Abstract
Introduction
The American College of Sports Medicine convened an International Multidisciplinary Roundtable on Exercise and Cancer in March 2018 to evaluate and translate the evidence linking physical activity and cancer prevention, treatment, and control. This paper discusses findings from the Roundtable in relation to the biologic and epidemiologic evidence for the role of physical activity in cancer prevention and survival.
Results
The evidence supports that there are a number of biologically plausible mechanisms whereby physical activity can influence cancer risk, and that physical activity is beneficial for the prevention of several types of cancer including breast, colon, endometrial, kidney, bladder, esophageal, and stomach. Minimizing time spent in sedentary behavior may also lower risk of endometrial, colon and lung cancers. Conversely, physical activity is associated with higher risk of melanoma, a serious form of skin cancer. Further, physical activity before and after a cancer diagnosis is also likely to be relevant for improved survival for those diagnosed with breast and colon cancer; with data suggesting that post-diagnosis physical activity provides greater mortality benefits than pre-diagnosis physical activity.
Conclusion
Collectively, there is consistent, compelling evidence that physical activity plays a role in preventing many types of cancer and for improving longevity among cancer survivors, although the evidence related to higher risk of melanoma demonstrates the importance of sun safe practices while being physically active. Together, these findings underscore the importance of physical activity in cancer prevention and control. Fitness and public health professionals and healthcare providers worldwide are encouraged to spread the message to the general population and cancer survivors to be physically active as their age, abilities, and cancer status will allow.
Keywords: physical activity, sedentary time, cancer, prevention, survival
INTRODUCTION
It was estimated that 18.1 million individuals were diagnosed with cancer in 2018 and 9.6 million individuals died from the disease—making cancer the second leading cause of mortality worldwide (1). In the United States (US) alone, the lifetime risk of developing cancer is 40% in men and 38% in women (2), and 1.74 million individuals were diagnosed with cancer in 2018 (3). There are also high direct and indirect costs related to the cancer burden; for example, in the US alone, the annual cost of cancer care is $158 billion (4), with billions of additional dollars lost to disability, lost work and lost household productivity (5). Thus, the burden of cancer remains a significant public health issue worldwide, and there is an increasing need to understand how modifiable health behaviors like physical activity may help prevent and control cancer in the population.
To begin to address the role of exercise in cancer, in 2008 the American College of Sports Medicine (ACSM) held the first Exercise and Cancer Roundtable that focused primarily on the role of exercise in cancer survivorship (6). At that time there was only limited evidence to show that physical activity may prevent malignancies other than breast or colon cancer, and studies suggesting that physical activity may extend survival after a cancer diagnosis were just emerging (7, 8). In the last decade there has been a substantial accumulation of new epidemiologic evidence indicating that physical activity is associated with the occurrence of many more types of cancer than previously thought, that sedentary behavior could also play a role, and that exercise may improve survival for those diagnosed with breast and colon cancer. Thus, when the ACSM convened a second Roundtable on Exercise and Cancer in March 2018, the objectives were expanded to review and summarize the biologic and epidemiologic evidence for the role of physical activity across the cancer prevention and control continuum. The purpose of this manuscript is to present the Roundtable conclusions focused on the role of physical activity as it pertains to risk of developing cancer and, among cancer survivors, as it relates to survival.
METHODS
The ACSM International Multidisciplinary Roundtable on Exercise and Cancer was held on March 12–13, 2018 in San Francisco, USA with 40 representatives from 20 organizations globally who were invited to participate based on their clinical and scientific expertise. Three topic areas were addressed, with subsequent resulting manuscripts summarizing the Roundtable conclusions on each topic. The present manuscript reviews the biologic and epidemiologic evidence related to physical activity, sedentary behavior, cancer risk and survival. The impact of exercise on the adverse effects of treatment is the focus of a companion publication (9), which together with this paper provides a comprehensive overview of the role of physical activity in reducing the risk of developing cancer and in improving the quality and quantity of life among cancer survivors. A third paper focuses on translation of the evidence base regarding exercise in cancer control into practice (10).
Herein, we first provide a brief overview of current concepts in cancer biology and a summary of human and animal/pre-clinical studies linking physical activity to processes thought to influence risk of developing and dying from cancer. Next, we summarize epidemiologic evidence for cancer risk based on recent review efforts including the Physical Activity Guidelines Advisory Committee (PAGAC) (11) and the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) (12), as well as a large pooling study (13), a book chapter (14), and updated meta-analyses(15–21), with consideration given to the inconsistencies between these recent reports. Finally, to reach conclusions for cancer survival, we reviewed observational studies of pre- and post-diagnosis physical activity, as well as results from randomized controlled trials of exercise interventions conducted in cancer survivors. Of note, because many observational studies capture data on “physical activity” whereas other study types, such as randomized controlled trials, generally measure “exercise”, here we use these terms interchangeably. Additionally, it should be noted this review focused on cancers diagnosed in adulthood as the evidence for physical activity and risk of childhood cancers is far more limited.
BIOLOGIC MECHANISMS
The process through which normal cells are transformed into invasive tumors and potentially lethal malignancies is complex (22, 23) and biological plausibility is essential for making strong inferences from epidemiologic evidence. There are more than 100 types of cancer that arise primarily from normal epithelial, connective and hematopoietic tissues (24). Cancer development is a multi-step process characterized by the transformation of normal tissues into pre-cancerous lesions and finally into malignant tumors for many cancers (22). From a molecular perspective, the process is propelled by genome instability (i.e., mutations and/or epigenetic alterations) in key growth regulatory genes (i.e., oncogenes, tumor suppressor genes). Both exogenous (e.g., tobacco smoke) and endogenous factors (e.g., hormones) promote the growth and survival of the transformed cells, facilitating their malignant progression (22). The hallmarks of cancer have been proposed to characterize key enabling factors and acquired capabilities that aid tumor growth and metastasis (25). The two enabling factors, genomic instability and tumor promoting inflammation, are thought to be general facilitators of the overall process. Acquired capabilities central to cancer development are related to cell proliferation (sustained proliferative signaling; evading growth suppressors); apoptosis (resisting cell death; enabling replicative immortality); angiogenesis; metabolic control (reprogramming cellular energy metabolism); and the immune response (evading immune destruction) (25). The final capability, invasion and metastasis, involves dissemination of cancer cells from the primary tumor into the circulatory system, successful migration to and penetration of distant tissues, and formation of new tumors. The ability to move into and thrive in specific tissues likely explains the preference of many common cancers to metastasize to organs that are vital for survival (e.g., lungs, brain, liver, bone) (22).
Physical activity is believed to affect the endogenous systemic milieu in a manner that influences cellular processes and tumor-growth (26). Several systemic factors have been proposed to modulate these processes including insulin/glucose metabolism, immune function, inflammation, sex hormones, oxidative stress, genomic instability, and myokines (26–30). Hyperinsulinemia may further play a role by reducing circulating IGF binding proteins and sex hormone binding globulins (SHBG) resulting in increased bioavailability of IGF-1 and sex hormones which can drive cell proliferation. Physical activity can also reduce cancer risk mediated through obesity (31, 32), since obesity is associated with increased risk of developing as many as 13 cancer types (33, 34) through similar biological mechanisms (35). For example, greater exercise-induced weight loss in postmenopausal women resulted in greater reductions in estradiol and C-reactive protein (27), changes that could reduce risk of breast and endometrial cancer. While more is known about the effects of exercise on these systemic endocrine factors, less is known about other biologic mechanisms (oxidative stress, DNA damage, epigenetic effects, myokines), and their possible interactions with different tumor subtypes within the same broad class of cancer (26–28). In making the link between circulating endogenous factors and cellular processes in tissues where cancer develops, studies in humans have shown that exercise can reduce cell proliferation (36) and increase markers of apoptosis in colorectal tissue (37), further demonstrating biological plausibility.
