Background
In dentistry, antibiotics are currently recommended for management of bacterial oral infections and for prophylaxis of infective endocarditis in patients with certain cardiac conditions and potentially in some immunocompromised patients. Increasing concerns about antibiotic resistance and antibiotic-associated adverse events make the identification of opportunities to improve antibiotic prescribing a public health priority. The Centers for Disease Control and Prevention (CDC) estimates that, in the United States, antibiotic-resistant infections annually affect at least 2 million people and cause 23,000 deaths.1 The primary driver of antibiotic resistance is the use of antibiotics, whether appropriate or inappropriate.2,3 Additionally, antibiotic use can lead to adverse drug events such as allergic reactions or Clostridium difficile infections, which are becoming increasingly common in the community setting and are often directly related to a recent antibiotic prescription or a visit to an outpatient healthcare facility.4–9 A recent study found that, among those with community-associated C. difficile infections (i.e. C. difficile infections occurring in individuals with no overnight healthcare facility stay within the previous 12 weeks), 40.7% reported that they had visited a doctor’s or dentist’s office in the preceding 12 weeks.9
Previously published studies have quantified the amount of antibiotic prescribing occurring in the outpatient setting in the United States and noted variability according to geographic region and provider specialty.10,11 Though the majority of antibiotic prescriptions in the outpatient setting were prescribed by primary care providers such as family practitioners (24%, 64.1 million prescriptions), pediatricians (12%, 32.4 million prescriptions), and internists (12%, 32.1 million prescriptions), in 2011, 10% (24.5 million prescriptions) of all antibiotics in the community were prescribed by general dentists.11 Despite the large contribution of dental prescribing to all antibiotic prescribing in the outpatient setting, not much is known about the details of antibiotic prescribing practices in dentistry. Recent changes in antibiotic prophylaxis recommendations and limited guidance from professional societies and other stakeholders regarding how to manage specific dental infections may be contributing to inappropriate antibiotic prescribing. To better understand how dentists are prescribing antibiotics in the United States, we conducted a study to characterize antibiotic prescribing by dentists according to antibiotic agent and class, patient demographics, and geographic region based on 2013 data.
Methods
Systemic, oral antibiotic prescriptions dispensed during 2013 were identified from the QuintilesIMS™ Xponent® database. QuintilesIMS™ captures greater than 75% of all outpatient prescriptions in the United States, reconciles them to wholesale deliveries, and projects to 100% coverage of all prescription activity using a patented projection methodology that is based upon a sample of patient de-identified prescription transactions, collected from pharmacies that report their entire pharmacy business to QuintilesIMS™ each week. These data represent all outpatient antibiotic prescriptions, across all payers, including community pharmacies and non-governmental mail service pharmacies with the exception of federal pharmacies and facilities. QuintilesIMS™ describes its projection methodology as standardizing these data into estimated prescription counts, and using geo-spatial methodology to align the ‘estimated’ prescriptions for the non-sample pharmacies to prescribers with observed prescribing behaviors for the same product in nearby sample pharmacies. The methodology is routinely validated at various levels of granularity by QuintilesIMS™ statistical and analytic teams.
Prescription counts were summarized by drug category, patient age and patient sex. Antibiotics were aggregated into categories according to the Uniform System of Classification (USC),_ENREF_20 a therapeutic classification system created by QuintilesIMS™ ,as follows: tetracyclines, cephalosporins, lincosamides (e.g. clindamycin), macrolides, narrow-spectrum penicillins (e.g. penicillin, amoxicillin), quinolones, trimethoprim/sulfamethoxazole, β –lactams with increased activity (e.g. amoxicillin-clavulanate), urinary anti-infectives (e.g. nitrofurantoin), and others. A list of all antibiotics and the respective USC class prescribed by dentists in 2013 can be found in Appendix A.
Provider specialties were based upon data collected by QuintilesIMS™ from multiple sources including the Drug Enforcement Agency and state licensing authorities. This analysis includes only those identified in the Xponent® data as practicing in general dentistry. Specialists, such as endodontists, periodontists and oral surgeons, were aggregated into broader specialty categories in the data we received and therefore were not included in this analysis. To estimate provider denominators and providers per capita, the QuintilesIMS™ Xponent® prescription database was used to extract the total number of prescribers in each provider specialty.
