Abstract
Preclinical studies using rodent models of stroke have had difficulty in translating their results to human patients. One possible factor behind this inability is the lack of studies utilizing aged rodents of both sexes. Previously, this lab showed that leukemia inhibitory factor (LIF) promoted recovery after stroke through antioxidant enzyme upregulation. This study examined whether LIF promotes neuroprotection in aged rats of both sexes. LIF did not reduce tissue damage in aged animals, but LIF-treated female rats showed partial motor skill recovery. The LIF receptor (LIFR) showed membrane localization in young male and aged rats of both sexes after stroke. Although LIF increased neuronal LIFR expression in vitro, it did not increase LIFR in the aged brain. Levels of LIFR protein in brain tissue were significantly downregulated between young males and aged males/females at 72 h after stroke. These results demonstrated that low LIFR expression reduces the neuroprotective efficacy of LIF in aged rodents of both sexes. Furthermore, the ability of LIF to promote motor improvement is dependent upon sex in aged rodents.
1. Introduction
Stroke is the fifth leading cause of death among Americans and a leading cause of major disability. One of the most damaging types of ischemic stroke, an emergent large vessel occlusion (ELVO), occurs when a thrombus impedes blood flow in a major cerebral artery. ELVO patients have a high mortality rate, and survivors are often left with profound deficits in motor and cognitive function (Malhotra et al., 2017). Intravenous administration of tissue plasminogen activator (tPA), the only FDA-approved drug for ischemic stroke, is often ineffective in ELVO patients (Broderick et al., 2013; Ciccone et al., 2013; Kidwell et al., 2013), and others are unable to undergo endovascular thrombectomy (EVT). The permanent intraluminal middle cerebral arterial occlusion (MCAO) model of stroke best represents ELVO without tPA administration or EVT. This model provides the opportunity to discover new treatments to decrease mortality and disability resulting from ELVO.
Treating large vessel stroke in rats requires an agent that promotes neuroprotection and reduces peripheral inflammation. Leukemia inhibitory factor (LIF), a cytokine in the IL-6 family, has shown efficacy in animal models for neurological diseases, such as multiple sclerosis and amyotrophic lateral sclerosis. Previous groups demonstrated that LIF reduces neurodegeneration through pro-survival PI3K/Akt signaling and by promoting the development of anti-inflammatory leukocytes (Azari et al., 2001; Butzkueven et al., 2002; Butzkueven et al., 2006; Duluc et al., 2007; Janssens et al., 2015; Slaets et al., 2008; Suzuki et al., 2005). This laboratory has shown that systemic LIF administration is effective in reducing infarct volume and improving functional recovery after MCAO (Rowe et al., 2014). This drug regimen entails an initial dose delivered intravenously starting at 6 h after MCAO followed by additional doses at 24 and 48 h with euthanization at 72 h. This regimen was initiated to be clinically relevant and to encompass the neuroinflammatory-induced neurodegeneration that occurs at 48 h in this model(Leonardo et al., 2010).
LIF protects neurons and oligodendrocytes from an ischemic environment through the upregulation of antioxidant enzymes (Davis et al., 2017; Rowe et al., 2014). LIF is also an anti-inflammatory cytokine that promotes the development of an anti-inflammatory phenotype in macrophages and T helper cells (Duluc et al., 2007; Gao et al., 2009; Janssens et al., 2015; Metcalfe et al., 2015). These combined actions lead to improved histological and functional outcomes in this severe stroke model.
Neuroprotective signaling begins with LIF binding to the LIF receptor (LIFR), a 190 kDa member of the type 1 cytokine receptor family (Gadina et al., 2001). To confer downstream signaling, LIFR must be localized to the plasma membrane and near other IL-6 cytokine receptor components. Besides LIF, this receptor transduces signals of two other cellular protective proteins, cardiotropin-1 and ciliary neurotrophic factor. This receptor is unusual in that it resides in the nuclei of neuronal cells until an injury occurs (Davis et al., 2017; Gardiner et al., 2002; Zhao et al., 2014). While LIFR plays a crucial role in transducing signals for these protective factors, the molecular and cellular roles of this receptor are not entirely understood.
Until now, pre-clinical studies aimed at promoting post-stroke recovery have primarily utilized primarily young male rodents. Unfortunately, results from these studies have not led to success in clinical trials. We have reported favorable results with the administration of LIF as a neuroprotective agent in young male rats (Davis et al., 2017; Konoeda et al., 2010; Rowe et al., 2014). In this study, we utilized the same drug regimen and experimental design using aged (18 mo) rats of both sexes. Our study shows that LIF treatment is less effective at promoting neuroprotection after MCAO in aged rats of both sexes, which coincides with significantly lower LIFR expression in the brain compared to young rats.
2. Results
2.1. Stroke-Related Mortality is not Altered by Sex or LIF Treatment
The rates of mortality among aged male and female rats after MCAO + PBS and MCAO + LIF treatment were compared using the Mantel-Cox test. Mortality rates after sham surgeries are shown as a negative control. There was no significant difference in survival between sexes and treatment groups (χ2 = 0.3448, df = 3, p = 0.9514; Fig. 1).
Figure 1.
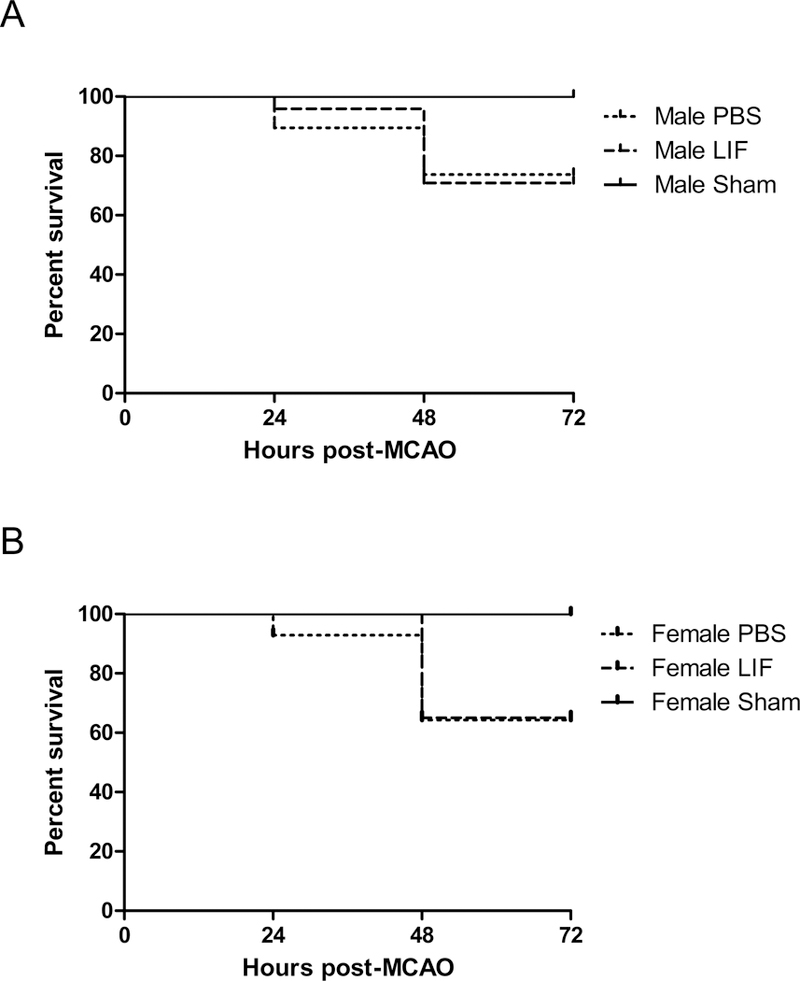
Kaplan-Meier curves show survival among (A) aged male rats and (B) aged female rats (sham-operated, PBS-treated, and LIF treated up to 72 h after MCAO. There was no significant difference in survival between sexes or treatment groups. n=9–17 animals per treatment group.
2.2. LIF Treatment does not Reduce Tissue Damage after MCAO
Before euthanizing animals, MRI scans were used to measure the volume of edema (T2) and infarcted tissue (DTI) in the brains of PBS and LIF-treated aged rats. Sham-operated animals of both sexes were included as a negative control for tissue damage. There was no significant difference in edema volume between sexes (F1,32 = 0.7368, p = 0.3971) or treatment groups F1,32 = 0.4469, p = 0.5086) at 72 h after MCAO (Fig. 2A). Likewise, there was no significant difference in the infarct volume between sexes (F1,25 = 0.1499; p = 0.7019) or treatment groups (F1,25 = 0.1372; p = 0.7142) at 72 h post- MCAO (Fig. 2B). However, there was a trend towards decreased edema volume and infarct volume after LIF treatment among aged female rats.
Figure 2.
