Abstract
Background
Many genera and species of Streptococcus-like bacteria (SLB) can cause infective endocarditis (IE), but little is known about the epidemiology of and the risk factors for IE in SLB-bacteremia. The aim of the study was to analyze this in a cohort of patients with SLB-bacteremia, focusing on Abiotrophia, Aerococcus, Gemella, and Granulicatella. We also evaluated whether published scoring systems generated for other Gram-positive bacteria known to cause IE (HANDOC for streptococci and NOVA and DENOVA for enterococci) could be used in SLB bacteremia to decide whether transesophageal echocardiography (TEE) could be omitted.
Methods
Positive blood cultures with SLB were retrieved from population-based registries in Sweden (3.2 million inhabitants), from January 2012 to December 2017. Clinical data were collected from medical records. Risk factors for IE were analyzed and the performances of the scoring systems were calculated.
Results
The incidence of bacteremia with the 4 SLB genera was 30 episodes/1 000 000 population per year, of which Aerococcus contributed with 18. Among 568 episodes of bacteremia, 32 cases of IE were identified (5.6%). Infective endocarditis was most common in bacteremia with Abiotrophia (4 of 19) followed by Granulicatella (9 of 124), Gemella (6 of 87), and Aerococcus (13 of 338). NOVA had 100% sensitivity to identify IE but a low specificity (15%). For HANDOC and DENOVA, the sensitivities were 97% and 91%, respectively, whereas specificities were 85% and 90%, respectively, and numbers needed to screen were 3.6 and 2.8, respectively.
Conclusions
Bacteremia with these SLB is relatively rare, and the decision whether TEE should be performed or not could be based on either HANDOC or DENOVA.
Keywords: bacteremia, echocardiography, endocarditis, management score
Through a retrospective population-based study, we describe the epidemiology of blood culture findings of the Streptococcus-like genera, Abiotrophia, Aerococcus, Gemella, and Granulicatella, clinical presentation, and propensity to cause endocarditis. Furthermore, we evaluate whether transesophageal echocardiography could be omitted.
Patients with suspected infective endocarditis (IE) pose a clinical challenge for healthcare systems all over the world [1]. Infective endocarditis is a severe infection of the heart valves that had a mortality of close to 100% in the pre-antibiotic era. Hence, a lot of effort has been put into developing the diagnostic work up and optimizing the management [2, 3], which is composed by a combination of long-time antimicrobial treatment and in some cases valve surgery. The 2 major criteria for the diagnosis is the identification of the causative bacterial pathogen in blood cultures and the demonstration of cardiac changes [4]. Transesophageal echocardiography (TEE) is more sensitive and specific than transthoracic echocardiography (TTE) and is the preferred modality to visualize the engagement of the heart valves [5], and it is a cornerstone in the diagnostic work up. However, TEE is a limited resource, and not without risks and discomfort for the patient, and it should be used rationally [6].
To guide the clinician, studies during the last years have developed algorithms to evaluate the risk of IE and the need for TEE in patients with bacteremia, based on readily available clinical data. The PREDICT [7] and VIRSTA [8] scores were developed for patients with Staphylococcus aureus bacteremia, whereas the NOVA score [9] can be used in enterococcal bacteremia. All 3 systems have high sensitivities but are afflicted with a relatively low specificity and are thus not easily applicable in settings where TEE is a limited resource. Two scoring systems with high sensitivity and specificity are the HANDOC for bacteremia with the different species of non-β-hemolytic streptococci [10] and the DENOVA designed for Enterococcus faecalis [11]. Furthermore, these scorings systems indicate the importance of the identification of the bacterium to the species, because the different species within a genus can exhibit different propensity to cause IE.
Both streptococci and enterococci belong to the order of Lactobacillales, which contains bacterial families of other genera and species known to cause IE. The genera Abiotrophia and Granulicatella are mentioned in guidelines for IE [2, 3], and their tendency to cause IE has been well documented [12–14]. Different species of Aerococcus have recently been increasingly recognized as a relatively common cause of bacteremia in certain age groups and tend to cause IE [15–17]. Not within the order, but related, is the genus Gemella that can also cause IE [18].