The total number of prescriptions, corresponding to the county-level location of the prescribing provider, and census denominators were used to calculate per-capita (1,000) prescribing rates by state and U.S. census region (https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html). In addition, prescribing by individual agent and drug class, as well as by patient demographics were analyzed. Indications and diagnoses were not available as these data capture dispensed prescriptions which to do not include this information. The total number of prescriptions was calculated for general dentists overall. All statistics were computed using SAS version 9.3 (SAS Institute, Cary, NC). This project was determined to be consistent with non-research program evaluation and monitoring by the Human Subjects Advisor in the National Center for Emerging and Zoonotic Infectious Diseases, and thus Institutional Review Board (IRB) review was not required.
Results
Dentists prescribed 24.5 million courses of outpatient antibiotics in 2013 for a prescribing rate of 77.5 prescriptions per 1,000 persons (Table 1). Females were prescribed more antibiotics than males by dentists in 2013 (females 13.7 million; 85.2 per 1,000 females; males 10.7 million; 69.2 per 1,000 males). The majority of antibiotics prescribed by dentists were for adults > 19 years of age; fewer than 10% of all antibiotics prescribed by dentists were for children < 19 years of age. Of the antibiotics prescribed for adults, the majority of these prescriptions were for those in the 40-64 year old age group (10.8 million prescriptions, 103.6 prescriptions per 1,000 persons). For children, the prescribing rate per 1,000 persons was 26.1 and 26.5 for ages 3-9 and 10-19, respectively. The prescribing rate for children < 2 years of age was only 2.8 prescriptions per 1,000 persons.
Table 1.
Antibiotic prescribing by general dentists according to antibiotic category, antibiotic agent and sex, age group, and geographic region for patients of all ages, 2013.
| Characteristic | Prescription | ||
|---|---|---|---|
| Number in millions | Percent | Per 1,000 persons | |
| Antibiotic category | |||
| Penicillins | 17.07 | 69.6 | 53.9 |
| Lincosamides | 3.57 | 14.6 | 11.3 |
| Macrolides | 1.33 | 5.4 | 4.2 |
| Cephalosporins | 1.24 | 5.1 | 3.9 |
| β –lactams, increased activity | 0.56 | 2.3 | 1.8 |
| Tetracycline | 0.47 | 1.9 | 1.5 |
| Quinolones | 0.21 | 0.8 | 0.6 |
| Trimethoprim/Sulfamethoxazole | 0.05 | 0.2 | 0.2 |
| Urinary anti-infectives | 0.02 | 0.1 | 0.1 |
| Other | 0.00 | 0.0 | 0.0 |
| Total | 24.52 | 100.0 | 77.5 |
| Antibiotic agent | |||
| Amoxicillin | 13.80 | 56.3 | 43.6 |
| Clindamycin | 3.57 | 14.4 | 11.3 |
| Penicillin V | 3.24 | 13.2 | 10.2 |
| Cephalexin | 1.19 | 4.9 | 3.8 |
| Azithromycin | 1.14 | 4.7 | 3.6 |
| Amoxicillin clavulanate | 0.56 | 2.3 | 1.8 |
| Doxycycline | 0.45 | 1.7 | 1.4 |
| Ciprofloxacin | 0.16 | 0.6 | 0.5 |
| Erythromycin | 0.15 | 0.4 | 0.5 |
| Sulfamethoxazole/trimethoprim | 0.05 | 0.2 | 0.2 |
| Others | 0.21 | 1.3 | 0.6 |
| Sex of patient | |||
| Male | 10.73 | 43.9 | 68.9 |
| Female | 13.70 | 56.1 | 85.2 |
| Age group of patient (years) | |||
