Abstract
Introduction
Hypertensive diseases in pregnancy are the leading medical problem during pregnancy. Some of the women affected remain hypertensive after pregnancy and the post-partum period. This study aimed to assess the factors associated to the persistence of hypertension after preeclampsia.
Methods
This was a retrospective cohort study which included all women who had preeclampsia. The minimal follow-up period was 12 months. We excluded from the study all women who had superimposed preeclampsia. Sociodemographic data and past history were recorded and a physical exam was performed for all participants. Multivariate logistic regression was used to determine factors independently associated to the persistence of hypertension.
Results
Our cohort consisted of 136 women. The mean follow-up period was 3.7 years. Thirty two women (23.53%) remained hypertensive. This represented an incidence rate of 2.85% per year. Old age (≥ 40 years), housewife occupation, multigravidity (> 4), onset of preeclampsia before 34 weeks' gestation, obesity and the presence of hypertension in siblings were factors independently associated to persistent hypertension.
Conclusion
Many women affected by preeclampsia remain hypertensive after pregnancy. It is important to provide adequate follow-up for this patients in order to intervene on the factors leading to this outcome.
Keywords: Associated factors, chronic hypertension, preeclampsia, Yaoundé, Cameroon
Introduction
Hypertension is defined by the World Health Organization (WHO) as a systolic blood pressure more or equal to 140 mmHg and/or a diastolic blood pressure more or equal to 90 mmHg [1]. It complicates about 10% of all pregnancies and represent the first medical problem according to the American College of Obstetricians and Gynecologists. Hypertensive diseases in pregnancy are divided in 4 categories: chronic hypertension, gestational hypertension, preeclampsia (PE) and chronic hypertension with superimposed PE [2]. Chronic hypertension is defined as hypertension that is diagnosed before pregnancy or before 20 weeks of gestation. Hypertension that is first diagnosed after 20 weeks' gestation and persists for greater than 12 weeks postpartum is also considered chronic hypertension. Gestational hypertension is defined as hypertension that develops in pregnancy after 20 weeks' gestation and resolves before 12 weeks postpartum in the absence of proteinuria (<300mg of protein in 24 h). PE is a syndrome and is typically characterized as new-onset hypertension and proteinuria (>300mg of protein in 24 h) diagnosed in pregnancy often after 20 weeks' gestation [3]. However, according to the latest definitions, other findings such as new-onset thrombocytopenia, renal impairment, neurological complications, liver involvement and fetal growth restriction may substitute to new-onset proteinuria. PE can be classified either as early-onset (before 34 weeks' gestation) or late-onset (at or after 34 weeks' gestation) [4]. Hypertensive diseases in pregnancy are one of the three leading causes of maternal death in the world together with hemorrhage and sepsis/infection. They cause approximately 14% of all maternal deaths worldwide and are more frequent in low and middle income countries where their incidence keeps rising [5]. Thus, in Cameroon Tebeu et al found 17.5% of maternal deaths related to hypertensive diseases in pregnancy in 2007 while Foumane et al found 22.4% in 2010 [6, 7]. These hypertensive diseases in pregnancy, especially PE, make the women affected to be more vulnerable to future cardiovascular disease. After a PE, there may be metabolic alterations causing endothelial dysfunction, sympathetic hyperactivity, peripheral vascular and renovascular resistance, insulin resistance, hyperlipidemia and obesity. All these can in turn lead to often silent diseases such as diabetes, kidney injury and hypertension [8]. The latter is the main risk factor of stroke and coronary artery disease, which are currently the leading causes of death in the World [9]. Many studies showed that women who had PE are at least twice as likely to have ischemic heart disease and stroke compared to those who had normotensive pregnancies and this risks seem to be mediated by a greater risk of future chronic hypertension after PE [4, 10-12]. It therefore appeared necessary to us to identify the factors associated to the occurrence of chronic hypertension after PE on which we may act in order to reduce this future cardiovascular risk in our context where two thirds of cardiovascular disease mortality is registered.
Methods
Type of study: we carried out a retrospective cohort study over an 8-year period from January 1st 2009 to January 1st 2016 in the obstetrics and gynecology department of the Yaoundé University Teaching Hospital.
Study population: all women who had PE during the study period were included in the study. The minimal follow-up period after pregnancy was 12 months. We excluded all women who had superimposed PE from the study.
Data collection: sociodemographic data and past medical history were collected from patients' medical records. We performed a physical examination of each participant including the measurement of weight, height and that of blood pressure with an automated blood pressure machine. The body mass index (BMI) was calculated from weight and height according to the formula BMI = WEIGHT/(HEIGHT)2.
