Abstract
Objective:
Diffusion kurtosis imaging (DKI) has emerged as a new acute stroke imaging approach, augmenting the routine diffusion weighted imaging (DWI). Whereas it has been shown that diffusion lesion without kurtosis abnormality is more likely to recover upon reperfusion while the kurtosis lesion shows poor response, little is known about the underlying pathophysiology between the kurtosis lesion and kurtosis/diffusion lesion mismatch.
Materials and methods:
We performed multiparametric MRI, including arterial spin labeling (ASL), pH-sensitive amide proton transfer (APT) and DKI in an embolic middle cerebral artery occlusion (MCAO) rodent model of acute stroke. Diffusion and kurtosis lesions were semiautomatically segmented, and multiparametric MRI indices were compared between kurtosis lesion, diffusion lesion, kurtosis/diffusion lesion mismatch and the contralateral normal area.
Results:
We confirmed a significant difference between diffusion and kurtosis lesion volumes (151 ± 65 vs. 125 ± 47 mm3, P<0.05). Although the ischemic lesions have significantly reduced cerebral blood flow (CBF) from the contralateral normal tissue, we did not find significant CBF difference between kurtosis lesion and the kurtosis/diffusion lesion mismatch (0.53±0.10 vs. 0.47±0.14 ml/g∙min, P>0.05). Importantly, pH in the kurtosis lesion was significantly lower from that of the lesion mismatch (6.81 ± 0.08 vs. 6.89±0.09, P<0.01).
Conclusions:
Our work confirmed that DKI provides an expedient approach for refining the heterogeneous DWI lesion that is associated with graded metabolic derangement, which is promising to improve infarction core definition and ultimately help guide stroke treatment.
Keywords: Acute stroke, Amide proton transfer (APT), Diffusion kurtosis imaging (DKI), Diffusion weighted imaging (DWI), pH
Introduction
Diffusion weighted imaging (DWI) has often been used to define the most severely damaged infarction core that is unlikely to recover.[1, 2] Perfusion weighted imaging (PWI), on the other hand, characterizes tissue hemodynamic state so that critically hypoperfused ischemic tissue can be identified.[3] The combined use of DWI and PWI provides a mismatch paradigm (i.e., DWI/PWI mismatch) that has been postulated as a practical imaging approximation of the salvageable ischemic tissue at risk of infarction (penumbra).[4, 5] The development of a reliable penumbra imaging approach is key to guide thrombolytic therapy, particularly in patients beyond the standard thrombolytic window[6]. Despite the initial enthusiasm, it has been recognized that the DWI/PWI mismatch paradigm is very crude. The perfusion lesion includes not only critically hypoperfused penumbral tissue but also benign oligemic area that is not at immediate risk of infarction.[7] More importantly, multiple reports have documented that the DWI lesion may partially reverse with early and effective recanalization, particularly in patients following endovascular recanalization.[8–12] Hence, the approximation of infarction core using the routine DWI may underestimate the penumbral tissue and restrict thrombectomy therapy to a subpopulation of stroke patients who could have benefited from late recanalization. As such, it is urgent to develop new imaging approaches to delineate the mildly injured and potentially reversible DWI lesion from the irreversibly damaged infarction core.
Although apparent diffusion coefficient (ADC) has been widely used, the severity of ADC drop is unable to predict DWI reversibility.[13] It has been shown that metabolic disruption within the DWI lesion is graded, yet the relatively low spatiotemporal resolution of lactate/phosphorus MR spectroscopy (MRS) and no readily access of positron emission tomography (PET) in the emergency setting limit their routine use. [14, 15] It is worth mentioning that amide proton transfer (APT) imaging is an emerging endogenous chemical exchange saturation transfer (CEST) MRI approach that probes pH-dependent exchange between amide protons from mobile proteins/peptides and bulk tissue water, which has been applied to map ischemic tissue acidification during acute stroke.[16–19] Indeed, pH MRI has revealed promising results in delineating the metabolic penumbra from benign oligemia.[20–22] Unfortunately, the spatiotemporal resolution of endogenous pH-sensitive APT MRI is not sufficient to directly resolve the tissue heterogeneity within the DWI stroke lesion, and new imaging means is urgently needed.