Little is known about the epidemiology, evaluation, and treatment of patients with bacteremia with these Streptococcus-like bacteria (SLB) genera or species and their propensity to result in IE. We aimed to study the epidemiology of the SLB causing IE and test whether the known scoring systems, for evaluating the need for TEE, are applicable on episodes of SLB bacteremia using a large population-based cohort.
METHODS
Statistics of species causing IE were collected from The Swedish Registry for Endocarditis. All consecutive blood cultures positive for Abiotrophia, Aerococcus, Gemella, and Granulicatella, from January 2012 through December 2017, were obtained from the databases of the Clinical Microbiology Laboratory in Skåne County (the only laboratory in the region with a catchment area of 1.3 million inhabitants and 9 hospitals) and from Karolinska University Laboratory, Karolinska University Hospital, Stockholm, Sweden (analyzing blood cultures from a population of 1.9 million inhabitants in the Stockholm County).
All medical records of patients with Abiotrophia, Aerococcus, Gemella, and Granulicatella bacteremia were studied retrospectively. Patients under the age of 18, or where medical records could not be retrieved, were excluded. Ethical approval was obtained from The Ethics Committee of Lund University (2017/1002) and from the Ethics committee review board in Stockholm (recordal 2015/1184-31).
Microbiology
Determination of genera and species was performed with matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS), using the direct transfer method as described previously [16]. If a MALDI-TOF MS score value >2.0 was obtained in the routine analysis, this was considered to be reliable to the species level. In cases in which a score of <2.0 but ≥1.8 was found and the second-best species had a score difference greater than 0.2, the identification to the species was considered reliable as previously shown for Aerococcus [19]. In other cases, a new MALDI-TOF MS analysis was made on stored isolates using the standard ethanol-formic acid extraction method described by the instrument manufacturer (Bruker, Bremen, Germany). The remaining isolates were analyzed by sequencing the 16S ribosomal ribonucleic acid (rRNA) gene and assigned a species as described previously [20]. The rest of the isolates, without a species assignment, were either not retrievable or did not generate a reliable assignment by 16S rRNA gene sequencing and were only assigned to the genus level.
Definitions
In the study, we use the term Streptococcus-like bacteria. Streptococcus-like bacteria is defined by the genera and species included in the order of Lactobacillales, except for species from the genera of Streptococcus and Enterococcus. Further included in SLB are species from the genus Gemella, attributed to the order of Bacillales, due to its clinical and bacteriological similarities.
An episode of SLB bacteremia was defined as a clinical situation in a patient resulting in blood cultures taken that showed growth of SLB. Multiple positive blood cultures taken on different days were included in the same episode if they were taken during the same clinical situation. To discriminate an episode from a recurrent bacteremia, an episode was delimited by at least 7 days of effective treatment.
Infective endocarditis was defined using the modified Duke criteria [4]. In the Duke criteria, the SLB were considered to be included in the viridans streptococci group.
Scoring of the patients, according to the following scoring systems, was performed using the information available to the clinician at the time when receiving the positive blood culture result analyzed to the genus or species. NOVA score parameters were defined as previously described by Bouza et al [9], with modification of number of cultures as described by Dahl et al [21], and DENOVA and HANDOC defined according to previous publications [10, 11]. The criteria for the scoring systems were as follows: two positive blood cultures were sufficient to fulfill the N variable of the scoring systems. Unknown origin of infection, O in NOVA [9], and DENOVA [11] was defined as lack of a focal infection, which was likely to be the point of entry for the bacteria. Thus, some types of focal infection, such as IE, spondylodiscitis, or septic arthritis, were not regarded as origins of infection. A focal infection was defined as previously described [11]. V (valve disease in NOVA and DENOVA) is defined as a history of any of the predisposing heart conditions that constitute a high or moderate risk of developing IE, including (1) native valve disease, (2) previous IE, or (3) the presence of a valve prosthesis [22, 23], and is in concordance with the minor criterion of predisposition (together with injection drug use) in the modified Duke criteria [4]. A cardiovascular implantable electronic device, pacemaker, or implantable cardioverter defibrillator was not regarded as a predisposing heart condition [22]. Auscultation of murmur (A in NOVA and DENOVA) included any murmur reported on admission or before scoring. H in HANDOC included either any valve disease or heart murmur. Duration of symptoms (D in DENOVA and HANDOC) was defined as ≥7 days before the first positive blood culture was taken with any symptom compatible with IE [24]. Embolization (E in DENOVA; vascular or immunological phenomena in the Duke criteria [4, 11]) was defined by signs on clinical examination or the findings using imaging techniques noted in the records on admission or to the time of scoring. Spondylodiscitis and septic arthritis were not regarded as signs of embolization. O in HANDOC refers to that only 1 species was isolated from the blood culture. Community- (C in HANDOC), nosocomial-, and healthcare-associated acquisition was defined as previously described [25, 26]. The A in HANDOC was calculated according to the reference, comparing the propensity of a genus or species to cause IE to the streptococcal cohort, excluding neutropenic patients [10]. A previous bacteremia was defined as any blood culture with growth of the same genus or species within the 90 days preceding an episode. Comorbidities were classified according to the Charlson index [27].