Statistical analysis: we used the software SPSS version 20.0. Student's t-test was used for comparison of means. The Chi-square test and multivariable logistic regression were used to measure the association between categorical variables. A p-value less than 0.05 was considered statistically significant.
Ethical considerations: the study was approved by the Institutional Ethics Committee of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I. A research authorization was obtained from the administration of the Yaoundé University Teaching Hospital and each participant gave informed consent before being included in the study.
Results
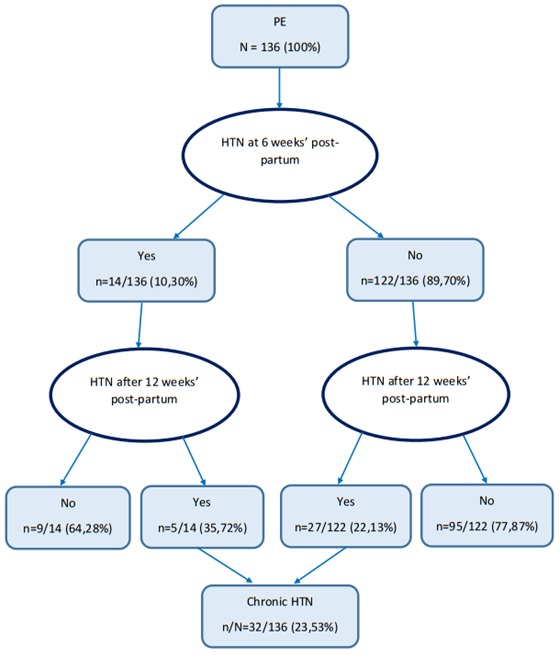
Our cohort included 140 women who had preeclampsia. Four of them were excluded from the study because they had superimposed preeclampsia. The mean follow-up period was 3.7 years. Thirty two women had persistent hypertension at the end of our study, which gave us a prevalence of 23.53% and an incidence rate of 2.85% per year. Table 1 show the baseline characteristics of our study population. We can see that the mean age, weight, BMI and gravidity were significantly greater for women who remains hypertensive. On the other hand, the onset of PE was significantly earlier in these last. Figure 1 shows blood pressures evolution after delivery. We see that of the thirty two women who remained hypertensive, five had persistent hypertension since delivery while the other first of all had a normalization of their blood pressure which later on raised up. After adjustment for confounders using multiple logistic regression (Table 2), the factors found to be associated to persistent hypertension were: age ≥ 40 years (OR = 20.7 (1.1 - 390.0); p = 0.043), housewife profession (OR = 21,.8 (3,4- 138.3); p = 0.001), gravidity > 4 (OR = 7.9 (1.0 - 59.1); p = 0.044), onset of PE before 34 weeks' gestation (OR = 9.3 (2.1 ' 42.0); p = 0.004), presence of hypertension in siblings (OR = 6.7 (1.0 - 44.2); p = 0.047) and obesity (OR = 16.5 (2.3 - 120.6); p = 0.006).
Table 1.
Baseline characteristics of the study population
| NormotensiveM ± σ | HypertensiveM ± σ | p | |
|---|---|---|---|
| N | 104 | 32 | |
| Age (years) | 31 ± 7 | 37 ± 6 | 0.000 |
| Weight (kg) | 67,34 ± 8,98 | 73,04 ± 10,39 | 0.003 |
| Height (cm) | 161 ± 4 | 160 ± 4 | 0.111 |
| BMI (kg/m2) | 25,93 ± 3,19 | 28,78 ± 4,03 | 0.000 |
| HR (bpm) | 77 ± 5 | 76 ± 6 | 0.409 |
| Gravidity | 3 ± 2 | 4 ± 2 | 0.000 |
| Gestational age (weeks) | 36 ± 3 | 33 ± 3 | 0.000 |
BMI: body mass index HR: heart rate M±σ: mean ± standard deviation
Figure 1.
Evolution of blood pressure after pregnancy
Table 2.
