Abstract
Background
Large numbers of perinatally HIV-infected children (PHIV) are aging into adolescence. We examined cognitive and behavioral outcomes in a longitudinal cohort of Asian youth.
Methods
We followed 231 PHIV, 125 perinatally HIV-exposed, uninfected (HEU), and 138 HIV-unexposed uninfected (HUU) adolescents (aged ≥10 years), matched by age/sex, in Thailand and Cambodia for 3 years. Executive function was assessed with Children’s Color Trails Tests 1 and 2 (CCTT-1 and -2), and the Design Fluency and Verbal Fluency Tests (DFT and VFT). Working memory (FDI) and processing speed (PSI) were assessed using WISC-III. Visual memory was assessed by design memory and recognition subtests of the Wide Range Assessment of Memory and Learning (WRAML-2), and behavioral problems using the Child Behaviour Checklist (CBCL). Generalised estimating equations examined adjusted odds ratios (aOR) of cognitive impairment (Z scores ≥2 SD below age-adjusted means of HUU group) and CBCL T-scores in the borderline-clinical range (T-Scores ≥60) in PHIV and HEU versus HUU youth, adjusting for ethnicity, household income, and caregiver characteristics.
Results
Median age at enrolment was 13.8 years, with 58% female and 63% Thai participants. PHIV youth had >86% virological suppression, and significantly higher impairment rates on CCTT-1 and 2 tests, DFT and VFT, Design Memory and CBCL Internalizing and Externalizing Problems. Results were mostly similar between HEU and HUU groups, apart from higher impairment rates on CCTT-1 and Internalizing Problems in HEU.
Conclusion
Asian adolescents with PHIV remain at risk for cognitive and mental health problems despite HIV treatment. Selective risks are observed among HEU youth.
Keywords: perinatally infected youth, executive function, mental health, HIV
INTRODUCTION
After successful combination antiretroviral therapy (cART) scale-up and consequent improvements in health and life expectancy, large numbers of children living with perinatal HIV infection (PHIV) in low-to-middle income countries are now aging into adolescence. Adolescence is a time of behavioral risk-taking when personal identity is forged while transitioning to adulthood. However, success in negotiating the world, building meaningful social relationships, and entering the workforce is in part dependent on the integrity of cognitive and behavioral functioning.
Children infected with HIV who have delayed cART have neuropsychological (NP) deficits compared with children who initiate cART soon after birth.1,2 Children enrolled in the PREDICT study of immediate versus deferred cART at a median age of 7 years had significantly lower indices of NP functioning than HIV-exposed but uninfected (HEU) children and HIV-unexposed, uninfected (HUU) children, and NP symptoms persisted in the early ART group despite long-term viral suppression.2 Even children from PREDICT with long-term nonprogressive HIV who maintained CD4 T-cell counts >350 cells/mm3 and plasma viral loads <10,000 copies/mL had cognitive deficits and emotional difficulties.3 By contrast, 5-year follow-up of PHIV children enrolled in the CHER study identified some improvement in neurodevelopmental outcomes when cART was initiated within 2 years after birth.4 Together, these studies suggest that children infected with HIV are at risk of delayed or atypical neurodevelopment independent of treatment history.
Thailand and Cambodia were among the first Asian countries where HIV spread among unborn children; approximately 14,800 children and youth aged 19 years and older were living with HIV in 2017.5 Both countries now provide free ART as part of their national program. Unfortunately, little is known about the long-term neurocognitive outcomes among Asian youth who started treatment at variable thresholds of immune compromise when they were younger, given that this cohort is only now reaching older ages in large enough numbers to be studied. Our aim in this article is to compare cognitive and behavioral functioning in a cohort of Thai and Cambodian adolescents who initiated ART during childhood and a group of HEU and HUU youth who served as comparison groups, using a longitudinal design. Identification of risk in cognitive, executive function, or behavioral development among Asian youth with PHIV could be critical to informing efforts to support these youth during their adolescence and transition to adulthood when neurocognitive skills, particularly executive function, play an important role.
METHODS
The Resilience Study enrolled participants from the PREDICT and PREDICT neurodevelopment substudy, which originally enrolled children from 7 sites in Thailand and 2 sites in Cambodia (Fig. 1fig1).2 PHIV and age-/sex-matched HEU and HUU youth, aged 10–24 years, were re-enrolled. Newly identified HEU (n = 40) and HUU (n = 22) youth were recruited to replace healthy youth previously lost to follow-up. The study was approved by the institutional review boards from each site. Written informed consent was obtained from caregivers and youth aged 18 years and older; assent was obtained from all youth younger than 18 years.
Figure 1.