Data Collection
Clinical data from each episode were collected from 90 days before its start until 360 days after it was completed. Thus, age, gender, comorbidities, previous bacteremia, symptoms, signs, performed radiology and its results, culture results other than blood cultures, hematuria, duration of symptoms, 30- and 360-day mortality, days hospitalized, and cultures or clinical conditions indicating therapeutic failure during follow up was registered. Furthermore, data on the following were collected: intravenous drug use, other predisposing heart conditions, fever, vascular or immunological phenomena, microbiological data fulfilling, or not fulfilling the prerequisites for minor or major criteria, and whether TTE or TEE was performed and if the diagnostic criteria for IE were met [4].
Missing data was registered as lack of result in that variable. No imputations were made.
Statistics
The analysis of the collected data was calculated in the statistical program Stata (StataCorp, College Station, TX). The odds ratios and their confidence intervals were calculated when applicable. The P value of Fisher’s exact test was used when the χ ₂ test was not applicable. Continuous variables were analyzed with Wilcoxon’s rank-sum test, because normal distribution could not be shown.
RESULTS
Epidemiology of Bacteremia and Infective Endocarditis
Data from the Swedish Registry for Infective Endocarditis (2014–2015) demonstrated that among 1486 reported episodes of IE, staphylococci, streptococci, and enterococci constituted 87%. Several cases of IE caused by Abiotrophia (n = 8), Aerococcus (n = 15), Gemella (n = 2), and Granulicatella (n = 12) were identified, whereas only 1 case of Rothia IE and no cases of IE caused by Leuconostoc, Lactobacillus, Lactococcus, or Globicatella had been reported.
Thus, episodes of bacteremia with Abiotrophia, Aerococcus, Gemella, and Granulicatella were chosen for the study, and positive blood cultures were identified. During the study period, a total of 843 positive blood cultures were identified from 601 patients with 611 episodes. Twenty-four episodes were excluded due to age below 18 years, and 19 were excluded because the medical records were not accessible. Thus, the study included 558 patients with 568 episodes. The Abiotrophia, Aerococcus, Gemella, and Granulicatella genera contributed with 19, 338, 87, and 124 episodes, and with 4, 13, 6, and 9 episodes of IE, respectively (Table 1). The proportion of bacteremia episodes that represented IE was 21% for Abiotrophia, 3.8% for Aerococcus, 6.9% for Gemella, and 7.3% for Granulicatella. With an underlying total population of 3.2 million and sampling period of 6 years, the incidence of bacteremia and IE of the different genera were calculated and shown in Table 1.
Table 1.
Cases of Bacteremia and Infective Endocarditis (IE) and the Incidencea
| Genus | Bacteremia, Cases | Bacteremia, Incidence | IE, Cases | IE, Incidence |
|---|---|---|---|---|
| Abiotrophia | 19 | 1.0 | 4 | 0.21 |
| Aerococcus | 338 | 18 | 13 | 0.68 |
| Gemella | 87 | 4.5 | 6 | 0.31 |
| Granulicatella | 124 | 6.5 | 9 | 0.47 |
aNumber per 1 000 000 inhabitants and year.