| 0-2 | 0.03 | 0.1 | 2.8 |
| 3-9 | 0.75 | 3.1 | 26.1 |
| 10-19 | 1.11 | 4.6 | 26.5 |
| 20-39 | 5.76 | 23.9 | 67.4 |
| 40-64 | 10.79 | 44.8 | 103.6 |
| 65+ | 5.63 | 23.4 | 126.0 |
| Region of prescriber | |||
| Northeast | 4.87 | 19.9 | 87.0 |
| Midwest | 5.43 | 22.1 | 80.3 |
| South | 8.96 | 36.6 | 75.6 |
| West | 5.26 | 21.4 | 70.7 |
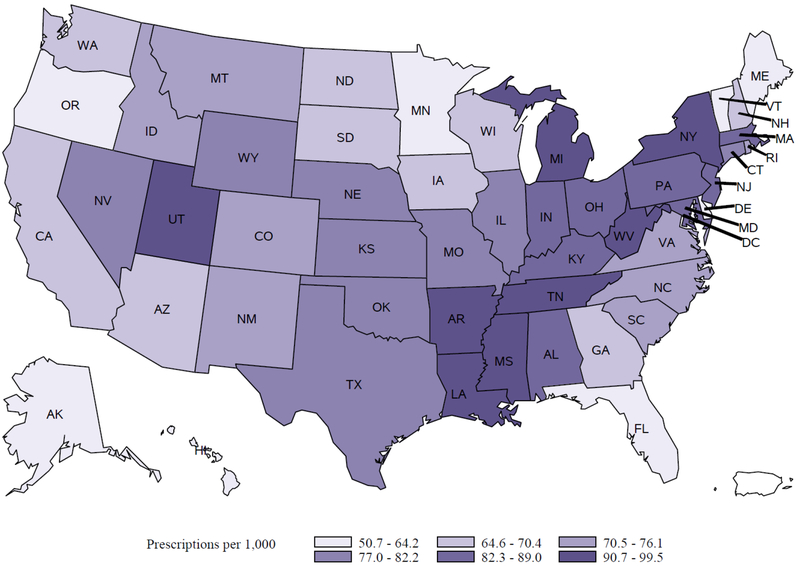
The geographic region with the most antibiotic prescriptions was the South census region (8.9 million, 36.6% of the total), but the Northeast region had the highest prescribing rate per 1,000 persons as compared to other regions of the country (Table 1). Amongst areas with the highest prescribing were the District of Columbia (99.5 prescriptions per 1,000 persons), Mississippi (97.3 per 1,000 persons) and Arkansas (96.7 per 1,000 persons). Areas with the lowest prescribing rates were Delaware (50.7 prescriptions per 1,000 persons), Alaska (55.3 per 1,000 persons) and Hawaii (57.2 per 1,000 persons) (Figure 1).
Figure 1:
Rates (per 1,000 persons) of antibiotics prescribed by dentists by state, 2013.
Penicillins were by far the most common antibiotic category prescribed (17 million prescriptions; 70% of all antibiotics), followed by lincosamides (3.6 million prescriptions; 15% of all antibiotics) and macrolides (1.3 million prescriptions; 5.4% of all antibiotics). Of note, some classes of antibiotics were prescribed by dentists that are not generally indicated in dentistry, including quinolones and urinary anti-infectives. The most commonly prescribed antibiotic agent was amoxicillin at a rate of 43.6 per 1,000 persons (13.8 million prescriptions), representing 56% of all antibiotics. Other commonly prescribed antibiotics were clindamycin (3.6 million prescriptions, 14.4% of all antibiotics); penicillin VK (3.2 million prescriptions; 13.2% of all antibiotics), cephalexin (1.2 million prescriptions; 4.9% of all antibiotic prescriptions) and azithromycin (1.1 million prescriptions; 4.7% of all antibiotic prescriptions). Amoxicillin-potassium clavulanate, doxycycline, ciprofloxacin, erythromycin and trimethoprim/sulfamethoxazole were also often prescribed by general dentists.
Discussion
We present the first broad description of outpatient antibiotic prescribing by general dentists in the United States, a critical first step to understanding how antibiotics are used in dentistry. The majority of antibiotic prescriptions were written for adult females, which is not surprising as previous studies have shown that females use health care services more than males in general.12,13 Also, when looking specifically at dental visits, females aged 20-64 had more dental visits than males of the same age.14 We also found that there is geographic variability in prescribing by dentists as has been shown in other studies looking at overall antibiotic prescribing in the United States.10, 11 Finally, while the most common class of drugs prescribed were the penicillins, which is in-line with available guidelines, some classes of drugs were prescribed that are not generally indicated in dentistry, which suggests opportunity for improvement.