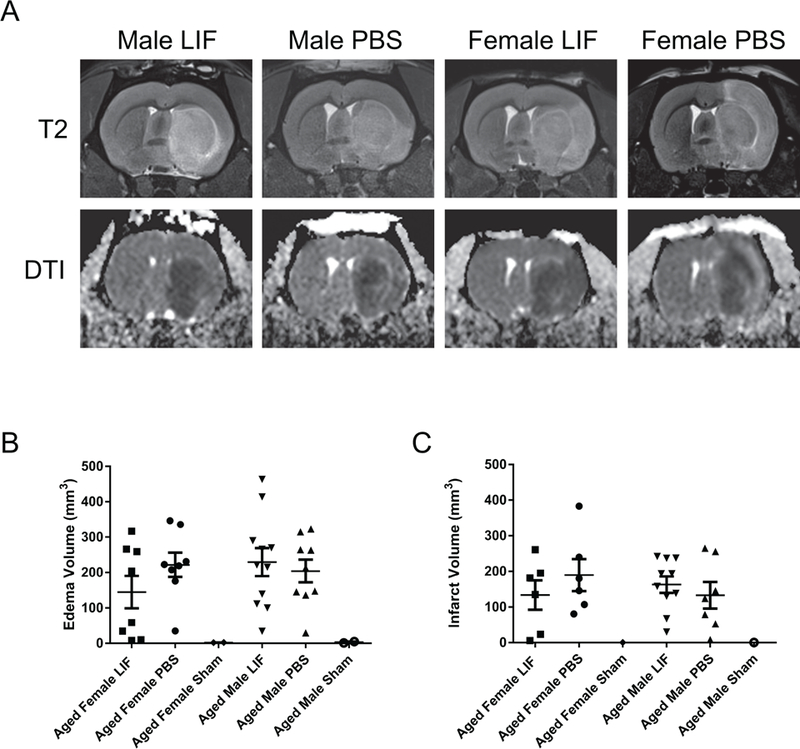
(A) MRI scans were used to assess edema (T2) and infarct volume (DTI) at 72 h after MCAO. Although there was not a significant overall difference in (B) edema or (C) infarct volume between PBS- or LIF-treated male and female rats, there was a trend towards decreased tissue damage after LIF treatment in aged female rats. n=9–17 animals per treatment group.
2.3. Aged Female Rats Show Partial Motor Recovery after LIF Treatment
Previously, this lab demonstrated that LIF administration after permanent large vessel stroke promotes motor skill recovery (Rowe et al., 2014). There was a trend towards improvement in LIF-treated female rats compared to PBS-treated female rats according to the Circling test that approached significance (χ2 = 2.524, df = 1, p = 0.056). However, male rats did not show decreased circling after LIF treatment (χ2 = 0.08184, df = 1, p = 0.3874; Fig. 3A). There was a significant two-way interaction between sex and drug treatment among the results for the EBST (F1,48 = 6.529, p = 0.0138). Upon further analysis, there was a significant decrease in swing bias between male and female LIF- treated rats (t = 3.646, df = 24, p = 0.0006). Female LIF-treated rats also showed a significant decrease in step bias compared to PBS-treated female rats (t = 2.548, df = 10, p = 0.0145; Fig. 3B). LIF-treated female rats also showed significant improvement according to the Paw Extension test compared to their PBS-treated counterparts (χ2 = 3.706, df = 1, p = 0.0271), but male LIF-treated rats did not show improvement compared to PBS-treated rats (χ2 = 1.299, df = 1, p = 0.1272; Fig 3C). The results of the Step Bias Test showed no significant improvement according to sex (F1,18 = 0.1409, p = 0.7118) or treatment (F1,18= 0.09829, p = 0.7575; Fig. 3D).
Figure 3.
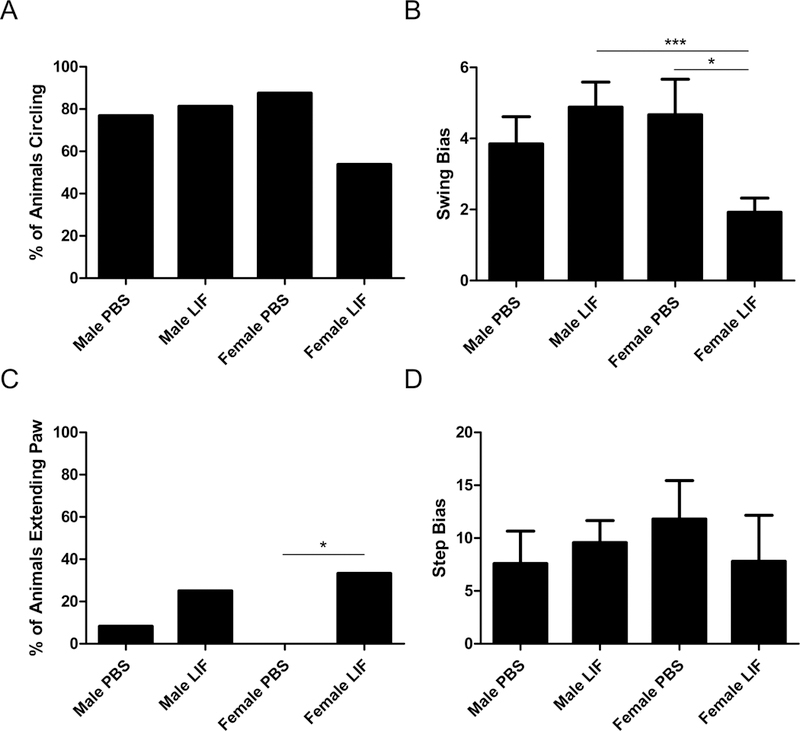
Four tests were used to determine motor skill function at 72 h after MCAO. (A) For the Circling test, there was a trend towards improvement in aged female LIF-treated rats that approached significance compared to PBS-treated females, but this trend was not observed in aged males. (B) A significant two-way interaction was observed between sex and drug treatment in the EBST results; LIF-treated rats performed significantly better than PBS-treated females and LIF-treated males. (C) LIF-treated females showed significant improvement compared to PBS-treated females in the Paw Extension test, but male LIF-treated rats showed no improvement compared to PBS-treated males. (D) Neither males nor females showed improvement according to the Step Test results.
2.4. LIF does not Upregulate SOD Activity in Aged Rat Brains after MCAO
LIF treatment decreases tissue damage after MCAO in young male rats by increasing SOD activity in the brain. SOD activity was measured in the ipsilateral tissue from aged male and female rats to determine whether LIF treatment promotes antioxidant activity in the aged brain. Tissue from sham rats of both sexes is shown as a negative control. There was no significant change in SOD activity between sexes (F1,12 = 0.5283 p = 0.4813) or treatment groups (F1,12 = 0.1919, p = 0.6691; Fig. 4).
Figure 4.
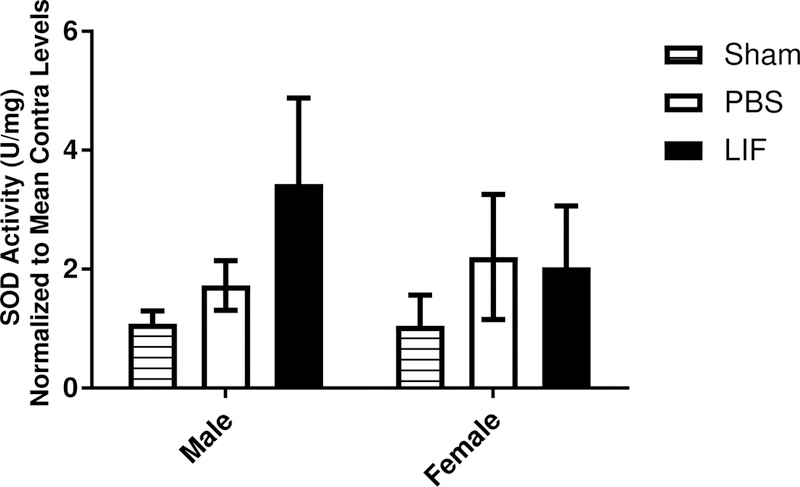
SOD activity was measured in the ipsilateral brain tissue samples from aged male and female rats. Neither drug treatment (PBS or LIF) had any significant effect on SOD enzyme activity at 72 h after MCAO.
2.5. LIFR Translocates to the Cell Membrane during Stroke
This lab previously showed that LIFR is localized to the nuclei of cortical neurons in vivo (Davis et al., 2017). However, LIFR has previously been shown to translocate from the nucleus to the membrane during neuronal injury to promote neuroprotective signaling (Gardiner et al., 2002). Representative tissue sections from young male rats were labeled with antibodies against LIFR (red) and MAP2 (green to visualize the localization of LIFR after MCAO. Punctate LIFR immunoreactivity was observed in the cytosol and membrane regions of cortical neurons in the ipsilateral tissue of PBS and LIF-treated young male rats at 72 h after MCAO (Fig. 5A). LIFR staining was also visualized in DAB-stained tissue sections from LIF-treated young male rats. Although LIFR remained localized to cellular nuclei in the contralateral cortex, membrane localization of LIFR was observed in the ipsilateral cortex (Fig. 5B).