Risk Factors for Infective Endocarditis
The results of the univariate analysis of the whole study material is shown in Table 2. Age, Charlson score, long duration of symptoms, predisposing heart disease, heart murmur, embolization, number of positive cultures, monomicrobial culture, and unknown origin of infection were all significantly associated with IE. However, community acquisition did not reach significance but was more common among IE episodes (Table 2). The variables were stratified to the different genera and analyzed (Supplementary Tables 2–5). Long duration, heart murmur, and growth in several blood culture bottles were all correlated to IE in all genera, but differences were seen also for other variables (Supplementary Tables 2–6). Receiver operating characteristic (ROC) curves and their area under the curve (AUC) are shown together with the results of tabulation with the cutoffs of the scores (Supplementary Figures 1–4). The propensities for each genus and species to cause IE are shown (Table 3).
Table 2.
Characteristics of the Cohort Including the Criteria of the Scores NOVA, DENOVA, and HANDOC
| Characteristics | IE (n = 32) | Non-IE (n = 536) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Age (mean) | 69 | 74 | n/a | .013 |
| Sex (male) | 22 (69) | 392 (73) | 0.81 (0.37–1.7) | .59 |
| Previous SLB-bacteremia | 2 (6) | 8 (1.5) | 4.4 (0.44–23) | .10 |
| Charlson score (mean) | 1.9 | 2.5 | n/a | .027 |
| Community acquired | 19 (59) | 231 (43) | 1.92 (0.93–4.0) | .072 |
| Healthcare associated | 11 (34) | 242 (45) | 0.64 (0.30–1.3) | .23 |
| Nosocomial | 2 (6) | 63 (12) | 0.50 (0.12–2.1) | .34 |
| Duration of symptoms ≥7 days | 17 (53) | 68 (13) | 7.8 (3.7–16) | <.001 |
| CIED | 3 (6) | 29 (5) | 1.8 (0.52–6.3) | .41 |
| Pacemaker | 2 (6) | 27 (5) | 1.3 (0.29–5.5) | .68 |
| ICD | 1 (3) | 2 (0.4) | 8.6 (0.76–98) | .16 |
| Predisposing heart condition | 12 (38) | 51 (10) | 5.7 (2.6–12) | <.001 |
| Prosthetic valve | 7 (22) | 12 (2) | 12.2 (4.4–34) | <.001 |
| Native valve disease | 5 (16) | 36 (7) | 2.6 (0.93–7.1) | .072 |
| Previous IE | 3 (9) | 6 (1) | 9.1 (2.2–38) | .011 |
| Intravenous drug user | 0 (0) | 2 (0.4) | n/a | 1.0 |
| Prosthetic vascular graft | 1 (3) | 26 (5) | 0.63 (0.08–4.8) | 1.0 |
| Heart murmur | 24 (75) | 54 (10) | 27 (11–63) | <.001 |
| Fever ≥38 degrees | 19 (59) | 347 (65) | 0.80 (0.38–1.6) | .57 |
| Embolization | 8 (25) | 7 (1.3) | 25 (8.4–75) | <.001 |
| Urinary tract disorders | 10 (31) | 193 (36) | 0.81 (0.37–1.7) | .70 |
| Urinary/suprapubic catheter | 3 (9) | 111 (21) | 0.40 (0.12–1.3) | .085 |
| Intermittent self-catheterization | 2 (6) | 18 (3) | 1.9 (0.43–8.7) | .31 |
| Number of positive cultures ≥2 | 30 (94) | 155 (29) | 37 (8.7–156) | <.001 |
| Only 1 species in culture | 31 (97) | 297 (55) | 25 (3.4–184) | <.001 |
| Origin of infection, any | 0 (0) | 121 (23) | n/a | .001 |
| Respiratory tract | 0 (0) | 27 (5) | n/a | .39 |
| Urinary tract | 0 (0) | 66 (12) | n/a | .040 |
| Gastrointestinal or biliary | 0 (0) | 23 (4) | n/a | .63 |
| Other origin of infection | 0 (0) | 5 (1) | n/a | 1.0 |
| Other focus | 1 (3) | 8 (1.5) | 2.1 (0.26–18) | .41 |
| Unknown origin of infection | 32 (100) | 415 (77) | n/a | .001 |
Significant correlation shown in bold face.
Abbreviations: CI, confidence interval; CIED, cardiovascular implantable electronic device; ICD, implantable cardioverter defibrillator; IE, infective endocarditis; n/a, not applicable; SLB, Streptococcus-like bacteria.
Table 3.