Factors independently associated to progression to chronic hypertension
| Normotensiven (%) | Hypertensiven (%) | ORa (95% CI) | Pa | |
|---|---|---|---|---|
| N (%) | 104 (100) | 32 (100) | ||
| Age (years) | ||||
| ≥ 40 | 11 (10,6) | 8 (25,0) | 20,7 (1,1 – 390,0) | 0.043 |
| < 40 | 93 (89,4) | 24 (75,0) | ||
| Profession | ||||
| Housewife | 14 (13,5) | 14 (43,8) | 21,8 (3,4 – 138,3) | 0,001 |
| Others | 90 (86,5) | 18 (56,2) | ||
| HTN in siblings | ||||
| Yes | 5 (4,8) | 9 (28,1) | 6,7 (1,0 – 44,2) | 0,047 |
| No | 99 (93,3) | 23 (71,9) | ||
| Gestational age | ||||
| < 34 | 25 (24,0) | 20 (62,5) | 9,3 (2,1 – 42,0) | 0,004 |
| ≥ 34 | 79 (76,0) | 12 (37,5) | ||
| Gravidity | ||||
| > 4 | 12 (11,5) | 17 (53,1) | 7,9 (1,0 – 59,1) | 0,044 |
| ≤ 4 | 92 (88,5) | 15 (46,9) | ||
| Obesity | ||||
| Yes | 7 (6,7) | 13 (40,6) | 16,5 (2,3 – 120,6) | 0,006 |
| No | 97 (93,3) | 19 (59,4) |
N: number %: percentage HTN: hypertension OR: odds ratio
Discussion
The aim of our study was to determine the prevalence, incidence and factors associated to the progression of PE to chronic hypertension. Thirty two women who had PE remained hypertensive at follow-up, giving us a prevalence of 23.5% in our study population. This finding is higher than the 14.8% found by Sibai et al in the United States of America and the 15.5% found by Festa et al. in Italy [13, 14]. This differences can be explained firstly by the differences in our study populations. Sibai et al included only primigravid women their study, but we know that the risk of PE and subsequent risk of chronic hypertension increases with parity especially when the previous pregnancies where preeclamptic. Festa et al. on their side included all women who had hypertensive disorders in pregnancy (gestational hypertension, chronic hypertension and preeclampsia). This may have reduce the prevalence of chronic hypertension after pregnancy which is mainly secondary to PE alone. Secondly, our study was done on an entirely black population which is more likely to develop hypertensive diseases in pregnancy and chronic hypertension. In this study housewife occupation was found to be independently associated to the progression to chronic hypertension. These women had significantly lower education levels and income (p< 0.05) and therefore low socioeconomic status. Silva et al. concluded in 2008 on the Generation R study that low maternal socioeconomic status is a strong risk factor for PE and that only a small part of this association can be explained by the mediating effects of established risk factors for PE [15]. So, higher risk of PE also leads to higher risk of chronic hypertension after pregnancy.
We found an association between the number of pregnancies (>4) and the risk of chronic hypertension. This can be explained by the fact that women who had a preeclamptic pregnancy are at higher risk of having PE in subsequent pregnancies. In addition in our study, women who had more than 4 pregnancies were more housewives (p<0.05). Low socioeconomic study may therefore be a mediating factor in this association. Women who had early onset PE had a 9 fold increased risk of progression to chronic hypertension. This result is consistent with those of Sibai et al. in the United States of America and Hwang et al in South Korea who also found early onset of PE as a risk factor of chronic hypertension [13, 16]. We assessed the link between family history of hypertension and the risk of future chronic hypertension after PE and found that the presence of hypertension in the siblings of the affected woman was independently associated to progression to chronic hypertension. The presence of a family history of hypertension especially in first degree relative is a well-known risk factor of chronic hypertension and once more points the fact that a genetic component is implicated in the pathogenesis of hypertensive diseases in pregnancy and primary hypertension. We also found that obese women who had PE were more prone to chronic hypertension. This result corroborates those of Hwang et al. in South Korea, Timpka et al. in the United States of America and Festa et al. in Italy who reached the same conclusion [14, 16, 17]. It can be justified firstly by the fact that obesity is an independent risk factor of PE and secondly by the fact that obesity is also associated to the risk of chronic hypertension. This results highlights the necessity to create strategies to specifically follow-up and take care of women who had PE and who have these risk factors of progression to chronic hypertension.
Conclusion
The occurrence of chronic hypertension following PE is common in Cameroon. Several clinical and sociodemographic and economic factors are linked to this risk namely age, high gravidity, obesity, early onset PE, history of hypertension in siblings and housewife occupation. It seem therefore important to us to put in place prevention strategies for affected women.
What is known about this topic
Women who had preeclampsia have increased risk of progression to chronic hypertension and cardiovascular disease after pregnancy.
What this study adds
In Cameroonian women who had preeclampsia, old age, housewife occupation, history of hypertension in siblings, early onset PE, high gravidity and obesity are independent factor linked to the risk of progression to chronic hypertension.
Competing interests
The authors declare no competing interests.
Acknowledgements
Our gratitude goes to the staff of the Obstetrics and Gynecology Department of the Yaoundé University Teaching Hospital and the Faculty of Medicine and Biomedical Sciences.