The states with the highest prescribing rates per 1,000 persons were nearly double the rates from the lowest prescribing states, suggesting differences in prescribing behaviors across the United States by dentists. As with previous studies examining antibiotic prescribing overall (all providers, all patient ages), why such large geographic variation exists within antibiotic prescriptions written by dentists is unclear. One partial explanation may be that the states with higher antibiotic prescribing by dentists have a population that is different than lower prescribing states in terms of access to dental care and the general health of the population. Data collected through the Behavioral Risk Surveillance System and reported by CDC’s Division of Oral Health shows that regular preventative dental care is less common in the southeast United States, and tooth loss due to infections are higher in this part of the country as well.15 It is also possible that this reflects variability in the quality of antibiotic prescribing and that dentists in higher prescribing states may prescribe differently or more frequently than dentists in lower prescribing states. Much like previous studies examining overall antibiotic prescribing in the United States,10, 11 further exploration of the factors driving the variability and perceived inappropriateness seen in dental antibiotic prescribing will be necessary in order to develop successful interventions and identify partners to work with to improve how antibiotics are being used.
The majority of antibiotic prescriptions written by dentists were for amoxicillin, which is expected considering current guidelines for both prophylaxis prior to dental procedures16,17 and treatment of many oral infections.18,19 In fact, of the ten most commonly prescribed antibiotics by general dentists in 2013 and the antibiotic classes represented, the majority are mentioned in either prophylaxis or treatment guidelines as acceptable options for care when antibiotic treatment or prophylaxis is recommended. Exceptions to these include ciprofloxacin and trimethoprim/sulfamethoxazole, while infrequently prescribed by dentists are not recommended treatment options in current guidelines.16–19 Additionally, when exploring the broader classes of antibiotics that are prescribed by dentists, it is unclear why urinary anti-infectives would be seen in this data and may be an indication of inappropriate use.
While treatment guidelines with a focus on improving antibiotic prescribing and decreasing resistance exist for both pediatric and adult patients presenting with oral wounds and/or infections, the guidelines lack specific recommendations on what antibiotic agent should be used as the first-line treatment before looking at reasonable alternative agents and when a procedure may be a better course of action than a prescription. Three separate Cochrane Reviews looked at 21 studies surrounding the therapeutic use of antibiotics for irreversible pulpitis, tooth extractions, apical periodontitis and acute apical abscess and mixed evidence to support the practice of prescribing in these instances.20–22 In addition, data about guideline adherence for therapeutic antibiotic use for dentists in the United States is not readily available and recent studies exploring this issue have been international.23,24 One study found that dentists adhere to guidelines 10-42% of the time in pediatric patients.25 Several studies indicate widespread misuse of antibiotics among dentists even though most dentists self-report adherence to guidelines.26–30 In the United Kingdom, a 2014 qualitative study indicated that dentists are aware that antibiotic resistance is a problem but disagree on the dental community’s role in creating and combating resistance.31 Revised treatment guidelines based on the most recent data and clinical knowledge regarding the best treatment options for common oral infections, including specific first-line and second line-treatment recommendations, could be extremely useful in ensuring that dentists have the most up-to-date and clinically relevant information available to make treatment decisions that will lead to the best outcomes for the patient. In many cases, the best course of treatment may not be an antibiotic prescription at all. In diagnoses where the use of antibiotics is clearly a benefit to the patient and clinically appropriate, additional guidance on antibiotic selection, dosing and duration of therapy could be useful as well.
Antibiotic prophylaxis is likely also playing a large role in antibiotic prescribing, not only by dentists but by internists, family practitioners, cardiologists, surgeons, pediatricians and others who may also be prescribing and recommending prophylactic antibiotics for dental infections and procedures. The actual amount of antibiotic prescribing by dentists for prophylaxis versus treatment of oral infections is unknown. Historically, much of the prophylactic prescribing of antibiotics by dentists stemmed from growing concern that transient bacteremia from dental procedures was causing infective endocarditis (IE).32,33 The initial guideline recommending antibiotic prophylaxis for IE was issued by the American Heart Association (AHA) in 1955 and included a lengthy list of conditions that warranted prophylaxis, including native heart valve disease, prosthetic heart valves and pacemakers, among others.34 A recent review found no evidence supporting any of these practices except for weak support for patients with certain cardiac conditions.35 Today the AHA recommends IE prophylaxis only for patients considered to be higher risk for IE – including a prior