Distribution of Genera and Species in the Cohort
| Pathogen | IE (n = 32) | Non-IE (n = 536) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Abiotrophia | 4 (12) | 15 (3) | 5.0 (1.5–16) | .018 |
| A defectiva | 4 (12) | 10 (2) | 7.5 (2.2–25) | .006 |
| A species | 0 (0) | 5 (1) | 1.5 (0.08–27) | 1.0 |
| Aerococcus | 13 (41) | 325 (61) | 0.44 (0.21–0.92) | .025 |
| A sanguinicola | 0 (0) | 57 (11) | n/a | .063 |
| A species | 0 (0) | 5 (1) | n/a | 1.0 |
| A urinae | 13 (41) | 226 (42) | 0.94 (0.45–1.9) | .84 |
| A viridans | 0 (0) | 37 (7) | n/a | .26 |
| A non-urinae | 0 (0) | 99 (18) | n/a | .003 |
| Gemella | 6 (19) | 81 (15) | 1.3 (0.52–3.2) | .61 |
| G bergeri | 1 (3) | 5 (1) | 3.4 (0.39–30) | .30 |
| G haemolysans | 1 (3) | 15 (3) | 1.1 (0.14–8.8) | .61 |
| G morbillorum | 3 (9) | 36 (7) | 1.4 (0.42–4.9) | .48 |
| G sanguinis | 1 (3) | 12 (2) | 1.4 (0.18–11) | .53 |
| G species | 0 (0) | 13 (2) | n/a | 1.0 |
| Granulicatella | 9 (28) | 115 (21) | 1.4 (0.61–3.2) | .38 |
| G adiacens | 8 (25) | 103 (19) | 1.4 (0.69–3.6) | .42 |
| G elegans | 1 (3) | 3 (0.6) | 5.7 (0.58–57) | .21 |
| G species | 0 (0) | 9 (2) | n/a | 1.0 |
Significant differences are shown in bold face.
Abbreviations: CI, confidence interval; IE, infective endocarditis; n/a, not applicable.
Predicting the Risk for Infective Endocarditis Using HANDOC, NOVA, and DENOVA
To use the “aetiology” variable (A) in HANDOC, the proportions of IE in bacteremia with Abiotrophia, Aerococcus, Gemella, and Granulicatella were compared to those obtained from the HANDOC study [10]. The propensities of the individual genera and species in this study were compared with the data from the HANDOC study (Supplementary Table 1). No differences were found between the bacteria investigated in HANDOC and the Gemella and Granulicatella genera and species. Bacteria from the genus Abiotrophia were more prone to cause IE; using Fisher’s exact test, the P value was .045 but the odds ratio included 1. However, when testing only isolates identified as Abiotrophia defectiva, a significant difference was demonstrated (P = .018). Species of Aerococcus non-urinae were significantly less prone to cause IE (Supplementary Table 1) as a group, but the individual species showed no significant association except for Aerococcus sanguinicola, which was less prone to cause IE. Thus, all species were given 0 points for A in the HANDOC-score calculation, except for A defectiva which was given 1 point and A sanguinicola where 1 point was subtracted.