Figure 5.
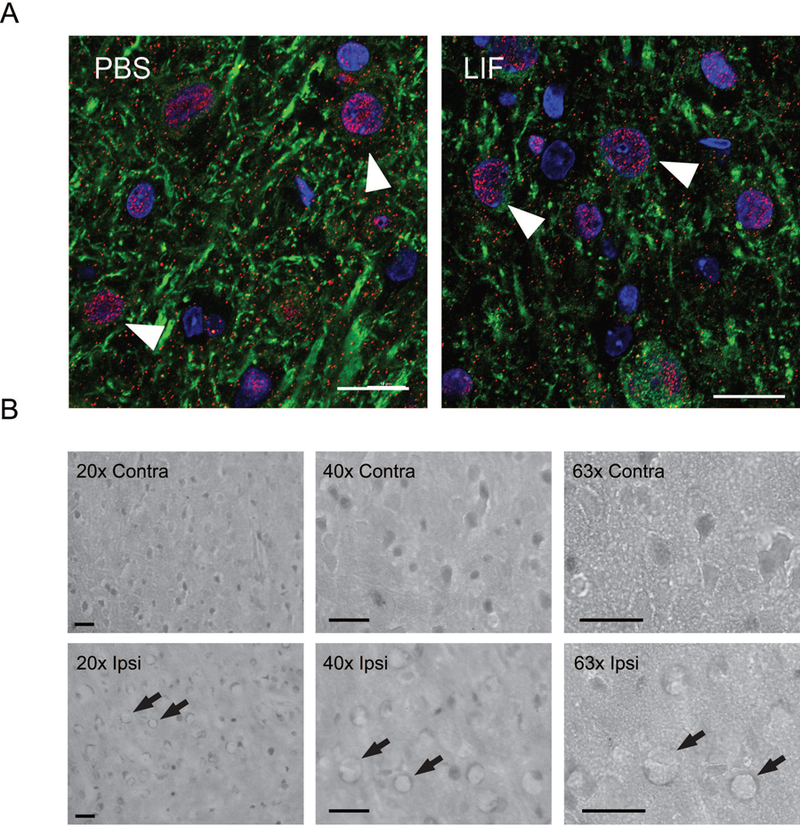
(A) Representative ipsilateral brain tissue sections from PBS and LIF-treated young rats were stained with antibodies against LIFR (red) and MAP2 (green). DAPI (blue) was used to label cell nuclei. LIFR immunoreactivity was apparent in the cytosol and membrane regions of neurons at 72 h after MCAO. Arrows indicate representative cells. Scale bar = 20 µm. (B) After LIF treatment, membrane localization of LIFR was apparent in the ipsilateral cortex, but nuclear localization of LIFR was more prominent in the contralateral cortex. Arrows indicate representative cells. Scale bar = 50 μm
Tissue sections from aged male and female rats were also stained with LIFR (red) and MAP2 (green) antibodies to visualize LIFR in the ipsilateral and contralateral cortices. Membrane localization of LIFR was observed in cortical neurons in the ipsilateral tissue of both sexes and treatment groups (Fig. 6A). DAB-stained tissue sections from aged male and female animals revealed a similar staining pattern to that of the young male rats. While LIFR was mainly localized to the neuronal nuclei in the contralateral cortices of male and female rats in both treatment groups, LIFR membrane localization was observed in the ipsilateral cortices of both sexes and treatment groups (Fig. 6B).
Figure 6.
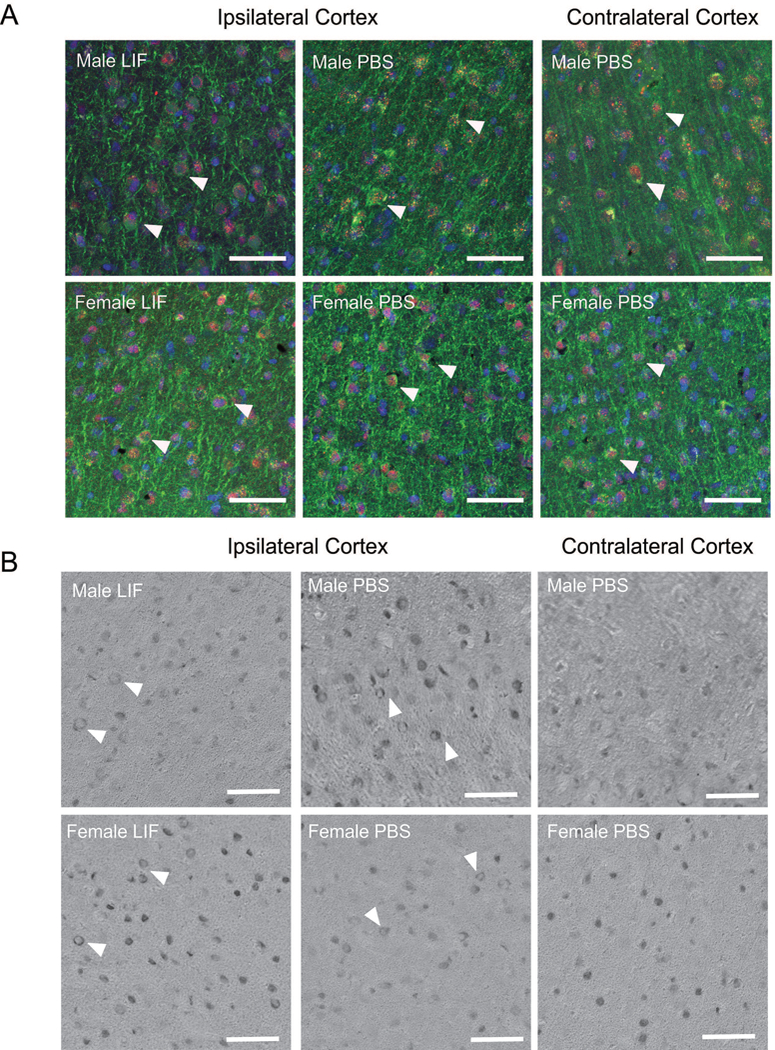
(A) Representative ipsilateral brain tissue sections from PBS- and LIF-treated aged male and female rats were stained with antibodies against LIFR (red) and MAP2 (green) to visualize LIFR localization after MCAO. Nuclei were labeled with DAPI (blue). Stained contralateral tissue served as a negative control. LIFR localization in neuronal nuclei and cytosolic/membrane regions was found in cortical neurons from rats in all treatment groups. Arrows indicate representative cells. (B) DAB-stained tissue sections also showed membrane localization of LIFR in the ipsilateral tissue of all treatment groups. Arrows indicate representative cells. Scale bar = 50 μm.
2.6. LIF Treatment Increases LIFR Expression in vitro but not in the Aged Brain
Previously, this lab demonstrated that 200 ng/ml LIF prior to 24 h OGD reduced cell death in cultured neurons and increased gene expression of SOD3 (Davis et al., 2017). Real-time PCR was used to assess levels of LIFR mRNA in cortical neurons treated with this concentration of LIF or PBS prior to 24 h OGD. LIF, but not PBS treatment increases in vitro expression of the LIFR gene (U = 0; p = 0.0286; Fig. 7A). Western blot was used to determine whether the upregulation of LIFR occurs after in vivo LIF administration. LIFR levels were not significantly altered by sex (F1,12 = 0.04023, p = 0.841) or treatment (F1,12 = 0.009275, p = 0.9249) at 72 h after MCAO (Fig. 7B).
Figure 7.
