Abstract
Frailty has emerged as a powerful predictor of outcomes in patients with cirrhosis and has inevitably made its way into decision making within liver transplantation. In an effort to harmonize integration of the concept of frailty among transplant centers, the AST and ASTS supported the efforts of our working group to develop this statement from experts in the field. Frailty is a multidimensional construct that represents the end-manifestation of derangements of multiple physiologic systems leading to decreased physiologic reserve and increased vulnerability to health stressors. In hepatology/liver transplantation, investigation of frailty has largely focused on physical frailty, which subsumes the concepts of functional performance, functional capacity, and disability. There was consensus that every liver transplant candidate should be assessed at baseline and longitudinally using a standardized frailty tool, which should guide the intensity and type of nutritional and physical therapy in individual liver transplant candidates. The working group agreed that frailty should not be used as the sole criterion for delisting a patient for liver transplantation, but rather should be considered one of many criteria when evaluating transplant candidacy and suitability. A road map to advance frailty in the clinical and research settings of liver transplantation is presented here.
Keywords: clinical research/practice, guidelines, liver transplantation/hepatology, nutrition, recipient selection
1 |. INTRODUCTION
Frailty has emerged as a fundamental force shaping the field of liver transplantation. Liver disease severity at transplantation is worsening, the proportion of older adults (≥65 years) awaiting transplantation is rising, and the prevalence of obesity-related liver disease is rapidly escalating—all of which are contributing to a cohort of liver transplant patients who are sicker, more medically complex, and increasingly being described as “frail.” Clinicians caring for these patients have long intuited the importance of frailty on health out-comes before and after liver transplantation, even removing patients from the waitlist for being “too frail for transplant.” Yet despite the fact that the concept of frailty has inevitably made its way into transplant decision-making, its integration into clinical transplant practice thus far has been haphazard, hindered by a lack of consensus on its definition, tools for assessment, and implications for transplant decision-making.1
To overcome these barriers, the American Society of Transplantation supported the efforts of our working group of experts in the field to develop this statement on frailty in liver transplantation. Our specific goals were to: (a) define frailty, (b) appraise tools for frailty measurement, and (c) develop an algorithm for practical incorporation of frailty into clinical practice. While much of this document applies to patients with cirrhosis, regardless of their transplant eligibility, this statement was primarily intended for the transplant setting; we have highlighted specific areas in which our recommendations may differ whether or not the patient is listed for liver transplantation.
One word of caution when implementing our recommendations: we do not support the use of a one-time assessment of frailty as the sole criterion for declining a patient for liver transplantation. Our goal with this document is to facilitate the systematic incorporation of a standardized frailty assessment for every patient at evaluation and longitudinally while awaiting liver transplantation in order to accurately capture progression of frailty on the waitlist as well as serve as the foundation for frailty intervention.
1.1. |. Defining “frailty” in the setting of liver transplantation
The concept of frailty is most commonly defined as a distinct biologic syndrome of decreased physiologic reserve and increased vulnerability to health stressors that predisposes one to adverse health outcomes.2 Frailty is a multidimensional construct, and represents the end-manifestation of derangements of multiple physiologic systems including all individual solid organ systems (eg, the liver, kidney, heart), inflammatory, endocrine, cognitive, and musculoskeletal systems, as well as psychosocial factors.
While frailty has generally been conceptualized in the geriatrics arena as distinct from functional status, in the fields of hepatology/liver transplantation, the term “frailty” has largely focused on physical frailty (the aspect of frailty related to functional impairment) due to considerations of measurement in the hepatology and transplant settings. To be clear, functional status refers to one’s ability to perform daily activities, fulfill social roles, and maintain health/well-being3and subsumes the concepts of functional performance, functional capacity, and disability. In the context of liver transplantation, the focus on the physical functional aspects of frailty has the advantage over a broader conceptualization of frailty (that includes cognitive, social, and emotional aspects) given the need for objectivity of measurement. Although cognitive frailty is predictive of outcome in cirrhosis,4,5 the lack of standardized tools for the assessment of cognitive dysfunction in cirrhosis and the overlap with hepatic encephalopathy makes it difficult to objectively evaluate this more encompassing definition of frailty at this time. Importantly, “physical frailty,” as investigated in patients with cirrhosis, is a critical determinant of adverse health outcomes in this population, including waitlist mortality,6–11 mortality after hospitalization and after liver transplantation,12–15 need for hospitalization, length of stay,14,16–18 and discharge location (ie, rehabilitation facility)13,14 (Table 1).
TABLE 1.