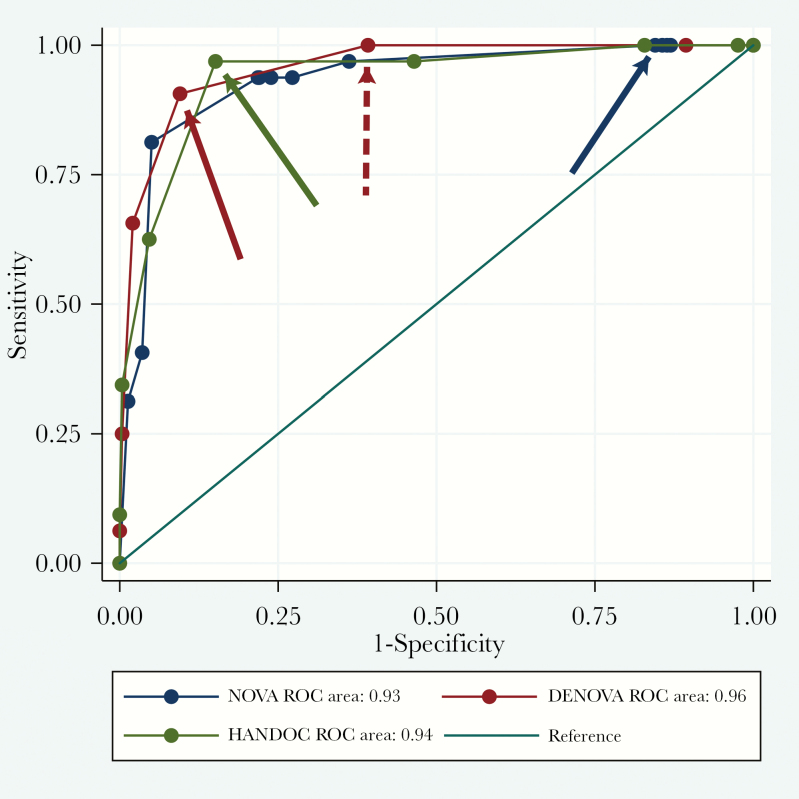
The NOVA, DENOVA, and HANDOC scores were calculated for all patients (Table 4), and the sensitivities and specificities are presented as a ROC curves (Figure 1). The AUC for the 3 different scores were not significantly different. However, the stipulated cutoffs generated very different specificities, 15%, 90%, and 85%, respectively. The sensitivities of all the scores were high (arrows in Figure 1 and Table 4), and the negative predictive values were high (Table 4). The positive predictive value for NOVA was 6.6% and for DENOVA and HANDOC it was 36% and 28%, respectively, resulting in numbers needed to screen of 15, 2.8, and 3.6 for the 3 scores.
Table 4.
The Result of the Testing of Scoring Systems Sensitivity, Specificity, PPV, NNS, and NPVa
| Scoring system | Cutoff | Sensitivity (%) | Specificity (%) | PPV (%) | NNS (%) | NPV (%) |
|---|---|---|---|---|---|---|
| NOVA | 4 | 100 | 15.5 | 6.6 | 15 | 100 |
| DENOVA | 3 | 90.6 | 90.5 | 36 | 2.8 | 99 |
| DENOVA | 2 | 100 | 60.8 | 13 | 7.6 | 100 |
| HANDOC | 3 | 96.9 | 84.9 | 28 | 3.6 | 100 |
Abbreviations: NNS, numbers needed to screen; NPV, negative predictive value; PPV, positive predicted value.
aThe cutoff values set in DENOVA, HANDOC, and NOVA reports were used, with addition of the cutoff of 2 for DENOVA.
Figure 1.
Receiver operating characteristic (ROC) curves for DENOVA, HANDOC, and NOVA of the entire cohort. Arrows in the same color as the curves showing the sensitivity and specificity in the plot where the cutoff values were set. Dotted red arrow showing the same features for DENOVA cutoff of 2.
Management and Outcome
Management of the patients and the outcome during the follow up are shown (Supplementary Table 7). The 30-day mortality was approximately 15%, whereas the 360-day mortality was higher in the non-IE group, although this was not statistically significant. The rate of TTE and TEE in the non-IE cohort was 25% and 12% (Supplementary Table 7), and the number of recurrent infections with the same bacterial species was low (Table 2).
DISCUSSION
We identified the risk for IE in bacteremia with the 4 genera of SLB most prone to cause IE. Abiotrophia had the highest propensity to cause IE (21%), and clearly IE has to be considered in cases of bacteremia with this pathogen. The risk of IE in Abiotrophia bacteremia is higher than in bacteremia with species more acknowledged for IE such as S aureus, E faecalis, and viridans group streptococci [7, 10, 28]. We recommend the use of DENOVA and HANDOC to evaluate whether TEE should be done in Abiotrophia bacteremia, but an alternative approach could be to do TEE in all cases with an increase in numbers needed to screen from 2 to 5 (data not shown). Infective endocarditis is more uncommon in bacteremia with the other investigated genera and species. The incidence of bacteremia with the studied bacterial genera is very diverse with aerococcal bacteremia being much more common than the others. However, the incidence of IE is similar between the 4 genera, 0.21–0.68/1 000 000 per year. To our knowledge, the present work is the largest population-based study on the epidemiology of bacteremia and IE caused by these bacteria [12–16, 29, 30], and the 2 regions represent approximately 30% of the Swedish population.