Preclinical animal studies have shown that physical activity can slow tumor growth across a wide range of cancer types (28, 38) and have started to provide important clues about the molecular processes underlying many of the hallmarks of cancer progression as described previously (26, 28, 38, 39). Many preclinical studies of physical activity, but not all (40), show substantial reductions in tumor growth in response to exercise with some studies citing reductions ranging between 31% and 67% (28, 38, 40). Repetitive exercise challenges whole-body homeostasis and has been shown to reduce cell proliferation, activate tumor suppressor genes (e.g., p53) and increase apoptosis in tumor tissue (28, 38, 39). Recent studies have also begun to investigate how exercise may influence mitochondrial metabolism in tumors (41). New insights regarding how exercise may affect angiogenesis in tumors have also emerged. Blood vessels in tumors are often structured abnormally which can limit tissue perfusion and increase hypoxia, which can lead to more aggressive tumors with a poorer prognosis (26). Studies using animal models of breast and prostate cancer have shown that exercise may normalize tumor blood vessels leading to greater tumor perfusion and oxygenation (26). It has been hypothesized that such changes could improve delivery of cytotoxic chemotherapies (28). Exercise combined with chemotherapy in mouse models has delayed tumor growth of breast cancer and melanoma more than chemotherapy alone (42, 43); suggesting that exercise could act synergistically with drug delivery to increase treatment efficacy. It has also been hypothesized that infiltration of cytotoxic immune cells into tumors may be enhanced by more normalized blood vessels (26), and evidence is emerging demonstrating associations between exercise, immune function and reduced tumor growth (28, 39). Exercise has demonstrable effects on immune function in humans (30), and a series of studies have suggested that interleukin-6 (IL-6) released from contracting skeletal muscle may facilitate epinephrine-dependent natural killer cell mobilization and subsequent immune cell infiltration into tumors (28). Two other putative myokines (oncostatin M, SPARC) have been linked to cancer in animal studies (28, 39), but relevance to humans remains uncertain (44).
Metastatic disease is responsible for 90% of all cancer deaths and the complex biology underlying the process of metastasis is not well understood (23). Thus, our understanding of how exercise may impede this process leading to reduced risk of dying from cancer is just emerging. Physical activity is thought to reduce the risk of recurrence and improve survival through similar mechanisms as have been investigated for cancer incidence. The most studied mechanisms in relation to cancer prognosis includes changes in adiposity, metabolic dysregulation, increasing circulating concentrations of adipokines and sex hormones, chronic low-grade inflammation, oxidative stress causing DNA damage with resulting gene mutations, and impaired immune surveillance and function (45). In addition to systemic endocrine factors that could facilitate development of metastatic tumors, it has been hypothesized that better tumor vasculature may impede release of tumor cells from the primary tumor and improved cytotoxic immune function could reduce survival of cancer cells and distant formation of metastatic lesions (28). Preliminary evidence from randomized controlled trials of exercise performed during cancer treatment have also suggested that treatment efficacy may be improved (45), and results from more definitive trials are pending. Thus, the mechanisms of action for exercise in relation to survival may include both improved treatment efficacy and other systemic adaptations to exercise which could generate additive or synergistic improvements in cancer outcomes. Although there is still much to be learned about the many and varied ways in which exercise can reduce the risk of developing or dying from cancer, there are many plausible biological mechanisms that may explain the benefits of exercise in cancer prevention and control described in the following sections.
CANCER PREVENTION
Cancer types associated with physical activity
Evidence that physical activity may play a large role in cancer prevention efforts has grown rapidly with new epidemiologic data on this topic accumulating over the past decade. At the time of the last ACSM Roundtable in 2008, there was only strong evidence linking physical activity with reduced risk of developing breast and colon cancer, and limited evidence linking physical activity with reduced risk of developing five other cancers (46) (Table 1). By 2018, sufficient evidence was available for a comprehensive review of physical activity and 16 cancer types by the Physical Activity Guidelines Advisory Committee (PAGAC) (11). The PAGAC review concluded that there is now strong evidence that physical activity lowers risk of seven types of cancer (namely colon, breast, kidney, endometrium, bladder, and stomach cancer, and esophageal adenocarcinoma), and moderate evidence for lung cancer (11), highlighting that increased physical activity may lower the risk of far more cancers than was thought just 10 years ago (Table 1).
Table 1.
The level of evidence linking physical activity with lower risk and sitting time with higher risk of cancer in 2008 [43] and 2018 [9] according to the Physical Activity Guidelines for Americans Advisory Committee.
| Cancer | Physical activity and lower risk, 2008 | Physical activity and lower risk, 2018 | Sitting time and higher risk, 2018 |
|---|---|---|---|
| Colon | Strong | Strong | Moderate |
| Breast | Strong | Strong | - |
| Kidney | - | Strong | - |
| Endometrial | Limited | Strong | Moderate |
| Bladder | - | Strong | - |
| Esophageal (adenocarcinoma) | - | Strong | - |
| Stomach (cardia) | - | Strong | - |
| Lung | Limited | Moderate | Moderate |
| Hematologic | - | Limited | - |
| Head and neck | - | Limited | - |
| Pancreas | - | Limited | - |
| Prostate | No effect (limited) | Limited | - |
| Ovary | Limited | Limited | - |
| Brain | - | Not assignable | - |
| Thyroid | - | No effect (limited) | - |
| Rectal | No effect (limited) | No effect (limited) |
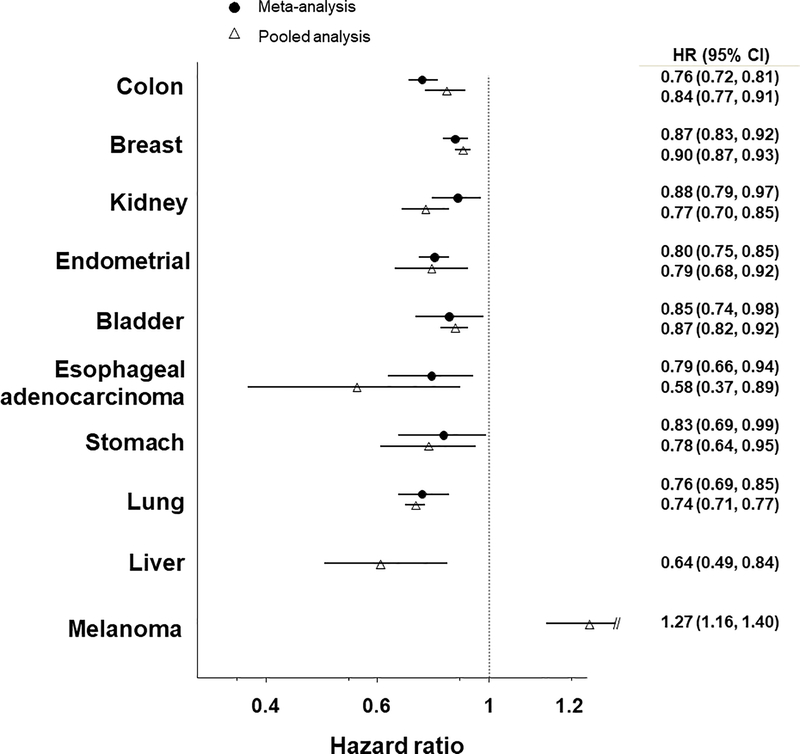
Although the 2018 PAGAC recently summarized evidence on physical activity and cancer prevention, the review effort for the Roundtable was undertaken in parallel and coincided with the PAGAC release. We based our evaluation of the evidence on systematic reviews (5, 15–20, 47–55) and a large pooled analysis of 12 prospective cohorts and 1.44 million study participants (13). Ultimately, we agreed with the majority of conclusions from the 2018 PAGAC report. We concur with the PAGAC for each of the seven types of cancer for which they concluded there was “strong” evidence for a protective effect of physical activity (Table 1). The magnitude of associations varied from 10–24% lower risks (hazard ratios (HR) 0.76–0.90) of these cancers (Figure 1) for higher versus lower level of activity, and there was great consistency in the observed associations comparing results from meta-analysis to the pooled analysis. We also concluded that there is more limited evidence for a protective effect of physical activity on hematologic cancers and cancers of the head and neck, pancreas, prostate, and ovary, though associations for the latter three types of cancer are more tenuous.
Figure 1. Multivariable hazard ratios (HRs) and 95% confidence intervals (CIs) for 10 types of cancer when comparing high versus low levels of physical activity.
The figure above depicts the hazard ratios for 10 types of cancer when comparing high versus low levels of physical activity. The hazard ratio for the most recent meta-analysis is shown by the black dot, and the hazard ratio for the large pooled analysis by Moore et al. (13) is shown by the open triangle. The top 8 types of cancer are those for which the Physical Activity Guidelines for Americans Advisory Committee (PAGAC) determined there was strong or moderate evidence for a protective effect of physical activity. The meta-analysis hazard ratios depicted here were derived from meta-analyses, as follows: colon (20), breast (5), kidney (47), endometrial (19), bladder (18), esophageal adenocarcinoma (15), stomach (cardia) (15), lung (17), liver (16). The bottom two cancers—liver cancer and melanoma—are those that we determined had substantial evidence from the pooled analysis but which the PAGAC report had not assigned a level of evidence.