Metrics of physical frailty, fitness, or disability studied in patients with cirrhosis (where the study included an adjustment for liver disease severity)
| Tool | Study | Details | N | Score | Association with outcomes (overall mortality unless otherwise specified) |
|---|---|---|---|---|---|
| ADL | Lai 20146 | Outpatient | 294 | ≥1 disability (24%) | HR: 1.23 95% Cl (0.91–1.66) |
| Samoylova 201728 | Outpatient | 458 | ≥1 disability (49%) | sHR: 1.8 95% Cl (1.4–2.4) | |
| Tapper 201514 | Inpatient | 734 | ADL < 12: 9.2% without HE and 24% with HE** | ADL < 12: HR 1.8 95% Cl (1.1–3.2) | |
| CFS Range 1–9 | Tandon 201616 | Outpatient | 300 | CFS > 4: 18% CFS > 3: 51% | OR (per 1 unit): 1.9 (1.4–2.6) |
| Ney20184 | Outpatient | 355 | MoCA-CFS score (cognitive + physical frailty) 0 1 2 |
OR of an HE-related hospitalization: 1 3.3 (1.5–7.7) 5.7 (1.9–17.3) |
|
| Karnofsky Performance Scale | Malinis 201429 | Transplant registry | 35 686 | KPS (B or C): 63.4% | 5-yr mortality: sHR 1.30 (1.23–1.37) |
| (range A-C or 0–100) | Orman 201611 | Transplant registry | 70 092 | KPS (B or C): 56% | 1-year mortality by KPS: A (11.4%), B (15.5%), C (27.4%) KPS B: HR 1.08 95% Cl (1.04–1.111) KPS C: HR 1.26 95% Cl (1.20–1.33) |
| Tandon 201715 | Hospitalized Decompensated Cirrhosis | 954 | KPS (B or C): 68% | 3-month postdischarge mortality: By KPS: A (5%), B (11%), C (23%) KPS (per 1-unit): OR 0.97 95% Cl (0.96–0.98) | |
| Braden Scale Range 6–23 | Tapper 201514 | Hospitalized Decompensated Cirrhosis | 734 | Moderate-to high-risk Braden Scale: ≤18 (28.1% HE, 13.7% without HE) | 90-day mortality Score 16–18: 2.71 95% Cl (1.88–3.90) Score < 16: 1.85 95% Cl (0.83–4.12) |
| Sundaram 201713 | Outpatient All transplant listed | 341 | Moderate-to high-risk Braden Scale: 16–18: (17%), <16 (20%) | Posttransplant mortality: insufficient outcomes | |
| FFP Range 0–5 | Lai 20146 | Outpatient All transplant listed | 294 | FFP ≥3: 17% | Per point: 1.45 95% Cl (1.04–2.02) |
| Tandon 201616 | Outpatient Cirrhosis |
300 | FFP ≥3: 35% | OR 4.0 | |
| Sinclair 201718 | Outpatient All transplant listed | 587 | FFP ≥3: 32% | Hospitalization days per 12 months IRR: 1.2 95% Cl (1.02–1.44) | |
| Tapper 20185 | Outpatient All transplant evaluated | 685 | FFP ≥3: 41% | Transplant-free survival HR per FFI point: Without HE: 1.37 (1.20–1.58) With HE: 1.14(0.98–1.33) | |
| 6MWD Meters walked | Carey 20109 | Outpatient All transplant listed | 121 | Mean 6MWD 69 ±122 m | Per 100 m: 0.58 95% Cl (0.37–0.93) |
| Yadav 201530 | Outpatient All transplant listed | 213 | Mean 6MWD 371±121m 12% ≤250 m | 250 m cutoff: HR 2.1 95% Cl (0.9–4.7) | |
| Faustini Pereira 201612 | Outpatient | 86 | Mean 6MWD 410 ± 27.8 m | <410 m walked (unadjusted): RR 4.21 95% Cl (1.25–6.41) | |
| Gait speed (meters/second) | Dunn17 | Outpatient | 373 | Mean gait speed 0.95 ± 0.25 m/s | Hospital bed-days Per 0.1 m/s: RR 0.85 (0.74–0.98) |
| SPPB Range 0–12 | Lai 20146 | Outpatient All transplant listed | 294 | SPPB < 9:31% | Per point: 1.19 95% Cl (1.07–1.32) |
| Tandon 201616 | Outpatient Cirrhosis |
300 | SPPB < 10:38% | OR 2.5 | |
| Liver Frailty Index per point | Lai 20177 | Outpatient All transplant listed | 529 | Median LFI: 3.8 (3.4–4.3) | Waitlist mortality Per point: HR 2.2 95% Cl (1.7–2.9) |
| Cardiopulmonary Exercise Testing mL/kg/min | Ney 201610 | Systematic review of: Outpatient All transplant listed | 1107 | Ventilatory anaerobic threshold (AT) Peak exercise oxygen uptake (peak V02) | Posttransplant mortality (mean difference between survivor and nonsurvivors) AT: 2.0 95% Cl (0.42–3.59) Peak V02: 0.77 95% Cl (−1.36–2.90) |
Abbreviations: 6MWT, 6-minute walk test; 6MWD, 6-minute walk test distance; ADL, Activities of Daily Living; AT, anaerobic threshold; CFS, Clinical Frailty Scale; CPET, cardiopulmonary exercise testing; FFP, Fried Frailty Phenotype; HE, hepatic encephalopathy; HR, hazard ratio; KPS, Karnofsky Performance Status; IADL, Instrumental Activities of Daily Living; IRR, incidence rate ratio; LFI, Liver Frailty Index; MoCA, Montreal Cognitive Assessment; OR, odds ratio; SPPB, Short Physical Performance Battery.