history of IE, patients with prosthetic cardiac valves, certain types of congenital heart disease, and cardiac transplantation recipients who have a cardiac valvulopathy.16 The guideline also outlines antibiotic selection for prophylaxis, and recommends amoxicillin as first-line therapy. It is important to note here that the AHA guidelines in the United States differ from prophylaxis guidelines developed by the National Institute for Health and Clinical Excellence (NICE) for IE in the United Kingdom, where prophylaxis for patients previously thought to be at risk for IE has not been recommended since 2008.36 It is unclear whether this recommendation has had a direct impact on IE in the UK, though a 2015 study reported sharp decreases in prophylaxis and concerning rises in IE incidences. This prompted a review of the guidelines by several organizations, including NICE, but ultimately no change to the recommendation in the UK regarding prophylaxis for IE.38
In addition to the prevention of IE, antibiotic prophylaxis was historically recommended for dental procedures in patients with prosthetic joints. In 2012, a panel from the American Academy of Orthopaedic Surgeons and the American Dental Association (ADA) reviewed the available evidence and released guidelines citing limited evidence to support prescribing prophylactic antibiotics to patients with prosthetic joints.39 However, after the release of this guideline, the ADA received many requests for clarification by dentists, and ultimately convened another panel in 2014. This recent panel issued revised guidance, which recommend against prophylactic antibiotics for dental procedures in patients with prosthetic joints.17
While we cannot be certain that antibiotics are being prescribed inappropriately, recent qualitative studies suggest that unnecessary prophylaxis is likely quite common. According to one qualitative British study, dentists still struggle with patient expectations regarding antibiotic prophylaxis, especially among those who have had IE.40 In the United States, a recent study regarding dentists’ views and attitudes toward the new AHA IE prophylaxis guideline revealed that close to 100% of dentists are aware of the guideline and more than three quarters are satisfied with it.41 However, 19% report that their antibiotic prophylaxis prescribing practices had not changed since the release of the new guidelines and 70% said they had patients that receive antibiotic prophylaxis inappropriately due to other physicians’ recommendations, patient preference, dentist recommendations, or for other reasons.41 It is unclear what the long-term impact of recent prophylaxis guideline updates will have on antibiotic prescribing by dentists, but it is clear that this is one area of antibiotic prescribing where further study, as well as education of both providers and patients, is warranted.
The organizations that are responsible for educating dentists and developing guidelines will be key in sharing information about antibiotic resistance, the threat of community-associated C. difficile infections, and the importance of appropriate antibiotic use. The ADA has committed to making this issue a future priority as part of their involvement in the White House Forum on Antibiotic Stewardship which took place in June 201548 and other professional societies focused on dentistry, such as the Organization for Safety, Asepsis and Prevention and the International College of Dentists have made this issue a priority among their members.
There are limitations associated with this study. Prescribing data may not accurately represent actual consumption of antibiotics, as we only captured prescriptions that were filled by pharmacies and patient medication adherence varies. 47We are unable to assess the appropriateness of the prescriptions, as information about the indication for the prescription was not available in this dataset. The estimate of 24.5 million prescriptions in the outpatient setting prescribed by dentists is likely an underestimate of antibiotic prescribing for dental conditions and prophylaxis, since other provider specialties may prescribe antibiotics for prophylaxis and dental infections (especially in emergency departments).42–47 Finally, because of the structure of this data, we were only able to identify prescriptions by general dentists, and were unable to include antibiotic prescriptions written by oral surgeons, endodontists, periodontists and other dental specialties.
Conclusions
All healthcare providers that prescribe antibiotics have an important role to play in improving how antibiotics are used in the United States. This study provides a closer look at the specific antibiotics prescribed and geographic variability associated with antibiotic prescribing by dentists. However, additional studies looking more closely at antibiotic prescribing in dentistry are needed especially in terms of adherence to updated prophylaxis guidelines. Also, identifying data sources to assess the appropriateness of antibiotic prescribing will be important in determining where there are opportunities to improve. The appropriate prescribing of antibiotics in dentistry, as in all specialties, is not only a critical public health issue, but also a patient safety and quality improvement issue that warrants immediate attention.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge the Division of Oral Health at the Centers for Disease Control and Prevention, Robert Hunkler (QuintilesIMS™) and Natalia Chalmers, DDS, PhD (DentaQuest Institute) for their review and thoughtful comments on this manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention.