It is worth mentioning that displacement profile of water molecules in the cerebral tissue is not exactly Gaussian due to the restricted diffusion and complex microstructures. As such, the routine ADC measurement is overly simplistic and may not capture the rich information embedded within the DWI dataset. The emerging diffusion kurtosis imaging (DKI) quantifies the degree of deviation from the standard Gaussian displacement profile, providing a new index that complements routine DWI and ADC analyses. [23–25] DKI thus provides more detailed information for an ischemic insult, in comparison to standard DWI MRI. [26–31] Indeed, experimental stroke studies have shown that kurtosis/diffusion lesion mismatch often renormalizes following prompt recanalization while kurtosis lesion shows poor recovery.[32] The recently developed fast DKI approach provides an expedient measurement of mean diffusion and kurtosis, facilitating in vivo evaluation.[33–36] Previously, we performed multiparametric MRI in a filament middle cerebral artery occlusion (MCAO) rodent model of acute stroke to investigate the underlying pathophysiological difference between kurtosis lesion and kurtosis/diffusion lesion mismatch. [37] Compared to filament model, embolic model more closely mimics the pathophysiology of human MCAO with heterogeneous tissue damage, the possibility to apply thrombolytic therapy and a prolonged therapeutic window [38, 39]. In this study, we further investigated the tissue perfusion and pH states of kurtosis/diffusion lesion mismatch in an embolic MCAO model, which more closely mimics acute stroke than filament model with graded hemodynamic and metabolic change. We applied the inherent correlation-based normalization (ICON) analysis to suppress intrinsic kurtosis heterogeneity not related to ischemia, and enabled semiautomatic lesion segmentation.[40] The tissue perfusion, pH, diffusion and relaxation indices were measured from the contralateral normal area, kurtosis lesion and the kurtosis/diffusion lesion mismatch. Although perfusion and relaxation were not significantly different between the kurtosis lesion and the kurtosis/diffusion mismatch, we documented significantly lower pH within the kurtosis lesion from that of the kurtosis/diffusion lesion mismatch, substantiating the postulation that DKI demarcates the heterogeneous DWI lesion of graded metabolic derangement.
Material and Methods
Rodents
In vivo studies have been approved by the local institutional animal care and use committee. Briefly, adult male Wistar rats (Charles River Laboratory, Wilmington, MA) were anesthetized with 1.5–2.0% isoflurane/air mixture throughout the study, with physiological parameters (e.g., respiratory and rectal temperature) monitored and maintained within their normal ranges. Fifteen acute stroke rats (n = 15) were imaged 1 hr after acute embolic MCAO. One animal showed a very small ischemic lesion likely due to clot autolysis, and was excluded from the study. Briefly, homologous blood clots were prepared the day before the occlusion and stored overnight at 4°C [41]. Two blood clots (2 cm/each) were injected through the right internal carotid artery (ICA) to block the middle cerebral artery (MCA) under the guidance of laser doppler flowmetry (LDF).
MRI
We performed multiparametric echo planar imaging (EPI) of 5 consecutive slices (slice thickness = 2 mm, field of view = 20×20 mm2, matrix = 48×48) at a 4.7 T Biospec MRI scanner (Bruker Biospin, Billerica, MA). Briefly, DWI was performed using single-shot isotropic diffusion-weighted MRI with two b-values of 250 and 1,000 s/mm2 (repetition time (TR)/echo time (TE) = 3250/54 ms, 16 averages, scan time=2 min) [42]. In addition, fast DKI protocol was obtained (gradient duration/diffusion time (δ/Δ) = 6/20 ms, repetition time (TR)/echo time (TE) = 2500/36.6 ms, 4 averages, scan time = 3 min).[33] The fast DKI protocol consisted of one b = 0 s/mm2 reference image, followed by three images of b = 1,000 s/mm2 along three gradient directions of (1,0,0), (0,1,0) and (0,0,1), and nine images of b = 2,500 s/mm2 along nine diffusion directions. We also collected amplitude-modulated ASL MRI (TR/TE = 6500/15 ms, B1 = 4.7 μT, time of saturation = 3250 ms, labeling distance of 15 mm, modulation frequency of 250 Hz, 32 averages and scan time = 7 min).[43, 44] In addition, we performed fast pH-weighted APT MRI with unevenly segmented RF irradiation (Recovery time = 3000 ms, primary RF saturation time = 3000 ms, secondary RF saturation time = 500 ms, and B1 = 0.75 μT).[45] The unsaturated control scan was signal-averaged 8 times, while the saturated images were averaged 32 times (scan time = 4 min). Moreover, T1-weighted images were acquired using inversion recovery EPI, with seven inversion delays from 250 to 3000 ms (recovery time = 6500 ms, TE = 15 ms, 4 averages, scan time = 4 min); T2-weigthed images were obtained with two TEs of 30 and 100 ms (TR = 3250 ms, 4 averages, scan time = 0.5 min).