We further determined that physical activity may also protect against risk of liver cancer. The pooled analysis (13) found that a high level of physical activity was associated with a 27% lower risk of liver cancer when compared with low levels of activity. This result was derived from 10 prospective cohorts and 1,384 liver cancer cases. No meta-analysis for liver cancer was available for the PAGAC report. Additionally, we found that physical activity may increase (not decrease) the risk of a serious form of skin cancer, melanoma. In the pooled analysis, a high level of physical activity was associated with a 27% greater risk of melanoma relative to low physical activity. Twelve cohorts with a total of 12,438 melanoma cases were evaluated and eight cohorts had hazard ratios of 1.2 or greater (range 1.23–1.90), which suggests a strong and highly consistent association. Given the unequivocal evidence for sun exposure as a cause of melanoma, it is likely that this association is attributable to the greater amount of time that physically active people spend outdoors (56), often in light clothing. These findings highlight that public health messages promoting physical activity should also emphasize the need for sun safety, but further research that directly addresses outdoor physical activity is needed to confirm this hypothesis.
Additionally, we evaluated evidence for two associations between physical activity and cancer that may be most susceptible to confounding, i.e. those for lung and endometrial cancers. The lung cancer association is complicated by the strong association of history of smoking with lung cancer risk, with hazard ratios exceeding 30-fold increased risk for current smoking vs. never smoking (57). It is debated whether statistical adjustments for smoking history are sufficient to remove confounding by such a powerful risk factor or whether restricting analyses to never smokers to guarantee that residual confounding by first-hand smoking is eliminated is necessary. Two of the three reviews and the well-powered pooled analysis found the physical activity-lung cancer association to be virtually null (HR of 0.96–1.05) among never smokers. The third review, by Zhong et al (58), found that the physical activity-lung cancer association was most likely inverse among never smokers, with the HR of 0.75 being virtually identical to the HR of 0.76 in current smokers and the HR of 0.77 in former smokers. However, the Zhong et al. review was highly influenced by a single outlying result from a small case-control study by Lin et al. (59). Without this study, the systematic reviews would be highly consistent in showing the physical activity-lung cancer association to be null in never smokers. Since no association between physical activity and lung cancer risk in never smokers appears to be evident, it remains unclear whether physical activity is in fact associated with lower lung cancer risk after accounting for the confounding effects of smoking; hence more research is needed to address this question more fully.
In our review, we also found some evidence suggesting that the association of physical activity with risk of endometrial cancer may be confounded by body weight. However, the association of physical activity with endometrial cancer may also be mediated, and not confounded by body weight given the fact that physical activity may act to reduce body weight. When the pooled analysis (13) adjusted for body mass index, the association of physical activity with endometrial cancer was completely abrogated. This finding differs from what was observed in a prior systematic review (19), where results were similar between studies that did and did not adjust for BMI. When compared with prior studies, though, the pooled analysis used a finer adjustment for BMI, including adjustment terms for each of the subcategories of obesity (30.0–34.9, 35.0–39.9, 40+ kg/m2), which would help to protect against any residual confounding. Additional research to address the role of body weight in the physical activity and endometrial cancer relationship is important for interpreting the endometrial cancer conclusion.
Type, Amount, Intensity and Timing of Physical Activity
Next we considered in more detail the dose-response relationships for the seven cancers with strong evidence that higher levels of physical activity are associated with lower risk, by examining results from available meta-analyses of colon (20, 60–66), breast (21, 64, 65, 67–69), endometrial (19, 70), kidney (47), bladder (18), esophageal (15, 71), and gastric cancers (15, 71–73). Specifically, we examined summary results in relation to the type, amount and intensity, and timing of physical activity. Results reported are derived from a review of the most recent and/or comprehensive studies and, when more than one source was available, the risk estimates reported were selected to be representative of the overall evidence. Differences in the strength of association by activity type or intensity should be interpreted cautiously given the mixture of study types (cohort, case-control) and the tendency for case-control studies to have stronger associations than those found in cohort studies (15, 18–20).
Physical Activity Type
Types of physical activity are determined by the life domain in which the activity occurs, and while the largest body of evidence is for leisure-time (i.e., active recreation, exercise, sports) and occupational physical activity, some information is available for household activity, active transportation, and walking. Table 2 summarizes meta-analytic risk estimates from selected studies for the seven cancer sites; including, the number of studies used to calculate the estimate and the different types of physical activity examined. The largest number of studies is available for leisure-time and occupational physical activity, with evidence of 10 to 20% lower risk comparing high to low activity levels. For kidney, bladder, gastric, and esophageal cancers a smaller number of occupational studies were available resulting in wider confidence intervals and non-statistically significant results for this exposure and these cancers. Protective associations were also noted for household, active transport and walking, but results were only available from a modest number of studies for colon, breast, and endometrial cancers in the available reviews. The smaller number of studies for some cancer sites and activity types other than leisure-time and occupational activity limits strong conclusions about the benefits of different types of physical activity but point to the need for more research to better understand these relationships. Evidence examining the association between incident cancer and strengthening activities (e.g., weight lifting), a key element of current physical activity recommendations (11), was unavailable in the reviews examined and is another understudied type of physical activity. Collectively, results are strongest for leisure-time and occupational physical activity, but different patterns of activity accumulation in these two domains suggest that a broad range of activity types may confer a lower risk of some cancers, although more definitive evidence is needed these for domains.
Table 2.
Summary of risk estimatesa for physical activity and risk of seven cancers with strong evidence, by physical activity type
| Physical Activity Type | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leisure-time | Occupational | Household | Transportation | Walking | ||||||
| Cancer Site | N | RR (95%CI) | N | RR (95%CI) | N | RR (95%CI) | N | RR (95%CI) | N | RR (95%CI) |
| Colon | 16 | 0.82 (0.75–0.87)[18] | 15 | 0.85 (0.77–0.93)[18] | 3 | 0.85 (0.71–1.02)[58] | 3 | 0.66 (0.45–0.98)[58] | -- | -- |
| Breast | 25 | 0.87 (0.83–0.91)[19] | 7 | 0.84 (0.73–0.96)[19] | 21 | 0.78 (0.69–0.89)[11] | -- | -- | 5 | 0.87 (0.79–0.96)[19] |
| Endometrial | 22 | 0.84 (0.78–0.91)[17] | 19 | 0.81 (0.75–0.87)[17] | 7 | 0.70 (0.47–1.02)[17] | -- | -- | 10 | 0.82 (0.69–0.97)[17] |
| Kidney | 19 | 0.88 (0.77–1.00)[44] | 14 | 0.91 (0.79–1.04)[44] | -- | -- | -- | -- | -- | -- |
| Bladder | 12 | 0.81 (0.66–0.99)[16] | 8 | 0.90 (0.76–1.07)[16] | -- | -- | -- | -- | -- | -- |
| Gastric | 22 | 0.80 (0.73–0.89)[13] | 11 | 0.84 (0.70–1.02)[13] | -- | -- | -- | -- | -- | -- |
| Esophageal | 10 | 0.72 (0.63–0.83)[13] | 8 | 0.91 (0.46–1.81)[13] | -- | -- | -- | -- | -- | -- |
Meta-analytic hazard ratio comparing the highest to lowest physical activity categories; ‘--’ indicates no summary estimates available in studies reviewed
Amount and Intensity
The “amount” of physical activity is conceptualized as the total volume of physical activity energy expenditure, or a weighted average of the frequency, intensity, and duration of physical activity (e.g., MET-hrs/wk). In most epidemiologic studies, intensity is determined via absolute intensity using metabolic equivalents (METs) and common energy expenditure thresholds (light, non-sedentary < 3 METs; moderate 3–5.9 METS; vigorous ≥ 6 METs (11)). Unfortunately, there are major challenges in translating results from available epidemiologic studies using meta-analytic techniques to estimate the optimal amount and intensity of physical activity associated with lower cancer risk, even though there is consistent evidence of a generalized dose-response relationship for a variety of cancers indicating higher levels of physical activity are associated with lower risk (13). This difficulty originates from the wide range of methods used for physical activity assessments and activity classification (e.g., tertiles, quartiles, quintiles) used in individual studies. Given the variation in methods between individual studies included in the various meta-analyses it is not possible to determine the minimum amount of leisure-time physical activity associated with lower risk of cancer, or to characterize the shape of the dose-response curve reliably. The current physical activity guidelines of 150 to 300 minutes/week of moderate or an equivalent amount of vigorous intensity aerobic activity (75 to 150 minutes/week) are recommended (32), although the precise amount needed for reduced risk of cancer remains to be determined.