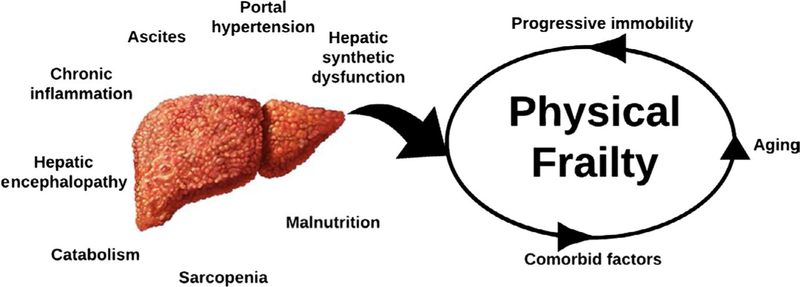
Major components of frailty in all patients include skeletal muscle mass depletion (sarcopenia), progressive immobility, decreased energy expenditure, and malnutrition.2 In patients with cirrhosis, there are multiple liver-specific factors that exacerbate and accelerate this cycle of frailty (Figure 1). Chronic inflammation from the underlying liver disease is often the initial insult. Hepatic synthetic dysfunction results in the impairment of muscle protein synthetic response that can rapidly lead to progressive muscle breakdown. Anorexia associated with malaise (from chronic inflammation) and early satiety (from ascites) leads to malnutrition, further accelerating muscle wasting. Hepatic encephalopathy and cognitive decline magnify the expression of frailty through multiple pathways, including altered taste perception, fatigue, immobility, and decreased energy expenditure. The obligatory shift of ammonia from liver to muscle for export as glutamine—diverting glutamate needed for muscle protein synthesis—is also recognized to be a pivotal driver of muscle wasting. Ammonia itself promotes muscle autophagy, directly impairs contractility, and triggers synthesis and release of myotoxins contributing to sarcopenia.19 In addition to these liver-related factors, patients with cirrhosis also experience non-liver-related factors including chronologic aging, non-hepatic comorbidities (eg, coronary artery disease, diabetic peripheral neuropathy), and age-related muscle wasting. The contributions of these non-liver-related factors are particularly important for transplant decision-making, as they are not modifiable and will not improve after transplantation.20
FIGURE 1.
Liver-related and non-liver-related factors that contribute to the development of physical frailty in patients with cirrhosis
While sarcopenia is a central and dominant component of frailty in patients with cirrhosis, the concept of frailty is more multifaceted than sarcopenia alone. The inclusion of functional measures (eg, chair stands, gait speed) in validated frailty metrics suggests that the influence of sarcopenia may be modified by factors related to muscle function rather than purely muscle mass. Furthermore, the influence of patient-reported outcomes (eg, exhaustion, sedentary time) implies that an individual’s experience of their frailty state may also influence health outcomes. This consensus statement only addresses sarcopenia as it relates to the over-all construct of frailty; a separate working group has been assembled to more specifically address sarcopenia as a single entity.
1.2 |. Measuring frailty in adult liver transplant patients
Table 2 lists the tools to capture the construct of frailty that have been studied in patients with cirrhosis, including those awaiting liver transplantation. We, again, emphasize that the studies in this patient population have largely focused on the physical contributors to frailty, including functional performance, functional capacity, and disability.
TABLE 2.