Data Analysis
MRI images were processed in Matlab (Mathworks, Framingham, MA). Specifically, for the fast DKI, mean diffusivity (MD) was calculated as the mean of MDx,y,z derived from the formula published by Jensen et al.[24]:
| (1) |
where , i = 1, j = 2, 3, and b1 = 0, b2 = 1,000, and b3 = 2,500 s/mm2. The mean kurtosis (MK) was obtained following the fast DKI quantification algorithm described by Hansen et al. [33]:
| (2) |
Parametric T1 map was obtained by least squares fitting of the signal as a function of the inversion time , where η is the inversion efficiency and TIi is the ith inversion time. ADC and T2w maps were calculated as and , where b1,2 and TE1,2 are two diffusion b values and TEs, respectively. In addition, cerebral blood flow (CBF) was calculated as , where Itag is the label image, Iref is the reference image, λ is the brain-blood partition coefficient for water, α is the degree of inversion with transient time correction, w is the post-labeling delay, and T1a is the arterial blood longitudinal relaxation time. Moreover, pH-weighted APT MRI was calculated as the difference between the magnetization transfer ratio (MTR) of the reference and label CEST scans (i.e. , where Iref and Ilabel are reference and label CEST scans with saturation applied at −3.5 and 3.5 ppm, respectively, and I0 is the control scan without pre-saturation). Tissue pH map was calibrated from APT MRI as published by Sun et al. [19]
| (3) |
where α is the labeling coefficient and σ is the spillover factor, calculated from relaxation images. In addition, fs is the amide proton concentration with respect to bulk tissue water, derived to be 1:867, and MTR’asym is the intrinsic asymmetry shift not due to pH-sensitive effect, being −7.44%*1.63/T1w.[19]
Because kurtosis images, unlike diffusion MRI, display notable intrinsic heterogeneity in the intact brain tissue, suppression of such non-ischemia related variation is necessary for reliable lesion segmentation. To achieve this, we performed inherent correlation-based normalization (ICON) analysis of the MK map based on magnetization transfer and relaxation images.[40] Briefly, MK from the contralateral normal brain was correlated with T1 and mean MTR () using Pearson’s correlation, excluding ventricular cavities. The difference between the experimentally measured MK and that estimated from regression analysis assuming no ischemia was calculated as ΔMRMK = MK-MKest, where MKest is the estimated MK map from the regression established from the contralateral normal brain tissue (i.e. MKest = C0+C1*R1+C2*MMTR+C3*R1*MMTR), and Cs are coefficients determined from the contralateral normal brain.[37] The diffusion and kurtosis lesions were determined using a K-means clustering-based algorithm.[22, 40] P values less than 0.05 were considered statistically significant.
Results
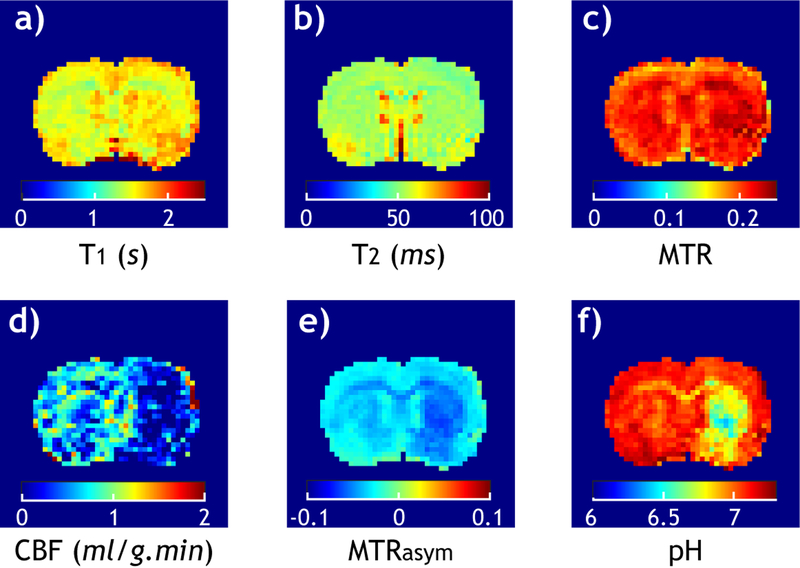
Fig. 1 shows multiparametric images from a representative acute stroke rat. T1 (Fig. 1a), T2 (Fig. 1b) and MMTR (Fig. 1c) images show relatively little change in the ipsilateral ischemic insult. In comparison, the CBF map (Fig. 1d) shows severe hypoperfusion in the right ipsilateral brain, evidencing successful MCAO. In addition, the pH-sensitive MTRasym map (Fig. 1e) captures noticeable hypointensity, predominantly within the ipsilateral striatum, from the contralateral normal area. Notably, MTRasym image shows non-negligible heterogeneity within the intact brain tissue, limiting its specificity to ischemia-induced pH change. To overcome this, tissue pH (Fig. 1e) was determined from pH-sensitive MTRasym and relaxation images by correction of non-pH related signal shift (MTR’asym), which confirmed severe pH drop within the striatum.[19]
Fig. 1.
Multiparametric images of a representative acute embolic MCAO rat. a) T1 map. b) T2 map. c) MMTR image. d) CBF map. e) pH-sensitive MTRasym image. f) Tissue pH map. Although relaxation and MT images show little signal change in the ipsilateral ischemic lesion, both perfusion and pH images reveal severe ischemia, evidencing acute MCAO.