In terms of physical activity intensity, most studies of leisure-time activity have focused on moderate-vigorous intensity activities, while the occupational studies were more varied in the intensity of activity considered. Meta-analytic estimates for associations for light, moderate, vigorous, and moderate-vigorous intensity activity suggest that moderate and vigorous intensity activity may be associated with lower risk for breast (21), and bladder (18) cancers, while results for endometrial cancer suggest benefit for light, moderate, and vigorous intensity activities (19) (see Table, SDC 1, Risk estimates for breast, endometrial, and bladder cancers by intensity of physical activity). The best available evidence from studies of leisure-time physical activity indicates that moderate-vigorous intensity physical activity is associated with reduced risk of many types of cancer, while evidence showing independent associations for light, moderate, or vigorous intensity activity is more limited.
Timing and Changes in Physical Activity
The transformation of normal cells into invasive tumors can take many years, hence, it is likely that long-term or lifetime participation in physical activity may be the most etiologically relevant for influencing cancer development (74). Previous reviews have examined the question of the relevant time period for physical activity influence on cancer etiology. Physical activity has been measured in different life-periods, including recent physical activity (e.g., past year), consistent participation over time (e.g., past 10 years, adulthood), and past activity participation in an earlier life-period (e.g., adolescence, early adulthood) (see Table, SDC 2, Risk estimates for renal, bladder, gastric, and esophageal cancers by timing (past, recent, and consistent over time) of physical activity). Recent physical activity appeared to be more strongly associated with lower risk of renal cancer compared to activity done earlier in life, while physical activity in many time periods was associated with lower risk for bladder and gastric cancer. In contrast, results suggest that longer-term participation rather than recent activity was linked to lower risk of esophageal cancer. Meta-analytic results were not available for colon and breast cancer, but narrative reviews have suggested that physical activity early, later, and throughout adulthood are associated with lower risk for these cancers (66, 69).
Cancer types associated with sedentary time
Emerging evidence supports that sitting time is a behavioral risk factor that is distinct from inadequate amounts of moderate-vigorous physical activity and could be an important additional target for intervention in the effort to increase daily physical activity in the population (75). In fact, accelerometry studies suggest that sitting time accounts for a significant proportion of the waking day and largely displaces light-intensity physical activity (76). As a result, interest in the amount of sitting time and chronic disease risk has grown rapidly in the past two decades and studies have documented higher risk of all-cause mortality (77–80), type II diabetes (81), cardiovascular disease (82) and some cancers (83, 84), independent of moderate-vigorous intensity physical activity.
Meta-analyses (84, 85) consistently have shown prolonged sitting time is associated with an approximate 30% higher risk of endometrial cancer after adjustment for moderate-to-vigorous physical activity, and the recent meta-analysis by Lynch et.al. (not included in the PAGAC or WCRF reports) reported 36% higher risk of endometrial cancer with the highest category of sitting time based on data from five prospective cohorts (85). Similarly, prolonged sitting time has been associated with a 30% higher risk of colorectal cancer (86), but it is unclear whether this association varies for colon and rectal cancer separately (84). In fact, a recent study found that sedentary time spent watching television increased the risk of rectal cancer to a greater extent than colon cancer in young-onset colorectal cancer (87). While both meta-analyses reviewed by the PAGAC reported 21–27% higher risk of lung cancer with the highest category of sitting time, a more recent meta-analysis (85) suggested the association may be a bit more modest (RR=1.13, 95% CI 0.94–1.36). The PAGAC concluded that there was moderate evidence for an association between increased sedentary time and risk of endometrial, colon, and lung cancers, but all other cancer sites were not assignable (Table 1) (11).
It is worth noting that results from studies for endometrial, colorectal, and lung cancers had sizeable heterogeneity between studies often due to study design (case-control vs. prospective cohort), type of sitting time examined (occupational vs. leisure-time vs. total), and, in regard, as previously discussed for physical activity, to fully assessing the role of potential confounders such as body weight and smoking status. There remains a paucity of evidence linking most cancer sites to prolonged sedentary time, and data on the dose-response relationship are sparse, limiting the ability to draw clear conclusions on the total amount of sitting time and/or breaks or bout length associations with any cancer type.
CANCER SURVIVAL
The consistent and strong evidence for an association between physical activity and a lower risk of developing several types of cancer has prompted investigation of the relationship between physical activity and mortality risk among cancer survivors. Research examining pre- and post-diagnosis physical activity and mortality has grown rapidly in the last decade. Herein, we discuss results for cancers with at least one observational study that evaluated the relationship between pre- and post-diagnosis physical activity and mortality (including cancer-specific and all-cause mortality) and provided sufficient data for inclusion in meta-analysis. Further, we also present exploratory analyses using data from randomized controlled trials of exercise conducted in cancer survivors that evaluated survival outcomes.
Observational studies on physical activity and cancer survival
At the time of the Roundtable at least 61 observational studies, involving breast, colorectal, prostate, endometrium, ovarian, kidney, lung, melanoma, lymphoma, childhood, esophageal, gastric, and malignant glioma cancer, had examined the association between the amount of physical activity and cancer survival, with median follow-up of 8–10 years (range: 2–43 years) post-diagnosis (Table 3). However, there were only sufficient data available from studies on patients with breast, colorectal and prostate cancer, to present findings across all four associations; that is, pre- and post-diagnosis physical activity and cancer-specific and all-cause mortality. Physical activity data were self-reported, mostly capturing recreational activity, and presented as relative units of physical activity per week, hours per week of physical activity and metabolic equivalent (MET)-hours per week. Pre-diagnosis physical activity represented a mix of recall of physical activity in the year prior to diagnosis and/or over a lifetime. Timing of post-diagnosis physical activity included assessment of pre-treatment levels, and levels within the first year of diagnosis, within the year preceding assessment or at one or more times between diagnosis and follow-up (up to 26 years post-diagnosis). Comparisons were typically made between lowest and highest levels of physical activity, with the cutpoints for the highest category of physical activity differing across studies.
Table 3.
Summary of risk estimates for pre- and post-diagnosis physical activity in relation to cancer-specific and all-cause mortality among cancer survivors.
| Cancer Site | Na | Pre-diagnosis Physical Activity | Post-diagnosis Physical Activity | |||||
|---|---|---|---|---|---|---|---|---|
| Cancer-Specific Mortality | All-Cause Mortality | Cancer-Specific Mortality | All-Cause Mortality | |||||
| RR (95% CI) | N | RR (95% CI) | N | RR (95% CI) | N | RR (95% CI) | ||
| Breast | 17 | 0.82 (0.73–0.92) | 17 | 0.79 (0.72–0.86) | 12 | 0.69 (0.56–0.84) | 13 | 0.59 (0.48–0.71) |
| Colorectal | 8 | 0.77 (0.68–0.87) | 7 | 0.75 (0.68–0.83) | 7 | 0.70 (0.54–0.90) | 9 | 0.62 (0.50–0.77) |
| Prostate | 6 | 0.99 (0.86–1.15) | 2 | 0.87 (0.80–0.96) | 4 | 0.67 (0.52–0.87) | 3 | 0.55 (0.40–0.76) |
| Endometrium | 2 | 1.04 (0.81–1.36) | 2 | 0.92 (0.77–1.10) | -- | -- | -- | -- |
| Ovarian | 2 | 1.01 (0.80–1.27) | 3 | 0.90 (0.71–1.13) | -- | -- | -- | -- |
| Kidney | 1 | 0.50 (0.27–0.93) | -- | -- | -- | -- | -- | -- |
| Lung | 1 | 0.78 (0.66–0.93) | -- | -- | -- | -- | 1 | 0.67 (0.31–1.48) |
| Melanoma | 1 | 1.09 (0.69–1.70) | -- | -- | -- | -- | -- | -- |
| NH Lymphomab | -- | -- | 1 | 0.85 (0.73–0.99) | 1 | 0.56 (0.31–1.03) | 1 | 0.63 (0.48–0.81) |
| Childhood cancers | -- | -- | -- | -- | 1 | 0.74 (0.39–1.42) | 1 | 0.79 (0.62–1.00) |
| Esophageal | -- | -- | -- | -- | 1 | 0.31 (0.22–0.43) | -- | 0.79 (0.55–1.13) |
| Gastric | -- | -- | -- | -- | -- | -- | 1 | 0.75 (0.61–0.93) |
| Malignant glioma | -- | -- | -- | -- | -- | -- | 1 | 0.64(0.46–0.91) |
All published papers on physical activity and cancer survival were identified to January 2018 and risk estimates for the highest versus lowest quantiles of physical activity and survival outcomes were extracted. A meta-analysis was conducted to provide overall summary risk estimates by cancer site.
