as a continuous variable, and to assess any differences in this relationship between patients in the CABG group and patients in the medical-therapy group.
Because the study protocol did not include a method for adjusting for multiplicity in testing, summary statistics are limited to point estimates and 95% confidence intervals. The widths of the intervals were not adjusted for multiplicity; therefore, the intervals should not be viewed as evidence for significant associations or treatment effects. P values of less than 0.05 were considered to indicate statistical significance. All the analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
RESULTS
STUDY POPULATION AND THE EFFECT OF CABG
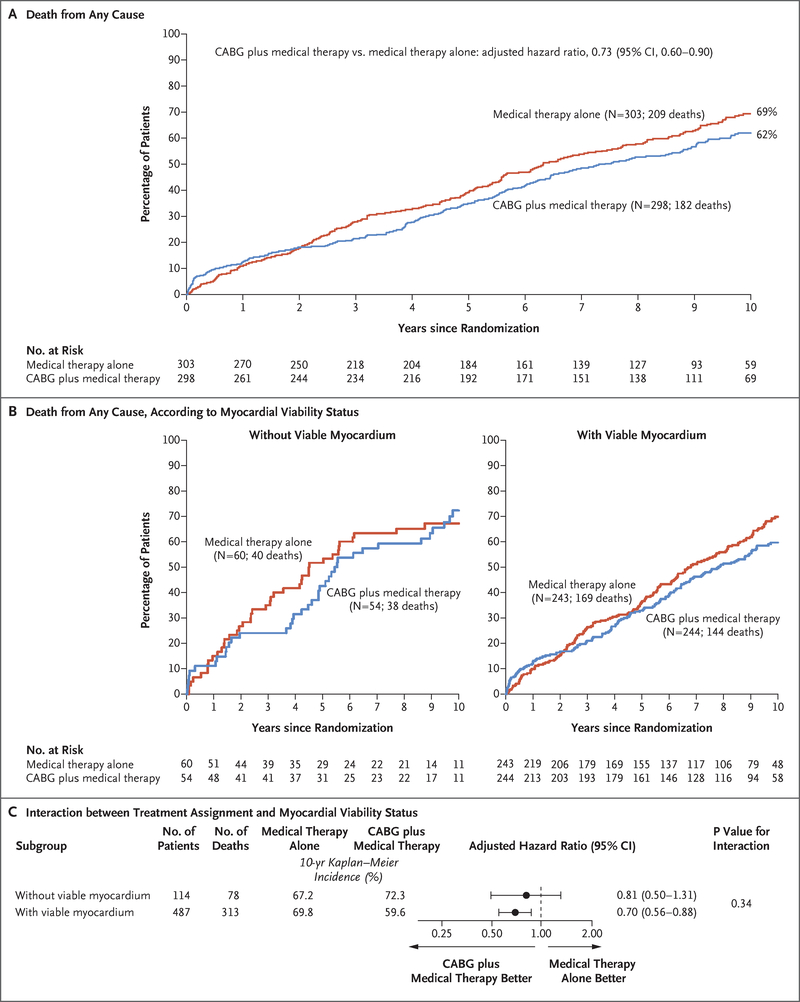
Of the 1212 patients enrolled in the STICH trial, 601 patients who underwent myocardial viability testing met the inclusion criteria for the substudy. Of these patients, 6 were lost to follow-up and 2 withdrew from the study (Fig. S1 in the Supplementary Appendix). The baseline characteristics of the 1212 patients overall and according to whether they underwent viability testing are shown in Table S2 in the Supplementary Appendix. No significant difference in the incidence of death from any cause or the incidence of death from cardiovascular causes was observed during the follow-up period between patients who underwent viability testing and those who did not (Fig. S2 in the Supplementary Appendix). Among the 298 patients randomly assigned to the CABG group, 279 underwent CABG before completion of the STICH trial, whereas 65 of the 303 patients randomly assigned to the medical-therapy group underwent CABG at any time before completion of the long-term follow-up; 32 of these 65 patients underwent CABG during the first year of follow-up. When outcomes were analyzed according to randomly assigned treatment, and independent of the presence or absence of myocardial viability, CABG plus medical therapy was associated with a lower incidence of death from any cause than medical therapy alone (182 deaths among 298 patients in the CABG group vs. 209 deaths among 303 patients in the medical-therapy group; adjusted hazard ratio, 0.73; 95% confidence interval [CI], 0.60 to 0.90) (Fig. 1A).
Figure 1 (facing page). Kaplan–Meier Analysis of the Incidence of Death from Any Cause.