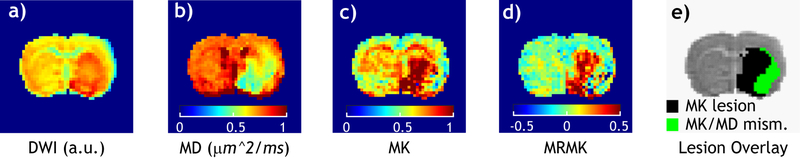
Fig. 2 shows diffusion and kurtosis images in a representative acute stroke rat. The DWI image shows hyperintensity in the striatum and ventral lateral cortex regions from the intact tissue (Fig. 2a). The MD map (Fig. 2b) reveals ipsilateral ischemic insult, similar as that captured by the routine DWI. In comparison, MK image (Fig. 2c) shows hyperintensity predominantly within the striatum without involving the lateral cortex. Similar as pH-sensitive MTRasym, the raw MK image is also susceptible to intrinsic tissue heterogeneity not related to ischemia. Fig. 2d shows magnetization transfer and relaxation normalized MK (MRMK) image that reasonably suppresses the intrinsic MK heterogeneity variation across the brain that has been dubbed ICON approach, enabling semi-automatic kurtosis lesion segmentation with MK and MD lesions shown in black and green, respectively (Fig. 2e). The MK and MD lesion volumes were determined to be 125±47 and 151±65 mm3, respectively, significantly different from each other (P<0.05, paired t-test).
Fig. 2.
Multiparametric diffusion images following acute embolic MCAO. a) DWI image (b=1000 s/mm2). b) MD map (µm2/ms). c) MK map. d) Magnetization transfer and relaxation-normalized MK image (MRMK). e) MK/MD lesion mismatch overlaid on the DWI image.
We analyzed perfusion, pH, diffusion, kurtosis and relaxation indices from the contralateral normal tissue, MD lesion, MK lesion and the MK/MD lesion mismatch (Table 1). Although CBF was reduced in the ischemic lesions (MD lesion, MK lesion and their mismatch), it did not show significant difference among intralesional regions (Fig. 3a). In addition to significant difference between normal tissue and ischemic lesions, pH decrease within the MK/MD lesion mismatch was significantly less than that of MD and MK lesions (Fig. 3b). Although MK lesion and MK/MD lesion mismatch have significantly different MD values from that of the MD lesion, MD did not differ between MK lesion and MK/MD lesion mismatch (Fig. 3c). Fig. 3d shows that MK in the MK lesion is significantly different from the contralateral normal, MD lesion and MK/MD lesion mismatch areas. It is important to point out that there was no significant MK difference between the contralateral normal and MK/MD lesion mismatch areas. This shows that MK/MD lesion mismatch has similar microstructural integrity as the normal tissue, indicating less injury than the MK lesion. Moreover, ischemic regions had significantly increased T1 from that of the contralateral normal area, yet without significant intralesional difference. Interestingly, we found that the MK lesion, not MD lesion and the MK/MD lesion mismatch, had significant T2 increase from that of the contralateral normal tissue, suggesting that the MK lesion has developed more prominent edema from other regions, consistent with worsened changes in pH and kurtosis.
Table 1:
Multiparametric MRI indexes for the normal, diffusion lesion, kurtosis lesion and their mismatch.
| Contralateral Normal | MD lesion | MK lesion | MD/MK mismatch | |
|---|---|---|---|---|
| MD (µm2/ms) | 0.79 ± 0.02 | 0.56 ± 0.02*§† | 0.60 ± 0.03*§ | 0.58 ± 0.03*† |
| MK | 0.65 ± 0.06 | 0.85 ± 0.06*§† | 0.97 ± 0.03*§† | 0.62 ± 0.06†‡ |
| CBF (ml/g.min) | 1.12 ± 0.18 | 0.43 ± 0.08* | 0.53 ± 0.10* | 0.46 ± 0.13* |
| pH | 7.04 ± 0.08 | 6.81 ± 0.09*† | 6.81 ± 0.09*‡ | 6.88 ± 0.09*†‡ |
| T1 (s) | 1.54 ± 0.05 | 1.67 ± 0.06* | 1.65 ± 0.08* | 1.67 ± 0.06* |
| T2 (ms) | 53.98 ± 0.86 | 54.83 ± 1.23 | 55.15 ± 1.64* | 54.51 ± 1.04 |
Repeated measures of ANOVA with Bonferroni’s multiple comparison analyses were performed with
P<0.05 indicating significant difference between contralateral normal and lesion regions,
P<0.05 indicating significant difference between MD lesion and MK/MD lesion mismatch,
P<0.05 indicating significant difference between MK lesion and MK/MD lesion mismatch, and
indicting significant difference between MD lesion and MK lesion, respectively.
Fig. 3.