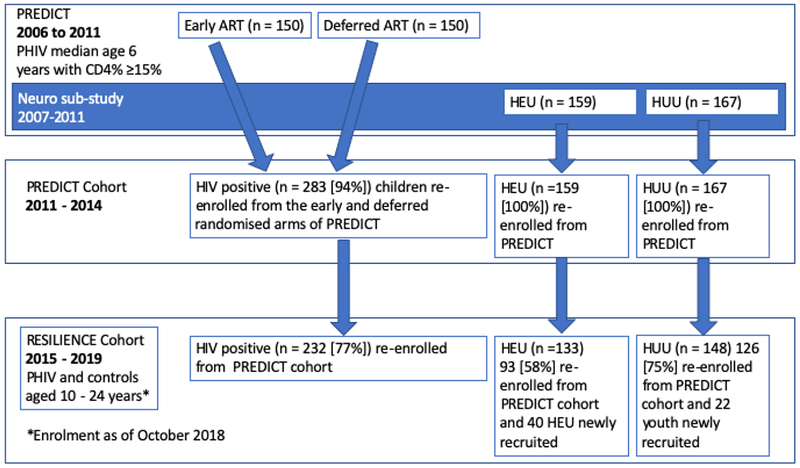
PREDICT, PREDICT Cohort, and RESILIENCE Study flow diagram. ART, antiretroviral therapy. Percentages in square brackets represent the percent of original PREDICT participants subsequently re-enrolled into PREDICT and RESILIENCE Cohorts.
Study Testing Procedures
Youth were assessed at enrollment and then yearly (±3 months) for 3 years. Physical and neurological examinations were performed at each visit. NP testing procedures (detailed below) were chosen to maximize sensitivity to previously identified HIV effects, while preserving high internal and cross-cultural validity. All NP tests were conducted at enrollment with executive function tests and emotional and behavioral functioning assessments repeated yearly. NP tests were administered by staff trained by US and Thai neuropsychologists, and quality assurance was conducted quarterly to reduce evaluator drift. Data accuracy was assured by cross-checking case report forms by designated study monitors.
Executive, Cognitive, and Motor Outcomes
Executive function was assessed with the Children’s Color Trails Test (CCTT) for youth younger than 17 years (PAR, Inc., Lutz, FL), the CTT for those aged 18 years and older (PAR, Inc), and the design fluency and verbal fluency (assessed in Thai or Khmer) subtests of the Delis–Kaplan Executive Function System (DKEFS).6 The CCTT is a test of alternating and sustained visual attention, sequencing, psychomotor speed, cognitive flexibility, planning, and inhibition–disinhibition. Age-norm–referenced standard scores for each trial completion time have a mean of 100 and SD of 15; higher scores indicate better performance. The design fluency and verbal fluency subtests assess verbal and nonverbal fluency, cognitive flexibility, inhibitory control, problem-solving and concept formation and provide age-referenced scaled scores with mean of 10 and SD of 3; higher scores reflect better performance.
Immediate and delayed visual memory abilities were assessed at enrollment using the design memory and design recognition subtests of the Wide Range Assessment of Memory and Learning (WRAML-2), a standardized measure of learning and memory for individuals 5–90 years of age.7 The design memory and recognition subtests require youth to draw geometric designs from memory and assess delayed recall (10 seconds) and delayed recognition (10–15 minutes). Age-adjusted scaled scores in this test have a mean of 10 and SD of 3.
Working memory was assessed by the Freedom from Distractibility Index (FDI) of the Wechsler Intelligence Scale for Children, Third Edition (WISC-III) (<17 years) and the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) (≥17 years).8,9 Processing speed was measured by the processing speed index (PSI) of the WISC-III and WAIS-III. Wechsler tests were conducted with Thai youth only. The WISC-III had previously undergone adaptation and validation in Thailand. The WISC-III and WAIS-III versions of the Wechsler scales were administered to maintain consistency with the preceding PREDICT study. Fine motor speed and dexterity was assessed by averaging the scores for the dominant and nondominant hands on the Grooved Pegboard.
Behavioral Outcomes
Emotional and behavioral functioning was assessed with the Child Behavior Checklist (CBCL), caregiver (<18 years) report.10 The CBCL has been adapted and validated to study children in multiple countries, including Thailand. Caregivers completed the CBCL in their primary language; T-scores in the borderline-clinical range (≥60) were used to define impairment. For children in orphanages, study sites requested that the same caregiver complete the CBCL at each study visit.
Statistical Analysis
Analysis was conducted with Stata 15.1 (Statacorp, College Station, TX). Sociodemographics were summarized by the HIV exposure group at the enrollment visit. For cognitive and behavioral tests, scaled scores were calculated by the study group at each study week. Performances on the NP measures were converted to z-scores based on the HUU sample, adjusted for age and sex. A conservative definition of NP impairment was used (≥2 SD below the mean of the HUU group) to minimize false-positive errors. We then summarized the frequency of impaired test performance at each study week by the exposure group and used impaired functioning on each specific test as an endpoint to calculate the odds of impairment in the PHIV and HEU relative to the HUU as a reference group. Estimates were adjusted for primary caregiver relationship (orphanage, grandparent, or other family versus parent), education of caregiver (college/university or high school diploma versus elementary or no education as a reference), participant ethnicity, and annual household income (<15,000 USD versus ≥15,000 USD as a reference), using generalized estimating equations with a logit link and exchangeable correlation matrix to account for within-subject clustering over the multiple visits.