Panel A shows Kaplan-Meier curves for the incidence of death from any cause among patients who underwent a myocardial viability test, according to treatment group; results were compared with the use of a Cox proportional-hazards model with adjustment for baseline covariates. Panel B shows Kaplan–Meier curves for the incidence of death from any cause among patients without viable myocardium (left panel) and among those with viable myocardium (right panel), according to treatment group. Panel C shows the results of a Cox proportional-hazards model that tested for the interaction between myocardial viability and treatment, with adjustment for baseline covariates. CABG denotes coronary-artery bypass grafting.
OUTCOMES IN PATIENTS WITH AND THOSE WITHOUT MYOCARDIAL VIABILITY
Among the 601 patients, 487 (81%) were considered to have myocardial viability, as defined in the protocol. The remaining 114 patients (19%) were classified as not having viability. Table 1 lists the demographic and clinical characteristics of the patients overall and according to the presence or absence of myocardial viability.
Table 1.
Baseline Characteristics of Patients Who Underwent Assessment of Myocardial Viability.*
| Characteristic | All Patients (N = 601) | Patients with Myocardial Viability (N = 487) | Patients without Myocardial Viability (N = 114) | ||||
|---|---|---|---|---|---|---|---|
| Medical- Therapy Group (N = 243) | CABG Group (N = 244) | P Value | Medical- Therapy Group (N = 60) | CABG Group (N = 54) | P Value | ||
| Age — yr | 60.7+9.4 | 60.0+9.7 | 61.5+9.2 | 0.05 | 61.6+8.5 | 60.0+9.2 | 0.34 |
| Male sex — no. (%) | 521 (87) | 205 (84) | 211 (86) | 0.51 | 55 (92) | 50 (93) | 1.00 |
| Previous myocardial infarction — no. (%) | 481 (80) | 190 (78) | 183 (75) | 0.41 | 56 (93) | 52 (96) | 0.68 |
| CCS angina class — no. (%)† | 0.60 | 0.03 | |||||
| 0 | 236 (39) | 101 (42) | 101 (41) | 18 (30) | 16 (30) | ||
| I | 94 (16) | 34 (14) | 34 (14) | 19 (32) | 7 (13) | ||
| II | 253 (42) | 104 (43) | 99 (41) | 23 (38) | 27 (50) | ||
| III | 14 (2) | 3 (1) | 8 (3) | 0 | 3 (6) | ||
| IV | 4 (1) | 1 (<1) | 2 (1) | 0 | 1 (2) | ||
| Highest NYHA functional class in 3 previous mo — no. (%)† | 0.51 | 0.25 | |||||
| I | 27 (4) | 15 (6) | 9 (4) | 0 | 3 (6) | ||
| II | 212 (35) | 94 (39) | 88 (36) | 14 (23) | 16 (30) | ||
| III | 275 (46) | 100 (41) | 111 (45) | 36 (60) | 28 (52) | ||
| IV | 87 (14) | 34 (14) | 36 (15) | 10 (17) | 7 (13) | ||
| Risk-at-randomization score‡ | 12.5+8.8 | 11.9+8.4 | 12.8+9.0 | 0.28 | 13.7+9.8 | 12.0+8.8 | 0.37 |
| Medications at baseline — no. (%) | |||||||
| Beta-blocker | 534 (89) | 221 (91) | 216 (89) | 0.38 | 52 (87) | 45 (83) | 0.62 |
| ACE inhibitor | 514 (86) | 202 (83) | 210 (86) | 0.37 | 54 (90) | 48 (89) | 0.85 |
| ARB | 46 (8) | 20 (8) | 20 (8) | 0.99 | 3 (5) | 3 (6) | 1.00 |
| ACE inhibitor or ARB | 554 (92) | 219 (90) | 227 (93) | 0.25 | 57 (95) | 51 (94) | 1.00 |
| Statin | 508 (85) | 212 (87) | 193 (79) | 0.02 | 56 (93) | 47 (87) | 0.26 |
| Aspirin | 513 (85) | 209 (86) | 205 (84) | 0.54 | 56 (93) | 43 (80) | 0.03 |
| No. of diseased vessels with ≥75% stenosis — no. (%) | 0.79 | 0.45 | |||||
| 0 | 12 (2) | 6 (2) | 3 (1) | 2 (3) | 1 (2) | ||
| 1 | 152 (25) | 62 (26) | 62 (25) | 17 (28) | 11 (20) | ||
| 2 | 221 (37) | 87 (36) | 92 (38) | 18 (30) | 24 (44) | ||
| 3 | 215 (36) | 88 (36) | 86 (35) | 23 (38) | 18 (33) | ||
| Left ventricular ejection fraction — % | 26.7+8.6 | 28.1+8.4 | 27.0+8.2 | 0.30 | 22.6+8.5 | 23.3+9.1 | 0.50 |
| Left ventricular end-diastolic volume index — ml/m2 of body-surface area | 122.8+41.9 | 117.8+37.9 | 116+35.1 | 0.63 | 152.3+51.3 | 140.0+53.8 | 0.16 |