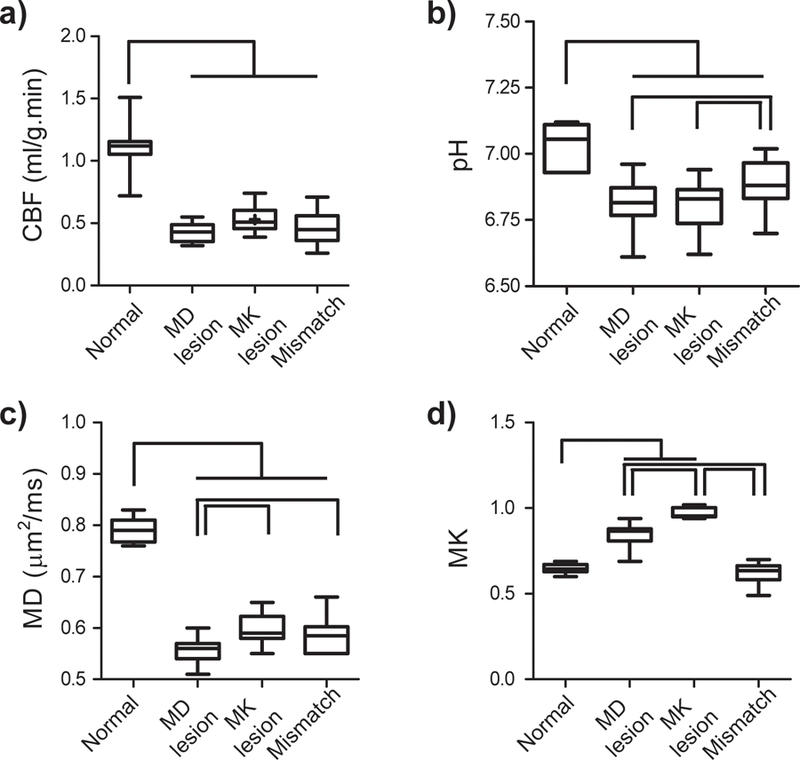
Regional analysis of multiparametric MRI indices of perfusion, pH, MD and MK values from the contralateral normal region, MD lesion, MK lesion and MK/MD lesion mismatch. a) CBF (ml/g.min). b) pH. c) MD (µm2/ms). d) MK.
Discussion
Our study confirmed significant kurtosis/diffusion lesion mismatch in an embolic MCAO model of acute stroke. We documented metabolic heterogeneity between kurtosis/diffusion lesion mismatch and kurtosis lesion, consistent with a prior report using a filament MCAO model.[37] Therefore, our study further supports the postulation that the kurtosis/diffusion lesion mismatch identifies portion of diffusion lesion of less severe ischemic injury.[32] By identifying the most severely injured ischemic lesion, DKI may augment routine DWI for improved definition of the infarction core, and ultimately aid imagining-guided stroke treatment (Fig. 4). It has also been shown that the outer boundary of ischemic penumbra can be refined with pH MRI. Specifically, hypoperfused tissue without measurable pH change likely represents ischemic tissue at no immediate risk of infarction (benign oligemia) while ischemic tissue with concomitant perfusion and pH decrease is at elevated risk of deterioration. Therefore, simultaneous acquisition of fast DKI and pH MRI provides a tangible means to demarcate the heterogeneous ischemic tissue into benign oligemia (hypoperfused tissue without pH change), metabolic penumbra (hypoperfused tissue without kurtosis abnormality) and infarction core (kurtosis lesion). Such a refined mismatch paradigm is consistent with that proposed by Kidwell et al., and it will be interesting to validate perfusion/pH/DKI-based tissue classification with acute histology and early reperfusion treatment.[46]
Fig. 4.
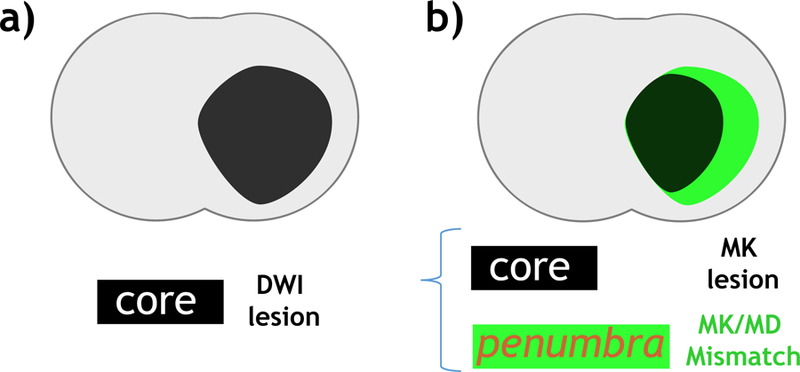
Refined MRI mismatch paradigm of infarction core. a) Infarction core (black) defined using routine DWI. b) The proposed refined mismatch paradigm that includes infarction core (black) defined by MK lesion and potentially reversible DWI lesion (penumbra) identified by MK/MD lesion match (green).