RESULTS
The Resilience Study began enrolling participants in June 2015, and data for this article were collected until October 2018. Sociodemographic characteristics of the youth are shown in Table 1tbl1. The median age of all participants was 13.8 (IQR: 11.8–16.1) years, and the median ages of the HEU and HUU youth were approximately 2 years younger compared with the PHIV youth. Approximately, 60% of all youth were women. More PHIV youth were cared for by relatives and in orphanages than HEU and HUU youth. The education level of caregivers was similar among PHIV and HEU groups, but HUU youth had a higher proportion of caregivers with a college/university education. Income adjusted for purchasing parity power to USD equivalents was similar between PHIV and HEU (median 12–13,000 USD per year) but was significantly lower in these 2 groups compared with HUU; yearly household income was <13,000 USD for 50% of PHIV and HEU families, whereas 50% of HUU had yearly incomes >34,000 USD.
Table 1.
Characteristics of youth at the baseline RESILIENCE study visit
| Characteristics | PHIV (n= 232) | HEU(n=133) | HUU (n=148) | P |
|---|---|---|---|---|
| Median (IQR) age (years) | 14.9 (12.7-16.7) | 13.3 (11.6-15.7) | 12.8 (10.9-15) | <0.001 |
| Female | 138 (59.5) | 77 (57.9) | 82 (55.4) | 0.74 |
| Thai:Cambodian ethnicity | 132 (56.9) : 100 (43.1) | 93 (69.9) : 40 (30.1) | 96 (64.9) : 52 (35.1) | 0.04 |
| Caregiver identity | <0.001 | |||
| Parent | 154 (66.4) | 121 (91) | 130 (87.8) | |
| Relative | 41 (17.7) | 8 (6) | 18 (12.2) | |
| Orphanage | 36 (15.5) | 1 (0.8) | 0 (0) | |
| Unknown | 1 (0.4) | 3 (2.3) | 0 (0) | |
| Education of caregiver | <0.001 | |||
| None/Elementary | 110 (47.4) | 75 (56.4) | 43 (29.1) | |
| High School Diploma | 94 (40.5) | 50 (37.6) | 52 (35.1) | |
| College/University | 24 (10.3) | 8 (6) | 53 (35.8) | |
| Unknown | 4 (1.7) | 0 (0) | 0 (0) | |
| Median (IQR) yearly household income in USD | 12,184 (7,310-19912) | 12,943 (7,467-24,368) | 34,115 (14,934-48,735) | <0.001 |
| Median (IQR) duration on cART (years) | 7.9 (6.4-8.8) | |||
| Median (IQR) CD4 count (cells/mm3) | 741 (585-985) | |||
| Median (IQR) CD4 percent | 29 (25 – 34)% | |||
| N (%) with viral load <50 copies/mL | 190/220 (86.4%) |
PHIV = perinatally HIV infected youth; HEU = HIV exposed, uninfected youth and HUU = HIV unexposed, uninfected youth.
Youth with PHIV initiated cART when the CD4 percent was ≥15%; the median duration of ART at the enrollment Resilience visit was 7.8 (IQR 6.2–8.8) years; and 190/220 (86.4%) participants with a plasma viral load available had plasma HIV-RNA <50 copies/mL. At weeks 48 and 96, 177/207 (85.5%) and 135/158 (85.4%) of the PHIV sample had plasma HIV-RNA <50 copies/mL, respectively. Ninety percent, 64%, and 20% of the cohort had completed week 48, 96, and 144 study visits; 2 youth with PHIV, but no HEU or HUU youth were deceased.
Executive Function
The standardized scores and the prevalence of impairment based on z-scores are shown in Table 2tbl2. At enrollment, the prevalence of impairment on the CCTT-1 or CTT-1 in the PHIV, HEU, and HUU groups was 14.4%, 4.8%, and 1.4%, respectively and impairment rates on CCTT2 or CTT2 scores were 15.9%, 11.1%, and 6.3%, respectively (Table 2). Over all follow-up visits, the adjusted odds ratios (aOR) of impairment in CCTT1/CT1 scores were 5.43 [95% confidence interval (CI): 2.23 to 13.2; P <0.001] in the PHIV and 3.24 (95% CI: 1.26 to 8.29, P = 0.01) in the HEU youth (Table 4). PHIV youth had a significantly increased adjusted odds of impaired CCTT2/CT2 scores relative to HUU youth [aOR = 3.01 (95% CI: 1.50 to 6.02)]; differences between HEU and HUU youth were not statistically significant [aOR = 1.79 (95% CI: 0.84 to 3.85); P = 0.13].
Table 2.