Non-Hodgkin Lymphoma
Pre-diagnosis physical activity and mortality
At the time of the review, the authors identified 31 studies that evaluated the relationship between pre-diagnosis physical activity and breast (n=17), colorectal (n=8) and prostate (n=6) cancer-specific survival and conducted a meta-analysis of available studies (Table 3). The highest level of physical activity pre-diagnosis was associated with an 18% lower breast cancer mortality risk (HR=0.82) and 23% lower colorectal cancer mortality risk (HR=0.77), with some consistency in effect size across the contributing studies. In contrast, current evidence suggests that there is no association between pre-diagnosis physical activity and cancer-specific survival in prostate cancer. Risk of all-cause mortality, as assessed in 17 breast, 7 colorectal and 2 prostate cancer cohort studies, was significantly lower and ranged between 13% lower for prostate cancer and 25% lower for colorectal cancer when comparing the highest versus the lowest level of pre-diagnosis physical activity.
Post-diagnosis physical activity and mortality
Twenty-three studies evaluated the relationship between post-diagnosis physical activity and cancer-specific mortality (n=12, 7 and 4 studies involving breast, colorectal and prostate cancer cohorts, respectively). Collectively, these data support a lower risk of cancer-specific mortality (ranging from 26–69%) when comparing those in the highest versus lowest post-diagnosis physical activity categories (Table 3). A consistent inverse association with all-cause mortality was also found for breast (n=13 studies), colorectal (n=9 studies) and prostate cancer (n=3 studies), with 21–45% lower mortality risk. Evidence was insufficient to comment on the association between pre- and post-physical activity and cancer-specific and all-cause mortality outside of these three cancer sites. Nonetheless, results from individual studies, particularly for those evaluating the relation between post-diagnosis PA and survival, are consistent with those reported for breast, colorectal and prostate, suggesting potential for benefit in additional groups of cancer survivors.
Timing of physical activity and results within population sub-groups
Taking into account the strength of associations observed for breast, colorectal and prostate cancer, the evidence suggests that post-diagnosis physical activity exerts greater effects on mortality outcomes, compared with pre-diagnosis physical activity. Results across these three cancers also suggest that both pre- and post-diagnosis physical activity may more strongly influence all-cause mortality when compared to cancer-specific mortality. This larger effect may be expected given that cardiovascular disease is a major cause of mortality for specific subgroups of cancer survivors, particularly of these cancers. When assessing the relationship between pre- and post-diagnosis physical activity and survival outcomes within cancer subgroups, preliminary results suggest that benefits accrue, irrespective of BMI or estrogen-receptor status within breast cancer cohorts, and irrespective of cancer subtype and sex within colorectal cancer. Nonetheless, there is a need to accumulate additional research to better evaluate the extent to which subgroups of cancer survivors may derive greater benefit through physical activity.
Dose-response and changes in physical activity
When considering findings from breast cancer cohort studies, evidence supports a dose-response relationship between physical activity levels and breast cancer outcomes. More specifically, 15% of studies examining pre-diagnosis physical activity and 71% of studies examining post-diagnosis physical activity, found evidence for a linear relationship between physical activity levels and cancer-specific mortality outcomes. Studies examining all-cause mortality had 50% and 88% of studies presenting evidence of a linear relationship between pre-diagnosis and post-diagnosis physical activity, respectively. The exact dose of physical activity needed to reduce cancer-specific or all-cause mortality is not yet known since too few studies have been conducted that have detailed data on the risk associations for specific doses of activity. Further, while absolute levels of pre- and post-diagnosis physical activity levels are associated with improved survival, data from three breast cancer cohort studies also suggest that increased physical activity during the pre- to post-diagnosis time-periods matters (88–90). Specifically, an increase in recreational physical activity was associated with a 36% lower all-cause mortality risk (HR: 0.64, 0.48–0.87).
Sedentary time and cancer survival
There are limited epidemiological data linking sedentary time and cancer survival; however, a modest 12–13% higher risk of cancer mortality for higher versus lower amounts of any type of sitting in studies including those with and without cancer has been reported in recent meta-analyses (85, 91). Given studies of cancer mortality could reflect the influence of sitting on cancer incidence and subsequent survival of disease, it is conceivable that an association with cancer survival would exist. In a recent review by Lynch et.al. (85), among colorectal cancer survivors greater time spent sitting both pre- (HR=1.38, 95% CI 1.08–1.75) and post-colon cancer diagnosis (HR=1.61, 95% CI 1.23–2.11) was associated with higher risk of colon cancer-specific mortality. Data related to other cancer survivor populations are largely absent.
Findings from clinical trial research
Over the past five years, findings from exploratory analysis of exercise performed during and following treatment on survival outcomes using existing clinical trial data have become available. The trials involved patients with breast cancer (n=2 studies) (92, 93), lymphoma or leukaemia (n=2 studies) (94, 95), or patients with bone metastasis following a range of cancers (n=1 study) (96). Sample sizes ranged from 60 to 337 participants, and the majority (65–100%) of them had completed or were currently receiving chemotherapy at the time of exercise intervention. Interventions evaluated included aerobic-only, resistance-only, and combined aerobic and resistance exercise, prescribed at moderate to vigorous intensity for at least three times per week. Findings from one breast cancer trial supported a statistically significant effect of exercise on all-cause mortality (HR: 0.45, 95% CI: 0.20–0.96) (93). Findings from two other trials also suggested a beneficial effect of exercise on all-cause mortality of at least 40% lower risk (92, 94), although results were not statistically significant. In contrast, results from the trials involving patients with metastatic disease and with lymphoma showed no mortality benefit (95, 96).
The two breast cancer trials also assessed the effect of exercise on disease-free survival, or the length of time the patient lives with no signs or symptoms of cancer. Despite one trial being an efficacy trial (highly supervised during chemotherapy) and the other being an effectiveness trial (mostly unsupervised during and following treatment) findings were remarkably similar favouring the exercise group (HR=0.68 [95% CI=0.37–1.24] n=242; 0.66 [0.38–1.17] n=337), although neither result was statistically significant (92, 93). While these findings are exploratory, and the original studies were not powered to test these hypotheses, findings to date are consistent with results of observational studies, particularly in the breast cancer setting. Collectively, these results suggest that influencing physical activity behavior through exercise intervention may be beneficial for cancer-specific survival outcomes.
SUMMARY AND FUTURE DIRECTIONS
As the body of evidence used to inform the relationship between physical activity and cancer incidence and other cancer-related outcomes continues to grow, several study design aspects require comment. Future observational research would benefit from: device-based measurement of physical activity and sedentary behavior; consideration of different types and intensities of physical activity (light, moderate and vigorous aerobic, resistance, balance), consideration of sedentary behavior and physical activity at multiple time points across the life course and across the cancer continuum; accounting for competing risks of mortality and the possibility of reverse causality; assessment of non-linear dose-response relationships between physical activity levels and cancer outcomes; and the use of standardized definitions for cancer outcomes such as recurrence, survival, progression-free survival, or other survival-related outcomes. There remains insufficient evidence to make comments regarding the quality of associations between physical activity and sedentary behavior for many types and subtypes of cancer. Importantly, significant gaps in understanding the underlying biologic mechanisms linking physical activity to the development and progression of cancer remain.
The existing gaps in the survival literature suggest the need for investigating causal associations in adequately powered, randomized controlled trials, involving cancer survivors with comparatively good five-year survival (e.g., prostate, breast and colorectal cancer), as well as cancers associated with poorer prognosis (ovarian, pancreatic, stage IV disease). In this regard, progress in science is already happening. One ongoing observational project of particular note, is the Alberta Moving Beyond Breast Cancer (AMBER) cohort study, which involves device-based assessment of physical activity, sedentary behavior, health-related fitness and breast cancer outcomes (target sample size: 1500) (97). Additionally, four randomized controlled exercise intervention trials, involving cancer cohorts of colon (n=962) (98), metastatic prostate (n=866) (99), ovarian (n=500) (100) and allogeneic haemopoietic stem cell transplant patients (n=256) (101), are currently open for recruitment, with target sample sizes providing adequate power to evaluate cancer outcomes. Future research could help address some important evidence gaps including whether there exists a minimum and maximum threshold for benefit from physical activity and, if so, what these limits are and whether timing of physical activity (pre-diagnosis, during treatment, post-treatment), cancer type (or subtype) or other factors influence these limits. Understanding components of physical activity (frequency, type, intensity and duration of physical activity bouts) and the relationship of total dose (MET-hours/week) to the make-up of this dose with cancer outcomes, will also help inform optimal design of physical activity interventions.