| Left ventricular end-systolic volume index — ml/m2 of body-surface area | 91.7+38.9 | 85.8+34.3 | 86.0+32.1 | 0.97 | 120.8+49.6 | 111.2+50.8 | 0.25 |
| Viability testing — no. (%) | |||||||
| SPECT | 471 (78) | 182 (75) | 197 (81) | 0.12 | 49 (82) | 43 (80) | 0.78 |
| DE | 280 (47) | 121 (50) | 108 (44) | 0.22 | 29 (48) | 22 (41) | 0.42 |
| SPECT and DE | 150 (25) | 60 (25) | 61 (25) | 0.94 | 18 (30) | 11 (20) | 0.24 |
Plus-minus values are means +SD. Complete data were available for all baseline covariates except for one missing value in the number of diseased vessels, one missing value for chronic renal insufficiency, and three missing values for mitral regurgitation. All these missing values were imputed with single imputation. Percentages may not total 100 because of rounding. ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker, CABG coronary-artery bypass grafting, DE dobutamine echocardiography, and SPECT single-photon-emission computed tomography.
The Canadian Cardiovascular Society (CCS) angina classes range from class 0, which indicates no symptoms, to class IV, which indicates angina at any level of physical exertion. New York Heart Association (NYHA) heart failure classes range from I to IV, with higher values indicating greater disability.
The risk-at-randomization score ranges from 1 to 32, with higher numbers indicating a higher risk of death. Among patients who received medical therapy alone, a score of 1 predicts a risk of 18% and a score of 32 predicts a risk of 99%.
During a median follow-up of 10.4 years, a total of 391 patients (65%) died. The overall incidence of death did not differ significantly between patients with viable myocardium (313 of 487 patients [64%]) and those without viable myocardium (78 of 114 patients [68%]) (hazard ratio, 0.81; 95% CI, 0.63 to 1.03; P = 0.09), even after adjustment for other relevant prognostic variables (P = 0.64) (Fig. S3 in the Supplementary Appendix).
Among the 487 patients with myocardial viability, 244 had been assigned to the CABG group and 243 to the medical-therapy group. Among the 114 patients without viability, 54 had been assigned to the CABG group and 60 to the medical-therapy group. Although CABG plus medical therapy was associated with a lower incidence of death from any cause than medical therapy alone (Fig. 1A), the interaction between the presence or absence of myocardial viability and the beneficial effect of CABG plus medical therapy over medical therapy alone was not significant (P = 0.34) (Fig. 1B and 1C). Similar findings were observed with respect to the secondary end points of death from cardiovascular causes and the composite of death from any cause or hospitalization for cardiovascular causes (Figs. S4 and S5 in the Supplementary Appendix).
LEFT VENTRICULAR EJECTION FRACTION AND SUBSEQUENT OUTCOMES
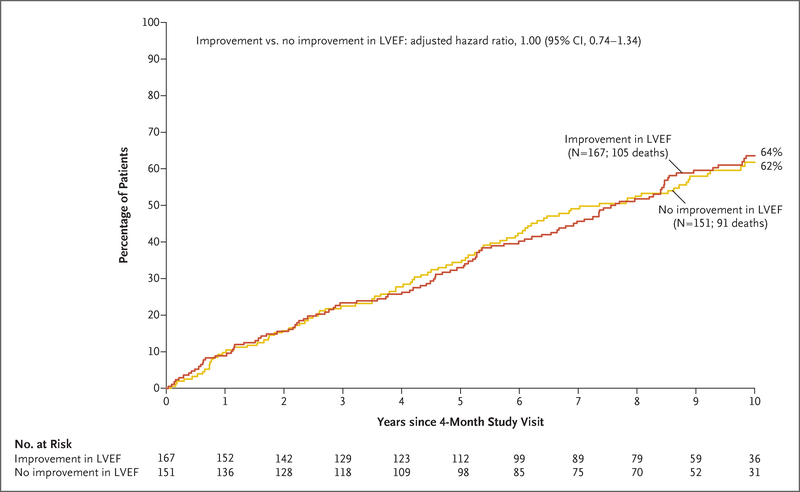
Among the 601 patients, 34 died during the first 4 months of follow-up. Of the remaining 567 patients, 318 (56%) had paired imaging at baseline and at 4 months for measurement of left ventricular ejection fraction. The baseline and follow-up values of left ventricular ejection fraction, as well as the left ventricular end-diastolic and end-systolic volume indexes, are shown in Table S3 in the Supplementary Appendix. Among these 318 patients, neither the incidence of death from any cause nor the incidence of death from cardiovascular causes differed significantly between patients who had improvement in left ventricular ejection fraction and those who did not have such improvement (Fig. 2, and Fig. S6 in the Supplementary Appendix).