Properties of the tools evaluating “frailty” that have been evaluated in patients with cirrhosisa
| Subjective ←-----------------------→ | Objective | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFS | KPS | ADL/IADL | Braden scale | FFP | SPPB | LFI | Grip strength | Gait speed | 6MWT | CPET | ||
| Subjectivity | Requires clinician judgment | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Can be biased by patient reporting | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Predictive validity | For pretransplant outcomes | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| For posttransplant outcomes | - | ✓ | - | - | - | - | - | - | - | - | ✓ | |
| Test characteristics | Reliability (internal consistency and repeatability) | ✓ | - | - | - | - | - | - | ✓ | ✓ | - | - |
| Responsiveness to change over time | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | - | - | |
| Clinical feasibility | Estimated time taken (minutes) | <1 | <1 | <2 | <5 | <10 | <5 | <5 | <i | <2 | <10 | <60 |
| Need for specialized equipment | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓✓ | |
| Need for highly trained personnel | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓✓ | |
Abbreviations: 6MWT, 6-minute walk test; ADL, Activities of Daily Living; CFS, Clinical Frailty Scale; CPET, cardiopulmonary exercise testing; IADL, Instrumental Activities of Daily Living; FFP, Fried Frailty Phenotype; KPS, Karnofsky Performance Status; LFI, Liver Frailty Index; SPPB, Short Physical Performance Battery.
Note: Double check mark indicates that these tests really need specialized technicians and equipment more so than the other tests that have only one check mark.
No data available; ✔ yes; ✗ no.
In the research arena, frailty indices that best capture the multidimensionality of frailty such as the Fried Frailty Phenotype2 or the Frailty Index (“deficit model”21) may be necessary to demonstrate construct validity of new tools in patients with cirrhosis. However, these “traditional” models of frailty have limited applicability to the clinical practice of liver transplantation in that they are not continuously scored, display strong ceiling and/or floor effects, or are too complex to use in a busy clinical practice.1
With respect to the application of frailty tools in the clinical arena, we recommend that every transplant center should incorporate a standardized tool to measure frailty in their liver transplant patients both at initial evaluation and longitudinally on the waitlist. This recommendation was based on evidence that standardized frailty metrics can improve the accuracy of the “eyeball test” and traditional liver disease metrics to predict mortality in patients with cirrhosis.5,7–14,21
Given that there is no single frailty tool that has emerged in the literature as suitable for evaluation of patients with cirrhosis in all clinical scenarios (outpatient vs. inpatient; transplant vs. nontransplant), we recommend a frailty tool kit to provide a range of tools that can be used depending upon the clinical setting, available resources, and intended clinical decisions that will be made based on the test result. Here, we offer several points for each center to consider when deciding on which standardized frailty tool(s) to incorporate into clinical practice:
Frailty tools have been best studied in the outpatient setting. Measures such as the Fried Frailty Phenotype2 or Liver Frailty Index7 have, to date, only been studied in the outpatient hepatology/liver transplant settings where patients are in their “steady state.” Hospitalized patients often have transient perturbations in physical and cognitive function, which limit the ability of these performance-based frailty assessments to represent true underlying physiologic reserve. However, while performance-based tests may have limited use in the inpatient setting, provider-and patient-assessed tools such as the Karnofsky Performance Status (KPS) and Activities of Daily Living (ADL) scale have been evaluated in the inpatient settings and demonstrated to predict nontransplant mortality,11,14‘15 re-admissions,14,16 and mortality after liver transplantation.11
Subjective tools for “screening” versus more objective frailty assessment. Because of the potential implications of frailty in the decision to proceed with transplant, there was a consensus that waitlisted patients require assessment with objective, performance-based frailty tools (eg, Liver Frailty Index, 6-minute walk test). Provider-or patient-assessed metrics of frailty (eg, KPS, ADLs, Clinical Frailty Scale), while simple and feasible to administer systematically in a busy clinical setting, may be insensitive to subtle, but prognostic, gradients of the frailty spectrum. That being said, in the larger population of patients in the nontransplant setting, a stepwise approach where patients are screened with an “easy-to-perform” test, followed by a more comprehensive test to either confirm or definitively rule out frailty may be the most practical.
Measurement of longitudinal changes in frailty is clinically relevant in the transplant setting and requires frailty tools that are sensitive to change. Longitudinal changes in frailty are predictive of wait-list mortality above and beyond a single assessment alone.22 Metrics such as the composite Liver Frailty Index, which is continuous, lacks a floor/ceiling and has been shown to be reliable/reproducible,23 are particularly well-suited for longitudinal measurement, although additional research is needed to validate the prognostic value of “Δfrailty” using the Liver Frailty Index. Identification of frailty tools that are sensitive to change is particularly relevant as an endpoint for clinical trials aiming to slow the progression of—or even reverse—frailty.