Standard scores of neuropsychological tests conducted at weeks 0, 48 and 96, and percent of youth with impairment
| Week 0 | Week 48 | Week 96 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | n/N (%) with impairment* | Mean (SD) | n/N (%) with impairment* | Mean (SD) | n/N (%) with impairment* | |||
| CCTT1 or CCT1 | ||||||||
| PHIV | 78. 3(17.7) | 30/208 (14.4) | 84.9 (19.1) | 19/188 (10.1) | 89.5 (19.7) | 10/141 (7.1) | ||
| HEU | 83.3 (18.0) | 6/126 (4.8) | 86.9 (18.6) | 11/101 (10.9) | 88 (21.4) | 6/57 (10.5) | ||
| HUU | 84.4 (16.1) | 2/142 (1.4) | 90.4 (16.8) | 4/124 (3.2) | 91.5 (16.9) | 2/84 (2.4) | ||
| CCTT2 or CCT2 | ||||||||
| PHIV | 84.4 (16.9) | 33/208 (15.9) | 88.7 (17.8) | 27/188 (14.4) | 93.2 (19.1) | 20/141 (14.2) | ||
| HEU | 86.4 (16.7) | 14/126 (11.1) | 92.8 (14.2) | 6/101 (5.9) | 94.3 (16.7) | 5/57 (8.8) | ||
| HUU | 91.2 (14.6) | 9/142 (6.3) | 93.5 (14.6) | 6/126 (4.8) | 97.6 (14.0) | 0/84 (0) | ||
| DKEFS Design Fluency | ||||||||
| PHIV | 8.4 (2.6) | 44/231 (19.1) | 9.3 (2.9) | 32/218 (14.7) | 9.6 (2.9) | 19/169 (11.2) | ||
| HEU | 9.7 (2.6) | 10/132 (7.5) | 10.6 (2.7) | 6/111 (5.4) | 10.8 (2.5) | 2/63 (3.2) | ||
| HUU | 10.4 (2.9) | 5/147 (3.4) | 11.3 (2.8) | 2/133 (1.5) | 11.9 (2.7) | 0/96 (0) | ||
| DKEFS Verbal Fluency | ||||||||
| PHIV | 10.1 (3.7) | 42/231 (18.2) | 9.6 (1.8) | 15 (6.9) | 10.7 (3.4) | 19/168 (11.3) | ||
| HEU | 12.2 (3.1) | 6/133 (4.5) | 10.7 (1.6) | 0 (0) | 12.3 (3.1) | 4/62 (6.5) | ||
| HUU | 12.3 (3.3) | 6/147 (4.1) | 11.1 (1.6) | 0 (0) | 12.0 (3.0) | 2/96 (2.1) | ||
Impairment defined as ≥2 SD below the age/sex adjusted mean of the HUU group
PHIV = perinatally HIV infected youth; HEU = HIV exposed, uninfected youth and HUU = HIV unexposed, uninfected youth.
CCTT = Children’s Color Trail Test for youth <17 years; CTT = Color Trails Test (ages >18 years); DKEFS = Delis-Kaplan Executive Function System.
Table 4.
Adjusted odds of impairment for PHIV and HEU versus the HUU youth*
| PHIV vs HUU | HEU vs HUU | |||
|---|---|---|---|---|
| Test | aOR (95%CI) | P | aOR (95%CI) | P |
| Tests conducted at all study visits | ||||
| Colortrail | ||||
| CCTT-1/CTT-1 | 5.43 (2.23 – 13.20) | 0.001 | 3.24 (1.26 – 8.29) | 0.01 |
| CCTT-2/ CTT-2 | 3.01 (1.50 – 6.02) | 0.01 | 1.79 (0.84 – 3.85) | 0.13 |
| DKEFS Design Fluency | 6.78 (2.67 – 17.26) | <0.001 | 2.69 (0.96 – 7.50) | 0.06 |
| DKEFS Verbal Fluency | 4.75 (1.94 – 11.65) | 0.001 | 1.21 (0.41 – 3.59) | 0.74 |
| CBCL | ||||
| Internalizing Problems | 3.25 (1.80 – 5.88) | <0.001 | 1.98 (1.08 – 3.64) | 0.03 |
| Externalizing Problems | 2.05 (1.40 – 1.80) | 0.05 | 0.98 (0.46 – 2.07) | 0.95 |
| Total problem score | 2.26 (1.16 – 4.41) | 0.02 | 1.16 (0.57 – 2.33) | 0.69 |
| Tests conducted at baseline only | ||||
| WISC-III/WAIS-III (Thai participants only) | ||||
| Processing speed index | 1.89 (0.36 – 9.79) | 0.45 | 0.66 (0.1 – 4.29) | 0.67 |
| Freedom from distractibility index | 3 (0.55 – 16.47) | 0.21 | ** | |
| WRAML-2 | ||||
| Design Memory | 5.94 (1.69 – 20.92) | 0.01 | 1.94 (0.47 – 8.06) | 0.36 |
Impairment defined as borderline-clinical range T-scores on the CBCL, or >2SD below age and sex adjusted means of the HUU group. Adjustment was made for primary carer, education of caregiver, participant ethnicity (with the exception of the WISC-III and WAIS) and annual household income.
Odds of impairment for PHIV and HEU versus HUU for WRAML Design Recognition, and Grooved Pegboard could not be calculated because no HUU had z-scores consistent with impairment. Odds of impairment for HEU versus HUU for FDI could not be calculated because no HEU had z-scores consistent with impairment.