We applied magnetization transfer and relaxation normalization ICON algorithm to enhance the specificity of MK elevation to ischemia for lesion segmentation. This avoids manual lesion outline that may introduce inter/intra-rater variability. MRMK minimizes intrinsic heterogeneity for automatic lesion outline, yet it may overcorrect certain pixels, resulting in lesion highlighted by MRMK not MD. The number of such pixels are significantly less than the MK/MD mismatch. In addition, most of such pixels appear abnormal on DWI but not MD map (dorsal part of the striatum in Fig. 2a and 2b). Therefore, it might also arise from the slight errors in MD and MK quantification determined by the fast DKI approach, which assumes negligible high order diffusion and Rician noise terms [34]. Moreover, the MK map showed a stronger percentage change in lesions than that of MD, which may detect lesion even beyond the MD deficit with the automatic segmentation. Further investigation is needed to identify the origins underlying this reversed mismatch.
Our study used a dual coil setup that includes a volume transmitter and a small surface receiver to maximize the detection sensitivity without sacrificing radiofrequency (RF) field homogeneity. Although this configuration does not affect calculation of post-processed parametric maps such as CBF and pH images, it does introduce spatial variation in raw images, particularly DWI, as a function of the distance between the tissue and receiver coil. To map the profile of detection sensitivity, we modeled the signal drop using a polynomial function along the dorsal-ventral dimension on a plain EPI (TR/TE=3250/30 ms) image, correction of which yielded DWI images void of otherwise gross signal drop not related to ischemic insult (Fig. 2a). This allows comparison of routine diffusion images (DWI and ADC) and those from fast DKI (MD and MK). We also would like to point out that our study chose a fast DKI protocol instead of tensor model-based DKI approach to minimize the scan time. Although fast DKI provides MK measurement at higher contrast to noise per unit time, it admittedly could not derive the axial and radial kurtosis, which may provide further information about the underlying tissue microstructural change.[35] More advanced DKI approach such as double diffusion encoding and symmetrized double pulsed field gradient MRI may provide novel insights of diffusion and kurtosis changes following stroke, which shall be investigated in the future.[47, 48]
Whereas DWI and MD images depicted similar lesion areas, MK map showed noticeably smaller lesion area than that defined from diffusion images (Fig. 2). Our results found that MK lesion and MK/MD lesion mismatch have similar MD values, suggesting that the MK elevation is likely attributable to diffusional heterogeneity within the voxel. In addition, the absolute percentage magnitude change in MK is significantly higher than that of MD lesion (50 ± 4% vs. 24 ± 3%, P<0.05, paired t-test). This suggests that although the magnitude of diffusion change (i.e., ADC and MD) may be associated with graded ischemic insult within the DWI lesion, kurtosis provides a more sensitive and hence reliable index for demarcating the DWI lesion, consistent with the findings that the severity of ADC decrease does not reliably predict DWI reversibility.[13]
It is worth pointing out that embolic MCAO model, despite its variability, provides a closer representation of human stroke than the commonly used filament stroke models. As such, our study here highlighted the translational potential of DKI in the acute stroke setting. Although we found kurtosis lesion is of worsened pH drop from the mismatch, perfusion did not show significant intralesional difference. One plausible explanation is that the normal perfusion level varies within the intact brain tissue such as white and gray matter while the intact brain tissue has a relatively uniform intracellular tissue pH.[49, 50] Therefore, tissue pH change may be more specific to ischemia than perfusion. Also, our study used amplitude modulated ASL MRI sequence without a separate tagging coil. Although this configuration is easy to implement, it is of relatively low sensitivity to perfusion. It is likely that dynamic susceptibly contrast (DSC) MRI may provide more reliable and comprehensive perfusion measurement than ASL MRI, which may be investigated in the future. To summarize, our study showed that fast DKI is promising to demarcate the heterogeneous DWI lesion, and the refined DKI lesion classification is associated with graded metabolic disruption, warranting further investigation of DKI-guided late thrombectomy in the acute stroke setting.
Acknowledgement:
This study was supported in part by grants from NIH (R21NS085574 and R01NS083654).
Footnotes
IRB Statement: The study has been approved by the local institutional animal care and use committee.
Financial Disclosure: No conflict of interest.