Based on these three criteria, we offer a parsimonious tool kit consisting of the KPS scale, ADL/IADLs, Liver Frailty Index, and the 6-minute walk test for transplant clinicians (Table 3). While no single tool is perfect for every clinical scenario, we selected these four tools specifically to balance the needs for speed, low-cost, patient-centeredness, and objectivity.
TABLE 3.
Suggested frailty tool kit
| Tool | Rationale for inclusion in the frailty tool kit |
Estimated time to assess |
Populations studied |
|---|---|---|---|
| Karnofsky performance status | Intuitive and instant | <10 s | Inpatient and |
| No cost | outpatient | ||
| Low floor effects | |||
| Can be assessed by the patient or the provider | |||
| Activities of daily living/instrumental activities of daily living | No cost | 2‐4 min | Inpatient and outpatient |
| Patient reported | |||
| Well‐accepted patient‐oriented outcome | |||
| Liver frailty index | Objective, performance‐based | 1‐3 min | Outpatient |
| Continuous scale without ceiling or floor effects | |||
| Quickly administered | |||
| Can be repeatedly performed in the outpatient setting | |||
| 6‐minute walk test | Objective, performance‐based | 6 min | Outpatient |
| Continuous scale without ceiling or floor effects | |||
| No need for specialized equipment |
1.3 |. Measuring frailty in pediatric liver transplantation
A recent 17-center study demonstrated that frailty assessment with the Fried Frailty Phenotype is feasible in school-aged children with chronic liver disease; nearly half of children with end-stage liver disease met criteria for being frail.24 It is not yet known the extent to which frailty measures impact mortality. Metrics that incorporate performance-based tests have limited application in infants and toddlers who may not be able to fully cooperate with testing instructions (eg, grip strength, chair stands). Frailty assessment in pediatric liver transplant patients < 5 years of age will likely require a combination of quantitative muscle mass measurement, laboratory and/or anthropometric nutritional biomarkers, and observed assessments of activity.
1.4 |. Incorporating frailty into clinical decisionmaking
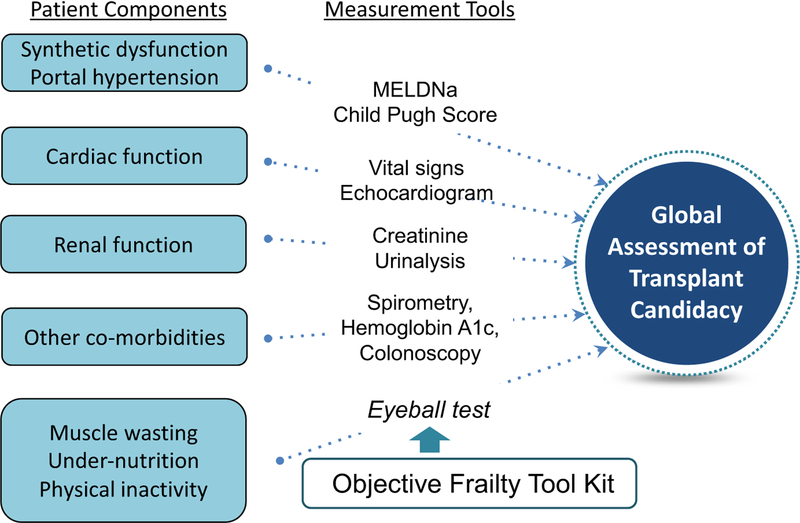
We believe that a single assessment of frailty should not be used as the sole criterion for removing a patient from the liver transplant waitlist, as there are no data to support a single frailty cutoff at which a patient should not undergo liver transplantation. Instead, we advocate that a standardized tool for frailty be considered as one of many objective components that are routinely incorporated into a clinician’s assessment of a patient’s global health status that ultimately determines his or her transplant candidacy (Figure 2).8
FIGURE 2.