All cases of IE with aerococci were due to infection with Aerococcus urinae, giving it a propensity to cause IE of 5.9%. The other aerococcal species did not contribute with any cases. However, despite the relatively large size of our study, we cannot draw the conclusion that TEE could be omitted in all cases of bacteremia with non-A urinae aerococci. Due to the limited number of IE cases for the other genera, we cannot draw any conclusion about the propensity of specific Gemella or Granulicatella species to cause IE.
We further show that SLB reveal similar clinical features associated with IE as streptococci and enterococci [10, 11], both regarded as a group and as separate genera. Thus, community acquisition, long duration of symptoms, predisposing heart condition, heart murmur, embolization, growth in many blood culture bottles, monomicrobial bacteremia, and unknown origin of the infection were associated with IE. The different genera exhibited some different features, but we recognize the risk of drawing any conclusions from the small sample upon which these correlations were based.
We proceeded with the initial plan to evaluate whether HANDOC, NOVA, or DENOVA could predict IE and guide in the decision on whether to perform TEE. We found that both DENOVA and HANDOC have favorable performance, and the ROC analysis indicates that the suggested cutoffs in the publications are applicable [10, 11]. The sensitivities and specificities were high, and the numbers needed to screen were low, allowing a rational and efficient use of TEE in bacteremia with Abiotrophia, Aerococcus, Gemella, and Granulicatella. Alternatively, a cutoff of 2 in DENOVA would yield even higher sensitivity (Table 4 and Figure 1) without too much loss of specificity (58%). However, the study is based on retrospective data and no imputations were made. When used prospectively, it is possible that the sensitivity could increase when more reliable data in the variables are acquired. As in other studies, we demonstrate that the NOVA score was hampered by a low specificity and was thus not applicable.
A weakness of this study is its retrospective design that limits the available data on each patient. The rate of TEE performed in this cohort was low, and thus some cases of IE could have been missed, but a recurrent infection caused by a missed IE would have been detected due to the long follow up of 12 months. All cultures and all clinical courses during the follow up were reviewed, including the patients that died. Only 1 of these cases fulfilled criteria for IE, and only this case aroused suspicion of IE. The only patient with a recurrent infection diagnosed to be a IE was a patient with A urinae isolated in 3 episodes with IE diagnosed during the third episode. The other recurrences were in patients with repeated urinary tract infections. We believe that the number of missed IE cases was low and that our conclusions are valid and potentially generalizable.
A strength of the study is that it is population-based, and it can thus establish the incidence of SLB bacteremia and IE. This also avoids the problem with selection of complicated cases, which is otherwise common in studies on IE from tertiary centers.
CONCLUSIONS
In conclusion, we suggest that clinicians facing bacteremia with the genera Abiotrophia, Aerococcus, Gemella, and Granulicatella can use either DENOVA or HANDOC to help them make a decision of whether to perform or omit TEE.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We acknowledge Lena Hyllebusk in Lund for help with laboratory database searches and Dr. Aina Iversen for the help in retrieving and revising the laboratory data from Stockholm. We are also greatly indebted to Dr. Elin Loo for her industrious search for missing isolates and her conscientious work with the species determination.