While we await the findings from ongoing and future observational, intervention, and experimental research, key messages with clinical ramifications can already be taken from the existing body of evidence. First, physical activity is beneficial for the prevention of a number of different types of cancer including breast, colon, endometrial, kidney, bladder, esophageal, and stomach. Decreasing time spent sedentary may also lower risk of some types of cancer including endometrial, colon and lung cancers. Second, physical activity pre- and post-diagnosis of breast, colorectal and prostate cancer is beneficial for survival outcomes. Post-diagnosis physical activity seems to exert greater effect on cancer outcomes compared with pre-diagnosis physical activity. Third, findings that physical activity is associated with an increased risk of melanoma highlights the importance of sun safety while engaging in physical activity outdoors.
Being physically active is one of the most important steps people of all ages and abilities can take for cancer prevention, treatment, and control. The findings from this Roundtable demonstrate strong evidence exists to support an association between physical activity and cancer risk and survival for many types of cancer. The prescription for physical activity and cancer benefit is becoming more clear though there is much to be learned about the optimal dosage of physical activity and sedentary behavior for which confers benefit across cancers and cancer subtypes. Approximately one-fourth of adults (102) globally are physically inactive, putting them at increased risk for development or progression of cancer. Moving forward, key constituent groups such as health care providers, and physical activity and public health professionals all can have a role in communicating and promoting the benefits of physical activity for cancer prevention and control. Furthermore, the scientific community can examine impactful questions underlying the relationship between physical activity and cancer. To achieve wide-scale impact, broad engagement from experts in cancer prevention, treatment, and control working together with other stakeholders could help realize a shared vision of a more physically active and thus a more healthy world.
Supplementary Material
Acknowledgments
Results of the paper do not constitute endorsement by the American College of Sports Medicine.
Funding for the Roundtable was provided by: American College of Sports Medicine, American Cancer Society, American Academy of Physical Medicine and Rehabilitation, American Physical Therapy Association, Canadian Society for Exercise Physiology, Exercise and Sports Science Australia, German Union for Health Exercise and Exercise Therapy (DVGS), McMillan, Royal Dutch Society for Physical Therapy (KVDP),Sunflower Wellness.
We thank Lee W. Jones for his participation in and contributions to the Roundtable. Dr. Sandra Hayes’ research position is supported by a Cancer Council Queensland Fellowship. We also thank Dr. Sarah Keadle for her contributions in reviewing and synthesizing the evidence for cancer prevention.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of the National Institutes of Health or the Centers for Disease Control and Prevention. Results of the paper do not constitute endorsement by the American College of Sports Medicine. Funding for the Roundtable was provided by: American College of Sports Medicine, American Cancer Society, American Academy of Physical Medicine and Rehabilitation, American Physical Therapy Association, Canadian Society for Exercise Physiology, Exercise and Sports Science Australia, German Union for Health Exercise and Exercise Therapy (DVGS), McMillan, Royal Dutch Society for Physical Therapy (KVDP), Sunflower Wellness.
Conflict of Interest
As an academic physician, Dr. Silver has published books and receives royalties from book publishers, and she gives professional talks such as Grand Rounds and medical conference plenary lectures and receives honoraria from conference organizers. Dr. Silver has grant funding from 1) The Arnold P. Gold Foundation (physician and patient care disparities research), and 2) Binational Scientific Foundation (culinary telemedicine research). Dr. Silver has personally funded the Be Ethical Campaign and proceeds from the campaign support disparities research.
Footnotes
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of the National Institutes of Health or the Centers for Disease Control and Prevention.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. Epub 2018/09/13. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Noone AM, Howlader N, Krapacho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2015 Bethesda, MD: National Cancer Institute; 2018. [cited 2018 July 18, 2018]. Available from: https://seer.cancer.gov/csr/1975_2015/results_merged/topic_lifetime_risk.pdf. [Google Scholar]
- 3.Siegel Rebecca L., Miller Kimberly D., Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the Cost of Cancer Care in the United States: 2010–2020. JNCI: Journal of the National Cancer Institute. 2011;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guy GP, Ekwueme DU, Yabroff RK, Dowling EC, Li C, Rodriguez JL, et al. Economic Burden of Cancer Survivorship Among Adults in the United States. Journal of Clinical Oncology. 2013;31(30):3749–57. doi: 10.1200/jco.2013.49.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise. 2010;42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 7.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–86. [DOI] [PubMed] [Google Scholar]
- 8.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(22):3527–34. Epub 2006/07/11. doi: 10.1200/jco.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 9.Campbell K Exercise Guidelines for Cancer Survivors: Consensus statement from International Multidisciplinary Roundtable. Medicine and science in sports and exercise. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz K Exercise Is Medicine in Oncology: Engaging clinicians to help patients move through cancer. CA: a cancer journal for clinicians. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2018 Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report. In: Services DoHaH, editor. Washington, D.C.2018. [Google Scholar]
- 12.World Cancer Research Fund/American Institute of Cancer Research (WCRF/AICR). Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report. 2018. [Google Scholar]
- 13.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA internal medicine. 2016;176(6):816–25. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thun MJ, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D (eds.). Schottenfeld and Fraumeni Cancer Epidemiology and Prevention. 4th edition. New York, NY: Oxford University Press; 2017. [Google Scholar]
- 15.Behrens G, Jochem C, Keimling M, Ricci C, Schmid D, Leitzmann MF. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. European journal of epidemiology. 2014;29(3):151–70. Epub 2014/04/08. doi: 10.1007/s10654-014-9895-2. [DOI] [PubMed] [Google Scholar]
- 16.Behrens G, Matthews CE, Moore SC, Freedman ND, McGlynn KA, Everhart JE, et al. The association between frequency of vigorous physical activity and hepatobiliary cancers in the NIH-AARP Diet and Health Study. European journal of epidemiology. 2013;28(1):55–66. doi: 10.1007/s10654-013-9767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner DR, Yannitsos DH, Farris MS, Johansson M, Friedenreich CM. Leisure-time physical activity and lung cancer risk: A systematic review and meta-analysis. Lung Cancer. 2016;95:17–27. doi: 10.1016/j.lungcan.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Keimling M, Behrens G, Schmid D, Jochem C, Leitzmann MF. The association between physical activity and bladder cancer: systematic review and meta-analysis. Br J Cancer. 2014;110(7):1862–70. Epub 2014/03/07. doi: 10.1038/bjc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. European journal of epidemiology. 2015;30(5):397–412. Epub 2015/03/25. doi: 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 20.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. British journal of cancer. 2009;100(4):611–6. Epub 2009/02/10. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Research and Treatment. 2013;137(3):869–82. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg RA. Multi-step Tumorigenesis The Biology of Cancer, 2nd Edition. 2nd ed. New York: Garland Science, Taylor and Francis Group; 2013. p. 439–510. [Google Scholar]
- 23.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging Biological Principles of Metastasis. Cell. 2017;168(4):670–91. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg RA. The Nature of Cancer The Biology of Cancer, 2nd Edition. 2nd ed. New York: Garland Science, Taylor and Francis Group; 2013. p. 31–69. [Google Scholar]
- 25.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nature Reviews Cancer. 2017;17:620. doi: 10.1038/nrc.2017.78. [DOI] [PubMed] [Google Scholar]