A conceptual model of some of the patient components that clinicians incorporate into their global assessment of a patient’s transplant candidacy and the tools that they use to inform this holistic assessment. An objective frailty tool kit should be used to inform clinicians’ assessments of muscle wasting, under-nutrition, and physical inactivity—which, together, form the major components of physical frailty—to improve objectivity and accuracy of the clinician’s global assessment of transplant candidacy for the purposes of transplant decision-making (adapted from Lai JC, AJG 2017)8
Incorporating frailty into transplant decision-making can offer the liver transplant community more than simply prognostication. What makes frailty such a unique risk factor for patients with cirrhosis is that, unlike more “traditional” transplant risk factors such as age, sex, or Model for End-Stage Liver Disease score, individual components of frailty (eg, physical function, sarcopenia, and malnutrition) are potentially modifiable with exercise and nutritional interventions.25,26
Recently, the concept of “prehabilitation” has gained significant momentum in transplant and nontransplant surgical fields.27 Prehabilitation refers to multidisciplinary “training” to enhance physical strength and nutritional status—with the theoretical benefit of improving physiologic reserve prior to surgery. Although data on the impact of prehabilitation in liver transplantation are limited to a small cohort at a single center,27 there is emerging evidence in studies of patients undergoing major abdominal surgeries that prehabilitation programs improve outcomes and reduce costs. Examples of specific interventions have included comprehensive physical activity programs, supervised and home-based exercises, educational/behavioral modification, and/or nutrition counseling.
Based on these data, we have developed a simple algorithm that leverages the potential “modifiability” of frailty through prehabilitation (Figure 3). Specifically, this algorithm uses a standardized frailty metric to guide recommendations regarding the intensity of prehabilitation for liver transplant candidates. While our working group agreed that all liver transplant candidates should be provided exercise and nutritional recommendations, in light of limited availability of outpatient physical therapy and dietician resources—not to mention limited reimbursement—our algorithm allows for intensification of resources in those patients who are most vulnerable (ie, frail). The specific goals of this algorithm were to: (a) increase physiologic reserve pretransplant so that patients may better withstand acute decompensating events, (b) improve clinical outcomes after liver transplantation, and (c) more efficiently and effectively allocate healthcare resources in liver transplantation.
FIGURE 3.
Algorithm to tailor prehabilitation recommendations based on frailty assessment
Our algorithm involves the following steps:
Step 1: Stratify risk by frailty status. All liver transplant candidates should undergo risk stratification using a standardized frailty assessment tool. Our proposed frailty stratification system, based on expert opinion, for a select number of tools, is presented in Table 4.
Step 2: Recommend a prehabilitation program based on risk stratum. The intensity of frailty intervention should be tailored to the degree of frailty. Patients with severe frailty may benefit from intensive prehabilitation, with consideration of referral to an inpatient rehabilitation center. We recommend that patients with a moderate degree of frailty engage in a home-based exercise program developed by a certified exercise professional that targets the patient’s greatest functional impairment(s) (eg, balance, chair stands) but also incorporates aerobic training and simulates ADLs (to improve quality of life). Patients with mild or no frailty should follow recommendations developed for the general population (ie moderate-intensity exercise ≥ 150 minutes per week), with gradual build up physical endurance and strength. Physical activity trackers (eg, accelerometers) may be considered to assess adherence.
Step 3: Reassess and re-stratify. Reversal of frailty among liver transplant candidates is feasible but has not been systematically studied. Lack of progression, however, is a clinically relevant achievement that should incentivize liver transplantation, particularly if early posttransplant rehabilitation will be provided. We recommend close monitoring of patients on the waitlist, with reassessment intervals based on the patient’s severity of frailty at the last available examination (Figure 3).
TABLE 4.
Recommended criteria to stage frailty in liver transplant candidates
| Stages of frailty |
|||
|---|---|---|---|
| Severe | Moderate | Mild/Absent | |
| ADL14,31 | Difficulty with ≥2 ADLs | Difficulty with 1 ADL | No difficulty with ADLs |
| Clinical Frailty Scale16 | ≥7 | 6 | 1‐5 |
| Fried Frailty Phenotype6 | ≥3 | 1‐2 | 0 |
| Karnofsky Performance Status Scale11,15 | 0‐40 | 50‐70 | ≥80 |
| Liver Frailty Index7 | ≥4.5 | 3.2‐4.4 | <3.2 |
| 6‐minute walk test9 | <250 m | <350‐250 m | >350 m |
Abbreviation: ADL, activities of daily living.