References
- 1.Moseley ME, Cohen Y, Mintorovitch J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magnetic resonance in medicine 1990; 14:330–346 [DOI] [PubMed] [Google Scholar]
- 2.Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology 1992; 42:1717–1723 [DOI] [PubMed] [Google Scholar]
- 3.Rosen B, Belliveau J, Vevea J, Brady T. Perfusion imaging with NMR contrast agents. Magnetic resonance in medicine 1990; 14:249–265 [DOI] [PubMed] [Google Scholar]
- 4.Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996; 199:391–401 [DOI] [PubMed] [Google Scholar]
- 5.Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology 1999; 53:1528–1537 [DOI] [PubMed] [Google Scholar]
- 6.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. The Lancet Neurology 2012; 11:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidwell CS, Alger JR, Saver JL. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke 2004; 35:2662–2665 [DOI] [PubMed] [Google Scholar]
- 8.Kidwell CS, Saver JL, Mattiello J, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Annals of neurology 2000; 47:462–469 [PubMed] [Google Scholar]
- 9.Yoo AJ, Hakimelahi R, Rost NS, et al. Diffusion weighted imaging reversibility in the brainstem following successful recanalization of acute basilar artery occlusion. Journal of neurointerventional surgery 2010; 2:195–197 [DOI] [PubMed] [Google Scholar]
- 10.Labeyrie MA, Turc G, Hess A, et al. Diffusion lesion reversal after thrombolysis: a MR correlate of early neurological improvement. Stroke 2012; 43:2986–2991 [DOI] [PubMed] [Google Scholar]
- 11.Yamada R, Yoneda Y, Kageyama Y, Ichikawa K. Reversal of large ischemic injury on hyper-acute diffusion MRI. Case reports in neurology 2012; 4:177–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molad JA, Findler M, Auriel E. Computed Tomography Perfusion-Based Decision Making for Acute Ischemic Stroke-Missing the Mismatch. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 2017; 26:e78–e79 [DOI] [PubMed] [Google Scholar]
- 13.Loh PS, Butcher KS, Parsons MW, et al. Apparent diffusion coefficient thresholds do not predict the response to acute stroke thrombolysis. Stroke 2005; 36:2626–2631 [DOI] [PubMed] [Google Scholar]
- 14.Nicoli F, Lefur Y, Denis B, Ranjeva JP, Confort-Gouny S, Cozzone PJ. Metabolic counterpart of decreased apparent diffusion coefficient during hyperacute ischemic stroke: a brain proton magnetic resonance spectroscopic imaging study. Stroke 2003; 34:e82–87 [DOI] [PubMed] [Google Scholar]
- 15.Guadagno JV, Warburton EA, Jones PS, et al. How affected is oxygen metabolism in DWI lesions?: A combined acute stroke PET-MR study. Neurology 2006; 67:824–829 [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature medicine 2003; 9:1085–1090 [DOI] [PubMed] [Google Scholar]
- 17.Sun PZ, Zhou J, Huang J, van Zijl P. Simplified quantitative description of amide proton transfer (APT) imaging during acute ischemia. Magnetic resonance in medicine 2007; 57:405–410 [DOI] [PubMed] [Google Scholar]
- 18.Sun PZ, Cheung JS, Wang E, Lo EH. Association between pH-weighted endogenous amide proton chemical exchange saturation transfer MRI and tissue lactic acidosis during acute ischemic stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2011; 31:1743–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun PZ, Wang E, Cheung JS. Imaging acute ischemic tissue acidosis with pH-sensitive endogenous amide proton transfer (APT) MRI--correction of tissue relaxation and concomitant RF irradiation effects toward mapping quantitative cerebral tissue pH. NeuroImage 2012; 60:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Detection of the ischemic penumbra using pH-weighted MRI. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2007; 27:1129–1136 [DOI] [PubMed] [Google Scholar]
- 21.Harston GW, Tee YK, Blockley N, et al. Identifying the ischaemic penumbra using pH-weighted magnetic resonance imaging. Brain : a journal of neurology 2015; 138:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Zhou IY, Chan ST, et al. pH-sensitive MRI demarcates graded tissue acidification during acute stroke - pH specificity enhancement with magnetization transfer and relaxation-normalized amide proton transfer (APT) MRI. NeuroImage 2016; 141:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. NeuroImage 2009; 45:386–392 [DOI] [PubMed] [Google Scholar]
- 24.Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR in biomedicine 2010; 23:698–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinberg F, Farrher E, Kaffanke J, Oros-Peusquens AM, Shah NJ. Non-Gaussian diffusion in human brain tissue at high b-factors as examined by a combined diffusion kurtosis and biexponential diffusion tensor analysis. NeuroImage 2011; 57:1087–1102 [DOI] [PubMed] [Google Scholar]
- 26.Jensen JH, Falangola MF, Hu C, et al. Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR in biomedicine 2011; 24:452–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber RA, Hui ES, Jensen JH, et al. Diffusional Kurtosis and Diffusion Tensor Imaging Reveal Different Time-Sensitive Stroke-Induced Microstructural Changes. Stroke 2015; 46:545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grinberg F, Ciobanu L, Farrher E, Shah NJ. Diffusion kurtosis imaging and log-normal distribution function imaging enhance the visualisation of lesions in animal stroke models. NMR in biomedicine 2012; 25:1295–1304 [DOI] [PubMed] [Google Scholar]