References
- 1.Antibiotic Resistance Threats in the United States. Atlanta, Ga.: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed September 29, 2016. [Google Scholar]
- 2.Turnidge J, Christiansen K. Antibiotic use and resistance--proving the obvious. Lancet 2005;365(9459):548–9. [DOI] [PubMed] [Google Scholar]
- 3.van de Sande-Bruinsma N, Grundmann H, Verloo D, et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis 2008;14(11):1722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis 2008;47(6):735–43. [DOI] [PubMed] [Google Scholar]
- 5.Kutty PK, Woods CW, Sena AC, et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis 2010;16(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beacher N, Sweeney MP, Bagg J. Dentists, antibiotics and Clostridium difficile-associated disease. British Dent J 2015;219(6):275–9. [DOI] [PubMed] [Google Scholar]
- 7.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372(9):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantes R, Mu Y, Hicks LA et al. Association between outpatient antibiotic prescribing practices and community-associated Clostridium difficilie infection. OFID 2015;2(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitnis AS, Holzbauer SM, Belflower RM et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med 2013; 173(14):1359–67. [DOI] [PubMed] [Google Scholar]
- 10.Hicks LA, Taylor TH, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013;368(15):1461–2. [DOI] [PubMed] [Google Scholar]
- 11.Hicks LA, Bartoces MG, Roberts RM, et al. U.S. outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015;60(9): 1308–16. [DOI] [PubMed] [Google Scholar]
- 12.Bertakis KD, Azari R, Helms LJ, et al. Gender differences in the utilization of health care services. J Fam Pract 2000;49(2):147–52. [PubMed] [Google Scholar]
- 13.Vaidya V, Partha G, Karmakar M. Gender differences in the utilization of preventive care services in the United States. J Womens Health (Larchmt) 2012;21(2):140–5. [DOI] [PubMed] [Google Scholar]
- 14.Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988–1994 and 1999–2004. National Center for Health Statistics. Vital Health Stat 2007;11(248):1–92. [PubMed] [Google Scholar]
- 15.Adults aged 18+ who have visited a dentist or dental clinic in the past year. Atlanta, Ga.: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. http://nccd.cdc.gov/oralhealthdata. Accessed September 29, 2016. [Google Scholar]
- 16.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 2008;139 Suppl:3S–24S. [DOI] [PubMed] [Google Scholar]
- 17.Sollecito TP, Abt E, Lockhart PB, et al. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: evidence-based clinical practice guideline for dental practitioners--a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 2015;146(1):11–16 e8. [DOI] [PubMed] [Google Scholar]
- 18.American Dental Association Council on Scientific Affairs. Combating antibiotic resistance. J Am Dent Assoc 2004;135(4):484–87. [DOI] [PubMed] [Google Scholar]
- 19.Guidelines for antibiotic therapy in pediatric patients. American Academy of Pediatric Dentist Council on Clinical Affairs; 2014. http://www.aapd.org/media/Policies_Guidelines/G_AntibioticTherapy.pdf. Accessed September 29, 2016. [Google Scholar]
- 20.Fedorowicz Z, van Zuuren EJ, Farman AG, et al. Antibiotic use for irreversible pulpitis. Cochrane Database Syst Rev 2013;12:CD004969. [DOI] [PubMed] [Google Scholar]
- 21.Lodi G, Figini L, Sardella A, et al. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst Rev 2012;11:CD003811. [DOI] [PubMed] [Google Scholar]
- 22.Cope A, Francis N, Wood F, et al. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Syst Rev 2014;6:CD010136. [DOI] [PubMed] [Google Scholar]
- 23.Marra F, George D, Chong M, et al. Antibiotic prescribing by dentists has increased: Why? J Am Dent Assoc 2016;147(5):320–7. [DOI] [PubMed] [Google Scholar]
- 24.Cope AL, Barnes E, Howells EP, et al. Antimicrobial prescribing by dentists in Wales, UK: findings of the first cycle of a clinical audit. Br Dent J 2016;221(1):25–30. [DOI] [PubMed] [Google Scholar]
- 25.Cherry WR, Lee JY, Shugars DA, et al. Antibiotic use for treating dental infections in children: a survey of dentists’ prescribing practices. J Am Dent Assoc 2012;143(1):31–8. [DOI] [PubMed] [Google Scholar]