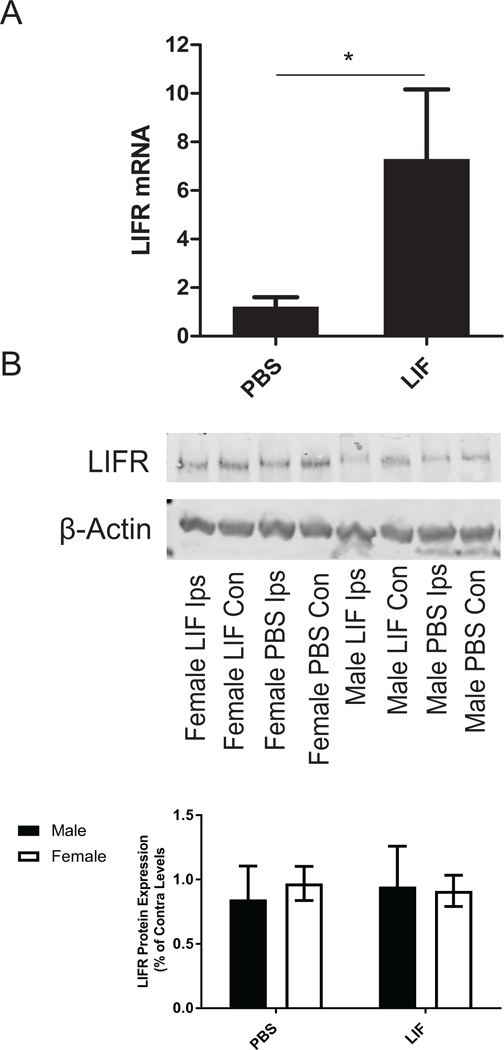
(A) Primary neurons were treated with PBS or LIF and subjected to 24 h OGD. LIFR mRNA was significantly elevated in neurons treated with LIF compared to those treated with PBS (*p<0.05). n= 3 wells per group. (B) However, LIFR expression in the ipsilateral tissue of aged rats of both sexes was not altered by LIF treatment at 72 h after MCAO.
2.7. Post-MCAO LIFR Expression Significantly Decreases with Age
LIF transport into the CNS during injury occurs due to LIFR being expressed by cerebral endothelial cells (Pan et al., 2000; Pan et al., 2006; Pan et al., 2008). Representative brain tissue sections from LIF-treated young male and aged animals of both sexes were labeled with antibodies against von Willebrand Factor (vWF; green), an endothelial cell marker, and LIFR (red) to determine LIFR expression in the cerebral vascular endothelium. Several LIFR+ endothelial cells were identified in the brain tissue from young male rats. However, there appears to be a notably lower number of LIFR+ endothelial cells in the cerebral vasculature of aged male and female rats that received LIF after MCAO (Fig. 8).
Figure 8.
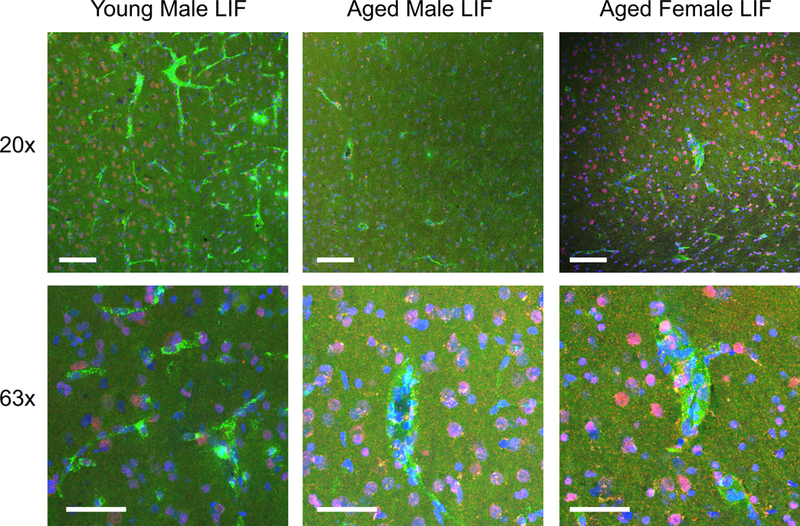
Brain tissue sections from young male and aged (male and female) LIF-treated rats were stained with antibodies against LIFR (red) and vWF (green) to visualize LIFR localization to cerebral endothelial cells. DAPI (blue) was used to label nuclei. LIFR- positive endothelial cells were visualized in after LIF-treated in the brains of young male rats. LIFR immunoreactivity was less prominent in the brains of LIF-treated aged male and female rats compared to young male rats. Scale bar = 50 μm.
Western blotting was also used to determine whether LIFR levels in the ischemic brain differed significantly between young male rats and their aged counterparts of both sexes. Levels of LIFR in the contralateral hemispheres of all age groups and sexes are shown as negative controls. There was a significant change in brain LIFR levels in the ipsilateral tissue from young male, aged male, and aged female rats at 72 h after MCAO (F2,10 = 32 p <0.0001). Young male rats had significantly higher levels of LIFR compared to aged male rats (p = 0.0003) and aged female rats (p = 0.0001). There was also a significant difference in LIFR levels between aged male and aged female rat brains (p = 0.0091). A representative western blot showing expression of LIFR in young and aged rat brains is shown in Fig. 9.
Figure 9.
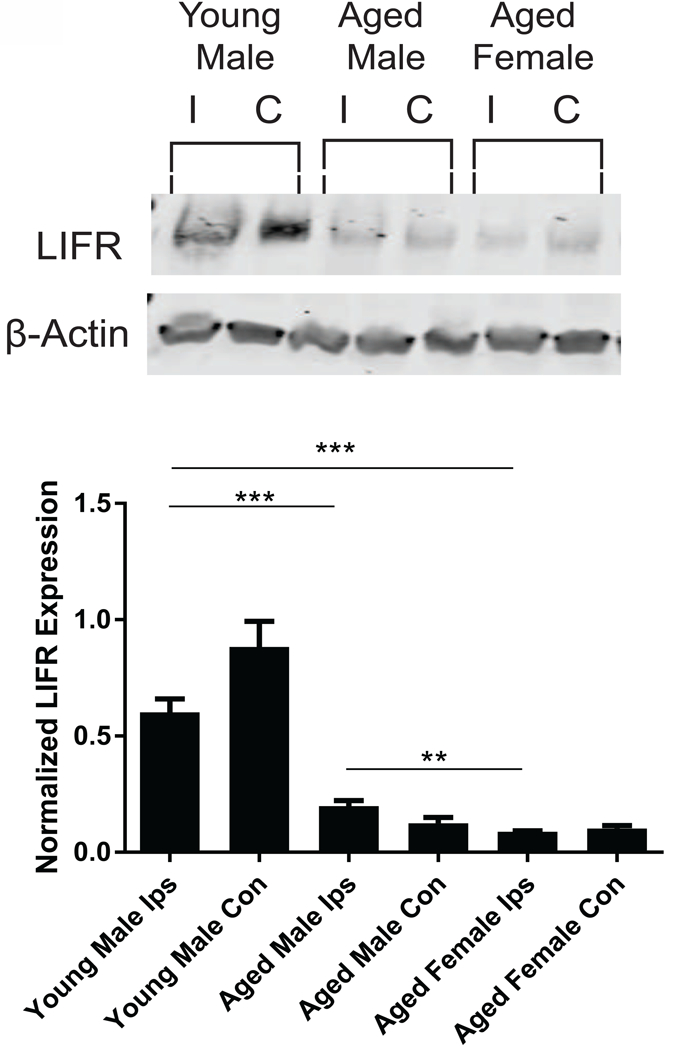
LIFR protein levels were measured in the ipsilateral brain tissue from the young male, aged male, and aged female rats after MCAO. LIFR levels were significantly decreased in aged male (*p<0.05) and aged female (*p<0.05) compared to young male rats. There was also a significant decrease in LIFR levels between aged male and aged female rats after MCAO. n = 4–6 animals per treatment group.
3. Discussion
Previously, we have reported the protective effects of LIF in young male rats after MCAO. Here we find that the same paradigm is not effective in aged male rats and has only a partially beneficial effect on functional recovery in aged females. Reduced expression of LIFR may be responsible for this discrepancy. While increased SOD3 expression and SOD activity are observed after LIF treatment in young male rats, this phenomenon is not found in aged male or female rats after MCAO and LIF treatment (Davis et al., 2017). The reduced expression of LIFR in the brain is possibly impairing the ability of LIF to induce antioxidant enzymes to reduce oxidative injury in brain tissue.
LIF treatment has shown efficacy in animal models of neurological disorders, including stroke (Suzuki et al., 2005), amyotrophic lateral sclerosis ALS (Azari et al., 2001; Azari et al., 2003; Konoeda et al., 2010) and multiple sclerosis (MS) (Butzkueven et al., 2002; Butzkueven et al., 2006). The application of LIF also mitigates the macrophage/microglial response to injury that contributes to the prolonged cell death in stroke (Hendriks et al., 2008). Anzari et al. revealed LIF decreased the degradation of motor neurons in the G93A SOD1 mouse model of familial ALS and reduced white matter damage after spinal cord injury (Azari et al., 2001; Azari et al., 2003; Azari et al., 2006). In an experimental MS model, administration of this agent directly affected white matter damage by inhibiting demyelinating injury (Azari et al., 2006; Emery et al., 2006). Following induction of experimental MS, oligodendrocytes upregulate LIFR in vivo, which appears to be an endogenous mechanism of sensitizing white matter to the protective effects of LIF (Butzkueven et al., 2002; Butzkueven et al., 2006; Hendriks et al., 2008; Laterza et al., 2013). LIF treatment also has shown a great of promise in treating MS (Metcalfe, 2011) and is being considered for clinical trials. However, these previously published studies utilized young rodents of both sexes. Furthermore, Suzuki et al. also reported the neuroprotective signaling of LlF after stroke, but this study only used young male rats. To our knowledge, these data are the first to test the neuroprotective efficacy of LIF in aged male and female rodents.