- 29.Guo Y-L, Li S-J, Zhang Z-P, et al. Parameters of diffusional kurtosis imaging for the diagnosis of acute cerebral infarction in different brain regions. Experimental and Therapeutic Medicine 2016; 12:933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui ES, Fieremans E, Jensen JH, et al. Stroke assessment with diffusional kurtosis imaging. Stroke 2012; 43:2968–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui ES, Du F, Huang S, Shen Q, Duong TQ. Spatiotemporal dynamics of diffusional kurtosis, mean diffusivity and perfusion changes in experimental stroke. Brain Res 2012; 1451:100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung JS, Wang E, Lo EH, Sun PZ. Stratification of heterogeneous diffusion MRI ischemic lesion with kurtosis imaging: evaluation of mean diffusion and kurtosis MRI mismatch in an animal model of transient focal ischemia. Stroke 2012; 43:2252–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen B, Lund TE, Sangill R, Jespersen SN. Experimentally and computationally fast method for estimation of a mean kurtosis. Magnetic resonance in medicine 2013; 69:1754–1760 [DOI] [PubMed] [Google Scholar]
- 34.Sun PZ, Wang Y, Mandeville E, Chan ST, Lo EH, Ji X. Validation of fast diffusion kurtosis MRI for imaging acute ischemia in a rodent model of stroke. NMR in biomedicine 2014; 27:1413–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Kim J, Chan ST, et al. Comparison of image sensitivity between conventional tensor-based and fast diffusion kurtosis imaging protocols in a rodent model of acute ischemic stroke. NMR in biomedicine 2016; 29:625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen B, Khan AR, Shemesh N, et al. White matter biomarkers from fast protocols using axially symmetric diffusion kurtosis imaging. NMR in biomedicine 2017; 30:e3741-n/a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang E, Wu Y, Cheung JS, et al. pH imaging reveals worsened tissue acidification in diffusion kurtosis lesion than the kurtosis/diffusion lesion mismatch in an animal model of acute stroke. Journal of Cerebral Blood Flow and Metabolism 2017; 37:3325–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Zhang ZG, Zhang RL, et al. Postischemic (6-Hour) treatment with recombinant human tissue plasminogen activator and proteasome inhibitor PS-519 reduces infarction in a rat model of embolic focal cerebral ischemia. Stroke 2001; 32:2926–2931 [DOI] [PubMed] [Google Scholar]
- 39.Henninger N, Sicard KM, Schmidt KF, Bardutzky J, Fisher M. Comparison of ischemic lesion evolution in embolic versus mechanical middle cerebral artery occlusion in Sprague Dawley rats using diffusion and perfusion imaging. Stroke 2006; 37:1283–1287 [DOI] [PubMed] [Google Scholar]
- 40.Zhou IY, Guo Y, Igarashi T, et al. Fast diffusion kurtosis imaging (DKI) with Inherent COrrelation-based Normalization (ICON) enhances automatic segmentation of heterogeneous diffusion MRI lesion in acute stroke. NMR in biomedicine 2016; 29:1670–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asahi M, Asahi K, Wang X, Lo E. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism 2000; 20:452–457 [DOI] [PubMed] [Google Scholar]
- 42.Mori S, van Zijl PCM. Diffusion weighting by the trace of the diffusion tensor within a single scan. Magnetic resonance in medicine 1995; 33:41–52 [DOI] [PubMed] [Google Scholar]
- 43.Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology 1998; 208:410–416 [DOI] [PubMed] [Google Scholar]
- 44.Utting JF, Thomas DL, Gadian DG, Helliar RW, Lythgoe MF, Ordidge RJ. Understanding and optimizing the amplitude modulated control for multiple-slice continuous arterial spin labeling. Magnetic resonance in medicine 2005; 54:594–604 [DOI] [PubMed] [Google Scholar]
- 45.Sun PZ, Cheung JS, Wang E, Benner T, Sorensen AG. Fast multislice pH-weighted chemical exchange saturation transfer (CEST) MRI with Unevenly segmented RF irradiation. Magnetic resonance in medicine 2011; 65:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Umesh Rudrapatna S, Wieloch T, Beirup K, et al. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. NeuroImage 2014; 97:363–373 [DOI] [PubMed] [Google Scholar]
- 47.Paulsen JL, Özarslan E, Komlosh ME, Basser PJ, Song Y-Q. Detecting compartmental non-Gaussian diffusion with symmetrized double-PFG MRI. NMR in biomedicine 2015; 28:1550–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw CB, Hui ES, Helpern JA, Jensen JH. Tensor estimation for double-pulsed diffusional kurtosis imaging. NMR in biomedicine 2017; 30:e3722-n/a [DOI] [PubMed] [Google Scholar]
- 49.Mason GF, Chu W-J, Vaughan JT, et al. Evaluation of 31P metabolite differences in human cerebral gray and white matter. Magnetic resonance in medicine 1998; 39:346–353 [DOI] [PubMed] [Google Scholar]
- 50.Eilaghi A, d’Esterre CD, Lee TY, et al. Toward Patient-Tailored Perfusion Thresholds for Prediction of Stroke Outcome. American Journal of Neuroradiology 2014; 35:472–477 [DOI] [PMC free article] [PubMed] [Google Scholar]