On the DKEFS design fluency test, impaired performance at week 0 was observed in 19.1%, 7.5%, and 3.4% of the PHIV, HEU, and HUU groups, respectively. The prevalence of impairment decreased over time but was higher at each study week in the PHIV youth relative to the HUU youth, with adjusted odds of impairment of 6.78 (95% CI: 2.67 to 17.26); P < 0.001. The prevalence of impairment was also higher in the HEU than the HUU group at each study week, but the difference was shy of statistical significance [aOR = 2.69 (95% CI: 0.96 to 7.50); P = 0.06]. DKEFS verbal fluency followed a similar pattern. Impaired performance at week 0 was observed among 18.2%, 4.5%, and 4.1% among PHIV, HEU and HUU groups, respectively, and the prevalence of impairment was higher in the PHIV youth than both comparison groups at each study week. Compared with the HUU group, the adjusted odds of impairment was significantly higher, aOR 4.75 (95% CI: 1.94 to 11.65; P = 0.001) in the PHIV youth, but not in the HEU youth, aOR = 1.21 (95% CI: 0.41 to 3.59; P = 0.74).
Processing Speed and Working Memory
The standard scores and the prevalence of impairment based on z-scores are shown in Table 3tbl3. Processing speed impairment rates, as measured by PSI, were 3.2% in both HUU and HEU and 7.6% in PHIV youth. Although the adjusted odds of impairment were approximately double in PHIV compared with HUU youth [aOR = 1.89 (95% CI: 0.36 to 9.79)], this was not significantly different, but the adjusted mean difference in PSI was significantly lower, indicating poorer performance [−7.9 (95% CI: −13.8 to −2.1); P = 0.008]. Neither impairment rates nor adjusted mean differences in standard PSI scores were significantly different in HEU versus HUU youth. Working memory impairment, as measured by FDI impairment rates, was 12.6% in PHIV and 3.6% in HUU; no youth in the HEU group met criteria for impairment in FDI. The scores showed a similar pattern between groups as with PSI: the adjusted odds of impairment in PHIV versus HUU youth were not significantly different, but the adjusted mean difference in FDI standard scores was significantly lower, indicating poorer performance [−7.8 (−12.9 to −2.6); P = 0.003] among PHIV youth. No HEU youth met criteria for impairment in FDI scores; adjusted mean FDI scores did not differ significantly between the HEU and HUU groups (Table 3).
Table 3.
Neuropsychological tests performed at study enrolment (week 0).
| Week 0 Standard Scores | *Adjusted Mean Difference (95%CI) vs HUU | ||
|---|---|---|---|
| Mean (SD) | n/N (%) with impairment** | ||
| Processing speed index | |||
| PHIV | 93.5 (16.7) | 10/131 (7.6) | −7.9 (−13.8 to −2.1) ; P = 0.008 |
| HEU | 103.7 (16.6) | 3/93 (3.2) | −0.2 (−5.7 to 5.4) ; P = 0.95 |
| HUU | 109.1 (19.3) | 3/94 (3.2) | Ref |
| Freedom from distractibility index | |||
| PHIV | 85.4 (13.3) | 11/87 (12.6) | −7.8 (−12.9 to −2.6); P = 0.003 |
| HEU | 95.4 (12.1) | 0/76 | −1.1 (−5.8 to 3.7) ; P = 0.66 |
| HUU | 100.8 (14.9) | 3/84 (3.6) | Ref |
| WRAML-2 Design Memory | |||
| PHIV | 6.4 (3.1) | 34/231 (14.7) | −1.2 (−1.9 to −0.4) ; P =0.005 |
| HEU | 7.5 (3.3) | 8/133 (6.0) | −0.1 (−0.9 to 0.7) ; P =0.83 |
| HUU | 8.0 (3.2) | 3/146 (2.1) | Ref |
| WRAML-2 Design Recognition | |||
| PHIV | 7.5 (2.5) | 18/231 (7.8) | −0.3 (−1.0 to 0.4) ; P = 0.35 |
| HEU | 8.7 (3.3) | 9/133 (6.8) | 0.6 (−0.2 to 1.3) ; P= 0.13 |
| HUU | 8.3 (3.1) | 0 /146 (0) | Ref |
| Grooved pegboard Successful pin placements | |||
| PHIV | 72.1 (19.5) | 5/231 (2.2) | 1.4 (−2.71 to 5.6); P =0.50 |
| HEU | 71.6 (12.3) | 1/132 (0.8) | 1.2 (−3.2 to 5.5) ; P = 0.60 |
| HUU | 72.8 (14.1) | 0/146 (0) | Ref |
PHIV = perinatally HIV infected youth; HEU = HIV exposed, uninfected youth and HUU = HIV unexposed, uninfected youth.
WISC-III/WAIS-III Processing Speed Index and Freedom from Distractibility Index were conducted only in Thai participants. WRAML = Wide Range Assessment of Memory and Learning
Adjusted for primary caregiver relationship (orphanage, grandparent or other family versus parent), education of caregiver (college/university or high school diploma versus elementary or no education as a reference), participant ethnicity, and annual household income (<15,000 USD versus ≥15,000 USD as a reference).
Impairment defined as ≥2 SD below the age/sex adjusted mean of the HUU group
WRAML design memory impairment was present in 14.7% of PHIV, 6.0% of HEU, and 2.1% of HUU youth. The adjusted odds of impairment in PHIV youth versus HUU youth was 5.94 (95% CI: 1.69 to 20.92; P = 0.01) in PHIV, with no significant differences between HUU and HEU youth. Design recognition impairment was present in 7.8% PHIV and 6.8% HEU youth but was not evident in HUU youth; thus, odds of impairment versus HUU youth could not be calculated. There were no group differences in adjusted mean scores for design recognition.