The nuclear localization of LIFR has been reported in a few studies including our laboratory’s report examining cortical neurons in vitro and in vivo (Couvreur et al., 2011; Davis et al., 2017; Gardiner et al., 2002; Gouin et al., 1999; Zhao et al., 2014). Several groups previously reported that CNTF and its receptor components, LIFR, gp130, and the ciliary neurotrophic factor receptor (CNTFR), were able to cross the nuclear envelope and resided primarily in the nucleus (Bajetto et al., 1999; Couvreur et al., 2011). Nuclear localization has previously been observed for cytokine receptors outside of IL-6 receptor family, such as the interferon gamma receptor 1 (Larkin III et al., 2000). In this study, LIFR migrated from the perinuclear region and cytosol towards the membrane after MCAO regardless of drug treatment. This movement of LIFR towards the membrane was observed in young male, aged male, and aged female rats that were administered LlF after MCAO (Figs. 5–6). These findings are consistent with a report published by Gardiner et al., which demonstrated that LIFR moved from the neuronal nuclei to the plasma membrane during injury (Gardiner et al., 2002).
In addition to its role as a mediator of downstream pro-survival signaling, LIFR acts as a transporter of LIF across endothelial cells. According to Pan et al., LIFR expressed by cerebral endothelial cells is responsible for mediating transcellular LIF transport across the blood-brain barrier. Furthermore, the rate of LIF transport into the central nervous system is increased by brain injury and pro-inflammatory cytokines such as tumor necrosis factor-α (Pan et al., 2000; Pan et al., 2006; Pan et al., 2008). This mechanism allows LIF to traverse the blood-brain barrier to enter the brain parenchyma to elicit its neuroprotective and anti-inflammatory effects. According to these data, LIFR expression is localized in some brain endothelial cells of aged rats, but not all endothelial cells in the cerebral microvasculature (Fig. 8). Loss of the transport of LIF in aged rats could be the basis of the lack of efficacy. Further studies are necessary to determine whether advanced age or sex differences affect the rate of transport of LIF across the blood-brain during injury.
Although the translocation of LIFR during stroke occurred in young and aged rats of both sexes, these data show an age-dependent downregulation of LIFR expression in brain tissue. Downregulation of neurotrophic receptors, such as the brain-derived neurotrophic factor and the nerve growth factor receptors, has previously been reported in the aged rodent brain (Gómez-Pinilla et al., 1989; Silhol et al., 2005; Silhol et al., 2007). Treatment with LIF prior to 24 h OGD does significantly increase expression of its receptor in neuronal cultures, but these neurons originate from rat fetuses at day 18 of embryonic development. While both sexes from aged rats failed to induce LIFR, the female rats did show functional improvement while the males did not. Moreover, LIF treatment did not increase SOD activity and expression in aged rats as it did in the young rats (Davis et al., 2017). The lower levels of LIFR could be due to age-dependent changes in the epigenetic regulation of the LIFR gene promoter. Previous reports have shown that LIFR transcription increases following acetylation of the promoter region (Johnson et al., 2016; Zeng et al., 2016) and through downregulation of the transcription factor hypoxia-inducible factor 1-α, which downregulates LIFR under hypoxic conditions (Jeong et al., 2007; Lee et al., 2010). Methylation of the LIFR promoter region suppresses transcription of the LIFR gene (Cho et al., 2011). Histone deacetylases, which are responsible for de-acetylating the LIFR promoter, have higher activity in the brain of the aged rat (dos Santos Sant’Anna et al., 2013). In addition, aberrant patterns of DNA methylation are associated with the aging process, which leads to dysregulated gene expression (Johnson et al., 2012). Changes in epigenetic regulation that occur in advanced age could explain the low expression of LIFR in the aged brain after stroke. Furthermore, lower levels of LIFR are a possible explanation for the poor neuroprotective efficacy by LIF in aged rats compared to their younger counterparts. Although direct neuroprotection was not observed in aged male or female rats after LIF treatment, our lab has shown LIF treatment attenuates the post-stroke peripheral inflammatory response in aged female rats but not aged males (Davis et al., 2018). This reduction in inflammation could explain why aged females fare better after LIF treatment despite having lower overall levels of LIFR in the CNS compared to aged males (Fig. 9).
However, more research is necessary to determine the sex-dependent effects of LIF on the post-stroke immune response. After LIF treatment, aged females have a better stroke outcome overall relative to the males indicating a sex difference in response to this anti-inflammatory cytokine. Differential responses to treatment have been observed in human patients. One such example is the use of uric acid as a treatment for stroke that is effective in women but not men (Chamorro et al., 2014). This finding was initially unnoticed in the clinical trial until results were broken out according to sex (Chamorro et al., 2014; Romanos et al., 2007). Furthermore, results from preclinical studies in aged rodents suggest that targeting the post-stroke inflammatory response is effective in aged female rodents (Chauhan et al., 2018).
Preclinical stroke studies have been less than successful at translating successful animal studies into new therapeutics. One problem has been the use of young male rodents in these studies. The vast majority of stroke patients are middle-aged to elderly individuals of both sexes, and the risk for stroke doubles every 10 years after the age of 55 (Chong and Sacco, 2005; Ovbiagele and Nguyen-Huynh, 2011). Not only is there an age mismatch between the typical stroke patient and animal models, but also a sex mismatch because most young rodents used in stroke studies are male. The use of young male rats is due to the confounding effect of estrogen in preclinical stroke studies (Hawk et al., 1998; Seifert and Offner, 2018; Simpkins et al., 1997; Zhang et al., 2010). According to several studies by Alkayed et al., young female rats (3–4 mo.) showed significantly smaller infarcts and less several functional damage compared to their age-matched male counterparts. Meanwhile, ovariectomized females did not show significantly smaller infarcts compared to male rats (Alkayed et al., 1998). In a subsequent report, aged female rats (16 mo.) did not show a significant difference in infarct size compared to age-matched males. In human patients, the severity of stroke-induced brain damage is often significantly higher in female patients compared to male patients (Appelros et al., 2009). These data show the necessity of performing preclinical studies in animals that best resemble their human counterparts; namely, aged male and female rodents.
Reduced LIFR expression decreases responsiveness to exogenous LIF and hinders the neuroprotective signaling from endogenous cytokines that use LIFR, such as ciliary neurotrophic factor and cardiotrophin-1. Further studies are needed to determine whether LIF promotes anti-inflammatory signaling in an age- and sex-dependent matter. The efficacy of LIF could potentially be improved through combination therapy with drugs that increase expression of LIFR. HDAC inhibitors have been shown to be neuroprotective after MCAO (Baltan et al., 2011; Kim et al., 2007). In addition, histone deacetylase inhibitors promote LIFR upregulation by increasing acetylation of its gene promoter (Johnson et al., 2016; Wirths et al., 2002; Zeng et al., 2016).
4. Conclusion
From this study, we show that aged rats are less responsive to LIF treatment after experimental stroke with females showing a trend towards decreased tissue damage and partial motor skill recovery and males showing no response to LIF treatment. The decline of the expression of LIFR would be responsible for the reduced neuroprotective signaling and transport into the brain. Furthermore, LIF does not promote protection against oxidative stress by increasing SOD activity in aged animals as it does in young, male rodents(Davis et al., 2017). The lack of responsiveness to exogenous LIF treatment may be explained by the significant downregulation of LIFR in the aged brain. These data demonstrate the importance of testing and optimizing potential stroke therapies in animal models that best represent the age and sex of stroke patients.
Methods and Materials
5.1. Animal Care
Retired breeding pairs of Sprague Dawley rats (9–10 mo.) were purchased from Envigo (Indianapolis, IN) and housed until between the age of 18–19 mo. Animals were housed in a climate-controlled room with access to food and water ad libitum and kept on a 12 h light/dark cycle (07:00–19:00 h). All animals were randomly assigned to treatment groups. All procedures were approved according to the University of Kentucky Institutional Animal Care and Use Committee.
5.2. Middle cerebral artery occlusion (MCAO)
To induce permanent large vessel stroke, the intraluminal middle cerebral artery occlusion (MCAO) method was performed as previously described (Ajmo et al., 2006). Briefly, animals were anesthetized using 5% isoflurane in 100% oxygen (1 L/min). Anesthesia was maintained at 3% isoflurane for the duration of the surgical procedure. An incision was made on the neck to expose the common carotid artery, and the external carotid was ligated and cut. A 40 mm monofilament was fed through the internal carotid artery and advanced to the origin of the middle cerebral artery (MCA). Laser Doppler measurement was used to confirm the reduction in blood flow. Animals with ≤ 60% reduction in cerebral blood flow were excluded from the study. For the sham procedure, the common carotid artery was exposed without occluding the MCA.