- 27.Neilson HK, Conroy SM, Friedenreich CM. The Influence of Energetic Factors on Biomarkers of Postmenopausal Breast Cancer Risk. Current nutrition reports. 2014;3:22–34. Epub 2014/02/25. doi: 10.1007/s13668-013-0069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell metabolism. 2018;27(1):10–21. Epub 2017/10/24. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes care. 2010;33(7):1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson RJ, Kunz H, Agha N, Graff R. Chapter Fifteen - Exercise and the Regulation of Immune Functions In: Bouchard C, editor. Progress in Molecular Biology and Translational Science. 135: Academic Press; 2015. p. 355–80. [DOI] [PubMed] [Google Scholar]
- 31.Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. The Lancet Oncology. 2017;18(8):e457–e71. Epub 2017/08/02. doi: 10.1016/s1470-2045(17)30411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.2018 Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 33.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. Bmj. 2017;356:j477 Epub 2017/03/02. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer — Viewpoint of the IARC Working Group. 2016;375(8):794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doerstling SS, O’Flanagan CH, Hursting SD. Obesity and Cancer Metabolism: A Perspective on Interacting Tumor–Intrinsic and Extrinsic Factors. Frontiers in Oncology. 2017;7(216). doi: 10.3389/fonc.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McTiernan A, Yasui Y, Sorensen B, Irwin ML, Morgan A, Rudolph RE, et al. Effect of a 12-Month Exercise Intervention on Patterns of Cellular Proliferation in Colonic Crypts: A Randomized Controlled Trial. Cancer Epidemiology Biomarkers Prevention. 2006;15(9):1588–97. [DOI] [PubMed] [Google Scholar]
- 37.Campbell KL, McTiernan A, Li SS, Sorensen BE, Yasui Y, Lampe JW, et al. Effect of a 12-month exercise intervention on the apoptotic regulating proteins Bax and Bcl-2 in colon crypts: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1767–74. Epub 2007/09/15. doi: 10.1158/1055-9965.Epi-07-0291. [DOI] [PubMed] [Google Scholar]
- 38.Figueira ACC, Cortinhas A, Soares JP, Leitao JC, Ferreira RP, Duarte JA. Efficacy of Exercise on Breast Cancer Outcomes: A Systematic Review and Meta-analysis of Preclinical Data. International journal of sports medicine. 2018;39(5):327–42. Epub 2018/03/23. doi: 10.1055/s-0044-101149. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Casado A, Martin-Ruiz A, Perez LM, Provencio M, Fiuza-Luces C, Lucia A. Exercise and the Hallmarks of Cancer. Trends in cancer. 2017;3(6):423–41. Epub 2017/07/19. doi: 10.1016/j.trecan.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer research. 2016;76(14):4032–50. Epub 2016/07/07. doi: 10.1158/0008-5472.Can-16-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu M, Sanderson SM, Zessin A, Ashcraft KA, Jones LW, Dewhirst MW, et al. Exercise inhibits tumor growth and central carbon metabolism in patient-derived xenograft models of colorectal cancer. Cancer & metabolism. 2018;6:14 Epub 2018/11/27. doi: 10.1186/s40170-018-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. Journal of the National Cancer Institute. 2015;107(5). Epub 2015/03/18. doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, Addai P, et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. 2016;7(40):65429–40. doi: 10.18632/oncotarget.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Songsorn P, Ruffino J, Vollaard NBJ. No effect of acute and chronic supramaximal exercise on circulating levels of the myokine SPARC. European Journal of Sport Science. 2017;17(4):447–52. doi: 10.1080/17461391.2016.1266392. [DOI] [PubMed] [Google Scholar]
- 45.Friedenreich CM, Shaw E, Neilson HK, Brenner DR. Epidemiology and biology of physical activity and cancer recurrence. J Mol Med (Berl). 2017;95(10):1029–41. Epub 2017/06/15. doi: 10.1007/s00109-017-1558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.2008 Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report. In: Services DoHaH, editor. Washington, D.C: 2008. [Google Scholar]
- 47.Behrens G, Leitzmann MF. The association between physical activity and renal cancer: systematic review and meta-analysis. British journal of cancer. 2013;108(4):798–811. Epub 2013/02/14. doi: 10.1038/bjc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jochem C, Leitzmann MF, Keimling M, Schmid D, Behrens G. Physical activity in relation to risk of hematologic cancers: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(5):833–46. doi: 10.1158/1055-9965.EPI-13-0699. [DOI] [PubMed] [Google Scholar]
- 49.Leitzmann MF, Koebnick C, Freedman ND, Park Y, Ballard-Barbash R, Hollenbeck AR, et al. Physical activity and head and neck cancer risk. Cancer Causes Control. 2008;19(10):1391–9. doi: 10.1007/s10552-008-9211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Behrens G, Jochem C, Schmid D, Keimling M, Ricci C, Leitzmann MF. Physical activity and risk of pancreatic cancer: a systematic review and meta-analysis. European journal of epidemiology. 2015;30(4):279–98. doi: 10.1007/s10654-015-0014-9. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Hu F, Li D, Wang F, Zhu L, Chen W, et al. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol. 2011;60(5):1029–44. doi: 10.1016/j.eururo.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Zhong S, Chen L, Lv M, Ma T, Zhang X, Zhao J. Nonoccupational physical activity and risk of ovarian cancer: a meta-analysis. Tumour Biol. 2014;35(11):11065–73. doi: 10.1007/s13277-014-2385-z. [DOI] [PubMed] [Google Scholar]
- 53.Niedermaier T, Behrens G, Schmid D, Schlecht I, Fischer B, Leitzmann MF. Body mass index, physical activity, and risk of adult meningioma and glioma: A meta-analysis. Neurology. 2015;85(15):1342–50. doi: 10.1212/WNL.0000000000002020. [DOI] [PubMed] [Google Scholar]
- 54.Schmid D, Behrens G, Jochem C, Keimling M, Leitzmann M. Physical activity, diabetes, and risk of thyroid cancer: a systematic review and meta-analysis. European journal of epidemiology. 2013;28(12):945–58. doi: 10.1007/s10654-013-9865-0. [DOI] [PubMed] [Google Scholar]
- 55.Robsahm TE, Aagnes B, Hjartaker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. Eur J Cancer Prev. 2013;22(6):492–505. doi: 10.1097/CEJ.0b013e328360f434. [DOI] [PubMed] [Google Scholar]
- 56.Lai JK, Lucas RM, Armstrong M, Banks E. Prospective observational study of physical functioning, physical activity, and time outdoors and the risk of hip fracture: a population-based cohort study of 158,057 older adults in the 45 and up study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(10):2222–31. Epub 2013/04/24. doi: 10.1002/jbmr.1963. [DOI] [PubMed] [Google Scholar]
- 57.Freedman ND, Abnet CC, Caporaso NE, Fraumeni JF Jr., Murphy G, Hartge P, et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int J Epidemiol. 2016;45(3):846–56. doi: 10.1093/ije/dyv175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong S, Ma T, Chen L, Chen W, Lv M, Zhang X, et al. Physical Activity and Risk of Lung Cancer: A Meta-analysis. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2016;26(3):173–81. doi: 10.1097/JSM.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 59.Lin Y, Cai L. Environmental and dietary factors and lung cancer risk among Chinese women: a case-control study in southeast China. Nutr Cancer. 2012;64(4):508–14. doi: 10.1080/01635581.2012.668743. [DOI] [PubMed] [Google Scholar]
- 60.Harriss DJ, Atkinson G, Batterham A, George K, Cable NT, Reilly T, et al. Lifestyle factors and colorectal cancer risk (2): a systematic review and meta-analysis of associations with leisure-time physical activity. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2009;11(7):689–701. Epub 2009/02/12. doi: 10.1111/j.1463-1318.2009.01767.x. [DOI] [PubMed] [Google Scholar]
- 61.Mahmood S, MacInnis RJ, English DR, Karahalios A, Lynch BM. Domain-specific physical activity and sedentary behaviour in relation to colon and rectal cancer risk: a systematic review and meta-analysis. Int J Epidemiol. 2017;46(6):1797–813. Epub 2017/10/13. doi: 10.1093/ije/dyx137. [DOI] [PubMed] [Google Scholar]
- 62.Shaw E, Farris MS, Stone CR, Derksen JWG, Johnson R, Hilsden RJ, et al. Effects of physical activity on colorectal cancer risk among family history and body mass index subgroups: a systematic review and meta-analysis. BMC cancer. 2018;18(1):71 Epub 2018/01/13. doi: 10.1186/s12885-017-3970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical Activity and Risks of Proximal and Distal Colon Cancers: A Systematic Review and Meta-analysis. JNCI: Journal of the National Cancer Institute. 2012;104(20):1548–61. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- 64.Liu L, Shi Y, Li T, Qin Q, Yin J, Pang S, et al. Leisure time physical activity and cancer risk: evaluation of the WHO’s recommendation based on 126 high-quality epidemiological studies. British journal of sports medicine. 2016;50(6):372–8. Epub 2015/10/27. doi: 10.1136/bjsports-2015-094728. [DOI] [PubMed] [Google Scholar]
- 65.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyle T Physical Activity and Colon Cancer: Timing, Intensity, and Sedentary Behavior. American Journal of Lifestyle Medicine. 2012;6(3):204–15. doi: 10.1177/1559827612436932. [DOI] [Google Scholar]