Motor Function
Motor performance impairment averaged over the dominant and nondominant hands was infrequent and did not differ by groups (2.2% in the PHIV group and <1% in the HEU group). The adjusted mean differences in successful pin placements were not significantly different between groups (Table 3).
Behavior
The prevalence of borderline-clinical CBCL internalizing, externalizing, and total problem scores at each study visit is shown in Figure 2fig2. A significantly higher proportion of PHIV and HEU youth had CBCL internalizing problem scores in the borderline-clinical range, compared with the HUU youth at each study week; the prevalence at enrollment was 20.4%, 19.5%, and 11.3% in the PHIV, HEU, and HUU groups, respectively. Internalizing T-scores in the borderline-clinical range improved at weeks 48 and 96 in PHIV and HUU youth. In the HEU group, the frequency of borderline-clinical scores decreased at week 48 and increased at week 96 in HEU youth. Compared with HUU youth, the overall adjusted odds of a borderline clinical internalizing score for PHIV youth was 3.25 (95% CI: 1.80 to 5.88), P < 0.001, and for HEU youth was 1.98 (1.08 to 3.64), P = 0.03, both significantly different (Table 4tbl4).
Figure 2.
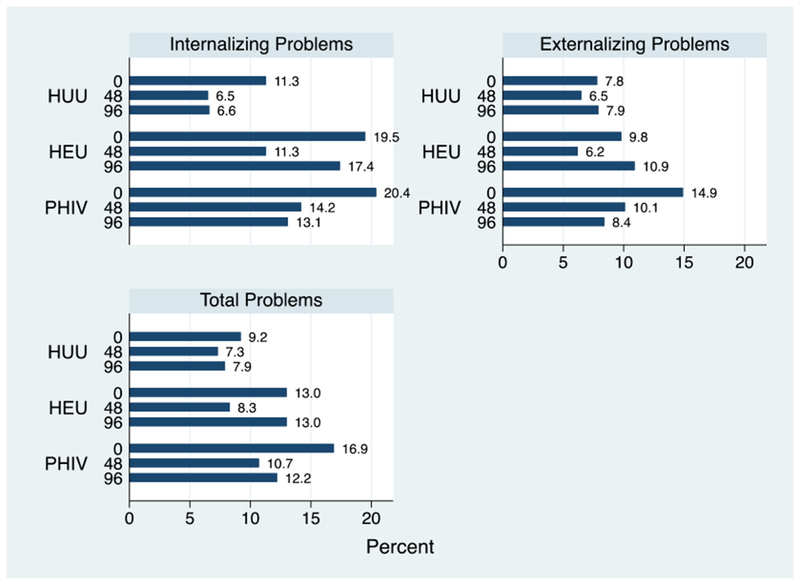
Prevalence of internalizing, externalizing, and total problem T-scores in the borderline-clinical range, by the study group and week. CBCL internalizing, externalizing, and total problem scores were based on caregiver reports in youth younger than 18 years. A total of 201, 169, and 107 PHIV, 123, 97, and 46 HEU, and 142, 123, and 76 youth are represented in the figure at weeks 0, 48, and 96, respectively.
Externalizing CBCL T-scores in the borderline clinical range were present at a lower frequency in each exposure group than internalizing T-scores and showed variability at each study week. At week 0, the prevalence of externalizing T-scores in the borderline-clinical range was 14.9%, 10.9%, and 7.8% in PHIV, HEU, and HUU groups, respectively. In PHIV and HEU youth, the prevalence of borderline-clinical externalizing T-scores over time mimicked the internalizing T-scores; in HUU youth, the scores were very similar over all study visits. Compared with HUU, the adjusted odds of borderline clinical externalizing T-scores was 2.05 (95% CI: 1.40 to 1.80), P = 0.05, in PHIV and 0.98 (95% CI: 0.46 to 2.07), P = 0.95, in HEU youth. Total problem scores that comprised internalizing, externalizing, and other problem scales showed a similar pattern to externalizing problems by the HIV exposure group and study week. Compared with the HUU group, PHIV youth were at higher risk of having total problem scores in the borderline-clinical range [aOR = 2.26 (95% CI: 1.16 to 4.41), P = 0.02], but the risk in HEU was not significantly different [aOR = 1.16 (95% CI: 0.57 to 2.33), P = 0.69].
Impairment Frequency at Enrollment Visit
One hundred forty-nine, 58, and 40 PHIV, HEU, and HUU youth, respectively, had impairment on at least 1 of the 12 NP measures at enrollment. Among these youth, the median (IQR) number of impairments in the PHIV youth was 2 (1–3), 2 (1–2) in the HEU youth and 1.5 (1–2) in the HUU youth.