Figure 2. Incidence of Death from Any Cause, According to Changes in Left Ventricular Ejection Fraction.
Shown are the results of a landmark analysis that included data from the 318 patients who underwent myocardial viability testing and had paired imaging (i.e., assessed with the same imaging method at each time point) at baseline and at 4 months for measurement of left ventricular ejection fraction (LVEF). Kaplan-Meier estimates of death from any cause among patients with improvement in LVEF and among patients without such improvement were compared with the use of a Cox proportional-hazards model with adjustment for baseline covariates.
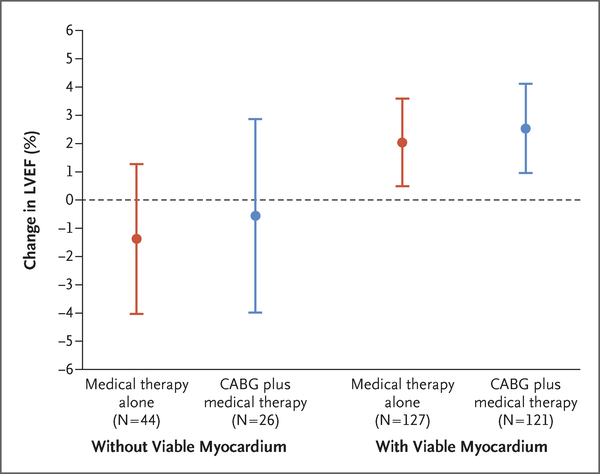
When changes in left ventricular ejection fraction were analyzed according to myocardial viability status, irrespective of treatment assignment, the patients with viable myocardium (248 patients) had a modest increase in left ventricular ejection fraction from baseline to month 4 (least-squares mean [±SE] change, 2.29±0.56). In contrast, among patients without viable myocardium (70 patients), no significant change in left ventricular ejection fraction was observed (least-squares mean change, −1.08±1.07). When the change in left ventricular ejection fraction was analyzed in the four subgroups of patients defined according to viability status and treatment assignment, improvements in left ventricular ejection fraction of similar magnitude were observed in the CABG group with viable myocardium and the medical-therapy group with viable myocardium. In contrast, among patients without myocardial viability, there was no improvement in left ventricular ejection fraction in either treatment group (Fig. 3).
Figure 3. Change in Left Ventricular Ejection Fraction, According to Myocardial Status and Treatment Group.
Shown is the least-squares mean change in LVEF from baseline to month 4 in the four subgroups of patients defined according to the presence or absence of viable myocardium and treatment assignment. I bars denote 95% confidence intervals.
When the extent of myocardial viability was analyzed quantitatively, no strong correlation was observed between the change in left ventricular ejection fraction and the amount of viable myocardium either when the entire cohort of patients was evaluated or when patients were evaluated separately according to treatment assignment (Fig. S7 in the Supplementary Appendix). Similarly, the change in left ventricular ejection fraction was not related to the degree of left ventricular remodeling, as measured by the baseline left ventricular end-systolic volume index, either in patients with myocardial viability or in those without (Fig. S8 in the Supplementary Appendix).
Finally, no significant interaction was observed between the presence or absence of myocardial viability and the improvement or lack of improvement in left ventricular ejection fraction with respect to the incidence of death from any cause and the incidence of death from cardiovascular causes (Fig. S9 in the Supplementary Appendix).
DISCUSSION
The findings of the current study do not support the concept that assessment of myocardial viability determines the likelihood of long-term benefit from surgical revascularization in patients with ischemic cardiomyopathy. Our study showed a lower incidence of death from any cause with CABG plus medical therapy than with medical therapy alone. However, the tests of interaction between myocardial viability and treatment effect of CABG were not significant for any of the three end points. Therefore, we must conclude that there is no statistical evidence of association between myocardial viability and benefit from CABG.