2 |. A ROADMAP TO ADVANCE FRAILTY IN THE CLINICAL AND RESEARCH SETTINGS OF LIVER TRANSPLANTATION
Frailty is now well-recognized in the scientific literature as a strong predictor of outcomes in patients with cirrhosis, including in the liver transplant setting. While the frailty literature in hepatology/liver transplantation is currently rich with high quality studies, many questions remain: (a) the impact of frailty on mortality after liver transplantation, (b) the impact of longitudinal changes in frailty on outcomes, and (c) the relationship between liver disease progression and frailty. Perhaps, the most exciting target for future investigation is the notion that frailty is actionable, and that its components can be arrested or even reversed. Here we propose a path forward to advance our understanding of frailty and improve the care of our patients:
Obtain funding for multicenter consortia for prospective studies on frailty in liver transplantation. Now is an opportune time for formal financial sponsorship of multicenter consortia to accelerate progress. Engagement with other teams studying frailty in other chronic diseases, geriatrics/gerontology, and other solid organ transplant disciplines may have a high value.
Implement evidence-based, objective frailty measurement as part of standard-of-care. Given its strong associations with health-related outcomes, frailty should be considered a vital sign and measured systematically and routinely during clinic visits.
Develop interventions targeting modifiable aspects of physical frailty through rigorous multicenter randomized clinical trials. Specific modifiable targets include muscle mass, muscle function, activity level, and nutrition. Interventions can focus on a single aspect or offer a more comprehensive approach (eg, prehabilitation program). Randomization should offer clinical equipoise: because we believe that all patients with cirrhosis would benefit from some form of activity and nutritional counseling, trials should explore varying intensities (eg, two times per week vs. daily) or types of intervention (eg, home-vs. center-based; telephone calls vs. text messages) rather than randomizing patients to a “no intervention” arm.
Investigate nonphysical aspects of frailty. These include cognitive, emotional, social, and environmental aspects that expand the concept of frailty beyond physical frailty alone.
Integrate the concept of frailty into training curricula for hepatology/surgery trainees and into national society guidelines for management of patients with cirrhosis. Educational modules should be developed to assess transplant trainees’ ability to objectively assess, document, and incorporate frailty into clinical decision-making. Assessment of frailty should be formally incorporated into national guidelines for evaluation of liver transplant candidates.
Include objective measurement of frailty into research studies and national registries. Frailty can be treated as a predictor, a confounder, or even an outcome in research studies. Inclusion of objective measurement of frailty into national registry data would accelerate research in this field and enable adjustment for frailty in any study evaluating pre-and posttransplant mortality. Based on the evidence to date and the need for uniformity of objective frailty measurement in this setting, we recommend use of the Liver Frailty Index for this purpose.
Key points.
Frailty is a multidimensional construct that represents the endmanifestation of derangements of multiple physiologic systems that leads to decreased physiologic reserve and increased vulner-ability to health stressors.
In hepatology/liver transplantation, the investigation of frailty has largely focused on physical frailty which subsumes the concepts of functional performance, functional capacity, and disability.
While sarcopenia is a primary driver of frailty in patients with cirrhosis, frailty is more multifaceted than sarcopenia alone, offering a comprehensive assessment of muscle function and the individual patient’s experience of their frailty state in addition to muscle mass.
Key points.
Every patient with cirrhosis awaiting liver transplantation should be assessed at baseline and longitudinally using a standardized frailty tool.
Frailty measurement with objective performance-based measures (eg, Liver Frailty Index) is best studied in the outpatient setting when patients are in their “steady state.” However, provider-and patient-assessed instruments (eg, KPS, ADLs) have prognostic value among hospitalized patients.
To date, the Liver Frailty Index has the broadest applicability among all the frailty instruments for practical frailty assessment in the liver transplant setting and has the advantages of being objective, performance-based, and suitable for longitudinal measurement.
Key points.
Standardized assessments of frailty may be used to tailor the intensity and type of nutritional and physical therapy in patients awaiting and undergoing liver transplantation.
Frailty should not be used as the sole criterion for delisting a patient for liver transplantation, but rather should be considered one of many criteria when evaluating transplant candidacy and suitability (Figure 2).
ACKNOWLEDGMENTS
This manuscript is a work product of the American Society of Transplantation’s Liver and Intestine Community of Practice and has been endorsed by the American Society of Transplantation and the American Society of Transplant Surgeons. This study was funded by NIH K23AG048337 (Lai), NIH R01AG059183 (Lai), NIH RO1GM119174 (Dasarathy); P50 AA024333 (Dasarathy); R21AR 71046 (Dasarathy); UO1 AA0026976 (Dasarathy); UO1DK061732 (Dasarathy); RO1DK113196 (Dasarathy); Mikati Foundation Grant (Dasarathy). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
Funding information
Mikati Foundation Grant; National Institute of General Medical Sciences, Grant/Award Number: R01GM119174; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: R01DK113196 and U01DK061732; Office of AIDS Research, Grant/Award Number: R21AR071046; National Institute on Aging, Grant/Award Number: K23AG048337 and R01AG059183; National Institute on Alcohol Abuse and Alcoholism, Grant/Award Number: P50AA024333 and U01AA0026976
Footnotes
DISCLOSURE
The authors of this manuscript have no conflict of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Lai JC. Advancing adoption of frailty to improve the care of patients with cirrhosis: time for a consensus on a frailty index. Am J Gastroenterol. 2016;111(12):1776–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):146–156. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society: Quality Life Resource. Functional Status. Available at: http://qol.thoracic.org/sections/key-concepts/functional-status.html Accessed February 23, 2018.