- 26.Dar-Odeh NS, Abu-Hammad OA, Al-Omiri MK, et al. Antibiotic prescribing practices by dentists: a review. Ther Clin Risk Manag 2010;6:301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sivaraman SS, Hassan M, Pearson JM. A national survey of pediatric dentists on antibiotic use in children. Pediatr Dent 2013;35(7):546–9. [PubMed] [Google Scholar]
- 28.Johnson TM, Hawkes J. Awareness of antibiotic prescribing and resistance in primary dental care. Prim Dent J 2014;3(4):44–7. [DOI] [PubMed] [Google Scholar]
- 29.Epstein JB, Chong S, Le ND. A survey of antibiotic use in dentistry. J Am Dent Assoc 2000;131(11):1600–9. [DOI] [PubMed] [Google Scholar]
- 30.Cope AL, Chestnutt IG. Inappropriate prescribing of antibiotics in primary dental care: reasons and resolutions. Prim Dent J 2014; 3(4):33–7. [DOI] [PubMed] [Google Scholar]
- 31.Cope AL, Wood F, Francis NA, et al. General dental practitioners’ perceptions of antimicrobial use and resistance: a qualitative interview study. Br Dent J 2014;217(5):E9. [DOI] [PubMed] [Google Scholar]
- 32.Lewis T, Grant, RT. Observations relating to subacute infective endocarditis. Heart 1923;10:21–77. [Google Scholar]
- 33.Taran LM. Rheumatic fever in its relation to dental disease. NY J Dent 1944;14(3):107–13. [Google Scholar]
- 34.Jones TD, Baumgartner L, Bellows MT, et al. Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation 1955; 11:317–320. [Google Scholar]
- 35.Lockhart PB, Loven B, Brennan MT, et al. The evidence base for the efficacy of antibiotic prophylaxis in dental practice. J Am Dent Assoc 2007;138(4):458–74; quiz 534,–5, 437. [DOI] [PubMed] [Google Scholar]
- 36.Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures. National Institute for Health and Clinical Excellence (UK); London; 2008. https://www.ncbi.nlm.nih.gov/books/NBK51793/. Accessed September 29, 2016. [PubMed] [Google Scholar]
- 37.Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000–13: a secular trend, interrupted time-series analysis. Lancet 2015;385(9974):1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thornhill MH, Dayer M, Lockhart PB, et al. Guidelines on prophylaxis to prevent infective endocarditis. Br Dent J 2016;220(2):51–6. [DOI] [PubMed] [Google Scholar]
- 39.Rethman MP, Watters W 3rd, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am 2013;95(8):745–7. [DOI] [PubMed] [Google Scholar]
- 40.Soheilipour S, Scambler S, Dickinson C, et al. Antibiotic prophylaxis in dentistry: part I. A qualitative study of professionals’ views on the NICE guideline. Br Dent J 2011;211(1):E1. [DOI] [PubMed] [Google Scholar]
- 41.Lockhart PB, Hanson NB, Ristic H, Menezes AR, Baddour L. Acceptance among and impact on dental practitioners and patients of American Heart Association recommendations for antibiotic prophylaxis. J Am Dent Assoc 2013;144(9):1030–5. [DOI] [PubMed] [Google Scholar]
- 42.Okunseri C, Okunseri E, Thorpe JM, et al. Medications prescribed in emergency departments for nontraumatic dental condition visits in the United States. Med Care 2012; 50(6):508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allareddy V, Nalliah RP, Hague M, et al. Hospital-based emergency department visits with dental conditions among children in the United States: nationalwide epidemiological data. Pediatr Dent 2014; 36(5):393–9. [PubMed] [Google Scholar]
- 44.Naik RJ, Patel NR, Wang M, et al. Infective endocarditis prophylaxis: current practice trend among paediatric cardiologists: are we following the 2007 guidelines? Cardiol Young 2016; 26(6): 1176–82. [DOI] [PubMed] [Google Scholar]
- 45.Roberts RZ, Erwin PC. Pediatricians and the oral health needs of children: one potential means for reducing oral healthcare inequities. J Tenn Dent Assoc 2015; 95(2)23–7. [PubMed] [Google Scholar]
- 46.Hong L, Liu Y, Hottel TL, et al. Neighborhood socio-economic context and emergency department visits for dental care in a U.S. Midwestern metropolis. Public Health 2015; 129(3):252–7. [DOI] [PubMed] [Google Scholar]
- 47.Mostajer Haqiqi A, Bedos C, Macdonald ME. The emergency department as a ‘last resort’: why parents seek care for their child’s nontraumatic dental problems in the emergency room. Community Dent Oral Epidemiol 2016; 44(5): 493–503. [DOI] [PubMed] [Google Scholar]
- 48.More Than 150 Animal and Health Stakeholders Join White House Effort to Combat Antibiotic Resistance. Washington, DC: The White House, 2015. https://www.whitehouse.gov/sites/default/files/docs/060215_private_sector_factsheet.pdf. Accessed September 29, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.