5.3. Drug Administration
To control for post-surgical pain, all rats were treated prophylactically with carprofen (5 mg/kg s.c.) with two additional doses of carprofen at 24 and 48 h post-MCAO. Atropine (0.04 mg/kg s.c.) was administered to reduce secretions in the upper and lower respiratory tract and improve breathing during surgery. Animals were randomly assigned to receive recombinant human LIF (125 μg/kg i.v.; ProSpec, Ness Ziona, Israel) or PBS (pH 7.4) treatment at 6, 24, and 48 h post-MCAO as previously described (Davis et al., 2017). Blinded personnel administered all drugs to animals.
5.4. Assessment of Motor Function
Functional sensorimotor deficits were measured as previously published using a battery of four tests immediately before euthanization (Rowe et al., 2014). The following tests were used: Circling, Elevated Body Swing Test (EBST), Paw Extension, and Step Bias tests. For the Circling test, animals were placed in an open field, and the presence or absence of circling behavior was recorded. For the EBST, animals were suspended by the tail, and the number of lateral “swings” toward the left or right side was recorded. For the Paw Extension test, animals were suspended by the tail, and the ability to extend the forelimb on the affected side was recorded. For the Step Bias test, each animal was balanced on one forepaw then drawn across the table. This process was repeated using the opposite forelimb, and the number of paw plants was recorded for each side. Measurements were recorded at baseline (3–4 days before surgery) and 72 h after MCAO. Results of the EBST and Step Tests were expressed as a change from baseline.
2.5. Magnetic Resonance Imaging (MRI)
MRI images were acquired using a 7T Bruker Clinscan horizontal bore system (7.0T, 30cm, 300Hz) equipped with a triple-axis gradient system (630 mT/m and 6300 T/m/s) with a closed cycle (Billerica, MA). Coronal Diffusion Tensor Imaging (DTI) images will were acquired with a fat saturated, double refocused echo planar sequence: 0.297 × 0.297 × 0.7 mm3, TR/TE 2200/34, 128 × 128 matrix, 3 av, 15 slices, four b=0 volumes, 256 directions with b=800, in 28:23 minutes. Coronal T2-weighted images were acquired with a fat saturated, turbo spin echo sequence: 0.125 × 0.125 × 0.4 mm3 resolution, TR/TE 6000/27, 192 × 192 matrix, 44 slices, is 9:03.
Male animals were anesthetized with an average of 2.25% isoflurane in oxygen, while female animals will be anesthetized with an average of 1.75% isoflurane in oxygen using MRI-compatible CWE Inc. equipment (Ardmore, PA). Animals were restrained on a Bruker scanning bed using a tooth bar and tape. Body temperature, heart rate, and respiratory rates were continuously monitored throughout the MRI scans (SA Instruments, Inc., Stony Brook, NY). Animals were maintained at 37° with a water heating system built into the scanning bed.
Infarct volume and edema volumes were measured using DTI and T2-weighted MR images. Volumes were counted and normalized to the number of images counted to provide a per section count. The volume of brain parenchyma demonstrating restricted diffusion (infarct volume) visibly affected by cerebral edema (edema volume) was calculated by manual segmentation using ITK-SNAP software as previously described (Yushkevich et al., 2006). The volume of brain parenchyma visibly affected by cerebral edema (edema volume) will be calculated similarly. Quantification of infarct and edema volumes was performed by a single individual who was blinded to the sex and treatment group of each animal.
5.6. Collection of Tissue
Animals were euthanized at 72 h post-MCAO via intraperitoneal injection of ketamine/xylazine solution (75 mg/kg and 7.5 mg/kg) (Seifert et al., 2012). Animals underwent trans-cardial perfusion with normal saline followed by 4% paraformaldehyde in phosphate buffer. Fixed brains were cryopreserved in 20% followed by 30% sucrose solutions and cut into 30 µm sections using a cryostat. Animals used for biochemical analysis were only perfused with normal saline before obtaining tissue. Fresh tissue was snap frozen and stored at −80°C until further processing. The brains were separated into the right (ipsilateral) and left (contralateral) hemispheres, snap-frozen, and stored at −80 °C. Brain tissue used in these experiments was located in the region between +1.7 to −3.3 mm from bregma.
5.7. Whole Cell Tissue Homogenization
To obtain whole cell extracts, ipsilateral and contralateral brain tissue was homogenized in whole cell lysis buffer containing the following: 50 mM Tris pH 8, 150 mM NaCl, 0.1% SDS, 1% Igepal CA-630, 1 mM PMSF, and a Complete Mini protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Briefly, tissue was disrupted using an electric homogenizer and allowed to incubate on ice for 10 min. Tissue was vortexed and pipetted to break up nuclei. Tissue was snap-frozen and stored at −80 °C.
5.8. Superoxide Dismutase Inhibition Assay
The activity of superoxide dismutase (SOD) enzymes in brain tissue was measured using a colorimetric assay kit purchased from Kamiya Biochemical (Tukwila, WA) (Davis et al., 2017). Briefly, SOD activity was measured in diluted brain tissue samples by measuring absorbance at 450 nm using a microplate spectrophotometer (Biotek, Winnoski, VT). A standard curve and the IC50 was calculated using the % inhibition at each dilution. The IC50 was used to calculate SOD activity (U/mg protein).
5.9. Western Blot Analysis
Western blotting was used for semi-quantitative measurement of protein expression using a previously described procedure (Davis et al., 2017). Briefly, whole cell lysates from ipsilateral brain tissue were run on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Contralateral tissue was included alongside ipsilateral tissue to serve as an internal negative control for the ischemic injury. Membranes were blocked with 5% nonfat milk in TBS and probed with the following antibodies: Rabbit anti-LIFR (C- 19) (1:100; Santa Cruz Biotechnology, Dallas, TX, US). Membranes were incubated in IRDye 800CW goat anti-rabbit antibodies (1:20,000; Li-Cor) for detection of protein bands. Membranes were visualized using the Odyssey CLx Imaging System (Li-Cor). To normalize for loading, membranes containing whole cell extracts were re-probed with mouse anti-β-actin (1:5000; Novus Biologicals) and IRDye 680RD goat anti-mouse antibodies (1:20,000; Li-Cor).
5.10. Immunohistochemical Staining
5.10.1. 3,3’-Diaminobenzidine Staining
3,3’- diaminobenzidine staining was performed as previously described (Hall et al., 2009) using rabbit anti-LIFR (1:200; Santa Cruz Biotechnology) and goat anti-rabbit biotinylated secondary antibodies (1:300; Vector Laboratories, Burlingame, CA). Images were acquired using a Zeiss AxioSkop 2 Microscope (Carl Zeiss, Oberkochen, Germany) or a Ti Inverted Microscope (Nikon, Tokyo, Japan).
5.10.2. Fluorescent Staining
Brain tissue sections were labeled as previously described (Hall et al., 2009) using the following antibodies: rabbit anti-LIFR (1:200; Santa Cruz Biotechnology), mouse anti- microtubule-associated protein 2 (MAP2) (1:200; EMD Millipore, Burlington, MA). Secondary detection was achieved using goat anti-mouse AlexaFluor 488 (1:500; Life Technologies) and goat anti-rabbit AlexaFluor 594 (1:500; Life Technologies). Sheep anti- von Willebrand Factor (vWF)-FITC (1:50; Abcam, Cambridge, UK) was used to label cerebral blood vessels. Images were acquired using a Ti Inverted Microscope.
5.11. Cell Culture
Primary neurons were isolated from E18 rat pups and cultured as previously described (Davis et al., 2017). Neurons used for experiments were used between 10 and 14 days in culture.
5.11.1. Oxygen-Glucose Deprivation
To induce in vitro ischemia, cultured neurons were subjected to 24 h of oxygen- glucose deprivation for 24 has previously described (Davis et al., 2017; Hall et al., 2009; Rowe et al., 2014). Neurons were incubated in glucose-free DMEM (Life Technologies, Carlsbad, CA) and treated with LIF (200 ng/ml) or PBS before being placed in an airtight chamber. Chamber was flushed with 94% N2 5% CO2 and 1% O2 for 15 min (2 L/min) and sealed for 24 h. Following incubation, cells were rinsed twice with PBS and pellets were collected and stored at −80 °C for RNA isolation.
5.12. Real-Time PCR
5.12.1. RNA Isolation and cDNA Synthesis
RNA isolation and was performed as previously described (Rowe et al., 2010). Total RNA was extracted from cells using an RNeasy Mini Kit (Qiagen, Germantown, MD). Total RNA was reverse-transcribed using an AffinityScript cDNA Synthesis Kit (Agilent Technologies, Santa Clara, CA) and cDNA was quantified using a Nanodrop Lite Spectrophotometer (Thermo-Fisher Scientific, Waltham, MA).