- 4.Ney M, Tangri N, Dobbs B, et al. Predicting hepatic encephalopathy-related hospitalizations using a composite assessment of cognitive impairment and frailty in 355 patients with cirrhosis. Am J Gastroenterol. 2018;113(10):1–10. [DOI] [PubMed] [Google Scholar]
- 5.Tapper EB, Konerman M, Murphy S, Sonnenday CJ. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. Am J Transplant. 2018;18(10):2566–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14(8):1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66(2):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai JC, Covinsky KE, McCulloch CE, Feng S. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol. 2017;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16(12):1373–1378. [DOI] [PubMed] [Google Scholar]
- 10.Ney M, Haykowsky MJ, Vandermeer B, Shah A, Ow M, Tandon P. Systematic review: pre-and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment Pharmacol Ther. 2016;44(8):796–806. [DOI] [PubMed] [Google Scholar]
- 11.Orman ES, Ghabril M, Chalasani N. Poor performance status is associated with increased mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(8):1189–1195.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faustini Pereira JXL, Galant LH, Rossi D, et al. Functional capacity, respiratory muscle strength, and oxygen consumption predict mortality in patients with cirrhosis. Can J Gastroenterol Hepatol. 2016;2016(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundaram V, Lim J, Tholey DM, et al. The Braden Scale, a standard tool for assessing pressure ulcer risk, predicts early outcomes after liver transplantation. Liver Transpl. 2017;23(9):1153–1160. [DOI] [PubMed] [Google Scholar]
- 14.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62(2):584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tandon P, Reddy KR, O’Leary JG, et al. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017;65(1):217–224. [DOI] [PubMed] [Google Scholar]
- 16.Tandon P, Tangri N, Thomas L, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol. 2016;111(12):1759–1767. [DOI] [PubMed] [Google Scholar]
- 17.Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol. 2016;111(12):1768–1775. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair M, Poltavskiy E, Dodge JL, Lai JC. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. 2017;23(5):899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65(6):1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai JC. A framework to determine when liver transplantation is futile. Clin Liver Dis. 2016;8(6):137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. [DOI] [PubMed] [Google Scholar]
- 22.Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional decline in patients with cirrhosis awaiting liver transplantation: results from the Functional Assessment in Liver Transplantation (FrAILT) study. Hepatology. 2016;63:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CW, Lebsack A, Chau S, Lai JC. The range and reproducibility of the Liver Frailty Index (published online ahead of print March 2019). Liver Transpl. 10.1002/lt.25449.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lurz E, Quammie C, Englesbe M, et al. Frailty in children with liver disease: a prospective multicenter study. J Pediatr. 2018;194:109–115. [DOI] [PubMed] [Google Scholar]
- 25.Duarte-Rojo A, Ruiz-Margáin A, Montano-Loza AJ, Macías-Rodríguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. 2017;24(1):122–139. [DOI] [PubMed] [Google Scholar]
- 26.Tandon P, Ismond KP, Riess K, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol. 2018;69:1164–1177. [DOI] [PubMed] [Google Scholar]
- 27.Volk ML, Sonnenday C. Patient-centered liver transplantation. Clin Liver Dis. 2016;8(1):24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samoylova ML, Covinsky KE, Haftek M, Kuo S, Roberts JP, Lai JC. Disability in patients with end-stage liver disease: results from the functional assessment in liver transplantation study. Liver Transpl. 2017;23(3):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malinis MF, Chen S, Allore HG, Quagliarello VJ. Outcomes among older adult liver transplantation recipients in the model of end stage liver disease (MELD) era. Ann Transplant. 2014;19:478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav A, Chang Y-H, Carpenter S, et al. Relationship between sarcopenia, six-minute walk distance and health-related quality of life in liver transplant candidates. Clin Transpl. 2015;29(2):134–141. [DOI] [PubMed] [Google Scholar]
- 31.Rakoski MO, McCammon RJ, Piette JD, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology. 2012;55(1):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]