Authors’ contributions
Sylvie Ndongo Amougou, Simon Maginot Mintya'a Mbita and Pierre-Marie Tebeu designed the study protocol. Simon Maginot Mintya'a Mbita assured data acquisition. Dieudonne Danwe and Simon Maginot Mintya'a Mbita did statistical analysis, interpretation and drafted the manuscript. Pierre-Marie Tebeu and Sylvie Ndongo Amougou revised the article and provided general supervision. All authors approved the final version of the manuscript.
References
- 1.WHO . A global brief on hypertension. WHO; 2013. Hypertension: the basic facts; pp. 17–21. [Google Scholar]
- 2.Jadhav V, Waydande S. A study of prevalence and clinical profile of the patients having hypertensive disorders of pregnancy. Int J Recent Trends Sci Technol. 2016;21(1):73–6. [Google Scholar]
- 3.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol. 2013;25(2):124–32. doi: 10.1097/GCO.0b013e32835e0ef5. [DOI] [PubMed] [Google Scholar]
- 4.Paauw ND, Luijken K, Franx A, Verhaar MC, Lely AT. Long-term renal and cardiovascular risk after preeclampsia: towards screening and prevention. Clin Sci. 2016;130(4):239–46. doi: 10.1042/CS20150567. [DOI] [PubMed] [Google Scholar]
- 5.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 6.Tebeu PM, Ngassa P, Kouam L, Major AL, Fomulu JN. Maternal mortality in Maroua Provincial Hospital, Cameroon (2003-2005) West Indian Med J. 2007;56(6):502–7. [PubMed] [Google Scholar]
- 7.Foumane P, Dohbit J, Ngo Um Meka E, Nkada M, Ze Minkande J, Mboudou E. Étiologies de la Mortalité Maternelle à l'Hôpital Gynéco-Obstétrique et Pédiatrique de Yaoundé: Une Série de 58 Décès. Health Sci Dis. 2015;16(3):1–5. [Google Scholar]
- 8.Williams D. Long-Term Complications of Preeclampsia. Semin Nephrol. 2011;31(1):111–22. doi: 10.1016/j.semnephrol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 9.OMS . Maladies cardiovasculaires. WHO; 2015. Accessed Sept 22 2017. [Google Scholar]
- 10.Leslie MS, Briggs LA. Preeclampsia and the Risk of Future Vascular Disease and Mortality: a review. J Midwifery Womens Health. 2016;61(3):315–24. doi: 10.1111/jmwh.12469. [DOI] [PubMed] [Google Scholar]
- 11.Mounier-Vehier C, Madika AL, Boudghène-Stambouli F, Ledieu G, Delsart P, Tsatsaris V. Hypertensions artérielles de la grossesse et devenir maternel. Presse Médicale. 2016;45(7-8):659–66. doi: 10.1016/j.lpm.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, et al. Hypertensive Disorders of Pregnancy and Maternal Cardiovascular Disease Risk Factor Development: an observational cohort study. Ann Intern Med. 2018;169(4):224. doi: 10.7326/M17-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155(5):1011–6. doi: 10.1016/0002-9378(86)90336-4. [DOI] [PubMed] [Google Scholar]
- 14.Festa C, Mattei L, Bitterman O, Pintaudi B, Framarino dei Malatesta M, Bianchi P, et al. Hypertensive disorders during pregnancy and 3 years after delivery in women with gestational hyperglycemia. J Endocrinol Invest. 2018;41(9):1075–82. doi: 10.1007/s40618-018-0833-0. [DOI] [PubMed] [Google Scholar]
- 15.Silva LM, Coolman M, Steegers EA, Jaddoe VW, Moll HA, Hofman A, et al. Low socioeconomic status is a risk factor for preeclampsia: the generation R study. J Hypertens. 2008;26(6):1200–8. doi: 10.1097/HJH.0b013e3282fcc36e. [DOI] [PubMed] [Google Scholar]
- 16.Hwang J, Park SJ, Oh S, Chang SA, Lee SC, Park SW, et al. The Risk Factors That Predict Chronic Hypertension After Delivery in Women With a History of Hypertensive Disorders of Pregnancy. Medicine (Baltimore) 2015;94(42):e1747. doi: 10.1097/MD.0000000000001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timpka S, Stuart JJ, Tanz LJ, Rimm EB, Franks PW, Rich-Edwards JW. Lifestyle in progression from hypertensive disorders of pregnancy to chronic hypertension in Nurses' Health Study II: observational cohort study. BMJ. 2017;358:j3024. doi: 10.1136/bmj.j3024. [DOI] [PMC free article] [PubMed] [Google Scholar]