Financial support. This work was funded by the Swedish Government Fund for Clinical Research (ALF); the foundations of Österlund, Skåne University Hospital, and the Royal Physiographic Society in Lund (to M. R.).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016; 387:882–93. [DOI] [PubMed] [Google Scholar]
- 2. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 3. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–128. [DOI] [PubMed] [Google Scholar]
- 4. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 5. Barton TL, Mottram PM, Stuart RL, et al. Transthoracic echocardiography is still useful in the initial evaluation of patients with suspected infective endocarditis: evaluation of a large cohort at a tertiary referral center. Mayo Clin Proc 2014; 89:799–805. [DOI] [PubMed] [Google Scholar]
- 6. Hilberath JN, Oakes DA, Shernan SK, et al. Safety of transesophageal echocardiography. J Am Soc Echocardiogr 2010; 23:1115–27; quiz 220–1. [DOI] [PubMed] [Google Scholar]
- 7. Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tubiana S, Duval X, Alla F, et al. The VIRSTA score, a prediction score to estimate risk of infective endocarditis and determine priority for echocardiography in patients with Staphylococcus aureus bacteremia. J Infect 2016; 72:544–53. [DOI] [PubMed] [Google Scholar]
- 9. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis 2015; 60:528–35. [DOI] [PubMed] [Google Scholar]
- 10. Sunnerhagen T, Törnell A, Vikbrant M, et al. HANDOC: a handy score to determine the need for echocardiography in non-β-hemolytic streptococcal bacteremia. Clin Infect Dis 2018; 66:693–8. [DOI] [PubMed] [Google Scholar]
- 11. Berge A, Krantz A, Östlund H, et al. The DENOVA score efficiently identifies patients with monomicrobial Enterococcus faecalis bacteremia where echocardiography is not necessary. Infection 2019; 47:45–50. [DOI] [PubMed] [Google Scholar]
- 12. Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev 2001; 14:177–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christensen JJ, Facklam RR. Granulicatella and Abiotrophia species from human clinical specimens. J Clin Microbiol 2001; 39:3520–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tellez A, Ambrosioni J, Llopis J, et al. Epidemiology, clinical features, and outcome of infective endocarditis due to abiotrophia species and granulicatella species: report of 76 cases, 2000–2015. Clin Infect Dis 2018; 66:104–11. [DOI] [PubMed] [Google Scholar]
- 15. Christensen JJ, Jensen IP, Faerk J, et al. Bacteremia/septicemia due to Aerococcus-like organisms: report of seventeen cases. Danish ALO Study Group. Clin Infect Dis 1995; 21:943–7. [DOI] [PubMed] [Google Scholar]
- 16. Senneby E, Göransson L, Weiber S, Rasmussen M. A population-based study of aerococcal bacteraemia in the MALDI-TOF MS-era. Eur J Clin Microbiol Infect Dis 2016; 35:755–62. [DOI] [PubMed] [Google Scholar]
- 17. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection 2016; 44:167–73. [DOI] [PubMed] [Google Scholar]
- 18. Zaidi SJ, Husayni T, Collins MA. Gemella bergeri infective endocarditis: a case report and brief review of literature. Cardiol Young 2018; 28:762–4. [DOI] [PubMed] [Google Scholar]
- 19. Senneby E, Nilson B, Petersson AC, Rasmussen M. Matrix-assisted laser desorption ionization-time of flight mass spectrometry is a sensitive and specific method for identification of aerococci. J Clin Microbiol 2013; 51:1303–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sonesson A, Oqvist B, Hagstam P, et al. An immunosuppressed patient with systemic vasculitis suffering from cerebral abscesses due to Nocardia farcinica identified by 16S rRNA gene universal PCR. Nephrol Dial Transplant 2004; 19:2896–900. [DOI] [PubMed] [Google Scholar]
- 21. Dahl A, Lauridsen TK, Arpi M, et al. Risk factors of endocarditis in patients with Enterococcus faecalis bacteremia: external validation of the NOVA score. Clin Infect Dis 2016; 63:771–5. [DOI] [PubMed] [Google Scholar]
- 22. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1990; 264:2919–22. [PubMed] [Google Scholar]
- 23. Steckelberg JM, Wilson WR. Risk factors for infective endocarditis. Infect Dis Clin North Am 1993; 7:9–19. [PubMed] [Google Scholar]
- 24. Fernández Guerrero ML, Goyenechea A, Verdejo C, et al. Enterococcal endocarditis on native and prosthetic valves: a review of clinical and prognostic factors with emphasis on hospital-acquired infections as a major determinant of outcome. Medicine (Baltimore) 2007; 86:363–77. [DOI] [PubMed] [Google Scholar]
- 25. Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 26. Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16:128–40. [DOI] [PubMed] [Google Scholar]
- 27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 28. Pinholt M, Ostergaard C, Arpi M, et al. Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006–2009: a population-based cohort study. Clin Microbiol Infect 2014; 20:145–51. [DOI] [PubMed] [Google Scholar]
- 29. Brouqui P, Raoult D. New insight into the diagnosis of fastidious bacterial endocarditis. FEMS Immunol Med Microbiol 2006; 47:1–13. [DOI] [PubMed] [Google Scholar]
- 30. Papakonstantinou PE, Samonis G, Andrianaki AM, et al. Epidemiology, microbiological and clinical features, treatment, and outcomes of infective endocarditis in Crete, Greece. Infect Chemother 2018; 50:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.