5.12.2. PCR Reaction
Real-time PCR was performed using PowerUp™ SYBR® Green Master Mix (Applied Biosystems, Foster City, CA) on a StepOnePlus Real-Time PCR System (Applied Biosystems). LIFR mRNA was amplified using primers purchased from Qiagen and normalized to levels of 18s rRNA (Qiagen). The following protocol was used: Hold at 50°C for 2 min, hold at 95°C for 2 min, and 40 cycles of 95°C for 15 s followed by 60°C for 1 min. Melt temp curve analysis was performed after all cycles were completed using the following protocol: 95°C for 15 s at a ramp rate of 1.6°C/s, 60°C for 1 min at a rate of 1.6°C/second, and 95°C for 15 s at a ramp rate of 0.15°C/s . Relative expression of LIFR was calculated using the ΔΔCt method as previously described (Davis et al., 2017).
5.13. Data Analysis
Data were analyzed using GraphPad Prism 5.0 Software (La Jolla, CA). Data from the Paw Extension and Circling tests were analyzed using a Chi-Square Analysis. All other data were analyzed using an ANOVA (one or two-way) followed by Fisher’s LSD post hoc test Student’s t-test was used to analyze data containing two treatment groups. The Welch correction used in the case of significant difference in variances, the Mann-Whitney U Test were used to analyze non-normal data. Statistical significance was denoted by a p-value ≥ 0.05. All reported p-values are one-tailed. The data in all figures are presented as the mean ± standard error of the mean.
LIF does not improve survival or decrease tissue damage after stroke in aged rats.
Aged female rats show partial motor skill recovery after LIF treatment.
LIF receptor localizes to cellular membranes in ischemic tissue to promote survival.
Brains of aged rats have much lower LIF receptor levels compared to young rats.
Acknowledgments
The authors would like to acknowledge Dr. Justin F. Fraser for providing his expertise in cerebrovascular disease and endovascular procedures. This work was supported by the National Institute of Neurological Disorders and Stroke [grant number 5R01NS091146–04, 2016] and the University of Kentucky.
Abbreviations
- EBST
elevated body swing test
- EVT
endovascular thrombectomy
- LIF(R)
leukemia inhibitory factor (receptor)
- MCA(O)
middle cerebral artery (occlusion)
- SOD(3)
superoxide dismutase (3)
- tPA
tissue plasminogen activator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest
The authors have no interests to disclose.
References
- Ajmo CT Jr., Vernon DO, Collier L, Pennypacker KR, Cuevas J, 2006. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res 3, 89–98. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD, 1998. Gender-linked brain injury in experimental stroke. Stroke 29, 159–166. [DOI] [PubMed] [Google Scholar]
- Appelros P, Stegmayr B, Terént A, 2009. Sex differences in stroke epidemiology: a systematic review. Stroke 40, 1082–1090. [DOI] [PubMed] [Google Scholar]
- Azari MF, Galle A, Lopes EC, Kurek J, Cheema SS, 2001. Leukemia inhibitory factor by systemic administration rescues spinal motor neurons in the SOD1 G93A murine model of familial amyotrophic lateral sclerosis. Brain Res 922, 144–147. [DOI] [PubMed] [Google Scholar]
- Azari MF, Lopes EC, Stubna C, Turner BJ, Zang D, Nicola NA, Kurek JB, Cheema SS, 2003. Behavioural and anatomical effects of systemically administered leukemia inhibitory factor in the SOD1 G93A G1H mouse model of familial amyotrophic lateral sclerosis. Brain Res 982, 92–97. [DOI] [PubMed] [Google Scholar]
- Azari MF, Profyris C, Karnezis T, Bernard CC, Small DH, Cheema SS, Ozturk E, Hatzinisiriou I, Petratos S, 2006. Leukemia inhibitory factor arrests oligodendrocyte death and demyelination in spinal cord injury. J Neuropathol Exp Neurol 65, 914–29. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Schettini G, Chimini G, 1999. Nuclear localization of ciliary neurotrophic factor in glial cells. Brain research 818, 565–569. [DOI] [PubMed] [Google Scholar]
- Baltan S, Murphy SP, Danilov CA, Bachleda A, Morrison RS, 2011. Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity. Journal of Neuroscience 31, 3990–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, Silver FL, 2013. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. New England Journal of Medicine 368, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzkueven H, Zhang JG, Soilu-Hanninen M, Hochrein H, Chionh F, Shipham KA, Emery B, Turnley AM, Petratos S, Ernst M, Bartlett PF, Kilpatrick TJ, 2002. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 8, 613–9. [DOI] [PubMed] [Google Scholar]
- Butzkueven H, Emery B, Cipriani T, Marriott MP, Kilpatrick TJ, 2006. Endogenous leukemia inhibitory factor production limits autoimmune demyelination and oligodendrocyte loss. Glia 53, 696–703. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Amaro S, Castellanos M, Segura T, Arenillas J, Marti-Fabregas J, Gallego J, Krupinski J, Gomis M, Canovas D, Carne X, Deulofeu R, Roman LS, Oleaga L, Torres F, Planas AM, Investigators U-I, 2014. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol 13, 453–60. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Al Mamun A, Spiegel G, Harris N, Zhu L, McCullough LD, 2018. Splenectomy protects aged mice from injury after experimental stroke. Neurobiol Aging 61, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YG, Chang X, PARk I-S, Yamashita K, Shao C, Ha PK, Pai SI, Sidransky D, Kim MS, 2011. Promoter methylation of leukemia inhibitory factor receptor gene in colorectal carcinoma. International journal of oncology 39, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JY, Sacco RL, 2005. Risk factors for stroke, assessing risk, and the mass and high-risk approaches for stroke prevention. CONTINUUM: Lifelong Learning in Neurology 11, 18–34. [Google Scholar]
- Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, Boccardi E, 2013. Endovascular treatment for acute ischemic stroke. New England Journal of Medicine 368, 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur O, Aubourg A, Crépin D, Degrouard J, Gertler A, Taouis M, Vacher CM, 2011. The anorexigenic cytokine ciliary neurotrophic factor stimulates POMC gene expression via receptors localized in the nucleus of arcuate neurons. American Journal of Physiology-Endocrinology and Metabolism 302, E458–E467. [DOI] [PubMed] [Google Scholar]
- Davis SM, Collier LA, Leonardo CC, Seifert HA, Ajmo CT, Pennypacker KR, 2017. Leukemia inhibitory factor protects neurons from ischemic damage via upregulation of superoxide dismutase 3. Molecular neurobiology 54, 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Collier LA, Martha SR, Powell DK, Pennypacker KR, 2018. Abstract WMP77: Anti-Inflammatory Signaling by Leukemia Inhibitory Factor is Suppressed in Aged Animals After Stroke. Vol., ed.^eds. Am Heart Assoc
- dos Santos Sant’Anna G, Elsner VR, Moysés F, Cechinel LR, Lovatel GA, Siqueira IR, 2013. Histone deacetylase activity is altered in brain areas from aged rats. Neuroscience letters 556, 152–154. [DOI] [PubMed] [Google Scholar]
- Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, Descamps P, Gamelin E, Gascan H, Hebbar M, Jeannin P, 2007. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 110, 4319–4330. [DOI] [PubMed] [Google Scholar]
- Emery B, Cate HS, Marriott M, Merson T, Binder MD, Snell C, Soo PY, Murray S, Croker B, Zhang JG, Alexander WS, Cooper H, Butzkueven H, Kilpatrick TJ, 2006. Suppressor of cytokine signaling 3 limits protection of leukemia inhibitory factor receptor signaling against central demyelination. Proc Natl Acad Sci U S A 103, 7859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadina M, Hilton D, Johnston JA, Morinobu A, Lighvani A, Zhou Y-J, Visconti R, O’Shea JJ, 2001. Signaling by type I and II cytokine receptors: ten years after. Current opinion in immunology 13, 363–373. [DOI] [PubMed] [Google Scholar]
- Gao W, Thompson L, Zhou Q, Putheti P, Fahmy TM, Strom TB, Metcalfe SM, 2009. Treg versus Th17 lymphocyte lineages are cross-regulated by LIF versus IL-6. Cell Cycle 8, 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner NJ, Cafferty WBJ, Slack SE, Thompson SWN, 2002. Expression of gp130 and leukaemia inhibitory factor receptor subunits in adult rat sensory neurones: regulation by nerve injury. J Neurochem 83, 100–109. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Cotman CW, Nieto-Sampedro M, 1989. NGF receptor immunoreactivity in aged rat brain. Brain Research 479, 255–262. [DOI] [PubMed] [Google Scholar]
- Gouin F, Couillaud S, Cottrel M, Godard A, Passuti N, Heymann D, 1999. Presence of leukaemia inhibitory factor (LIF) and LIF-receptor chain (gp190) in osteoclast-like cells cultured from human giant cell tumour of bone. Ultrastructural distribution. Cytokine 11, 282–289. [DOI] [PubMed] [Google Scholar]