- 67.Pizot C, Boniol M, Mullie P, Koechlin A, Boniol M, Boyle P, et al. Physical activity, hormone replacement therapy and breast cancer risk: A meta-analysis of prospective studies. European Journal of Cancer. 2016;52:138–54. doi: 10.1016/j.ejca.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 68.Neilson HK, Farris MS, Stone CR, Vaska MM, Brenner DR, Friedenreich CM. Moderate-vigorous recreational physical activity and breast cancer risk, stratified by menopause status: a systematic review and meta-analysis. Menopause. 2017;24(3):322–44. doi: 10.1097/gme.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 69.Friedenreich CM. The role of physical activity in breast cancer etiology. Seminars in oncology. 2010;37(3):297–302. Epub 2010/08/17. doi: 10.1053/j.seminoncol.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 70.Keum N, Ju W, Lee DH, Ding EL, Hsieh CC, Goodman JE, et al. Leisure-time physical activity and endometrial cancer risk: dose-response meta-analysis of epidemiological studies. International journal of cancer. 2014;135(3):682–94. Epub 2014/01/01. doi: 10.1002/ijc.28687. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Yu C, Li Y. Physical activity and risks of esophageal and gastric cancers: a meta-analysis. PloS one. 2014;9(2):e88082–e. doi: 10.1371/journal.pone.0088082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Psaltopoulou T, Ntanasis-Stathopoulos I, Tzanninis IG, Kantzanou M, Georgiadou D, Sergentanis TN. Physical Activity and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2016;26(6):445–64. Epub 2016/10/27. doi: 10.1097/jsm.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 73.Singh S, Devanna S, Edakkanambeth Varayil J, Murad MH, Iyer PG. Physical activity is associated with reduced risk of esophageal cancer, particularly esophageal adenocarcinoma: a systematic review and meta-analysis. BMC gastroenterology. 2014;14:101-. doi: 10.1186/1471-230X-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White E, Hunt JR, Casso D. Exposure measurement in cohort studies: the challenges of prospective data collection. Epidemiologic reviews. 1998;20(1):43–56. Epub 1998/10/08. [DOI] [PubMed] [Google Scholar]
- 75.Keadle SK, Conroy DE, Buman MP, Dunstan DW, Matthews CE. Targeting Reductions in Sitting Time to Increase Physical Activity and Improve Health. Med Sci Sports Exerc. 2017;49(8):1572–82. Epub 2017/03/09. doi: 10.1249/mss.0000000000001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Craft LL, Zderic TW, Gapstur SM, Vaniterson EH, Thomas DM, Siddique J, et al. Evidence that women meeting physical activity guidelines do not sit less: an observational inclinometry study. The international journal of behavioral nutrition and physical activity. 2012;9:122 Epub 2012/10/05. doi: 10.1186/1479-5868-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ekelund E, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388:1302–10. [DOI] [PubMed] [Google Scholar]
- 78.Chau JY, Grunseit AC, Chey T, Stamtakis E, Brown WJ, Matthews CE, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS One. 2013;8:e80000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel AV, Bernstein L, Deka A, Feigelson HS, Campbell PT, Gapstur SM, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol. 2010;172:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matthews CE, George SM, Moore SC, Bowles HR, Blair A, Park Y, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. The American Journal of Clinical Nutrition. 2012;95(2):437–45. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 82.Ford ES, Caspersen CJ. Sedentary behaviour and cardiovascular disease: a review of prospective studies. International journal of epidemiology. 2012;41(5):1338–53. doi: 10.1093/ije/dys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel AV, Hildebrand JS, Campbell PT, Teras LR, Craft LL, McCullough ML, et al. Leisure-Time Spent Sitting and Site-Specific Cancer Incidence in a Large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1350–9. Epub 2015/07/02. doi: 10.1158/1055-9965.epi-15-0237. [DOI] [PubMed] [Google Scholar]
- 84.Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. Journal of the National Cancer Institute. 2014;106(7). Epub 2014/06/18. doi: 10.1093/jnci/dju098. [DOI] [PubMed] [Google Scholar]
- 85.Lynch B, Mahmood S, Boyle T. Sedentary behaviour and cancer In: Leitzmann MFea, editor. Sedentary Behaviour Epidemiology: Springer International Publishing; 2018. [Google Scholar]
- 86.Shen D, Mao W, Liu T, Lin Q, Lu X, Wang Q, et al. Sedentary behavior and incident cancer: a meta-analysis of prospective studies. PloS one. 2014;9(8):e105709–e. doi: 10.1371/journal.pone.0105709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chan AT, Fuchs CS, Giovannucci EL, Wu K, Ng K, Nguyen LH, et al. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectrum. 2019;2(4). doi: 10.1093/jncics/pky073%JJNCICancerSpectrum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The Health, Eating, Activity, and Lifestyle study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(24):3958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the Women’s Health Initiative. Cancer Prev Res. 2011;4(4):522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ammitzboll G, Sogaard K, Karlsen RV, Tjonneland A, Johansen C, Frederiksen K, et al. Physical activity and survival in breast cancer. Eur J Cancer. 2016;66:67–74. Epub 2016/08/17. doi: 10.1016/j.ejca.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 91.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Annals of internal medicine. 2015;162(2):123–32. Epub 2015/01/20. doi: 10.7326/m14-1651. [DOI] [PubMed] [Google Scholar]
- 92.Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, Friedenreich CM, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46(9):1744–51. Epub 2014/03/19. doi: 10.1249/MSS.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 93.Hayes SC, Steele ML, Spence RR, Gordon L, Battistutta D, Bashford J, et al. Exercise following breast cancer: exploratory survival analyses of two randomised, controlled trials. Breast Cancer Res Treat. 2018;167(2):505–14. Epub 2017/10/25. doi: 10.1007/s10549-017-4541-9. [DOI] [PubMed] [Google Scholar]
- 94.Wiskemann J, Kleindienst N, Kuehl R, Dreger P, Schwerdtfeger R, Bohus M. Effects of physical exercise on survival after allogeneic stem cell transplantation. International journal of cancer. 2015;137(11):2749–56. Epub 2015/06/11. doi: 10.1002/ijc.29633. [DOI] [PubMed] [Google Scholar]
- 95.Courneya KS, Friedenreich CM, Franco-Villalobos C, Crawford JJ, Chua N, Basi S, et al. Effects of supervised exercise on progression-free survival in lymphoma patients: an exploratory follow-up of the HELP Trial. Cancer Causes Control. 2015;26(2):269–76. Epub 2014/12/11. doi: 10.1007/s10552-014-0508-x. [DOI] [PubMed] [Google Scholar]
- 96.Rief H, Bruckner T, Schlampp I, Bostel T, Welzel T, Debus J, et al. Resistance training concomitant to radiotherapy of spinal bone metastases - survival and prognostic factors of a randomized trial. Radiat Oncol. 2016;11:97 Epub 2016/07/29. doi: 10.1186/s13014-016-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Courneya KS, McNeely ML, Culos-Reed SN, Vallance JK, Bell GJ, Mackey JR, et al. The Alberta Moving Beyond Breast Cancer (AMBER) Cohort Study: recruitment, baseline assessment, and description of the first 500 participants. BMC cancer. 2016;16:481 Epub 2016/07/16. doi: 10.1186/s12885-016-2534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Courneya KS, Booth CM, Gill S, O’Brien P, Vardy J, Friedenreich CM, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008;15(6):279–85. Epub 2008/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Newton RU, Kenfield SA, Hart NH, Chan JM, Courneya KS, Catto J, et al. Intense Exercise for Survival among Men with Metastatic Castrate-Resistant Prostate Cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open. 2018;8(5):e022899 Epub 2018/05/17. doi: 10.1136/bmjopen-2018-022899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hayes SC, Friedlander M, Obermair A, Mileshkin L, Janda M, Gordon L, et al. Exercise during Chemotherapy for Ovarian Cancer (ECHO): study design features and outcomes of a Cancer Australia and Cancer Council Australia funded randomized, controlled trial 15th Biennial meeting of the International Gynecological Society 2014. [Google Scholar]
- 101.Wiskemann J, Kuehl R, Dreger P, Huber G, Kleindienst N, Ulrich CM, et al. Physical Exercise Training versus Relaxation in Allogeneic stem cell transplantation (PETRA Study) - Rationale and design of a randomized trial to evaluate a yearlong exercise intervention on overall survival and side-effects after allogeneic stem cell transplantation. BMC cancer. 2015;15:619 Epub 2015/09/09. doi: 10.1186/s12885-015-1631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Organization WH. Global Health Observatory (GHO) data. Prevalence of Insufficient Physical Activity (2016). 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.