A number of various possibilities, alone or in combination, may account for the negative results of our study. It is certainly possible that a true biologic interaction exists between myocardial viability and the benefit of revascularization and that we were unable to unveil it because of the relatively small number of patients, especially patients without myocardial viability, included in our study. A complementary explanation is that the physiological complexity underpinning the potential therapeutic benefit of surgical revascularization cannot be surmised from the results of a single test of myocardial viability, particularly when those results are expressed in a dichotomous fashion (i.e., patients having or not having viability). In fact, previous results from the STICH trial showed that the degree of left ventricular systolic dysfunction and remodeling and the number of stenotic coronary arteries appear to be stronger determinants of the benefit of revascularization than myocardial viability.19 Patients with ischemic cardiomyopathy often present with complex and challenging clinical scenarios. Therefore, the results of tests for detection of viable myocardium must be interpreted within the context of the multiplicity of factors necessary to reach the best treatment decision for each patient. These factors include not only the results of functional tests but also the anatomical extent of coronary and myocardial disease, as well as the regional correspondence between segmental left ventricular dysfunction and the likelihood of successful revascularization of the corresponding coronary arteries.
A putative mechanism that mediates the benefit of CABG among patients with myocardial viability is the improvement in left ventricular systolic function that results from revascularization. In our study, a modest but significant increase in left ventricular ejection fraction at 4 months was indeed observed among patients with viable myocardium. This increase was similar in patients in the CABG group and those in the medical-therapy group, a finding that is consistent with the known relationship between myocardial viability and improvement in systolic function, both with revascularization and with beta-blocker therapy, that has been reported in previous studies.20–22 Nevertheless, when the amount of viable myocardium was assessed as a continuous variable, no association was observed between the extent of viability and the magnitude of improvement in left ventricular function either with or without CABG, findings that indicate that the quantitative relationship between these variables is weak.
There was no association between improvement in left ventricular ejection fraction at 4 months and subsequent survival, nor was there a significant interaction between myocardial viability and changes in left ventricular ejection fraction with regard to death from any cause or death from cardiovascular causes. Overall, these findings are consistent with previous observations23 and deemphasize the relevance of changes in left ventricular ejection fraction as a determinant of long-term prognosis in patients with ischemic cardiomyopathy. Thus, our results suggest that abatement or reversal of left ventricular systolic dysfunction is not a critical mechanism involved in mediating the beneficial effect of CABG in these patients.
Certain limitations of this study must be acknowledged. First, only half the patients enrolled in the STICH trial had an assessment of myocardial viability. However, among the patients included in the myocardial viability substudy, about half had been randomly assigned to the CABG group and half had been assigned to the medical-therapy group, which shows the lack of biased interaction between inclusion in the substudy and treatment assignment. Moreover, despite certain differences in baseline characteristics, the incidence of death from any cause and the incidence of death from cardiovascular causes during the entire follow-up period was similar among patients who had undergone viability testing and those who had not. Second, the assessment of left ventricular ejection fraction was made at a relatively early point (i.e., after 4 months of follow-up) during the 10-year follow-up period. Consequently, the development and potential prognostic effect of changes in left ventricular function that might have occurred at a later time could not be assessed as part of this study. It must also be noted that the assessment of changes in left ventricular ejection fraction was performed only among patients with paired studies at baseline and 4 months, with the resultant reduction in the number of observations. Finally, our findings are based on the assessment of myocardial viability with either SPECT or dobutamine echocardiography. The study did not include the routine performance of cardiac magnetic resonance imaging, which has become an accepted technique for the assessment of myocardial scarring.
In conclusion, this 10-year follow-up study of the STICH trial does not confirm the hypothesis that the presence of substantial amounts of viable myocardium is associated with the long-term beneficial effect of CABG. At the same time, our findings indicate that improvement in left ventricular ejection fraction is more likely to occur among patients with myocardial viability, is not restricted to patients who undergo revascularization, and is not an important mechanism for the long-term survival of patients with ischemic cardiomyopathy treated medically or surgically.