DISCUSSION
In one of few cohort studies following Asian youth longitudinally, we found significantly higher rates of impairment in all assessed measures of executive function and behavioral problems in PHIV youth, compared with HUU youth, after adjustment for relevant sociodemographic factors. Adjusted rates of memory impairment were not significantly higher in PHIV than HUU youth, apart from WRAML design memory scores; however, the mean adjusted PSI and FDI standard scores were significantly lower, which is consistent with previous work in this cohort when they were younger.2 Adjusted impairment rates in HEU compared with HUU youth were not significantly higher for most measures, with the exception of Children’s Color Trails 1 and CBCL internalizing problems, and motor function assessed by the Grooved pegboard was similar in all groups.
Results from the US-based Pediatric HIV/AIDS Cohort Study (PHACS) have indicated impairment in executive function in PHIV compared with HEU youth, with impairment significantly worse in those with previous AIDS-defining illnesses and higher peak viral loads.11,12 Verbal fluency (DKEFS) impairment rates were variable in PHACS, ranging from 0% to 28% in PHIV youth over 2 years of follow-up compared with our rates that ranged from 4.1% to 18.2%; visual fluency impairment was low among PHIV in PHACS (0%–2.6%) compared with a range of 3.4%–19.1% in our cohort. PHACS also reported that CCTT1 and CCTT2 scores were more likely impaired in PHIV youth with a previous CDC class C diagnosis.12 The enrollment ages of PHIV youth in PHACS and our study were similar, but the PHACS study had a high proportion of youth who started ART at CD4 <15% and after receiving a CDC category C diagnosis, given the epidemic in the United States began before cART; these children had poorer test scores than those whose immune status was better when starting ART.11 By contrast, given that the aims of the PREDICT study were to examine the impact of delayed treatment, our cohort initiated ART when CD4 was ≥15%, and only 1 participant had a CDC category C illness.
Previous examination of performance IQ and verbal IQ scores in our cohort when they were younger found significantly worse scores in the PHIV children compared with the HEU and HUU youth, and these scores did not change significantly in the 3 years after ART initiation.2 The current data included some of the same measures, namely PSI and FDI subtests of the WISC-III at week 0, with mean scores in PHIV youth showing an identical trend, although the odds of impairment was not significantly different compared with HUU youth. Visual memory functioning (WRAML design memory), also measured only at week 0, demonstrated significantly higher odds of impairment in PHIV youth, as was found among youth in PHACS.11
All behavioral outcomes, measured by CBCL, were worse among PHIV than HUU youth, but HEU also had statistically significantly higher rates of internalizing problems compared with HUU youth over all follow-up periods. In a cross-sectional analysis of our cohort when the uninfected groups were younger (mean age 7.6 years), the prevalence of borderline-clinical internalizing scores of HEU and HUU children was 18.4% and 18.5%, respectively13; a longitudinal analysis of CBCL scores when youth were younger found that HEU children were the least likely to have scores in a borderline clinical range.14 In this current analysis, when the youth were in adolescence, the prevalence of internalizing problems in HUU youth decreased but progressively increased in the HEU youth. Both PHIV and HEU youth in the United States have also been shown to be at risk of mental health problems, using similar measures.15–17 In the United States, this is believed to be at least partly due to living situations, with HEU youth more likely to live in single-parent households with high exposure to stressful life events, limited resources, and/or parental histories of substance use. In our study, psychological disorders among family members of PHIV and HEU participants were reported by 3% and 4.6% respectively, whereas 1.3% of PHIV and 1.5% of HEU caregivers reported family members taking recreational drugs. These observations suggest that lower socioeconomic status and its consequences, common to PHIV and HEU in both cohorts, play an important role in these adverse behavioral outcomes. Importantly, both groups are affected by parental HIV, and associated stigma and/or loss of a parent can significantly impact the development of internalizing (depression, anxiety, and withdrawal) problems.
Our finding that PHIV youth are more compromised than HEU and HUU across multiple NP measures supports the conclusion that HIV-infection status is an important contributor to these problems. The PHIV youth in this study received ART for a median of 7.9 years and were virologically well controlled. More than 95% of the PHIV youth in this study initiated ART after 2 years of age, an age after which the most vulnerable would have already died. Nevertheless, HIV infects the brain soon after systemic infection, and chronic or intermittent brain inflammation over a long period, combined with genetic and sociodemographic factors, may have effects on brain development and integrity, with executive function and visual memory particularly at risk. The deficits observed in this study, coupled with lower socioeconomic status and variable family resources, have potential to exert a cumulative toll on competency in youth and their successful transition to adulthood and functional skill development. Executive function and mental health issues increase the risk of poor adherence to treatment and patient care, with downstream effects on HIV transmission to their children and sexual partners, if viral suppression is not maintained.
Our study has a number of limitations. First, the PHIV youth were approximately 2 years older than the HEU and HUU youth. Second, it is possible that the differences between the study groups were at least partly due to socioeconomic differences. Cognition and health outcomes are significantly influenced by socioeconomic status,18,19 and HUU youth had significantly higher household incomes and had parents and carers who had completed higher levels of education compared with PHIV and HEU. Third, the measures used in our study were designed for and normed using English-speaking US youth. Adjustment for age and use of z-scores based on the HUU control children partially mitigate these problems. Fourth, PSI and FDI were not administered to Cambodian youth, resulting in reduced power for making statistical comparisons between study groups. Fifth, there is the possibility of practice effects on the tests that were administered yearly, but we might expect that these practice effects would be common to all study groups and the clustered data adjusted for both intraindividual and interindividual variability. Despite these limitations, the longitudinal nature of the cohort and the diverse battery of NP and behavioral tests allowed us to comprehensively characterize the cognitive and emotional health of youth in this cohort with 2 comparison groups and all groups relatively unaffected by maternal substance use. In addition, to date, there are few studies of cognitive and behavioral function over time among older youth with PHIV in Asian countries, and the results can inform support services during adolescence for other youth in the region.