- Hall AA, Guyer AG, Leonardo CC, Ajmo CT Jr., Collier LA, Willing AE, Pennypacker KR, 2009. Human umbilical cord blood cells directly suppress ischemic oligodendrocyte cell death. J Neurosci Res 87, 333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk T, Zhang Y, Rajakumar G, Day AL, Simpkins JW, 1998. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain research 796, 296–298. [DOI] [PubMed] [Google Scholar]
- Hendriks JJ, Slaets H, Carmans S, de Vries HE, Dijkstra CD, Stinissen P, Hellings N, 2008. Leukemia inhibitory factor modulates production of inflammatory mediators and myelin phagocytosis by macrophages. J Neuroimmunol 204, 52–7. [DOI] [PubMed] [Google Scholar]
- Janssens K, Van den Haute C, Baekelandt V, Lucas S, van Horssen J, Somers V, Van Wijmeersch B, Stinissen P, Hendriks JJ, Slaets H, Hellings N, 2015. Leukemia inhibitory factor tips the immune balance towards regulatory T cells in multiple sclerosis. Brain Behav Immun 45, 180–8. [DOI] [PubMed] [Google Scholar]
- Jeong C-H, Lee H-J, Cha J-H, Kim JH, Kim KR, Kim J-H, Yoon D-K, Kim K-W, 2007. Hypoxia-inducible Factor-1α Inhibits Self-renewal of Mouse Embryonic Stem Cells in Vitro via Negative Regulation of the Leukemia Inhibitory Factor- STAT3 Pathway. Journal of Biological Chemistry 282, 13672–13679. [DOI] [PubMed] [Google Scholar]
- Johnson AA, Akman K, Calimport SR, Wuttke D, Stolzing A, De Magalhaes JP, 2012. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation research 15, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RW, Finger EC, Olcina MM, Vilalta M, Aguilera T, Miao Y, Merkel AR, Johnson JR, Sterling JA, Wu JY, 2016. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nature cell biology 18, 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, Feng L, Meyer BC, Olson S, Schwamm LH, 2013. A trial of imaging selection and endovascular treatment for ischemic stroke. New England Journal of Medicine 368, 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong J-S, Chen P-S, Chuang D-M, 2007. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. Journal of Pharmacology and Experimental Therapeutics 321, 892–901. [DOI] [PubMed] [Google Scholar]
- Konoeda F, Shichita T, Yoshida H, Sugiyama Y, Muto G, Hasegawa E, Morita R, Suzuki N, Yoshimura A, 2010. Therapeutic effect of IL-12/23 and their signaling pathway blockade on brain ischemia model. Biochemical and biophysical research communications 402, 500–506. [DOI] [PubMed] [Google Scholar]
- Larkin III J, Johnson HM, Subramaniam PS, 2000. Differential nuclear localization of the IFNGR-1 and IFNGR-2 subunits of the IFN-γ receptor complex following activation by IFN-γ. Journal of Interferon & Cytokine Research 20, 565–576. [DOI] [PubMed] [Google Scholar]
- Laterza C, Merlini A, De Feo D, Ruffini F, Menon R, Onorati M, Fredrickx E, Muzio L, Lombardo A, Comi G, Quattrini A, Taveggia C, Farina C, Cattaneo E, Martino G, 2013. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat Commun 4, 2597. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Lee KY, Lee YM, 2010. Downregulation of a tumor suppressor RECK by hypoxia through recruitment of HDAC1 and HIF-1α to reverse HRE site in the promoter. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1803, 608–616. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Hall AA, Collier LA, Ajmo CTJ, Willing AE, Pennypacker KR, 2010. Human umbilical cord blood cell therapy blocks the morphological change and recruitment of CD-11b-expressing isolectin-binding proinflammatory cells after middle cerebral artery occlusion. J Neurosci Res 88, 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra K, Gornbein J, Saver JL, 2017. Ischemic Strokes Due to Large-Vessel Occlusions Contribute Disproportionately to Stroke-Related Dependence and Death: A Review. Front Neurol 8, 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe SM, 2011. LIF in the regulation of T-cell fate and as a potential therapeutic. Genes Immun 12, 157–68. [DOI] [PubMed] [Google Scholar]
- Metcalfe SM, Strom TB, Williams A, Fahmy TM, 2015. Multiple Sclerosis and the LIF/IL-6 Axis: Use of Nanotechnology to Harness the Tolerogenic and Reparative Properties of LIF. Nanobiomedicine 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovbiagele B, Nguyen-Huynh MN, 2011. Stroke Epidemiology: Advancing Our Understanding of Disease Mechanism and Therapy. Neurotherapeutics 8, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, Brennan JM, 2000. Saturable entry of leukemia inhibitory factor from blood to the central nervous system. J Neuroimmunol 106, 172–80. [DOI] [PubMed] [Google Scholar]
- Pan W, Cain C, Yu Y, Kastin AJ, 2006. Receptor-mediated transport of LIF across blood–spinal cord barrier is upregulated after spinal cord injury. J Neuroimm 174, 119–125. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu C, Hsuchou H, Zhang Y, Kastin AJ, 2008. Neuroinflammation facilitates LIF entry into brain: role of TNF. Am J Physiol Cell Physiol 294, C1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanos E, Planas AM, Amaro S, Chamorro Á, 2007. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. Journal of Cerebral Blood Flow & Metabolism 27, 14–20. [DOI] [PubMed] [Google Scholar]
- Rowe DD, Leonardo CC, Hall AA, Shahaduzzaman MD, Collier LA, Willing AE, Pennypacker KR, 2010. Cord blood administration induces oligodendrocyte survival through alterations in gene expression. Brain Res 1366, 172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DR, Collier LA, Seifert HA, Chapman CB, Leonardo CC, Willing AE, Pennypacker KR, 2014. Leukemia inhibitory factor promotes functional recovery and oligodendrocyte survival in rat models of focal ischemia. Eur J Neurosci 40, 3111–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Leonardo CC, Hall AA, Rowe DD, Collier LA, Benkovic SA, Willing AE, Pennypacker KR, 2012. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis 27, 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Offner H, 2018. The splenic response to stroke: from rodents to stroke subjects. Journal of neuroinflammation 15, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L, 2005. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience 132, 613–624. [DOI] [PubMed] [Google Scholar]
- Silhol M, Arancibia S, Maurice T, Tapia-Arancibia L, 2007. Spatial memory training modifies the expression of brain-derived neurotrophic factor tyrosine kinase receptors in young and aged rats. Neuroscience 146, 962–973. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang Y-Q, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL, 1997. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. Journal of neurosurgery 87, 724–730. [DOI] [PubMed] [Google Scholar]
- Slaets H, Dumont D, Vanderlocht J, Noben JP, Leprince P, Robben J, Hendriks J, Stinissen P, Hellings N, 2008. Leukemia inhibitory factor induces an antiapoptotic response in oligodendrocytes through Akt-phosphorylation and up- regulation of 14–3-3. Proteomics 8, 1237–47. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Yamashita T, Tanaka K, Hattori H, Sawamoto K, Okano H, Suzuki N, 2005. Activation of cytokine signaling through leukemia inhibitory factor receptor (LIFR)/gp130 attenuates ischemic brain injury in rats. J Cereb Blood Flow Metab 25, 685–93. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Czech C, Feldmann N, Blanchard V, Tremp G, Beyreuther K, Pradier L, Bayer T, 2002. Intraneuronal APP/Aß trafficking and plaque formation in ß-amy;oid precursor protein and presenilin-1 transgenic mice. Brain Pathol 12, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G, 2006. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128. [DOI] [PubMed] [Google Scholar]
- Zeng H, Qu J, Jin N, Xu J, Lin C, Chen Y, Yang X, He X, Tang S, Lan X, 2016. Feedback activation of leukemia inhibitory factor receptor limits response to histone deacetylase inhibitors in breast cancer. Cancer cell 30, 459–473. [DOI] [PubMed] [Google Scholar]
- Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, Migliati E, Lewis AD, Vandenbark AA, Offner H, Hurn PD, 2010. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J Immunol 184, 4087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JW, Dyson SC, Kriegel C, Tyers P, He X, Fahmy TM, Metcalfe SM, Barker RA, 2014. Modelling of a targeted nanotherapeutic ‘stroma’ to deliver the cytokine LIF, or XAV939, a potent inhibitor of Wnt-beta-catenin signalling, for use in human fetal dopaminergic grafts in Parkinson’s disease. Dis Model Mech 7, 1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]