Supplementary Material
Acknowledgments
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
The STICH trial was supported by cooperative agreements (U01-HL-069009, HL-069010, HL-069011, HL-069012, HL-069013, HL-069015, HL-070011, and HL-072683) with the National Heart, Lung, and Blood Institute. The STICHES trial was supported by a separate grant (R01-HL105853/NCT00023595) from the National Institutes of Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Julio A. Panza, Westchester Medical Center, New York Medical College, Valhalla
Alicia M. Ellis, Duke Clinical Research Institute, Durham, NC
Hussein R. Al-Khalidi, Duke Clinical Research Institute, Durham, NC
Thomas A. Holly, Northwestern University, Chicago
Daniel S. Berman, Cedars Sinai Medical Center, Los Angeles
Jae K. Oh, Mayo Clinic, Rochester, MN
Gerald M. Pohost, University of Southern California, Los Angeles
George Sopko, National Heart, Lung, and Blood Institute, Bethesda, MD
Lukasz Chrzanowski, Medical University of Lodz, Lodz, Poland
Daniel B. Mark, Duke Clinical Research Institute, Durham, NC
Tomasz Kukulski, Medical University of Silesia, Silesian Center for Heart Diseases, Zabrze, Poland
Liliana E. Favaloro, University Hospital Favaloro Foundation, Buenos Aires
Gerald Maurer, Medical University of Vienna, Vienna
Pedro S. Farsky, Instituto Dante Pazzanese de Cardiologia, São Paulo
Ru-San Tan, National Heart Center, Singapore
Federico M. Asch, MedStar Washington Hospital Center, Washington, DC
Eric J. Velazquez, Yale University School of Medicine, New Haven, CT
Jean L. Rouleau, Montreal Heart Institute, Montreal
Kerry L. Lee, Duke Clinical Research Institute, Durham, NC
Robert O. Bonow, Northwestern University, Chicago
REFERENCES
- 1.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol 2002;39:210–8. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation 2006;114:1202–13. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EJ, Lee KL, O’Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg 2007;134:1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonow RO, Maurer G, Lee KL, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med 2011;364:1617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol 2002; 39:1151–8. [DOI] [PubMed] [Google Scholar]
- 7.Schinkel AFL, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol 2007; 32:375–410. [DOI] [PubMed] [Google Scholar]
- 8.Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation 2008;117: 103–14. [DOI] [PubMed] [Google Scholar]
- 9.Anavekar NS, Chareonthaitawee P, Narula J, Gersh BJ. Revascularization in patients with severe left ventricular dysfunction: is the assessment of viability still viable? J Am Coll Cardiol 2016;67:2874–87. [DOI] [PubMed] [Google Scholar]
- 10.Mielniczuk LM, Toth GG, Xie JX, De Bruyne B, Shaw LJ, Beanlands RS. Can functional testing for ischemia and viability guide revascularization? JACC Cardiovasc Imaging 2017;10:354–64. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee K, Swan HJC, Parmley WW, Sustaita H, Marcus H, Matloff J. Depression of left ventricular function due to acute myocardial ischemia and its reversal after aortocoronary saphenous-vein bypass. N Engl J Med 1972;286:1117–22. [DOI] [PubMed] [Google Scholar]
- 12.Bax JJ, Visser FC, Poldermans D, et al. Time course of functional recovery of stunned and hibernating segments after surgical revascularization. Circulation 2001;104:Suppl 1:I314–I318. [DOI] [PubMed] [Google Scholar]
- 13.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011;364:1607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonow RO, Castelvecchio S, Panza JA, et al. Severity of remodeling, myocardial viability, and survival in ischemic LV dysfunction after surgical revascularization. JACC Cardiovasc Imaging 2015;8:1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh JK, Velazquez EJ, Menicanti L, et al. Influence of baseline left ventricular function on the clinical outcome of surgical ventricular reconstruction in patients with ischaemic cardiomyopathy. Eur Heart J 2013;34:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc B 1972;34:187–220. [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94: 496–509. [Google Scholar]
- 19.Panza JA, Velazquez EJ, She L, et al. Extent of coronary and myocardial disease and benefit from surgical revascularization in ischemic LV dysfunction [Corrected]. J Am Coll Cardiol 2014;64:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53. [DOI] [PubMed] [Google Scholar]
- 21.Bello D, Shah DJ, Farah GM, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation 2003;108:1945–53. [DOI] [PubMed] [Google Scholar]
- 22.Cleland JG, Pennell DJ, Ray SG, et al. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet 2003;362:14–21. [DOI] [PubMed] [Google Scholar]
- 23.Samady H, Elefteriades JA, Abbott BG, Matter a JA, McPherson CA, Wackers FJ. Failure to improve left ventricular function after coronary revascularization for ischemic cardiomyopathy is not associated with worse outcome. Circulation 1999; 100:1298–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.