Asian adolescents with PHIV are at risk of cognitive and mental health problems despite relatively early HIV treatment. Targeted education and care when problems emerge may reduce the functional impact of disease mechanisms on cognitive function in youth with PHIV. HEU youth are also at increased risk of mental health problems, but are unfortunately, often invisible to health care systems as has been documented in previous studies.15,16 Finally, it is critical to note that, despite being born with HIV and the challenges this entails, many youth born with PHIV or HEU were not cognitively impaired in the current study, and we saw a decline in impairment rates on several test measures over successive years of enrollment, which may relate to ongoing brain development/learning during adolescence as well as supportive educational, counseling, social services, and medical care available for youth with HIV. Thus, many of these youth are likely to successfully manage transition to new adult responsibilities as they age, particularly with adequate family support, giving us reason for optimism for their futures.
ACKNOWLEDGMENTS
The authors thank the children and families who participated in the RESILIENCE multisite study in Thailand and Cambodia and are grateful to the investigators, clinical centers, and staff for their contributions.
Supported by the U.S. National Institute of Allergy and Infectious Diseases (U19 AI053741) and the U.S. National Institute of Mental Health (R01MH089722 and R01MH102151). Antiretroviral drugs for PREDICT were provided by ViiV Healthcare (AZT, 3 TC), Boehringer Ingelheim (NVP), Merck (EFV), Abbott (RTV), and Roche (NFV).
Footnotes
The authors have no conflicts of interest to disclose.
An earlier analysis of this work was presented at the 22nd International AIDS Conference (AIDS 2018); July 23–27, 2018; Amsterdam, the Netherlands.
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, the Department of Defense, or the National Institutes of Health, the Department of Health and Human Services.
REFERENCES
- 1.Crowell CS, Huo Y, Tassiopoulos K, et al. Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS. 2015;29:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected Children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr Infect Dis J. 2013;32:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul R, Apornpong T, Prasitsuebsai W, et al. Cognition, emotional health, and immunological markers in children with long-term nonprogressive HIV. J Acquir Immune Defic Syndr. 2018;77:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughton B, Cornell M, Kidd M, et al. Five year neurodevelopment outcomes of perinatally HIV-infected children on early limited or deferred continuous antiretroviral therapy. J Int AIDS Soc. 2018;21:e25106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNICEF. Children, HIV and AIDS. Regional Snapshot: East Asia and the Pacific. 2018. [Google Scholar]
- 6.Delis D, Kaplan E, Kramer J. The Delis-Kaplan Executive Function System. San Antionio, TX: The Psychological Corportaion; 2001. [Google Scholar]
- 7.Sheslow D, Adams W. In: Wilmington DE, ed. Wide Range Assessment of Memory and Learning. 2nd ed. Wide Range Inc; 2003. [Google Scholar]
- 8.Wechsler D Manual for the Wechsler Adult Intelligence Scale. 3rd ed. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 9.Wechsler D Manual for the Wechsler Intelligence Scale for Children. 3rd ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 10.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth and Families; 2001. [Google Scholar]
- 11.Malee KM, Chernoff MC, Sirois PA, et al. Impact of perinatally acquired HIV disease upon longitudinal changes in memory and executive functioning. J Acquir Immune Defic Syndr. 2017;75:455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols SL, Chernoff MC, Malee KM, et al. Executive functioning in children and adolescents with perinatal HIV infection and perinatal HIV exposure. J Pediatr Infect Dis Soc. 2016;5(suppl 1):S15–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr SJ, Puthanakit T, Vibol U, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care. 2014;26:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malee K, Kerr S, Paul R, et al. Emotional and behavioral resilience among children with perinatally acquired HIV in Thailand and Cambodia. AIDS. 2019;33(suppl 1):S17–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malee KM, Tassiopoulos K, Huo Y, et al. Mental health functioning among children and adolescents with perinatal HIV infection and perinatal HIV exposure. AIDS Care. 2011;23:1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellins CA, Tassiopoulos K, Malee K, et al. Behavioral health risks in perinatally HIV-exposed youth: co-occurrence of sexual and drug use behavior, mental health problems, and nonadherence to antiretroviral treatment. AIDS Patient Care STDS. 2011;25:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkington KS, Robbins RN, Bauermeister JA, et al. Mental health in youth infected with and affected by HIV: the role of caregiver HIV. J Pediatr Psychol. 2011;36:360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: how do they relate to adolescent health? Health Psychol. 2006;25:704–714. [DOI] [PubMed] [Google Scholar]
- 19.Mani A, Mullainathan S, Shafir E, et al. Poverty impedes cognitive function. Science. 2013;341:976–980. [DOI] [PubMed] [Google Scholar]


